Submitted:
03 May 2024
Posted:
07 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moscone, E.A.; Scaldaferro, M.A.; Grabiele, M.; Cecchini, N.M.; García, Y.S.; Jarret, R.; Davinã, J.R.; Ducasse, D.A.; Barbosa, G.E.; Ehrendorfer, F. The evolution of the chili pepper (Capsicum – Solanaceae): a cytogenetic perspective. Acta Hortic. 2007, 745, 137–169. [Google Scholar] [CrossRef]
- Pickersgill, B. Genetic resources and breeding of Capsicum spp. Euphytica 1997, 96, 129–133. [Google Scholar] [CrossRef]
- Nascimento, N.F.F.; Rêgo, E.R.; Nascimento, M.F.; Bruckner, F.L.; Finger, F.L.; Rêgo, M.M. Combining ability for yield and fruit quality in the pepper Capsicum annuum. Genet. Mol. Res. 2014, 13, 3237–3249. [Google Scholar] [CrossRef] [PubMed]
- Rêgo, E.R.; Rêgo, M.M.; Finger, F.L. Methodological basis and advances for ornamental pepper breeding program in Brazil. Acta Hortic. (ISHS) 2015, 1087, 309–314. [Google Scholar] [CrossRef]
- Rêgo, ER.; Rêgo, M.M. Genetics and Breeding of Chili Pepper Capsicum spp., in Production and Breeding of Chilli Peppers (Capsicum spp.). ed. Rêgo, E.R.; Rêgo, M.M.; Finger, F. L. Springer International Publishing, Switzerland, 2016, 1-129.
- Rêgo, E.R.; Rêgo, M.M. Ornamental Pepper, in Ornamental Crops. Ed. Van Huylenbroeck, J. Springer International Publishing, Switzerland, 2018, 529-565.
- Tafolla-Arellano, J.C.; Báez-Sañudo, R.; Tiznado-Hernández, M.E. The cuticle as a key factor in the quality of horticultural crops. Scientia Horticulturae 2018, 232, 145–152. [Google Scholar] [CrossRef]
- Wang, J.; Shan, Q.; Yi, T.; Ma, Y.; Zhou, X.; Pan, L.; Liu, F. Fine mapping and candidate gene analysis of CaFCD1 affecting cuticle biosynthesis in Capsicum annuum L. Theoretical and Applied Genetics 2023, 136, 46. [Google Scholar] [CrossRef] [PubMed]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf life potential and the fruit cuticle: the unexpected player. Frontiers in Plant Science 2019, 10, 460894. [Google Scholar] [CrossRef]
- Banaras, M.; Lownds, N.K.; Bosland, P.W. Relationship of physical properties to postharvest water loss in pepper fruits (Capsicum annuum L.). Pakistan J. Bot. 1994, 26, 321–326. [Google Scholar]
- Maalekuu, K.; Elkind, Y.; Tuvia-Alkalai, S.; Shalom, Y.; Fallik, E. Quality evaluation of three sweet pepper cultivars after prolonged storage. Advances in horticultural science 2003, 17, 1000–1005. [Google Scholar]
- Rêgo, E.R.; Rêgo, M.M.; Finger, F.L.; Cruz, C.D.; Casali, V.W.D. A diallel study of yield components and fruit quality in chilli pepper (Capsicum baccatum). Euphytica 2009, 168, 275–287. [Google Scholar] [CrossRef]
- Martínez, S.; Curros, A.; Bermúdez, J.; Carballo, J.; Franco, I. The composition of Arnoia peppers (Capsicum annuum L.) at different stages of maturity. International journal of food sciences and nutrition 2007, 58, 150–161. [Google Scholar] [PubMed]
- Ilić, Z.S.; Sunic, L.; Mirecki, N.; Fallik, E. Cultivars differences in keeping quality and bioactive constituents of bell pepper fruit during prolonged storage. Journalof Advances in Biotechnology 2014, 31, 313–318. [Google Scholar] [CrossRef]
