1. Introduction
Saline water poses a significant challenge to agricultural sustainability in Tunisia, a country characterized by arid and semi-arid climates. With limited freshwater resources, Tunisia heavily relies on alternative water sources for irrigation, including saline water (Hill and Woodland, 2003). Saline water, defined as water containing high concentrations of dissolved salts, presents both opportunities and challenges for agriculture in Tunisia. The use of saline water for irrigation is widespread in Tunisia, driven by the scarcity of freshwater resources and the increasing demands of agriculture [
1]. Saline water sources include groundwater with elevated levels of dissolved salts, as well as treated wastewater from various sources [
2]. In some regions, desalination plants have also been established to convert seawater into usable irrigation water [
3]. Saline water requires careful management to avoid negative impacts on soil quality and crop productivity [
4].
One of the main challenges associated with saline water irrigation is soil salinization. When saline water is applied to the soil, salts accumulate over time, increasing soil salinity levels. High soil salinity can have detrimental effects on soil structure, nutrient availability, and plant growth [
5]. It can also contribute to soil erosion and degradation, further exacerbating land degradation issues in Tunisia [
6]. In addition to soil salinization, saline water irrigation can directly impact crop health and productivity. Many crops are sensitive to high salt concentrations in the soil and irrigation water. Excessive salt levels can cause physiological stress in plants, leading to reduced growth, yield losses, and even crop failure [
7]. Certain crops, such as tomatoes, are particularly sensitive to salinity stress, making them susceptible to yield reductions when irrigated with saline water [
8]. Despite these drawbacks, saline water irrigation also has some benefits for agriculture in Tunisia. Saline water sources are often more readily available and less expensive than freshwater sources, providing an alternative water supply for irrigation during periods of water scarcity [
9].
Modeling irrigation with saline water is crucial for understanding its effects and implementing effective mitigation strategies. Modeling can be performed using hydrological models such as Hydrus-1D [
10]. These models simulate water movement and solute transport in soils, allowing researchers and farmers to predict how saline water affects soil salinity, crop growth, and yields over time [
11]. Using different data for soil hydraulic properties, irrigation practices, and water quality, these models can simulate various scenarios and assess the effectiveness of different management strategies [
12]. Modeling can also aid in identifying the most suitable crops for water irrigation with a given salinity. Indeed, some crops have higher salt tolerance than others, and modeling can help determine which crops are best suited for cultivation in saline environments. Additionally, models can evaluate the impact of different irrigation strategies, such as drip irrigation or leaching, on soil salinity levels and crop productivity [
13].
The objectives of this study are: (i) to integrate the effects of soil matric and osmotic potentials into modeling water and salts transport, (ii) to study the effects of saline water irrigation on tomatoes cultivated under semi-arid conditions to provide a framework for understanding the complex interactions between soil, water, and crops in saline environments, and (iii) to evaluate the effects of an increase in mean air temperature on soil water and salts dynamics and tomatoes root water uptake and ultimately yield. Haut du formulaire
2. Materials and Methods
2.1. Experiment Design and Measurements
The experimental trials were conducted on a land parcel (36°51'36.791''N, 10°11'36.795''E) in the Ariana region, a semi-arid area in north Tunisia, during the year 2018. The soil is loamy clay. The study focused on a tomato variety, Rio Grande, commonly cultivated in Tunisia. Planting was done on 16/03/2018 in the pot experiment and on 17/04/2018 in the field experiment. A spacing of 25 cm between plants and 50 cm between rows was used in the field experiment. Tomato harvesting occurred on 10/06/2018 in pot cultivation and on 03/08/2018 in field cultivation. The water requirements of the crops were estimated using climate data from the past 10-year period. The average values were used to estimate reference evapotranspiration using the Penman-Monteith formula (FAO-56). For calculating actual evapotranspiration, crop coefficient values for the three growth stages were taken from those proposed by Allen et al. (1998). These values are as follows:
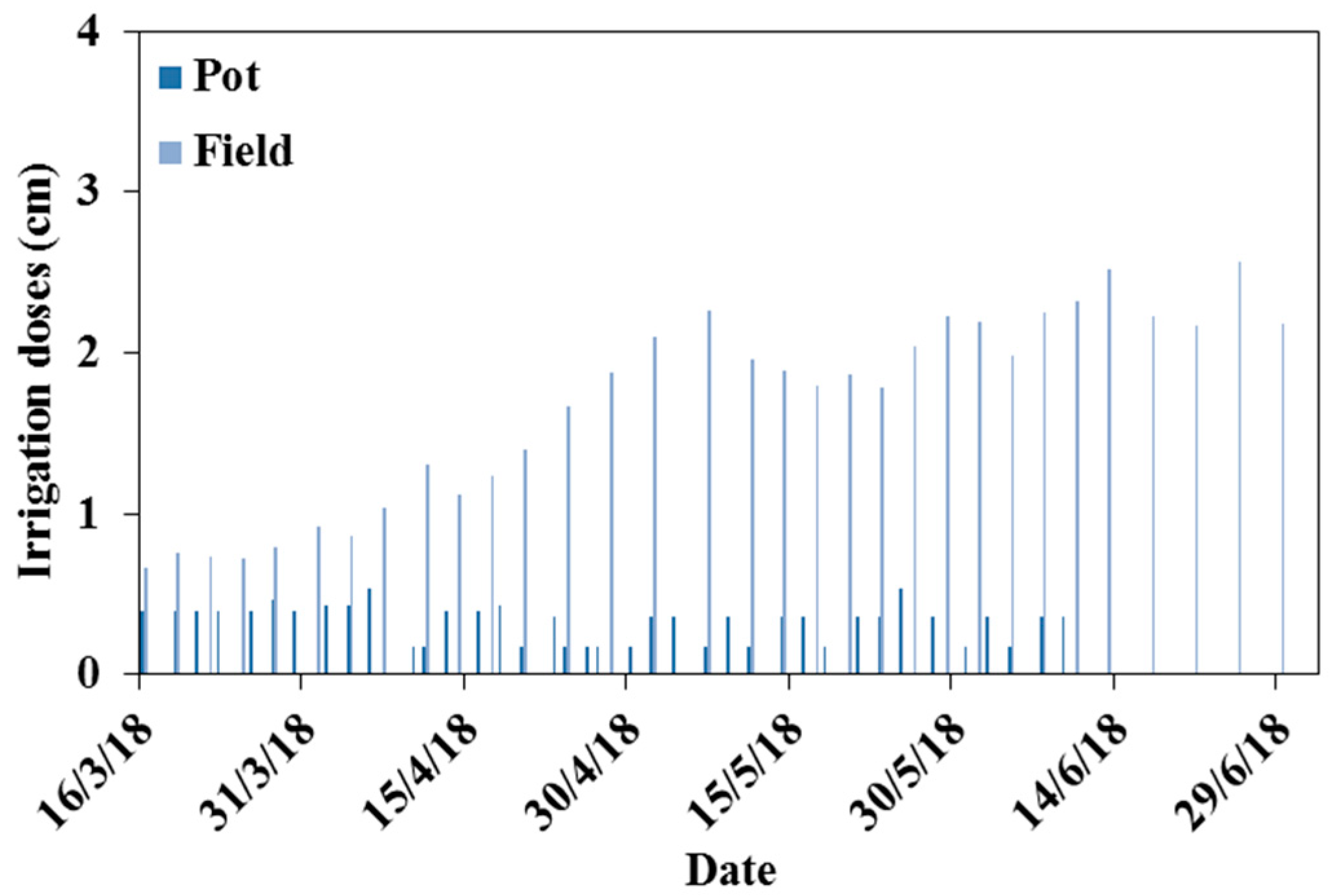
Kc = 0.6 (initial stage), 1.15 (mid-stage), 0.8 (final stage). The CROPWAT 8.0 software (FAO, 1998; FAO, 2009) was utilized to establish the irrigation schedule. Water requirements for tomato cultivation are approximately 720 mm. The irrigation schedule in the pot and field experiments is presented in
Figure 1. Irrigation in the pot experiments was supplied until the soil water content reached field capacity (34%). The amounts of water added were recorded for each pot. Irrigation water provided to the plants had three different qualities: distilled water with a salinity of 0 dS/m, saline water with a salinity of 3.5 dS/m, and saline water with a salinity of 7 dS/m. The soil water content was measured using the gravimetric method, and soil salinity was measured using the saturated paste extract method. Soil samples were taken every ten days until the end of the tomato plant cycle in the pot experiment. In the field experiment, the soil water content was measured on days 1, 30, 60, and 109 after planting every 20 cm down to a depth of 80 cm
.
2.2. Hydrus-1D Model
2.2.1. Theory
Hydrus-1D [
14,
15] uses the Galerkin finite-element method to solve the Richards equation governing water dynamics in the unsaturated zone:
where
h is the water pressure head [L],
θ is the soil volumetric water content [L
3L
−3],
t is time [T],
z is the depth measured from the land surface (positive downward) [L],
S is a sink term [L
3L
−3T
−1], and
K is the soil unsaturated hydraulic conductivity [LT
−1].
Hydrus-1D uses the van Genuchten-Mualem soil-hydraulic functions to represent soil hydraulic properties. The soil water retention [
16] and hydraulic conductivity [
17] functions are as follows:
where
θr is the residual water content [L
3L
−3],
θs is the saturated water content [L
3L
−3],
α [L
−1],
n (n > 1), and
m=1−1/
n are shape parameters,
Se is the effective saturation, and
Ks is the saturated hydraulic conductivity [LT
−1].
Hydrus-1D uses the Galerkin Finite element method to solve the advection-dispersion equations (ADE) governing solute transport:
where
C is the solute concentration of the liquid phase [ML
−3],
D is the dispersion coefficient [L
2T
−1], and
q is Darcy velocity [LT
−1]. When neglecting molecular diffusion, the dispersion coefficient is defined as:
where λ is the soil longitudinal dispersivity [L].
The sink term,
S, represents the volume of water removed from a unit volume of soil per unit time due to plant water uptake. The Feddes model, as described by [
18], was employed to simulate root water uptake. Three stress response function models available in Hydrus-1D are evaluated for the simulation of salt stress: the Additive model (Additive), the Threshold model (T-Model), and the S-shape model (S-Model). The last two models are multiplicative models, combining the Feddes model [
18], accounting for saturation stress, with either the "Threshold and Slope" model of Mass [
19] or the S-shape model of van Genuchten [
20], accounting for salinity stress. The theoretical underpinnings of these models are elaborated in the Hydrus-1D manual, with specific parameter values provided for tomato crops.
