1. Introduction
Critical illness results in characteristic changes to iron metabolism. Up to 75% of critically ill patients show elevated ferritin but low serum iron levels at admission to the intensive care unit (ICU) [
1]. Most of the critically ill COVID-19 patients showed significantly decreased levels of serum iron during the first 24 hours of ICU stay [
2,
3]. Moreover, as the most common nutritional deficiency worldwide, iron deficiency including absolute iron deficiency (AID) and functional iron deficiency (FID) represents a risk factor for developing critical illness [
1,
4]. Generally, AID is characterized by reduced iron stores and inadequate iron supply, whereas FID means that iron stores are adequate, but iron supply is insufficient [
5]. The combination of pre-existing disturbances of iron metabolism and regulatory changes of iron metabolism in critical illness represents a diagnostic dilemma for the management of iron metabolism in critically ill patients. In general, iron replacement therapy is not considered during the acute phase of critical illness, because more accessible iron is beneficial for invading microbes and oxidative stress [
1,
6]. However, during the recovery phase of critical illness, clinicians are facing the question of when to (re-)start iron supplementation. This review summarizes the existing knowledge about the changes in iron metabolism during critical illness as the basis for decision-making for iron supplementation in the recovery phase of critical illness.
2. The Role of Iron in Human Health
Iron plays an important role in human health (for review see [
7]). It is necessary for the transport of oxygen, mitochondrial function, and the synthesis of deoxyribonucleic acid. Iron is also involved in the immune response due to its role in the Fenton reaction, which is required for the formation of oxygen-free radicals (OFR), a main inflammatory mediator [
8]. Iron levels must be tightly regulated because of the potentially detrimental effects of iron overload. Iron uptake is under metabolic control by various enzymes such as hepcidin [
9]. Surprisingly, humans do not have an active iron excretion pathway. The organism downregulates the available iron pool by reducing the uptake and shifting iron to the intracellular space [
10]. Therapeutic iron supplementation needs to consider the dynamics of iron homeostasis.
3. Iron Metabolism in Critically Ill Patients
Critical illness is accompanied by characteristic changes in iron metabolism, with microcytic anemia as the leading clinical symptom [
11,
12,
13]. FID without anemia is also common in critical illness. FID, measured by red cell hypochromasia on flow cytometry and presence of zinc protoporphyrin, represents an insufficient biological availability of iron in the presence of adequate storage reserves [
14,
15]. In a prospective observational study, it was reported that the incidence of FID reached up to 35% in patients at admission [
4]. In a cohort of 314 patients from an interdisciplinary ICU, about 28.3% of patients were diagnosed with FID [
16]. Most investigators concurred that during the early phase of critical illness, hepcidin is upregulated due to the release of pro-inflammatory mediators which leads to decreased activity of ferroportin, a cell surface protein exporting iron form cells into blood circulation, and results in higher intracellular iron levels [
1,
17]. This causes a decline in serum iron levels.
An increasing body of evidence from both animal and human studies suggests that iron levels may play an important role in the outcome of critical illness. A prospective single-center study demonstrated that iron and serum transferrin saturation (TSAT) levels are strong predictors of outcome in ICU patients [
18]. Interestingly, iron and TSAT levels were significantly decreased in sepsis survivors, and transferrin levels were lower in non-survivors [
18]. Potential reasons for higher serum iron levels in non-survivors were reviewed previously [
18]. Insufficient activation of hepcidin is considered as one of the main reasons. Severe sepsis is often accompanied by liver dysfunction, which reduces hepatic hepcidin synthesis [
19]. Secondly, non-survivors show increased catabolism with iron being released from the elevated turnover of erythrocytes and other cells [
20,
21]. Thirdly, to improve tissue perfusion, frequent blood transfusions will further increase iron levels from aged and damaged erythrocytes. Therefore, serum iron levels can also be used as a marker of disease severity and prognosis [
22]. In a two-center retrospective and prospective observational study with COVID-19 patients, the authors demonstrated that serum iron levels were significantly decreased in patients with mild respiratory failure (RF) compared to those without RF, but there were no significant differences in iron levels between the non-RF and severe RF groups, so there is an U-shaped relationship between serum iron levels and disease severity [
23]. Another study revealed that serum iron levels were an independent risk factor for 90-day mortality [
24]. In this study including total of 1,891 patients diagnosed with sepsis according to the Sepsis-3 criteria, the results showed that after adjusting for confounding variables, a higher serum iron level on ICU admission was associated with increased 90-day mortality.
On the other hand, free iron, which is generally referred to as non-transferrin-bound iron or non-ferritin-bound iron, may be more meaningful than conventional serum iron levels to forecast adverse clinical outcomes and in-hospital mortality [
25]. Free iron accelerates the Fenton reaction, resulting increased levels of reactive oxygen species cause tissue damage. Catalytic iron, also known as non-transferrin bound iron, is a kind of free iron and positively associated with oxidative stress, vascular injury, and risk of mortality in critically ill patients [
26,
27]. Also, in another study involving 806 patients with acute coronary syndrome, the authors evaluated the role of catalytic iron in predicting mortality. The results showed that higher catalytic iron levels were associated with higher mortality [
28]. Similarly, a study focused on critically ill COVID-19 patients also highlighted that higher plasma concentrations of catalytic iron were significantly correlated with in-hospital mortality [
25]. Therefore, elevated catalytic iron appears to be a reliable parameter to predict unfavorable clinical outcomes in critically ill patients.