- Ziv, C.; Lers, A.; Fallik, E.; Paran, I. Genetic and biotechnological tools to identify breeding targets for improving postharvest quality and extending shelf life of peppers. Current Opinion in Biotechnology 2022, 78, 102794. [Google Scholar] [CrossRef] [PubMed]
- Parsons, E.P.; Popopvsky, S.; Lohrey, G. T.; Alkalai-Tuvia, S.; Perzelan, Y.; Bosland, P.; Bebeli, P. J.; Paran, I.; Fallik, E.; Jenks, M. Fruit cuticle lipid composition and water loss in a diverse collection of pepper (Capsicum). Physiologia plantarum 2013, 149, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Lannes, S.D.; Finger, F.L; Schuelter, A.R.; Casali, V.W.D. Growth and quality of Brazilian accessions of Capsicum chinense fruits. Scientia Horticulturae 2007, 112, 266–270. [Google Scholar] [CrossRef]
- Bazakos, C.; Hanemian, M.; Trontin, C.; Jiménez-Gómez, J.M.; Loudet, O. New strategies and tools in quantitative genetics: how to go from the phenotype to the genotype. Annual review of plant biology 2017, 68, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Bres, C.; Mauxion, J.; Bakan, B.; Rothan, C. Breeding for cuticle-associated traits in crop species: traits, targets, and strategies. Journal of experimental botany 2017, 68, 5369–5387. [Google Scholar] [CrossRef] [PubMed]
- Popovsky-Sarid, S.; Borovsky, Y.; Faigenboim, A.; Parsons, E.P.; Lohrey, G.T.; Alkalai-Tuvia, S.; Fallik, E.; Jenks, M.A.; Paran, I. Genetic and biochemical analysis reveals linked QTLs determining natural variation for fruit postharvest water loss in pepper (Capsicum). Theor Appl Genet. 2017, 130, 445–459. [Google Scholar] [CrossRef] [PubMed]
- IPGRI. Descriptors for Capsicum. Rome, International plant genetic resources institute, 1995; 49p.
- Cruz, C. D. Genes: a software package for analysis in experimental statistics and quantitative genetics. Acta Scientiarum Agronomy 2013, 35, 271–276. [Google Scholar] [CrossRef]
- Griffing, B. Concept of general and specific combining ability in relation to diallel crossing systems. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef]
- Silva, A.R.; Rêgo, E.R.; Pessoa, A.M.S.; Rêgo, M.M. Correlation network analysis between phenotypic and genotypic traits of chili pepper. Pesquisa Agropecuária Brasileira 2016, 51, 372–377. [Google Scholar] [CrossRef]
- Rêgo, E.R.; Rêgo, M.M.; Cruz, C.D.; Finger, F.L.; Casali, V.W. Phenotypic diversity, correlation and importance of variables for fruit quality and yield traits in Brazilian peppers (Capsicum baccatum). Genet. Resour. Crop. Evol. 2011, 58, 909–918. [Google Scholar] [CrossRef]
- Legg, P.D.; Lippert, L.F. Estimates of genetic and environmental variability in a cross between two strains of pepper (Capsicum annuum L.). Proc. Am. Soc. Hort. Proc. Sci. 1966, 89, 443–448. [Google Scholar]
- Marin, O.; Lippert, L.F. Combining ability analysis of anatomical components of the dry fruit in chili pepper. Crop Sci. 1975, 15, 326–329. [Google Scholar]
- Ahmed, N.; Tanki, M.I.; Jabeen, N. Heterosis and combining ability studies in hot pepper (Capsicum annuum L.). Applied Biological Research 1999, 1, 11–14. [Google Scholar]