2.2.2. Soil Hydraulic Properties and Solute Transport Parameters
The estimation of the soil hydrodynamic parameters, namely the van Genuchten parameters in the water content-pressure head (Eq. 2) and conductivity-saturation (Eq. 3) relationships, was based on soil column evaporation experiments. The procedure is detailed by [
21].
The dispersion coefficient, a crucial parameter in solute transport in unsaturated soils, cannot be measured, and we thus resort to indirect methods. Experiments on soil columns were conducted using PVC tubes with a diameter of 15 cm filled with 10 cm of soil. The columns were slowly saturated from the top with distilled water. A Mariotte bottle was placed at the top of the column to maintain a constant pressure head of about 3 cm and steady-state flow conditions. A pulse (50 ml) of a potassium chloride solution (0.8 M, 8.4 dS/m) was manually applied [
22,
23]. Effluent samples of approximately 100 cm
3 were used to measure soil electrical conductivity and determine the solute breakthrough curve (BTC). The experimental BTC values were fitted using the analytical solution of the ADE using CXTFIT 2.1 software [
24] to estimate the dispersion coefficient.
2.3. Statistical evaluation of modeling results
Simulation results were evaluated graphically and statistically. In the graphical method, the measured and simulated volumetric water contents were plotted as a function of soil depth. The statistical approach involved calculating the root mean square error (RMSE):
where
si are simulated values,
mi are measured values,
is the average observed value, and
n is the number of observations.
3. Results
3.1. Soil Water Content Dynamics
The variations in the soil water content in the pot experiments are presented in
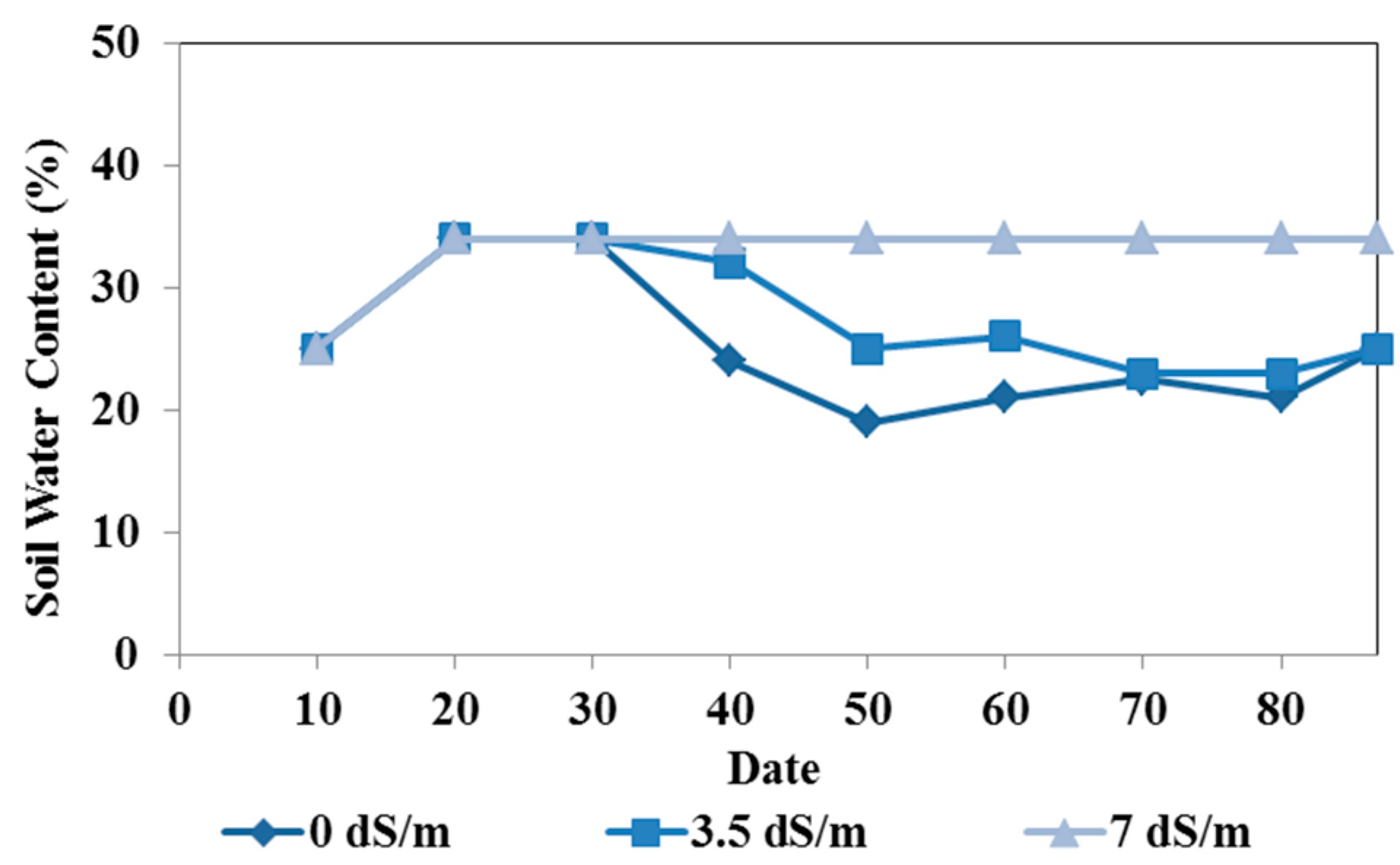
Figure 2. During the first month after the start of irrigation, the water content gradually increased to reach the field capacity (34%) in all three pots irrigated with different water qualities. The water content of the soil irrigated with the saltiest water (7 dS/m) remained at the field capacity. As the soil salinity increased with frequent irrigations, osmotic pressure also increased, preventing root water uptake. Irrigations with the other two water qualities did not block water extraction by the plant roots, and the soil water content decreased over time. The soil irrigated with fresh water (0 dS/m) had the lowest water contents, as the plant roots extracted water without any osmotic potential constraints.
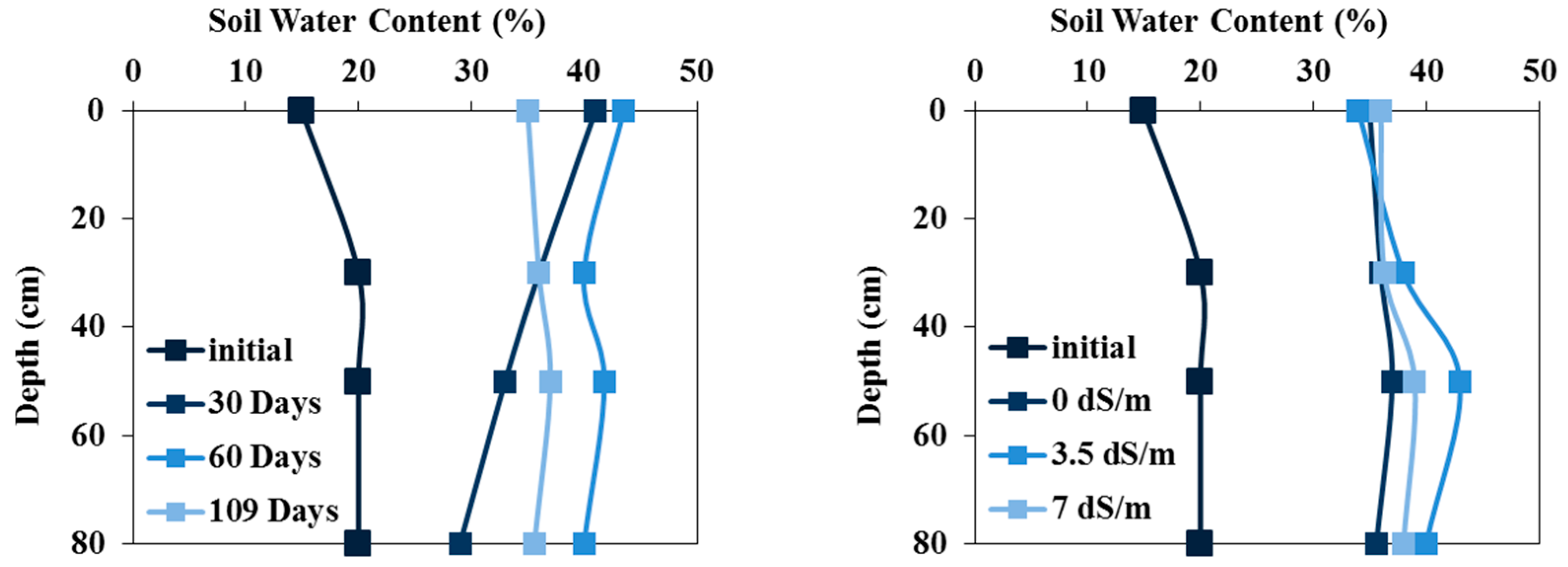
The soil water profiles measured every 30 days in the field experiment with tomatoes irrigated with fresh water are illustrated in
Figure 3a. This figure shows the variations in soil water content without significant osmotic potential effects. Indeed, the soil water content profiles show dynamics reproducing infiltration (wetting) and evaporation (drying) episodes. After 109 irrigation days, water contents exceeded field capacity, indicating that the roots did not undergo water stress.
Figure 3b shows the water content profiles measured after 109 irrigation days in field experiments irrigated with waters of different qualities. The water content in the surface layers is very close to field capacity (34%), while it increases considerably with depth, indicating deep water infiltration. The irrigation strategy based on calculating water needs using climatic parameters from the previous ten years to estimate actual evapotranspiration succeeded in not subjecting the crop to water stress. However, deep percolation shows that crop water needs were overestimated.