4. Iron Metabolism in the Recovery Phase from Critical Illness
4.1. Ferritin
Ferritin, a molecular weight of 450 kDa, is an iron storage protein found in the circulation, cytosol and within the mitochondria [
29]. Ferritin’s role, used to evaluate body iron stores, is well established in iron metabolism, and its value as a marker of body iron to assess iron status in infection, inflammation and malignancy has been extensively studied. For example, a recent systematic review reported that serum ferritin can be considered as a diagnostic biomarker for iron storage with ferritin concentration in patients with iron depletion as low as 80 mcg/L, and in patients with iron overload was nearly 500 mcg/L [
30]. Recently, serum ferritin has also been used for assessing iron metabolism and organ dysfunction in critically ill patients. In a prospective study in ICU patients, serum ferritin increase was higher in nonsurvivors than survivors, and there was a significant positive correlation between serum ferritin and Sequential Organ Failure Assessment (SOFA) score [
31]. The researchers suggested that serum ferritin levels are linked to the presence of inflammation [
31,
32]. Based on the relationship between ferritin in serum and urine, Gerday et al. reported that measuring urinary ferritin was a potential noninvasive screening test for iron metabolism in neonatal intensive care unit (NICU) patients, and the results showed that in order to detect iron-limited erythropoiesis, the amended urine ferritin below 12 ng/mL had 82% sensitivity and 100% specificity, and the positive predictive value was 100% [
33].
In COVID-19 patients admitted to ICU, serum ferritin was used to distinguish the severity of the disease. Increased serum levels of ferritin were associated with more severe COVID-19 [
34,
35,
36,
37,
38]. Serum ferritin was also an independent predictor of in-hospital COVID-19-related mortality. Zhou et al. reported that the mortality rate for patients with raised serum levels of ferritin (> 300 μg/L) was significantly higher [
39]. Increased levels of serum ferritin may enhance the inflammatory response, so as to exert a pathogenic role in viral infection [
40]; On the other hand, excessive ferritin can contribute to the production of reactive oxygen species and oxidative stress [
41,
42,
43]. Furthermore, a number of retrospective and prospective studies have verified that the state of hyperferritinemia is accompanied by a worse prognosis of COVID -19 both in acute and post-acute phase [
34,
44,
45,
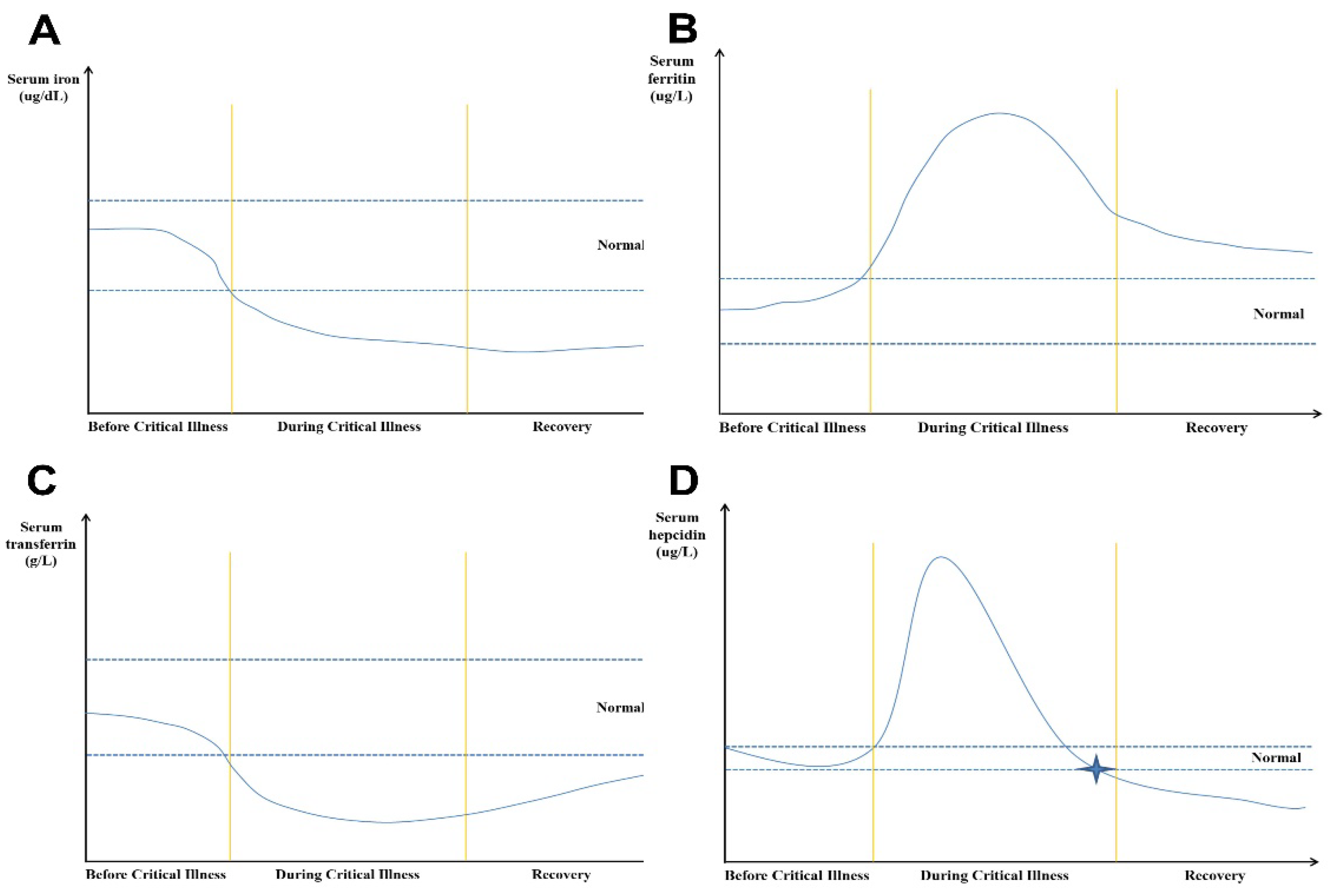
46]. Therefore, during recovery phase of ICU patients, serum ferritin is still an important marker of iron metabolism, and if the concentration of serum ferritin continues to be high, it indicates that the inflammatory response of the body has not been fully controlled, and even if the serum iron concentration is low, it is not the time to supplement iron. Based on the information above, the serum iron and ferritin dynamics in surviving ICU patients with long ICU stay can be summarized in
Figure 1.
