Submitted:
05 May 2024
Posted:
06 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Detailed medical history needs to be collected in order to answer the question if there are there any symptoms (syncope, exercise intolerance, palpitations, symptoms of heart failure).
- Physical examination ought to check the signs of bradycardia and heart failure.
- Echocardiography should be performed to assess the presence of valvular heart disease and to assess the size and function of the heart. In some patients, long-term AV block may cause left ventricular dyssynchrony.
- Exercise ECG should be performed and chronotropic capacity and exercise tolerance should be assessed.
- ECG monitoring using the Holter method to assess the average, minimum and maximum rhythm rate, assess whether there is ventricular arrhythmia and inhibitions and interruptions in the leading rhythm and QT interval duration [11].
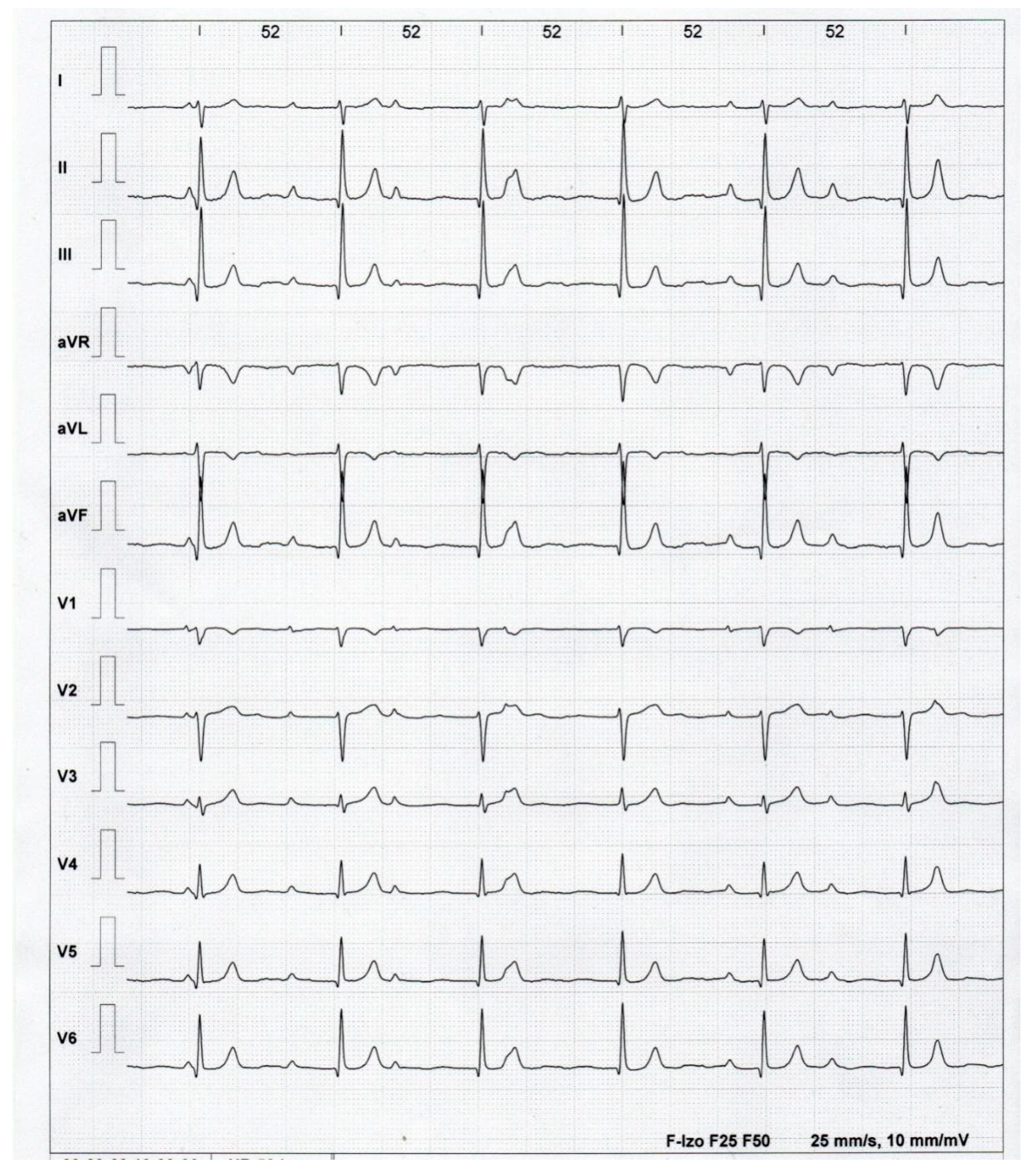
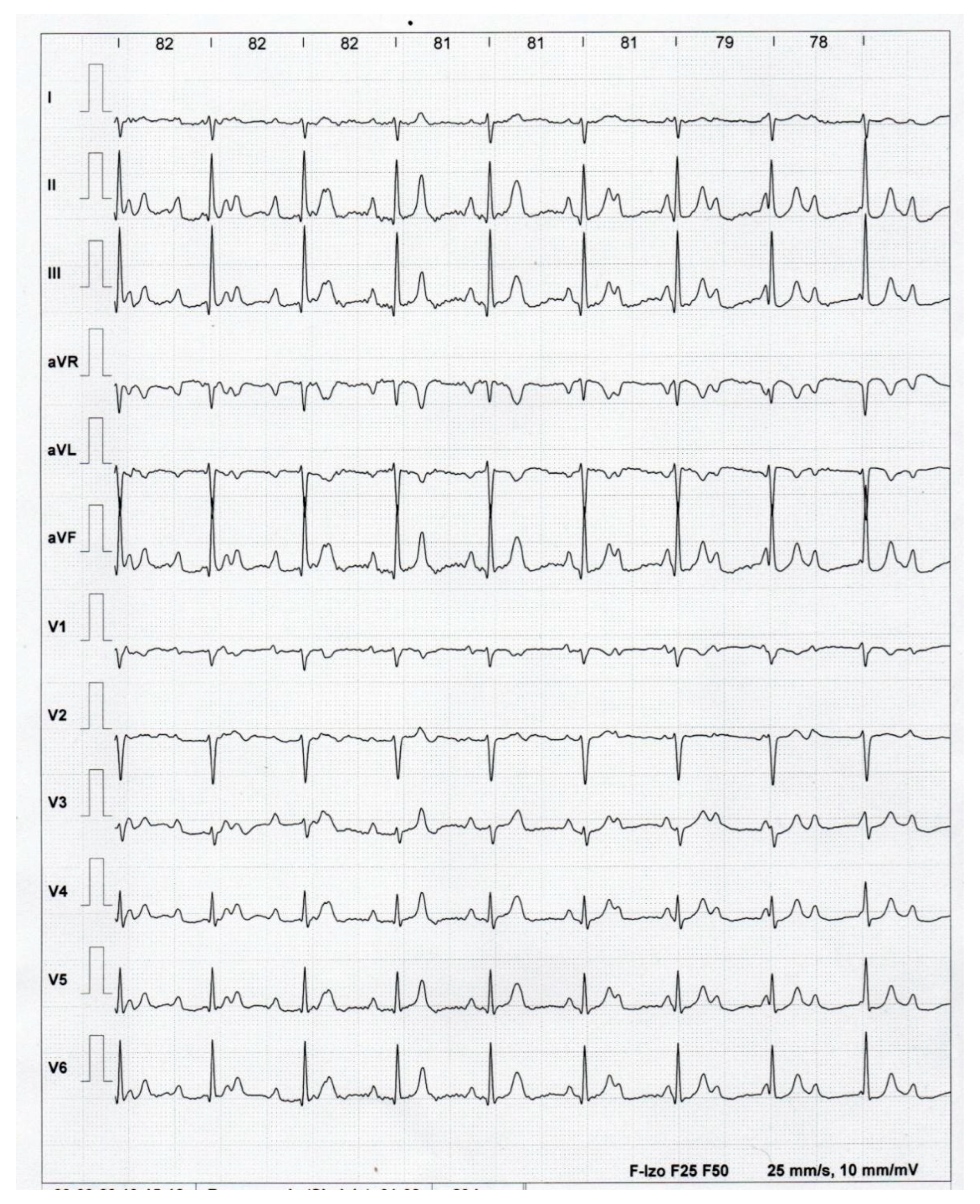
