Introduction
Pregnancy signifies a transformative phase wherein the placenta, an intricate organ, assumes a central role in orchestrating the exchange of nutrients, water, and waste materials between the mother and fetus. This intricate interplay is indispensable for sustaining the developing fetus and regulating placental functions, thereby influencing critical aspects of fetal growth (Peter et al. 2017; Santos et al. 2017). Beyond its conventional role, the placenta drives as an immune-endocrine organ, mediating the effects of metabolic and environmental stressors on fetal development (Diaz et al. 2014). It is critical to understand the developmental changes that may affect embryo-fetal growth and development since the placenta experiences significant morphological and structural changes during gestation to promote fetal growth.
The bovine (cattle), characterized by a gestation period comparable to humans and the gestation of a single conceptus with a similar growth trajectory and maturity at birth stands as an invaluable model for biomedical research (Andersen and Plum 1965; Gatford et al. 2020). Moreover, the longstanding practice of in vitro fertilization and somatic cell nuclear transfer cloning in this species enhances its utility beyond reproductive biology. This underscores the significance of employing domestic cows as models in biomedical research, given their biological similarities and the advanced reproductive technologies employed in their study (Chen et al. 2013; Xiang et al. 2014; Estrella et al. 2017).
During the pregnancy, a dynamic interplay of vital processes, encompassing the generation of hormones and growth factors, fetal growth and development, maternal-fetal exchange, lactation, pregnancy management, and parturition, relies heavily on the availability of energy, potentially leading to elevated reactive oxygen species (ROS) production and oxidative stress. In bovine, the most prevalent calving-related complication is retention of placenta (RP), characterized by the inability to expel fetal membranes within 6–12 hours of calving (Dervishi et al. 2016; Jemal 2016; Fisher et al. 2019). The occurrence of RP in crossbred cattle has adverse implications for both productive and reproductive performance, manifesting in reduced milk yield, extended open days, and an elevated service per conception rate. Although the precise etiology of RP remains elusive, factors such as hormonal imbalances, nutritional disturbances, immunosuppression, environmental stress, and oxidative stress have been identified as contributors to its progression (Hanafi et al. 2011; Kumari et al. 2015; Rao et al. 2018; Keywanloo et al. 2021). The bulk of cellular energy production takes place in mitochondria, which are also responsible for controlling several vital cellular processes, including fat metabolism, steroid synthesis, and apoptosis. Furthermore, mitochondria are probably crucial for placental implantation, growth, and development (Polei et al. 2020; de Lima et al. 2023). Numerous studies have delved into unraveling the intricacies of placental physiology, exploring pathologies, understanding the mechanisms of fetal membrane separation, and identifying various factors contributing to oxidative stress in the context of RP (Dimri et al. 2010; Kumari et al. 2015; Santos et al. 2017; Elsayed et al. 2020). Even that, none of the studies explored mitochondrial contribution in the context of separation and expulsion of a fetal membrane in crossbred cattle. Thus, it is important to know that mitochondrial dysfunction impacts the concern of RP, the same, as various reproductive disorders (Dimri et al. 2010).
It is well-known mitochondria are widely recognized for their remarkable sensitivity and adaptability to environmental stress, serving as both targets and orchestrators of adaptive cellular responses. An integral determinant of embryo competence during fertilization and development is associated with the mitochondrial capacity to regulate ATP demand and supply. ATP, crucial for sustaining the placenta's energy requirements, growth, endocrine function, and transport, heavily relies on mitochondrial energy. Beyond ATP production, mitochondria play a pivotal role in hormone synthesis, with the steroidogenic acute regulatory (StAR) protein residing in mitochondria, governing cholesterol uptake and conversion into pregnenolone. Furthermore, mitochondria serve as the primary intracellular site for the synthesis of steroid hormones (Miller 2013). The CYP19 (aromatase) catalyzes the conversion of androgens to estrogens in hormone synthesis, a process facilitated by the StAR protein's role in mediating cholesterol transport to the mitochondria for steroidogenesis (Ghai et al. 2012). The maturation of the placenta, coupled with inadequate antioxidant mechanisms, contributes to the elevated generation of ROS within mitochondria. These dysfunctions in mitochondrial activity play a central role in the pathophysiology of diverse diseases across multiple organ systems. This significance is underscored by the direct influence of mitochondrial function on critical processes such as biosynthesis, energy production, redox signaling, antioxidant defense, adaptation to low oxygen levels, inflammatory responses, and apoptotic signaling (Spinelli and Haigis 2018).
Despite numerous investigations, the exact cause of RP remains elusive. Surprisingly, there is a notable gap in research regarding the mitochondrial role in the detachment and expulsion of fetal membranes in crossbred cattle. Conversely, mitochondria play a crucial role in maintaining homeostasis in energy metabolism through OXPHOS machinery and contribute significantly to placental formation. Thus, ensuring the proper functioning of placental mitochondria is essential for optimal fetal growth and development. Mitochondrial DNA encodes the 13 subunits of the OXPHOS complexes, serving as a primary cellular energy producer (Jia et al. 2015). Unfortunately, mtDNA is highly susceptible to damage from ROS, as the mitochondrial electron transport chain is a major ROS source, and lacks effective repair mechanisms and rendering it susceptible to mutations (Linnane et al. 1989; Spinazzi et al. 2012; May-Panloup et al. 2014; Jia et al. 2015). Aditionally, study from our lab revealed the tissues specific changes in mitochondrial and nuclear encoded mitochondrial protein coding genes as per the energy requirement of the specific tissue or organ (Sadeesh et al. 2023; Sadeesh et al. 2024). Given this context, our study aims to seek the presence or abnormalities in mitochondrial activity, content, and gene expression related to RP in comparison to normal placenta in crossbred cattle.
Materials and Methods
The study was conducted on Karan Fries crossbred (Holstein Friesian × Tharparkar) cows maintained at the Livestock Research Centre, National Dairy Research Institute (NDRI), Karnal, situated in the Trans-Gangetic plain region of India. All experiments adhered to ethical standards and received approval from the Institutional Animal Ethics Committee (IAEC) at ICAR-NDRI, Karnal, with approval number 50-IAEC-23-01. Chemicals utilized in the study were sourced from Sigma-Aldrich (St. Louis, MO, USA), while reagents for molecular biology procedures were obtained from Qiagen (Hilden, Germany), Thermo Fisher Scientific (USA), and Macherey-Nagel (Germany). Any deviations from this sourcing information will be explicitly noted.
Placenta of the animals that expelled their fetal membranes naturally within 6-12 h of parturition was considered as normal placenta. The animals that did not expel their fetal membranes naturally but the placenta that was ejected manually without any medicinal treatment were considered as RP (Dervishi et al. 2016; Jemal 2016). Tissue samples were collected from cows within the same age group, encompassing both normal (n = 3) and RP (n = 3) conditions, and a comprehensive gross morphological analysis of the collected placental specimens was conducted. Subsequently, three samples from each group were used for further experimentation. The gross morphological examination persisted until a total of 6 cows exhibited placental retention, while another set of 6 cows displayed normal placental expulsion. Immediately after expulsion, the cotyledons from the placenta were excised and washed in chilled phosphate buffer saline (PBS) containing antibiotics to eliminate contamination and blood. Tissue samples were transported to the laboratory in chilled PBS as early as possible. In cases of RP, a manual removal procedure was performed and the fetal membrane was separated, and done the same as normal placenta. After that, all the tissues were washed three times in sucrose tris EGTA buffer. Subsequently, each tissue sample was bifurcated into two segments: one destined for RNA and DNA isolation, preserved in RNA later, and the other allocated for complex activity measurement, preserved in chilled sucrose tris EGTA buffer, and stored at -80°C for subsequent processing. This systematic and meticulous approach ensured the preservation of sample quality and integrity throughout the experimental pipeline.
Following the expulsion or manual removal of the placenta, we performed a comprehensive morphological examination of the fetal membrane. Throughout this investigation, we revealed anomalies in the lesions, shape, and number of cotyledons within each placenta. Phenotypic data pertaining to the included crossbred cattle are presented in
Table 1.
Mitochondria were isolated from placental tissue specimens through differential centrifugation methods. In brief, placental cotyledons were extracted from the fetal side of both normal and RP samples. Approximately 1g of tissue specimen was finely minced and homogenized in ice-cold sucrose-tris-EGTA buffer (STE). The resulting homogenate underwent centrifugation at 1500× g for 10 minutes at 4°C. The supernatant was collected and subjected to further centrifugation at 4000× g for 15 minutes at 4°C to produce the pellet of enriched mitochondria. The resulting pellet was collected (Fraction 1), and the supernatant was again centrifuged at 12000× g for 15 min at 4°C to produce a second pellet of enriched mitochondria (Fraction 2).
