Submitted:
06 May 2024
Posted:
07 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
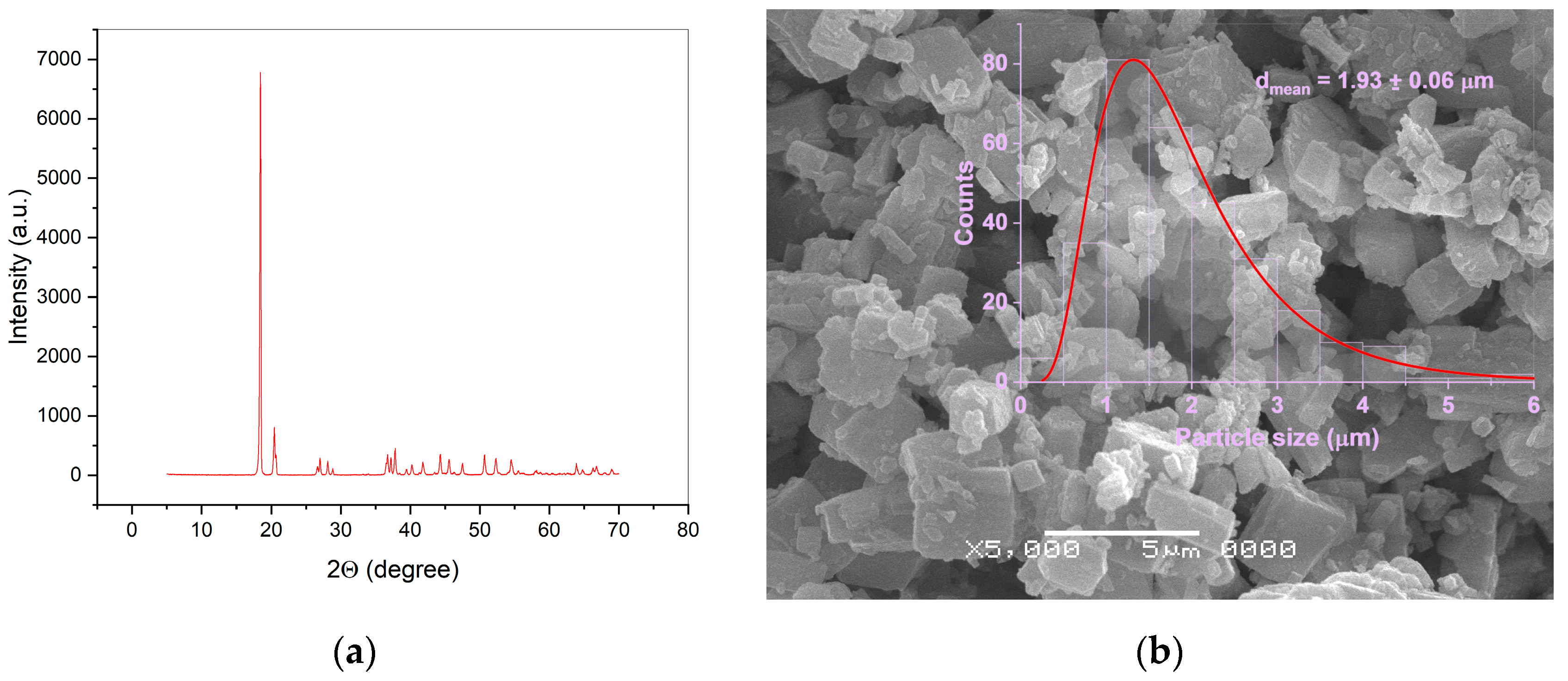
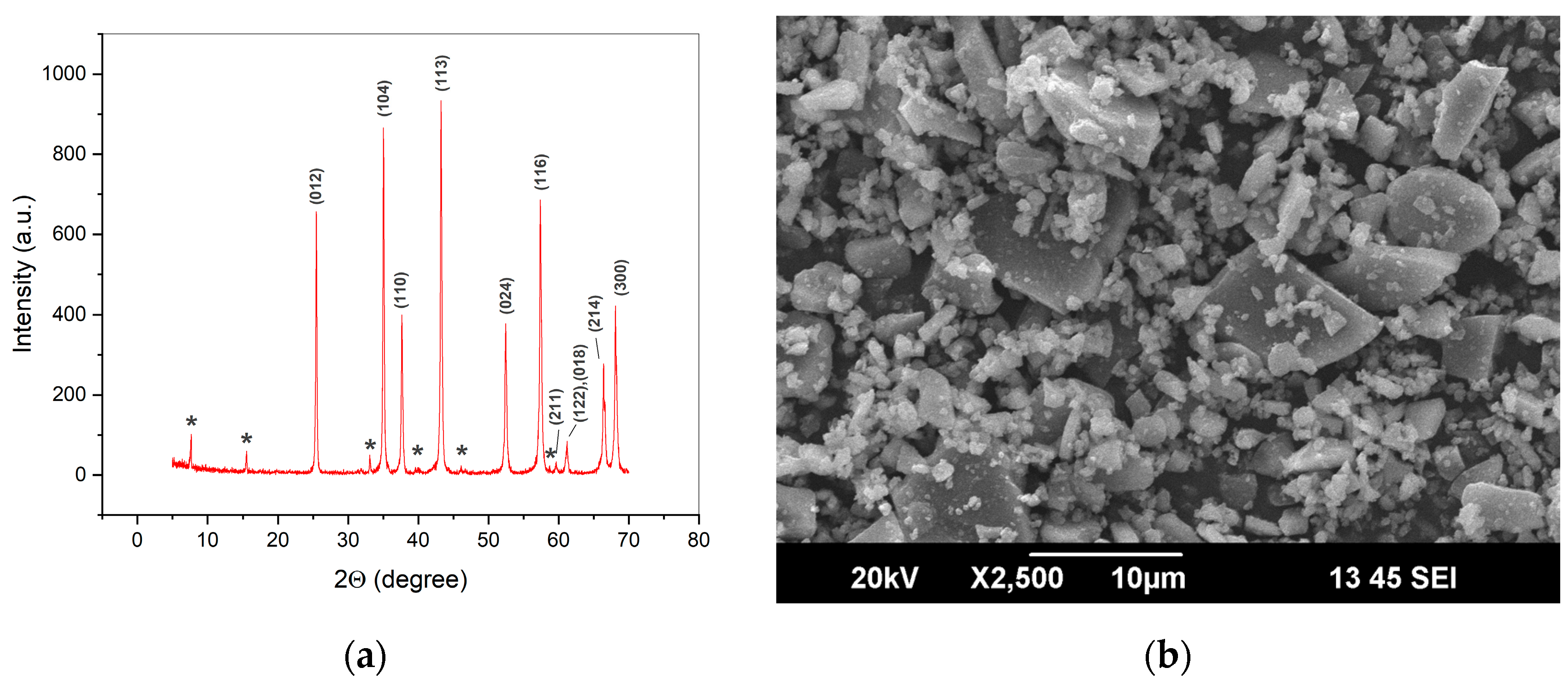
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- (1)
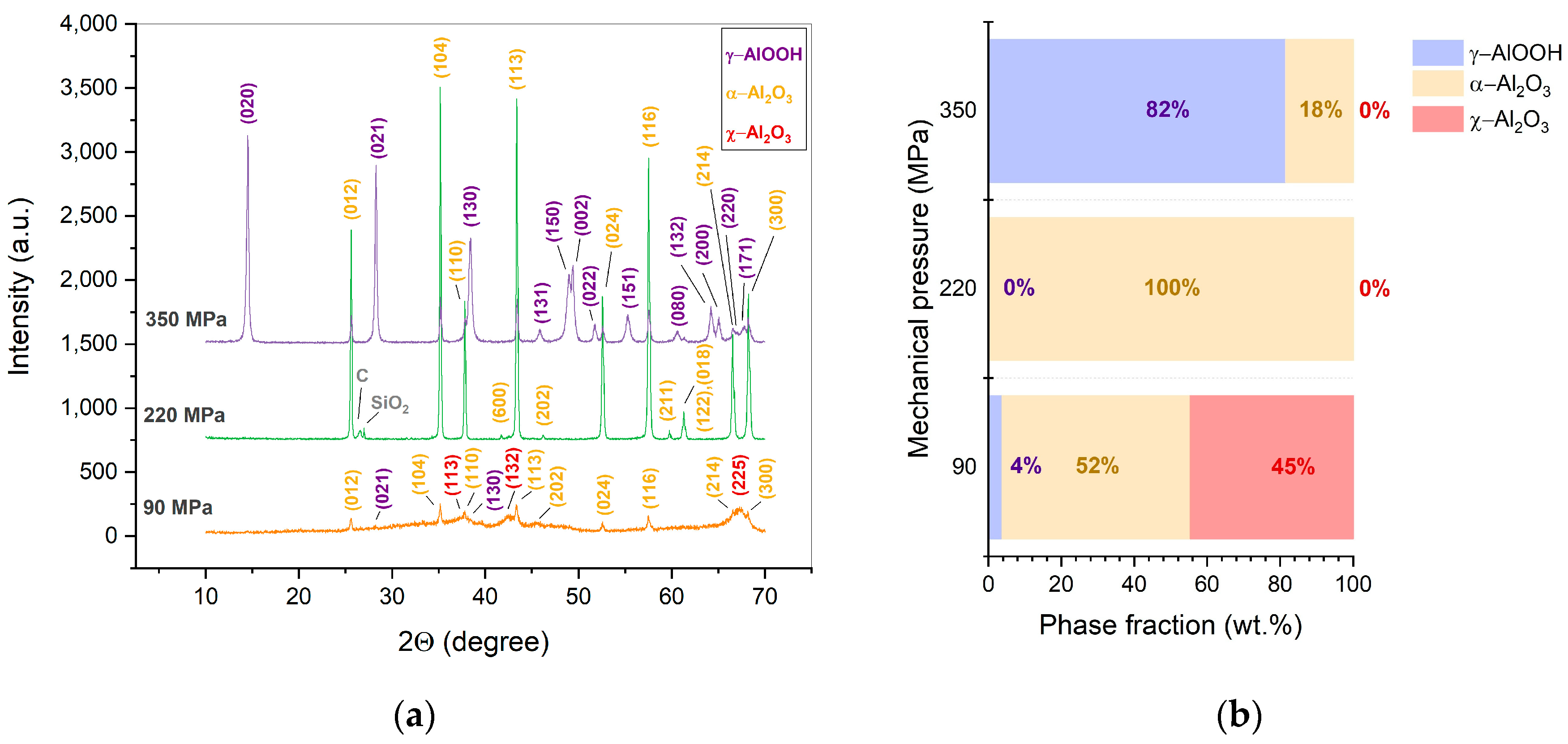
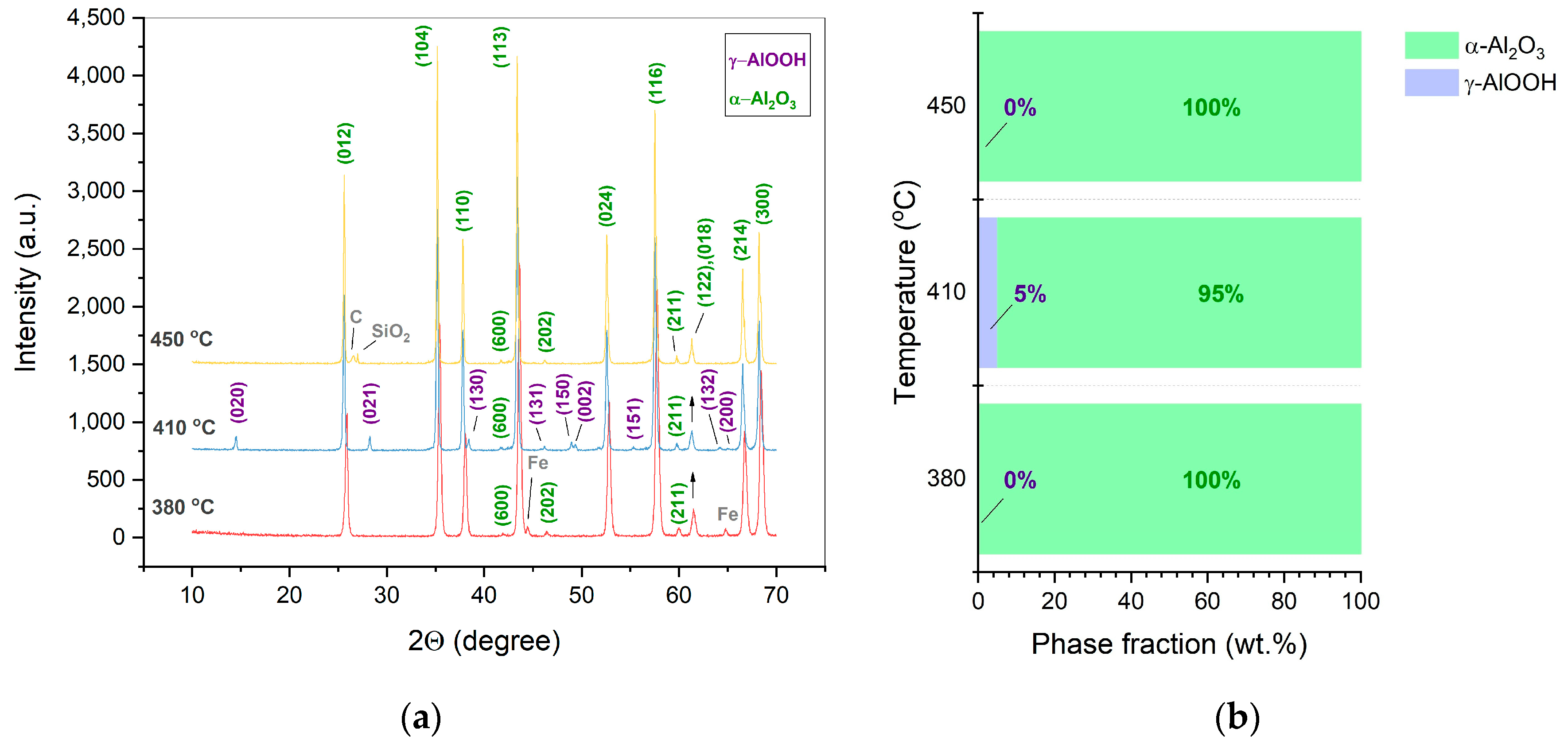