4.2. Hepcidin
Hepcidin is a master iron regulatory hormone, which is synthesized in the liver, circulates in the blood stream and is excreted in the urine. Hepcidin regulates intestinal absorbed, macrophage recycled or liver stored iron efflux from cells into the blood circulation through regulating the expression of ferroportin on the cell surfaces. It is reported that hepcidin is positively associated with ferritin, interleukin-6, RBC transfusion and C-reactive protein, and negatively correlated with iron, total iron binding capacity, transferrin, and reticulocyte response [
47]. In a prospective single-center clinical non-interventional study, which included one hundred adult surgical ICU patients, the authors found that the levels of hepcidin in serum showed a time-dependent course (
Figure 1D). Hepcidin serum concentrations were markedly increased on ICU admission and decreased significantly over the course of the ICU stay [
47]. During the recovery phase of the ICU stay, most of the patients’ reticulocyte response was increased, and inflammatory response was decreased. Therefore, the hepcidin concentrations decreased. This suggests that measurements of hepcidin levels may be useful for managing iron deficiency in critically ill patients during the recovery phase [
48]. In fact, AID was defined when hepcidin was below 20 µg/L, whereas FID was defined as a hepcidin level from 20 µg/L to 41 µg/L [
49].
In addition, serum hepcidin can predict mortality of patients in ICU during recovery phase. Hepcidin was suggested as a clinical biomarker for COVID-19 severity, since the levels of serum hepcidin was significantly increased in ICU non-survive patients, compared with ICU survivors. This indicates that in critical patients admitted to ICU, high hepcidin levels is positively associated with the mortality [
50]. Also, some studies showed that the higher baseline levels of serum hepcidin in hospitalized COVID-19 patients, the higher possibility of mechanical ventilation and kidney replacement for these patients [
36,
50]. However, Yağcı et al. reported that critically ill COVID-19 patients in ICU had lower serum hepcidin levels than healthy patients [
35]. It was speculated there was a similar structure between SARS-CoV-2-spiked glycoprotein cytoplasmic tail and hepcidin protein, so SARS-CoV-2 can directly increase the levels of circulating and tissue ferritin, induce serum iron deficiency and hemoglobin deficiency, and suppress hepatic hepcidin synthesis [
35]. Moreover, in a prospective multicenter study including 2087 patients and 28 ICUs, the authors used plasma hepcidin levels to diagnose iron deficiency and assess the association of iron deficiency with outcomes during the recovery phase after an intensive care unit stay. They showed that iron deficiency diagnosed based on hepcidin concentration < 20 ng/l at ICU discharge was associated with increased one-year mortality, and severe iron deficiency defined as hepcidin < 10 ng/l was an independent predictor of poor one-year physical recovery [
48]. Therefore, to accelerate the speed of physical recovery, during the recovery phase of ICU patients, dynamic monitoring of hepcidin levels might be useful to guide the timeline in starting iron supplementation.
4.3. Transferrin and Transferrin Saturation
As the primary iron transport protein, transferrin binds to transferrin receptors on cell surfaces and delivers iron into the cells of target organs and tissues via receptor-mediated endocytosis. Since transferrin can stand without binding of iron, transferrin saturation (TSAT) reflects the amount of iron bound to transferrin, and was used as a reliable marker of systemic iron status [
29]. Recent findings demonstrated that parameters of iron metabolism, particularly transferrin and TSAT can be utilized as strong outcome predictors for diverse groups of critically ill patients [
18]. Brandtner et al. designed a prospective study to investigate the putative impact of serum iron parameters on the outcome of sepsis, and the results showed that low transferrin concentrations and higher TSAT levels were associated with reduced survival [
51]. Therefore, they suggested that TSAT can serve as a stand-alone predictor of sepsis survival and improve the prediction power of the SOFA score [
51].