2. Case Presentation
3. Discussion
- occurrence of the symptoms mentioned earlier
- left ventricular systolic dysfunction
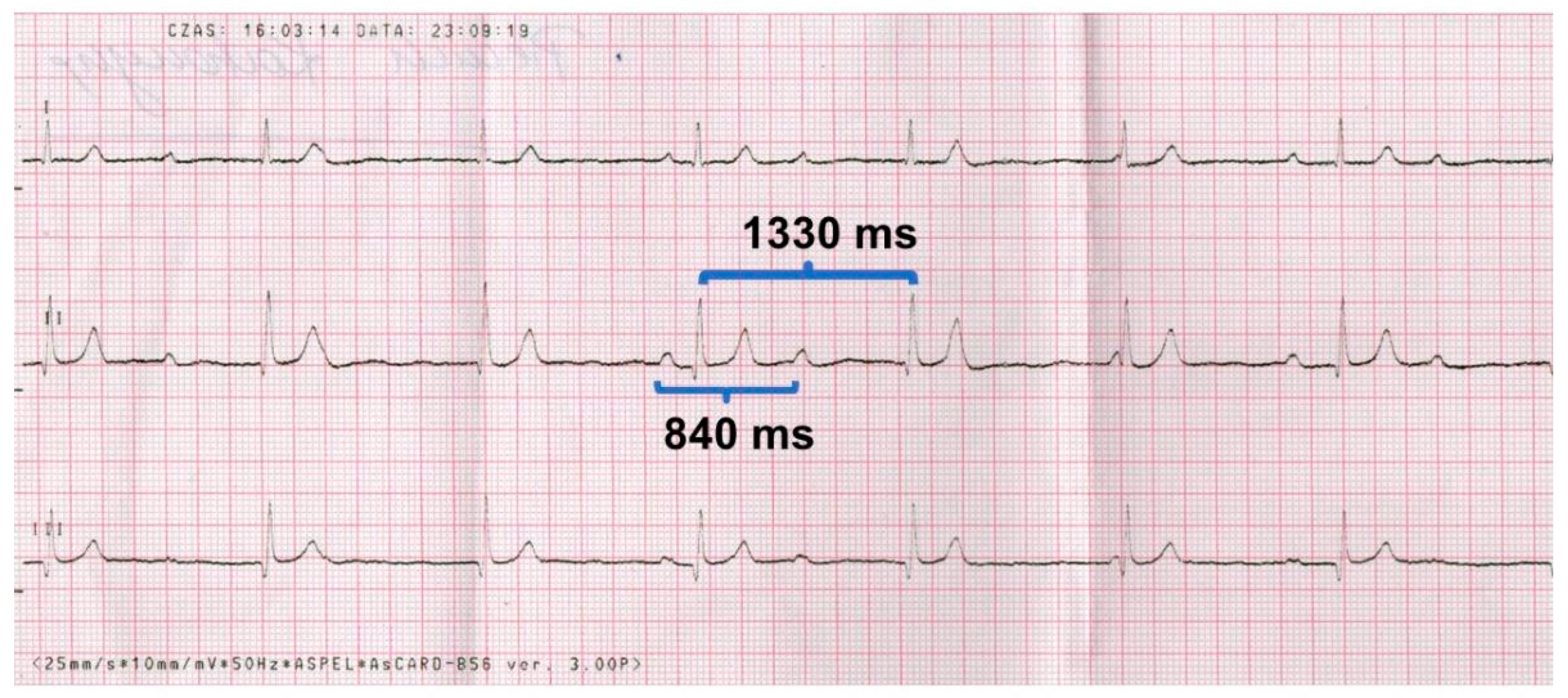
- pauses > 3 times the basic rhythm (e.g. in our case the average cycle is 1330 ms, so the pause > 3.990 ms (> ~ 4 seconds)
- escape rhythm with wide QRS complexes
- prolonged QTc interval; it should be remembered that Bazett's correction rule overestimates QTc for rhythms above 100 bpm and underestimates QTc for rhythms below 50 bpm.