- For the first time, porous α-Al2O3 ceramics were processed directly by CSP at a temperature of 380-450 °C, a pressure of 220 MPa during 30 min. in a presence of pure water. The materials were characterized by predominately open-type porosity reaching 36 %.
- (2)
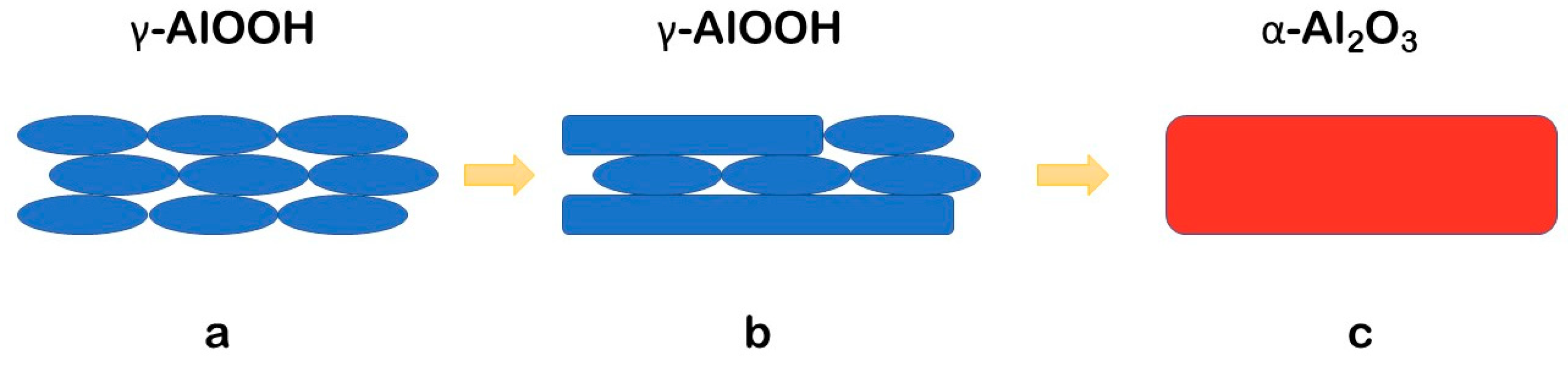
- Under the studied CSP conditions, the initial γ-Al(OH)3 powder undergoes dehydration leading to γ-AlOOH and possible minor χ-Al2O3 phases. Further processed, γ-AlOOH forms elongated grains, which coalesce into plate-like ones and transform into the grains of α-Al2O3.