Recently, a growing body of evidence demonstrated the relationship between transferrin (or TSAT) and disease severity in patients with liver dysfunction. It was reported that iron metabolism was disrupted in patients with acute-on-chronic liver failure (ACLF), and a prospective study showed that low transferrin concentration and high TSAT related to the severity of ACLF and increased short-term mortality [
52,
53]. A similar correlation between TSAT and disease severity was also reported in decompensated cirrhosis patients [
54].
In COVID-19-related studies, transferrin levels were low at the time of hospitalization, especially in patients with serious medical conditions, such as high oxygen demand, the decreased levels of transferrin were even more intense [
29]. For example, Hippchen et al. reported that inpatients and critically ill patients showed significantly lower transferrin levels than outpatients, indicating that low transferrin levels can predict increased inflammation and disease severity [
55]. On the other hand, there was also a significant relationship between TSAT and COVID-19 severity. Similarly, TSAT in patients with severe respiratory failure was markedly higher than in patients with mild or no respiratory failure; for example, intubated patients had higher TSAT than non-intubated patients [
23,
35]. Moreover, the levels of TSAT in COVID-19 patients showed a dynamic pattern. Generally, in the early stage of infection, such as at ICU admission, the levels of TSAT were not very high but increased between the 3rd and 6th day of admission, then in the later stage, generally within 7-18 days in the hospital, returned to normal [
56]. Therefore, because the changes of transferrin is mainly regulated by the availability of iron, during the recovery phase of critically ill patients, the levels of transferrin especially TSAT can be used as an important reference to guide the timing of iron supplementation. The serum transferrin dynamics in critically ill patients with long ICU stay is shown in
Figure 1C.
4.4. Iron Supplementation
In the recovery phase from critical illness, iron supplementation should be considered for critically ill patients once the diagnosis of iron deficiency is established. Appropriate iron supplementation is safe and beneficial to the recovery of critically ill patients. Giving intravenous iron to patients without iron deficiency increases the risk of toxic side effects and iron overload [
57].
Iron deficiency and anemia are frequent and may impair recovery in critically ill patients. High-dose intravenous iron can be administered safely under the guidance of hepcidin levels. During the recovery phase of critically ill patients, if the serum hepcidin level is below 20 μg/L, it may be an appropriate time to start iron supplementation (
Figure 1D).
5. Harm of Dysregulated Iron Metabolism in the Recovery Phase of Critical Illness
Despite protective effects during the critical phase of critical illness, low iron levels in the recovery phase of critically ill patients are harmful [
20,
21,
22,
23,
24]. Moreover, hypoferremia may reflect a detrimental intracellular iron overload during inflammatory processes and impair oxygen delivery to peripheral tissues by limiting erythropoiesis [
24,
26].
5.1. Anemia
Anemia is a common condition in critically ill patients in intensive care, with a mean prevalence of up to 65% of all patients at the time of admission and nearly 97% after a length of stay of 8 days [
16]. Anemia has been associated with worse outcomes including increased stay lengths and mortality. The etiology of anemia in critically ill patients is often multifactorial and complex, influenced by underlying chronic diseases and complicated through relevant blood loss for diagnostic investigations, surgery, or hemolysis. However, the most common reason for anemia in hospitalized patients is iron-restricted erythropoiesis, which is usually caused by iron deficiency [
58]. During the recovery phase of critical illness, reduced uptake of nutritional iron and retention of iron into the reticulohistiocytic system (RHS) are the characteristic features of iron-deficiency anemia [
16,
59]. Early clinical trials have demonstrated that iron supplementation can elevate serum transferrin and increase erythropoiesis in critically ill patients, so transfusion of red cells and supplementation with iron are the usual therapeutic strategies [
60]. Recently, Litton et al. also reported that combined supplementation of iron and erythropoietin could promote the recovery of intensive care patients with anemia (hemoglobin <100 g/L) [
61]. Conversely, a multicenter randomized trial in trauma patients found that iron supplementation significantly increased the serum ferritin concentration, but had no effect on transferrin saturation, iron-deficient erythropoiesis, hemoglobin concentration, or packed RBC transfusion requirement [
62].
5.2. Cognitive Dysfunction
Cognitive dysfunction is common in survivors of critical illness, affecting more than 25% of patients and often persisting after physical recovery [
63]. For example, in a prospective trial including 821 patients with respiratory failure or shock, cognitive and functional status was assessed at 3 and 12 months after discharge. The results showed that among survivors of critical illness at 3 months post-discharge, 40% of patients’ scores are comparable to scores seen in patients after mild to moderate traumatic brain injury, and 26% is comparable to scores of patients with mild Alzheimer’s disease. The effect persisted at those levels in 34% and 24% of patients at 12 months [
64]. Although the pathophysiology of cognitive dysfunction after critical illness is multifactorial and the exact mechanism is not very clear and needs more investigations, the lack of iron is a potentially important contributing factor. It was reported that iron deficiency is associated with poor health and severe neurological impairment such as mental, motor, social, emotional, neurophysiological, and neurocognitive dysfunction [
65]. The mechanisms of how iron deficiency affects behavior include changes in the hippocampus, the corpus striatum, certain neurotransmitters, redox balance, and myelination [
66]. Especially iron is essential for neurotransmitter synthesis, uptake, and degradation and is necessary for mitochondrial function in metabolically active brain tissue [
67]. Some researchers also considered that in line with dopaminergic dysfunction, iron deficiency has the same effect on cerebral and behavioral, such as poor inhibitory control and diminished executive and motor function [
68]. Although some research data shows that iron supplementation is associated with improved quality-of-life and cognitive function for iron deficiency patients [
64,
65], studies on the effects of iron supplementation on cognitive outcomes in patients recovering from critical illness are currently lacking and will need to consider the optimal timing, dose, and duration of therapy.
