Submitted:
03 May 2024
Posted:
07 May 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Sample Collection
Demographic Data Recruitment
Characteristics of Isolates
Bacterial Reactivation
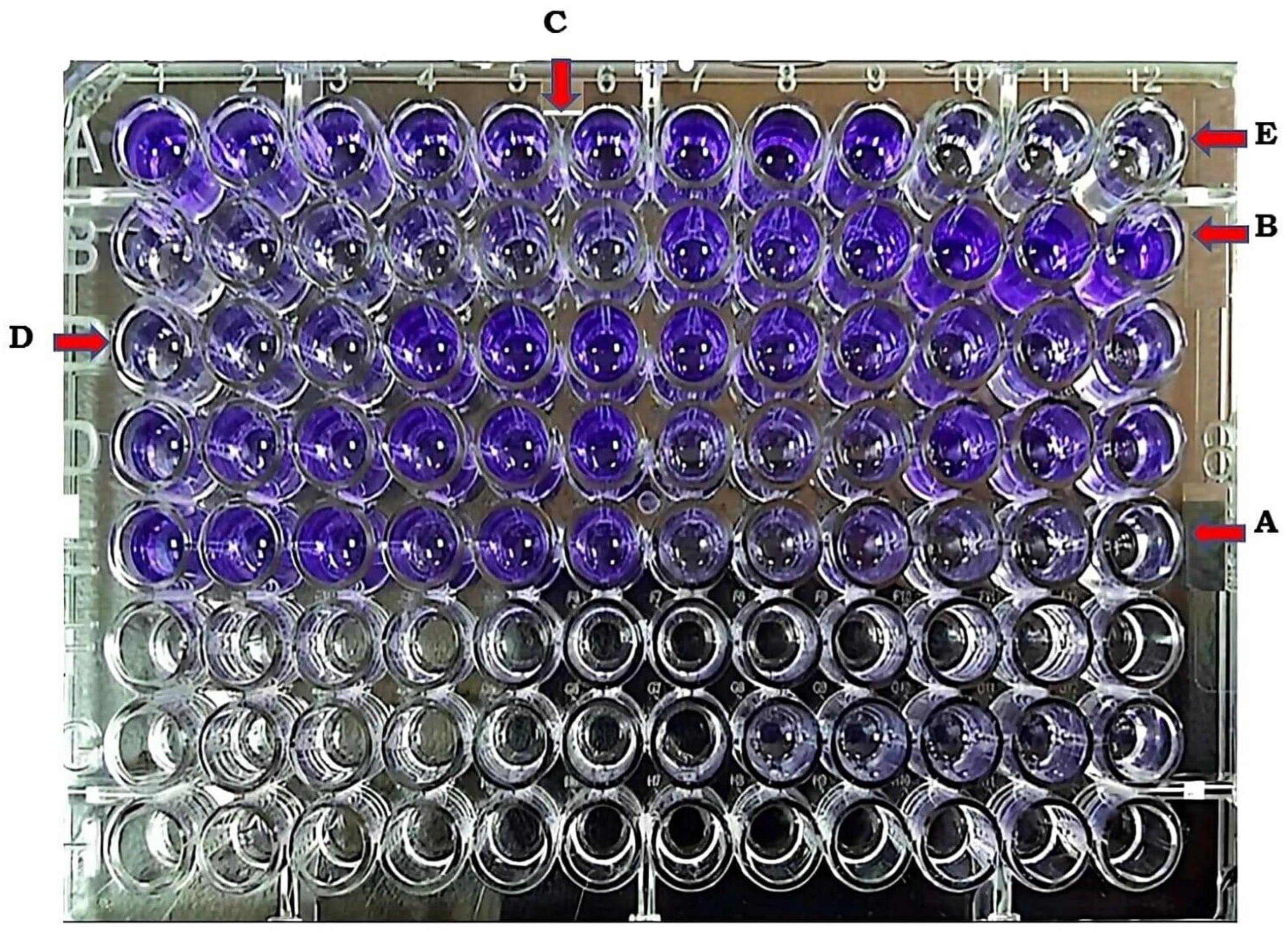
Detection of Biofilm Production
Polymerase Chain Reaction for Seven Virulence Genes
DNA Extraction
Primers
PCR Assay