The pellet containing the mitochondria was suspended in the same solution of ice-cold STE buffer, and subjected to additional centrifugation at 12000× g for 15 minutes at 4°C, with this step being repeated twice. Ultimately, the pellet containing mitochondria was resuspended in 200 µl ice-cold STE buffer and promptly utilized for bioactivity assays. The total protein concentration of the mitochondrial fraction was quantified using the Bradford Assay. A portion of the mitochondrial sample was used for subsequent OXPHOS complex enzyme activity measurements
Mitochondrial OXPHOS enzyme complex I activity in isolated mitochondrial suspensions was assessed in normal and RP tissues, employing a protocol derived from Spinazzi et al. (2012) with slight modifications. The assessment focused on measuring NADH oxidation at 340 nm, utilizing 10 µg of protein from isolated mitochondrial suspension. To prepare the reaction mixture, 0.5 M PBS (pH 7.5), 50 mg/ml fatty acid-free BSA, 50 mM sodium azide, and 5 mM NADH were taken, and the final volume was adjusted to 1 ml using distilled water. The thoroughly mixed solution was then subjected to baseline measurement at 340 nm for 2 min on a multimode plate reader (TECAN, Infinite M200 PRO, and Switzerland). The reaction was initiated by adding 10 mM of ubiquinone, and absorbance at 340 nm was monitored for 2 min. Simultaneously, a separate reaction was executed to determine inhibitor-resistant activity with the same reagents and sample by adding 1 mM rotenone solution. The enzymatic activity of OXPHOS complex I was quantified as nmol/min/mg of protein according to the equation: enzyme activity (nmol/min/mg) = (∆ Absorbance/min × 1,000)/ (extinction coefficient × volume of sample used in ml) × (sample protein concentration in mg/ ml). The specific activity of complex I was calculated by subtracting the total complex I activity (without rotenone) from rotenone-resistant activity (with rotenone), and the result was expressed as nmol/min/mg of total proteins.
Placental tissues (30 mg) were weighed and transferred to sterilized 2 ml Eppendorf tubes. Subsequently, total genomic DNA extraction was carried out using the DNeasy Blood and Tissue Kit (Qiagen), following the manufacturer's instructions. In brief, 180 μl ATL buffer, 20 μl proteinase K, and 10 μl RNase were added to the weighed tissue sample. The mixture was vortexed and incubated at 56°C until complete tissue lysis occurred. Following this, an equal volume (200 μl) of absolute ethanol and AL buffer was added, and thoroughly mixed by vortexing. The resulting mixture was transferred into a DNeasy spin column, and the column was placed in a collection tube. Centrifugation was performed at ≥ 6000× g at room temperature for 1 min. The flow-through, along with the collection tube, was discarded, and the spin column was relocated to a new collection tube. Subsequently, 500 μl of wash buffer AW1 was added to the spin column and centrifuged at ≥ 6000× g at room temperature for 1 min. The collection tube was then discarded, and the spin column was placed in a new collection tube. Following this, 500 μl of Buffer AW2 was added, followed by centrifugation at 20000× g for 3 min at room temperature. The flow-through, along with the collection tube, was discarded, and the spin column was placed in a clean 2.0 ml microcentrifuge tube. Following these steps, 200 μl of buffer AE was added to the DNeasy membrane and incubated at room temperature for 1 minute. Subsequent centrifugation was performed at ≥ 6000× g for 1 min at room temperature. This step was repeated for optimal purity. The elute tube contained the purified DNA. The DNA was quantified spectrophotometrically using a NanoQuant reader (ND1000, Thermo Scientific, USA), and DNA samples with an A260/280 ratio in the range of 1.8–2.0 were considered acceptable. Then, the isolated total DNA sample used for the downstream processes.
The
mtDNA copy number was quantified through real-time qPCR using the Applied Biosystems 7500 Real-Time PCR (Thermo Scientific) from both normal and RP tissues of crossbred cattle. The mt-
Cytb, a protein part of complex III of the electron transport chain and ß-2-microglobulin (
B2M), a single-copy nuclear-encoded gene were used as the endogenous controls. A 10 ng/ul of total
DNA was diluted with MilliQ water. The reaction mixtures comprised 2x QIAgen Sybr green PCR master mix, 1 µl of each primer (forward and reverse), 10 ng of template
DNA, and nuclease-free water. The total reaction volume in each well of the 96 well plates was 20 µl. Cycling conditions were set as follows: 5 min at 94 °C and 40 cycles of 15 s at 94 °C and 30s at 58 °C. Each qPCR reaction included three replicates for samples sourced from the same
DNA origin. The data analysis was performed by using the comparative Ct method and ΔCt values were obtained by subtracting the Ct values for the test gene (mt-
Cyt b) and from those of the reference housekeeping gene (
B2M), thereby normalizing the nuclear DNA. The 2
-ΔCt was calculated for each sample, and
mtDNA copy numbers were analyzed using the formula: 2 × 2
-ΔCt (Livak and Schmittgen 2001). The analyzed data were graphically represented as relative
mtDNA copy numbers in the log2 scale. The melting curve, indicating the specificity of the primers, is illustrated in
Figure S1.
Total RNA was isolated from 100 mg placental cotyledons using the TRIzol method with slight modification. Then, cDNA synthesis was executed by utilizing the appropriate amount of RNA in 20 μl of reaction volume using a Biodyne SOLIScript RT cDNA synthesis kit (CAT# 06-37-0000S).
The transcript level of 13 mitochondrial protein-coding genes (PCGs) from a normal and RP that encode for the subunits of respiratory chain complexes: NADH dehydrogenase subunits (
ND1, ND2, ND3, ND4L, ND4, ND5 and
ND6), cytochrome c oxidase subunits (
COX1, COX2 and
COX3),
Cytb, and ATP synthase F0 subunits (
ATP6 and
ATP8) were quantified. Relative gene expression was analyzed in 100ng of
cDNA using specific primers developed in a prior exhaustive study conducted by our lab (
Table S1) (Sadeesh et al. 2023). The mRNA levels were quantified by qRT-PCR, utilizing the 12s gene as a housekeeping gene to normalize the transcript levels of the target genes. The reaction was carried out in a total volume of 12 μl, comprising 6 μl Maxima SYBR Green qPCR master mix with ROX (Thermo Scientific, CAT No # K0251), 2 μl cDNA (100 ng), 3 μl H
2O, and 0.5 μl each of forward and reverse primers. Amplification conditions were set as an initial denaturation at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 5 s and annealing/extension at 60 °C for 34 s. Subsequently, a stepwise increase in temperature from 60 °C to 95 °C was implemented to obtain the melting curve data, facilitating post-amplification scrutiny for single-product amplification with consistent melting temperatures. The entire procedure was performed in triplicate to ensure precision and reproducibility. Melting point curves were presented for each primer after amplification to confirm qRT-PCR product identity (
Figure S2). The relative transcript levels of mitochondrial PCGs in normal and RP were assessed through the 2
−ΔΔCt method and relative expression of the normal placenta was set as 1 (Livak and Schmittgen 2001).
To undertake an extensive correlation analysis encompassing all 13 mitochondrial encoded PCGs (MtMP) genes, we employed a dual analytical approach consisting of Mantel's test and Pearson's correlation analyses. This rigorous methodological combination, implemented within the powerful statistical computing environment R, allowed for a thorough exploration of the relationships and interactions between the MtMP genes. By harnessing Mantel's test, which is particularly adept at assessing spatial correlations or associations between matrices, alongside Pearson's correlation analyses, renowned for measuring linear relationships, we gained a nuanced understanding of the intricate interplay among the MtMP genes. This comprehensive analysis not only elucidated the direct correlations between individual genes but also provided insights into potential indirect associations and network dynamics within the mitochondrial protein-coding landscape.
Data are shown as the mean values ± standard error of the mean (SEM). All statistics were performed by Prism 8.0 (GraphPad). The activity assay, mtDNA copy number, phenotypic data, gene transcript levels, and the interaction of these factors were determined through an Independent t-test. A p-value < 0.05 was considered significantly different from the normal placenta group and was presented as follows: Ns: p > 0.05, *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001. A graphical representation of the data was generated using Graphpad Prism 8.0 software. Additionally, advanced analyses including cluster heatmap, volcano plot, and Principal Component Analysis (PCA) were conducted utilizing R and SRplot software. To understand and confirm the biological functions of significantly different genes between retained vs normal placenta, a cluster profiler was used to perform Gene Ontology (GO) and KEGG pathway enrichment analyses. A value of p<0.05 was defined as a significant enrichment result.
Results
In this study, all subjects were categorized into two distinct groups: Normal (n=6) and Retained Placenta (n=6). The RP was considered as the failure of the fetal membrane to expel out after calving. Comparative analysis of phenotypic data from normal and RP groups was conducted. The mean age, gestation period, and parity did not exhibit statistically significant differences when comparing normal and RP groups. However, a notable finding emerged from the analysis of crossbred cattle with retained placenta, revealing statistically significant variations in service per conception in comparison to those with normal placenta (
Table 1). There was no significant difference observed in the calf weight of both groups of normal and RP.