- (3)
- An increase in the applied pressure (up to 350 MPa) prevents the dehydration of γ-AlOOH and impedes the formation of the final α-Al2O3.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Randall, C.A.; Guo, J.; Baker, A.; Lanagan, M.T.; Guo, H. Cold Sintering Ceramics and Composites 2017.
- Huang, Y.; Huang, K.; Zhou, S.; Lin, C.; Wu, X.; Gao, M.; Zhao, C.; Fang, C. Influence of Incongruent Dissolution-Precipitation on 8YSZ Ceramics during Cold Sintering Process. Journal of the European Ceramic Society 2022, 42, 2362–2369. [Google Scholar] [CrossRef]
- Zahabi, M.; Said, A.; Memari, A. Cold Sintering of Calcium Carbonate for Construction Material Applications. ACS Omega 2021, 6, 2576–2588. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Hashimoto, S. Mechanism of Densification of Calcium Carbonate by Cold Sintering Process. Journal of the European Ceramic Society 2022, 42, 6048–6055. [Google Scholar] [CrossRef]
- Smirnov, A.V.; Ivakin, Yu.D.; Kornyushin, M.V.; Kholodkova, A.A.; Vasin, A.A.; Ayudinyan, S.; Kirakosyan, H.V. Effect of Activating Additives on the Cold Sintering Process of (MnFeCoNiCu)3O4 High-Entropy Ceramics. Fine Chem. Technol. 2022, 17, 439–449. [Google Scholar] [CrossRef]
- Galotta, A.; Sglavo, V.M. The Cold Sintering Process: A Review on Processing Features, Densification Mechanisms and Perspectives. Journal of the European Ceramic Society 2021, 41, 1–17. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Hashimoto, S. Effect of Phase Transformation in Cold Sintering of Aluminum Hydroxide. Journal of the European Ceramic Society 2024, 44, 2754–2761. [Google Scholar] [CrossRef]
- Thabet, K.; Quarez, E.; Joubert, O.; Le Gal La Salle, A. Application of the Cold Sintering Process to the Electrolyte Material BaCe0.8Zr0.1Y0.1O3-δ. Journal of the European Ceramic Society 2020, 40, 3445–3452. [Google Scholar] [CrossRef]
- Guo, H.; Bayer, T.J.M.; Guo, J.; Baker, A.; Randall, C.A. Cold Sintering Process for 8 mol%Y2O3-Stabilized ZrO2 Ceramics. Journal of the European Ceramic Society 2017, 37, 2303–2308. [Google Scholar] [CrossRef]
- Lai, Q.; Chen, J.; Chang, F.; Pei, J.; Liang, Y.; Chen, X.; Feng, Q.; Cen, Z.; Luo, N. Cold Sintering Process Assisted Sintering for 8YSZ Ceramic: A Way of Achieving High Density and Electrical Conductivity at a Reduced Sintering Temperature. Ceramics International 2023, 49, 14744–14749. [Google Scholar] [CrossRef]
