Submitted:
06 May 2024
Posted:
07 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
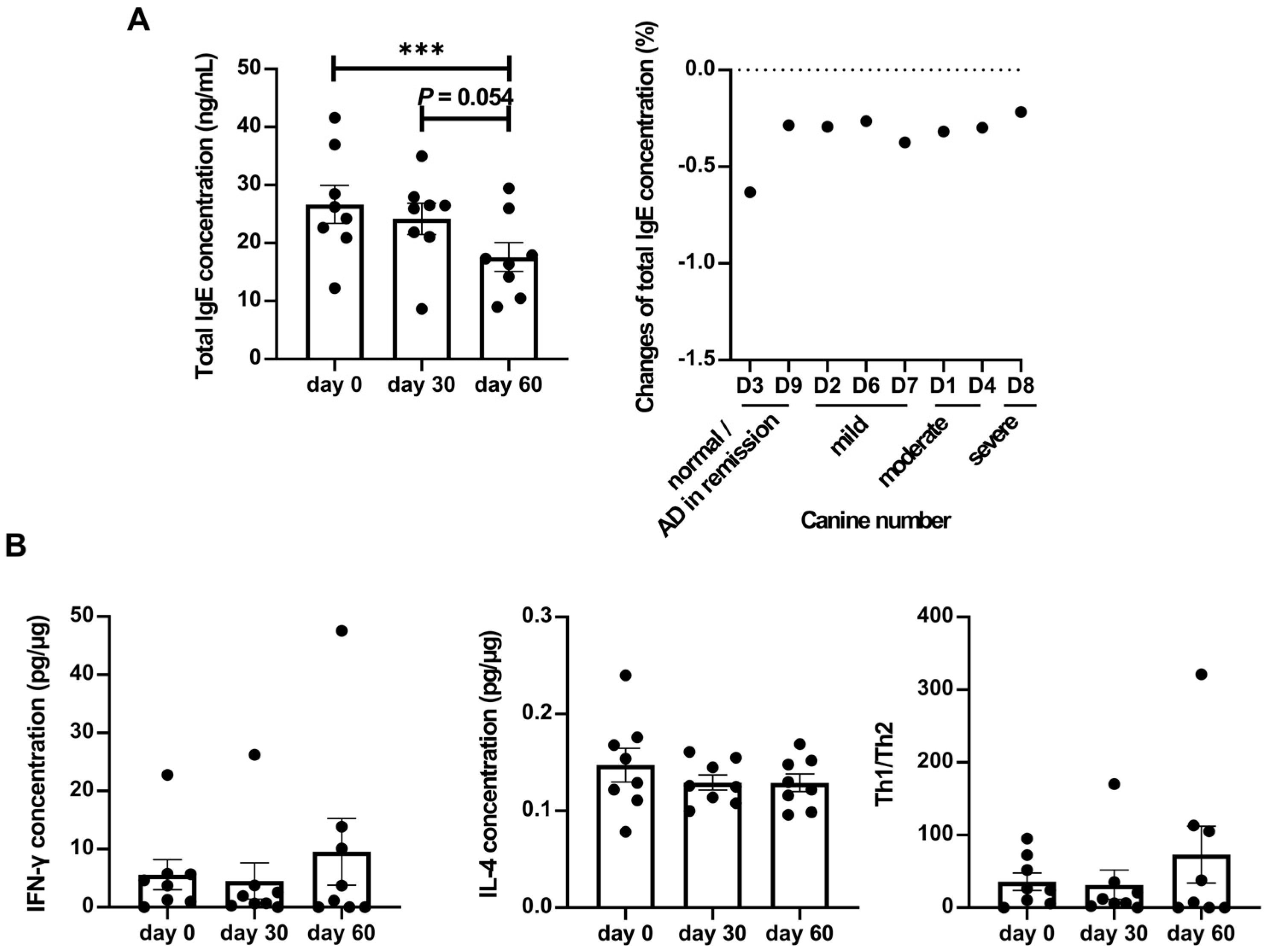
2.1. Administrating LCP Reduced the Symptoms of Atopic Dermatitis in Dogs via Modulating the Immune System
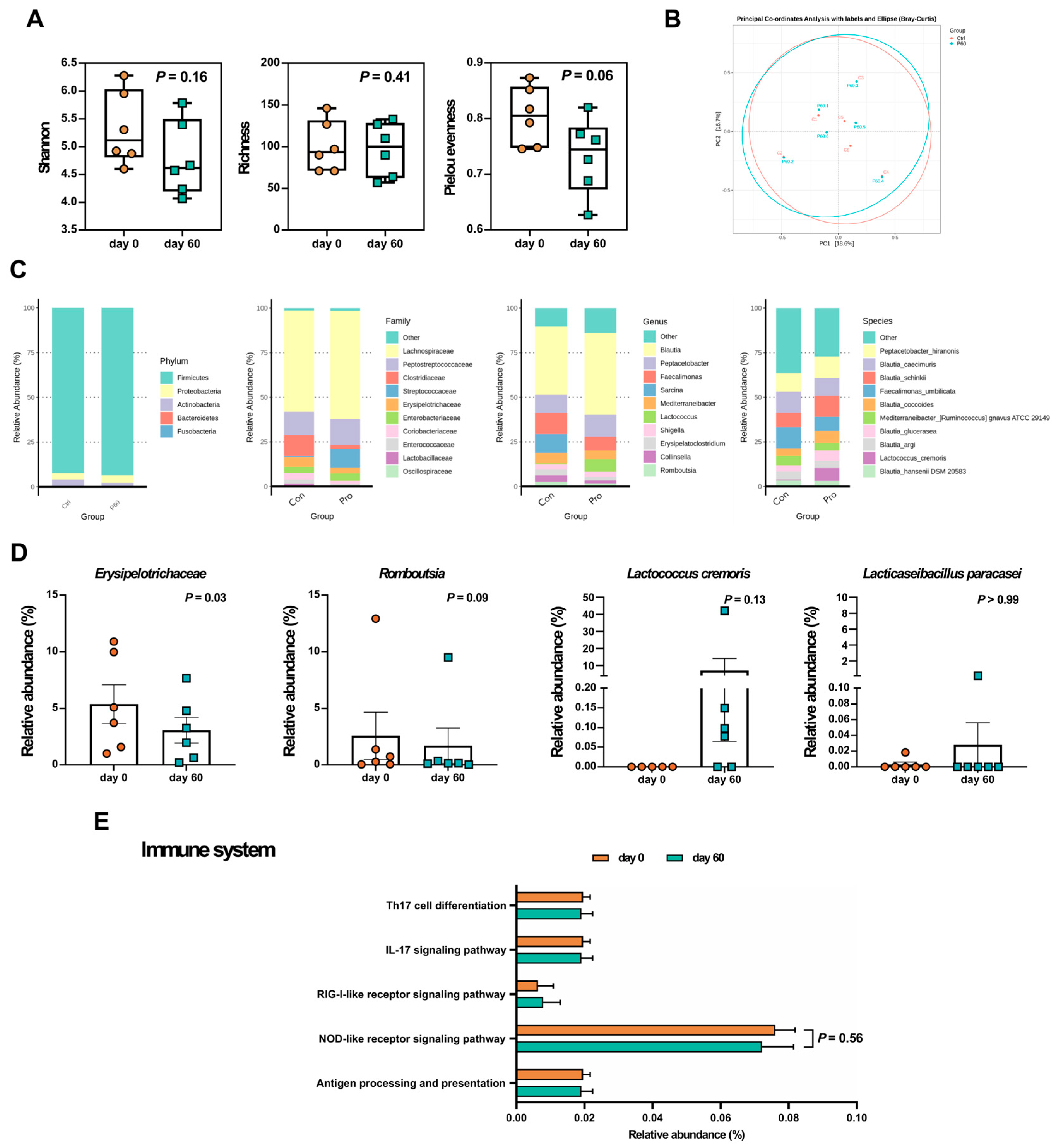