Agarose gel Electrophoresis
Statistical Analysis
Results
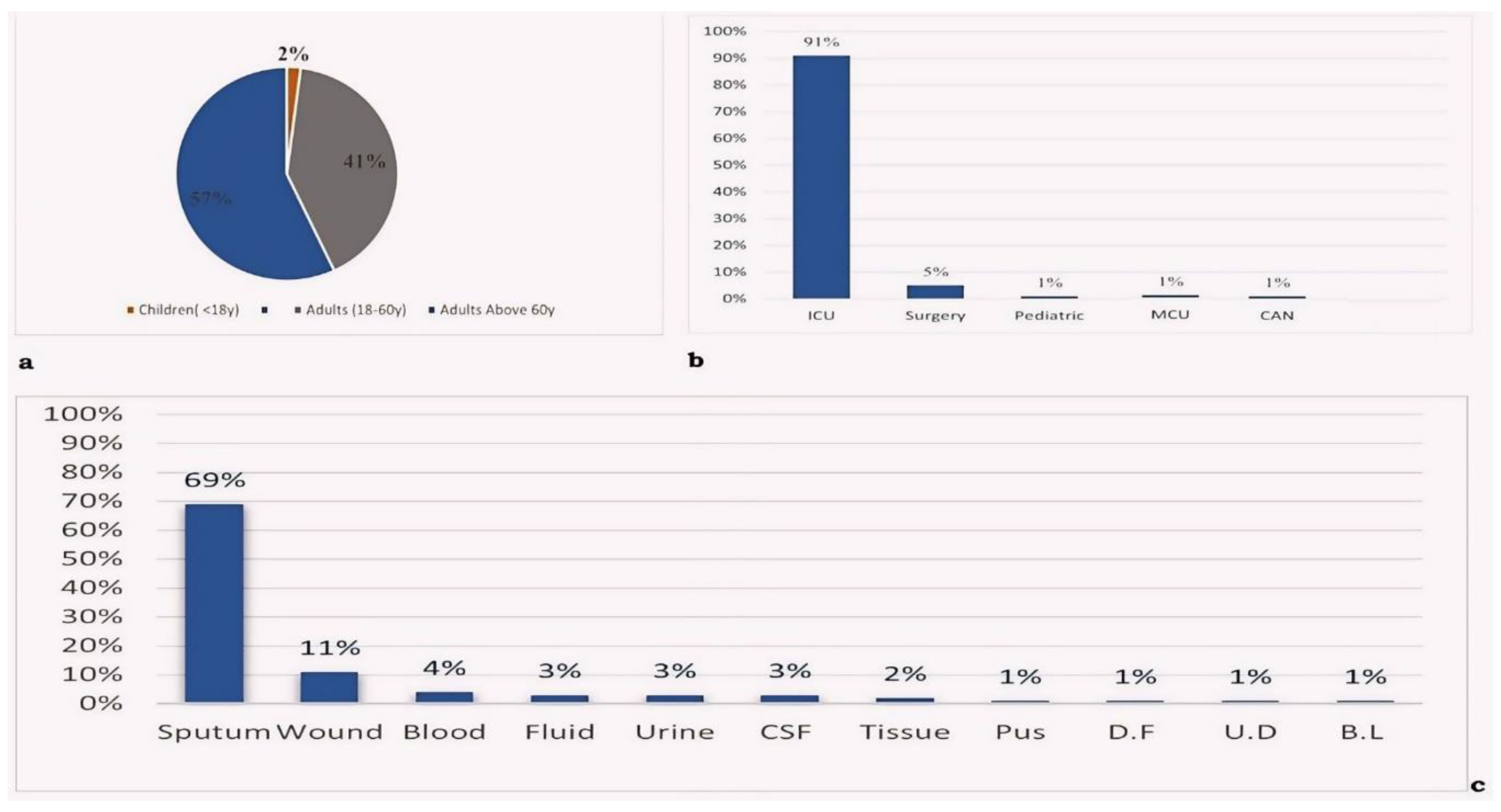
Demographic Data
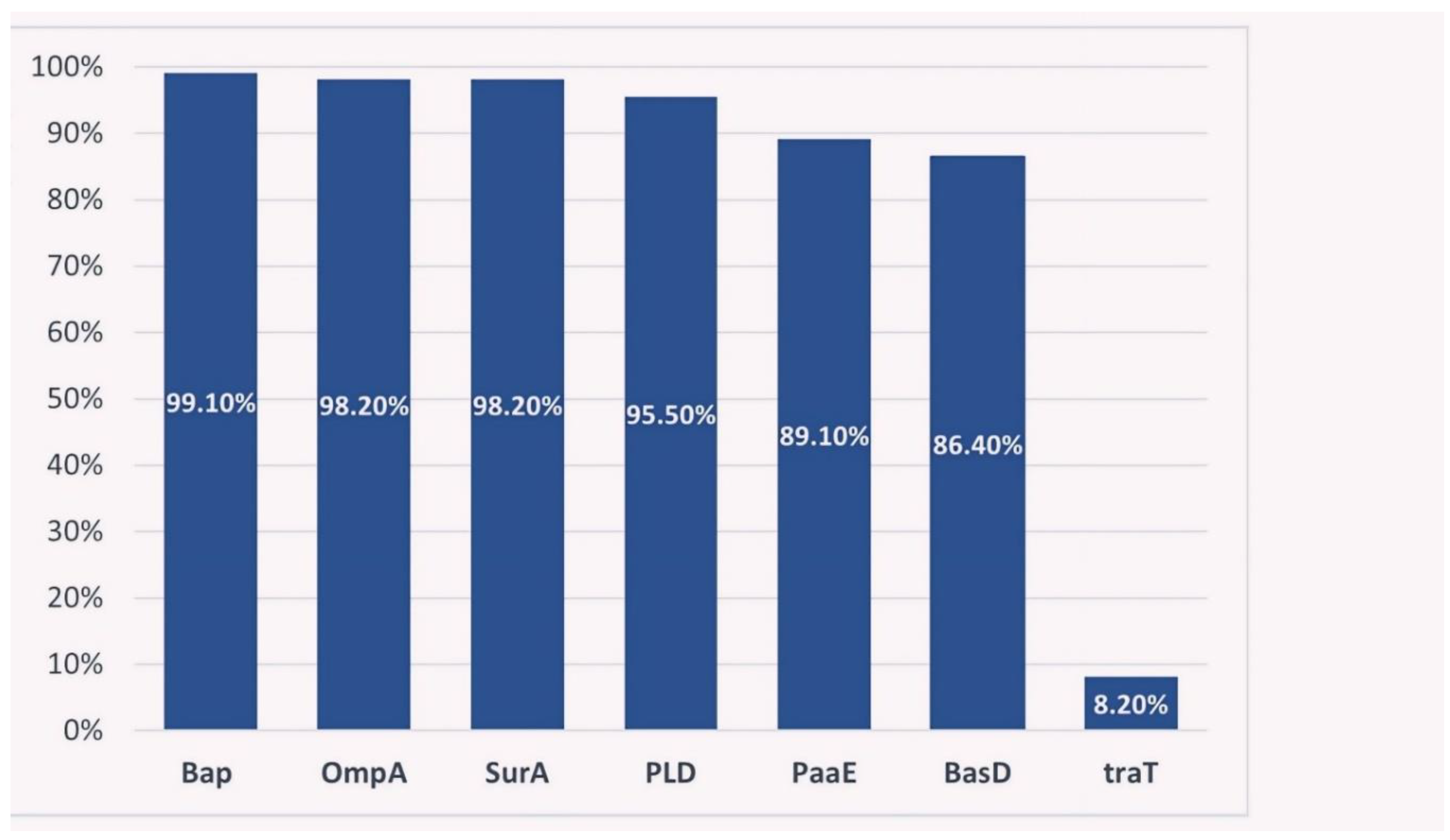
Distribution of Virulence Genes among CRAB Isolates
Biofilm Detection by Microtiter Plate Method (MTP) in CRAB
Statistical Associations
Discussion
Conclusion
Author’s Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumann, P. Isolation of Acinetobacter from Soil and Water. J. Bacteriol. 1968, 96, 39–42. [CrossRef]
- Towner A, Dworkin M, Falkow S, Rosenberg E, Schleifer K, Stackebrandt E. The Prokaryotes. Springer; 2006.
- Munoz-Price LS, Weinstein RA. Acinetobacter infection. New England Journal of Medicine. 2008;358(12):1271-81.
