1. Introduction
According to the World Health Organization (WHO), preterm birth occurs before 37 weeks gestation (before 259 days from the date of the last menstrual period) [
1]. It is a condition defined by the failure to reach the expected gestational time and not by the presence of specific symptoms or signs [
2]. Thus, preterm birth can be seen as a result of complications during pregnancy that anticipate the time of birth or as a medical alternative to avoid a greater risk to the fetus or the mother [
2].
Gestational age is the most frequent criterion for the classification of prematurity, grouped into: late when it occurs between 36 and 32 weeks of gestation, moderate, between 31 and 29 weeks, and extreme, when it occurs before 28 weeks. Although the risk of survival and morbidity increases the lower the gestational age, chances are present in all categories [
1]. Its etiology is heterogeneous, characterized by a complex interaction between factors that include five components: 1) maternal conditions before or during labor, 2) fetal conditions, 3) pathological placental conditions, 4) signs of the onset of labor, and 5) type of delivery [
3,
4]. According to data from international organizations such as the WHO, and the United Nations, about 30% of cases occur due to early labor or cesarean section for medical or personal reasons. Meanwhile, approximately 70% occur spontaneously. Among the most frequent causes are multiple pregnancies, infectious or chronic maternal health conditions, and genetic factors. However, in many cases, the reason is not determined [
1,
2].
Prematurity is a frequent phenomenon and represents the leading cause of perinatal mortality. Different studies have reported figures suggesting that between 10% and 16% of births occurring annually in the world are premature, representing about 15 million cases, most of these occurring in regions such as South Asia and sub-Saharan Africa [
5,
6,
7].
The statistics in Mexico are similar to those reported worldwide, with 7.04% of total births registered in 2014 [
8]. However, it is essential to note that these data correspond to births registered in health institutions, so figures on births occurring outside health facilities are unknown. Studying the risk factors for pre- and perinatal brain damage, including prematurity, is of interest to neuroscience and neuropsychology since they occur during a critical period for brain maturation and, therefore, cognitive, socioemotional, and physical development. Furthermore, the findings suggest that some disabilities and cognitive impairments originate in the early stages of life due to biological, psychosocial, and environmental risks. These conditions are exacerbated in developing countries since it is estimated that about 43% of children under five years old do not reach their potential, increasing the likelihood of presenting mental health problems [
9,
10].
In the particular case of children with a history of prematurity, it is recognized that although mortality rates have decreased in recent decades, the morbidity associated with long-term neurocognitive problems has remained [
11]. The hypomyelination, neuronal, and axonal damage that characterize the preterm brain has a high potential to cause high prevalence but low severity cognitive impairments are frequently observed. Studies suggest that these difficulties begin to be more noticeable from school age onwards and are wide-ranging, including motor problems, alterations in visuospatial processing, memory, language, executive functioning, and intelligence [
12,
13,
14,
15,
16,
17].
Statistics suggest that about 70% of children with these backgrounds require exceptional education support, representing a significant economic and social burden on families and communities [
18]. Developmental monitoring of preterm infants during infancy usually includes measurements of general intellectual functioning. IQ measures are generally the basis for cognitive assessment, which can guide the search for possible deficits in more specific functions. A linear relationship between weeks of gestation and IQ has been reported, and the rate of severe intellectual disability in this population is estimated to be around 10-50% of cases [
3,
16,
19,
20,
21].
The cognitive alterations described in preterm infants as a consequence of medical and neurological complications have repercussions on the quality of life of individuals in the medium and long term. They have been associated with the presence of symptoms of neurodevelopmental disorders such as attention deficit hyperactivity disorder, autism spectrum disorder, learning problems, poor academic performance, and risk behaviors in adolescence such as substance abuse, involvement in criminal activities, early pregnancies, school dropout, etc. [
12,
22,
23,
24,
25].
Most of these studies come from cross-sectional designs at different stages of development in different participants, so conclusions on development are based on indirect inferences. Longitudinal studies in preterm samples are scarce due to the inherent difficulties of this research design; however, one of their advantages is that they allow detection, and measure of the evolutionary change in cognitive development.
This study aimed to longitudinally study the impact of prematurity and risk factors for perinatal brain damage on intelligence development in a sample of children from 6 to 8 years of age with and without a history of prematurity.
2. Materials and Methods
The data presented in this research article belong to a prospective longitudinal study of a group of preterm children with risk factors for perinatal brain damage, and a group of children born at term without risk factors, who were evaluated annually from 6 to 8 years old.
2.1. Participants
A total of 74 six years children were recruited at the beginning of the study; 39 of them formed the preterm group born between 28 and 36 weeks of gestation (31.7 ±.8), and the remaining 35 formed the control group born between 38 and 40 weeks of pregnancy (38.7 ±.85). The participants in the preterm group were part of the general research protocol of the Neurodevelopment Research Unity "Augusto Fernández Guardiola" (UIND by its Spanish acronym) of the Neurobiology Institute (NBI), in the Autonomous University of México, and were selected under the following criteria: birth between 28 and 36 weeks of gestation, obtaining a score classified in the categories of normal development or mild-moderate delay in the cognitive and motor scales of the Bayley Development Scale (BSID-II) at one year of age, without severe motor or sensory disability and having a structural MRI in the first year of age without evidence of clinically significant brain lesions.
The control group participants were recruited through public and private preschool, and elementary schools, as well as direct invitation from the NBI staff. The criteria for the selection of participants in this group were: birth between 37 and 41 weeks of gestation, no history of risk factors for perinatal brain damage, no known psychiatric or neurological diagnosis, attendance at a regular school with an average level of performance, no severe motor or sensory deficits, and no behavioral problems. Table 1, shows the demographic characteristics of the participants. Each participant’s parent properly signed informed consent, agreed to voluntarily participate in the study and informed about their right to leave the investigation at any moment.
