Submitted:
03 May 2024
Posted:
06 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. The Phenolic Compounds Composition of Grape Pomace Extracts
2.2. Encapsulation Efficiency of Total Phenolic Compounds from Grape Pomace Extracts
2.3. Physicochemical Characterization of Microencapsulated Powder
2.3.1. Morphology of Microencapsulated Powder
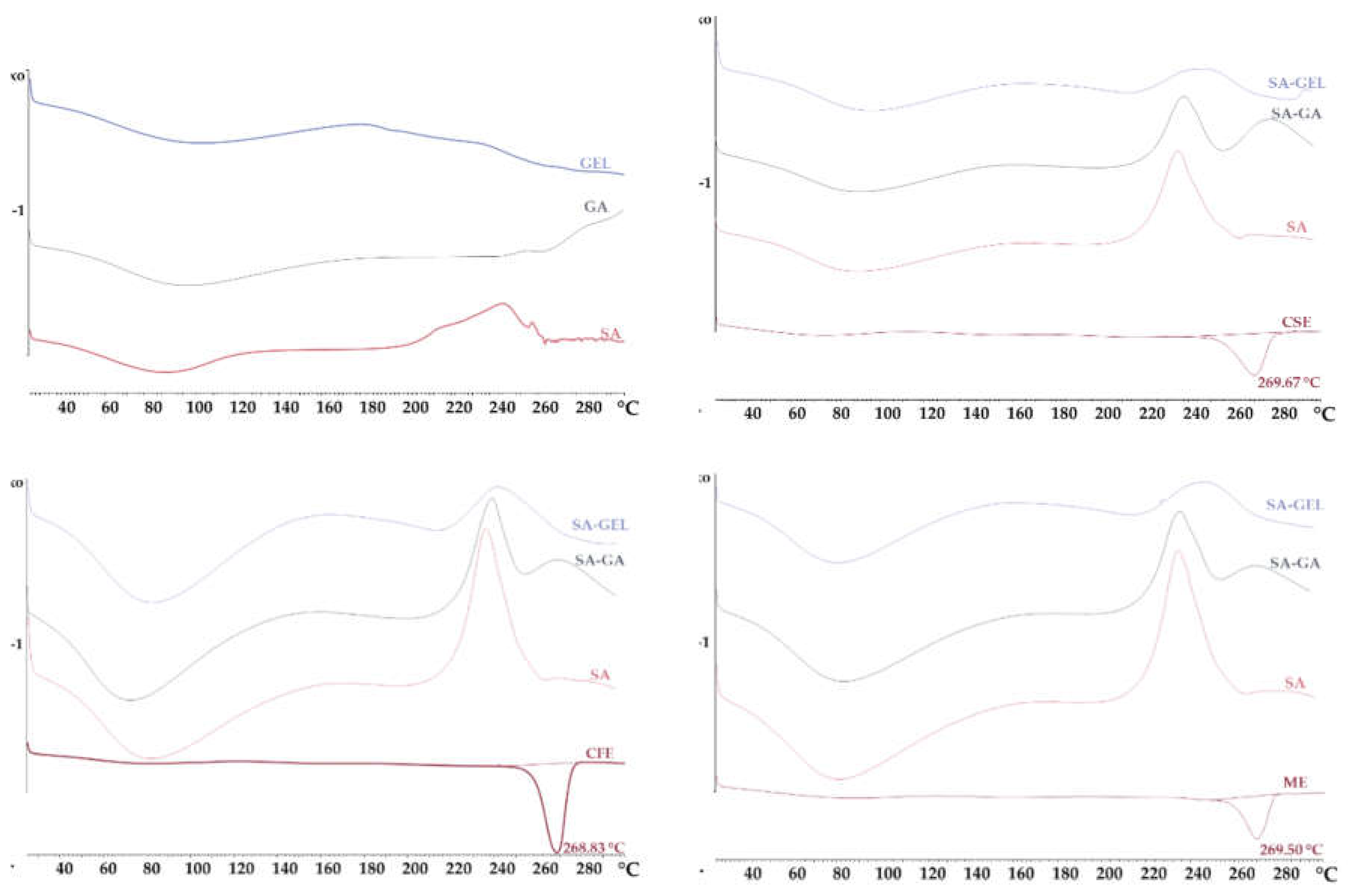
2.3.2. X-Ray Powder Diffraction and Differential Scanning Calorimetry Analysis
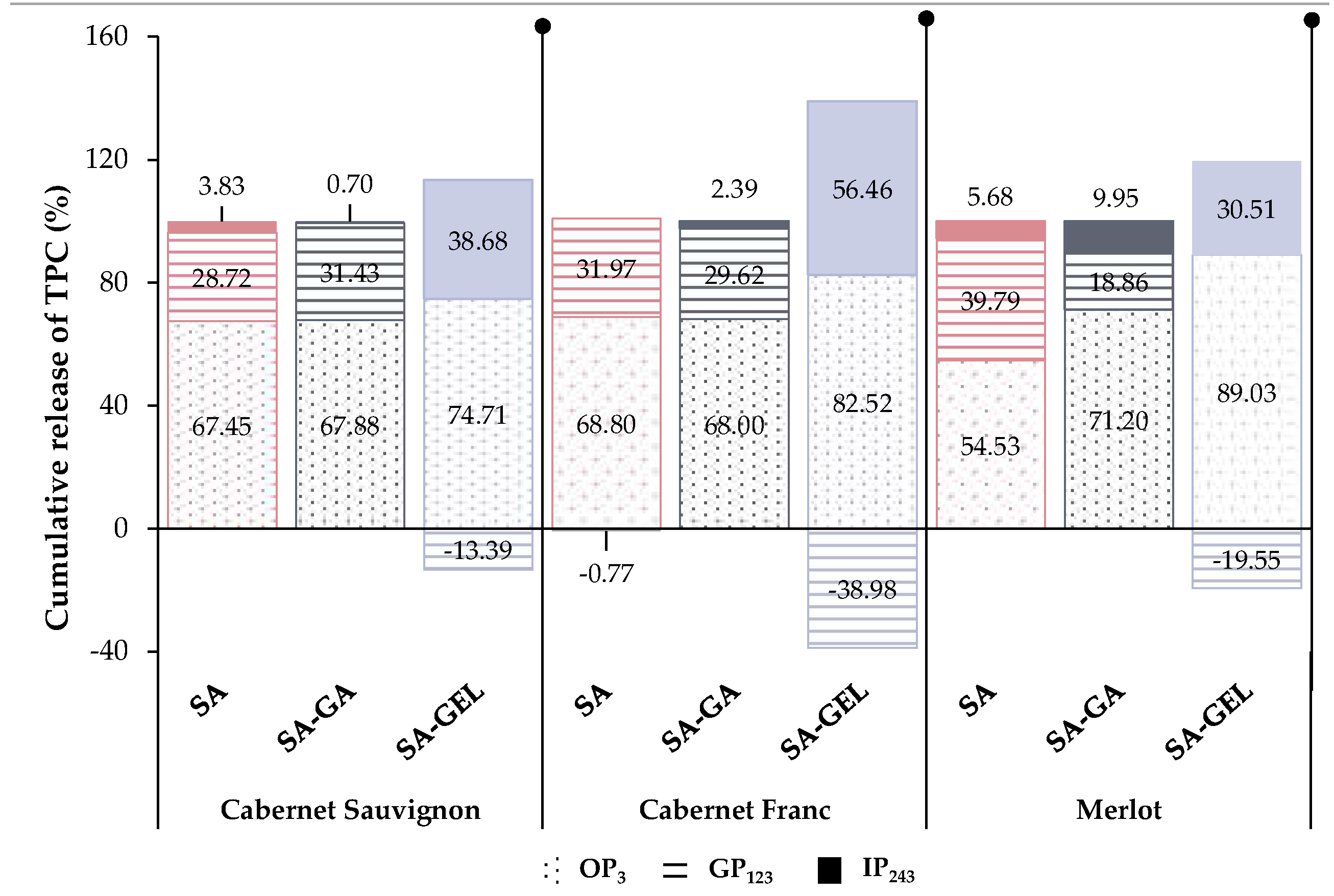
2.4. In Vitro Release of Total Phenolic Compounds from Microencapsulated Powders
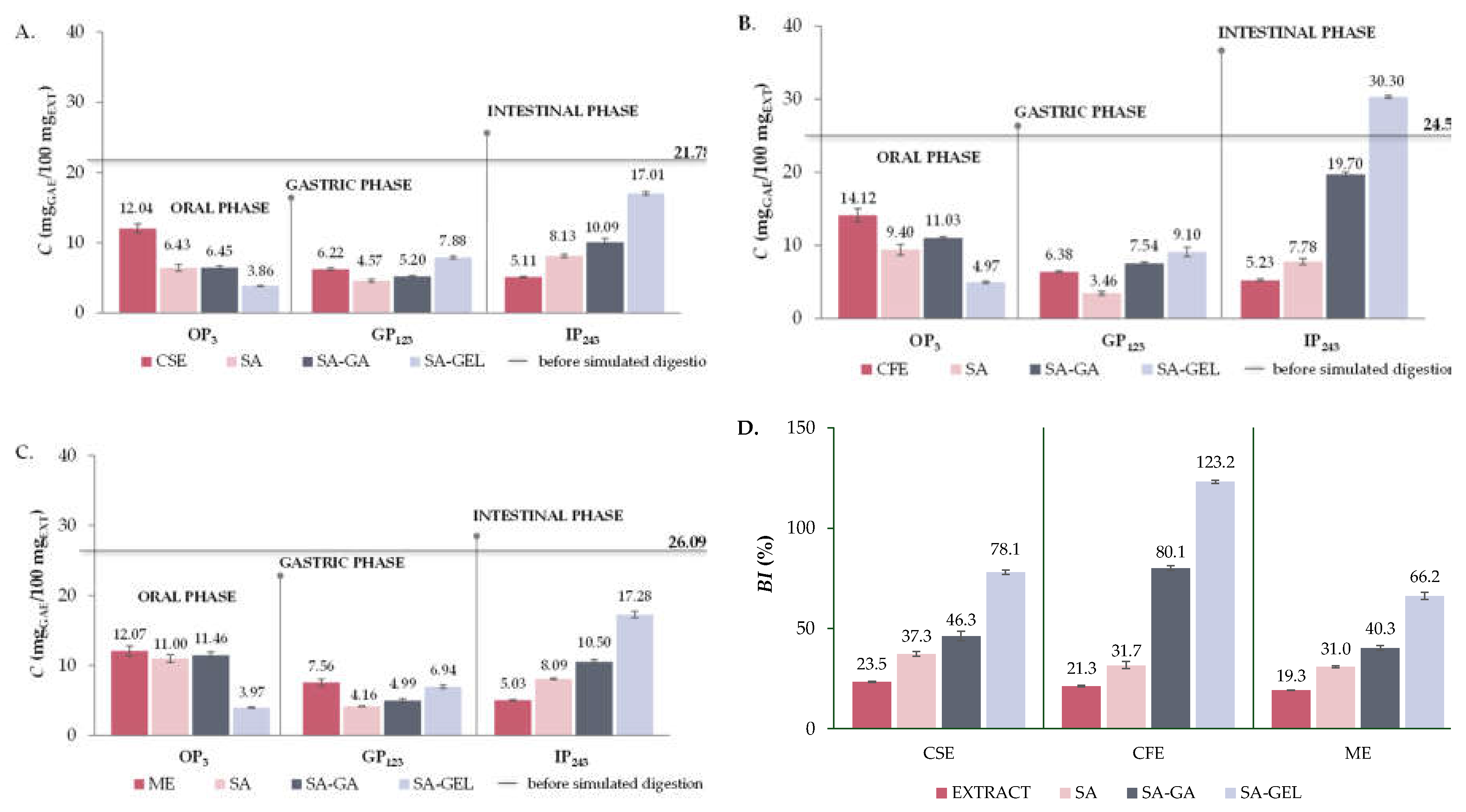
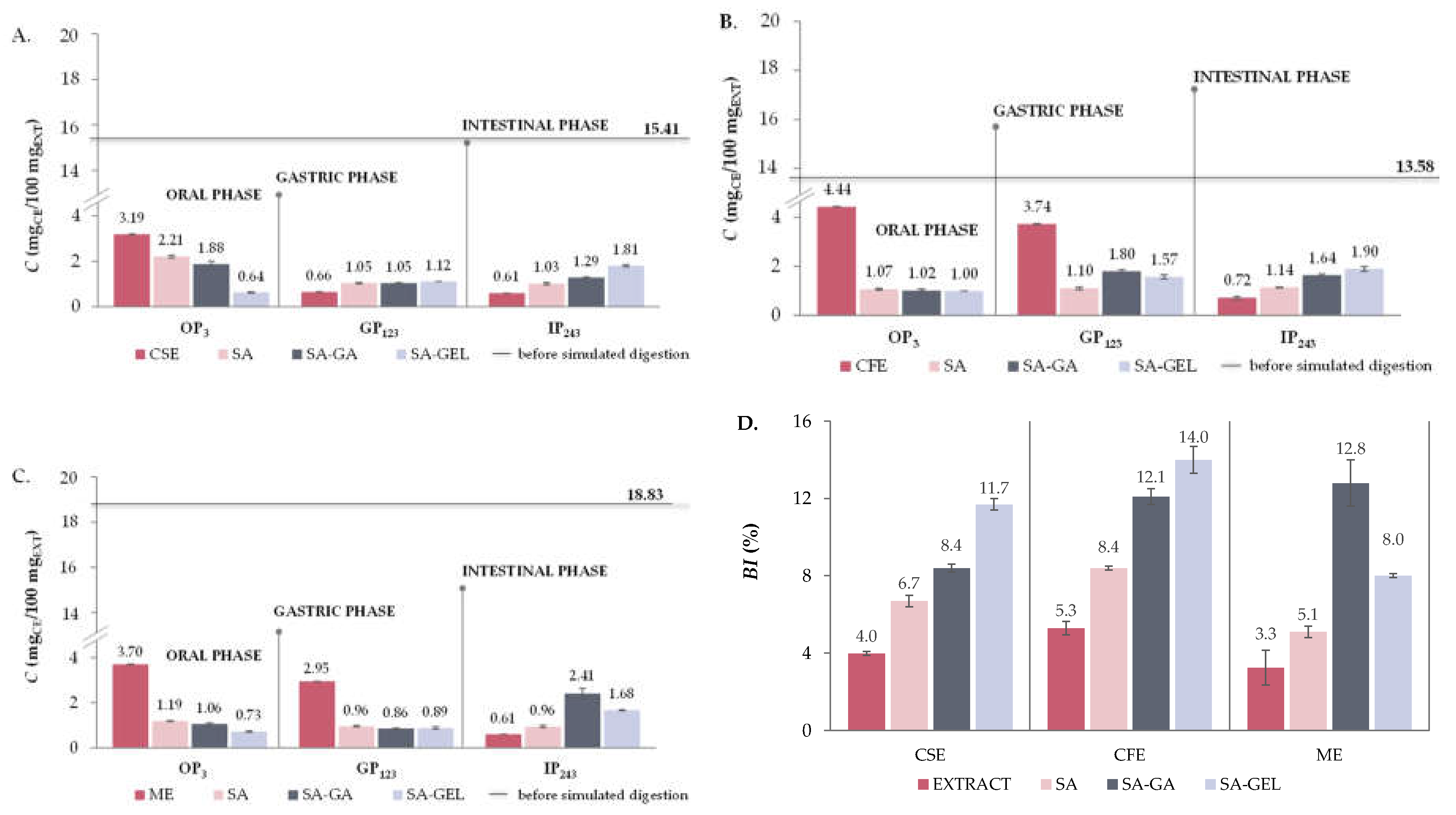
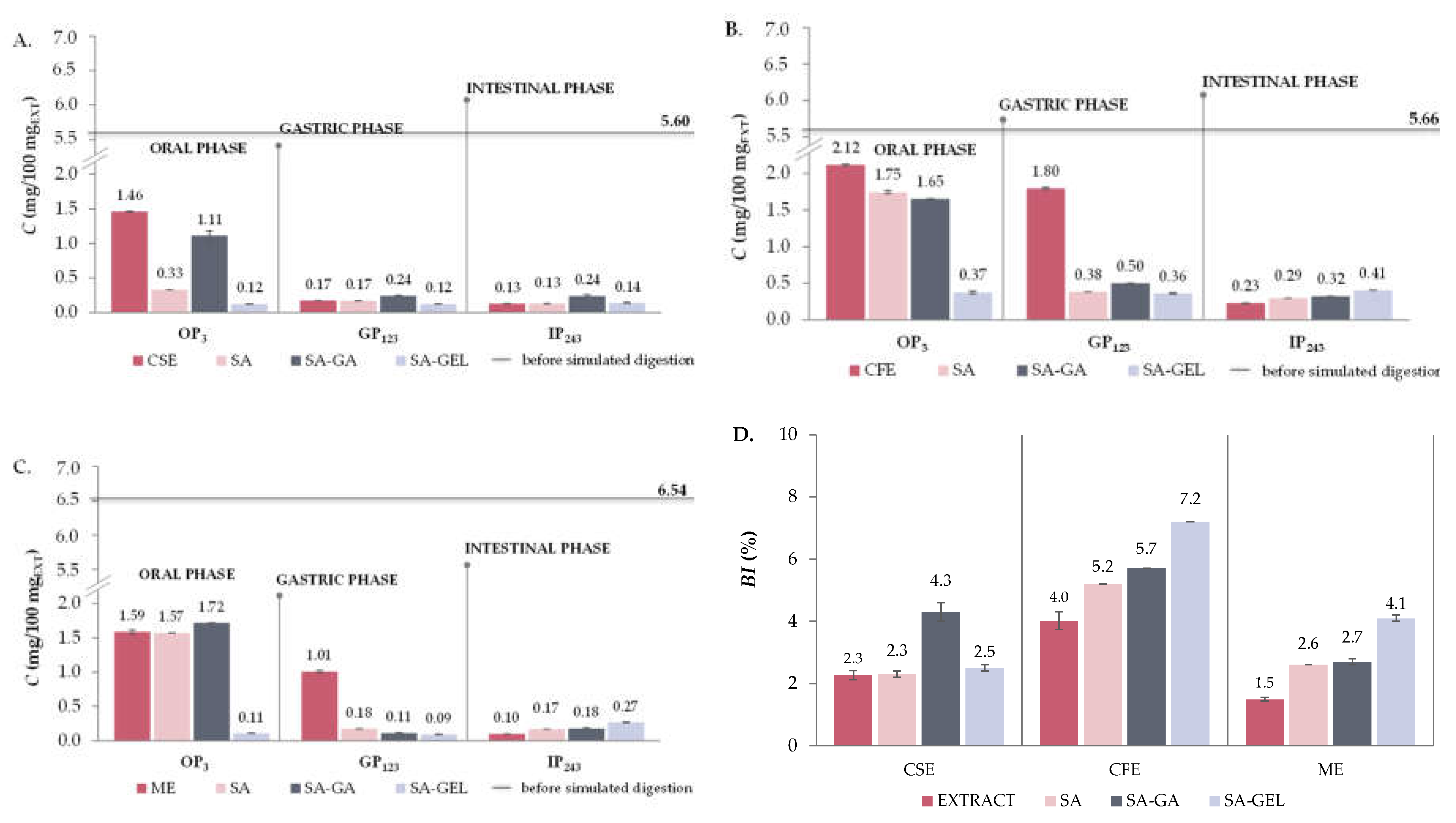
2.5. In Vitro Simulated Digestion and Bioaccessibility Index of Phenolic Compounds
2.5.1. Total Phenolic Compounds, Total Flavonoid Compounds and Total Extractable Proanthocyanidins
