Submitted:
02 May 2024
Posted:
07 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Case Description
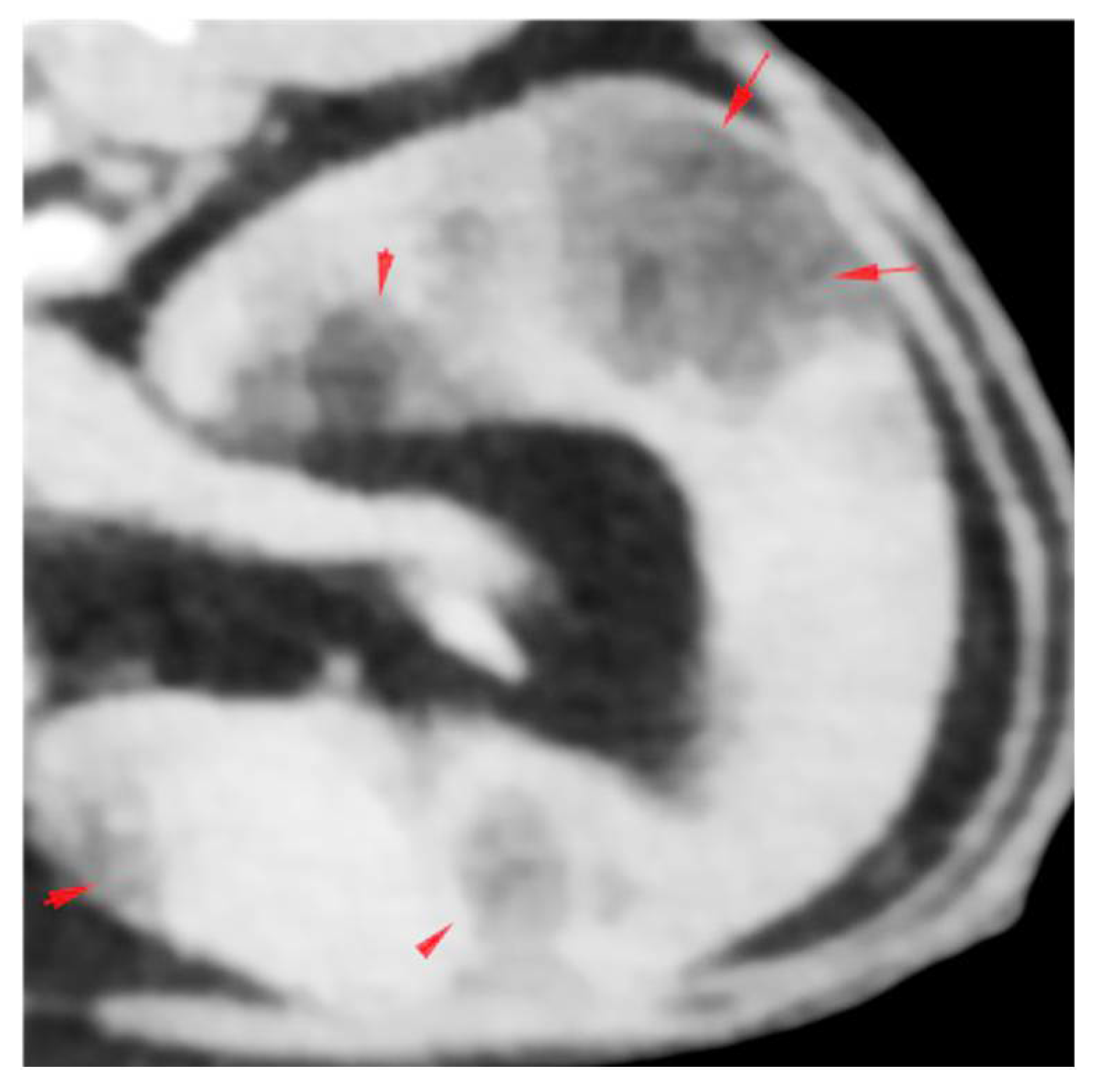
2.1. History, Clinical Findings and Diagnostic Investigations
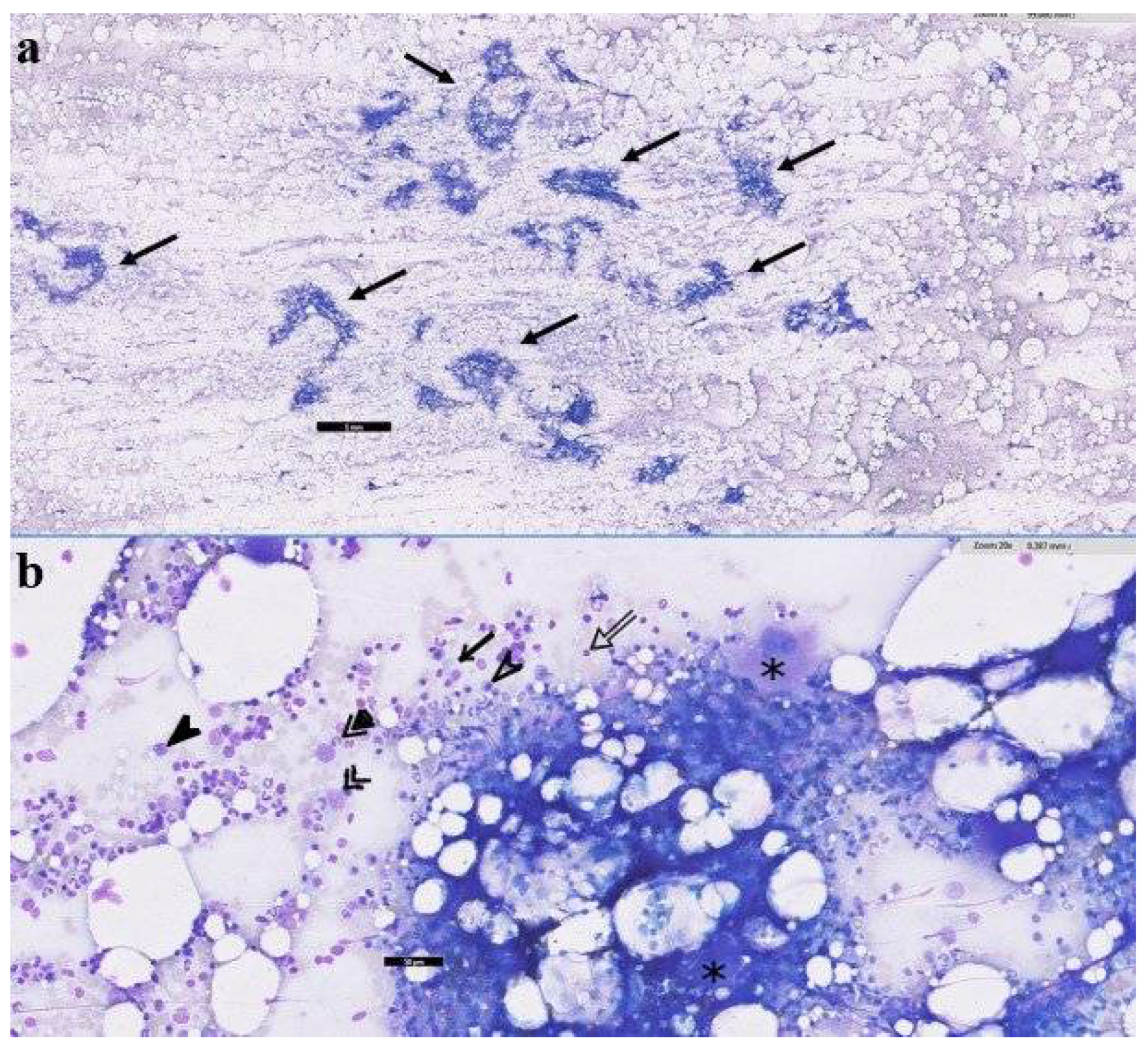
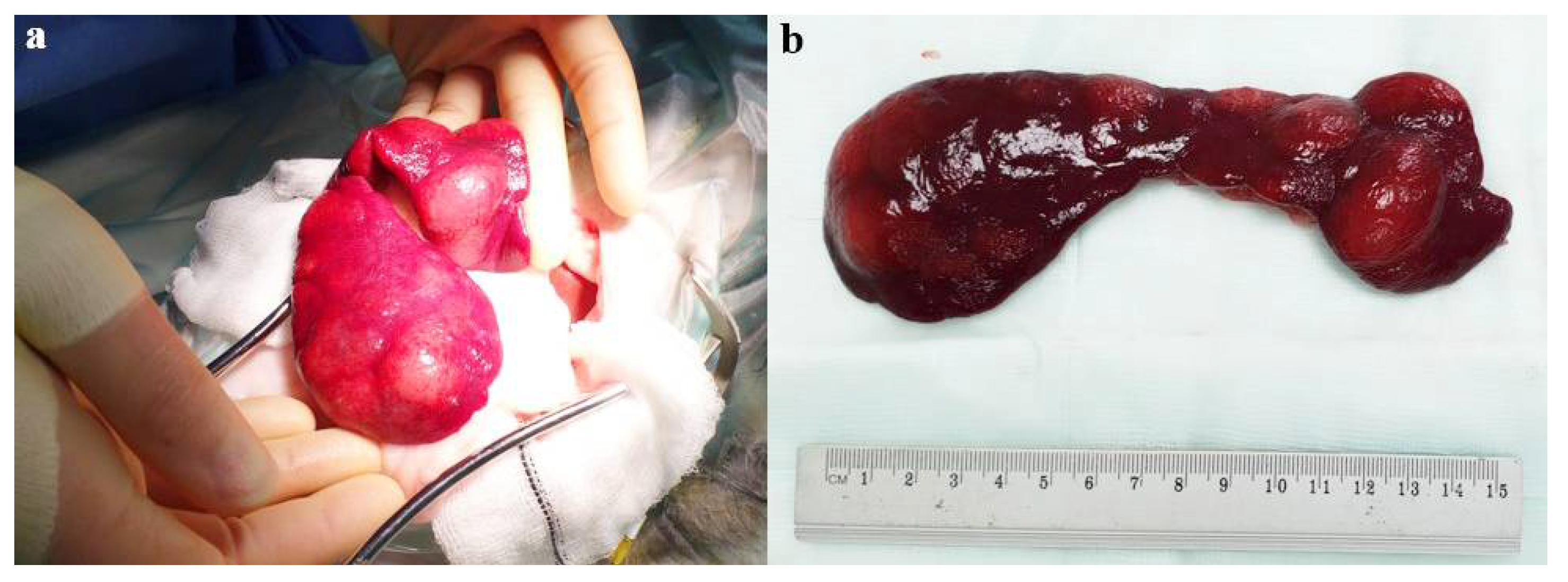
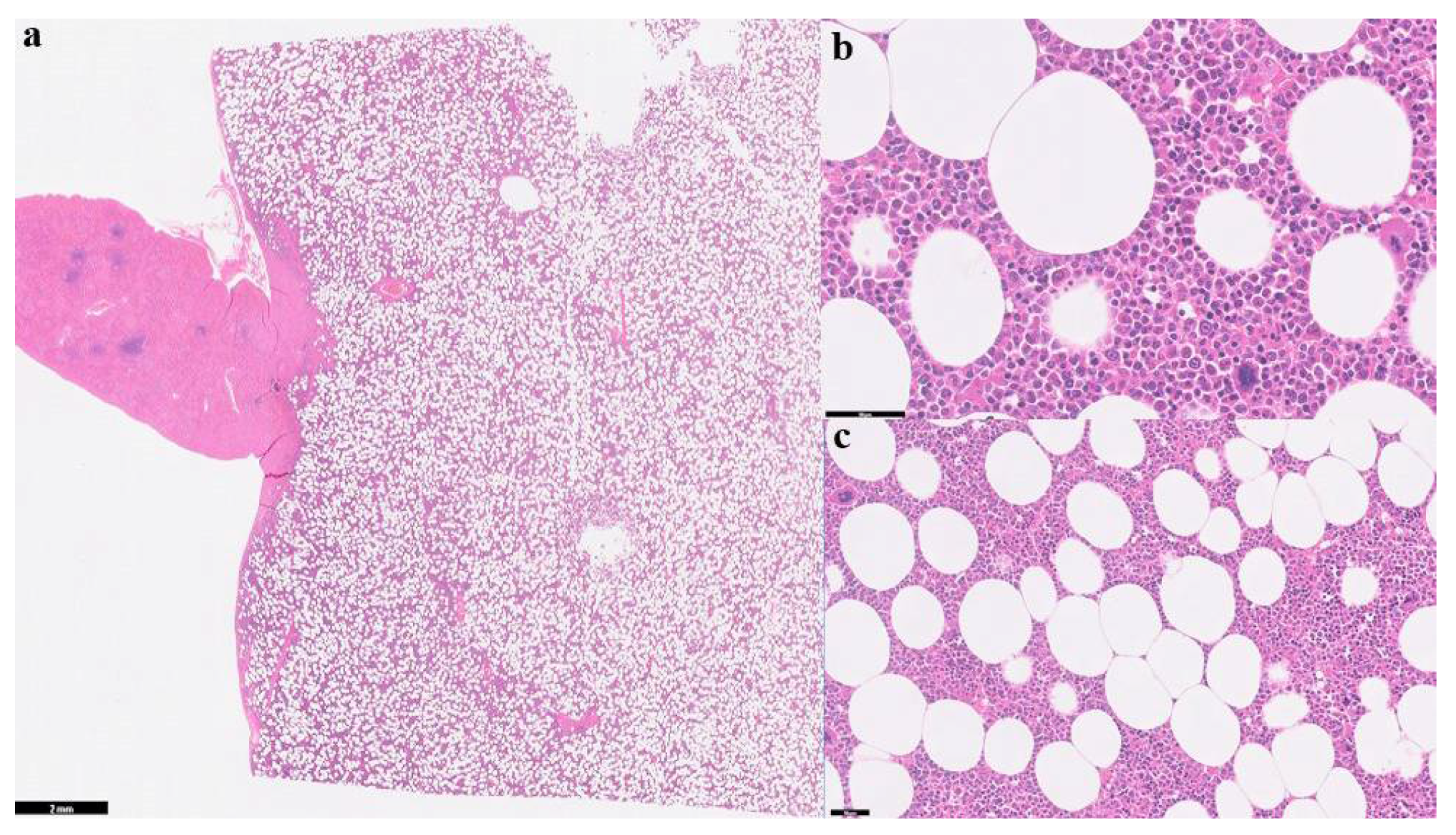
2.2. Surgical Case Management and Outcome
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Feng, C., et al., Adrenal myelolipoma: a mingle of progenitor cells? Med Hypotheses, 2013. 80(6): p. 819-22.
- Thomas, R.M. and A.J. Fischetti, What is your diagnosis? Myelolipoma. J Am Vet Med Assoc, 2012. 241(7): p. 881-3.
- Sander, C.H. and R.F. Langham, Myelolipoma of the spleen in a cat. J Am Vet Med Assoc, 1972. 160(8): p. 1101-3.