3.2. Soil Salinity Dynamics
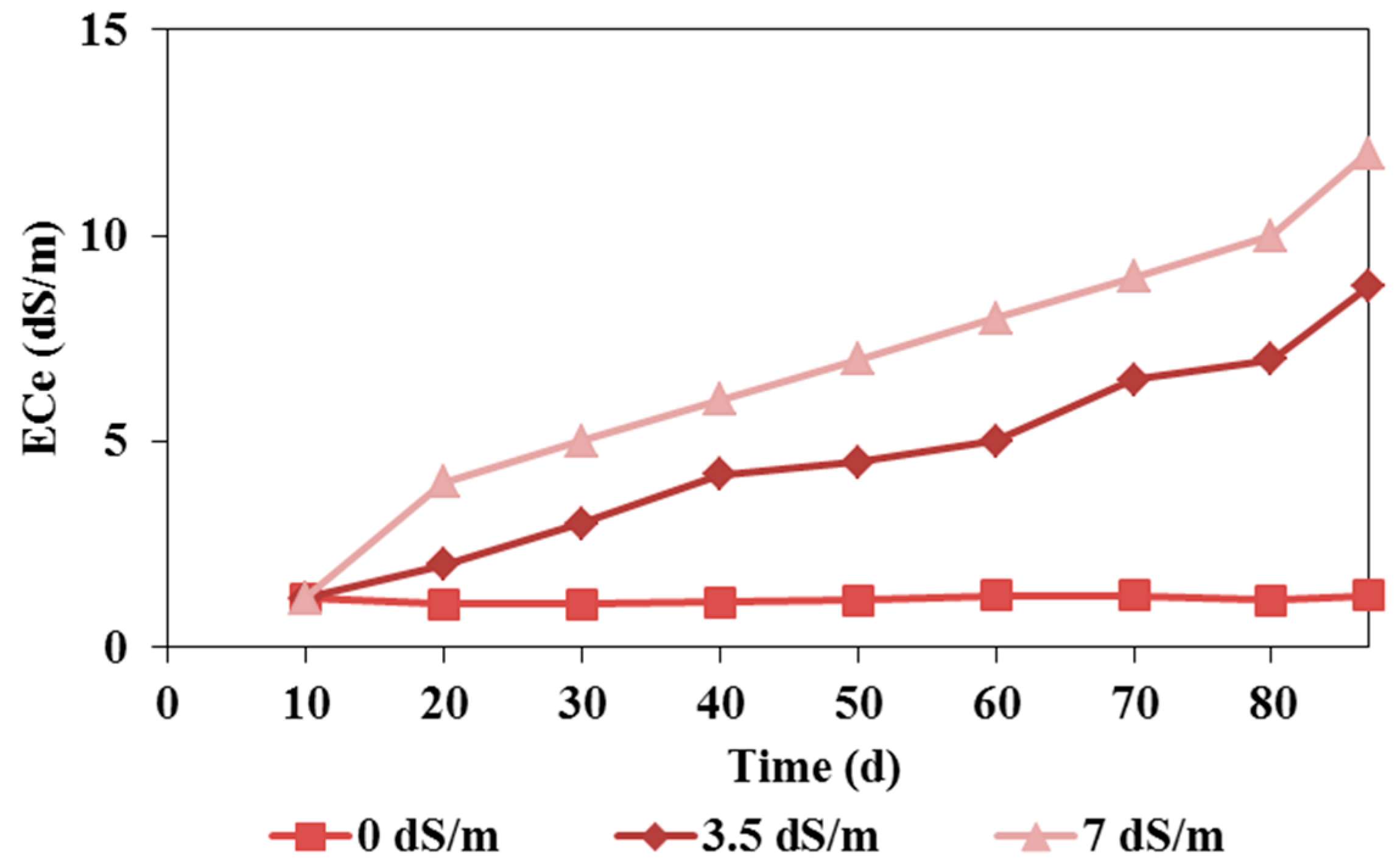
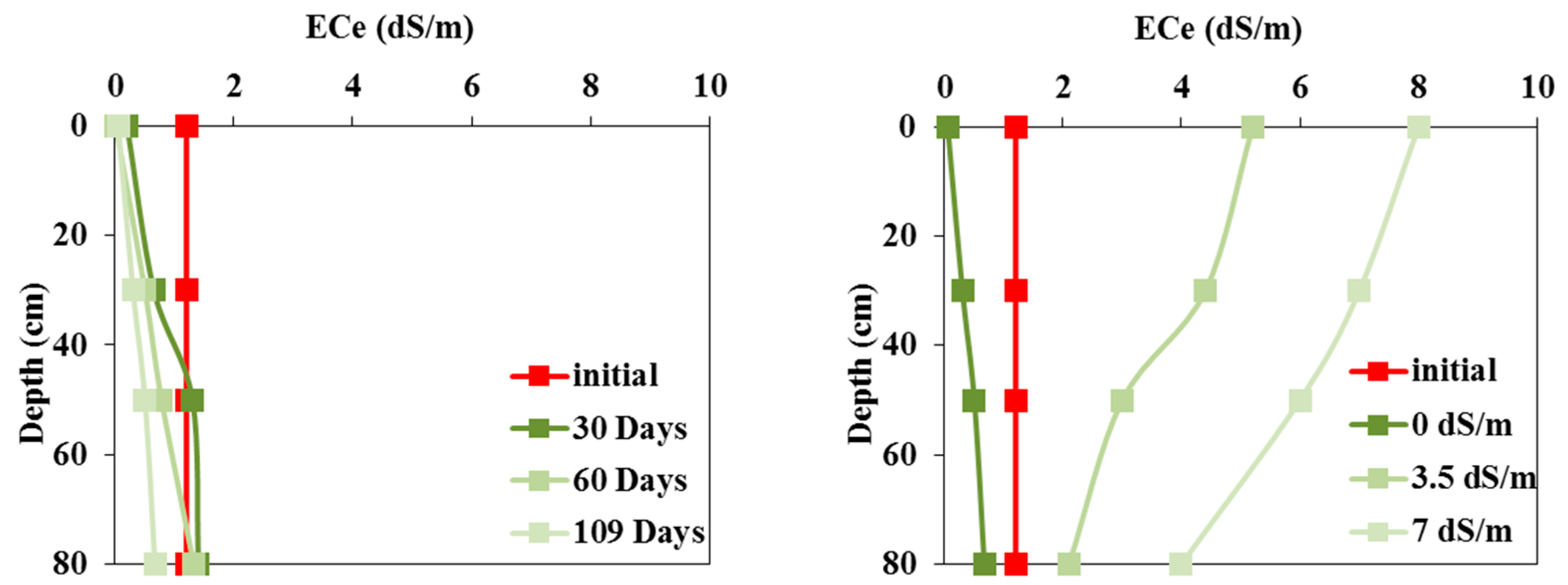
The variations in the average soil salinity in the pot experiments irrigated with waters of different qualities are shown in
Figure 4. This figure shows:
A nearly constant level of salinity in soils irrigated with fresh water,
A gradual increase in soil salinity in soils irrigated with lower-quality waters, reaching 8 dS/m when 3.5 dS/m water was used and 12 dS/m when 7 dS/m water was used.
In the field experiment irrigated with fresh water, the variations in soil salinity profiles measured every thirty days indicated continuous leaching of the initial salinity (
Figure 5a). In contrast, surface salinity significantly increased during irrigations with saline waters of 3.5 dS/m and 7 dS/m, reaching 7 dS/m and 12 dS/m, respectively, at the end of the crop cycle (
Figure 5b). Soil salinity is higher near the surface than at depth, mainly due to evapotranspiration causing salt accumulation near the soil surface.
3.2. Numerical Modeling of Water and Salt Dynamics with Root Water Uptake
3.2.1. Inputs to Hydrus-1D
The soil was considered homogeneous in pot experiments, consisting of a single layer of 30 cm. In the field experiment, water content and salinity measurements were taken every 20 cm down to a depth of 80 cm. Four soil layers (horizons) of 20cm thickness were considered in the simulations. The values of the van Genuchten-Mualem parameters for the four soil layers are presented in
Table 1 . The simulation period lasted 87 days in the pot experiments, with three output dates of 30, 60, and 87 days. On the other hand, the field trial lasted 109 days, and the output dates were 30, 60, and 109 days.
The dispersivity of different soil layers varied around 5 cm, except for the 60-80 cm layer, where it was equal to 3 cm. The distribution coefficient of the adsorption isotherm ranged between 0.1 g/cm
3 and 0.3 g/cm
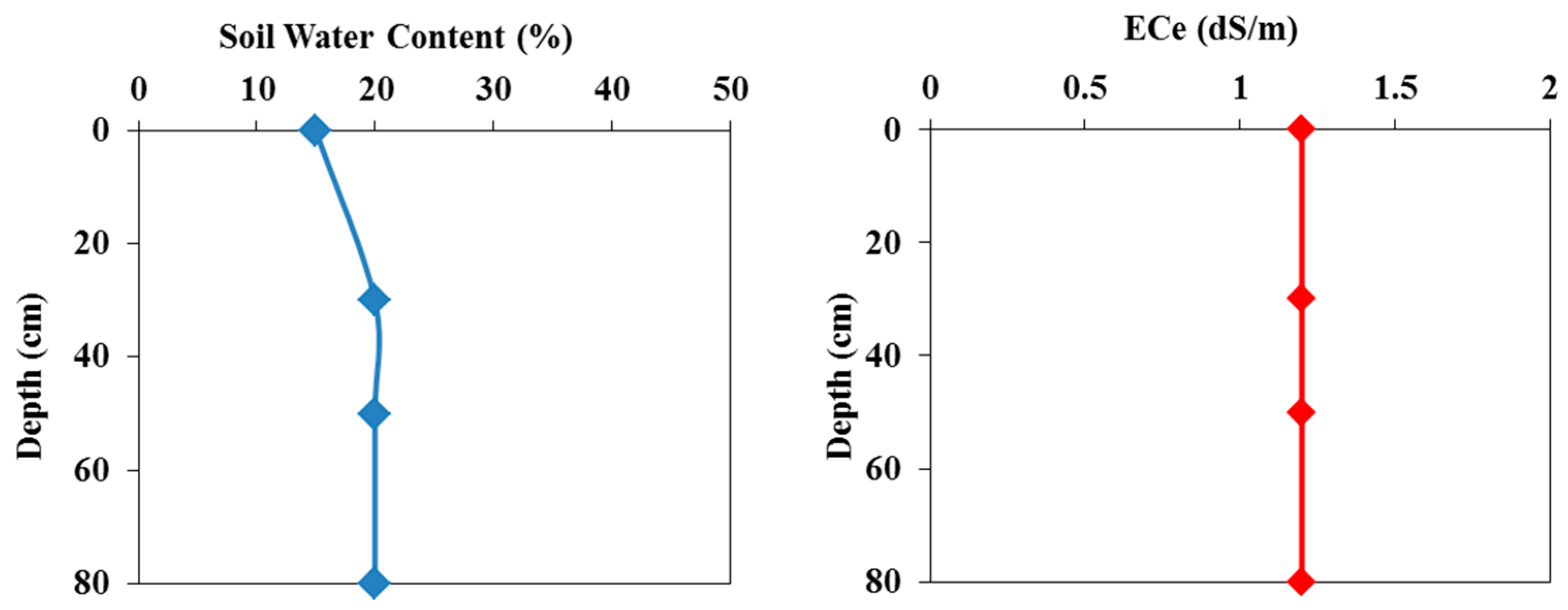
3 for all soil layers. Hydrus-1D includes a database that provides stress response function values for many crops, including tomatoes, which were considered here. The initial soil water contents and soil salinities as a function of depth in the field experiment are shown in
Figure 6. In the pot experiment, the initial water content was 20%, and the initial soil salinity was 1.2 dS/m.