- Grady, Z.; Ndayishimiye, A.; Randall, C. A Dramatic Reduction in the Sintering Temperature of the Refractory Sodium Β′′-Alumina Solid Electrolyte via Cold Sintering. J. Mater. Chem. A 2021, 9, 22002–22014. [Google Scholar] [CrossRef]
- Guo, N.; Liu, M.; Shen, J.-Y.; Shen, H.-Z.; Shen, P. Surface Hydrate-Assisted Low- and Medium-Temperature Sintering of MgO. Scripta Materialia 2022, 206, 114258. [Google Scholar] [CrossRef]
- Amrute, A.P.; Jeske, K.; Łodziana, Z.; Prieto, G.; Schüth, F. Hydrothermal Stability of High-Surface-Area α-Al2O3 and Its Use as a Support for Hydrothermally Stable Fischer–Tropsch Synthesis Catalysts. Chem. Mater. 2020, 32, 4369–4374. [Google Scholar] [CrossRef]
- Huang, C.-L.; Wang, J.-J.; Huang, C.-Y. Sintering Behavior and Microwave Dielectric Properties of Nano Alpha-Alumina. Materials Letters 2005, 59, 3746–3749. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Gkekas, I.; Kastrinaki, G.; Prigione, A.; Zaspalis, V.T.; Petrakis, S. Biocompatibility of α-Al2O3 Ceramic Substrates with Human Neural Precursor Cells. JFB 2020, 11, 65. [Google Scholar] [CrossRef]
- Hashimoto, H.; Kojima, S.; Sasaki, T.; Asoh, H. α-Alumina Membrane Having a Hierarchical Structure of Straight Macropores and Mesopores inside the Pore Wall. Journal of the European Ceramic Society 2018, 38, 1836–1840. [Google Scholar] [CrossRef]
- Rytter, E.; Borg, Ø.; Enger, B.C.; Holmen, A. α-Alumina as Catalyst Support in Co Fischer-Tropsch Synthesis and the Effect of Added Water; Encompassing Transient Effects. Journal of Catalysis 2019, 373, 13–24. [Google Scholar] [CrossRef]
- Liu, Y.; Ng, Z.; Khan, E.A.; Jeong, H.-K.; Ching, C.; Lai, Z. Synthesis of Continuous MOF-5 Membranes on Porous α-Alumina Substrates. Microporous and Mesoporous Materials 2009, 118, 296–301. [Google Scholar] [CrossRef]
- Dong, J.; Payzant, E.A.; Hu, M.Z.C.; Depaoli, D.W.; Lin, Y.S. Synthesis of MFI-Type Zeolite Membranes on Porous α-Alumina Supports by Wet Gel Crystallization in the Vapor Phase. Journal of Materials Science 2003, 38, 979–985. [Google Scholar] [CrossRef]
- Ohji, T.; Fukushima, M. Macro-Porous Ceramics: Processing and Properties. International Materials Reviews 2012, 57, 115–131. [Google Scholar] [CrossRef]