2.2. LCP Intervention Altered the Composition of Specific Bacterial Species in the Fecal Microbiota Of Dogs Suffering from Atopic Dermatitis
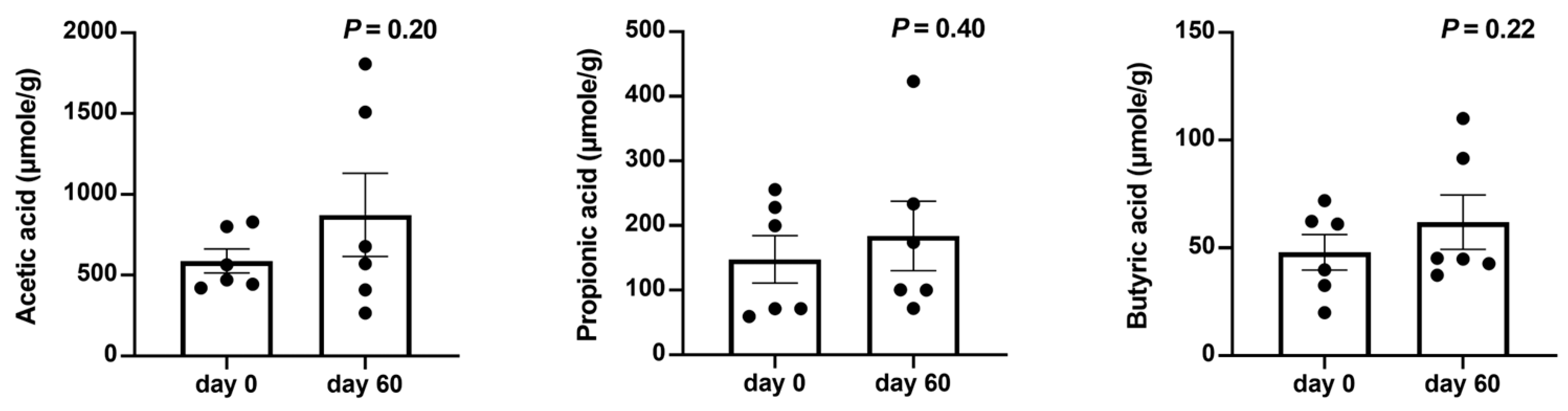
2.3. LCP Intervention Altered the Gut Microbial Function
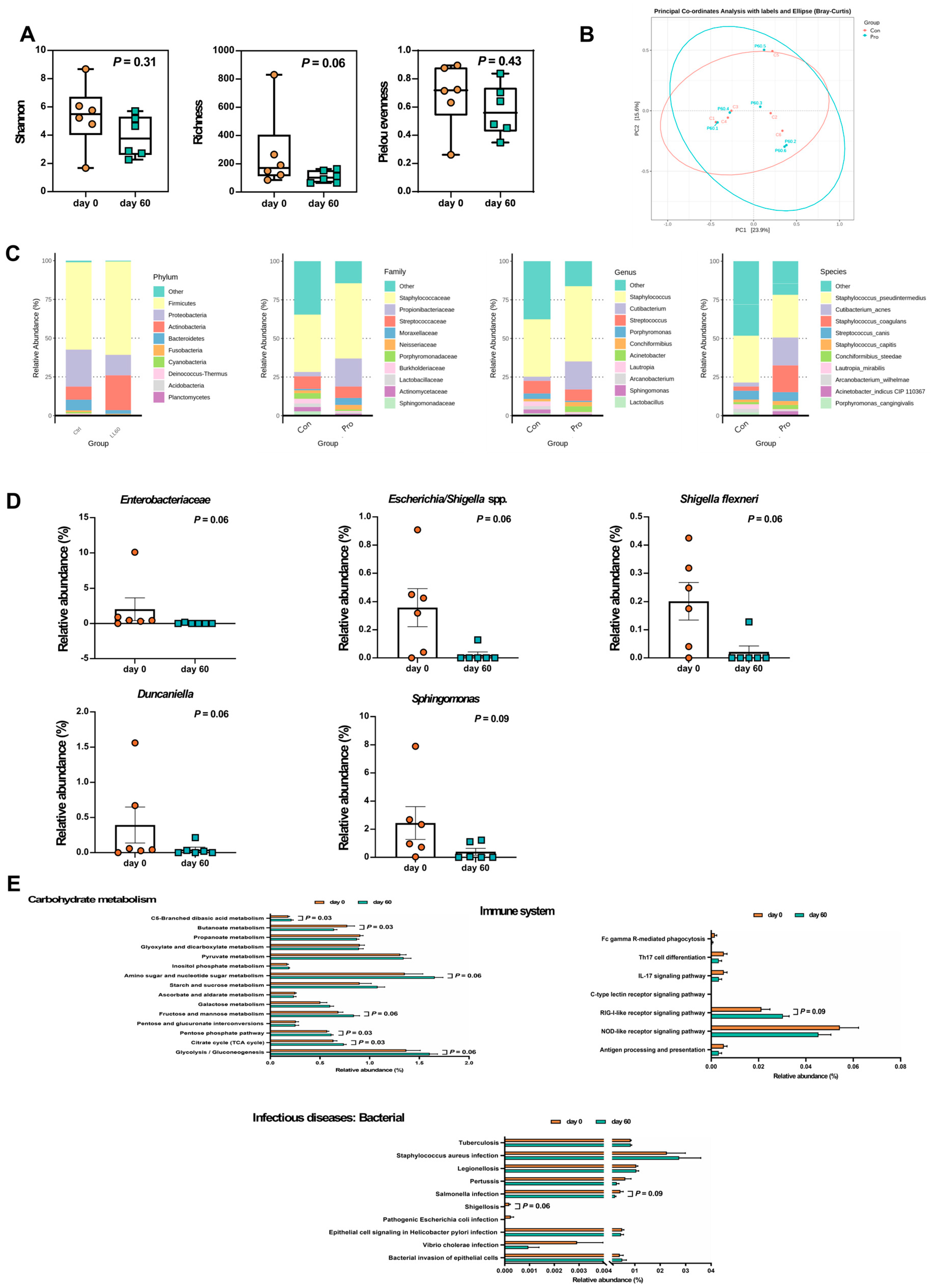
2.4. LCP Intervention Altered the Composition of Specific Bacterial Species