For the simulation of field experiments, we used the rainfall and evapotranspiration data from the National Institute of Meteorology and crop coefficients from [
25].
3.2.2. Simulation Results
The Hydrus-1D model was used to simulate the movement of water and salts in both pot and field soils, considering root water uptake under the combined effects of soil matric and osmotic potentials.
Model Calibration
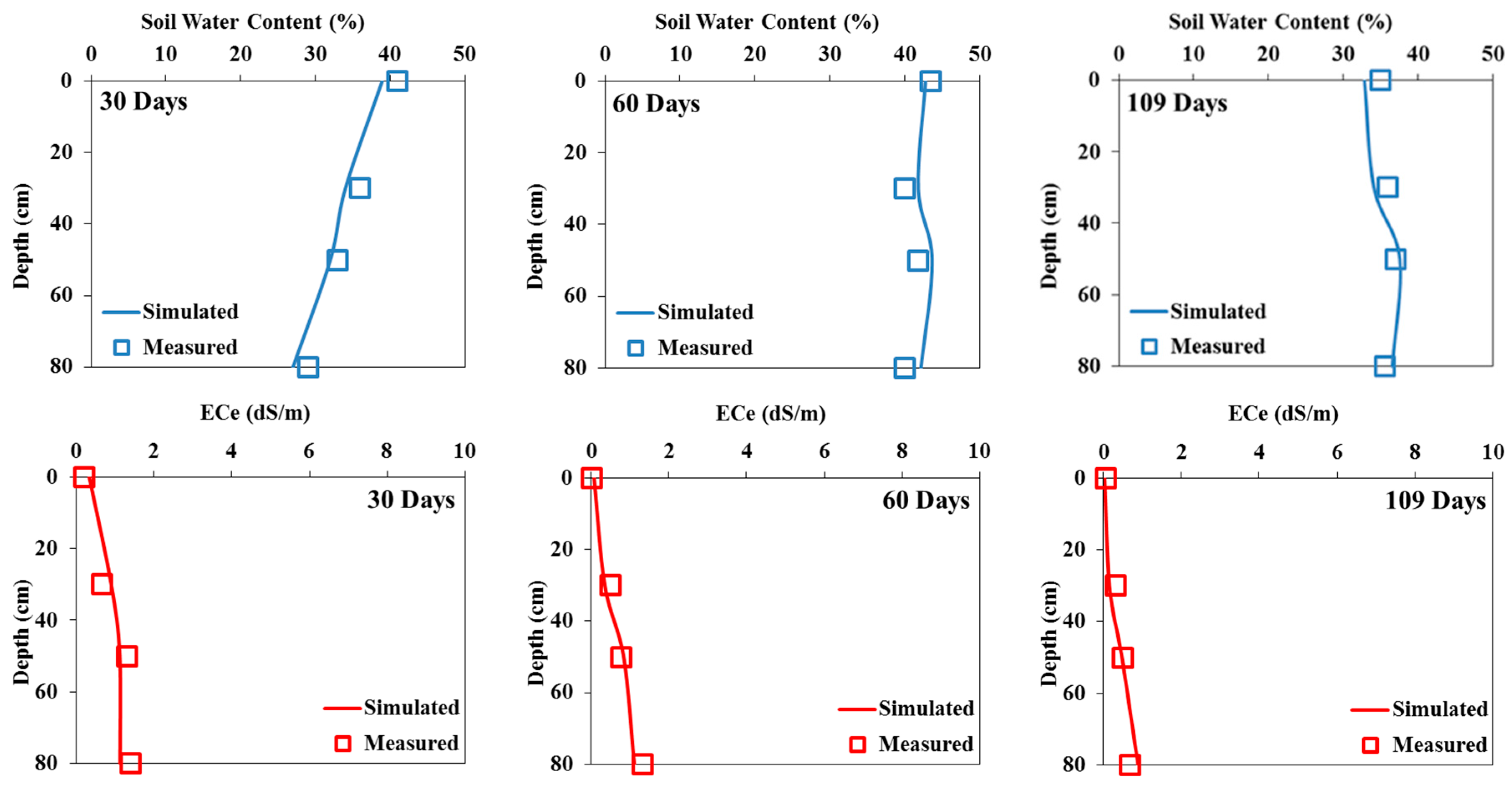
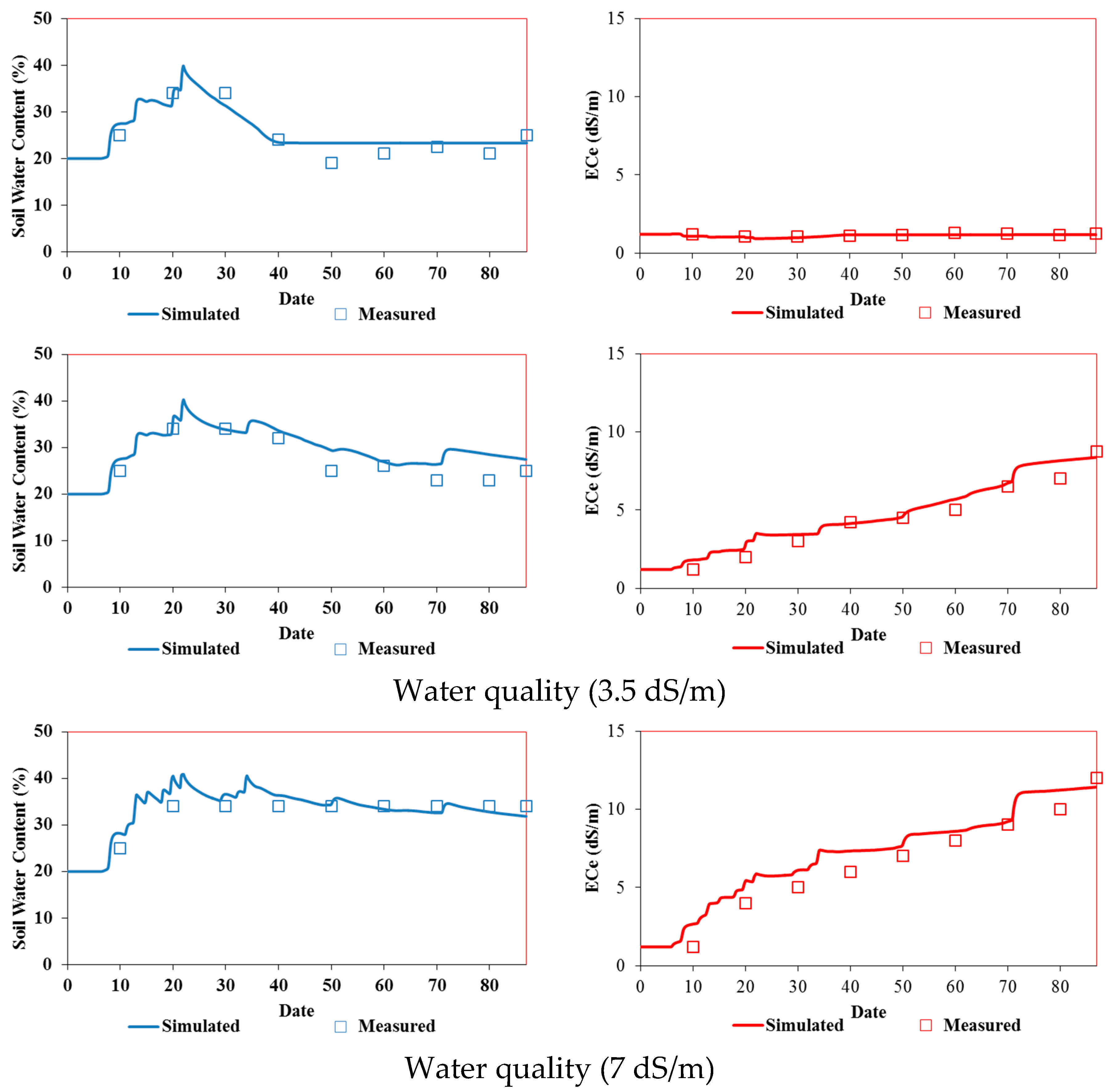
The Hydrus-1D model was calibrated using measured soil water content and salinity profiles in both pots and field experiments irrigated with fresh water (i.e., 0 dS/m). The measured and simulated water contents and salinities for both experiments are presented in
Figure 7,
Figure 8 and
Figure 9. The measured soil water contents are quite close to the simulated values. In the field experiment (
Figure 7 and
Figure 8), the Hydrus-1D model underestimated the measured water contents on day 30 and overestimated them on the other output dates. The model slightly overestimated the volumetric water contents in the pot experiment (
Figure 9).