- Al-Bahri, S., et al., Giant bilateral adrenal myelolipoma with congenital adrenal hyperplasia. Case Rep Surg, 2014. 2014: p. 728198. [CrossRef]
- Bokhari, M.R., et al., Adrenal Myelolipoma, in StatPearls. 2024, StatPearls Publishing: Treasure Island (FL).
- Nitz, J.A., et al., Symptomatic Extra-Adrenal Myelolipoma in the Spleen. Case Rep Surg, 2020. 2020: p. 8839178. [CrossRef]
- Wood, W.G., et al., Myelolipoma in the spleen: a rare discovery of extra-adrenal hematopoietic tissue. J Surg Case Rep, 2013. 2013(3). [CrossRef]
- Cina, S.J., B.M. Gordon, and N.S. Curry, Ectopic adrenal myelolipoma presenting as a splenic mass. Arch Pathol Lab Med, 1995. 119(6): p. 561-3.
- Zeng, Y., et al., Giant Myelolipoma in the Spleen: A Rare Case Report and Literature Review. Int J Surg Pathol, 2016. 24(2): p. 177-80.
- McCaw, D.L., J.M. da Silva Curiel, and D.P. Shaw, Hepatic myelolipomas in a cat. J Am Vet Med Assoc, 1990. 197(2): p. 243-4. [CrossRef]
- Wani, N.A., et al., Giant adrenal myelolipoma: Incidentaloma with a rare incidental association. Urol Ann, 2010. 2(3): p. 130-3. [CrossRef]
- Daneshmand, S. and M.L. Quek, Adrenal myelolipoma: diagnosis and management. Urol J, 2006. 3(2): p. 71-4.
- Liu, H.P., et al., Intra-abdominal bleeding with hemorrhagic shock: a case of adrenal myelolipoma and review of literature. BMC Surg, 2017. 17(1): p. 74. [CrossRef]
- Thomas, R.M. and A.J. Fischetti, What Is Your Diagnosis? Journal of the American Veterinary Medical Association, 2012. 241(7): p. 881-883.
- Haak, C.E., et al., Nontraumatic hemoabdomen and pancytopenia secondary to myelolipoma in a cat. J Vet Emerg Crit Care (San Antonio), 2022. 32(2): p. 260-266. [CrossRef]
- Nabi, J., et al., Incidental detection of adrenal myelolipoma: a case report and review of literature. Case Rep Urol, 2013. 2013: p. 789481. [CrossRef]
- Cardy, R.H. and R.E. Bostrom, Multiple Splenic Myelolipomas in a Cheetah (Acinonyx jubatus). Veterinary Pathology, 1978. 15(4): p. 556-558. [CrossRef]
- Kamiie, J., et al., Multicentric myelolipoma in a dog. J Vet Med Sci, 2009. 71(3): p. 371-3. [CrossRef]
- Gourley, I.M., J.A. Popp, and R.D. Park, Myelolipomas of the liver in a domestic cat. J Am Vet Med Assoc, 1971. 158(12): p. 2053-7.