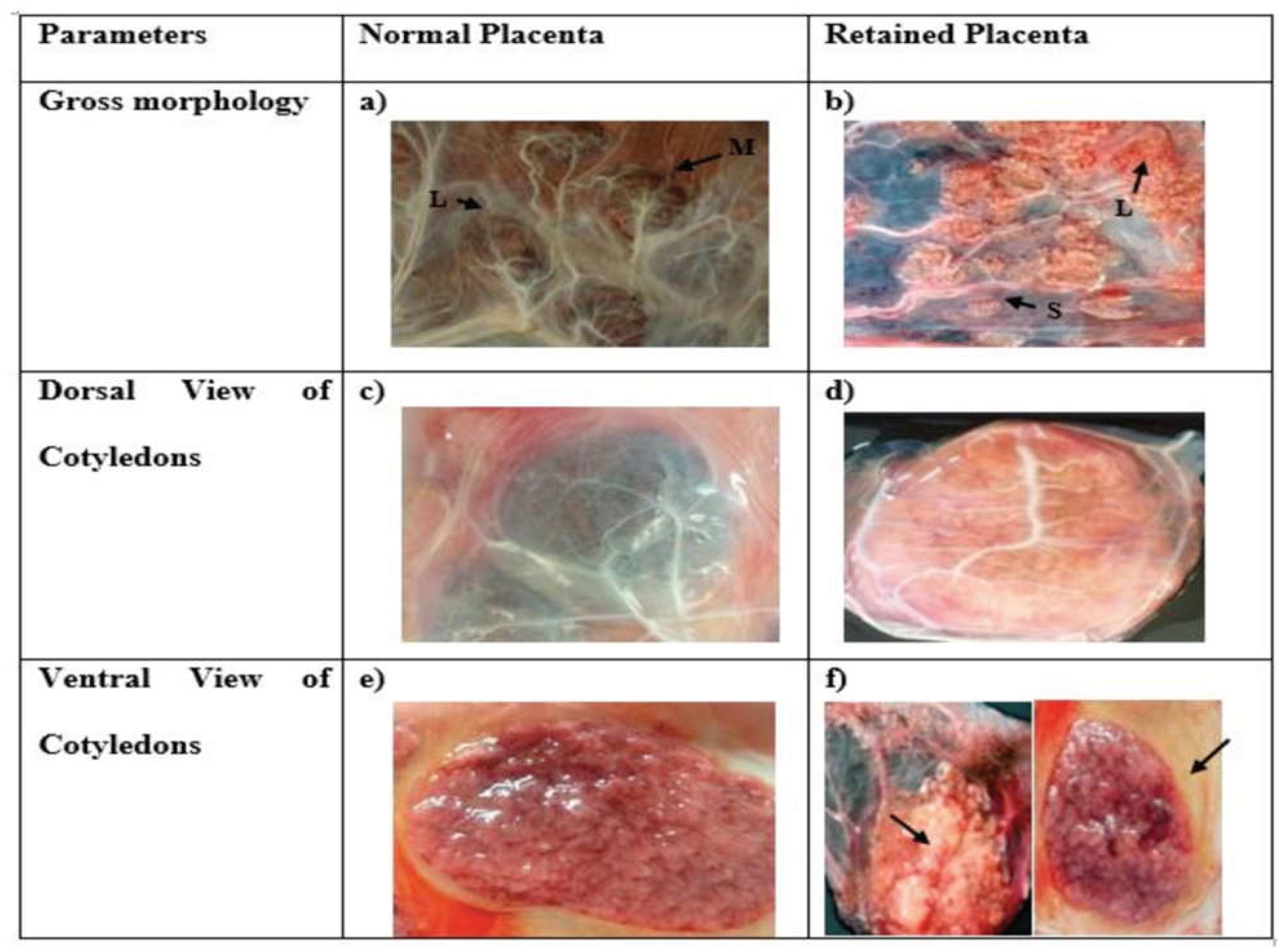
Anticipated in light of the dynamic alterations in fetal weight and the pivotal role of the placenta in fetal growth and development, our investigation revealed noticeable distinctions in the morphology and dimensions of cotyledons between normal and retained placental tissues. Predominantly, cotyledons in the normal placenta exhibited a characteristic convex shape. In stark contrast, cotyledons within the RP group displayed notable variability, presenting as either flat or dispersed in comparison to their normal placenta samples. Intriguingly, within the RP samples, we observed thickening and necrosis of villi, a phenomenon absent in the normal placental tissue, as visually depicted in
Figure 1. A profound dissimilarity emerged in terms of cotyledon numbers between the two groups. Specifically, the RP group exhibited a significant decrease in cotyledon count relative to the normal placenta group. In the normally expelled placenta group, each bovine specimen showed 96.67 ± 1.89 cotyledons with diverse dimensions. Conversely, the RP group presented with 87.5 ± 3.51 cotyledons, as detailed in
Table 1.
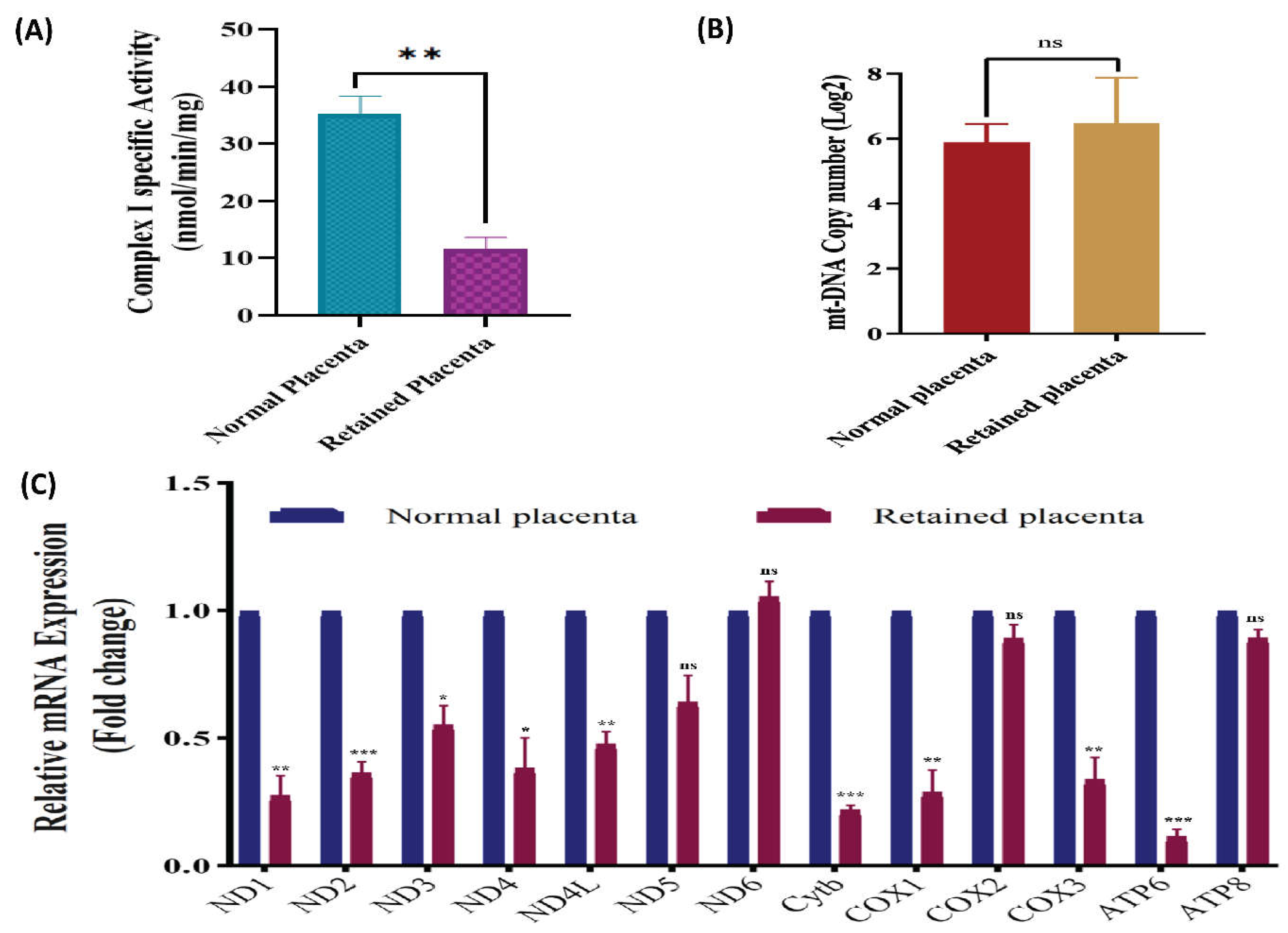
The investigation into the specific activity of OXPHOS Complex I (NADH: Ubiquinone Oxidoreductase) unveiled significant disparities between the normal and RP groups. The enzymatic activity of the OXPHOS complex I was quantified and the results are graphically depicted in
Figure 2A. Specifically, the retained placenta group exhibited markedly lower activity in comparison to the normal placenta group, with values of 11.455±4.68 and 35.168±5.84, respectively, at a significance level of p<0.01. These findings underscore the physiological differences in mitochondrial function between normal and RP scenarios, with implications for understanding cellular energy metabolism in these contexts.