Table 1.
Participants’ demographics.
Table 1.
Participants’ demographics.
| |
Preterm (n = 39) |
|
Full term (n = 35) |
|
X2
|
| Variable |
n |
% |
n |
% |
|
| Sex |
|
|
|
|
.525 |
| Girls |
20 |
51.3 |
15 |
42.9 |
|
| Boys |
19 |
48.7 |
20 |
57.1 |
|
| Dominance |
|
|
|
|
1.79 |
| Right handed |
33 |
84.6 |
33 |
94.3 |
| Left handed |
2.29 |
.970 |
2.62 |
.651 |
| Schooling at beginning |
|
|
|
|
12.7* |
| 3rd Kinder garden |
25 |
64 |
23 |
8 |
|
| 1st Elementary |
14 |
36 |
77 |
27 |
|
| |
Mean |
SD |
Mean |
SD |
Mann-Whitney’sU
|
| Gestational age |
31.7 |
2.8 |
38.7 |
.85 |
12.0* |
| Birth weight (grams) |
1645.77 |
463 |
3204.4 |
553 |
0.0* |
| Maternal education (yrs) |
15.1 |
4.1 |
15.3 |
4 |
675.5 |
| Risk factors (frequency) |
6 |
3 |
- |
- |
- |
| Age of assessment (months) |
| 1st Assessment |
74.7 |
4.3 |
76.3 |
5.5 |
546.5 |
| 2nd Assessment |
87 |
3.7 |
87.8 |
4.4 |
595.5 |
| 3rd Assessment |
100.1 |
3.4 |
100.1 |
3.5 |
671.5 |
2.2. Instruments and Procedure
Intelligence assessment: this function was evaluated using the Wechsler Intelligence Scale for Children, fourth edition (WISC-IV) in its version with norms for Mexico [
26]. This test consists of 15 subscales that are organized into four indexes: the Verbal Comprehension Index (VCI), the Perceptual Reasoning Index (PRI), the Working Memory Index (WMI), and the Processing Speed Index (PSI), as well as a total intelligence quotient (IQ). The test applies to children between the ages of 6 and 16 years 11 months. The ten mandatory scales were used in standard form to obtain the indexes (similarities, vocabulary, comprehension, cube design, concepts with drawings, matrices, digit repetition, number and letter ordering, clues, and symbol search). The neuropsychologist completed the study application, and two psychology students were about to graduate. The reliability coefficients for the subscales ranged from .70 to .89, while the composite scores ranged from .88 to .97.
The sample for the premature infant group was selected from an exhaustive review of the database of children who entered the UIND protocol, 102 children were pre-selected who met the previously mentioned inclusion criteria, and the parents were contacted by telephone to invite them to participate; 50 of them agreed to participate. The evaluation sessions were conducted individually in a psychological office within the UIND facilities. Six of the 50 children initially evaluated were excluded because they did not meet the inclusion criteria, so the sample in the first evaluation period comprised 44 participants. In the 7-year evaluation, it was possible to contact only 40 families, and the procedure was the same as in the initial evaluation.
Finally, for the 8-year evaluation, 39 children participated, and the same procedure was followed to apply the instruments. In the three evaluation periods, a written report of the results was given to the participant’s parents. To recruit the control group, three regular schools in Querétaro were contacted and asked to pre-select among the students in the third grade of preschool and first grade of elementary school with regular academic performance. Then, the children’s parents were invited to participate in the study. Thus, the total number of participants in the initial period (six years) who met the requirements and whose parents signed the informed consent was 49. The evaluation was carried out in designated cubicles within the facilities of the participating schools and the UIND in individual sessions. In each period evaluated, a written report of the results was given to the participant’s parents.
2.3. Data Analysis
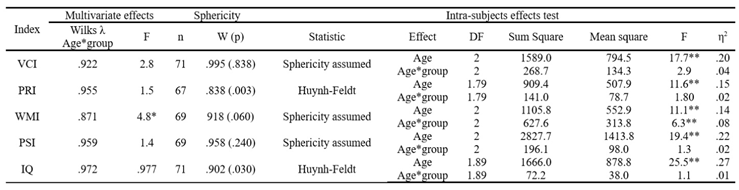
Data analysis was performed using SPSS.25 statistical program (IBM). An exploration of the data was carried out, and the cases whose values were identified as extreme in the box plots were eliminated; the sample distribution was standard in each of the cognitive variables evaluated according to the results of the Shapiro-Wilk test (p > .05). A repeated measure mixed ANOVA (2X3) was performed to determine the existence of significant differences in the scores obtained in the indices measured by the test: verbal comprehension, perceptual reasoning, working memory, processing speed, and total IQ between the groups (preterm and full-term) and throughout the three evaluation periods (6, 7 and 8 years). Univariate and multivariate normality assumptions were tested. The equality of the covariance matrices and the absence of multicollinearity and post hoc analysis with Bonferroni adjustment were performed for each measured index.
2.4. Ethical Considerations
The information from participants was anonymously driven and only known by the principal investigator of this research; the participants’ parents were informed about the objectives of the study and were free to participate or decline the procedures voluntarily. The protocol was approved by the Autonomous University of Baja California, Faculty of Administrative, Social and Engineering Sciences Institutional Review Board.
3. Results
The results obtained by each of the groups in the WISC-IV in the three evaluation phases are shown in Table 2.