- Santos, R.M.C.; Rêgo, E.R.; Borém, A.; Nascimento, M.F.; Nascimento, N.F.F.; Funger, F.L.; Rêgo, M.M. Epistasis and inheritance of plant habit and fruit quality traits in ornamental pepper (Capsicum annuum L.). Genetics and Molecular Research 2014, 13, 8876–8887. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.Q.; Rodrigues, R.; Bento, C. S.; Pimenta, S. Heterosis and combining ability for ornamental chili pepper. Horticultura Brasileira 2017, 35, 349–357. [Google Scholar] [CrossRef]
- Lownds, N.K.; Banaras, M.; Bosland, P.W. Postharvest water loss and storage quality of nine pepper (Capsicum) cultivars. HortScience 1994, 29, 191–193). [Google Scholar] [CrossRef]
- Banaras, M.; Lownds, N.K.; Bosland, P.W. Relationship of physical properties to postharvest water loss in pepper fruits (Capsicum annuum L.). Pakistan Journal of Botany 1994, 26, 321–326. [Google Scholar]
- Parsons, E.P.; Popopvsky, S.; Lohrey, G.T.; Lu, S.; Alkalai-Tuvia, S.; Perzelan, Y.; Paran, I.; Fallik, E.; Jenks, M.A. Fruit cuticle lipid composition and fruit post-harvest water loss in an advanced backcross generation of pepper (Capsicum sp.). Physiologia plantarum 2012, 146, 15–25. [Google Scholar] [CrossRef]
- Silva-Neto, J.J.; Rêgo, E.R.; Nascimento, M.F.; Silva Filho, V.A.L.; Almeida Neto, J. X.; Rêgo, M.M. Variabilidade em população base de pimenteiras ornamentais (Capsicum annuum L.). Rev. Ceres 2014, 61, 84–89. [Google Scholar] [CrossRef]
- Lownds, N.K., Banaras, M.; Bosland, P.W. Relationships between postharvest water loss and physical properties of pepper fruit (Capsicum annuum L.). HortScience 1993, 28, 1182-1184.
- Kissinger, M.; Tuvia-Alkalai, S.; Shalom, Y.; Fallik, E.; Elkind, Y.; Jenks, M.A.; Goodwin, M.S. Characterization of Physiological and Biochemical Factors Associated with Postharvest Water Loss in Ripe Pepper Fruit during Storage. J. Amer. Soc. Hort. Sci. 2005, 130, 735–741. [Google Scholar] [CrossRef]
- Kissinger, M. et al. Characterization of physiological and biochemical factors associated with postharvest water loss in ripe pepper fruit during storage. Journal of the American Society for Horticultural Science. 130, 735-741 (2005).
- Arumugam, V.; Balamohan, T.N. Wax coating affects postharvest shelf-life of non-cooled sweet pepper. Journal of Spices and Aromatic Crops 2014, 23, 98–101. [Google Scholar]
- Vilarinho, L.B.O. Silva, D.J.H.; Greene, A.; Salazar, K.D.; Alves, C.; Eveleth, M.; Nichols, B.; Tehseen, S.; Khoury Jr, J. K.; Johnson, J.V.; Sargent, S.A.; Rathinasabapathi, B. Inheritance of fruit traits in Capsicum annuum: Heirloom cultivars as sources of quality parameters relating to pericarp phape, color, thickness, and total soluble solids. Journal of the American Society for Horticultural Science. 140, 597-604 (2015).
- Bosland, P.W. Capsicums: innovative use of an ancient crop. In: Janick, J. (Ed.) Progress in New Crops. ASHS Press, Arlington, 2016, 479-487.
- Rêgo, E.R.; Finger, F.L.; Rêgo, M.M. “Types, Uses and Fruit Quality of Brazilian Chili Peppers”, in Spices: Types, Uses and Health Benefits. Ed. Johnathan F. (Nova Sci. Pub.), 2012, 1-70.