in the Skin Microbiota of Dogs Suffering from Atopic Dermatitis
2.5. LCP Intervention Altered the Skin Microbial Function
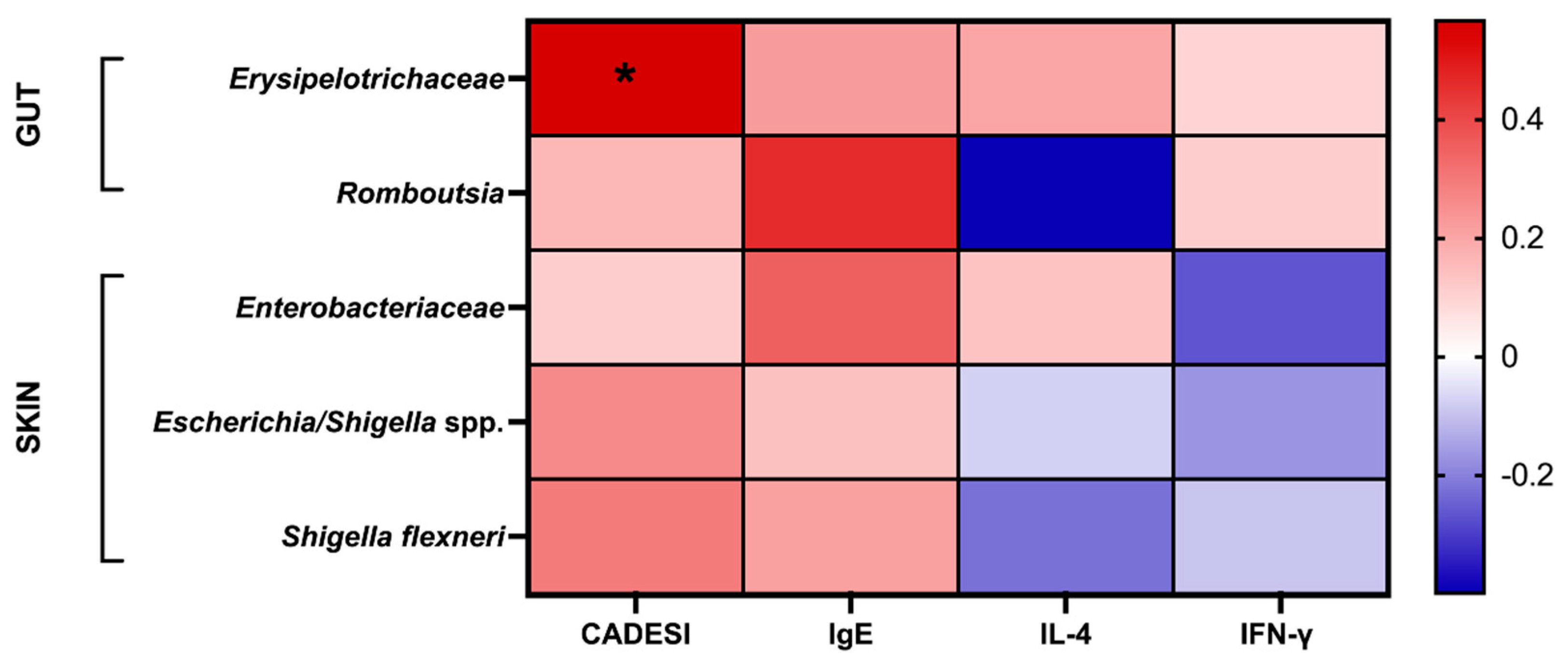
2.6. Correlation Analysis between Fecal and Skin Microbiota and Atopic Dermatitis Indicators
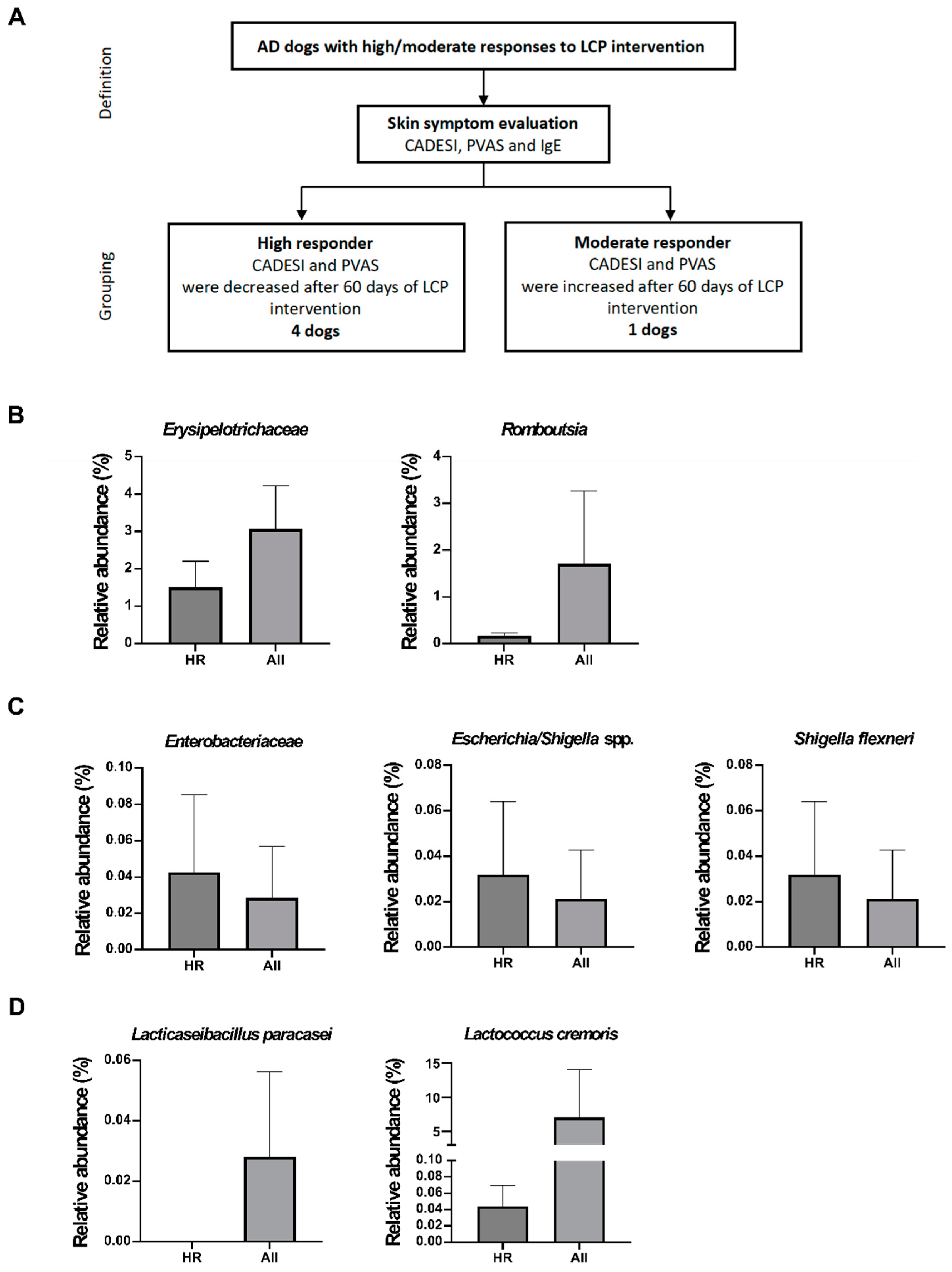