2.5.2. Individual Phenolic Compounds
Phenolic Acids
Stilbenes
Flavanols
Flavonols
Anthocyanins
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Grape Pomace Samples
4.3. Preparation of Grape Pomace Phenol-Rich Extracts
4.4. Determination of Total Phenolic Compounds
4.5. Determination of Individual Phenolic Compounds
4.6. Encapsulation of Grape Pomace Extracts by Freeze-Drying Method
4.7. Determination of Encapsulation Efficiency
4.8. Physicochemical Characterization of Grape Pomace Extracts, Coatings and Microencapsulated Powder
4.8.1. Scanning Electron Microscopy (SEM)
4.8.2. X-ray Powder Diffraction (XPRD)
4.8.3. Differential Scanning Calorimetry (DSC)
4.9. Release Studies of Phenolic Compounds
4.9.1. Enzyme-Free Release Study and Release Kinetics
4.9.2. Simulated Digestion In Vitro and Bioaccessibility Index (BI)
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green Processing and Biotechnological Potential of Grape Pomace: Current Trends and Opportunities for Sustainable Biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef] [PubMed]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; Da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manage. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic Compounds in Fruits – an Overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, X.; Zheng, H.; Li, J.; Wu, X.; Xu, J.; Zhen, Z.; Du, C. The Application of Encapsulation Technology in the Food Industry: Classifications, Recent Advances, and Perspectives. Food Chem. 2024, 21, 101240. [Google Scholar] [CrossRef]
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier, 2015; ISBN 978-0-08-100092-2.