The analysis of RT-qPCR data revealed no statistically significant variation in
mtDNA copy numbers within the RP, in comparison to the control group (
Figure 2B). Conversely, differential variations in
mtDNA copy numbers were observed among species within both normal and RP groups. Despite the apparent variations, a conclusive and statistically significant disparity in
mtDNA copy number between the two groups could not be established. In the RP group, the median
mtDNA copy number was 38.85, while in the normal placenta group, it was 60.69. Nevertheless, the disparity in median
mtDNA copy numbers failed to attain statistical significance, as indicated by a p-value of 0.461. Notably, a skewed distribution of
mtDNA copy numbers was evident in the RP group of cattle.
In this study, we delved into the transcript levels of the 13
MtMP genes in both normal and RP of crossbred cattle, employing a meticulously conducted analysis. Our findings elucidate that the transcript levels of the mitochondrial PCGs in the RP were significantly reduced or unchanged compared to those in the normal placenta. To quantify these alterations, mean values for gene expression in normal placenta were normalized to 1.0 ± SEM, serving as a reference, while values for RP were expressed relative to normal placenta. Remarkably, the transcript levels of nine PCGs exhibited a significant decrease, particularly in complex I genes (
ND1, ND2, ND3, ND4L and ND4,), cytochrome b (
Cyt b), and
COX1, COX3 of complex IV, as well as ATP6, when compared to normal placenta. Explicitly, the transcript levels of
ND5,
COX2, and
ATP8 in RP exhibited a downward trend, though not statistically significant when compared to normal placenta. Conversely,
ND6 exhibited a transcript level expression in RP of crossbred cattle that was approximately equivalent to that observed in normal placenta (
Figure 2C). Subsequent investigation unveiled a substantial reduction in the transcript levels of mitochondrial genes
ND1, ND2, ND3, ND4L and ND4 in RP, amounting to 0.278±0.075, 0.367±0.04, 0.554±0.073, 0.480±0.044 and 0.384±0.118 times, respectively, in comparison to NP. Furthermore, the transcripts of
Cyt b, COX1, COX3, and
ATP6 in the RP were significantly lower by 0.222±0.014, 0.292±0.082, 0.34±0.083 and 0.118±0.025 fold, respectively, in contrast to their levels in normal placental. While non-significant reduction in gene expression was seen in
ND5,
COX3, and
ATP8 in RP tissues.
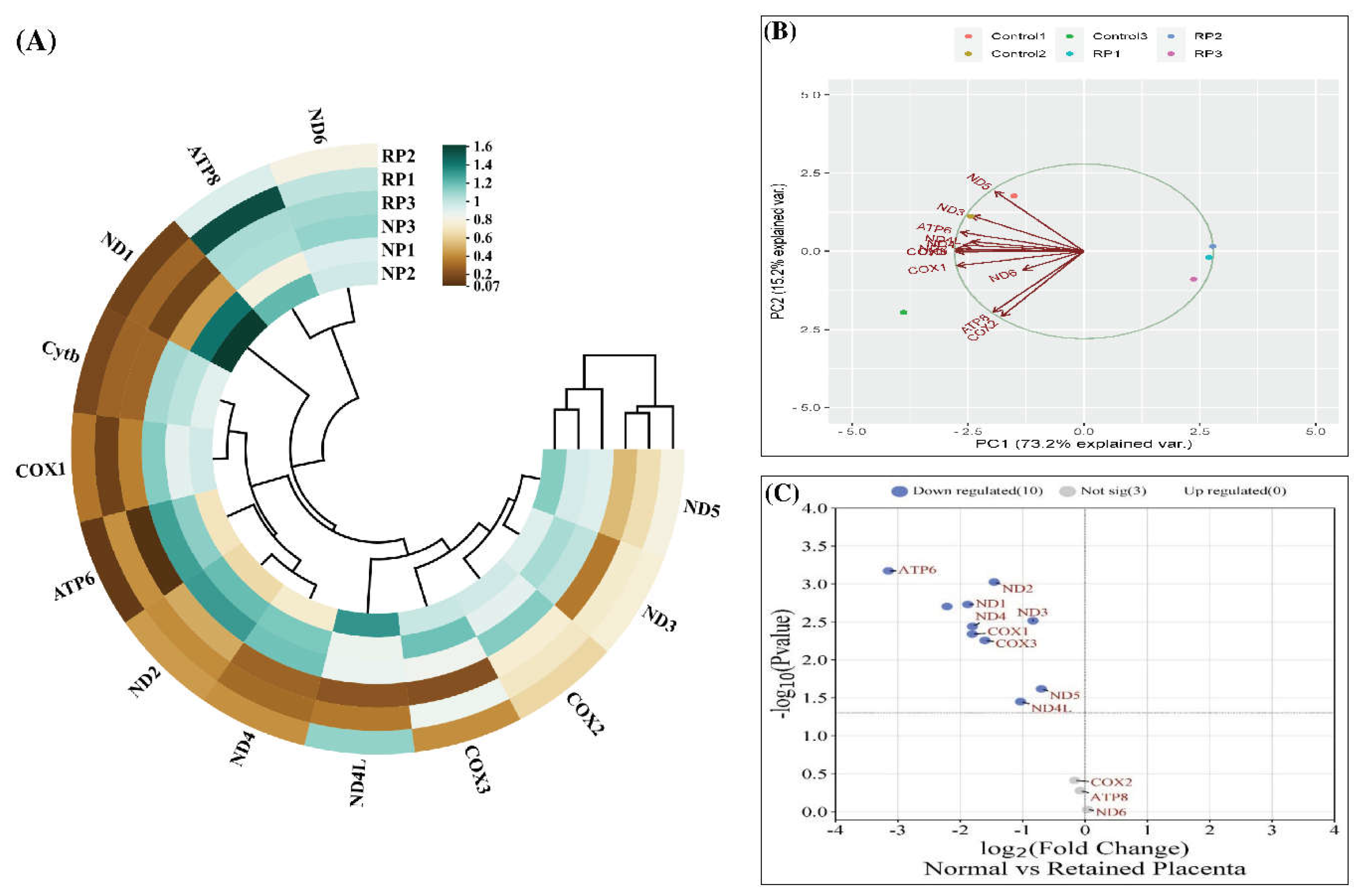
The cluster heatmap and PCA plot, depicted in
Figure 3A and B, respectively, serve as visual representations of the gene expression relationships between samples. Notably, RP samples exhibited a higher correlation with each other, with RP1 showing a stronger correlation with RP3. However, the correlations with RP2 were less pronounced. Likewise, regarding the gene expression between samples in the PCA plot, a control1-control2/RP1-RP2-RP3 clustering could be deduced. The percentage of variations obtained by the principal components is specified on each axis. Moreover, the volcano plot (
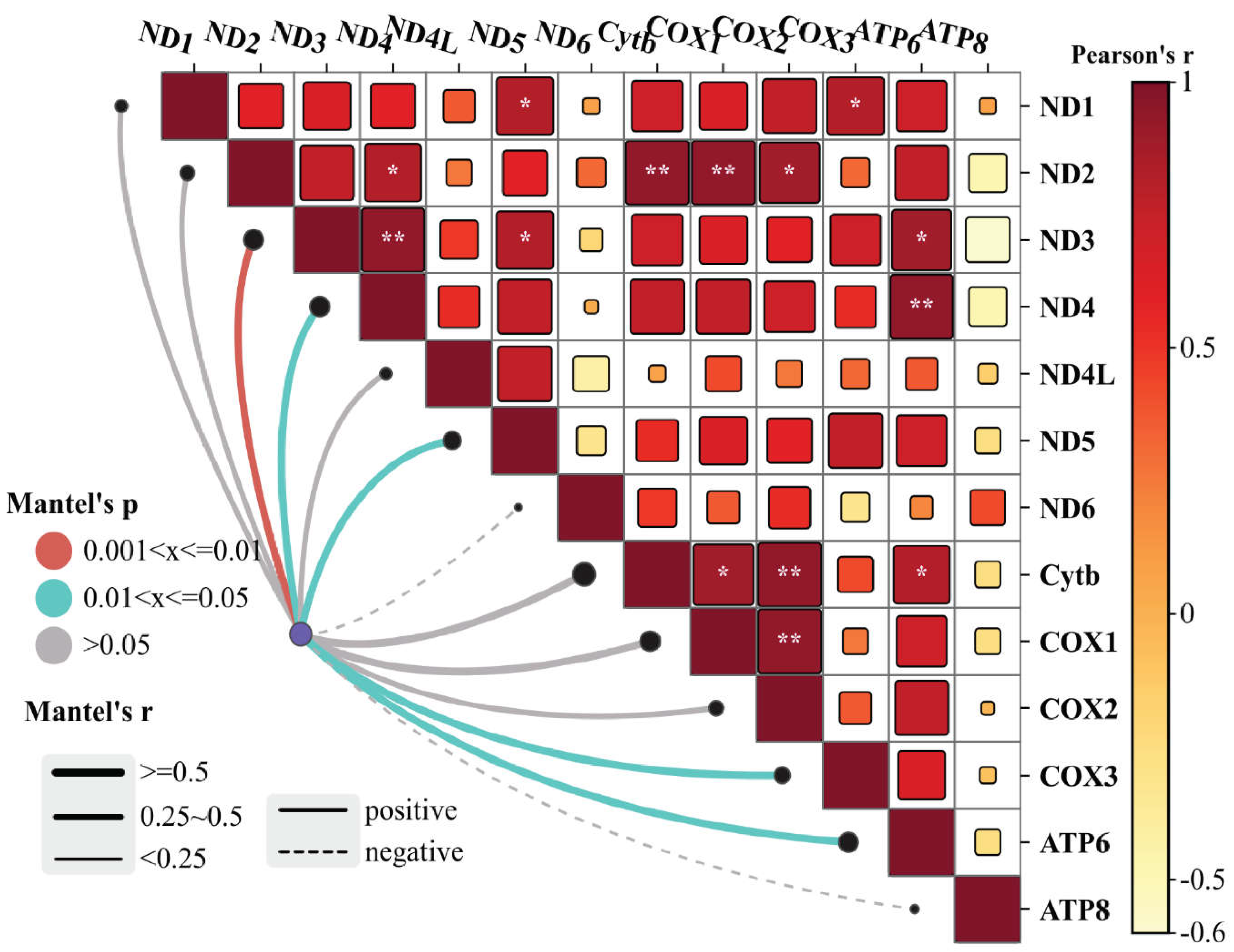
Figure 3C) illustrates the distribution of analyzed genes based on their log2 fold change (x-axis) and -log10 P value (y-axis). The Mantel’s test was used to examine the correlation and associations of the
MtMP gene expression among the normal and RP (
Figure 4). The results of the correlation study revealed that
Cytb,
COX1, and
COX2 showed a strong positive correlation with the
ND2 gene of complex I. Similarly,
ATP6 gene expression also exhibited a positive correlation with
ND3, ND4 of complex I, and
Cytb of complex III. The GO and KEGG pathway analyses reveal the main functions of these genes (
Figure 5A), and we believe that these genes are highly correlated with the course of various functions and energy production mechanisms. We also verified the role of mitochondrial PCGs by conducting a functional enrichment analysis of all the genes associated with RP (
Figure 5B–D).
Discussion
In this study, for the first time, we conducted a comprehensive analysis of mitochondrial OXPHOS activity, content, and MtMP gene expression in the cotyledon samples from the crossbred cows with or without RP. Simultaneously, phenotypic data analysis and gross morphological studies were carried out in both groups. This study represents the inaugural exploration into the interplay of mitochondrial OXPHOS dynamics and MtMP gene expression within the context of RP, shedding light on their potential implications for the separation and evacuation of fetal membranes.
First, the phenotypic traits were meticulously examined to elucidate the variations in each parameter between the RP and normal placenta. In this regard, age, gestation length, and parity of the cows exhibited non-significant differences between the two groups, showing that these variables may not exert a substantial impact on the placental expulsion status in the crossbred cows. However, a significantly lower average service per conception was observed in cases of RP compared to the normal placenta. Furthermore, a detailed gross morphological analysis of retained and normal placenta was conducted. The bovine cotyledonary placenta, characterized by its organization into 100 to 120 placentomes, comprises fetal cotyledons interconnected with maternal caruncle tissues (Haeger et al. 2016). The number of cotyledons initially increases with placental growth and development but remains constant from mid-gestation onwards (Estrella et al. 2017). In line with this notion, our result showed a significant difference in a number of cotyledons, accompanied by variations in size, and shape.