Table 2.
Composite scores in the Wechsler Intelligence Scale for Children (WISC-IV).
Table 2.
Composite scores in the Wechsler Intelligence Scale for Children (WISC-IV).
| |
|
|
Preterm (n = 39) |
|
|
Full term (n = 35) |
|
| Index |
Age |
Mean |
SD |
CI %95 |
Mean |
SD |
CI %95 |
| VCI |
|
|
|
|
|
|
|
| |
6 years |
80.9 |
10.9 |
77.3-84.4 |
98.1 |
7.4 |
95.5-100.6 |
| |
7 years |
86.2 |
10.2 |
82.9-89.5 |
103.2 |
7.9 |
100.5-105.9 |
| |
8 years |
84.5 |
10.7 |
81-88 |
105.7 |
11.4 |
101.5-109.4 |
| PRI |
|
|
|
|
|
|
|
| |
6 years |
87.3 |
11.1 |
83.6-90.9 |
99.3 |
7.8 |
96.6-102 |
| |
7 years |
93.0 |
11.7 |
89.1-96.8 |
104.3 |
9.5 |
101.1-107.6 |
| |
8 years |
93.6 |
13.9 |
89.1-98.2 |
103.8 |
11.5 |
99.9-107.8 |
| WMI |
|
|
|
|
|
|
|
| |
6 years |
81.3 |
16.2 |
76-86.5 |
94.4 |
6.3 |
92.2-96.6 |
| |
7 years |
88.1 |
12.5 |
84.1- 92.2 |
96.2 |
9.0 |
93.1-99.3 |
| |
8 years |
89.8 |
14.2 |
85.2-94.4 |
97.2 |
9.0 |
94.1-100.3 |
| PSI |
|
|
|
|
|
|
|
| |
6 years |
93.3 |
13.4 |
88.9-97.6 |
103.9 |
11.1 |
100-107.7 |
| |
7 years |
101.8 |
16.5 |
96.4-107.2 |
110 |
12.1 |
105.9-102.7 |
| |
8 years |
95.2 |
15.0 |
90.3-100.1 |
101.5 |
10.1 |
98-105 |
| IQ |
|
|
|
|
|
|
|
| |
6 years |
81.8 |
12.4 |
77.8-85.8 |
98.77 |
7.1 |
96.3-101.2 |
| |
7 years |
89.4 |
12.5 |
85.4-93.5 |
104.9 |
9.0 |
101.8-108.0 |
| |
8 years |
87.9 |
13.8 |
83.4-92.4 |
103.6 |
9.5 |
100.3-106.9 |
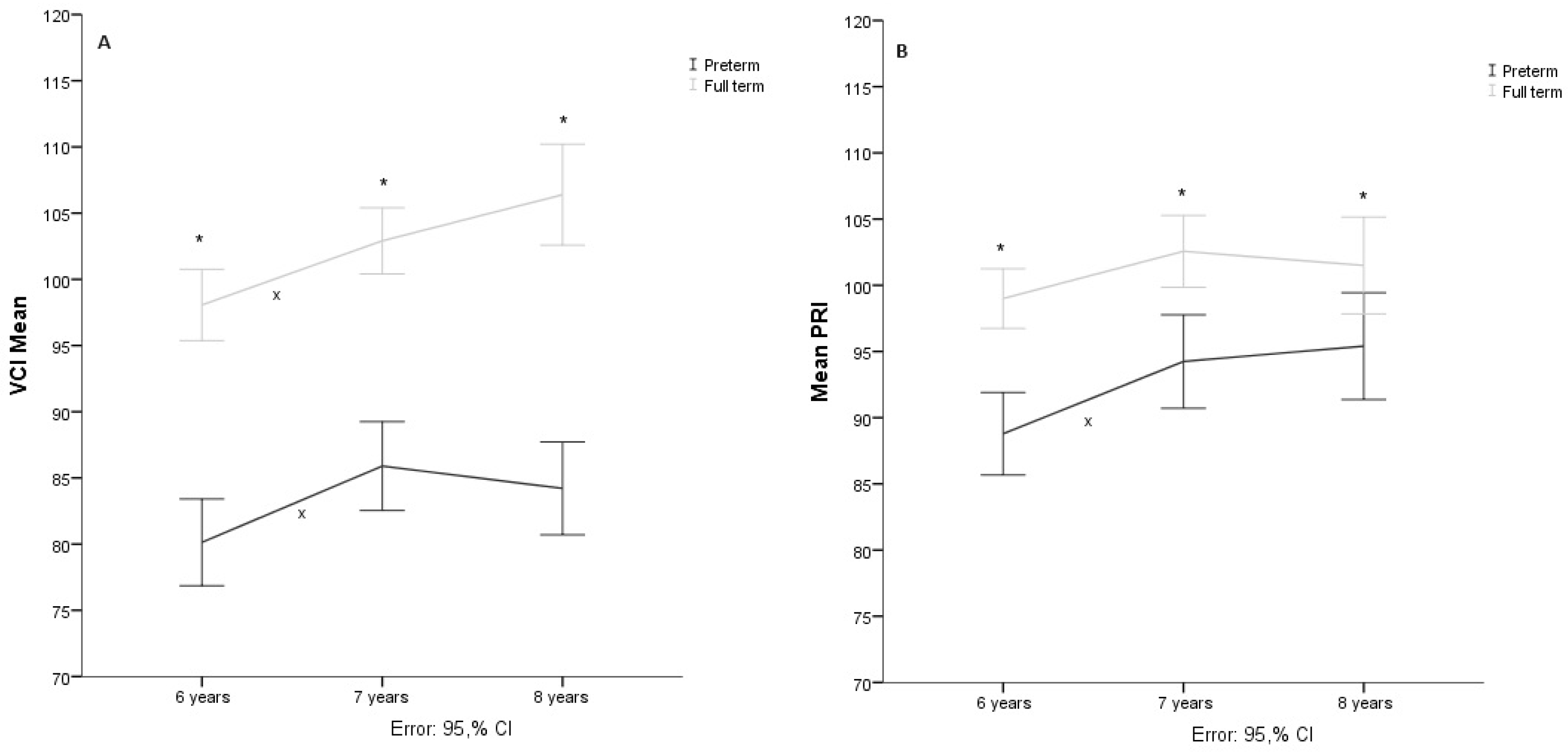
3.1. Verbal Comprehension
Three cases with extreme values were eliminated, so the sample comprised 71 participants (38 preterm and 33 terms). The Shapiro-Wilk test showed a normal distribution (
p > .05). Therefore, the sphericity of the data measured through Mauchly’s test of sphericity (
X2 = 0.353,
p = 0.838) could be assumed. The repeated measures mixed ANOVA showed that the age at which the evaluation was performed produced significant changes in the verbal comprehension index score (F(138,6190.5) = 17.712
p < .05,
= .204). However, the group * age interaction analysis did not show significant effects (F(2, 268.7) = 2.996,
p = 0.053,
= .204). Post-hoc analysis by Bonferroni adjustment showed significant differences (
p < .05) between the groups in the three evaluation phases, as well as intragroup differences (
p < .05) between 6 and 7 years in both groups (see
Figure 1A).
3.2. Perceptual Reasoning
Data from 67 of the participants (37 preterms and 30 terms) were analyzed since seven cases were eliminated for presenting extreme values, the Shapiro-Wilk test showed a normal distribution (
p > .05). However, the sphericity of the data measured by Mauchly’s test
X2 = 11.322,
p = 0.003 could not be assumed, so the correction of the degrees of freedom with the Huynh-Feldt sphericity estimate (
= .895) was considered. The repeated measures mixed ANOVA showed that age at evaluation significantly changed the IRP score (F(116.38,5091.88) = 11.609,
p < .001,
=.152). At the same time, the group*age interaction analysis showed no significant changes (F(1.791, 141.03) = 1.8,