- Peppas, N.A.; Narasimhan, B. Mathematical Models in Drug Delivery: How Modeling Has Shaped the Way We Design New Drug Delivery Systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonçalves, A.E.D.S.S.; Fett, R. Phenolic Compounds Content and Antioxidant Activity in Pomace from Selected Red Grapes (Vitis Vinifera L. and Vitis Labrusca L.) Widely Produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Iora, S.R.F.; Maciel, G.M.; Zielinski, A.A.F.; Da Silva, M.V.; Pontes, P.V.D.A.; Haminiuk, C.W.I.; Granato, D. Evaluation of the Bioactive Compounds and the Antioxidant Capacity of Grape Pomace. Int. J. Food Sci. Technol. 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic Compounds, Antioxidant, and Antibacterial Properties of Pomace Extracts from Four Virginia-grown Grape Varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef]
- Jin, Q.; O’Hair, J.; Stewart, A.C.; O’Keefe, S.F.; Neilson, A.P.; Kim, Y.-T.; McGuire, M.; Lee, A.; Wilder, G.; Huang, H. Compositional Characterization of Different Industrial White and Red Grape Pomaces in Virginia and the Potential Valorization of the Major Components. Foods 2019, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef]
- Ramachandran, V.; Raja, B. Protective Effects of Syringic Acid against Acetaminophen-Induced Hepatic Damage in Albino Rats. J. Basic Clin. Physiol. Pharmacol. 2010, 21. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Kang, H.; Lee, K.; Yang, G.; Ham, I.; Bu, Y.; Kim, H.; Choi, H.-Y. The Aerial Part of Taraxacum Coreanum Extract Has an Anti-Inflammatory Effect on Peritoneal Macrophages In Vitro and Increases Survival in a Mouse Model of Septic Shock. J. Ethnopharmacol. 2013, 146, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, D.; Yi, Y.; Qi, H.; Gao, X.; Fang, H.; Gu, Q.; Wang, L.; Gu, L. Syringic Acid Extracted from Herba Dendrobii Prevents Diabetic Cataract Pathogenesis by Inhibiting Aldose Reductase Activity. J. Evid. Based Complementary Altern. Med. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Güven, M.; Aras, A.B.; Topaloğlu, N.; Özkan, A.; Şen, H.M.; Kalkan, Y.; Okuyucu, A.; Akbal, A.; Gökmen, F.; Coşar, M. The Protective Effect of Syringic Acid on Ischemia Injury in Rat Brain. Turk. J. Med. Sci. 2015, 45, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, C.; Li, W.; Adu-Frimpong, M.; Wang, Q.; Yu, J.; Xu, X. Preparation and Characterization of Syringic Acid–Loaded TPGS Liposome with Enhanced Oral Bioavailability and In Vivo Antioxidant Efficiency. AAPS PharmSciTech 2019, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New Progress in the Pharmacology of Protocatechuic Acid: A Compound Ingested in Daily Foods and Herbs Frequently and Heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Kowczyk-Sadowy, M.; Świsłocka, R.; Lewandowska, H.; Piekut, J.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H- and 13C-NMR), Theoretical and Microbiological Study of Trans o-Coumaric Acid and Alkali Metal o-Coumarates. Molecules 2015, 20, 3146–3169. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic Studies on the Antioxidant Activity of P-Coumaric Acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A. LC-ESI-MS/MS Analysis and Extraction Method of Phenolic Acids from Gluten-Free Precooked Buckwheat Pasta. Food Anal. Methods 2016, 9, 3063–3068. [Google Scholar] [CrossRef]
- Anderson, J.W. Whole Grains and Coronary Heart Disease: The Whole Kernel of Truth. Am. J. Clin. Nutr. 2004, 80, 1459–1460. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C. Dietary Intake of Antioxidant Nutrients and the Risk of Incident Alzheimer Disease in a Biracial Community Study. J. Am. Med. Assoc. 2002, 287, 3230. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef] [PubMed]
- Heptinstall, S.; May, J.; Fox, S.; Kwik-Uribe, C.; Zhao, L. Cocoa Flavanols and Platelet and Leukocyte Function: Recent In Vitro and Ex Vivo Studies in Healthy Adults: J. Cardiovasc. Pharmacol. 2006, 47, S197–S205. [Google Scholar] [CrossRef] [PubMed]
- Yunhai, L.; Jianguo, F.; Ting, L.; Wenqing, W.; Aihua, L. Anti-Endotoxic Effects of Syringic Acid ofRadix Isatidis. Curr. Med. Sci. 2003, 23, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Du, P.; Meiser, P.; Jacob, C. Proanthocyanidins: Oligomeric Structures with Unique Biochemical Properties and Great Therapeutic Promise. Nat. Prod. Commun. 2012, 7, 1934578X1200700. [Google Scholar] [CrossRef]
- Beecher, G.R. Proanthocyanidins: Biological Activities Associated with Human Health. Pharm. Biol. 2004, 42, 2–20. [Google Scholar] [CrossRef]
- Nandakumar, V.; Singh, T.; Katiyar, S.K. Multi-Targeted Prevention and Therapy of Cancer by Proanthocyanidins. Cancer Lett. 2008, 269, 378–387. [Google Scholar] [CrossRef]
- Shen, D.; Feng, Y.; Zhang, X.; Gong, L.; Liu, J.; Li, Y.; Liao, H. Antiosteoporosis Studies of 20 Medicine Food Homology Plants Containing Quercetin, Rutin, and Kaempferol: TCM Characteristics, In Vivo and In Vitro Activities, Potential Mechanisms, and Food Functions. J. Evid. Based Complement. Altern. Med. 2022, 2022, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Biais, B.; Richard, T.; Puertas, B.; Waffo-Teguo, P.; Merillon, J.-M.; Cantos-Villar, E. Grapevine Cane’s Waste Is a Source of Bioactive Stilbenes. Ind. Crops Prod. 2016, 94, 884–892. [Google Scholar] [CrossRef]
- Castellanos-Gallo, L.; Ballinas-Casarrubias, L.; Espinoza-Hicks, J.C.; Hernández-Ochoa, L.R.; Muñoz-Castellanos, L.N.; Zermeño-Ortega, M.R.; Borrego-Loya, A.; Salas, E. Grape Pomace Valorization by Extraction of Phenolic Polymeric Pigments: A Review. Processes 2022, 10, 469. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef] [PubMed]
- Chagas, M. do S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential Anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell. Longev. 2022, 2022, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q. Gelatin-Based Composite Films and Their Application in Food Packaging: A Review. J. Food Eng. 2022, 313. [Google Scholar] [CrossRef]
- Li, Y.; Lim, L.-T.; Kakuda, Y. Electrospun Zein Fibers as Carriers to Stabilize (−)-Epigallocatechin Gallate. J. Food Sci. 2009, 74, C233–C240. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation Techniques, Factors Influencing Encapsulation Efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Perković, G.; Bucić-Kojić, A. The Release of Grape Pomace Phenolics from Alginate-Based Microbeads during Simulated Digestion In Vitro: The Influence of Coatings and Drying Method. Gels 2023, 9, 870. [Google Scholar] [CrossRef]
- Mariod, A.A. Functional Properties of Gum Arabic. In Gum Arabic; Elsevier, 2018; pp. 283–295 ISBN 978-0-12-812002-6.