5.3. Fatigue
Critical illness fatigue is a frequent symptom of prolonged acute illness. For example, a prospective study among a broader population of ICU survivors reported a high prevalence of fatigue at 12-month follow-up among medical, urgent surgery, and elective surgery ICU survivors as follows: 36%, 45%, and 24%, respectively [
69]. In addition, critical illness myopathy or weakness is often prolonged with many patients experiencing decreased exercise capacity and compromised quality of life years after the acute event [
70]. Among the complex variety of causes, it was reported that iron deficiency has been associated with fatigue and muscular weakness and may thus impair post-ICU rehabilitation [
71]. In a prospective observational study in cardiac surgery patients, the score of physical fatigue in iron deficiency patients on day 7 was higher than non-iron deficiency patients [
72]. However, some animal studies and clinical reports indicated that the relationship between iron deficiency and fatigue seems independent of anemia [
73,
74,
75]. For example, Zhou et al. revealed that through positively regulating skeletal muscle-specific mitochondrial biogenesis and energy production, long-term iron supplementation in combination with vitamin B6 led to less body weight gained and increased maximal oxygen uptake in rats [
76]. Therefore, giving the catabolic state conferred by critical illness and the role of iron in myoglobin and muscle oxidative metabolism, further evaluation of the relationship between iron deficiency and critical illness fatigue may provide insight into the role of iron supplementation in improving physical recovery after acute severe illness. Furthermore, because physiological iron uptake is quite low (approximately 1 to 2 mg/day), correction of iron deficiency after an ICU stay should be prolonged [
1].
5.4. Cardiopulmonary Dysfunction
It is well-known that iron is an essential co-factor for the normal functioning of many enzymes participating in vital cellular and organismal functions, particularly iron is very important for some high energy demand cells, such as cardiomyocytes, hepatocytes and skeletal cells [
77]. As a necessary co-factor for hypoxia-inducible factor (HIF), iron combined with HIF can also mediate the systemic cardiovascular response to hypoxia. Therefore, iron plays an important role in cardiopulmonary recovery in critically ill patients.
Independent of anemia, iron deficiency is associated with an increased risk of death in patients with heart failure [
78]. Although the underlying causes of iron deficiency in heart failure are poorly characterized, several factors have gained attention to explain the high prevalence of iron deficiency in heart failure, including advanced age, kidney failure, female gender, malnutrition, chronic inflammation, reduced iron absorption, increased iron loss, and heart failure severity [
77]. Recently, Mordi et al. demonstrated that in chronic heart failure, clinical outcomes were worse in non-anemic iron deficiency patients than in anemic iron-repleted patients, suggesting that iron deficiency can affect the heart directly and in a manner that is different from the effects of anemia [
79]. Results from animal experiments are similar. E.g., in a mouse model lacking cardiomyocyte transferrin receptor 1 (TfR1), severely reduced iron levels in the cardiomyocytes resulted in fatal heart failure by the second week of age, in part due to failure of mitochondrial respiration [
80]. Another study showed that critical illness may impair cardiac iron availability through a variety of mechanisms, including catecholamine-induced downregulation of myocardial TfR1, and upregulation of hepcidin [
81]. Importantly, high-quality evidence has suggested that intravenous iron for patients with systolic heart failure improved exercise capacity, survival, and quality of life [
82]. However, it was also reported that among participants with heart failure and iron deficiency, high-dose oral iron did not improve exercise capacity over 16 weeks [
83]. Therefore, larger controlled studies are required to confirm iron supplementation’s effectiveness in the rehabilitation of heart failure patients with iron deficiency.
Iron deficiency is also present in 33%-46% of patients with pulmonary artery hypertension (PAH), which is common in patients admitted to the ICU with severe acute respiratory distress syndrome [
84]. Moreover, PAH has been associated with reduced exercise capacity, compromised oxygen handling, deterioration of right ventricular function, and even mortality [
85]. Both oral and intravenous iron supplementation in patients with PAH provided better clinical outcomes [
84]. In a retrospective study, researchers analyzed the long-term effects of iron supplementation with ferric carboxymaltose (FCM) on iron status and clinical parameters in patients with PAH and iron deficiency. The results showed a significant improvement in exercise capacity and World Health Organization functional class. Hospitalizations for worsening PAH over 12 months were also significantly reduced [
86].