- Seifert, H.; Dijkshoorn, L.; Gerner-Smidt, P.; Pelzer, N.; Tjernberg, I.; Vaneechoutte, M. Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic identification methods. J. Clin. Microbiol. 1997, 35, 2819–2825. [CrossRef]
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [CrossRef]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [CrossRef]
- Jung, J.; Park, W. Acinetobacter species as model microorganisms in environmental microbiology: current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 2533–2548. [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [CrossRef]
- Thummeepak R, Kongthai P, Leungtongkam U, Sitthisak SJIM. Distribution of virulence genes involved in biofilm formation in multi-drug resistant Acinetobacter baumannii clinical isolates. International microbiology. 2016;19(2):121-9.
- Dehbanipour, R.; Ghalavand, Z. Acinetobacter baumannii : Pathogenesis, virulence factors, novel therapeutic options and mechanisms of resistance to antimicrobial agents with emphasis on tigecycline. J. Clin. Pharm. Ther. 2022, 47, 1875–1884. [CrossRef]
- Brossard, K.A.; Campagnari, A.A. The Acinetobacter baumannii Biofilm-Associated Protein Plays a Role in Adherence to Human Epithelial Cells. Infect. Immun. 2012, 80, 228–233. [CrossRef]
- Loehfelm, T.W.; Luke, N.R.; Campagnari, A.A. Identification and Characterization of an Acinetobacter baumannii Biofilm-Associated Protein. J. Bacteriol. 2008, 190, 1036–1044. [CrossRef]
- Conde-Pérez, K.; Vázquez-Ucha, J.C.; Álvarez-Fraga, L.; Ageitos, L.; Rumbo-Feal, S.; Martínez-Guitián, M.; Trigo-Tasende, N.; Rodríguez, J.; Bou, G.; Jiménez, C.; et al. In-Depth Analysis of the Role of the Acinetobactin Cluster in the Virulence of Acinetobacter baumannii. Front. Microbiol. 2021, 12. [CrossRef]
- Depka, D.; Bogiel, T.; Rzepka, M.; Gospodarek-Komkowska, E. The Prevalence of Virulence Factor Genes among Carbapenem-Non-Susceptible Acinetobacter baumannii Clinical Strains and Their Usefulness as Potential Molecular Biomarkers of Infection. Diagnostics 2023, 13, 1036. [CrossRef]
- Mozafari, H.; Mirkalantari, S.; Kalani, B.S.; Amirmozafari, N. Prevalence Determination of Virulence Related and Biofilm Formation Genes in Acinetobacter baumannii Isolates from Clinical Respiratory Samples in Imam Khomeini Hospital, Tehran, Iran in 2018. Iran. J. Med Microbiol. 2021, 15, 266–280. [CrossRef]
- Liu, C.; Chang, Y.; Xu, Y.; Luo, Y.; Wu, L.; Mei, Z.; Li, S.; Wang, R.; Jia, X. Distribution of virulence-associated genes and antimicrobial susceptibility in clinical Acinetobacter baumannii isolates. Oncotarget 2018, 9, 21663–21673. [CrossRef]
- Howard A, O’Donoghue M, Feeney A, Sleator RD. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3(3):243-50.
- Doi, Y.; Murray, G.L.; Peleg, A.Y. Acinetobacter baumannii: Evolution of Antimicrobial Resistance-treatment Options. Semin. Respir. Crit. Care Med. 2015, 36, 85–98. [CrossRef]
- Joly-Guillou, M.-L. Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 2005, 11, 868–873. [CrossRef]
- Poirel L, Naas T, Nordmann P. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrobial agents and chemotherapy. 2010;54(1):24-38.
- Morris, S.; Cerceo, E. Trends, Epidemiology, and Management of Multi-Drug Resistant Gram-Negative Bacterial Infections in the Hospitalized Setting. Antibiotics 2020, 9, 196. [CrossRef]
- Abadi, A.T.B.; Rizvanov, A.A.; Haertle, T.; Blatt, N.L. World Health Organization Report: Current Crisis of Antibiotic Resistance. BioNanoScience 2019, 9, 778–788. [CrossRef]
- Medioli F, Bacca E, Faltoni M, Burastero GJ, Volpi S, Menozzi M, et al. Is It Possible to Eradicate Carbapenem-Resistant Acinetobacter baumannii (CRAB) from Endemic Hospitals? Antibiotics. 2022;11(8):1015.