p = 0.175,
= .027). On the other hand, posthoc analysis showed significant differences (
p < .05) between the groups in the three evaluation phases, as well as significant differences (
p < .05) within groups between 6 and 7 years for the preterm group but not for the term group (see
Figure 1B).
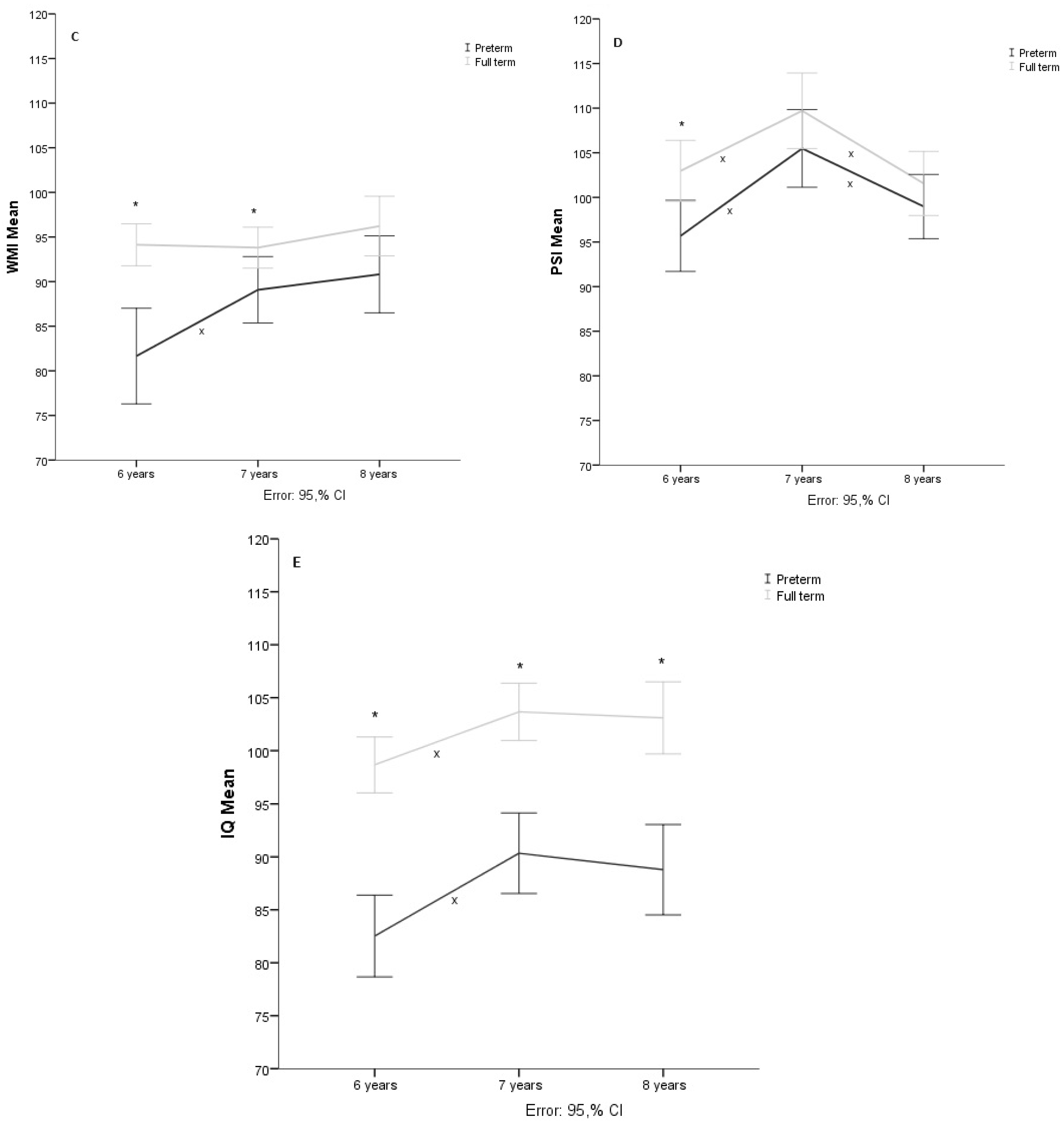
3.3. Working Memory
The results of 69 participants (38 preterms and 31 terms) were analyzed, and the Shapiro-Wilk test showed a normal distribution (
p > .05). Sphericity of the data was assumed measured through Mauchly’s test of sphericity (
X2 = 5.620,
p = .060). The repeated measures mixed ANOVA showed that age at evaluation produced significant changes (F(134, 6655.57)=11.13,
p < 0.001,
= .142). In addition, the group*age interaction showed a significant effect (F(2, 627.63) = 6.31, = 0.002,
= .086). On the other hand, the results of the post hoc analysis showed significant differences between the groups in the first two evaluation stages. The preterm group showed significantly different scores (
p < .05) between 6 and 7 years of age but not from 7 to 8 years of age, while the group of children born at term did not show significant differences (
p > .05) between ages (See
Figure 1C).
3.4. Processing Speed
The analysis was performed with 69 participants (35 preterms and 34 terms) after five cases were eliminated for presenting extreme values. The Shapiro-Wilks test showed that there is a normal distribution (
p < .05). The results of the Mauchly test showed that the sphericity is fulfilled (
X2 = 2.854,
p = .240). Repeated measures mixed ANOVA showed that age at evaluation produced significant changes (F(134, 9716.20)= 19.499,
p < .001,
= .225), but the group*age interaction yielded no evidence of substantial changes (F(2, 196.176) = 1.353,
p = 0.262,
2 = .020). Post hoc analyses revealed significant differences (
p < .05) between groups at six years. In addition, both the preterm and term group obtained significantly different scores (
p < .05) between the three ages (see
Figure 1D).
3.5. Total Intelligence Quotient
The results of 71 of the participants (38 preterm and 33 term) were analyzed. The results of the Shapiro-Wilk test showed a normal distribution (
p > .05). The sphericity of the data could not be assumed according to Mauchly’s test (
X2 = 6.999
p = .030), so degrees of freedom were corrected with Huynh-Feldt’s estimate of sphericity (
= .948). The repeated measures mixed ANOVA showed that there is an effect of age (F(130.8, 4506.198) = 25.511,
p < .001,
= .270), but not of the age*group interaction (F(1.896, 72.205) = 1.106,
p = .332,
= .016). Post hoc analysis evidenced significant (
p < .05) intragroup changes in total IQ in the two groups between the ages of six and seven. At the same time, intergroup differences were significantly (
p < .05) present in the three ages evaluated (see
Figure 1E).
Figure 1.