2.7. The Effectiveness of LCP Intervention in Alleviating Canine Atopic Dermatitis Involves Changes in the Abundance of Biomarkers Indicated in Both Skin and Fecal Samples
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Preparation of Probiotic Capsule
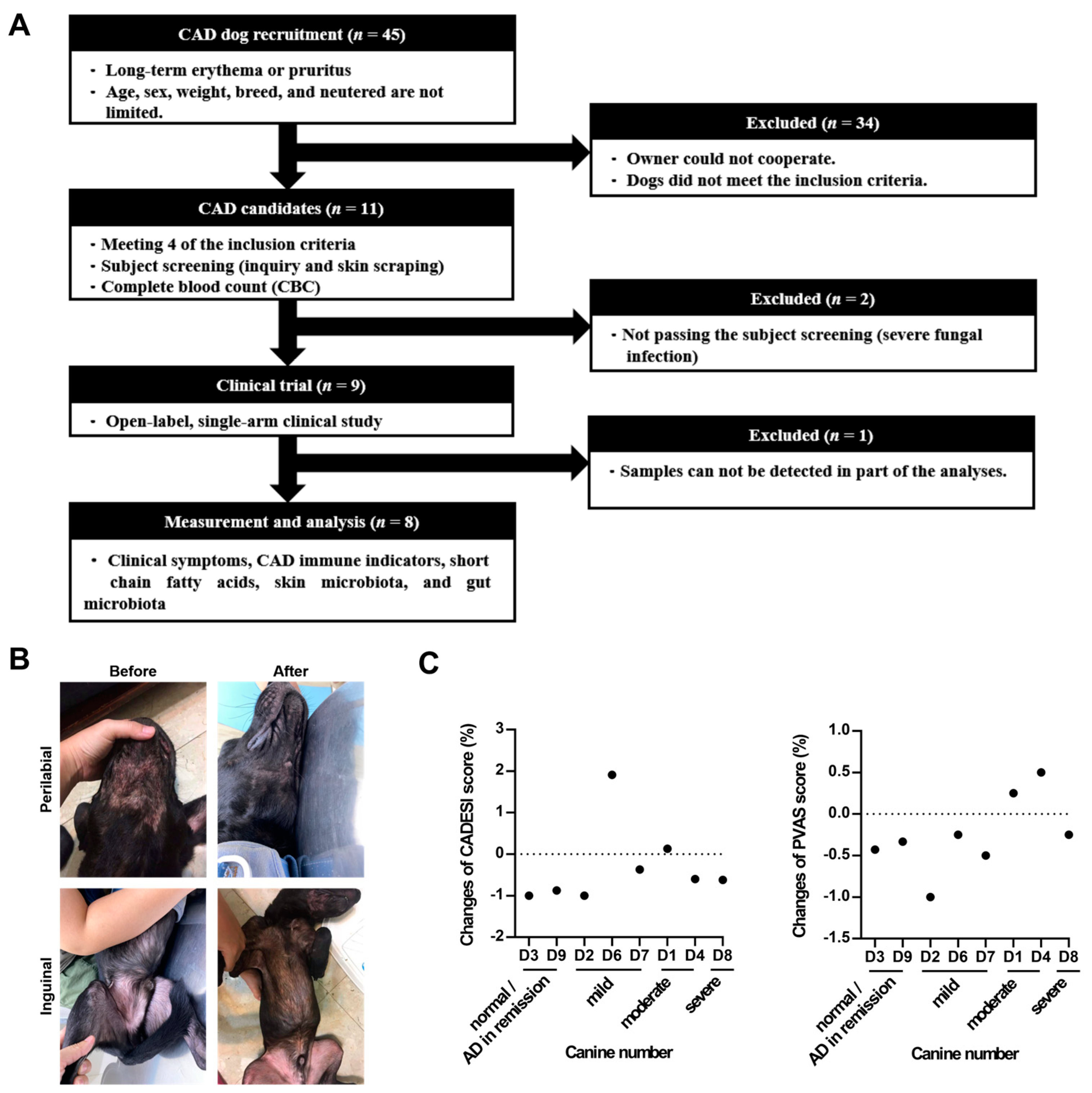
4.3. Clinical Canine Trial
- The initial onset of symptoms must have occurred at or before 3 years of age.
- The dog primarily resided indoors. Itching symptoms were significantly improved by steroids
- Skin lesions were primarily located on the ears, interdigital spaces, around the mouth, eyes, dorsal surfaces of the joints, and inguinal areas.