As per the previous studies, oxidative stress, hormonal imbalance, and an inflammatory state are well-known features of retention of placenta (Kumari et al. 2015; Dervishi et al. 2016; Santos et al. 2017). Although the role of oxidative stress in RP pathology has been extensively studied, this is the first study to demonstrate reduced bioenergetics (OXPHOS complex activity) from freshly isolated placental mitochondria from RP compared to normal placenta in bovine. The mitochondrial genome encodes several subunits of electron transport chain complexes that are involved in normal cellular metabolism and energy production (Nolfi-Donegan et al. 2020). Among these, the respiratory chain's largest and most intricate subunit link is Complex I. The Complex I activities, and genes, which include ND1-6, serve as the foundation for cellular respiration. It employs the two electrons it releases from the oxidation of NADH-produced in the mitochondrial matrix to convert ubiquinone to ubiquinol. The cytochrome bc1 complex reoxidizes, ubiquinol, transferring electrons to water at complex IV to reduce molecular oxygen. These oxidation-reduction cascades are maintained until Complex V produces ATP. As the main point of entry for electrons into the respiratory chain, complex I is thought to be the rate-limiting step in respiration overall. It is also important for energy metabolism and has been linked to the control of ROS, which are molecules involved in several signaling pathways, including apoptosis (Sazanov 2015; Bhatti et al. 2017). The organization of OXPHOS complexes determines mitochondrial energy efficiency. We observed a significant reduction in the OXPHOS complex I activity in RP compared with normal placenta suggesting that there may be effects on mitochondrial efficiency and ATP production. As per the published report, changes in OXPHOS complex activities could disrupt the formation of mitochondrial supercomplexes and result in increased production of ROS and oxidative stress (Zhang et al. 2023). Therefore, reduced complex I activity may have led to the effect of mitochondrial content and energy production, which increases RP risk.
Next, we examined the association of mtDNA copy number with odds of RP as compared to normal placenta. This study tried to decipher the difference between mtDNA copy numbers in retained and normal placenta. This difference was reported to be non-significant, but a lower copy number in combination with OXPHOS Complex I activity regulating the production of ATP indicates the difference in energy requirement of the placental tissue during expulsion of the placenta. However, we also observed variation in mtDNA copy number among the individual animals of both groups, which may arise due to energy demand variation, genetic mutations, or environmental factors, potentially influencing placental function and contributing to RP’s. The mt-DNA is particularly vulnerable to oxidative damage, owing to factors such as the lack of histones or DNA-binding proteins, limiting DNA repair mechanisms, the lack of introns in mitochondrial genes, swift replication, and an insufficient and inaccurate proofreading system. It has been shown that damage to mtDNA, particularly deletions that happen in the circular mitochondrial genome's regulatory sections and are indicated by variations in the copy number of mtDNA, might interfere with the expression of mitochondrial genes. Because of this disturbance, there is less oxidative phosphorylation (Alghoul et al. 2023; de Lima et al. 2023). Additionally, oxidative stress is known to be an important pathogenesis causative factor implicated in RP (Amin and Hussein, 2022; Elmetwally et al. 2022; Martini et al. 2023), and may contribute to alterations in mitochondrial functions and downtrend mtDNA copy numbers through several mechanisms, it is plausible that higher levels of systematic ROS may damage mitochondrial metabolic functions, biogenesis and content or disrupt cellular structural elements, including the lipid membranes of mitochondria (Hu et al. 2020). In conjunction, our investigation posits that the correlation between mtDNA copy number and the incidence of retained placenta is inherently plausible from a biological standpoint. In agreement with our findings, extant research on pathologies associated with compromised perfusion, such as intrauterine growth restriction (IUGR) and preeclampsia, has recently demonstrated a discernible link between oxidative conditions and alterations in mitochondrial content across human, mice, and pig models, encompassing both augmented and diminished states (Luo et al. 2018; Jahan et al. 2023). The results obtained from this preliminary study on the retained placental tissue may be used as an impetus for future studies. This study lays the foundation for future endeavors aimed at unraveling the nuanced biology underpinning placental dynamics during parturition.