Note. A. Verbal Comprehension Index (VCI), B: Perceptive Reasoning Index (PRI), C: Working Memory Index (WMI), D: Processing Speed Index (PSI), E: Full Scale Intellectual Quotient (IQ). * Shows intergroup significative differences p < .05, x shows intragroup significative differences p < .05 in the post hoc analysis.
Figure 1.
Note. A. Verbal Comprehension Index (VCI), B: Perceptive Reasoning Index (PRI), C: Working Memory Index (WMI), D: Processing Speed Index (PSI), E: Full Scale Intellectual Quotient (IQ). * Shows intergroup significative differences p < .05, x shows intragroup significative differences p < .05 in the post hoc analysis.
Table 3.
Repeated Measures Mixed ANOVA.
Table 3.
Repeated Measures Mixed ANOVA.
4. Discussion
The objective of this research was to longitudinally study the impact of prematurity and risk factors for perinatal brain damage on intelligence development. A preterm and term children sample was evaluated using the WISC-IV at 6, 7, and 8 years. A comparison of the demographic data showed that the groups were different in the expected variables (weeks of gestation, birth weight, and risk factors). No differences were observed in variables such as the ages at which they were evaluated in each period or maternal schooling, which guarantees that the differences between the groups are not due to more significant maturation due to age or disparities in socioeconomic level when choosing the sample.
Among the indicators considered in the evaluation of this study, the working memory index showed a significant effect of the interaction between age and group. The data showed significant changes in the scores obtained in this index throughout the three ages in the preterm participants but not in the control group. In addition, directionality analyses showed that in all three assessments, the control group’s scores were significantly higher than those of the preterm infants.
Previous studies have reported such differences in preterm and non-preterm children. An example is the study conducted by Fitzpatrick and collaborators [
27], who reported significant differences in the performance of a spatial working memory task between moderate preterm and full-term children at 11 years of age. Similar findings are reported by Korpela et al. [
28] who compared the performance of 95 preterm children at 11 years of age on working memory tasks and the WISC-IV; in their study, the group was divided into three subgroups according to the degree of brain pathology (identified on neonatal MRI): normal, minor, or severe. In addition to comparisons between the three groups, performance was compared against the norms of the different tests. This study showed that preterm infants (regardless of a subgroup) with an average level of cognitive performance on the intelligence test performed significantly worse than test norms, particularly on tasks related to the central executive and visuospatial agenda. Meanwhile, the group with the most severe neonatal brain lesions had the worst results.