- Tyuftin, A.A.; Kerry, J.P. Gelatin Films: Study Review of Barrier Properties and Implications for Future Studies Employing Biopolymer Films. Food Packag. Shelf Life 2021, 29, 100688. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of Antioxidant Phenolic Compounds Extracted from Spent Coffee Grounds by Freeze-Drying and Spray-Drying Using Different Coating Materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Ravichai, K.; Muangrat, R. Effect of Different Coating Materials on Freeze-drying Encapsulation of Bioactive Compounds from Fermented Tea Leaf Wastewater. J. Food Process. Preserv. 2019, 43. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Shimoni, E. Chapter 2: Overview of Microencapsulates for Use in Food Products or Processes and Methods to Make Them. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedovic, V., Eds.; Springer New York: New York, NY, 2010; ISBN 978-1-4419-1007-3. [Google Scholar]
- Papoutsis, K.; Golding, J.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.; Scarlett, C.; Bowyer, M. Encapsulation of Citrus By-Product Extracts by Spray-Drying and Freeze-Drying Using Combinations of Maltodextrin with Soybean Protein and ι-Carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, P.; Wang, J.; Wu, Y.; Han, Y.; Zhou, J. Combining Various Wall Materials for Encapsulation of Blueberry Anthocyanin Extracts: Optimization by Artificial Neural Network and Genetic Algorithm and a Comprehensive Analysis of Anthocyanin Powder Properties. Powder Technol. 2017, 311, 77–87. [Google Scholar] [CrossRef]
- Arab, K.; Ghanbarzadeh, B.; Ayaseh, A.; Jahanbin, K. Extraction, Purification, Physicochemical Properties and Antioxidant Activity of a New Polysaccharide from Ocimum Album L. Seed. Int. J. Biol. Macromol. 2021, 180, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Archana, G.; Sabina, K.; Babuskin, S.; Radhakrishnan, K.; Fayidh, M.A.; Babu, P.A.S.; Sivarajan, M.; Sukumar, M. Preparation and Characterization of Mucilage Polysaccharide for Biomedical Applications. Carbohydr. Polym. 2013, 98, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Kakati, D.K. Smart Porous Microparticles Based on Gelatin/Sodium Alginate Polyelectrolyte Complex. J. Food Eng. 2013, 117, 193–204. [Google Scholar] [CrossRef]
- Boostani, S.; Jafari, S.M. A Comprehensive Review on the Controlled Release of Encapsulated Food Ingredients; Fundamental Concepts to Design and Applications. Trends Food Sci. Tech. 2021, 109, 303–321. [Google Scholar] [CrossRef]