Mitochondrial DNA encodes several subunits of electron transport chain complexes that are involved in normal cellular metabolism and energy production (Sazanov 2015). Thus, further, we have examined all 13 mitochondrial PCGs expressions using the qRT-PCR method to deduce the gene expression in the normal and RP. The results indicates a significant down regulation (p value= 0.01) of mitochondrial OXPHOS complex genes (ND1, ND2, ND3, ND4L, ND4, Cytb, COX1, COX3 and ATP6) in RP. These genes are reported to be the essential components of the electron transfer chain and help in ATP production. Generally, the downregulation of the complex I genes in our study directly implies reduced ATP production and stress on mitochondria for energy production in the RP. Complex III is a membrane-bound enzyme that catalyzes the transfer of electrons from ubiquinol to cytochrome c. It does this by connecting the activity to the intracellular translocation of protons and helps to create the proton gradient that is needed for the creation of ATP across the mitochondrial membrane. In addition, the terminal enzyme of the ETC is cyclooxygenase, or COX, which is a member of OXPHOS complex IV. It takes electrons from the reduced form of Cyt C and moves them to the reduced form of molecular oxygen, which is water. The movement of protons by COX from the matrix into the inter-membrane gap is linked to this process. The downtrend of genes of both Complex III and IV ultimately impacts the proton gradient across the inter-membrane space of the mitochondria. Specifically, the low level of COX leads to a hypoactive state culminating in energy depletion (Sazanov 2015). In agreement with our result, Luo et al. (2018) showed that IUGR placental mitochondria of pigs exhibit low mtDNA-encoded gene expression in comparison to normal intrauterine gestation, and further showed that ND1, ND4L and COX3 gene expression in IUGR placental mitochondria was significantly reduced.
Further, the proton gradient created by the transfer of reduction equivalents, initiates the last link in the respiratory chain, ATP synthase (F1F0 ATPase/ complex V). F1F0-ATPases are the primary ATP generators in mitochondria, chloroplasts, and bacterial plasma membranes. The enzyme complex V creates ATP by using a stream of protons that flow through the mitochondria's inner membrane (Grzybowska-Szatkowska et al. 2014). The proper assembly of complex V holoenzyme is the responsibility of subunit 8 of the mitochondrial F1F0-ATP synthase (ATP8), an inner membrane polypeptide of the F0 component, while, ATP6 functions as the integral component of the proton channel (Zhang et al. 2017). An interesting finding of this study that significant reduction in the ATP6, which directly regulates the energy production in the RP. The correlation analysis conducted on the gene expressions of different complexes in our study provides robust support for the intricate relationships within mitochondrial protein-coding gene networks. We delved deeply into the interconnectedness of gene expressions across various complexes. The findings not only confirm direct correlations between specific gene expressions but also unveil broader patterns of coordination and interaction among the complexes. This comprehensive understanding underscores the coordinated regulation and functional integration of mitochondrial protein-coding genes, shedding light on the underlying mechanisms governing mitochondrial biology and cellular processes. We further substantiated the functional roles of mitochondrial PCGs by performing comprehensive GO, KEGG pathway, and functional enrichment analyses on the entire set of genes associated with RP. The downregulation observed in the expression of these complex V genes implies an energy-deficient state in various cells and tissues, consistent with findings in previous studies (Dorgi et al. 2020; Sadeesh et al. 2023). Notably, the significant alteration in ATP6 observed in pigs with IUGR lends support to our results (Luo et al. 2018). Additionally, our observations align with previously published reports in humans, where investigations demonstrated abnormalities in mitochondrial structure and perturbations in molecular pathways regulating mitochondrial content and function within the placenta under the conditions of IUGR and preeclampsia (Lefebvre et al. 2018; Vangrieken et al. 2021). These collective findings underscore the significance of mitochondrial dysfunction in the context of RP, shedding light on potential connections and shared pathways across species and reproductive complications.
Conclusion
Within the context of RP, the study reveals noteworthy observations: (1) a declining trend in OXPHOS complex I activity, accompanied by discernible alterations in the transcript levels of numerous mitochondrial protein-coding genes. These changes detrimentally impact energy production, compromise complex activities, and intensify oxidative stress. (2) Variability in mtDNA copy numbers among individual subjects may be correlated with elevated energy requirements or mitochondrial inadequacy, thereby elevating associated risks. To conclude, this investigation elucidates the association between RP and significant eccentricities within the placental mitochondria. These eccentricities may intricately connect with disruptions in energy production, hormonal balance, and the exacerbation of oxidative stress, ultimately influencing the process of fetal membrane separation and evacuation, which may increase the risk of RP.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
MSL assumed responsibility for conducting the experiments and analyzing the data. Additionally, MSL drafted the manuscript in collaboration with SEM. SEM played a pivotal role in providing insightful guidance, conceptualizing the study, and designing the research. All authors actively participated in reviewing the manuscript and contributed valuable feedback during both the preparation and revision phases.
Funding
This study was financially supported by ICAR-NDRI Karnal.
Acknowledgements
We express gratitude to the Indian Council of Agricultural Research (ICAR) for financial support through the ICAR-National Dairy Research Institute. The authors extend their appreciation to the Director of ICAR-National Dairy Research Institute for facilitating the essential resources required to conduct this study. Furthermore, the author acknowledges ICAR-National Dairy Research Institute for granting the Institute fellowship to Madhuri.
Data Availability Statement
Data generated from this study is presented in the manuscript. Further details are presented in additional files. Information not included would be shared by the corresponding author on reasonable request.
Funding
This work was supported by the National Dairy Research Institute, Karnal, India.
Data Availability
Data generated from this study is presented in the manuscript. Further details are presented in additional files. Information not included would be shared by the corresponding author on reasonable request.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Consent to Participate
This article does not contain any individual person’s data in any form.
Consent for Publication
All authors agreed to publish the research in this journal.
Conflict of Interest Statement
The authors declare that they have no competing interests
References
- Alghoul E, Basbous J, Constantinou A (2023) Compartmentalization of the DNA damage response: Mechanisms and functions. DNA repair 103524.
- Amin Y A, Hussein H A (2022) Latest update on predictive indicators, risk factors and ‘Omic’technologies research of retained placenta in dairy cattle–A review. Reprod Domest Anim 57(7): 687-700. [CrossRef]
- Andersen H, Plum M (1965) Gestation length and birth weight in cattle and buffaloes: A review. J Dairy Sci 48(9): 1224-1235. [CrossRef]
- Bhatti J S, Bhatti G K, Reddy P H (2017) Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis 1863(5): 1066-1077. [CrossRef]
- Chen Z, Robbins K M, Wells K D, Rivera RM (2013) Large offspring syndrome: A bovine model for the human loss-of-imprinting overgrowth syndrome Beckwith- Wiedemann. Epigenetics 8(6): 591-601. [CrossRef]
- de Lima C B, Martin H, Pecora Milazzotto M, Sirard M A (2023) Genome-wide methylation profile of mitochondrial DNA across bovine preimplantation development. Epigenetics 18(1): 2241010. [CrossRef]
- Dervishi E, Zhang G, Hailemariam D, Dunn S M, Ametaj BN (2016) Occurrence of retained placenta is preceded by an inflammatory state and alterations of energy metabolism in transition dairy cows. J Anim Sci Biotechnol 7(1): 1-13. [CrossRef]
- Díaz P, Powell T L, Jansson T (2014) The role of placental nutrient sensing in maternal-fetal resource allocation. Biol Reprod 91(4): 82-1. [CrossRef]
- Dimri U, Ranjan R, Sharma M C, Varshney V (2010) Effect of vitamin E and selenium supplementation on oxidative stress indices and cortisol level in blood in water buffaloes during pregnancy and early postpartum period. Trop Anim Health Prod 42(3): 405–410. [CrossRef]
- Dorji J, Vander Jagt C J, Garner J B, Marett L C, Mason B A, Reich C M, Daetwyler H D (2020) Expression of mitochondrial protein genes encoded by nuclear and mitochondrial genomes correlate with energy metabolism in dairy cattle. BMC Genom 21(1): 1-17. [CrossRef]
- Elmetwally M A, Shalapy SA, Montaser A (2022) Predictive biochemical and oxidative markers for dairy cows with and without retained fetal placenta. https://doi.org/10.21203/rs.3.rs-2227667/v1. [CrossRef]
- Elsayed D H, Abdelrazek HMA, El-Azzazi FE, Ismail SAA, Mahmoud YK (2020) Hormonal and metabolic profiles related to placental retention with emphasis on oxidative stress and serotonin receptors in pluriparous buffaloes. Reprod Domest Anim 1–10. [CrossRef]
- Estrella C A S, Kind K L, Derks A, Xiang R, Faulkner N, Mohrdick M, ... Hiendleder S (2017) Remodeling of the bovine placenta: Comprehensive morphological and histomorphological characterization at the late embryonic and early accelerated fetal growth stages. Placenta 55: 37-46. [CrossRef]
- Fisher J J, Bartho LA, Perkins AV, Holland OJ (2019) Placental mitochondria and reactive oxygen species in the physiology and pathophysiology of pregnancy. Clin Exp Pharmacol Physiol 47(1): 176-184. [CrossRef]
- Gatford K L, Andraweera P H, Roberts C T, Care AS (2020) Animal models of preeclampsia: Causes, consequences, and interventions. Hypertension 75(6): 1363-1381. [CrossRef]
- Ghai S, Monga R, Mohanty TK, Chauhan MS, Singh D (2012) Term placenta shows methylation independent down regulation of Cyp19 gene in animals with retained fetal membranes. Res Vet Sci 92(1): 53-59. [CrossRef]
- Grzybowska-Szatkowska L, Ślaska B, Rzymowska J, Brzozowska A, Floriańczyk B (2014) Novel mitochondrial mutations in the ATP6 and ATP8 genes in patients with breast cancer. Mol Med Rep 10(4): 1772-1778. [CrossRef]
- Haeger JD, Hambruch N, Pfarrer C (2016) The bovine placenta in vivo and in vitro. Theriogenology 86(1): 306-312.