Ford et al. [
29] evaluated 35 preterm children aged 7 to 11 years and 37 term-born children of the same ages. The tests used the DSM-IV regression digit repetition task (the same task we used in our study). According to the results of the statistical analyses, there were significant differences between the groups in this domain, suggesting differences in prospective memory.
More recent similar results were reported by Kaul et al. [
30], who contrasted the WICS-IV performance of 359 extremely preterm children with the performance of 367 non-preterm children. The mean age of the participants was 6.5 years, and the results showed that the most significant differences between the groups were perceptual reasoning and working memory.
The indices of verbal comprehension, perceptual reasoning, processing speed, and total IQ only showed significant effects of age. Differences between the groups were present at all three ages in the verbal comprehension index, perceptual reasoning, and total IQ, while working memory and processing speed showed differences only at 6 and 7 years and six years, respectively. These data are consistent with studies noting that preterm infants tend to have significantly lower scores than full-term infants on intelligence tests [
30,
31,
32,
33,
34]; furthermore, studies report that these differences are maintained over time holding even into adulthood [
35,
36]. A domain that may be particularly interesting is processing speed since the main neurological alterations reported in preterm infants are associated with lesions in the white matter [
37,
38].
In our study, no interactions between age and group were found, and in both groups, a pattern of decreasing tendency was found between the ages of 7 and 8. These changes described in processing speed contradict the findings of other studies indicating that processing speed increases substantially during middle childhood and less markedly in late childhood. In both groups, scores on this index tended to decline. However, given that these changes remain within the parameters of normality in both groups, it is possible to affirm that this is not a pathological phenomenon but a change in the dynamic interaction between cognitive processes. Thus, for example, it has been described that processing speed may decrease in the face of changes in the executive control demands of the task [
39,
40].
In general, the results of our study, related to intellectual activity suggest that intelligence and its different components change throughout childhood, regardless of the condition at birth. However, it is essential to note that although the trajectories found have similar characteristics between the groups, in the case of the term infants, the mean scores for each of the indicators evaluated always remained within a performance classification within the normal parameters for their age (range of 94.4 to 110) according to the test, while in the preterm group, the mean scores were mainly in the low average (range from 80.9 to 101.8). These data are consistent with research work suggesting that preterm intelligence is within the parameters of normal but significantly below-term births [
3,
16,
41,
42].
Roze et al. [
43] suggest that other cognitive deficits often accompany low IQ scores obtained by preterm infants, making it relevant to perform comprehensive neuropsychological assessments systematically throughout development. These characteristics of cognitive functioning are associated with structural changes and brain connectivity. Low intelligence test scores correlate with decreased connectivity between the left lateral occipital region and parietal lobes [
44].
Finally, we consider that it is crucial to expand the knowledge we have about the neurocognitive development of premature infants both in specific cognitive domains and in age ranges so that the information obtained helps to predict the probability of presenting cognitive alterations from early stages and, therefore to implement intervention strategies and programs based on scientific evidence and complemented in their design by clinical experience, empirical and theoretical knowledge of the different professionals involved in infant cognitive intervention.
We also find it relevant to consider the interaction of the changes found with other psychosocial variables, such as maternal schooling, which may have a modulating effect as has been reported in works such as that of Wang et al. [
17]; the analyses of our data in this regard, which are not presented in this paper, are indicative in this sense.
5. Limitations and Future Considerations
The generalization of the data may be limited by two conditions of this study: the sample size in both groups and the fact that the participants of the preterm group belong to a broader research protocol that involves the follow-up of their neurodevelopment and the implementation of neurohabilitation techniques for their intervention from two months of age, a condition that is not common to the preterm population in Mexico and the results regarding the alterations presented throughout the period studied may be attenuated by that factor. Nevertheless, these limitations open the door for future research in which neuropsychological data are correlated with data obtained by means of techniques such as electrophysiology or structural and functional neuroimaging so that the changes observed at the behavioral level can be explained by the changes that occurred in the nervous system.
Author Contributions
Conceptualization, T.G.C., and R.G.J.; methodology, T.G.C., and R.G.J; formal analysis, T.G.C.; investigation, T.G.C., and R.G.J.; data curation, T.G.C. and A.N.D.; writing original draft preparation, T.G.C., A.N.D., and R.G.J.; writing review and editing, T.G.C. and G.A.G.; visualization, G.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The protocol was approved by the Institutional Review Board of the Faculty of Administrative, Social, and Engineering Sciences from the Autonomous University of Baja California, code number POSG/024-1-01.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available upon request from the first author.
Acknowledgments
In this section, you can acknowledge any support not covered by the author’s contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Born too soon. The global action report on preterm birth. Eds. CP Howson, MV Kinney, JE Lawn. 2012.
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best practice & research. Clinical obstetrics & gynaecology 2018, 52, 3 – 12.
- Sansavini, A.; Guarini, A.; Caselli, M.C. PRETERM BIRTH: NEUROPSYCHOLOGICAL PROFILES AND ATYPICAL DEVELOPMENTAL PATHWAYS. Developmental Disabilities Research Reviews 2011, 17, 102 – 113.