- Luchese, C.L.; Uranga, J.; Spada, J.C.; Tessaro, I.C.; De La Caba, K. Valorisation of Blueberry Waste and Use of Compression to Manufacture Sustainable Starch Films with Enhanced Properties. Int. J. Biol. Macromol. 2018, 115, 955–960. [Google Scholar] [CrossRef]
- Shahidi, F.; Dissanayaka, C.S. Phenolic-Protein Interactions: Insight from in-Silico Analyses – a Review. Food Prod. Process. Nutr. 2023, 5, 2. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static In Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, Ana. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Comp. Rev. Food Sci. Food Safe 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello-Perez, L.A.; Álvarez-Parrilla, E.; De La Rosa, L.A.; González-Córdova, A.F.; González-Aguilar, G.A. Dietary Fiber and Phenolic Compounds as Functional Ingredients: Interaction and Possible Effect after Ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef]
- Fuloria, S.; Sekar, M.; Khattulanuar, F.S.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Subramaniyan, V.; Jeyabalan, S.; Begum, M.Y.; Chidambaram, K.; et al. Chemistry, Biosynthesis and Pharmacology of Viniferin: Potential Resveratrol-Derived Molecules for New Drug Discovery, Development and Therapy. Molecules 2022, 27, 5072. [Google Scholar] [CrossRef]
- González-Manzano, S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Extraction of Flavan-3-Ols from Grape Seed and Skin into Wine Using Simulated Maceration. Anal. Chim. Acta 2004, 513, 283–289. [Google Scholar] [CrossRef]
- Kamonpatana, K.; Giusti, M.M.; Chitchumroonchokchai, C.; MorenoCruz, M.; Riedl, K.M.; Kumar, P.; Failla, M.L. Susceptibility of Anthocyanins to Ex Vivo Degradation in Human Saliva. Food Chem. 2012, 135, 738–747. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Martinović, J.; Perković, G.; Bucić-Kojić, A. Bioconversion of Grape Pomace with Rhizopus Oryzae under Solid-State Conditions: Changes in the Chemical Composition and Profile of Phenolic Compounds. Microorganisms 2023, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons Inc.: New York, NY, USA, 2001; pp. I1.1.1–I1.1.8. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total Phenolics And Total Flavonoids In Bulgarian Fruits And Vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, Proanthocyanidins, Flavones and Flavonols in Some Plant Materials and Their Antioxidant Activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Šelo, G.; Zelić, B.; Planinić, M.; Tišma, M. Recovery of Phenolic Acid and Enzyme Production from Corn Silage Biologically Treated by Trametes Versicolor. Appl. Biochem. Biotechnol. 