- Hanafi E M, Ahmed W M, ElKhadrary H H, Zabaal MM (2011) An overview of placental retention in farm animals. J Sci Res 7(5): 643-651. [CrossRef]
- Hu C, Yang Y, Deng M, Yang L, Shu G, Jiang Q, ... Wu G (2020) Placentae for low birth weight piglets are vulnerable to oxidative stress, mitochondrial dysfunction, and impaired angiogenesis. Oxid Med Cell Longev 2020. [CrossRef]
- Jahan F, Vasam G, Green AE, Bainbridge SA, Menzies KJ (2023) Placental Mitochondrial Function and Dysfunction in Preeclampsia. Int J Mol Sci 24(4): 4177. [CrossRef]
- Jemal J Y (2016) A review on retention of placenta in dairy cattle. Int J Vet Sci 5(4): 200- 207.
- Jia Y, Song H, Gao G, Cai D, Yang X, Zhao R (2015) Maternal betaine supplementation during gestation enhances expression of mtDNA-encoded genes through D-loop DNA hypomethylation in the skeletal muscle of newborn piglets. J Agric Food Chem 63(46): 10152–60. [CrossRef]
- Keywanloo M, Ramroodi A, Jebell Javan A, Narenji Sani R, Ahmadi-hamedani M (2021) Comparison of total antioxidant capacity, total oxidation capacity and oxidative stress index in cows with and without retained fetal membranes. J Ve Lab Res 12(2): 141-149. [CrossRef]
- Kumari S, Prasad S, Kumaresan A, Manimaran A, Patbandha TK, Pathak R, ... Ravi S K (2015) Risk factors and impact of retained fetal membranes on performance of dairy bovines reared under subtropical conditions. Trop Anim Health Prod 47(2): 285–290. [CrossRef]
- Lefebvre T, Roche O, Seegers V, Cherif M, Khiati S, Gueguen N, .. Gascoin G (2018) Study of mitochondrial function in placental insufficiency. Placenta 67:1-7. [CrossRef]
- Linnane A, Ozawa T, Marzuki S, Tanaka M (1989) Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet 333(8639): 642-645. [CrossRef]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real- time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4): 402- 8. https://doi.org/10.1006/meth.2001.1262. [CrossRef]
- Luo Z, Luo W, Li S, Zhao S, Sho T, Xu X, ...Xu J (2018) Reactive oxygen species mediated placental oxidative stress, mitochondrial content, and cell cycle progression through mitogen-activated protein kinases in intrauterine growth restricted pigs. Reprod Biol 18(4): 422-431. [CrossRef]
- Martini S, Aceti A, Della Gatta AN, Beghetti I, Marsico C, Pilu G, Corvaglia L (2023) Antenatal and Postnatal Sequelae of Oxidative Stress in Preterm Infants: A Narrative Review Targeting Pathophysiological Mechanisms. Antioxidants 12(2): 422. [CrossRef]
- May-Panloup P, Desquiret V, Moriniere C, Ferré-L'Hôtellier V, Lemerle S, Boucret L, ... Reynier P (2014) Mitochondrial macro-haplogroup JT may play a protective role in ovarian ageing. Mitochondrion 18: 1-6. [CrossRef]
- Miller L W (2013) Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol 379(1–2): 62-73. [CrossRef]
- Nolfi-Donegan D, Braganza A, Shiva S (2020) Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol 37: 101674. [CrossRef]
- Peter AT, Beg MA, Ahmad E, Bergfeld DR (2017) Trophoblast of domestic and companion animals: Basic and applied clinical perspectives. Anim Reprod 14: 1209-1224. [CrossRef]
- Polei M, Gunther J, Koczan D, Furbass R (2020) Trophoblast cell differentiation in the bovine placenta: Differentially expressed genes between uninucleate trophoblast cells and trophoblast giant cells are involved in the composition and remodeling of the extracellular matrix and O-glycan biosynthesis. BMC mol cell boil 21: 1. [CrossRef]
- Rao TKS, Kumar B, Chaurasia S, Sharma VK, Baishya A, Singh A, Patel NB (2018) Fetal Membranes and Associated Complications in Dairy Animals: A Review. Theriogenology 8(3): 125-136.
- Sadeesh E M, Lahamge MS, Malik A N, Ampadi A (2024) Differential Expression of Nuclear-Encoded. [CrossRef]
- Genes of ATP Synthase Across Different Tissues of Female Buffalo. Mol Biotechnol 10.1007/s12033-024-01085-x. [CrossRef]
- Sadeesh E M, Singla N, Lahamge M S, Kumari S, Ampadi A N, Malik A (2023) Tissue heterogeneity of mitochondrial activity, biogenesis and mitochondrial protein gene expression in buffalo. Mol Biol Rep 50(6): 5255-5266. [CrossRef]
- Santos R B, Silva JM, Beletti ME (2017) Ultrastructure of bovine placenta during all gestational periods. Arq Bras Med Vet Zootec 69(6): 1376-1384. [CrossRef]
- Sazanov L A (2015) A giant molecular proton pump: Structure and mechanism of respiratory complex I. Nat Rev Mol Cell Biol 16(6): 375-388. [CrossRef]
- Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C (2012) Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc 7(6): 1235-1246. [CrossRef]
- Spinelli J B, Haigis MC (2018) The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol 20(7): 745-754. [CrossRef]
- Vangrieken P, Al-Nasiry S, Bast A, Leermakers PA, Tulen CB, Schiffers PM, ... Remels AH (2021) Placental mitochondrial abnormalities in preeclampsia. Reprod Sci 28: 2186-2199. [CrossRef]
- Xiang R, Lee AM, Eindorf T, Javadmanesh A, Ghanipoor-Samami M, Gugger M, ... Hiendleder S (2014) Widespread differential maternal and paternal genome effects on fetal bone phenotype at mid-gestation. J Bone Miner Res 29(11): 2392- 2404. [CrossRef]
- Zhang H, Zheng Y, Liu X, Zha X, Elsabagh M, Ma Y, ... Wang M (2023) Autophagy attenuates placental apoptosis, oxidative stress and fetal growth restriction in pregnant ewes. Environ Int 173 : 107806. [CrossRef]
- Zhang R, Wang Y, Ye K, Picard M, Gu Z (2017) Independent impacts of aging on mitochondrial DNA quantity and quality in humans. BMC Genom. 18(1): 1-14. [CrossRef]
Figure 1.
A-B) Placental gross morphology of crossbred cow in normal and retention of placenta condition (L) large size (M) Medium size (S) small size cotyledon; C-D) Dorsal view of fetal cotyledons with feto–maternal interface of cotyledon via villi E-F) Ventral view of fetal cotyledons (thickening and necrosis of villi in cotyledons indicated by arrow in retained placental cotyledon).
Figure 1.
A-B) Placental gross morphology of crossbred cow in normal and retention of placenta condition (L) large size (M) Medium size (S) small size cotyledon; C-D) Dorsal view of fetal cotyledons with feto–maternal interface of cotyledon via villi E-F) Ventral view of fetal cotyledons (thickening and necrosis of villi in cotyledons indicated by arrow in retained placental cotyledon).
Figure 2.
Placental mitochondrial OXPHOS enzymatic activity, mitochondrial content and relative gene expression in normal and retained placenta (n=3 per group). (A) OXPHOS complex I specific activity (B) mtDNA copy number. **P < 0.01, compared with the normal placenta. (C) Relative mRNA expression levels of protein-coding mitochondrial genes NADH dehydrogenase subunits (ND1, ND2, ND3, ND4, ND4L, ND5 and ND6), cytochrome b (Cytb), cytochrome c oxidase subunits (COX1, COX2, COX3), and ATP synthase F0 subunits (ATP6 and ATP8) in normal and retained placenta of crossbred cattle. Mean values for normal placenta animals were set to 1.0 and values for retained placenta animals are expressed relative to normal placenta. Statistical significance was assessed with an Independent t-test, asterisks indicate significantly different expression *P< 0.05, **P< 0.01, ***P<0.001.