- Villar, J.; Papageorghiou, A.T.; Knight, H.E.; Gravett, M.G.; Iams, J.; Waller, S.A.; Kramer, M.; Culhane, J.F.; Barros, F.C.; Conde-Agudelo, A.; et al. The preterm birth syndrome: a prototype phenotypic classification. American journal of obstetrics and gynecology 2012, 206, 119 – 123.
- Beck, S.; Wojdyla, D.; Say, L.; Betran, A.P.; Merialdi, M.; Requejo, J.H.; Rubens, C.; Menon, R.; Van Look, P.F.A. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization 2010, 88, 31 – 38.
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Garcia, C.V.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012, 379, 2162 – 2172.
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. The Lancet. Global health 2019, 7, e37 – e46.
- World Health Organization. Global Preterm Birth Estimates. Retrieved June 2, 2021, from https://ptb.srhr.org/ 2018.
- Ferreira, R.d.C.; Alves, C.R.L.; Guimarães, M.A.P.; Menezes, K.K.P.d.; Magalhães, L.d.C. Effects of early interventions focused on the family in the development of children born preterm and/or at social risk: a meta-analysis. Jornal de pediatria 2020, 96, 20 – 38.
- Forigua, J.C. Aproximaciones de las neurociencias a la conducta. Revista Latinoamericana de Psicología 2007, 39, 627 – 628.
- Montaño-Pérez, C.M.; Cázarez-Ortiz, M.; Juárez-Astorga, A.; Ramírez-Moreno, M.A. Morbilidad y mortalidad en recién nacidos menores de 1,000 gramos en una institución pública de tercer nivel en México. Revista Mexicana de Pediatria 2019, 86, 108 – 111.
- Aarnoudse-Moens, C.S.H.; Duivenvoorden, H.J.; Weisglas-Kuperus, N.; Van Goudoever, J.B.; Oosterlaan, J. The profile of executive function in very preterm children at 4 to 12 years. 54, 247 – 253.
- Gnigler, M.; Neubauer, V.; Griesmaier, E.; Zotter, S.; Kager, K.; Kiechl-Kohlendorfer, U. Very preterm children are at increased risk of reduced processing speed at 5 years of age, predicted by typical complications of prematurity and prenatal smoking. Acta paediatrica (Oslo, Norway : 1992) 2015, 104, e124 – e129.
- Linsell, L.; Malouf, R.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Prognostic Factors for Poor Cognitive Development in Children Born Very Preterm or With Very Low Birth Weight: A Systematic Review. JAMA pediatrics 2015, 169, 1162 – 1172.
- Pérez-Pereira, M.; Fernández, M.P.; Gómez-Taibo, M.L.; Martínez-López, Z.; Arce, C. A Follow-Up Study of Cognitive Development in Low Risk Preterm Children. International journal of environmental research and public health 2020, 17.
- Stålnacke, J.; Lundequist, A.; Böhm, B.; Forssberg, H.; Smedler, A.C. Individual cognitive patterns and developmental trajectories after preterm birth. Child Neuropsychology 2015, 21, 648 – 667.
- Wang, L.W.; Chu, C.H.; Lin, Y.C.; Huang, C.C. Trends in Gestational Age-Related Intelligence Outcomes of School-Age Children Born Very Preterm from 2001 to 2015 in Taiwan. The Journal of pediatrics 2023, 261, 113584.
- Qasemzadeh, M.; Pirnia, S.; Mohebi, S.; Matin, S.; Ebrahimi, H.; Ebrahimi, H.; Jangholi, E.; Gharehbeglou. Correlation of intelligence quotient (IQ) of children younger than 12 years old with history of preterm birth. Galen Medical Journal 2013, 3, 120–125.
- Heuvelman, H.; Abel, K.; Wicks, S.; Gardner, R.; Johnstone, E.; Lee, B.; Magnusson, C.; Dalman, C.; Rai, D. Gestational age at birth and risk of intellectual disability without a common genetic cause. European journal of epidemiology 2018, 33, 667 – 678.
- Twilhaar, E.S.; Wade, R.M.; de Kieviet, J.F.; van Goudoever, J.B.; van Elburg, R.M.; Oosterlaan, J. Cognitive Outcomes of Children Born Extremely or Very Preterm Since the 1990s and Associated Risk Factors: A Meta-analysis and Meta-regression. JAMA pediatrics 2018, 172, 361 – 367.
- van Veen, S.; van Wassenaer-Leemhuis, A.G.; Oosterlaan, J.; van Kaam, A.H.; Aarnoudse-Moens, C.S.H. Eight-year-old very and extremely preterm children showed more difficulties in performance intelligence than verbal intelligence. Acta paediatrica (Oslo, Norway : 1992) 2020, 109, 1175 – 1183.
- Chen, L.W.; Wang, S.T.; Wang, L.W.; Kao, Y.C.; Chu, C.L.; Wu, C.C.; Hsieh, Y.T.; Chiang, C.H.; Huang, C.C. Behavioral characteristics of autism spectrum disorder in very preterm birth children. Molecular autism 2019, 10, 32.
- Linsell, L.; Malouf, R.; Johnson, S.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Prognostic Factors for Behavioral Problems and Psychiatric Disorders in Children Born Very Preterm or Very Low Birth Weight: A Systematic Review. Journal of developmental and behavioral pediatrics : JDBP 2016, 37, 88 – 102.
- Sun, J.; Buys, N. Early executive function deficit in preterm children and its association with neurodevelopmental disorders in childhood: a literature review. International journal of adolescent medicine and health 2012, 24, 291 – 299.