- Al-Tamimi, M.; Albalawi, H.; Alkhawaldeh, M.; Alazzam, A.; Ramadan, H.; Talalwih, M.; Alma’aitah, A.; Al Balawi, D.; Shalabi, S.; Abu-Raideh, J.; et al. Multidrug-Resistant Acinetobacter baumannii in Jordan. Microorganisms 2022, 10, 849. [CrossRef]
- Batarseh, A.; Al-Sarhan, A.; Maayteh, M.; Al-Khatirei, S.; Alarmouti, M. Antibiogram of multidrug resistant Acinetobacter baumannii isolated from clinical specimens at King Hussein Medical Centre, Jordan: a retrospective analysis.. East. Mediterr. Heal. J. 2015, 21, 828–834. [CrossRef]
- Smitran A, Lukovic B, Bozic L, Jelic D, Jovicevic M, Kabic J, et al. Carbapenem-Resistant Acinetobacter baumannii: Biofilm-Associated Genes, Biofilm-Eradication Potential of Disinfectants, and Biofilm-Inhibitory Effects of Selenium Nanoparticles. Microorganisms. 2023;11(1).
- Nguyen M, Joshi SG. Carbapenem resistance in Acinetobacter baumannii, and their importance in hospital-acquired infections: a scientific review. Journal of applied microbiology. 2021 Dec 1;131(6):2715-38.
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [CrossRef]
- Obenhuber, T.; Scheier, T.; Stutz, T.; Hug, M.; Fontein, D.; Kaiser, A.; Schoene, S.; Steiger, P.; Brugger, S.; Zingg, W.; et al. An outbreak of multi-drug-resistant Acinetobacter baumannii on a burns ICU and its control with multi-faceted containment measures. J. Hosp. Infect. 2024, 146, 102–108. [CrossRef]
- Raddaoui A, Mabrouk A, Chebbi Y, Frigui S, Al-Gallas N, Abbassi MS, et al. Outbreak caused by pandrug-resistant blaOXA-69/blaOXA-23/blaGES harboring Acinetobacter baumannii ST2 in an intensive care unit. Acta Microbiologica et Immunologica Hungarica. 2024.
- Al-Sheboul, S.A.; Al-Moghrabi, S.Z.; Shboul, Y.; Atawneh, F.; Sharie, A.H.; Nimri, L.F. Molecular Characterization of Carbapenem-Resistant Acinetobacter baumannii Isolated from Intensive Care Unit Patients in Jordanian Hospitals. Antibiotics 2022, 11, 835. [CrossRef]
- Gharaibeh, M.H.; Abandeh, Y.M.; Elnasser, Z.A.; Lafi, S.Q.; Obeidat, H.M.; Khanfar, M.A. Multi-drug Resistant Acinetobacter baumannii: Phenotypic and Genotypic Resistance Profiles and the Associated Risk Factors in Teaching Hospital in Jordan. J. Infect. Public Heal. 2024, 17, 543–550. [CrossRef]
- Ibrahim, S.; Al-Saryi, N.; Al-Kadmy, I.M.S.; Aziz, S.N. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol. Biol. Rep. 2021, 48, 6987–6998. [CrossRef]
- Zhang, X.; Li, F.; Awan, F.; Jiang, H.; Zeng, Z.; Lv, W. Molecular Epidemiology and Clone Transmission of Carbapenem-Resistant Acinetobacter baumannii in ICU Rooms. Front. Cell. Infect. Microbiol. 2021, 11. [CrossRef]
- Bostanghadiri, N.; Narimisa, N.; Mirshekar, M.; Dadgar-Zankbar, L.; Taki, E.; Navidifar, T.; Darban-Sarokhalil, D. Prevalence of colistin resistance in clinical isolates of Acinetobacter baumannii: a systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2024, 13, 1–17. [CrossRef]
- Karakonstantis, S.; Rousaki, M.; Vassilopoulou, L.; Kritsotakis, E.I. Global prevalence of cefiderocol non-susceptibility in Enterobacterales, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia: a systematic review and meta-analysis. Clin. Microbiol. Infect. 2024, 30, 178–188. [CrossRef]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [CrossRef]
- Wang, M.; Ge, L.; Chen, L.; Komarow, L.; Hanson, B.; Reyes, J.; Cober, E.; Alenazi, T.; Zong, Z.; Xie, Q.; et al. Clinical Outcomes and Bacterial Characteristics of Carbapenem-resistant Acinetobacter baumannii Among Patients From Different Global Regions. Clin. Infect. Dis. 2023, 78, 248–258. [CrossRef]
- Abarca-Coloma, L.; Puga-Tejada, M.; Nuñez-Quezada, T.; Gómez-Cruz, O.; Mawyin-Muñoz, C.; Barungi, S.; Perán, M. Risk Factors Associated with Mortality in Acinetobacter baumannii Infections: Results of a Prospective Cohort Study in a Tertiary Public Hospital in Guayaquil, Ecuador. Antibiotics 2024, 13, 213. [CrossRef]
- Park SM, Suh JW, Ju YK, Kim JY, Kim SB, Sohn JW, et al. Molecular and virulence characteristics of carbapenem-resistant Acinetobacter baumannii isolates: a prospective cohort study. Scientific Reports. 2023;13(1):19536.