- occurrence of complex ventricular arrhythmias
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melim, C.; Pimenta, J.; Areias, J.C. Congenital atrioventricular heart block: From diagnosis to treatment. Rev. Port. Cardiol. 2022, 41, 231–240. [Google Scholar] [CrossRef]
- Baruteau, A.E.; Pass, R.H.; Thambo, J.B.; Behaghel, A.; Le Pennec, S.; Perdreau, E.; Combes, N.; Liberman, L.; McLeod, C.J. Congenital and childhood atrioventricular blocks: Pathophysiology and contemporary management. Eur. J. Pediatr. 2016, 175, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zeron, P.; Izmirly, P.M.; Ramos-Casals, M.; Buyon, J.P.; Khamashta, M.A. The clinical spectrum of autoimmune congenital heart block. Nat. Rev. Rheumatol. 2015, 11, 301–312. [Google Scholar] [CrossRef]
- Ho, A.; Gordon, P.; Rosenthal, E.; Simpson, J.; Miller, O.; Sharland, G. Isolated complete heart block in the fetus. Am. J. Cardiol. 2015, 116, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, E.T.; Hamilton, R.M.; Silverman, E.D.; Zamora, S.A.; Hornberger, L.K. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution's experience of 30 years. J. Am. Coll. Cardiol. 2002, 39, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Michaëlsson, M.; Jonzon, A.; Riesenfeld, T. Isolated congenital complete atrioventricular block in adult life. A prospective study. Circulation 1995, 92, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Clark, T.J.; Tan, J.H.; Delaney, S.; Jolley, J.A. Ultrasound findings in fetal congenital heart block associated with maternal anti-Ro/SSA and anti-La/SSB antibodies. Ultrasound Q. 2015, 31, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Moak, J.P.; Barron, K.S.; Hougen, T.J.; Wiles, H.B.; Balaji, S.; Sreeram, N.; Cohen, M.H.; Nordenberg, A.; Van Hare, G.F.; Friedman, R.A.; et al. Congenital heart block: Development of late-onset cardiomyopathy, a previously underappreciated sequela. J. Am. Coll. Cardiol. 2001, 37, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, G.; Arnold, J.; Coles, D.M. Torsade de pointes and asystole in a child with complete heart block and prolonged QT interval. Aust. Paediatr. J. 1983, 19, 187–191. [Google Scholar] [CrossRef]
- Shah, M.J.; Silka, M.J.; Avari Silva, J.N.; Balaji, S.; Beach, C.M.; Benjamin, M.N.; Berul, C.I.; Cannon, B.; Cecchin, F.; Cohen, M.I.; Dalal, A.S.; et al. 2021 PACES expert consensus statement on the indications and management of cardiovascular implantable electronic devices in pediatric patients. Cardiol. Young 2021, 31, 1738–1769. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Wahren-Herlenius, M. Congenital heart block: Evidence for a pathogenic role of maternal autoantibodies. Arthritis Res. Ther. 2012, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Buyon, J.P.; Heibert, R.; Copel, J.; Craft, J.; Friedman, D.; Katholi, M.; Lee, L.A.; Provost, T.T.; Reichlin, M.; Rider, L.; et al. Autoimmune-associated congenital heart block: Mortality, morbidity, and recurrence rates obtained from a national neonatal lupus registry. J. Am. Coll. Cardiol. 1998, 31, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.S.; Rausa, J.; Villarreal, E.; Farias, J.S.; Flores, S. Postoperative atrioventricular block in pediatric patients: Impact of congenital cardiac malformations and medications. Pediatr. Cardiol. 2024, 45, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Brugada, J.; Blom, N.; Sarquella-Brugada, G.; Blomstrom-Lundqvist, C.; Deanfield, J.; Janousek, J.; Abrams, D.; Bauersfeld, U.; Brugada, R.; Drago, F.; et al. Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. Europace 2013, 15, 1337–1382. [Google Scholar] [CrossRef] [PubMed]