- The edge skin of the ears appeared normal
- The skin of the lower back was normal
4.4. Assessment of the Degree of Atopic Dermatitis and Pruritus
4.5. Cytokine Production from Peripheral Blood Mononuclear Cells (PBMCs)
4.6. IgE Production in Serum
4.7. Microbiota Analyses in Skin and Fecal
4.8. Short-Chain Fatty Acids Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gedon, N.K.Y.; Mueller, R.S. Atopic dermatitis in cats and dogs: a difficult disease for animals and owners. Clin. Transl. Allergy 2018, 8, 1–12. [CrossRef]
- Outerbridge, C.A.; Jordan, T.J. Current knowledge on canine atopic dermatitis: pathogenesis and treatment. Adv. Small Anim. Care 2021, 2, 101–115.
- Couceiro, G.A.; Ribeiro, S.M.M.; Monteiro, M.M.; Meneses, A.M.C.; Sousa, S.K.S.; Coutinho, L.N. Prevalence of canine atopic dermatitis at the Veterinary Hospital of the “Universidade Federal Rural da Amazônia” in Belém/Pará, Brazil. Pesqui. Vet. Bras. 2021, 41.
- Humeniuk, P.; Dubiela, P.; Hoffmann-Sommergruber, K. Dendritic Cells and Their Role in Allergy: Uptake, Proteolytic Processing and Presentation of Allergens. Int. J. Mol. Sci. 2017, 18, 1491. [CrossRef]
- Marsella, R.; Sousa, C.A.; Gonzales, A.J.; Fadok, V.A. Current understanding of the pathophysiologic mechanisms of canine atopic dermatitis. J. Am. Veter- Med Assoc. 2012, 241, 194–207. [CrossRef]
- Nguyen, H.L.; Anderson, K.R.; Tollefson, M.M. New and Emerging Therapies for Pediatric Atopic Dermatitis. Pediatr. Drugs 2019, 21, 239–260. [CrossRef]
- Gonzales, A.J.; Bowman, J.W.; Fici, G.J.; Zhang, M.; Mann, D.W.; Mitton-Fry, M. Oclacitinib (APOQUEL®) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J. Veter- Pharmacol. Ther. 2014, 37, 317–324. [CrossRef]
- Bağci, I.S.; Ruzicka, T. IL-31: A new key player in dermatology and beyond. J. Allergy Clin. Immunol. 2018, 141, 858–866. [CrossRef]
- Chrobak-Chmiel, D.; Golke, A.; Kwiecień, E.; Biegańska, M.J.; Dembele, K.; Dziekiewicz-Mrugasiewicz, M.; Czopowicz, M.; Kizerwetter-Świda, M.; Rzewuska, M. Is Vitamin D3 a Worthy Supplement Protecting against Secondary Infections in Dogs with Atopic Dermatitis?. Pathogens 2023, 12, 145. [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut–Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [CrossRef]
- Pfefferle, P.I.; Keber, C.U.; Cohen, R.M.; Garn, H. The Hygiene Hypothesis – Learning From but Not Living in the Past. Front. Immunol. 2021, 12. [CrossRef]
- Dou, J.; Zeng, J.; Wu, K.; Tan, W.; Gao, L.; Lu, J. Microbiosis in pathogenesis and intervention of atopic dermatitis. Int. Immunopharmacol. 2019, 69, 263–269. [CrossRef]
- E Fujimura, K.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [CrossRef]
- Fang, Z.; Li, L.; Zhang, H.; Zhao, J.; Lu, W.; Chen, W. Gut Microbiota, Probiotics, and Their Interactions in Prevention and Treatment of Atopic Dermatitis: A Review. Front. Immunol. 2021, 12, 720393. [CrossRef]
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; Sivamani, R.K. The Skin and Gut Microbiome and Its Role in Common Dermatologic Conditions. Microorganisms 2019, 7, 550. [CrossRef]
- Kwon, H.-K.; Lee, C.-G.; So, J.-S.; Chae, C.-S.; Hwang, J.-S.; Sahoo, A.; Nam, J.H.; Rhee, J.H.; Hwang, K.-C.; Im, S.-H. Generation of regulatory dendritic cells and CD4 + Foxp3 + T cells by probiotics administration suppresses immune disorders. Proc. Natl. Acad. Sci. 2010, 107, 2159–2164. [CrossRef]
- Shin, J.-H.; Chung, M.-J.; Seo, J.-G. A multistrain probiotic formulation attenuates skin symptoms of atopic dermatitis in a mouse model through the generation of CD4+Foxp3+T cells. Food Nutr. Res. 2016, 60, 32550. [CrossRef]
- Kim, W.-K.; Jang, Y.J.; Han, D.H.; Seo, B.; Park, S.; Lee, C.H.; Ko, G. Administration of Lactobacillus fermentum KBL375 Causes Taxonomic and Functional Changes in Gut Microbiota Leading to Improvement of Atopic Dermatitis. Front. Mol. Biosci. 2019, 6, 92. [CrossRef]