6. Potential Therapeutics for Iron Deficiency in the Recovery Phase from Critically Illness
Due to its effectiveness, safety, and low cost, oral iron, such as ferrous sulfate, is the first-line treatment for most patients with iron deficiency. However, the oral drug delivery route presents a number of disadvantages in critically ill patients, such as bad absorption of iron and a high incidence of gastrointestinal adverse reactions [
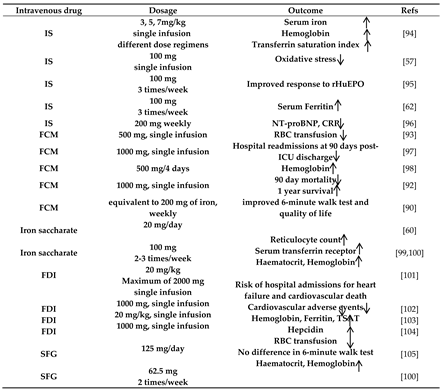
87]. Moreover, intravenous iron supplementation can accomplish the need for a fast replenishment of large demand. Therefore, parenteral iron administration is common in clinical practice to overcome limitations and risks related to oral iron. Several formulations for intravenous administration are being used: high and low molecular weight iron dextran, iron gluconate (IG), iron sucrose (IS), iron ferrumoxytol (FO), ferric carboxymaltose (FCM) and ferric derisomaltose (FDI) [
88]. Although these intravenous preparations have the same structure, the size of the core and density of the surrounding carbohydrate significantly differ from each other. The latter three (FCM, FO, FDI) are new-generation products with pharmacokinetic parameters allowing for a single high-dose iron administration without the need for a test dose. Several studies revealed that FCM is the most successful treatment for iron deficiency and iron deficiency related anemia [
87,
89,
90,
91].
Because increased levels of iron have been shown to promote bacterial growth in vitro, there is a significant concern about the risk of infection with using iron in critically ill patients. In a multicenter, interventional study, the results revealed that iron infusion by intravenous did not induce more nontransferrin-bound iron, lipid, or protein oxidation in patients with anemia compared with volunteers. Conversely, iron administration decreased antioxidant levels, compatible with higher oxidative stress in volunteers than in critically ill patients [
57]. Moreover, intravenous FCM for patients with cardiac failure showed improved functional capacity and quality of life with no increased infection [
90]. A systematic review and network meta-analysis of randomized controlled trials showed high-quality evidence regarding the efficacy and safety of available iron formulations, suggesting that all currently available intravenous iron preparations appear to be safe and effective, but FCM seems to provide a better benefit for iron deficiency patients [
87]. Therefore, during the recovery phase of critically ill patients with iron deficiency, considering iron dysmetabolism may be of substantial therapeutic benefit in improving functional recovery with the right type of drug, the best route and optimal timing of administration. Intravenous iron formulaitons used in critically ill patients are summarized in
Table 1.
For the optimal timing of iron therapy, precise diagnosis of iron deficiency in critically ill patients during convalescence is important. As the tests routinely used to screen for iron deficiency, such as ferritin, transferrin and TSAT, are confounded by the presence of inflammation, iron deficiency diagnosis is challenging. As a key regulator of iron metabolism, serum hepcidin has recently opened avenues for improved diagnosis and management of anemia in critically ill patients. It was confirmed that if iron deficiency were diagnosed at low hepcidin concentration (< 20 ng/l) at ICU discharge, there would be a significant association with increased one-year mortality [
48]. And importantly, hepcidin levels would accurately guide the treatment of iron deficiency in critically ill anemic patients after a prolonged ICU stay and affect the post-ICU outcomes [
92]. Furthermore, a prospective observational study demonstrated that serum hepcidin concentration can be used to predict the responsiveness to iron therapy in critically ill patients with anemia, and intravenous iron supplementation can decrease the RBC transfusion requirement [
93]. Therefore, it is recommended that serum hepcidin may provide a better marker of iron deficiency than the routine biochemical tests in future use.
7. Conclusions
In summary, critical illness can exacerbate pre-existing iron deficits since iron absorption and recycling is reduced and stored iron is less accessible for use. Iron deficiency is common in the recovery phase of critically ill patients. Although low levels of serum iron may be beneficial in the early phase of critical illness, persistent iron deficiency may lead to iron dysmetabolism in which reduced iron availability contributes to impaired end-organ function. Therefore, iron supplementation may substantially benefit patients with prolonged ICU stay in improving functional recovery. Recent studies suggest that hepcidin levels can accurately guide the treatment of iron deficiency in critically ill patients after a prolonged ICU stay and affect post-ICU outcomes. However, iron administration's optimal dosing and timing in critically ill patients require further studies.