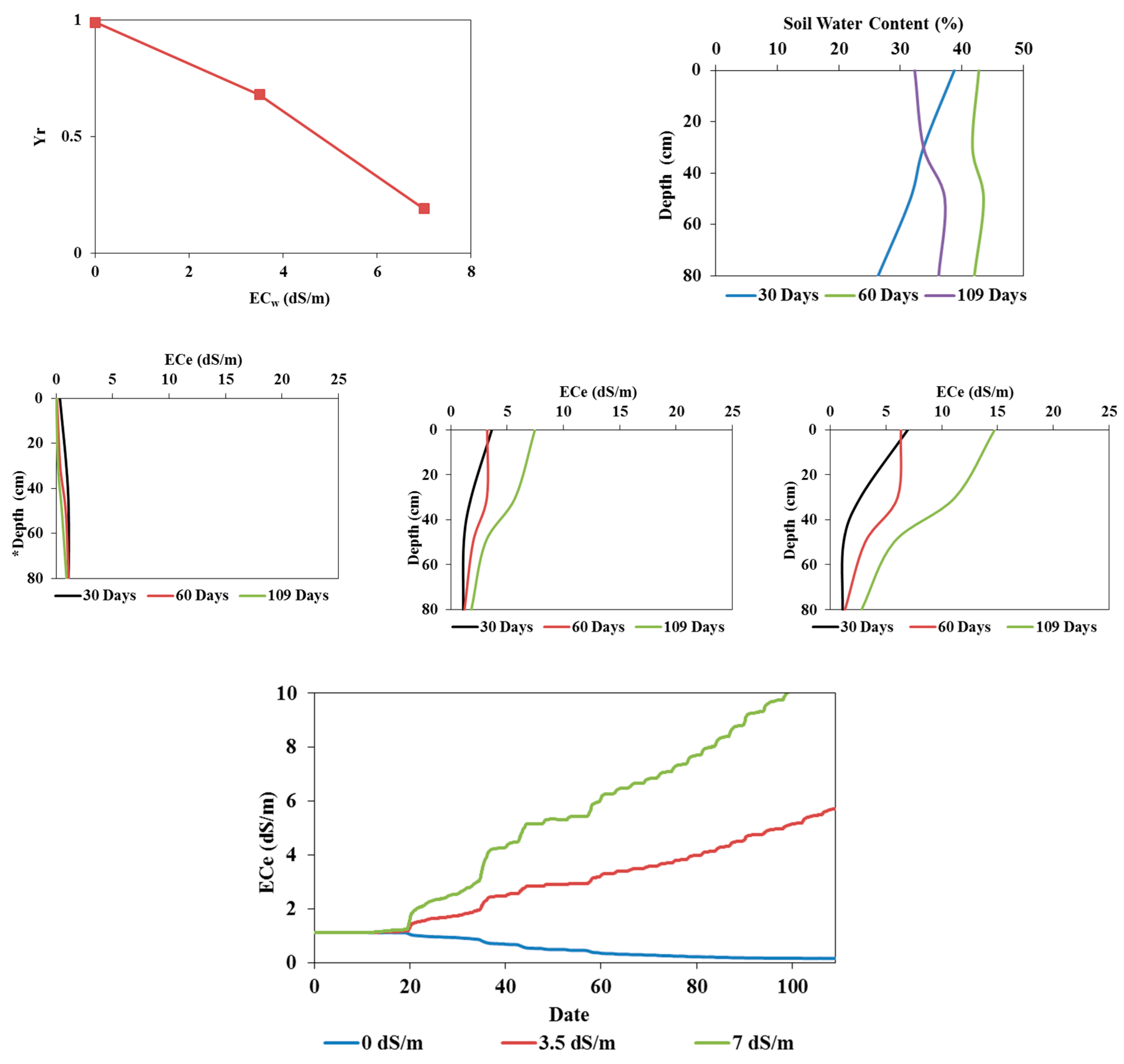
The measured soil salinity profiles during the two pot and field experiments with tomatoes irrigated with saline waters and simulated using the Hydrus-1D model are presented in
Figure 9. Overall, measured and simulated values are similar on all three output dates. The simulated soil electrical conductivities generally overestimated measured values in both experiments.
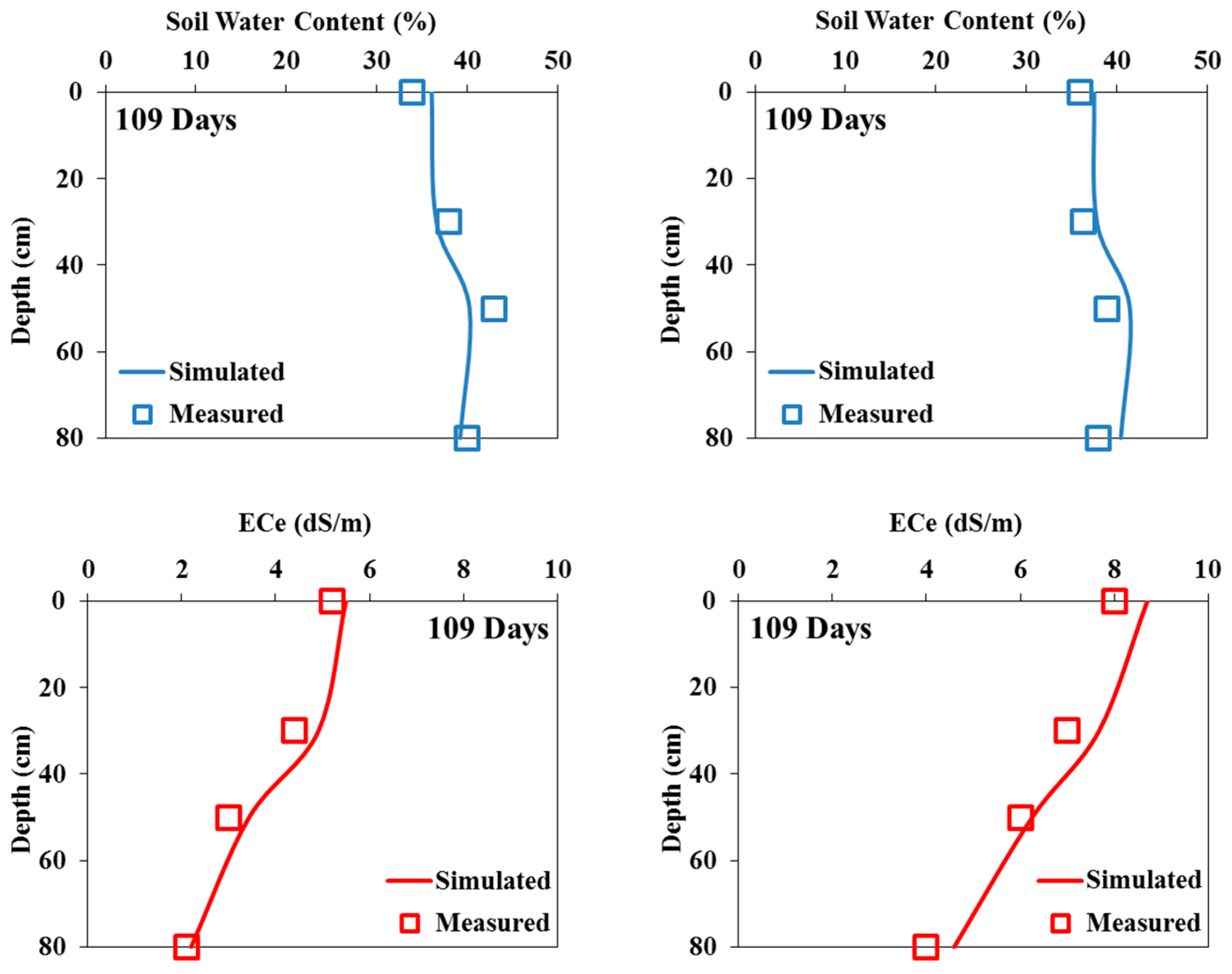
Model Validation
The validation of the Hydrus-1D model was carried out by keeping the same input parameters and only changing the water quality in both cases of this study (pot and field experiments).
Figure 6 and
Figure 7 show that the simulated soil water content profiles agree well with the measured profiles for both pot and field experiments. Indeed, for all the output dates, the simulated profiles are very close to the measured ones.
The model's performance was evaluated qualitatively (
Figure 7,
Figure 8 and
Figure 9) and assessed using statistical evaluations and the RMSE values (
Table 2 and
Table 3). These values are low (<10%) and highlight the model's reliability for simulating water and salt dynamics for both experiments and during the calibration and validation processes.
3.2.3. Crop Yield
The Hydrus-1D model cannot directly estimate the crop yield. However, according to Oster et al. (2012), the relative yield (
Yr) can be estimated as the ratio between actual and potential (or maximum) transpiration, which are both provided in the model's output files.