- Twilhaar, E.S.; Pierrat, V.; Marchand-Martin, L.; Benhammou, V.; Kaminski, M.; Ancel, P.Y. Profiles of Functioning in 5.5-Year-Old Very Preterm Born Children in France: The EPIPAGE-2 Study. Journal of the American Academy of Child and Adolescent Psychiatry 2022, 61, 881 – 891.
- Wechsler, D. Wechsler Intelligence Scale for Children (WISC-IV). Manual Moderno. 2007.
- Fitzpatrick, A.; Carter, J.; Quigley, M.A. Association of Gestational Age With Verbal Ability and Spatial Working Memory at Age 11. Pediatrics 2016, 138.
- Korpela, S.; Nyman, A.; Munck, P.; Ahtola, A.; Matomäki, J.; Korhonen, T.; Parkkola, R.; Haataja, L. Working memory in very-low-birthweight children at the age of 11 years. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence 2018, 24, 338 – 353.
- Ford, R.M.; Griffiths, S.; Neulinger, K.; Andrews, G.; Shum, D.H.K.; Gray, P.H. Impaired prospective memory but intact episodic memory in intellectually average 7- to 9-year-olds born very preterm and/or very low birth weight. Child Neuropsychology 2017, 23, 954 – 979.
- F Kaul, Y.; Johansson, M.; Månsson, J.; Stjernqvist, K.; Farooqi, A.; Serenius, F.; B Thorell, L. Cognitive profiles of extremely preterm children: Full-Scale IQ hides strengths and weaknesses. Acta paediatrica (Oslo, Norway : 1992) 2021, 110, 1817 – 1826.
- Arpi, E.; D’Amico, R.; Lucaccioni, L.; Bedetti, L.; Berardi, A.; Ferrari, F. Worse global intellectual and worse neuropsychological functioning in preterm-born children at preschool age: a meta-analysis. Acta paediatrica (Oslo, Norway : 1992) 2019, 108, 1567 – 1579.
- Begega, A.; Méndez-López, M.; de Iscar, M.J.; Cuesta-Izquierdo, M.; Solís, G.; Fernández-Colomer, B.; Álvarez, L.; Méndez, M.; Arias, J.L. Assessment of the global intelligence and selective cognitive capacities in preterm 8-year-old children. Psicothema 2010, 22, 648 – 653.
- Loe, I.M.; Lee, E.S.; Luna, B.; Feldman, H.M. Executive function skills are associated with reading and parent-rated child function in children born prematurely. Early human development 2012, 88, 111 – 118.
- Sejer, E.P.F.; Bruun, F.J.; Slavensky, J.A.; Mortensen, E.L.; Schiøler Kesmodel, U. Impact of gestational age on child intelligence, attention and executive function at age 5: a cohort study. BMJ open 2019, 9, e028982.
- Hollanders, J.J.; Schaëfer, N.; van der Pal, S.M.; Oosterlaan, J.; Rotteveel, J.; Finken, M.J.J. Long-Term Neurodevelopmental and Functional Outcomes of Infants Born Very Preterm and/or with a Very Low Birth Weight. Neonatology 2019, 115, 310 – 319.
- Jaekel, J.; Sorg, C.; Baeuml, J.; Bartmann, P.; Wolke, D. Head Growth and Intelligence from Birth to Adulthood in Very Preterm and Term Born Individuals. Journal of the International Neuropsychological Society : JINS 2019, 25, 48 – 56.
- Soria-Pastor, S.; Gimenez, M.; Narberhaus, A.; Falcon, C.; Botet, F.; Bargallo, N.; Mercader, J.M.; Junque, C. Patterns of cerebral white matter damage and cognitive impairment in adolescents born very preterm. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 2008, 26, 647 – 654.
- Zubiaurre-Elorza, L.; Soria-Pastor, S.; Junqué, C.; Fernandez-Espejo, D.; Segarra, D.; Bargalló, N.; Romano-Berindoague, C.; Macaya, A. Thalamic changes in a preterm sample with periventricular leukomalacia: correlation with white-matter integrity and cognitive outcome at school age. Pediatric research 2012, 71, 354 – 360.
- Cepeda, N.J.; Blackwell, K.A.; Munakata, Y. Speed isn’t everything: complex processing speed measures mask individual differences and developmental changes in executive control. Developmental Science 2013, 16, 269 – 286.
- Wehrle, F.M.; Kaufmann, L.; Benz, L.D.; Huber, R.; O’Gorman, R.L.; Latal, B.; Hagmann, C.F. Very preterm adolescents show impaired performance with increasing demands in executive function tasks. Early human development 2016, 92, 37 – 43.
- Bruggink, J.L.M.; Van Braeckel, K.N.; Bos, A.F. The early motor repertoire of children born preterm is associated with intelligence at school age. Pediatrics 2010, 125, e1356 – e1363.
- Eryigit Madzwamuse, S.; Baumann, N.; Jaekel, J.; Bartmann, P.; Wolke, D. Neuro-cognitive performance of very preterm or very low birth weight adults at 26 years. Journal of Child Psychology & Psychiatry 2015, 56, 857 – 864.
- Roze, E.; Reijneveld, S.A.; Stewart, R.E.; Bos, A.F. Multi-domain cognitive impairments at school age in very preterm-born children compared to term-born peers. BMC pediatrics 2021, 21, 169.
- Kim, S.Y.; Kim, E.K.; Song, H.; Cheon, J.E.; Kim, B.N.; Kim, H.S.; Shin, S.H. Association of Brain Microstructure and Functional Connectivity With Cognitive Outcomes and Postnatal Growth Among Early School-Aged Children Born With Extremely Low Birth Weight. JAMA network open 2023, 6, e230198.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).