| Parents | Color | Cuticle thickness | Exocarp thickness | Width | Length | Total soluble solids | Dry matter content | Wall thickness |
|---|---|---|---|---|---|---|---|---|
| µm | µm | mm | mm | % | % | mm | ||
| UFV - 04 | red | 25 | 145 | 53 | 46 | 9.6 | 14 | 3 |
| UFV - 24 | red | 22 | 110 | 32 | 142 | 10.2 | 15 | 2.3 |
| UFV - 38 | red | 22 | 65 | 19 | 69 | 8.7 | 15 | 1.7 |
| UFV - 44 | yellow | 25 | 95 | 15 | 74 | 9.4 | 17 | 2.3 |
| UFV - 46 | red | 20 | 90 | 14 | 57 | 9.6 | 23 | 1.5 |
| UFV - 50 | red | 25 | 130 | 37 | 44 | 8.8 | 18 | 2.9 |
| UFV - 56 | red | 25 | 95 | 10 | 47 | 12.9 | 28 | 0.9 |
| UFV - 58 | red | 35 | 105 | 17 | 66 | 10.7 | 16 | 1.9 |
| CV (%) | 18.1 | 23.6 | 59.8 | 46.8 | 13.5 | 24.4 | 34.3 |
| SV | DF | Water loss | Cuticle thickness | Exocarp thickness | Fruit width | Fruit length | Total soluble solids | Dry matter content | Fruit wall thickness | |
|---|---|---|---|---|---|---|---|---|---|---|
| % | µm | µm | mm | mm | % | % | mm | |||
| Treatment | 35 | 373.97** | 43.83** | 1,073.92** | 202.83** | 1,355. 95** | 3.83** | 26.86** | 1.20** | |
| GCA | 7 | 1,222. 04** | 85.89* | 1369.19ns | 769.60** | 5,890. 07** | 10.04** | 107.65** | 4.97** | |
| SCA | 28 | 161.95ns | 33.31** | 1,000.10** | 61.13** | 222.43** | 2.35** | 6.67 ns | 0.26** | |
| Error | 36 | 131.05 | 12.67 | 50.35 | 6.91 | 55.75 | 0.32 | 3.94 | 0.05 | |
| 2g | 54.55 | 10.93 | 3,035.35 | 38.13 | 291.71 | 0.48 | 5.18 | 0.24 | ||
| 2s | 15.45 | 48.55 | 35,862.19 | 27.11 | 83.33 | 1.01 | 1.34 | 0.1 | ||
| 2g/ 2s | 3.53 | 0.22 | 0.08 | 1.4 | 3.5 | 0.47 | 3.86 | 2.4 | ||
| Mean | 37.04 | 25.55 | 87.43 | 21.57 | 72.59 | 8.88 | 17.63 | 2.14 | ||
| h2b (%) | 94.01 | 71.08 | 95.31 | 93.2 | 95.88 | 91.73 | 85.32 | 95.19 | ||
| h2n (%) | 63.26 | 21.8 | 21 | 74.6 | 81.7 | 48 | 71.8 | 80.27 |