Author Contributions
Conceptualization, C.L., X.Y.Z.; software, X.Y.Z.; validation, J.Z, B.E.H. and C.L.; writing—original draft preparation, X.Y.Z.; writing—review and editing, X.Y.Z., J.Z. and C.L.; supervision, C.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Litton, E.; Lim, J. Iron Metabolism: An Emerging Therapeutic Target in Critical Illness. Crit. Care 2019, 23, 81. [Google Scholar] [CrossRef]
- Shah, A.; Frost, J.N.; Aaron, L.; Donovan, K.; Drakesmith, H. Systemic hypoferremia and severity of hypoxemic respiratory failure in COVID-19. Crit. Care 2020, 24, 1–4. [Google Scholar] [CrossRef]
- Jabara, H.H.; Boyden, S.E.; Chou, J.; Ramesh, N.; Massaad, M.J.; Benson, H.; Bainter, W.; Fraulino, D.; Rahimov, F.; Sieff, C.A.; et al. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat. Genet. 2015, 48, 74–78. [Google Scholar] [CrossRef]
- Bellamy, M.; Gedney, J. Unrecognised iron deficiency in critical illness. Lancet 1998, 352, 1903–1903. [Google Scholar] [CrossRef]
- Aksan, A.; Farrag, K.; Aksan, S.; Schroeder, O.; Stein, J. Flipside of the Coin: Iron Deficiency and Colorectal Cancer. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Grange, C.; Lux, F.; Brichart, T.; David, L.; Couturier, A.; Leaf, D.E.; Allaouchiche, B.; Tillement, O. Iron as an emerging therapeutic target in critically ill patients. Crit. Care 2023, 27, 1–14. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Holbein, B.E.; Lehmann, C. Iron Chelation as a Potential Therapeutic Approach in Acute Lung Injury. Life 2023, 13, 1659. [Google Scholar] [CrossRef]
- Haschka, D.; Hoffmann, A.; Weiss, G. Iron in immune cell function and host defense. Semin. Cell Dev. Biol. 2020, 115, 27–36. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef]
- Kühn, L.C. Iron regulatory proteins and their role in controlling iron metabolism. Metallomics 2015, 7, 232–243. [Google Scholar] [CrossRef]
- Vincent, J.L.; Baron, J.F.; Reinhart, K.; Gattinoni, L.; Thijs, L.; Webb, A.; Meier-Hellmann, A.; Nollet, G.; Peres-Bota, D.; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA 2002, 288, 1499–1507. [Google Scholar] [CrossRef]
- Corwin, H.L.; Gettinger, A.; Pearl, R.G.; Fink, M.P.; Levy, M.M.; Abraham, E.; MacIntyre, N.R.; Shabot, M.M.; Duh, M.-S.; Shapiro, M.J. The CRIT Study: Anemia and blood transfusion in the critically ill—Current clinical practice in the United States*. Crit. Care Med. 2004, 32, 39–52. [Google Scholar] [CrossRef]
- Piagnerelli M, Vincent JL. Role of iron in anaemic critically ill patients: it's time to investigate! Critical care (London, England) 2004, 8, 306–307.
- Patteril, M.V.; Davey-Quinn, A.P.; Gedney, J.A.; Murdoch, S.D.; Bellamy, M.C. Functional Iron Deficiency, Infection and Systemic Inflammatory Response Syndrome in Critical Illness. Anaesth. Intensiv. Care 2001, 29, 473–478. [Google Scholar] [CrossRef]
- Făgărășan, V.; Andraș, D.; Amarinei, G.; Seicean, R.I.; Bințințan, V.V.; Dindelegan, G.C.; Căinap, C.I. Absolute and Functional Iron Deficiency in Colon Cancer: A Cohort Study. Medicina 2022, 58, 1202. [Google Scholar] [CrossRef]
- Zuther, M.; Rübsam, M.-L.; Zimmermann, M.; Zarbock, A.; Hönemann, C. Improved Diagnosis of Iron Deficiency Anemia in the Critically Ill via Fluorescence Flowcytometric Hemoglobin Biomarkers. Cells 2022, 12, 140. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, J.; Zhang, X.; Wu, X.; Zhao, Y.; Ren, J. Iron homeostasis and disorders revisited in the sepsis. Free. Radic. Biol. Med. 2021, 165, 1–13. [Google Scholar] [CrossRef]
- Tacke, F.; Nuraldeen, R.; Koch, A.; Strathmann, K.; Hutschenreuter, G.; Trautwein, C.; Strnad, P. Iron Parameters Determine the Prognosis of Critically Ill Patients*. Crit. Care Med. 2016, 44, 1049–1058. [Google Scholar] [CrossRef]
- Pietrangelo A, Caleffi A, Corradini E. Non-HFE hepatic iron overload. Seminars in liver disease 2011, 31, 302–318.
- Heming, N.; Montravers, P.; Lasocki, S. Iron deficiency in critically ill patients: highlighting the role of hepcidin. Crit. Care 2011, 15, 210–7. [Google Scholar] [CrossRef]
- Retter, A.; Wyncoll, D.; Pearse, R.; Carson, D.; McKechnie, S.; Stanworth, S.; Allard, S.; Thomas, D.; Walsh, T. ; British Comm Stand British Committee for Standards in Haematology Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br. J. Haematol. 2012, 160, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Huang, J.; Dai, D.; Feng, Y.; Liu, L.; Nie, S. Serum Iron Level as a Potential Predictor of Coronavirus Disease 2019 Severity and Mortality: A Retrospective Study. Open Forum Infect. Dis. 2020, 7, ofaa250. [Google Scholar] [CrossRef] [PubMed]
- Tojo, K.; Sugawara, Y.; Oi, Y.; Ogawa, F.; Higurashi, T.; Yoshimura, Y.; Miyata, N.; Hayami, H.; Yamaguchi, Y.; Ishikawa, Y.; et al. The U-shaped association of serum iron level with disease severity in adult hospitalized patients with COVID-19. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Pan, K.-H.; Wang, S.-J.; Shi, Q.-C.; Fu, Y.; Chen, Y.; Jiang, Y.; Hua, X.-T.; Zhou, J.-C.; Yu, Y.-S. High Serum Iron level is Associated with Increased Mortality in Patients with Sepsis. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Chakurkar, V.; Rajapurkar, M.; Lele, S.; Mukhopadhyay, B.; Lobo, V.; Injarapu, R.; Sheikh, M.; Dholu, B.; Ghosh, A.; Jha, V. Increased serum catalytic iron may mediate tissue injury and death in patients with COVID-19. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lele S, Shah S, McCullough PA, Rajapurkar M. Serum catalytic iron as a novel biomarker of vascular injury in acute coronary syndromes. EuroIntervention : Journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 2009, 5, 336–342.