- Niu, L.; Qin, R.; Liu, Y.; Xin, J.; Wu, X.; Zhang, F.; Li, X.; Shao, C.; Li, X.; Liu, Y. Hierarchical Porous Alumina Ceramics as Multi-Functional Support with Excellent Performance. Ceramics International 2024, 50, 2611–2622. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Mao, X.; An, L.; Liu, Y.; Wang, S.; Zhang, J.; Feng, K. Preparation and Properties of Porous Alumina Ceramics for Ultra-Precision Aerostatic Bearings. Ceramics International 2022, 48, 13311–13318. [Google Scholar] [CrossRef]
- Zhang, A.; Sang, K.; Zeng, D.; Liu, Q.; Guo, Y. Preparation and Properties of Porous Alumina with Inter-Locked Platelets Structure. Ceramics International 2022, 48, 25918–25922. [Google Scholar] [CrossRef]
- Dong, X.; Chua, B.W.; Li, T.; Zhai, W. Multi-Directional Freeze Casting of Porous Ceramics with Bone-Inspired Microstructure. Materials & Design 2022, 224, 111344. [Google Scholar] [CrossRef]
- Chen, H.; Pan, Y.; Chen, B.; Li, J.; Gui, Z.; Chen, J.; Yan, H.; Zeng, Y.; Chen, J. Fabrication of Porous Aluminum Ceramics beyond Device Resolution via Stereolithography 3D Printing. Ceramics International 2023, 49, 18463–18469. [Google Scholar] [CrossRef]
- Moshkovitz, M.Y.; Paz, D.; Magdassi, S. 3D Printing Transparent γ -Alumina Porous Structures Based on Photopolymerizable Sol–Gel Inks. Adv Materials Technologies 2023, 8, 2300123. [Google Scholar] [CrossRef]
- Hérisson de Beauvoir, T.; Estournès, C. Translucent γ-AlOOH and γ-Al2O3 Glass-Ceramics Using the Cold Sintering Process. Scripta Materialia 2021, 194, 113650. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, X.; Guo, J.; Liang, J.; Sun, J.; Yang, Y.; Yang, L.; Liao, R.; Randall, C.A. Thermal-Assisted Cold Sintering Study of Al2O3 Ceramics: Enabled with a Soluble γ-Al2O3 Intermediate Phase. Journal of the European Ceramic Society 2023, 43, 478–485. [Google Scholar] [CrossRef]
- Suleiman, B.; Zhang, H.; Ding, Y.; Li, Y. Microstructure and Mechanical Properties of Cold Sintered Porous Alumina Ceramics. Ceramics International 2022, 48, 13531–13540. [Google Scholar] [CrossRef]
- Gao, J.; Ding, Q.; Yan, P.; Liu, Y.; Hu, Y.; Ren, Y.; Wang, X.; Mustafa, T.; Fan, Y.; Jiang, W. Direct Cold Sintering of Translucent Gamma-Al2O3 Ceramics. Journal of the European Ceramic Society 2024, 44, 4225–4231. [Google Scholar] [CrossRef]