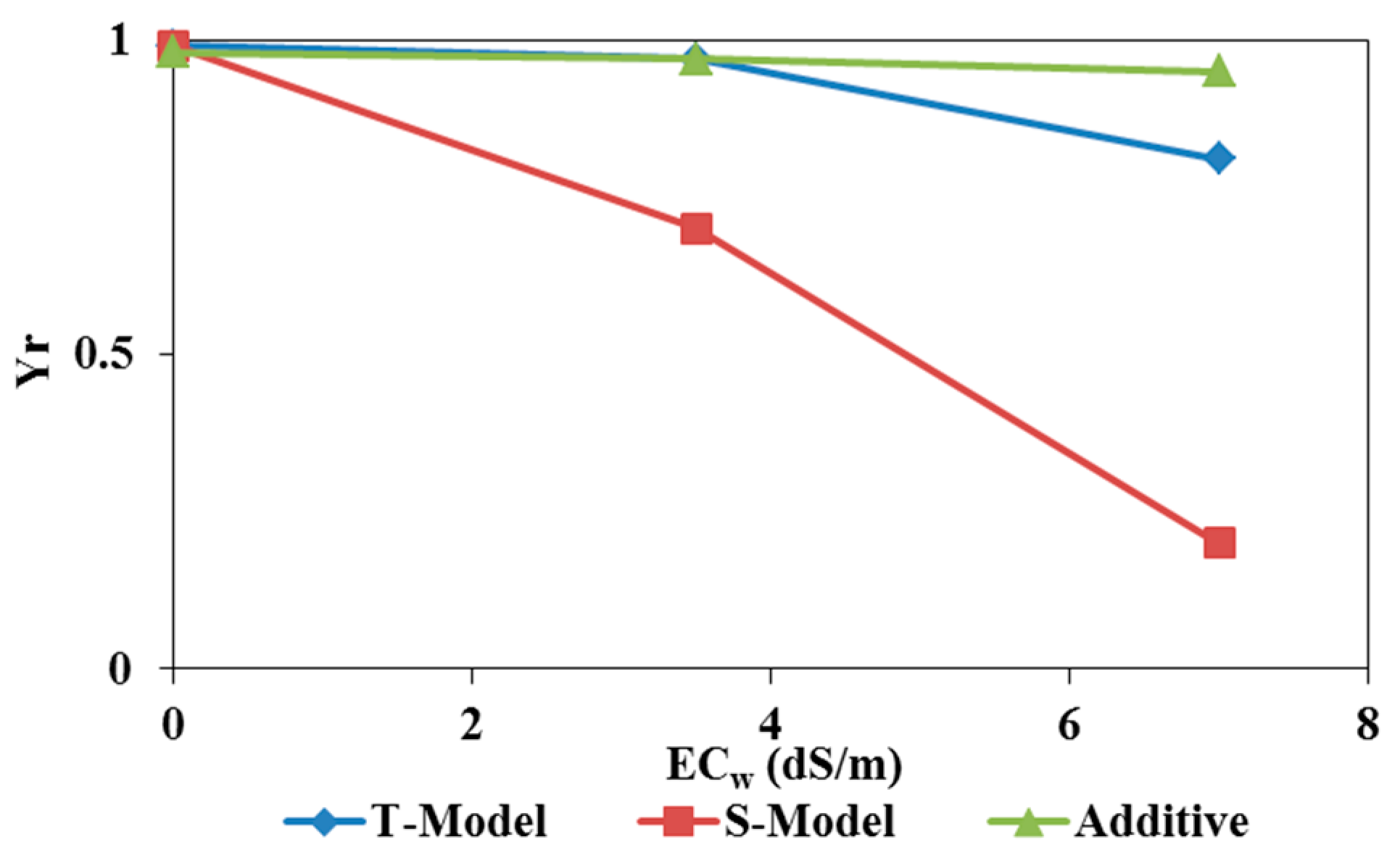
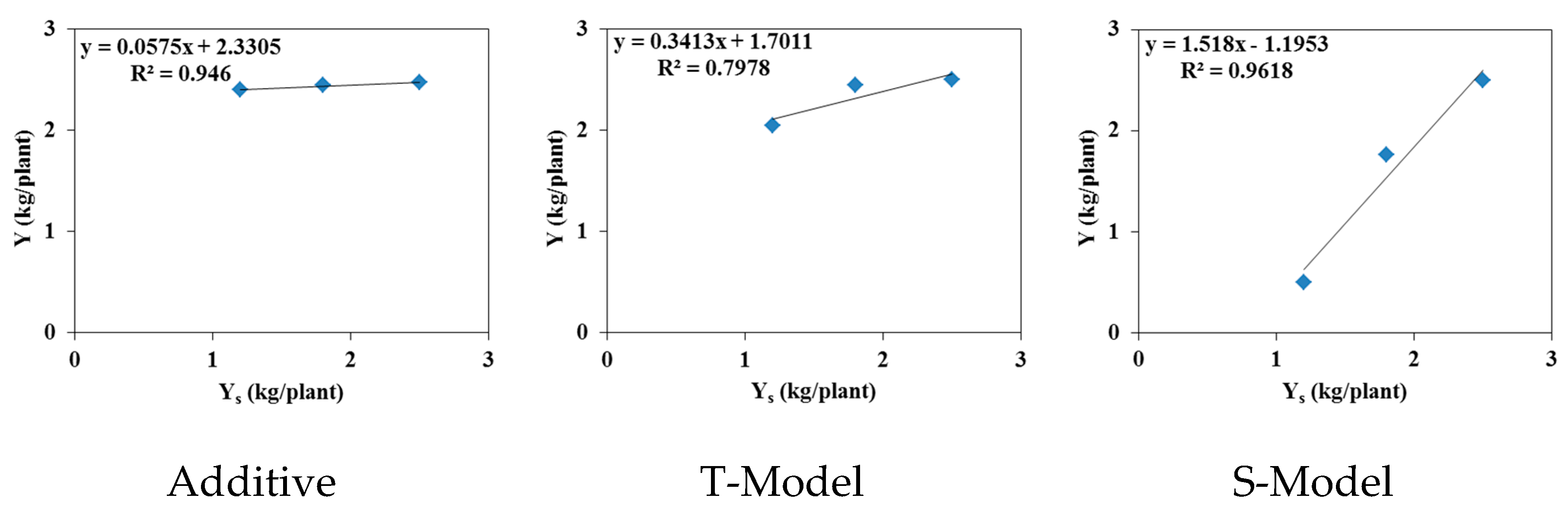
Figure 10 and
Figure 11 show relative yields for tomatoes irrigated with waters of different qualities for both experimental pot and field trials simulated using the three models mentioned above (Additive, T-Model, and S-Model) to represent the effects of osmotic and matric potentials on crop root water uptake. Simulated relative yields decrease with increasing irrigation water salinity (EC
w) for all three stress response functions (
Figure 10). The relative yield is 0.98 in the field experiment with tomatoes irrigated with fresh water (0 dS/m) for all three stress response functions. However, in the pot case,
Yr equals 0.33 for freshwater irrigation. The reduced root development in pots compared to the field accounts for this reduction in relative yield.
The actual yields measured in the two trials with different irrigation water qualities were compared to the simulated yields to identify the most suitable stress response function model for studying the combined effects of osmotic and matric potentials. According to FAO Note 66 (2012), Yr = Y / Ymax, where Y is the actual yield and Ymax is the maximum (or potential) yield. In our experiments, Ymax is calculated for irrigations with fresh water. The Ymax value equals 2.53 kg/plant for the field experiment and 4.16 kg/plant for the pot trial. The values of simulated relative yields were calculated using these values.
Figure 10 and
Figure 11 also present linear correlations between measured and simulated relative yields. The relative yields simulated using the additive model (Feddes) and the multiplicative T-Model (combining the Feddes et al. [
19] model for saturation stress with the threshold and slope model of Mass [
20] for osmotic stress) were least correlated with the measured relative yields in both pot and field trials. The multiplicative S-Model (combining the Feddes model [
19] for saturation stress with the S-shape model of van Genuchten [
21] for osmotic stress) is the only model capable of reproducing the measured relative yields with a correlation coefficient
R² close to 1 for both pot and field experiments.
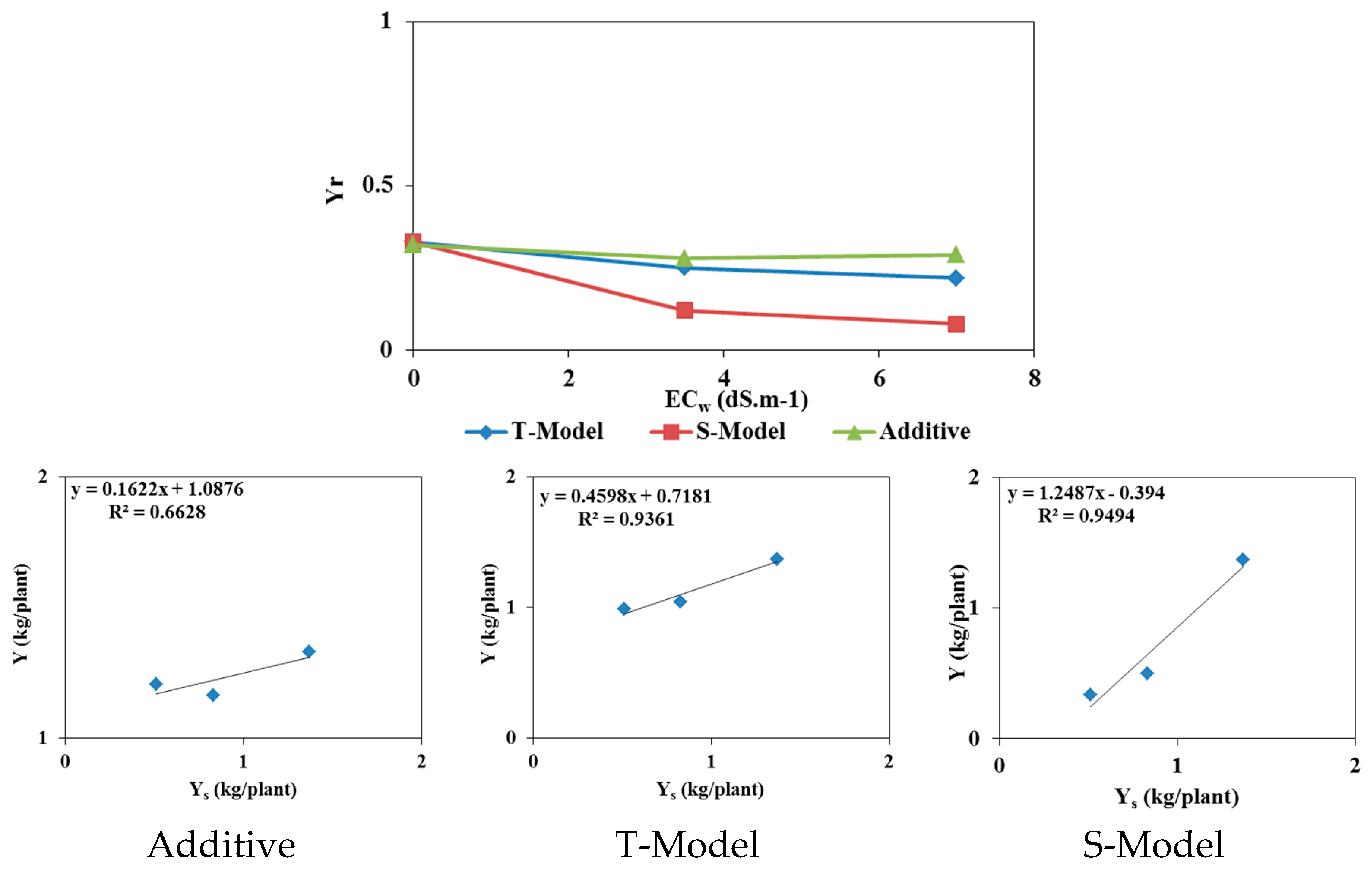
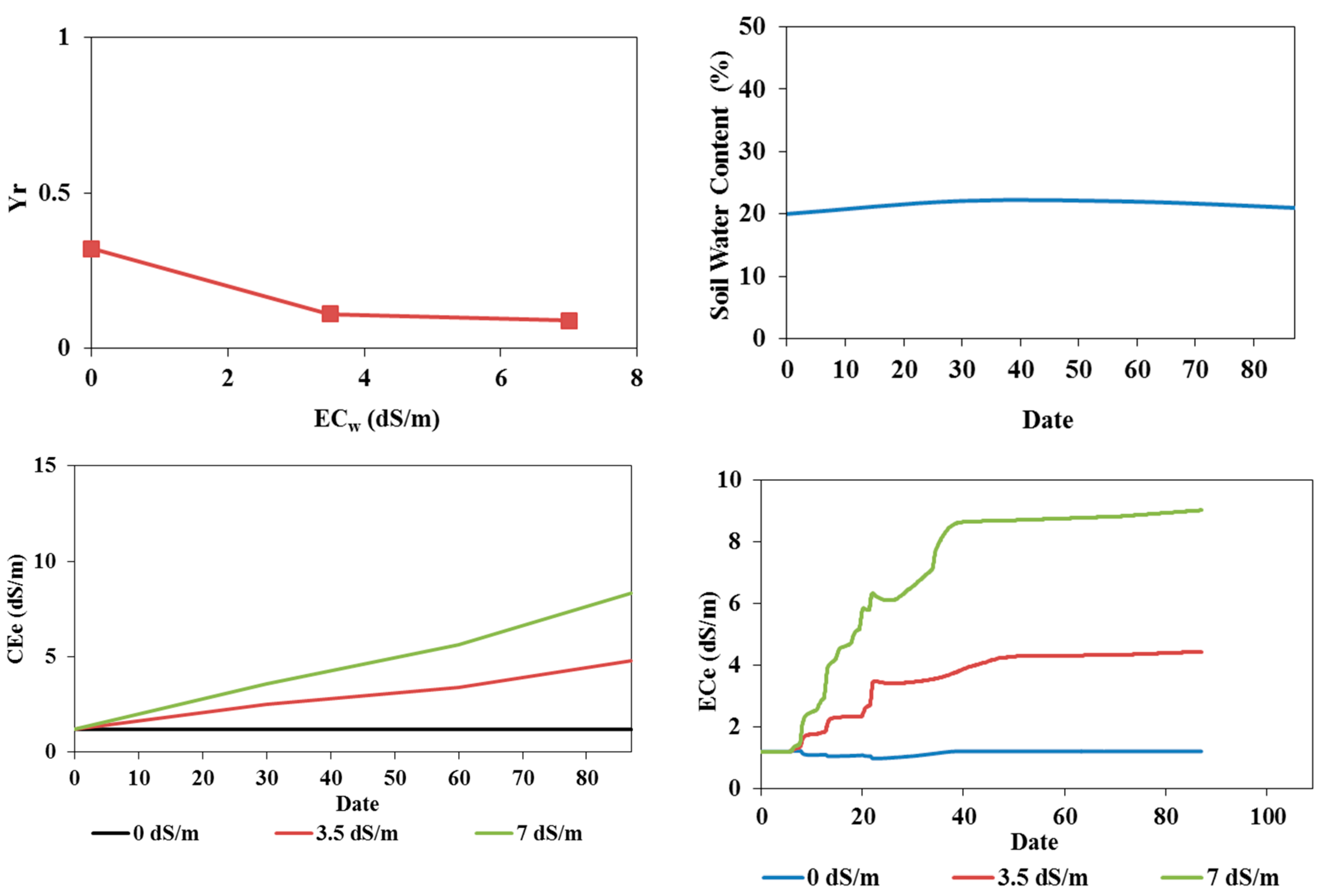
3.3. Effect of Temperature Increase
According to the IPCC (2021), temperatures in Tunisia are expected to increase by 2°C over the coming decades. The effects of this increase on tomato cultivation were studied by increasing the average temperature by 2°C. To this aim, the reference evapotranspiration (
ET) values were recalculated, and simulations were rerun using the updated
ET values while all other model parameters were kept unchanged. The results of these simulations are presented in
Figure 12 and
Figure 13, which show:
- -
In the field experiment, soil water contents exceeded field capacity in the root zone. In deeper layers between 40 cm and 80 cm, soil water contents decreased after the 60th day, highlighting infiltration beyond the root zone.
- -
In the pot experiment, water contents remained constant and below field capacity.
- -
An accumulation of salts in the surface layer due to irrigation with saline waters and increased evaporation.
- -
An increase in salinity in the root zone, reaching an average value of 6 dS/m.
- -
The relative yields estimated using the S-Model have not changed compared to the measured relative yields. An increase in temperature does not significantly affect the tomato yields in both trials.
4. Conclusions
The aridity of regions like Tunisia has prompted the intensification of irrigation practices. However, the scarcity of good-quality water resources has led to the necessity of using brackish water in agriculture. The accumulation of salts due to irrigation with such waters has detrimental effects on both soil and crop yields. Experiments conducted using tomatoes grown in pots and the field and irrigated with three different water qualities ranging in salinity from 0 dS/m to 7 dS/m provided data to study the variations in soil water and salt dynamics. In both pot and field trials, the water content in the root zone (0-30 cm) remained close to field capacity throughout the crop cycle. The variations in salt profiles showed progressive soil salinization depending on irrigation water salinity, reaching 12 dS/m in the pot soil and 8 dS/m in the field soil. Crop reactions to these water qualities were evaluated using water movement and salt transfer modeling using the Hydrus-1D model. The combined effects of osmotic and matric potentials were evaluated using three stress response function models: the additive model and two multiplicative models: T-Model and S-Model. The estimated relative crop yields showed that only the S-Model could reproduce the measured relative yields. A hypothetical 2°C temperature increase did not significantly affect crop yields. Soil salinization slightly increased due to increased evaporation. Adopting a modeling approach based on soil geochemical characterization would be an interesting alternative to this study, where the risks of soil alkalization and salinization could be more thoroughly explored for strategic crops like tomatoes.
Author Contributions
Conceptualization, Sabri Kanzari, Issam Daghari, and Khouloud Ben Ali; Data curation, Sabri Kanzari, Khouloud Ben Ali, Sana Ben Mariem and Samir Ghannem; Formal analysis, Sabri Kanzari, Jiří Šimůnek, and Sana Ben Mariem; Funding acquisition, Sabri Kanzari, and Sana Ben Mariem; Investigation, Sabri Kanzari, Jiří Šimůnek, Issam Daghari, Anis Younes, Khouloud Ben Ali, Sana Ben Mariem and Samir Ghannem; Project administration, Sabri Kanzari, and Sana Ben Mariem; Resources, Sabri Kanzari, and Sana Ben Mariem; Software, Jiří Šimůnek; Supervision, Sabri Kanzari, and Sana Ben Mariem; Visualization, Sabri Kanzari, Jiří Šimůnek, Issam Daghari, Anis Younes, Khouloud Ben Ali, Sana Ben Mariem, and Samir Ghannem; Writing – original draft, Sabri Kanzari, and Jiří Šimůnek; Writing – review & editing, Sabri Kanzari, Jiří Šimůnek, Issam Daghari, Anis Younes, Khouloud Ben Ali, Sana Ben Mariem, and Samir Ghannem.