Figure 2.
Placental mitochondrial OXPHOS enzymatic activity, mitochondrial content and relative gene expression in normal and retained placenta (n=3 per group). (A) OXPHOS complex I specific activity (B) mtDNA copy number. **P < 0.01, compared with the normal placenta. (C) Relative mRNA expression levels of protein-coding mitochondrial genes NADH dehydrogenase subunits (ND1, ND2, ND3, ND4, ND4L, ND5 and ND6), cytochrome b (Cytb), cytochrome c oxidase subunits (COX1, COX2, COX3), and ATP synthase F0 subunits (ATP6 and ATP8) in normal and retained placenta of crossbred cattle. Mean values for normal placenta animals were set to 1.0 and values for retained placenta animals are expressed relative to normal placenta. Statistical significance was assessed with an Independent t-test, asterisks indicate significantly different expression *P< 0.05, **P< 0.01, ***P<0.001.
Figure 3.
A) Cluster heatmap shows the different levels of gene expression and the relationship between different samples and genes. B) PCA plot illustrating gene expression variations among samples. Arrows represent variables, while circles represent samples and are labeled by their abbreviations. Axis percentages indicate the variance explained by each principal component. C) The volcano plot shows the distribution of the DEGs. Genes with –log10 p-value >1.3 and log2 fold change > 0.59 or < -0.59 are considered as significant (shown with red and blue circles, respectively). Genes (upregulated or downregulated) with the highest relative expression (Normal vs Retained) have been specified with their gene symbol.
Figure 3.
A) Cluster heatmap shows the different levels of gene expression and the relationship between different samples and genes. B) PCA plot illustrating gene expression variations among samples. Arrows represent variables, while circles represent samples and are labeled by their abbreviations. Axis percentages indicate the variance explained by each principal component. C) The volcano plot shows the distribution of the DEGs. Genes with –log10 p-value >1.3 and log2 fold change > 0.59 or < -0.59 are considered as significant (shown with red and blue circles, respectively). Genes (upregulated or downregulated) with the highest relative expression (Normal vs Retained) have been specified with their gene symbol.
Figure 4.
Mantel tests and Pearson's correlation matrix illustrate the relationships among the gene expression across normal and retained placenta. Mantel's r (coefficient of correlation) represents the correlation in which the thickness of the line represents the level of correlation and the color of the lines represents the statistical significance. Color shed represents the positive and negative correlations and square size and color intensity reflect correlation strength. (*) denotes the level of significance of correlation coefficient * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 4.
Mantel tests and Pearson's correlation matrix illustrate the relationships among the gene expression across normal and retained placenta. Mantel's r (coefficient of correlation) represents the correlation in which the thickness of the line represents the level of correlation and the color of the lines represents the statistical significance. Color shed represents the positive and negative correlations and square size and color intensity reflect correlation strength. (*) denotes the level of significance of correlation coefficient * p < 0.05, ** p < 0.01, *** p < 0.001.
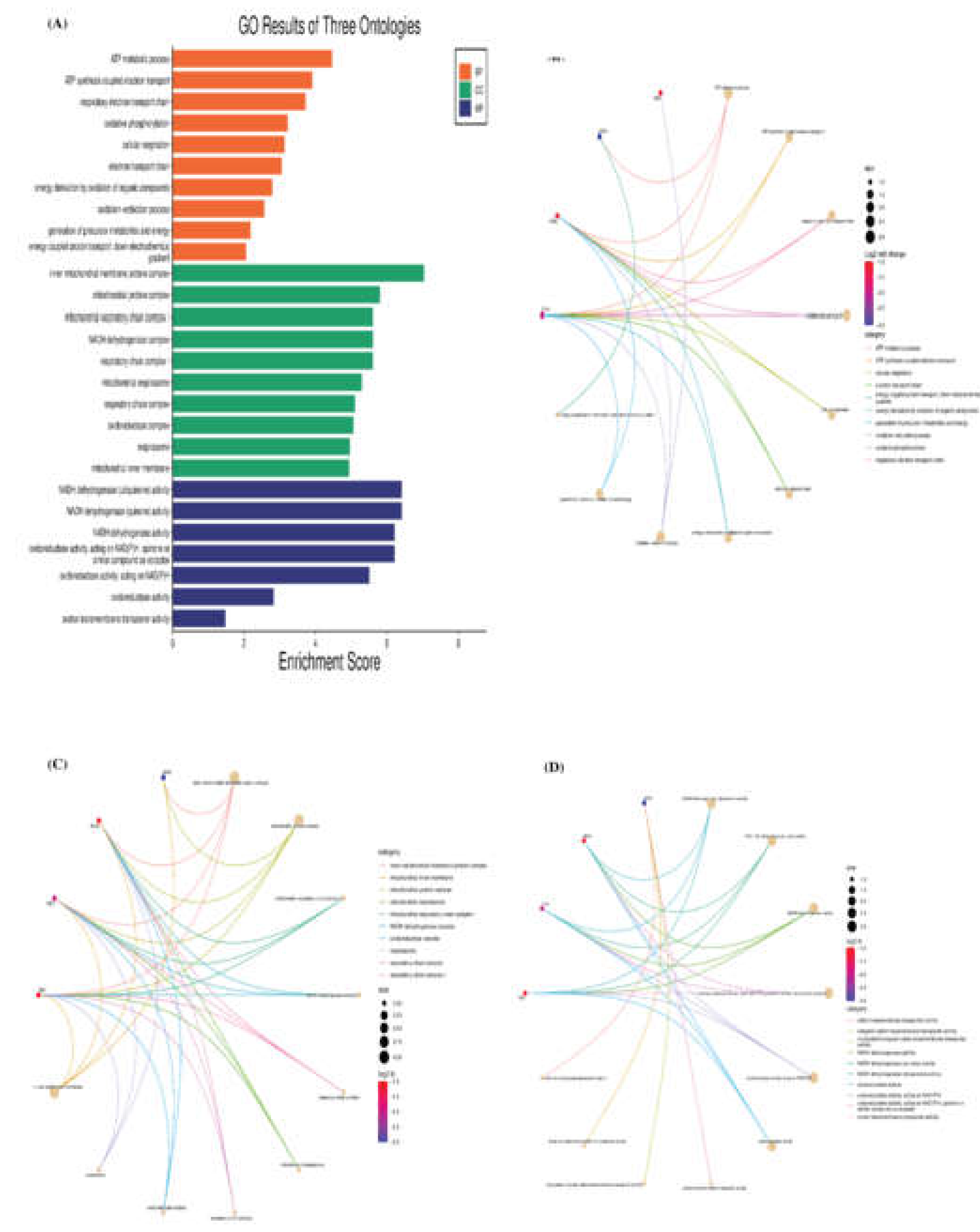
Figure 5.
Gene Ontology and Functional Enrichment Analysis of Differentially Expressed Mitochondrial Protein-Coding Genes in Retained Samples. (A) Bar Graph illustrating GO Annotation of significant genes in retained placenta. The bar graph highlights the significant enrichment of genes in various biological processes (BP), cellular components (CC), and molecular functions (MF) between the two groups. The functional GO enrichment results are presented as gene-concept networks for (B) BP, (C) CC, and (D) MF. Each connection between a gene and a term signifies the gene's involvement in that particular GO term.
Figure 5.
Gene Ontology and Functional Enrichment Analysis of Differentially Expressed Mitochondrial Protein-Coding Genes in Retained Samples. (A) Bar Graph illustrating GO Annotation of significant genes in retained placenta. The bar graph highlights the significant enrichment of genes in various biological processes (BP), cellular components (CC), and molecular functions (MF) between the two groups. The functional GO enrichment results are presented as gene-concept networks for (B) BP, (C) CC, and (D) MF. Each connection between a gene and a term signifies the gene's involvement in that particular GO term.
Table 1.
Comparative morphological features in normal (n=6) and retained placenta (n=6) of crossbred cattle, Mean ± SEM; *Significant at p< 0.05 between the group.
Table 1.
Comparative morphological features in normal (n=6) and retained placenta (n=6) of crossbred cattle, Mean ± SEM; *Significant at p< 0.05 between the group.
| Parameter |
Normal Placenta (Mean±SE) |
Retained Placenta
(Mean±SE) |
p value
|
| Age (yrs) |
4.7 ± 0.54 |
5.82 ± 0.47 |
Ns |
| Gestation Period (days) |
280.83 ± 1.796 |
278 ± 2.77 |
Ns |
| Parity |
3 ± 0.63 |
3.83 ± 0.401 |
Ns |
| Service per conception |
1.33 ± 0.21 |
2.67 ± 0.21 |
* |
| Calf weight (Kg) |
26.17±1.47 |
23.83 ± 2.23 |
Ns |
| Cotyledon number |
96.67±1.89 |
87.5 ± 3.51 |
* |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).