- Chen, H.-Y.; Chen, Y.-T.; Li, K.-Y.; Huang, H.-W.; Lin, Y.-C.; Chen, M.-J. A Heat-Killed Probiotic Mixture Regulates Immune T Cells Balance and IgE Production in House Dust Mite Extraction-Induced Atopic Dermatitis Mice. Microorganisms 2022, 10, 1881. [CrossRef]
- Harvey, N.D.; Craigon, P.J.; Shaw, S.C.; Blott, S.C.; England, G.C. Behavioural Differences in Dogs with Atopic Dermatitis Suggest Stress Could Be a Significant Problem Associated with Chronic Pruritus. Animals 2019, 9, 813. [CrossRef]
- Linek, M.; Favrot, C. Impact of canine atopic dermatitis on the health-related quality of life of affected dogs and quality of life of their owners. Veter- Dermatol. 2010, 21, 456–462. [CrossRef]
- Udoye, C.C.; Rau, C.N.; Freye, S.M.; Almeida, L.N.; Vera-Cruz, S.; Othmer, K.; Korkmaz, R..; Clauder, A.-K.; Lindemann, T.; Niebuhr, M.; et al. B-cell receptor physical properties affect relative IgG1 and IgE responses in mouse egg allergy. Mucosal Immunol. 2022, 15, 1375–1388. [CrossRef]
- Carballo, I.; Alonso-Sampedro, M.; Gonzalez-Conde, E.; Sanchez-Castro, J.; Vidal, C.; Gude, F.; Gonzalez-Quintela, A. Factors Influencing Total Serum IgE in Adults: The Role of Obesity and Related Metabolic Disorders. Int. Arch. Allergy Immunol. 2021, 182, 220–228. [CrossRef]
- Chaudhary, S.K.; Singh, S.K.; Kumari, P.; Kanwal, S.; Soman, S.P.; Choudhury, S.; Garg, S.K. Alterations in circulating concentrations of IL-17, IL-31 and total IgE in dogs with atopic dermatitis. Vet. Dermatol. 2019, 30, 383–e114.
- Reddel, S.; Del Chierico, F.; Quagliariello, A.; Giancristoforo, S.; Vernocchi, P.; Russo, A.; Fiocchi, A.; Rossi, P.; Putignani, L.; El Hachem, M. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci. Rep. 2019, 9, 1–10. [CrossRef]
- Sugita, K.; Shima, A.; Takahashi, K.; Ishihara, G.; Kawano, K.; Ohmori, K. Pilot evaluation of a single oral fecal microbiota transplantation for canine atopic dermatitis. Sci. Rep. 2023, 13, 1–11. [CrossRef]
- Ye, S.; Yan, F.; Wang, H.; Mo, X.; Liu, J.; Zhang, Y.; Li, H.; Chen, D. Diversity analysis of gut microbiota between healthy controls and those with atopic dermatitis in a Chinese population. J. Dermatol. 2020, 48, 158–167. [CrossRef]
- Uchiyama, J.; Osumi, T.; Mizukami, K.; Fukuyama, T.; Shima, A.; Unno, A.; Takemura-Uchiyama, I.; Une, Y.; Murakami, H.; Sakaguchi, M. Characterization of the oral and faecal microbiota associated with atopic dermatitis in dogs selected from a purebred Shiba Inu colony. Lett. Appl. Microbiol. 2022, 75, 1607–1616. [CrossRef]
- Wang, Y.; Hou, J.; Tsui, J.C.-C.; Wang, L.; Zhou, J.; Chan, U.K.; Lo, C.J.Y.; Siu, P.L.K.; Loo, S.K.F.; Tsui, S.K.W. Unique Gut Microbiome Signatures among Adult Patients with Moderate to Severe Atopic Dermatitis in Southern Chinese. Int. J. Mol. Sci. 2023, 24, 12856. [CrossRef]
- You, I.; Kim, M.J. Comparison of Gut Microbiota of 96 Healthy Dogs by Individual Traits: Breed, Age, and Body Condition Score. Animals 2021, 11, 2432. [CrossRef]
- Turpin, W.; Bedrani, L.; Espin-Garcia, O.; Xu, W.; Silverberg, M.S.; Smith, M.I.; Garay, J.A.R.; Lee, S.-H.; Guttman, D.S.; Griffiths, A.; et al. Associations ofNOD2polymorphisms with Erysipelotrichaceae in stool of in healthy first degree relatives of Crohn's disease subjects. BMC Med Genet. 2020, 21, 1–8. [CrossRef]
- Duan, W.; Mehta, A.K.; Magalhaes, J.G.; Ziegler, S.F.; Dong, C.; Philpott, D.J.; Croft, M. Innate signals from Nod2 block respiratory tolerance and program TH2-driven allergic inflammation. J. Allergy Clin. Immunol. 2010, 126, 1284–1293.e10. [CrossRef]
- Girardin, S.E.; Boneca, I.G.; Viala, J.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 Is a General Sensor of Peptidoglycan through Muramyl Dipeptide (MDP) Detection. J. Biol. Chem. 2003, 278, 8869–8872. [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9.
- Maeda, S.; Hsu, L.C.; Liu, H.; Bankston, L.A.; Iimura, M.; Kagnoff, M.F.; Eckmann, L.; Karin, M.. Nod2 mutation in Crohn's disease potentiates NF-κB activity and IL-1ß processing. Science 2005, 307, 734–738.