Data Availability Statement
The data supporting the findings of this research are available in the manuscript.
References
- Besser, H.; Dhaouadi, L.; Hadji, R.; Hamed, Y.; Jemmali, H. Ecologic and Economic Perspectives for Sustainable Irrigated Agriculture under Arid Climate Conditions: An Analysis Based on Environmental Indicators for Southern Tunisia. J. Afr. Earth Sci. 2021, 177, 104134. [Google Scholar] [CrossRef]
- Srivastava, A.; Parida, V.K.; Majumder, A.; Gupta, B.; Gupta, A.K. Treatment of Saline Wastewater Using Physicochemical, Biological, and Hybrid Processes: Insights into Inhibition Mechanisms, Treatment Efficiencies and Performance Enhancement. J. Environ. Chem. Eng. 2021, 9, 105775. [Google Scholar] [CrossRef]
- Martínez-Alvarez, V.; González-Ortega, M.J.; Martin-Gorriz, B.; Soto-García, M.; Maestre-Valero, J.F. The Use of Desalinated Seawater for Crop Irrigation in the Segura River Basin (South-Eastern Spain). Desalination 2017, 422, 153–164. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D. Crop and Irrigation Management Strategies for Saline-Sodic Soils and Waters Aimed at Environmentally Sustainable Agriculture. Sci. Total Environ. 2004, 323, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.-H.; Li, L.; Li, W.-J. Soil Salinity and Drought Tolerance: An Evaluation of Plant Growth, Productivity, Microbial Diversity, and Amelioration Strategies. Plant Stress 2024, 11, 100319. [Google Scholar] [CrossRef]
- Deeb, M.; Smagin, A.V.; Pauleit, S.; Fouché-Grobla, O.; Podwojewski, P.; Groffman, P.M. The Urgency of Building Soils for Middle Eastern and North African Countries: Economic, Environmental, and Health Solutions. Sci. Total Environ. 2024, 917, 170529. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Prokisch, J.; Mansour, H.; Bayoumi, Y.A.; Shalaby, T.A.; Veres, S.; Brevik, E.C. Review of Crop Response to Soil Salinity Stress: Possible Approaches from Leaching to Nano-Management. Soil Systems 2024, 8, 11. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; He, P.; Chen, D.; Dai, X.; Jin, Q.; Su, X. The Optimal Irrigation Water Salinity and Salt Component for High-Yield and Good-Quality of Tomato in Ningxia. Agric. Water Manag. 2022, 274, 107940. [Google Scholar] [CrossRef]
- Khondoker, M.; Mandal, S.; Gurav, R.; Hwang, S. Freshwater Shortage, Salinity Increase, and Global Food Production: A Need for Sustainable Irrigation Water Desalination—A Scoping Review. Earth 2023, 4, 223–240. [Google Scholar] [CrossRef]
- Li, P.; Ren, L. Evaluating the Saline Water Irrigation Schemes Using a Distributed Agro-Hydrological Model. J. Hydrol 2021, 594, 125688. [Google Scholar] [CrossRef]
- Yu, Q.; Kang, S.; Hu, S.; Zhang, L.; Zhang, X. Modeling Soil Water-Salt Dynamics and Crop Response under Severely Saline Condition Using WAVES: Searching for a Target Irrigation Volume for Saline Water Irrigation. Agric. Water Manag. 2021, 256, 107100. [Google Scholar] [CrossRef]
- Kanzari, S.; Jaziri, R.; Ali, K.B.; Daghari, I. Long-Term Evaluation of Soil Salinization Risks under Different Climate Change Scenarios in a Semi-Arid Region of Tunisia. Water Supply 2021, 21, 2463–2476. [Google Scholar] [CrossRef]
- Majeed, A.; Stockle, C.O.; King, L.G. Computer Model for Managing Saline Water for Irrigation and Crop Growth: Preliminary Testing with Lysimeter Data. Agric. Water Manag. 1994, 26, 239–251. [Google Scholar] [CrossRef]
- Šimůnek, J.; van Genuchten, M.T.; Šejna, M. Recent Developments and Applications of the HYDRUS Computer Software Packages. VZJ 2016, 15, vzj2016–04. [Google Scholar] [CrossRef]
- Šimůnek, J.; Brunetti, G.; Jacques, D.; van Genuchten, M.T.; Šejna, M. Recent developments and applications of the HYDRUS computer software packages since 2016. VZJ 2024, 23, e20310. [Google Scholar]
- van Genuchten, M.T. A Closed-Form Equation for Predicting the Hydraulic Conductivity of Unsaturated Soils. SSSAJ 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Mualem, Y. A New Model for Predicting the Hydraulic Conductivity of Unsaturated Porous Media. Water Resour. Res. 1976, 12, 513–522. [Google Scholar] [CrossRef]
- Feddes, R.A.; Kowalik, P.J.; Zaradny, H. Simulation of Field Water Use And Crop Yield. In Simulation Monographs; Pudoc.: Wageningen, The Netherlands, 1978; p. 189. [Google Scholar]
- Maas, E.V. Crop salt tolerance. In Agricultural salinity assessment and management; Tanji, K.K., Ed.; ASCE Manuals and Reports on Engineering Practice: New York, 1990; No 71. [Google Scholar]
- van Genuchten, M.T. A numerical model for water and solute movement in and below the root zone; Unpublished Research Report; U.S. Salinity Laboratory, USDA, ARS: Riverside, CA, 1987. [Google Scholar]
- Kanzari, S.; Rezig, M.; Ben Nouna, B. Estimating Hydraulic Properties of Unsaturated Soil Using a Single Tensiometer. American Journal of Geophysics, Geochemistry and Geosystems 2017, 3, 1–4. [Google Scholar]
- Vanclooster, M.; Mallants, D.; Diels, J.; Feyen, J. Determining Local-Scale Solute Transport Parameters Using Time Domain Reflectometry (TDR). J. Hydrol 1993, 148, 93–107. [Google Scholar] [CrossRef]
- Mallants, D.; Vanclooster, M.; Meddahi, M.; Feyen, J. Estimating Solute Transport in Undisturbed Soil Columns Using Time-Domain Reflectometry. J. Contam. Hydrol. 1994, 17, 91–109. [Google Scholar] [CrossRef]
- Toride, N.; Leij, F.J.; van Genuchten, M.T. The CXTFIT code for estimating transport parameters from laboratory or field tracer experiment; Research Report N°137; US Salinity Laboratory: Riverside, California, 1999; 119p. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration: Guide-lines for computing crop water requirements. In FAO Irrigation and Drainage Paper No. 56; FAO: Rome, Italy, 1998; 300p. [Google Scholar]
Figure 1.