| Parents/Hybrids | Water Loss | Cuticle thickness | Exocarp thickness | Width | Length | Total soluble solids | Dry matter content | Wall thickness |
|---|---|---|---|---|---|---|---|---|
| % | µm | µm | mm | mm | % | % | mm | |
| 4 | 31.47 b† | 25.00 b | 145.00 a | 62.15 a | 51.10 f | 7.50 c | 13.70 c | 2.80 a |
| 04 x 24 | 18.64 b | 27.50 a | 45.00 f | 25.80 c | 95.25 c | 8.65 d | 14.45 c | 3.15 a |
| 04 x 38 | 33.31 b | 32.50 a | 85.00 d | 31.70 b | 60.75 f | 8.50d | 14.50 c | 2.75 a |
| 04 x 44 | 17.66 b | 35.00 a | 105.00 c | 19.85 d | 61.10 f | 9.50 c | 15.05 c | 2.35 b |
| 04 x 46 | 45.44 a | 25.00 b | 100.00 c | 20.25 d | 55.10 f | 7.60 d | 17.35 c | 2.80 a |
| 04 x 50 | 36.99 b | 30.00 a | 115.00 b | 33.20 b | 52.40 f | 7.90 d | 16.20 c | 3.25 a |
| 04 x 56 | 31.27 b | 30.00 a | 60.00 e | 13.50 e | 59.00 f | 10.20 c | 20.15 b | 1.10 d |
| 04 x 58 | 14.47 b | 30.00 a | 65.00 e | 25.65 c | 84.50 d | 8.30 d | 14.45 c | 2.60 b |
| 24 | 49.25 a | 22.50 b | 110.00 c | 23.75 d | 173.50 a | 11.90 b | 13.70 c | 2.85 a |
| 24 x 38 | 22.60 b | 30.00 a | 90.00 d | 19.70 d | 128.30 b | 7.80 d | 12.60 c | 1.86 c |
| 24 x 44 | 32.74 b | 27.50 a | 70.00 e | 20.55 d | 107.00 c | 10.55 c | 15.80 c | 2.45 b |
| 24 x 46 | 58.28 a | 30.00 a | 70.00 e | 21.35 d | 88.95 d | 8.85 d | 16.80 c | 2.40 b |
| 24 x 50 | 25.17 b | 23.75 b | 120.00 b | 32.50 b | 105.65 c | 9.80 c | 14.45 c | 3.25 a |
| 24 x 56 | 57.77 a | 30.00 a | 95.00 d | 12.20 e | 70.75 e | 11.40 b | 23.25 b | 1.55 c |
| 24 x 58 | 25.67 b | 20.00 b | 75.00 e | 21.30 d | 99.30 c | 7.70 d | 16.00 c | 2.35 b |
| 38 | 31.98 b | 22.50 b | 65.00 e | 19.20 d | 85.40 d | 9.05 c | 16.00 c | 1.70 c |
| 38 x 44 | 46.10 a | 17.50 b | 65.00 e | 16.25 e | 73.05 e | 7.35 d | 18.70 c | 1.65 c |
| 38 x 46 | 43.01 a | 21.25 b | 92.50 d | 17.15 d | 70.60 e | 9.35 c | 19.75 b | 2.30 b |
| 38 x 50 | 25.63 b | 20.00 b | 65.00 e | 26.7 c | 78.40 e | 7.65 d | 14.70 c | 2.25 b |