- Kanter, R.J.; Pfeiffer, R.; Hu, D.; Barajas-Martinez, H.; Carboni, M.P.; Antzelevitch, C. Brugada-like syndrome in infancy presenting with rapid ventricular tachycardia and intraventricular conduction delay. Circulation 2012, 125, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Makita, N.; Seki, A.; Sumitomo, N.; Chkourko, H.; Fukuhara, S.; Watanabe, H.; Shimizu, W.; Bezzina, C.R.; Hasdemir, C.; Mugishima, H. A connexin40 mutation associated with a malignant variant of progressive familial heart block type I. Circ. Arrhythm. Electrophysiol. 2012, 5, 163–172. [Google Scholar] [CrossRef] [PubMed]
- McCulley, D.J.; Black, B.L. Transcription factor pathways and congenital heart disease. Curr. Top. Dev. Biol. 2012, 100, 253–277. [Google Scholar] [CrossRef]
- Forrester, J.D.; Mead, P. Third-degree heart block associated with lyme carditis: Review of published cases. Clin. Infect. Dis. 2014, 59, 996–1000. [Google Scholar] [CrossRef]
- Barra, S.N.; Providência, R.; Paiva, L.; Nascimento, J.; Marques, A.L. A review on advanced atrioventricular block in young or middle-aged adults. Pacing Clin. Electrophysiol. 2012, 35, 1395–1405. [Google Scholar] [CrossRef]
- Yildirim, I.; Karagöz, T.; Ertuğrul, I.; Karagöz, A.H.; Özer, S. Efficacy and safety of cryoablation of parahissian accessory pathways in children: A single institution study. Pacing Clin. Electrophysiol. 2013, 36, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Salinas, C.A.; Ezzeddine, F.M.; Mulpuru, S.K.; Asirvatham, S.J.; Sharaf, B.A. Cardiac implantable electronic devices in female patients: Esthetic, breast implant, and anatomic considerations. J. Cardiovasc. Electrophysiol. 2024, 35, 747–761, Online ahead of print. [Google Scholar] [CrossRef]
- Ranasinghe, I.; Labrosciano, C.; Horton, D.; Ganesan, A.; Curtis, J.P.; Krumholz, H.M.; McGavigan, A.; Hossain, S.; Air, T.; Hariharaputhiran, S. Institutional variation in quality of cardiovascular implantable electronic device implantation: A cohort study. Ann. Intern. Med. 2019, 171, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Winkler, F.; Von Felten, S.; Gass, M.; Berger, F.; Weber, R.; Dave, H.; Balmer, C. Lead and generator dysfunction in children and adolescents with epicardial pacemaker and implantable cardioverter defibrillator systems: The challenge of early recognition. Pacing Clin. Electrophysiol. 2024, 47, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Thambo, J.B.; Bordachar, P.; Garrigue, S.; Lafitte, S.; Sanders, P.; Reuter, S.; Girardot, R.; Crepin, D.; Reant, P.; Roudaut, R.; et al. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation 2004, 110, 3766–3772. [Google Scholar] [CrossRef] [PubMed]
- Wiliński, J.; Czarnecka, D.; Kloch-Badełek, M. Cardiac resynchronization therapy in the treatment of chronic heart failure. Przew. Lek. 2008, 4, 48–53. [Google Scholar]
- Wiliński, J.; Czarnecka, D.; Wojciechowska, W.; Kloch-Badełek, M.; Jastrzębski, M.; Bacior, B.; Sondej, T.; Kusak, P.; Przybyła, A.; Kawecka-Jaszcz, K. Clinical and classic echocardiographic features of patients with, and without, left ventricle reverse remodeling following the introduction of cardiac resynchronization therapy. Cardiol. J. 2011, 18, 157–164. [Google Scholar] [PubMed]
- Chung, M.K.; Patton, K.K.; Lau, C.P.; Dal Forno, A.R.J.; Al-Khatib, S.M.; Arora, V.; Birgersdotter-Green, U.M.; Cha, Y.M.; Chung, E.H.; Cronin, E.M. ; 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure. Heart Rhythm 2023, 20, e17–e91. [Google Scholar] [CrossRef]
- Takeuchi, D.; Tomizawa, Y. Pacing device therapy in infants and children: A review. J. Artif. Organs. 2013, 16, 23–33. [Google Scholar] [CrossRef]
- Wilhelm, B.J.; Thöne, M.; El-Scheich, T.; Livert, D.; Angelico, R.; Osswald, B. Complications and risk assessment of 25 years in pediatric pacing. Ann. Thorac. Surg. 2015, 100, 147–153. [Google Scholar] [CrossRef]