Irrigation scheduling for tomatoes in the pot and field experiments.
Figure 1.
Irrigation scheduling for tomatoes in the pot and field experiments.
Figure 2.
Measured soil water contents (in %) in the pot experiments irrigated with different water qualities (0, 3, and 7 dS/m).
Figure 2.
Measured soil water contents (in %) in the pot experiments irrigated with different water qualities (0, 3, and 7 dS/m).
Figure 3.
Measured soil water contents (in %) in the field experiments with tomatoes irrigated with a) fresh water (at 30, 60, and 109 d) and b) waters of different salinities (0, 3, and 7 dS/m) (at 109 days).
Figure 3.
Measured soil water contents (in %) in the field experiments with tomatoes irrigated with a) fresh water (at 30, 60, and 109 d) and b) waters of different salinities (0, 3, and 7 dS/m) (at 109 days).
Figure 4.
Soil salt contents (ECe) in the pot experiments irrigated with waters of different salinities (0, 3, and 7 dS/m).
Figure 4.
Soil salt contents (ECe) in the pot experiments irrigated with waters of different salinities (0, 3, and 7 dS/m).
Figure 5.
Measured soil salt contents in the field experiments with tomatoes irrigated with a) fresh water (at 30, 60, and 109 d) and b) waters of different salinities (0, 3, and 7 dS/m) (at 109 days).
Figure 5.
Measured soil salt contents in the field experiments with tomatoes irrigated with a) fresh water (at 30, 60, and 109 d) and b) waters of different salinities (0, 3, and 7 dS/m) (at 109 days).
Figure 6.
Measured initial a) water contents and b) salinities as a function of soil depth in the field experiment.
Figure 6.
Measured initial a) water contents and b) salinities as a function of soil depth in the field experiment.
Figure 7.
Measured and simulated (calibration) soil water contents (in %) (a) and salinities (in dS/m) (b) in the field experiment irrigated with fresh water on 30 (left), 60 (middle), and 109 (right) days.
Figure 7.
Measured and simulated (calibration) soil water contents (in %) (a) and salinities (in dS/m) (b) in the field experiment irrigated with fresh water on 30 (left), 60 (middle), and 109 (right) days.
Figure 8.
Measured and simulated (validation) soil water contents (in %; top) and salinities (in dS/m; bottom) in the field experiments irrigated with saline waters of 3.5 dS/m (left) and 7 dS/m (right) at the end of the experiment.
Figure 8.
Measured and simulated (validation) soil water contents (in %; top) and salinities (in dS/m; bottom) in the field experiments irrigated with saline waters of 3.5 dS/m (left) and 7 dS/m (right) at the end of the experiment.
Figure 9.
Measured and simulated time series of soil water content (%) (left) and salinity (dS/m) (right) in the pot experiments irrigated with (a) freshwater (top) (calibration) and (b) saline waters of 3.5 dS/m (middle) and 7 dS/m (bottom) (validation).
Figure 9.
Measured and simulated time series of soil water content (%) (left) and salinity (dS/m) (right) in the pot experiments irrigated with (a) freshwater (top) (calibration) and (b) saline waters of 3.5 dS/m (middle) and 7 dS/m (bottom) (validation).
Figure 10.
The effect of saline irrigation water on tomatoes relative yield (Yr) [-] (top) and the relationships between actual (Y) and simulated (Ys) yields (kg/plant) (bottom) using the three stress response models (additive, T-Model, and S-Model) incorporated in Hydrus-1D (field experiments).
Figure 10.
The effect of saline irrigation water on tomatoes relative yield (Yr) [-] (top) and the relationships between actual (Y) and simulated (Ys) yields (kg/plant) (bottom) using the three stress response models (additive, T-Model, and S-Model) incorporated in Hydrus-1D (field experiments).
Figure 11.
The effect of saline irrigation water on tomatoes relative yield (Yr) [-] (top) and the relationships between actual (Y) and simulated (Ys) yields (kg/plant) (bottom) using the three stress response models (additive, T-Model, and S-Model) incorporated in Hydrus-1D (pot experiments).
Figure 11.
The effect of saline irrigation water on tomatoes relative yield (Yr) [-] (top) and the relationships between actual (Y) and simulated (Ys) yields (kg/plant) (bottom) using the three stress response models (additive, T-Model, and S-Model) incorporated in Hydrus-1D (pot experiments).
Figure 12.
Effects of a temperature increase of 2°C on tomatoes cultivated in field conditions: relative yield as a function of irrigation water salinity (top left), soil water content profiles at 30, 60, and 109 days when irrigated with freshwater (top right), soil salinity profiles at 30, 60, and 109 days (middle row) when irrigated with fresh water (left) and saline waters of 3.5 (middle) and 7 (right) dS/m, and average root zone salinity when irrigated with fresh water and saline waters of 3.5 and 7 dS/m (bottom).
Figure 12.
Effects of a temperature increase of 2°C on tomatoes cultivated in field conditions: relative yield as a function of irrigation water salinity (top left), soil water content profiles at 30, 60, and 109 days when irrigated with freshwater (top right), soil salinity profiles at 30, 60, and 109 days (middle row) when irrigated with fresh water (left) and saline waters of 3.5 (middle) and 7 (right) dS/m, and average root zone salinity when irrigated with fresh water and saline waters of 3.5 and 7 dS/m (bottom).
Figure 13.
Effects of a temperature increase of 2°C on tomatoes cultivated in pot conditions: relative yield as a function of irrigation water salinity (top left), average soil water content when irrigated with fresh water (top right), average soil salinity in the 0-30-cm soil layer (bottom left), and root zone salinity (at 5 cm depth) for irrigations with fresh water and saline waters of 3.5 and 7 dS/m (bottom right).
Figure 13.
Effects of a temperature increase of 2°C on tomatoes cultivated in pot conditions: relative yield as a function of irrigation water salinity (top left), average soil water content when irrigated with fresh water (top right), average soil salinity in the 0-30-cm soil layer (bottom left), and root zone salinity (at 5 cm depth) for irrigations with fresh water and saline waters of 3.5 and 7 dS/m (bottom right).
Table 1.
The soil hydraulic parameters of different soil layers.
Table 1.
The soil hydraulic parameters of different soil layers.
| Layer (cm) |
θr (cm3.cm-3) |
θs (cm3.cm-3) |
α (cm-1) |
n (-) |
Ks (cm.d-1) |
| Pot experiment |
| - |
0.1 |
0.41 |
0.27 |
1.11 |
6.41 |
| Field experiment |
| 0-20 cm |
0.078 |
0.546 |
0.07 |
1.067 |
8.87 |
| 20-40 cm |
0.078 |
0.544 |
0.07 |
1.079 |
8.87 |
| 40-60 cm |
0.078 |
0.445 |
0.10 |
1.073 |
12.6 |
| 60-80 cm |
0.078 |
0.443 |
0.03 |
1.078 |
12.5 |
Table 2.
Root mean square error (RMSE) values assessing the Hydrus-1D calibration process.
Table 2.
Root mean square error (RMSE) values assessing the Hydrus-1D calibration process.
| Variable |
Experiment |
Date 1 |
Date 2 |
Date 3 |
| Soil Water Profile |
Field |
9.30 |
7.30 |
6.40 |
| Pot |
10.30 |
11.10 |
8.30 |
| Soil Salinity Profile |
Field |
5.30 |
2.10 |
4.60 |
| Pot |
2.00 |
4.50 |
1.70 |
Table 3.
Root mean square error (RMSE) values assessing the Hydrus-1D validation process.
Table 3.
Root mean square error (RMSE) values assessing the Hydrus-1D validation process.
| Variable |
Experiment |
Irrigation Water Quality |
RMSE (%)
(on the final day) |
| Soil Water Profile |
Field |
3.5 dS/m |
9.10 |
| 7 dS/m |
1.10 |
| Soil Water Profile |
Pot |
3.5 dS/m |
3.00 |
| 7 dS/m |
1.10 |
| Soil Salinity Profile |
Field |
3.5 dS/m |
10.20 |
| 7 dS/m |
5.70 |
| Soil Salinity Profile |
Pot |
3.5 dS/m |
5.20 |
| 7 dS/m |
3.00 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).