- Sung, J.Y.; Koo, S.H.; Kim, S.; Kwon, G.C. Persistence of Multidrug-Resistant Acinetobacter baumannii Isolates Harboring blaOXA-23 and bap for 5 Years. J. Microbiol. Biotechnol. 2016, 26, 1481–1489. [CrossRef]
- Fallah, A.; Rezaee, M.A.; Hasani, A.; Barhaghi, M.H.S.; Kafil, H.S. Frequency of bap and cpaA virulence genes in drug resistant clinical isolates of Acinetobacter baumannii and their role in biofilm formation. Iranian journal of basic medical sciences 2017, 20, 849–855. [CrossRef]
- Altınok Ö, Boral B, Ergin MU, Eser ÖZ. Existence of biofilm and biofilm-associated virulence genes in multi-drug resistant invasive acinetobacter baumannii isolates Çok İlaca dirençli İnvaziv acinetobacter baumannii İzolatlarında biyofilm ve biyofilm İlişkili virülans genlerinin varlığı. Mikrobiyoloji Bulteni. 2020;54(1).
- Khalil, M.A.F.; Ahmed, F.A.; Elkhateeb, A.F.; Mahmoud, E.E.; Ahmed, M.I.; Ahmed, R.I.; Hosni, A.; Alghamdi, S.; Kabrah, A.; Dablool, A.S.; et al. Virulence Characteristics of Biofilm-Forming Acinetobacter baumannii in Clinical Isolates Using a Galleria mellonella Model. Microorganisms 2021, 9, 2365. [CrossRef]
- Santajit, S.; Bhoopong, P.; Kong-Ngoen, T.; Tunyong, W.; Horpet, D.; Paehoh-Ele, W.; Zahedeng, T.; Pumirat, P.; Sookrung, N.; Hinthong, W.; et al. Phenotypic and Genotypic Investigation of Carbapenem-Resistant Acinetobacter baumannii in Maharaj Nakhon Si Thammarat Hospital, Thailand. Antibiotics 2023, 12, 580. [CrossRef]
- Hazhirkamal, M.; Zarei, O.; Movahedi, M.; Karami, P.; Shokoohizadeh, L.; Taheri, M. Molecular typing, biofilm production, and detection of carbapenemase genes in multidrug-resistant Acinetobacter baumannii isolated from different infection sites using ERIC-PCR in Hamadan, west of Iran. BMC Pharmacol. Toxicol. 2021, 22, 1–7. [CrossRef]
- Khoshnood S, Savari M, Abbasi Montazeri E, Farajzadeh Sheikh A. Survey on Genetic Diversity, Biofilm Formation, and Detection of Colistin Resistance Genes in Clinical Isolates of Acinetobacter baumannii. Infection and Drug Resistance. 2020;13:1547-58.
- Longo, F.; Vuotto, C.; Donelli, G. Biofilm formation in Acinetobacter baumannii. New Microbiology 2014, 37, 119–27.
- Abdi-Ali, A.; Hendiani, S.; Mohammadi, P.; Gharavi, S. Assessment of Biofilm Formation and Resistance to Imipenem and Ciprofloxacin among Clinical isolates of Acinetobacter baumannii in Tehran. Jundishapur J. Microbiol. 2014, 7, e8606. [CrossRef]
- Bardbari, A.M.; Arabestani, M.R.; Karami, M.; Keramat, F.; Alikhani, M.Y.; Bagheri, K.P. Correlation between ability of biofilm formation with their responsible genes and MDR patterns in clinical and environmental Acinetobacter baumannii isolates. Microb. Pathog. 2017, 108, 122–128. [CrossRef]
- zkul C, Hazırolan G. Oxacillinase gene distribution, antibiotic resistance, and their correlation with biofilm formation in Acinetobacter baumannii bloodstream isolates. Microbial Drug Resistance. 2021;27(5):637-46.