| 38 x 56 | 36.75 b | 20.00 b | 65.00 e | 14.4 e | 61.75f | 8.70 d | 21.00 b | 1.00 d |
| 38 x 58 | 30.23 b | 22.50 b | 40.00 f | 18.35 d | 68.85 e | 7.50 d | 16.0 c | 1.65 c |
| 44 | 45.58 a | 25.00 b | 95.00 d | 14.00 e | 56.70 f | 7.00 d | 18.60 c | 1.80 c |
| 44 x 46 | 41.63 a | 20.00 b | 82.50 e | 13.80 e | 60.05 f | 9.60 c | 21.35 b | 1.65 c |
| 44 x 50 | 35.11 b | 22.50 b | 102.50 c | 30.75 b | 56.90 f | 7.65 d | 15.35 c | 3.05 a |
| 44 x 56 | 44.48 a | 22.50 b | 85.00 d | 13.30 e | 64.05 f | 9.35 c | 22.75 b | 1.15 d |
| 44 x 58 | 26.46 b | 32.50 a | 90.00 d | 15.50 e | 84.60 d | 8.10 d | 17.10 c | 1.85 c |
| 46 | 62.85 a | 20.00 b | 90.00 d | 12.40 e | 48.15 f | 10.35 c | 22.30 b | 1.70 c |
| 46 x 50 | 40.33 a | 22.50 b | 110.00 c | 25.75 c | 56.65 f | 9.40 c | 18.10 c | 2.90 b |
| 46 x 56 | 62.41 a | 22.5 b | 92.50 d | 10.50 e | 45.70 f | 13.30 a | 27.55 a | 0.85 d |
| 46 x 58 | 27.22 b | 27.50 a | 97.50 d | 13.30 e | 63.45 f | 8.15 d | 22.80 b | 1.40 c |
| 50 | 32.35 b | 25.00 b | 130.00 b | 36.05 b | 66.65 f | 8.20 d | 13.65 c | 3.10 a |
| 50 x 56 | 48.52 a | 20.00 b | 55.00 f | 26.10 c | 47.05 f | 8.35 d | 16.00 c | 3.20 a |
| 50 x 58 | 13.75 b | 27.50a | 75.00 e | 31.40 b | 54.90 f | 8.40 d | 15.85 c | 3.05 a |
| 56 | 67.67 a | 25.00 b | 95.00 d | 7.85 e | 42.40 f | 10.25 c | 26.05 a | 0.50 d |
| 56 x 58 | 36.21 b | 30.00 a | 100.00 c | 13.40 e | 61.80 f | 7.85 d | 16.05 c | 1.10 d |
| 58 | 34.71 b | 35.00 a | 105.00 c | 17.00 d | 73.5 e | 8.20 d | 16.70 c | 2.00 c |
| Water loss | Cuticle thickness | Exocarp thickness | Width | Length | Total soluble solids | Dry matter content | Wall thickness | |
|---|---|---|---|---|---|---|---|---|
| % | µm | µm | mm | mm | % | % | mm | |
| Parents | ||||||||
| 4 | -7.26** | 3.00** | 7.81** | 10.01** | -8.30** | -0.43** | -1. 92** | 0.43ns |