2017, 181, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Bucić-Kojić, A.; Fernandes, F.; Silva, T.; Planinić, M.; Šelo, G.; Šibalić, D.; Pereira, D.M.; Andrade, P.B. Enhancement of the Anti-Inflammatory Properties of Grape Pomace Treated by Trametes Versicolor. Food. Funct. 2020, 11, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Vergara, C.; Saavedra, J.; Sáenz, C.; García, P.; Robert, P. Microencapsulation of Pulp and Ultrafiltered Cactus Pear (Opuntia Ficus-Indica) Extracts and Betanin Stability during Storage. Food Chem. 2014, 157, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. In Vitro Bioaccessibility Assessment of Phenolic Compounds from Encapsulated Grape Pomace Extract by Ionic Gelation. Molecules 2023, 28, 5285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J PharmSciTech 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Ozkan, G.; Isik, H.; Horoz, O.; Van Camp, J.; Capanoglu, E. Black Carrot Pomace as a Source of Polyphenols for Enhancing the Nutritional Value of Cake: An In Vitro Digestion Study with a Standardized Static Model. LWT - Food Sci. Technol. 2017, 77, 475–481. [Google Scholar] [CrossRef]
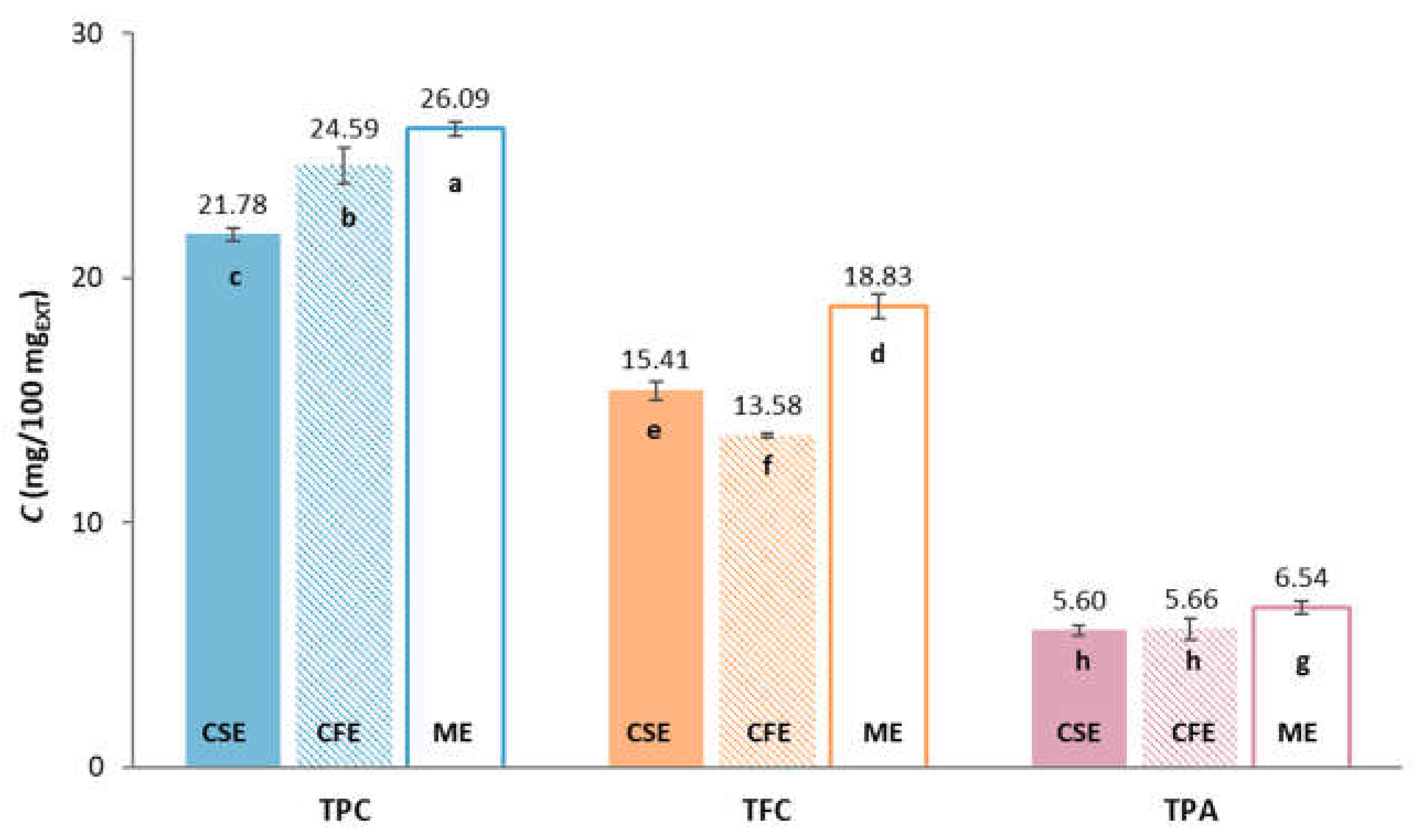
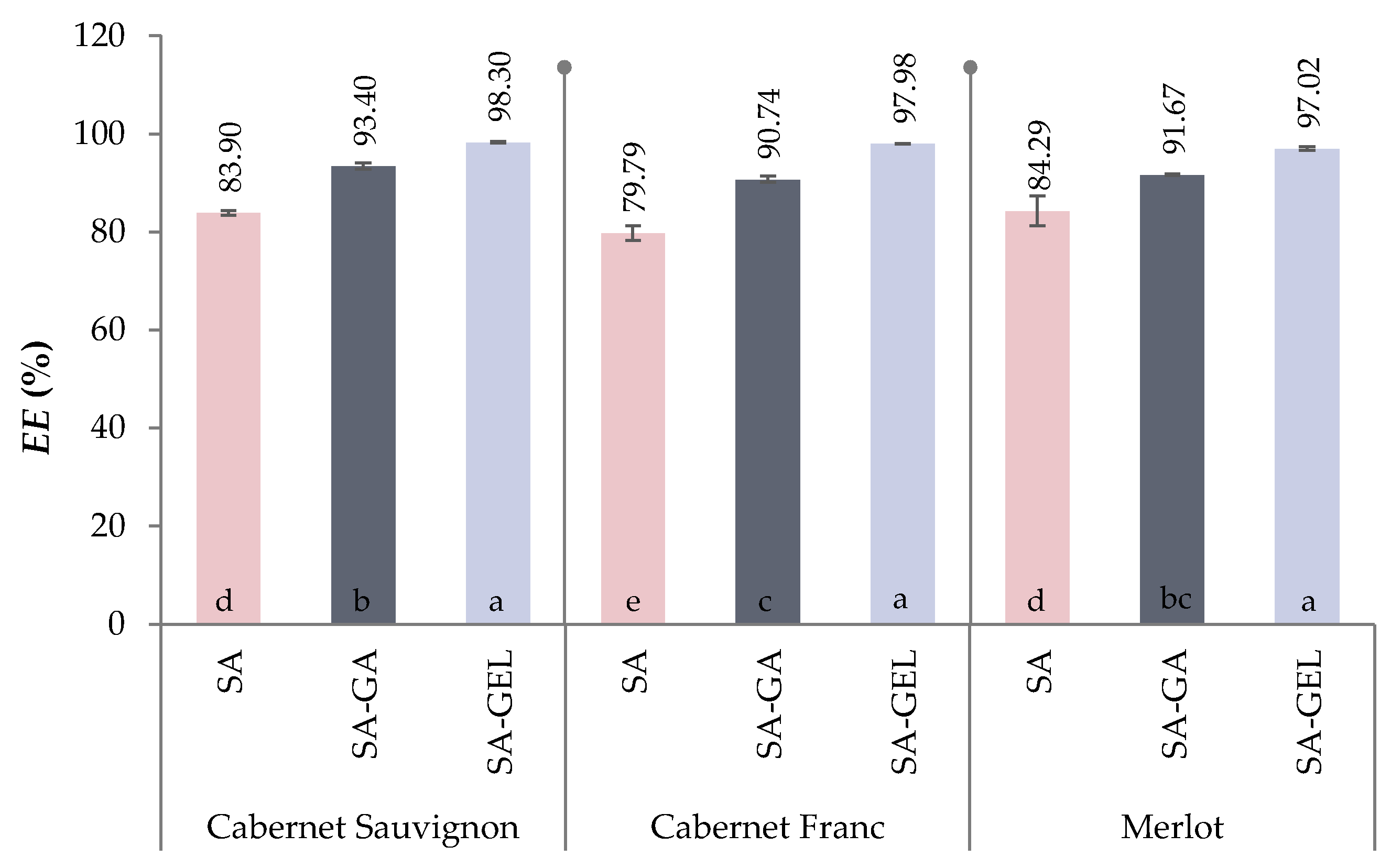
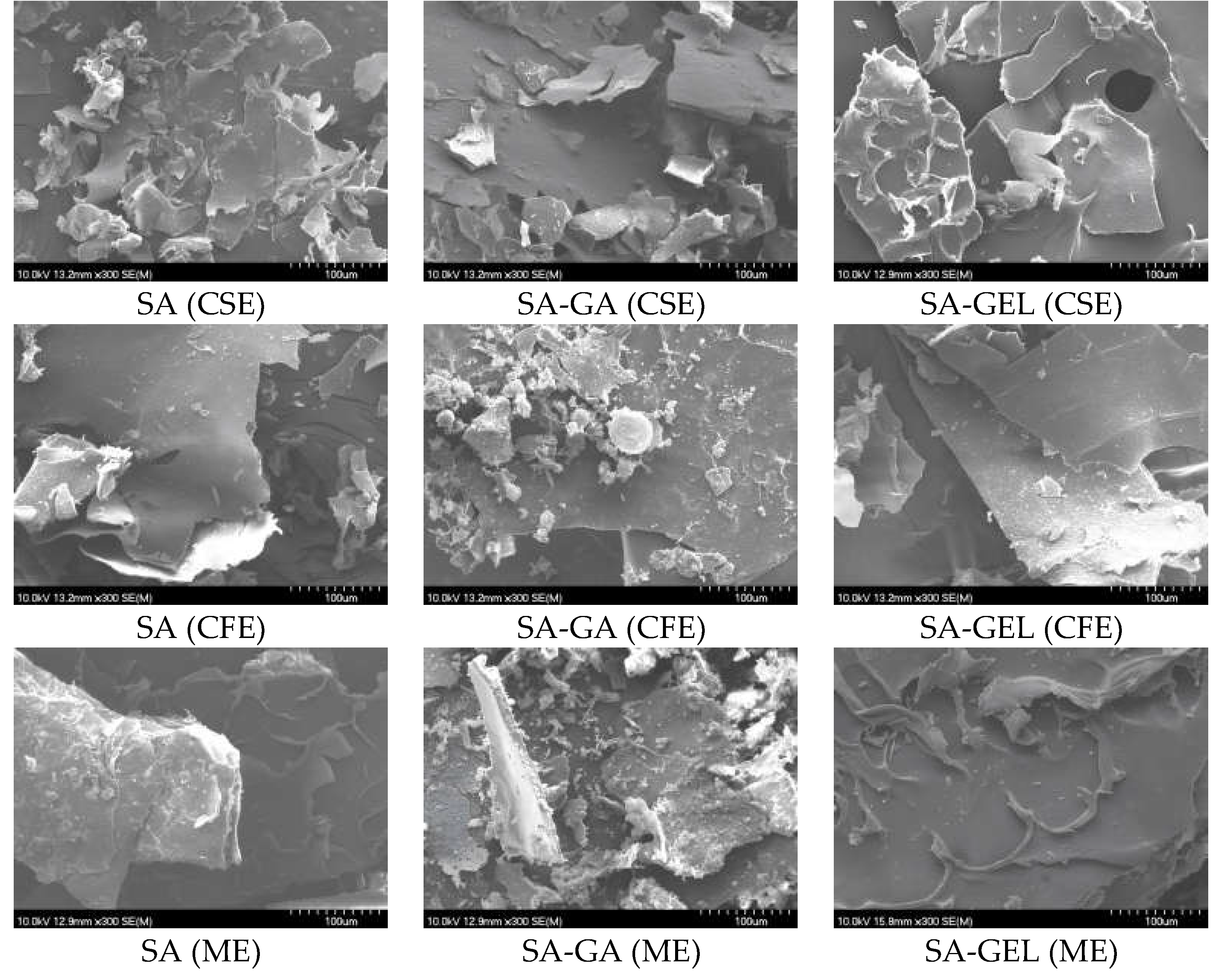
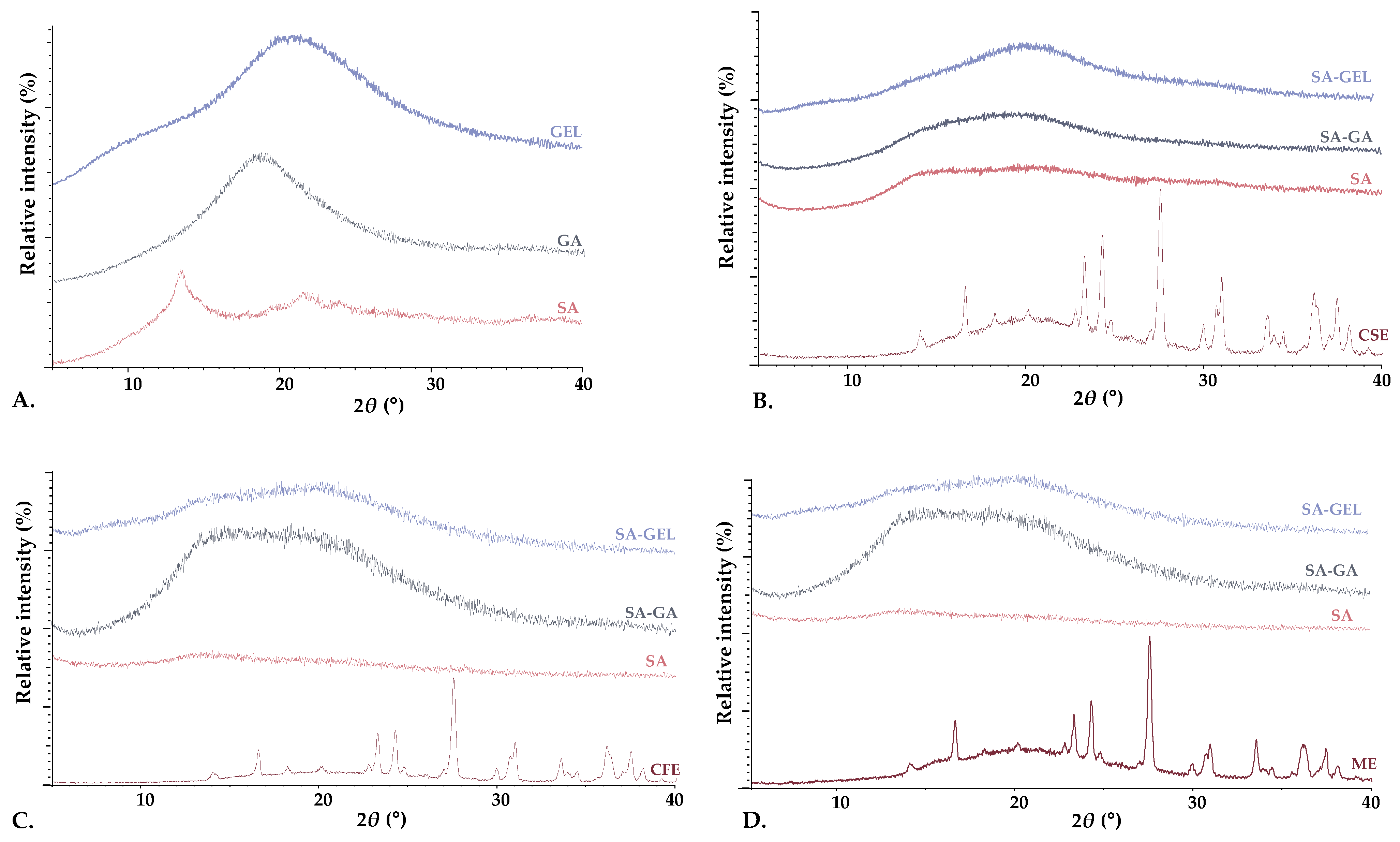
| CSE | CFE | ME | |
|---|---|---|---|
| Phenolic acids (mg/100 mgEXT) | |||
| Gallic acid | 161.99 ± 31.68 | 130.00 ± 10.98 | 207.79 ± 10.15 |
| 3,4-Dihydroxybenzoic acid | 62.02 ± 11.60 | 24.09 ± 1.97 | 75.63 ± 5.51 |
| p-Hydroxybenzoic acid | 2.97 ± 0.54 | 1.00 ± 0.04 | 2.10 ± 0.09 |
| Syringic acid | 88.88 ± 20.39 | 114.36 ± 3.02 | 51.89 ± 9.07 |
| Vanillic acid | 10.45 ± 0.80 | 14.83 ± 0.74 | 12.34 ± 0.25 |
| Ellagic acid | 94.72 ± 10.39 | 81.33 ± 1.23 | 16.32 ± 0.12 |
| Caffeic acid | 1.40 ± 0.04 | 1.04 ± 0.02 | 0.75 ± 0.01 |
| Ferulic acid | 0.97 ± 0.01 | 2.52 ± 0.25 | 0.97 ± 0.08 |
| o-Coumaric acid | 7.46 ± 1.39 | 19.48 ± 0.58 | 9.60 ± 0.60 |
| p- Coumaric acid | 3.43 ± 0.71 | 1.44 ± 0.04 | 3.31 ± 0.20 |
| Flavanols (mg/100 mgEXT) | |||
| Epicatechin | 100.71 ± 11.65 | 547.27 ± 25.23 | 248.31 ± 9.17 |
| Catechin | 240.87 ± 13.61 | 527.59 ± 28.62 | 272.26 ± 0.42 |
| Epicatechin gallate | 5.78 ± 2.74 | 32.46 ± 1.65 | 8.29 ± 0.04 |
| Gallocatechin gallate | 72.53 ± 21.84 | 127.23 ± 9.54 | 76.11 ± 4.85 |
| Procyanidin B1 | 118.52 ± 25.63 | 317.42 ± 2.59 | 197.93 ± 16.51 |
| Procyanidin B2 | 46.57 ± 21.88 | 126.51 ± 23.32 | 62.19 ± 2.76 |
| Flavonols (mg/100 mgEXT) | |||
| Quercetin | 214.33 ± 24.44 | 146.04 ± 3.93 | 120.95 ± 1.84 |
| Kaempferol | 13.22 ± 1.34 | 10.40 ± 1.00 | 5.19 ± 0.06 |