- Eze, E.C.; Chenia, H.Y.; El Zowalaty, M. Acinetobacter baumannii biofilms: effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect. Drug Resist. 2018, ume 11, 2277–2299. [CrossRef]
| Virulence factor | Gene | Role during pathogenesis | Ref. |
|---|---|---|---|
| biofilm-associated protein | Bap | Have a key role in the development, thickening of mature biofilm structure, and its intercellular adhesion. It enhances the persistence of A. baumannii in the hospital environment, and promotes antibiotic resistance and tolerance to other inhibitors | [12,13] |
| Surface antigen protein | surA1 | Surface antigen protein is a periplasmic chaperone protein necessary for cellular invasion. | |
| Iron acquisition system | BasD | Iron acquisition system is essential for bacterial survival and growth in a host under iron-limited conditions | [14] |
| Phospholipase D | Pld | Phospholipase D enhances A. baumannii ability to thrive in serum and invade epithelial cells | [15] |
| Outer membrane protein A | OmpA | Outer membrane protein A (OmpA) is an adhesion protein enhances bacterial attachment to eukaryotic epithelial cells and formation of biofilms | [16] |
| Phenylalanine catabolic pathway | PaaE | A. baumannii exhibits a unique enzymatic pathway encoded by PaaE gene for degrading phenylacetic acid and a carbon source to enhance its adaptability and survival in various environments | [17] |
| Surface-exposed lipoprotein | traT | A surface-exposed lipoprotein linked with serum resistance and evasion of the host immune response and may contribute to the ability to survive and infect the host | [17] |
| Gene | Sequence | Annealing temp (ºC) | Ref. |
|---|---|---|---|
| Bap | F:AGTTAAAGAAGGGCAAGAAG | 50 | [17] |
| R:GGAGCACCACCTAACTGA | |||
| surA1 | F:CAATTGGTAGCTGGCGATCA | 55 | [17] |
| R:TTAGGCGGGACTCAGCTTTT | |||
| BasD | F: CTCTTGCATGGCAACACCAC | 65 | [17] |
| R:CCAACGAGACCGCTTATGGT | |||
| Pld | F:CGTCAATTACGCCAAGCTG | 64.7 | [17] |
| R:CTGACGCTACCTGACGGTTT | |||
| OmpA | F:CGCTTCTGCTGGTGCTGAAT | 50 | [10] |
| R:CGTGCAGTAGCGTTAGGGTA | |||
| PaaE | F:CTATTTAGGCGTTGCTGCGG | 64.5 | [17] |
| R:CCTTACAACGACAGGTCGCA | |||
| traT | F:GGTGTGGTGCGATGAGCACAG | 67.9 | [17] |
| R:CACGGTTCAGCCATCCCTGAG |
| Biofilm production | |||||
|---|---|---|---|---|---|
| Strong | Moderate | Weak | Non | Total | |
| Frequency | 24 | 40 | 31 | 15 | 110 |
| Percent | 21.8% | 36.4% | 28.2% | 13.6% | 100% |
| Carbapenem resistance gene | Biofilm production | p-value | ||
|---|---|---|---|---|
| Negative | Positive | |||
| VIM | Negative | 13 | 68 | 0.218 |
| Positive | 2 | 27 | ||
| OXA-23 | Negative | 1 | 0 | 0.011 |
| Positive | 14 | 95 | ||
| Virulence gene | Frequency in Jordan (this study) | Study country | Frequency range | Ref |
|---|---|---|---|---|
| Bap | 99.1% | S. Korea, Iran Serbia, Thailand | 48-100% | [10,27,42,43] |
| OmpA | 98.2% | Iran, S. Korea, | 77.1-100% | [16,41] |
| surA | 98.2% | Iran, China | 95-98% | [16,17] |
| PLD | 95.5% | China, Poland | 87.5-99% | [15,17] |
| PaaE | 89.1% | China | 88.6% | [17] |
| basD | 86.4% | China, Poland | 92-95% | [17,15] |
| traT | 8.2% | China, Iran | 0-80% | [17,43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