| 24 | 0.59ns | 0.37ns | -0.18ns | 0.67ns | 38.88** | 0.85** | -1.80** | 0.33ns |
| 38 | -3.18ns | -2.12* | -15.43** | -1.15* | 5.92** | -0.50** | -0.95* | -0.25ns |
| 44 | 0.19ns | 0.00ns | 0.31ns | -3.61** | -3.31* | -0.38** | 0.46ns | -0.15ns |
| 46 | 11.05** | -2.12* | 3.81** | -4.72** | -11.65** | 0.70** | 2.96** | -0.16ns |
| 50 | -4.32ns | -1.62ns | 11.56** | 8.43** | -6.81** | -0.44** | -2.10** | 0.78ns |
| 56 | 11.93** | -0.50ns | -4.43** | -7.51** | -15.84** | 0.97** | 4.01** | -0.84* |
| 58 | -9.00** | 3.00** | -3.43* | -2.12** | 1.11ns | -0.76** | -0.71ns | -0.13ns |
| Hybrids | ||||||||
| 4 x 24 | -11.73ns | -1.43ns | -50.05** | -6.45** | -7.92ns | -0.66** | 0.53ns | 0.23ns |
| 4 x 38 | 6.71ns | 6.06** | 5.19ns | 1.26ns | -9.45** | 0.54ns | -0.27ns | 0.42** |
| 4 x 44 | -12.30* | 6.44** | 9.44* | -8.11** | 0.13ns | 1.43** | -1.13ns | -0.06ns |
| 4 x 46 | 4.61ns | -1.43ns | 0.94ns | -6.61** | 2.46ns | -1.55** | -1.33ns | 0.38** |
| 4 x 50 | 11.53ns | 3.06ns | 8.19* | -6.82** | -5.08ns | -0.11ns | 2.56* | -0.11ns |
| 4 x 56 | -10.43ns | 1.94ns | -30.80** | -10.57** | 10.55* | 0.78* | 0.42ns | -0.63** |
| 4 x 58 | -6.30ns | -1.55ns | -26.80** | -3.81* | 19.10** | 0.60ns | -0.56ns | 0.15ns |
| 24 x 38 | -1.86ns | 6.19** | 18.19** | -1.39ns | 10.90* | -1.44ns | -2.29* | -0.38 |
| 24 x 44 | -5.09ns | 1.56 | -17.55** | 1.91ns | -1.16ns | 1.19** | -0.50ns | 0.12ns |
| 24 x 46 | 9.59ns | 6.19** | -21.05** | 3.82* | -10.87* | -1.59ns | -1.99ns | 0.07ns |
| 24 x 50 | -8.15ns | -0.55ns | 21.19** | 1.81ns | 0.97ns | 0.50ns | 0.69ns | -0.01ns |
| 24 x 56 | 8.19ns | 4.56* | 12.19** | -2.54ns | -24.89** | 0.69* | 3.40** | -1.10ns |
| 24 x 58 | -2.95ns | -8.93** | -8.80* | 1.17ns | -13.29** | -1.29** | 0.87ns | -0.002ns |
| 38 x 44 | 12.03ns | -3.43ns | -7.30ns | -0.56ns | -2.14ns | -0.65* | 1.55ns | -0.10ns |