| Rutin | 13.14 ± 3.60 | 65.08 ± 5.10 | 24.13 ± 0.95 |
| Stilbenes (mg/100 mgEXT) | |||
| Resveratrol | 7.91 ± 0.45 | 8.82 ± 0.40 | 14.16 ± 0.37 |
| ε-Viniferin | 22.75 ± 0.58 | 13.72 ± 0.06 | 8.53 ± 0.31 |
| Anthocyanins (mg/100 mgEXT) | |||
| Oenin chloride | 91.28 ± 0.72 | 794.37 ± 21.84 | 32.83 ± 0.36 |
| Myrtillin chloride | 12.09 ± 0.04 | 35.15 ± 0.00 | 2.81 ± 0.01 |
| Kuromanin chloride | 2.29 ± 0.00 | 4.88 ± 0.03 | 1.11 ± 0.15 |
| Petunidin chloride | 1.89 ± 0.08 | 7.77 ± 0.01 | 0.67 ± 0.03 |
| Callistephin chloride | nd | 2.27 ± 0.03 | nd |
| Peonidin-3-O-glucoside chloride | 9.76 ± 0.02 | 77.75 ± 7.28 | 7.17 ± 0.09 |
| Matematical models | Release rate constants and statistical criteria of model approximation success | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cabernet Sauvignon | Cabernet Franc | Merlot | ||||||||
| SA | SA-GA | SA-GEL | SA | SA-GA | SA-GEL | SA | SA-GA | SA-GEL | ||
|
First order model |
R2adj | -0.464 | -0.073 | -0.641 | -0.558 | 0.021 | -0.178 | -0.364 | -0.079 | -1.479 |
| AIC | 134.382 | 131.764 | 138.136 | 136.299 | 130.475 | 133.887 | 134.748 | 130.362 | 147.222 | |
| MSC | -2.332 | -1.580 | -1.568 | -2.248 | -1.492 | -0.784 | -1.684 | -1.656 | -2.129 | |
| k1 | 0.040 | 0.048 | 0.031 | 0.041 | 0.049 | 0.013 | 0.026 | 0.043 | 0.024 | |
|
Higuchi model |
R2adj | -1.004 | -0.501 | -0.538 | -1.118 | -0.477 | 0.121 | -0.498 | -0.463 | -1.226 |
| AIC | 138.896 | 136.550 | 137.223 | 140.826 | 136.275 | 129.785 | 136.079 | 134.624 | 145.714 | |
| MSC | -2.655 | -1.922 | -1.503 | -2.571 | -1.906 | -0.491 | -1.779 | -1.961 | -2.021 | |
| kH | 8.639 | 8.851 | 7.996 | 8.811 | 8.839 | 6.725 | 8.473 | 8.474 | 9.054 | |
| HixsonCrowell model | R2adj | -1.273 | -0.790 | -0.913 | -1.380 | -0.826 | -0.224 | -0.635 | -0.800 | -1.668 |
| AIC | 140.664 | 139.010 | 140.282 | 142.449 | 139.223 | 134.428 | 137.308 | 137.528 | 148.251 | |
| MSC | -2.781 | -2.098 | -1.722 | -2.687 | -2.116 | -0.823 | -1.867 | -2.168 | -2.202 | |
| kHC | 0.007 | 0.007 | 0.006 | 0.007 | 0.007 | 0.003 | 0.006 | 0.007 | 0.006 | |
| Korsmeyer-Peppas model | R2adj | 0.963 | 0.873 | 0.611 | 0.910 | 0.922 | 0.462 | 0.889 | 0.951 | - |
| AIC | 83.525 | 102.775 | 118.761 | 97.381 | 95.742 | 123.825 | 100.523 | 87.613 | - | |
| MSC | 1.300 | 0.490 | -0.195 | 0.532 | 0.989 | -0.065 | 0.761 | 1.397 | - | |
| kKP | 73.698 | 72.207 | 75.257 | 77.047 | 67.023 | 48.962 | 61.468 | 64.742 | - | |
| n | 0.056 | 0.050 | 0.014 | 0.049 | 0.078 | 0.093 | 0.084 | 0.077 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