- Barge, P., et al., Cholangiocarcinoma and hepatic myelolipoma incarcerated in a peritoneopericardial diaphragmatic hernia with pulmonary metastasis and carcinomatosis in a cat. JFMS Open Rep, 2019. 5(1): p. 2055116919835081. [CrossRef]
- Zimmer, M.A. and F.L. Stair, Splenic Myelolipomas in Two Dogs. Veterinary Pathology, 1983. 20(5): p. 637-638. [CrossRef]
- Ikede, B.O. and R.S. Downey, Case report. Multiple hepatic myelolipomas in a cat. Can Vet J, 1972. 13(7): p. 160-3.
- Schuh, J.C., Hepatic nodular myelolipomatosis (myelolipomas) associated with a peritoneo-pericardial diaphragmatic hernia in a cat. J Comp Pathol, 1987. 97(2): p. 231-5. [CrossRef]
- Nemanic, S., N.C. Nelson, and L.P. Guiot, What Is Your Diagnosis? Journal of the American Veterinary Medical Association, 2013. 243(5): p. 627-629.
- Hamidi, O., et al., Clinical course of adrenal myelolipoma: A long-term longitudinal follow-up study. Clin Endocrinol (Oxf), 2020. 93(1): p. 11-18. [CrossRef]
- Ramirez, M. and S. Misra, Adrenal myelolipoma: To operate or not? A case report and review of the literature. Int J Surg Case Rep, 2014. 5(8): p. 494-6. [CrossRef]
- Wouda, R.M., et al., Hepatic myelolipoma incarcerated in a peritoneopericardial diaphragmatic hernia in a cat. Aust Vet J, 2010. 88(6): p. 231-5. [CrossRef]
- Rolph, K.E., B. Vidana, and E. Field, Giant splenic myelolipoma in a cat with hyperthyroidism. JFMS Open Rep, 2022. 8(2): p. 20551169221127889. [CrossRef]
- Griffin, S., Feline abdominal ultrasonography: What's normal? What's abnormal? The spleen. J Feline Med Surg, 2021. 23(3): p. 241-255. [CrossRef]
- Rao, P., et al., Imaging and pathologic features of myelolipoma. Radiographics, 1997. 17(6): p. 1373-85. [CrossRef]
- Hasan, M., F. Siddiqui, and M. Al-Ajmi, FNA diagnosis of adrenal myelolipoma: a rare entity. Diagn Cytopathol, 2008. 36(12): p. 925-6. [CrossRef]
- Hernández-Amate, A., et al., Cytological diagnosis of a presacral myelolipoma: a case report diagnosed by fine-needle aspiration. Diagn Cytopathol, 2008. 36(12): p. 921-2. [CrossRef]
- Tursi, M., et al., Adrenal Myelolipoma in a Dog. Veterinary Pathology, 2005. 42(2): p. 232-235. [CrossRef]
- Finch, N.C., Hypercalcaemia in cats: The complexities of calcium regulation and associated clinical challenges. J Feline Med Surg, 2016. 18(5): p. 387-99.
- Gordon, S.S., et al., Outcome following splenectomy in cats. J Feline Med Surg, 2010. 12(4): p. 256-61. [CrossRef]
- Kraus, K.A., et al., Outcome and Prognostic Indicators in Cats Undergoing Splenectomy for Splenic Mast Cell Tumors. J Am Anim Hosp Assoc, 2015. 51(4): p. 231-8. [CrossRef]
- Evans, B.J., et al., Treatment outcomes and prognostic factors of feline splenic mast cell tumors: A multi-institutional retrospective study of 64 cases. Vet Comp Oncol, 2018. 16(1): p. 20-27. [CrossRef]
- Brodbelt, D., Perioperative mortality in small animal anaesthesia. Vet J, 2009. 182(2): p. 152-61. [CrossRef]
- Brodbelt, D.C., et al., The risk of death: the confidential enquiry into perioperative small animal fatalities. Vet Anaesth Analg, 2008. 35(5): p. 365-73. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