| 38 x 46 | -1.90ns | -0.05ns | 16.69** | 1.45ns | 3.74ns | 0.27ns | 0.10ns | 0.56** |
| 38 x 50 | -3.91ns | -4.30* | -18.55** | -2.13ns | 6.70ns | -0.29ns | 0.08ns | -0.43** |
| 38 x 56 | -9.05ns | -2.93ns | -2.55ns | 1.48ns | -0.92ns | -0.65* | 0.30ns | -0.06ns |
| 38 x 58 | 5.37ns | -3.93* | -28.55* | 0.05ns | -10.77* | -0.13ns | 0.02ns | -0.12ns |
| 44 x 46 | -6.65ns | -3.43ns | -9.05* | 0.56ns | 2.43ns | 0.40ns | 0.30ns | -0.18ns |
| 44 x 50 | 2.18ns | -1.43ns | 3.19ns | 4.35** | -5.57ns | -0.41ns | -0.67ns | 0.27* |
| 44 x 56 | -4.69ns | -2.55ns | 1.69ns | 2.84ns | 10.61ns | -0.11ns | 0.64ns | -0.001ns |
| 44 x 58 | -1.76ns | 3.94* | 5.69ns | -0.33ns | 14.21** | 0.36ns | -0.29ns | -0.006ns |
| 46 x 50 | -3.44ns | 0.69ns | 7.19ns | 0.46ns | 2.52ns | 0.26ns | -0.42ns | 0.13ns |
| 46 x 56 | 2.37ns | -0.43ns | 5.69ns | 1.15ns | 0.60ns | 2.75** | 2.94* | -0.29* |
| 46 x 58 | -11.98ns | 1.06ns | 9.69* | -1.42ns | 1.40ns | -0.68* | 2.91* | -0.45** |
| 50 x 56 | 3.85ns | -3.43ns | -39.55** | 3.59* | -2.89ns | 1.06** | -3.57** | 1.11** |
| 50 x 58 | -9.95ns | 0.56ns | -20.55** | 3.51* | -11.99** | 0.71* | 0.99ns | 0.25** |
| 56 x 58 | -3.76ns | 1.94ns | 20.44** | 1.45ns | 3.94ns | -1.24** | -4.89** | -0.07ns |
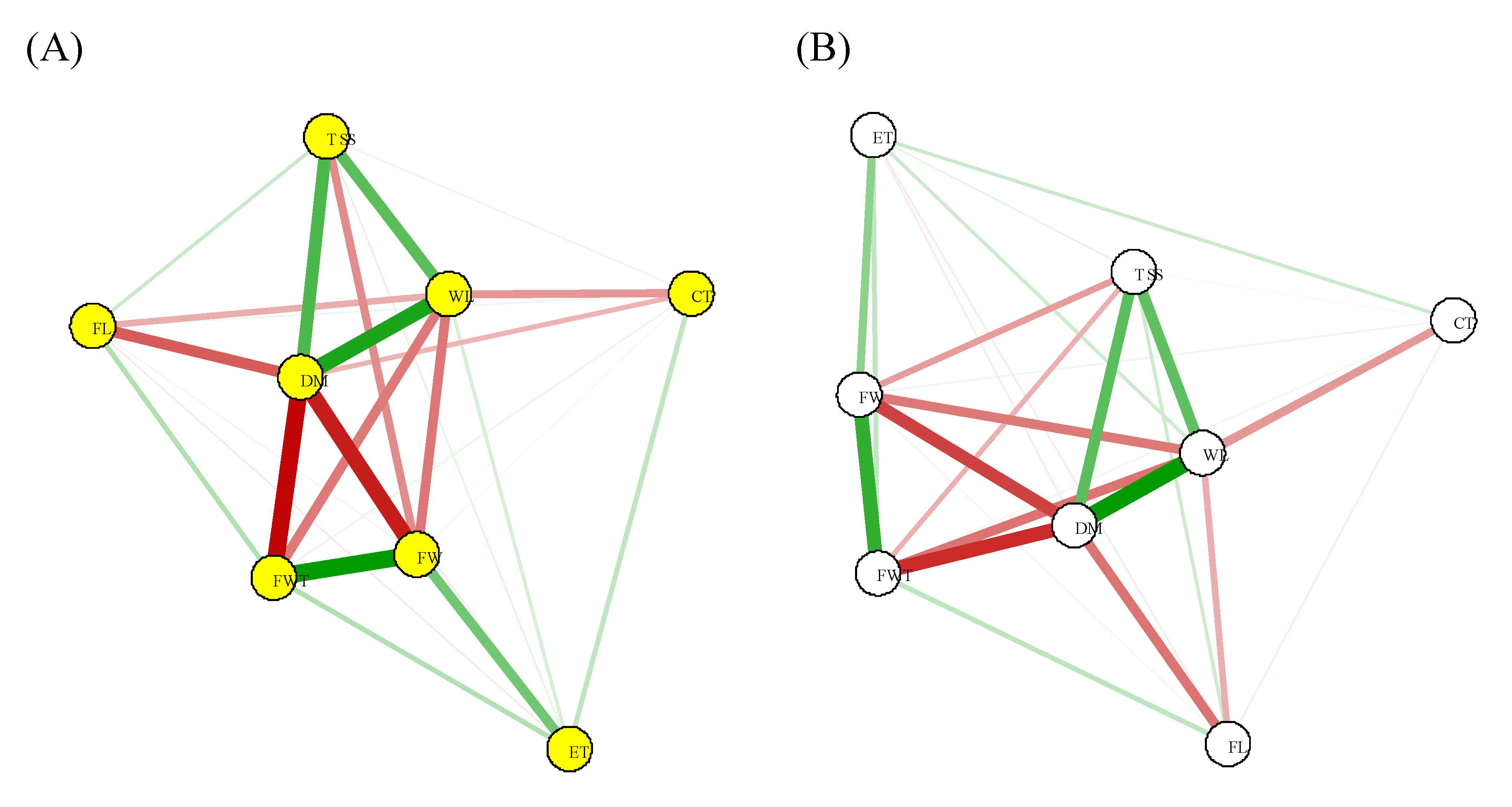
| CT | ET | FW | FL | TSS | DM | FWT | Genetic Correlation Coefficient | |
| CT | -0.56 | 0.09 | -0.03 | -0.04 | -0.01 | 0.18 | -0.01 | -0.38 |
| ET | -0.11 | 0.47 | -0.25 | 0.04 | 0.06 | 0.03 | -0.05 | 0.19 |
| FW | -0.03 | 0.2 | -0.57 | -0.02 | -0.24 | 0.34 | -0.18 | -0.50 |
| FL | -0.04 | -0.03 | -0.02 | -0.51 | 0.12 | 0.25 | -0.06 | -0.29 |
| TSS | 0.01 | 0.04 | 0.21 | -0.09 | 0.65 | -0.29 | 0.07 | 0.60 |
| DM | 0.21 | -0.03 | 0.4 | 0.27 | 0.39 | -0.48 | 0.18 | 0.94 |
| FWT | -0.03 | 0.11 | -0.44 | -0.12 | -0.19 | 0.38 | -0.23 | -0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