- Su, Y.-J.; Luo, S.-D.; Hsu, C.-Y.; Kuo, H.-C. Differences in gut microbiota between allergic rhinitis, atopic dermatitis, and skin urticaria. Medicine 2021, 100, e25091. [CrossRef]
- Li, R.; Yao, Y.; Gao, P.; Bu, S. The Therapeutic Efficacy of Curcumin vs. Metformin in Modulating the Gut Microbiota in NAFLD Rats: A Comparative Study. Front. Microbiol. 2021, 11. [CrossRef]
- Wang, H.; Zhang, M.; Wen, X.; He, L.; Zhang, M.; Zhang, J.; Yang, X. Cepharanthine ameliorates dextran sulphate sodium-induced colitis through modulating gut microbiota. Microb. Biotechnol. 2022, 15, 2208–2222. [CrossRef]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [CrossRef]
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [CrossRef]
- Trompette, A.; Pernot, J.; Perdijk, O.; Alqahtani, R.A.A.; Domingo, J.S.; Camacho-Muñoz, D.; Wong, N.C.; Kendall, A.C.; Wiederkehr, A.; Nicod, L.P.; et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 2022, 15, 908–926. [CrossRef]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 1–15. [CrossRef]
- Glaeser, S.P.; Kämpfer, P. The family sphingomonadaceae. In The Prokaryotes: Alphaproteobacteria and Betaproteobacteria, 4th ed.; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin, Germany, 2014; Volume 8, pp. 641–707.
- Gamage, H.K.; Vuong, D.; Minns, S.A.; Chen, R.; Piggott, A.M.; Lacey, E.; Paulsen, I.T. The composition and natural variation of the skin microbiota in healthy Australian cattle. Research Square 2022, submitted.
- Imamura, R.; Wang, Y.; Kinoshita, T.; Suzuki, M.; Noda, T.; Sagara, J.; Taniguchi, S.; Okamoto, H.; Suda, T. Anti-Inflammatory Activity of PYNOD and Its Mechanism in Humans and Mice. J. Immunol. 2010, 184, 5874–5884. [CrossRef]
- Lautz, K.; Damm, A.; Menning, M.; Wenger, J.; Adam, A.C.; Zigrino, P.; Kremmer, E.; Kufer, T.A. NLRP10 enhancesShigella-induced pro-inflammatory responses. Cell. Microbiol. 2012, 14, 1568–1583. [CrossRef]
- Mirza, N.; Sowa, A.S.; Lautz, K.; Kufer, T.A. NLRP10 Affects the Stability of Abin-1 To Control Inflammatory Responses. J. Immunol. 2019, 202, 218–227. [CrossRef]
- Fritz, J.H.; Le Bourhis, L.; Sellge, G.; Magalhaes, J.G.; Fsihi, H.; Kufer, T.A.; Collins, C.; Viala, J.; Ferrero, R.L.; Girardin, S.E.; et al. Nod1-Mediated Innate Immune Recognition of Peptidoglycan Contributes to the Onset of Adaptive Immunity. Immunity 2007, 26, 445–459. [CrossRef]
- Aoyama, M.; Kotani, J.; Usami, M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 2010, 26, 653–661.
- Carrion, S.L.; Sutter, C.H.; Sutter, T.R. Combined treatment with sodium butyrate and PD153035 enhances keratinocyte differentiation. Exp. Dermatol. 2014, 23, 211–214. [CrossRef]
- Wang, Y.; Kao, M.-S.; Yu, J.; Huang, S.; Marito, S.; Gallo, R.L.; Huang, C.-M. A Precision Microbiome Approach Using Sucrose for Selective Augmentation of Staphylococcus epidermidis Fermentation against Propionibacterium acnes. Int. J. Mol. Sci. 2016, 17, 1870. [CrossRef]
- Favrot, C.; Steffan, J.; Seewald, W.; Picco, F. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Veter- Dermatol. 2010, 21, 23–31. [CrossRef]
- Terada, Y.; Nagata, M.; Murayama, N.; Nanko, H.; Furue, M. Clinical comparison of human and canine atopic dermatitis using human diagnostic criteria (Japanese Dermatological Association, 2009): Proposal of provisional diagnostic criteria for canine atopic dermatitis. J. Dermatol. 2011, 38, 784–790. [CrossRef]
- Olivry, T.; Saridomichelakis, M.; Nuttall, T.; Bensignor, E.; Griffin, C.E.; Hill, P.B. Validation of the Canine Atopic Dermatitis Extent and Severity Index (CADESI)-4, a simplified severity scale for assessing skin lesions of atopic dermatitis in dogs. Veter- Dermatol. 2014, 25, 77–e25. [CrossRef]
- Rybníček, J.; Lau-Gillard, P.J.; Harvey, R.; Hill, P.B. Further validation of a pruritus severity scale for use in dogs. Veter- Dermatol. 2009, 20, 115–122. [CrossRef]
- Watanabe, K.; Fujimoto, J.; Sasamoto, M.; Dugersuren, J.; Tumursuh, T.; Demberel, S. Diversity of lactic acid bacteria and yeasts in Airag and Tarag, traditional fermented milk products of Mongolia. World J. Microbiol. Biotechnol. 2007, 24, 1313–1325. [CrossRef]
- Torii, T.; Kanemitsu, K.; Wada, T.; Itoh, S.; Kinugawa, K.; Hagiwara, A. Measurement of short-chain fatty acids in human faeces using high-performance liquid chromatography: specimen stability. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2010, 47, 447–452. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