- Vouliotis, A.I.; Roberts, P.R.; Dilaveris, P.; Gatzoulis, K.; Yue, A.; Tsioufis, K. Leadless pacemakers: Current achievements and future perspectives. Eur. Cardiol. 2023, 18, e49. [Google Scholar] [CrossRef]
- Pachon-M, J.C.; Ortencio, F.A.; Pachon-M, E.I.; Lobo, T.; Santillana-P, T.G.; Pachon, C.T. , Cunha-P, M.Z.; Pachon-M, J.C.M.; Zerpa-A, J.C.; Amarante, R.C. Treatment of symptomatic functional atrioventricular block by cardioneuroablation as an alternative to pacemaker implantation. JACC Case Rep. 2022, 4, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Kukla, P.; Jastrzębski, M.; Fijorek, K.; Stec, S.; Bryniarski, L.; Czarnecka, D.; Baranchuk, A. Electrocardiographic parameters indicating worse evolution in patients with acquired long QT syndrome and torsades de pointes. Ann. Noninvasive Electrocardiol. 2016, 21, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, R.; Wojciechowski, D.; Kozłowski, D.; Kukla, P.; Kurpesa, M.; Lelakowski, J.; Maciejewska, M.; Średniawa, B.; Wranicz, J.K. Electrocardiographic criteria for diagnosis of the heart chamber enlargement, necrosis and repolarization abnormalities including acute coronary syndromes. Experts' group statement of the Working Group on Noninvasive Electrocardiology and Telemedicine of the Polish Cardiac Society. Kardiol Pol. 2016, 74, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cuneo, B.F.; Strasburger, J.F.; Huhta, J.C.; Gotteiner, N.L.; Wakai, R.T. Electrophysiological characteristics of fetal atrioventricular block. J. Am. Coll. Cardiol. 2008, 51, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Strasburger, J.F.; Eckstein, G.; Butler, M.; Noffke, P.; Wacker-Gussmann, A. Fetal arrhythmia diagnosis and pharmacologic management. J. Clin. Pharmacol. 2022, 62, S53–S66. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Silverman, E.; Golding, F.; Guerra, V.; Hiraki, L.; Thakur, V.; Jaeggi, E. Effects of transplacental dexamethasone therapy on fetal immune-mediated complete heart block. Fetal Diagn. Ther. 2021, 48, 183–188. [Google Scholar] [CrossRef]
- Friedman, D.M.; Llanos, C.; Izmirly, P.M.; Brock, B.; Byron, J.; Copel, J.; Cummiskey, K.; Dooley, M.A.; Foley, J.; Graves, C.; et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: Results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. 2010, 62, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Ruffatti, A.; Cerutti, A.; Favaro, M.; Del Ross, T.; Calligaro, A.; Hoxha, A.; Marson, P.; Leoni, L.; Milanesi, O. Plasmapheresis, intravenous immunoglobulins and bethametasone - a combined protocol to treat autoimmune congenital heart block: A prospective cohort study. Clin. Exp. Rheumatol. 2016, 34, 706–713. [Google Scholar]
- De Caluwé, E.; Van De Bruaene, A.; Willems, R.; Troost, E.; Gewillig, M.; Rega, F.; Budts, W. Long-term follow-up of children with heart block born from mothers with systemic lupus erythematosus: A retrospective study from the database pediatric and congenital heart disease in University Hospitals Leuven. Pacing Clin. Electrophysiol. 2016, 39, 935–943. [Google Scholar] [CrossRef]
- Kusumoto, F.H.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2019, 140, e382–e482. [Google Scholar] [CrossRef] [PubMed]
- Practical Medicine, Drug Index, Krakow, Poland. Available online: https://indeks.mp.pl (accessed on 25 April 2024).
- Hondo, H.; Kowase, S.; Shunichi, A.; Osada, J.; Aoki, H.; Yumoto, H. Theophylline to treat prolonged paroxysmal complete atrioventricular block without conduction disorder or structural heart disease after COVID-19 infection: A case report. Heart Rhythm Case Rep. 2022, 8, 229–232. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