- Kholodkova, A.A.; Kornyushin, M.V.; Pakhomov, M.A.; Smirnov, A.V.; Ivakin, Y.D. Water-Assisted Cold Sintering of Alumina Ceramics in SPS Conditions. Ceramics 2023, 6, 1113–1128. [Google Scholar] [CrossRef]
- Ivakin, Yu.D.; Danchevskaya, M.N.; Muravieva, G.P. Induced Formation of Corundum Crystals in Supercritical Water Fluid. Russ. J. Phys. Chem. B 2015, 9, 1082–1094. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Hirao, K.; Kanzaki, S. Fabrication of Low Cost Fine-Grained Alumina Powders by Seeding for High Performance Sintered Bodies. Journal of the European Ceramic Society 2004, 24, 325–330. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Physica B: Condensed Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Vaitkus, A.; Merkys, A.; Sander, T.; Quirós, M.; Thiessen, P.A.; Bolton, E.E.; Gražulis, S. A Workflow for Deriving Chemical Entities from Crystallographic Data and Its Application to the Crystallography Open Database. J Cheminform 2023, 15, 123. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J Appl Crystallogr 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Temuujin, J.; Okada, K. Thermal Decomposition of Mechanically Activated Gibbsite. Thermochimica Acta 1999, 327, 103–108. [Google Scholar] [CrossRef]
- Lamouri, S.; Hamidouche, M.; Bouaouadja, N.; Belhouchet, H.; Garnier, V.; Fantozzi, G.; Trelkat, J.F. Control of the γ-Alumina to α-Alumina Phase Transformation for an Optimized Alumina Densification. Boletín de la Sociedad Española de Cerámica y Vidrio 2017, 56, 47–54. [Google Scholar] [CrossRef]
- Chen, B.; Xu, X.; Chen, X.; Kong, L.; Chen, D. Transformation Behavior of Gibbsite to Boehmite by Steam-Assisted Synthesis. Journal of Solid State Chemistry 2018, 265, 237–243. [Google Scholar] [CrossRef]
- Suchanek, W.L. Hydrothermal Synthesis of Alpha Alumina (α-Al2O3) Powders: Study of the Processing Variables and Growth Mechanisms. Journal of the American Ceramic Society 2010, 93, 399–412. [Google Scholar] [CrossRef]
- Ingram-Jones, V.J.; Slade, R.C.T.; Davies, T.W.; Southern, J.C.; Salvador, S. Dehydroxylation Sequences of Gibbsite and Boehmite: Study of Differences between Soak and Flash Calcination and of Particle-Size Effects. J. Mater. Chem. 1996, 6, 73. [Google Scholar] [CrossRef]
- Hayashi, H.; Hakuta, Y. Hydrothermal Synthesis of Metal Oxide Nanoparticles in Supercritical Water. Materials 2010, 3, 3794–3817. [Google Scholar] [CrossRef] [PubMed]
- Ivakin, Yu.D.; Danchevskaya, M.N.; Muravieva, G.P. Recrystallization of Zinc Oxide in a Sub- and Supercritical Water Medium. Russ. J. Phys. Chem. B 2019, 13, 1189–1200. [Google Scholar] [CrossRef]
- Plyasunov, A.V. Predicting Solubility of Oxides of Metals and Metalloids in Supercritical Water. Ind. Eng. Chem. Res. 2020, 59, 970–980. [Google Scholar] [CrossRef]
- Smirnov, A.V.; Kornyushin, M.V.; Kholodkova, A.A.; Melnikov, S.A.; Stepanov, A.D.; Fesik, E.V.; Ivakin, Y.D. Cold Sintering Process of Zinc Oxide Ceramics: Powder Preparation and Sintering Conditions Effects on Final Microstructure. Inorganics 2022, 10, 197. [Google Scholar] [CrossRef]
- Ndayishimiye, A.; Fan, Z.; Mena-Garcia, J.; Anderson, J.M.; Randall, C.A. Coalescence in Cold Sintering: A Study on Sodium Molybdate. Open Ceramics 2022, 11, 100293. [Google Scholar] [CrossRef]
- Marmier, A.; Parker, S.C. Ab Initio Morphology and Surface Thermodynamics of α − Al2O3 Phys. Rev. B 2004, 69, 115409. [Google Scholar] [CrossRef]
- Alzukaimi, J.; Jabrah, R. The Preparation and Characterization of Porous Alumina Ceramics Using an Eco-friendly Pore-forming Agent. Int J Applied Ceramic Tech 2019, 16, 820–831. [Google Scholar] [CrossRef]
- Kerolli Mustafa, M.; Gabelica, I.; Mandić, V.; Veseli, R.; Ćurković, L. Reusing Waste Coffee Grounds in the Preparation of Porous Alumina Ceramics. Sustainability 2022, 14, 14244. [Google Scholar] [CrossRef]
- Vemoori, R.; Bejugama, S.; Khanra, A.K. Fabrication and Characterization of Alumina and Zirconia-Toughened Alumina Porous Structures. Ceramics International 2023, 49, 21708–21715. [Google Scholar] [CrossRef]

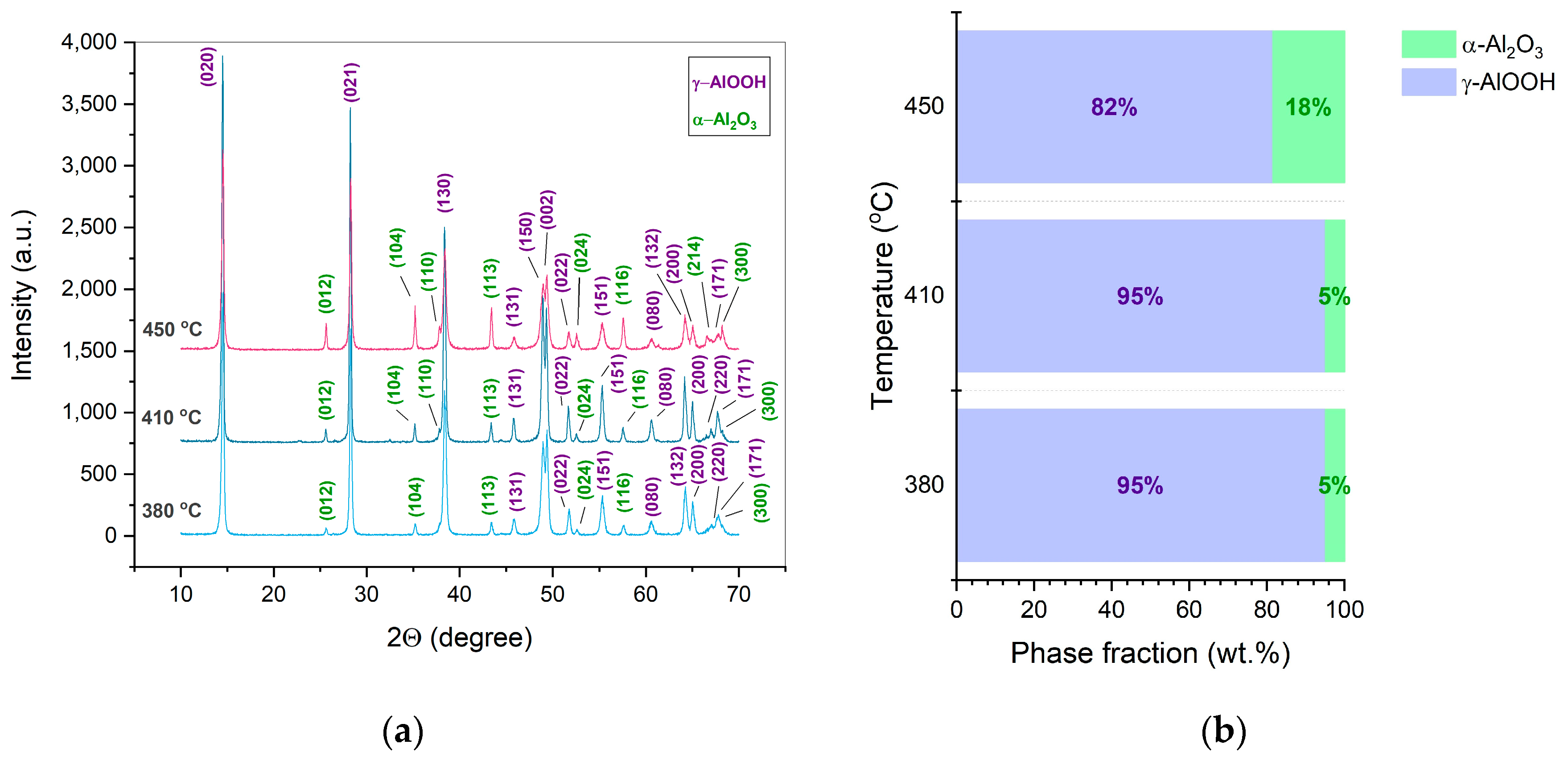
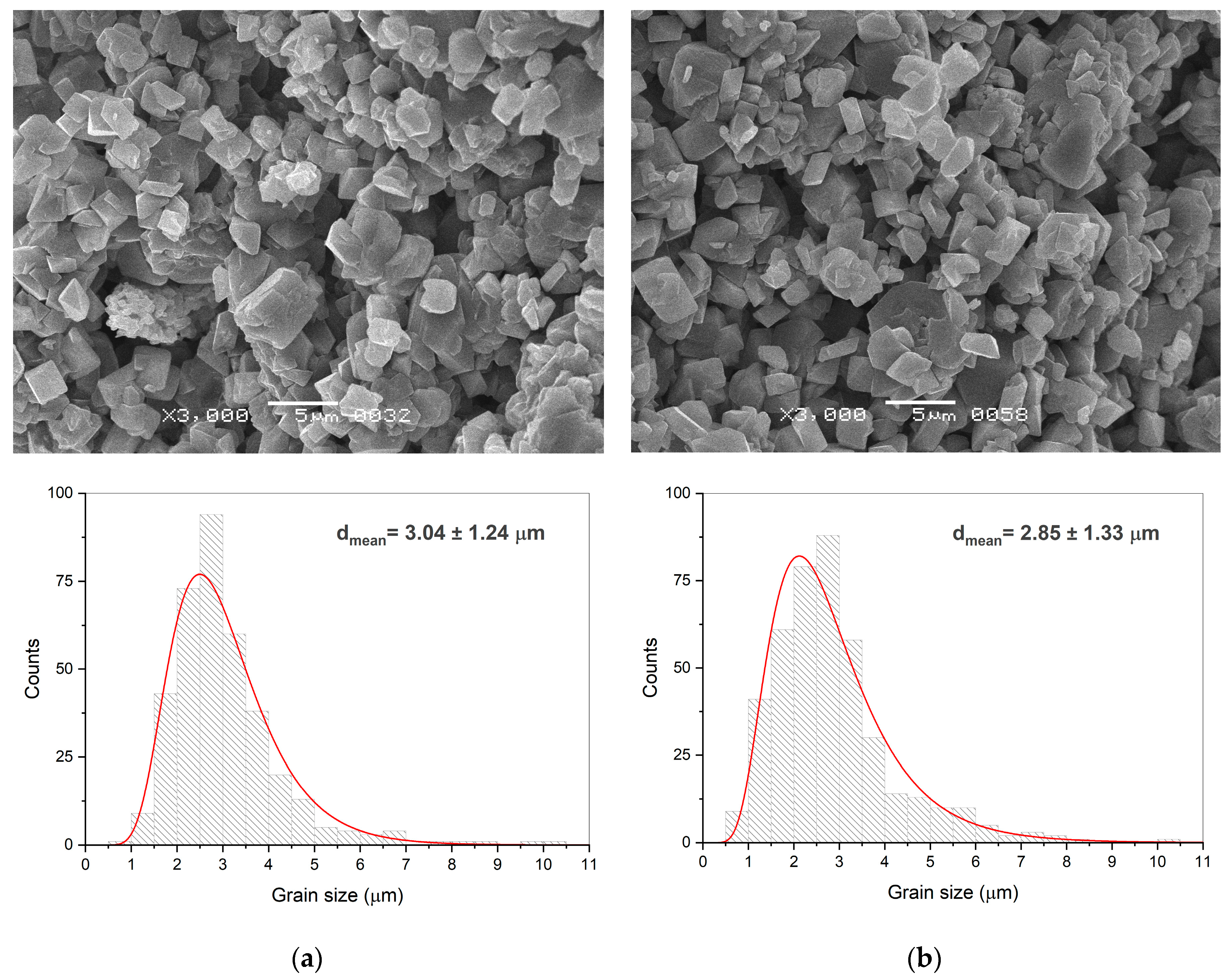
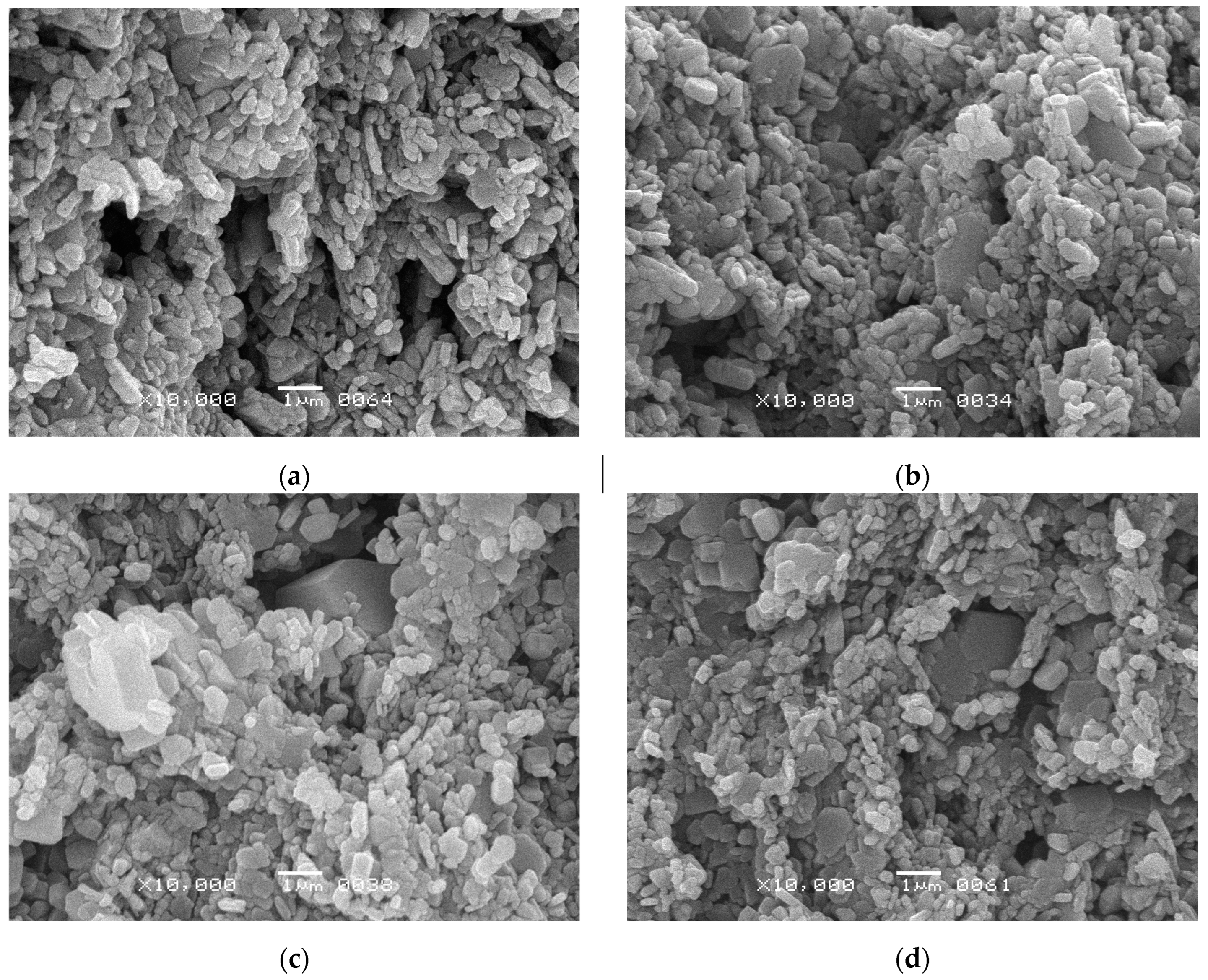
| Mechanical pressure (MPa) | Temperature (°C) | Average crystallite size (nm) | Density (g cm-1) | Relative density (%) | Open porosity (%) | |
|---|---|---|---|---|---|---|
| γ-AlOOH | α-Al2O3 | |||||
| 90 | 450 | - | - | 2.23 | 58.2 | 41.0 |
| 220 | 380 | - | 4.20 | 2.53 | 63.7 | 36.0 |
| 410 | 6.48 | 5.68 | 2.51 | 64.0 | 35.7 | |
| 450 | - | 6.68 | 2.58 | 65.0 | 34.6 | |
| 350 | 380 | 4.17 | 6.08 | 1.95 | 62.6 | 35.3 |
| 410 | 4.94 | 7.17 | 1.98 | 63.6 | 28.4 | |
| 450 | 3.66 | 5.49 | 2.08 | 64.4 | 22.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