- Leaf DE, Rajapurkar M, Lele SS; et al. Iron, Hepcidin, and Death in Human AKI. Journal of the American Society of Nephrology : JASN 2019, 30, 493–504.
- Lele, S.S.; Mukhopadhyay, B.N.; Mardikar, M.M.; Patel, T.A.; Vasavada, A.K.; Banker, D.N.; Kapasi, K.D.; Chauhan, V.C.; Chawla, K.C.; Raju, S.R.; et al. Impact of catalytic iron on mortality in patients with acute coronary syndrome exposed to iodinated radiocontrast—The Iscom Study. Am. Hear. J. 2013, 165, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Suriawinata, E.; Mehta, K.J. Iron and iron-related proteins in COVID-19. Clin. Exp. Med. 2022, 23, 969–991. [Google Scholar] [CrossRef]
- Garcia-Casal MN, Pasricha SR, Martinez RX, Lopez-Perez L, Peña-Rosas JP. Are Current Serum and Plasma Ferritin Cut-offs for Iron Deficiency and Overload Accurate and Reflecting Iron Status? A Systematic Review. Archives of medical research 2018, 49, 405–417.
- Rusu, D.; Blaj, M.; Ristescu, I.; Patrascanu, E.; Gavril, L.; Lungu, O.; Siriopol, I.; Buzincu, I.; Grigoraș, I. Outcome Predictive Value of Serum Ferritin in ICU Patients with Long ICU Stay. Medicina 2020, 57, 1. [Google Scholar] [CrossRef] [PubMed]
- Moen, I.W.; Bergholdt, H.K.; Mandrup-Poulsen, T.; Nordestgaard, B.G.; Ellervik, C. Increased Plasma Ferritin Concentration and Low-Grade Inflammation—A Mendelian Randomization Study. Clin. Chem. 2018, 64, 374–385. [Google Scholar] [CrossRef]
- Gerday, E.; Brereton, J.B.; Bahr, T.M.; Elmont, J.O.; Fullmer, S.; Middleton, B.A.; Ward, D.M.; Ohls, R.K.; Christensen, R.D. Urinary ferritin; a potential noninvasive way to screen NICU patients for iron deficiency. J. Perinatol. 2021, 41, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Boehm, A.; Sahanic, S.; Pizzini, A.; Aichner, M.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: a prospective observational cohort study. Respir. Res. 2020, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yagci, S.; Serin, E.; Acicbe, O.; Zeren, M.I.; Odabasi, M.S. The relationship between serum erythropoietin, hepcidin, and haptoglobin levels with disease severity and other biochemical values in patients with COVID-19. Int. J. Lab. Hematol. 2021, 43, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Zhou C, Chen Y, Ji Y, He X, Xue D. Increased Serum Levels of Hepcidin and Ferritin Are Associated with Severity of COVID-19. Medical science monitor : International medical journal of experimental and clinical research 2020, 26:e926178.
- Dahan, S.; Segal, G.; Katz, I.; Hellou, T.; Tietel, M.; Bryk, G.; Amital, H.; Shoenfeld, Y.; Dagan, A. Ferritin as a Marker of Severity in COVID-19 Patients: A Fatal Correlation. IMAJ 2020, 22, 494–500. [Google Scholar] [PubMed]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 28;395(10229):1054-1062. [CrossRef]
- Ruscitti P, Berardicurti O, Barile A; et al. Severe COVID-19 and related hyperferritinaemia: More than an innocent bystander? Annals of the rheumatic diseases 2020, 79, 1515–1516.
- Gomes, A.C.; Moreira, A.C.; Mesquita, G.; Gomes, M.S. Modulation of Iron Metabolism in Response to Infection: Twists for All Tastes. Pharmaceuticals 2018, 11, 84. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Ruddell RG, Hoang-Le D, Barwood JM; et al. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology (Baltimore, Md) 2009, 49, 887–900.
- Bianconi, V.; Mannarino, M.R.; Figorilli, F.; Cosentini, E.; Batori, G.; Marini, E.; Banach, M.; Sahebkar, A.; Pirro, M. The detrimental impact of elevated Ferritin to Iron ratio on in-hospital prognosis of patients with COVID-19. Expert Rev. Mol. Diagn. 2022, 22, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.T.; Attwood, M.; Barratt, S.; Morley, A.; Elvers, K.T.; McKernon, J.; Donald, C.; Oates, A.; Noel, A.; MacGowan, A.; et al. Predicting outcomes of COVID-19 from admission biomarkers: a prospective UK cohort study. Emerg. Med. J. 2021, 38, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Elhadi, M.; Alsoufi, A.; Abusalama, A.; Alkaseek, A.; Abdeewi, S.; Yahya, M.; Mohammed, A.; Abdelkabir, M.; Huwaysh, M.; Amkhatirah, E.; et al. Epidemiology, outcomes, and utilization of intensive care unit resources for critically ill COVID-19 patients in Libya: A prospective multi-center cohort study. PLOS ONE 2021, 16, e0251085. [Google Scholar] [CrossRef] [PubMed]
- Cherry-Bukowiec JR, Engoren M, Wiktor A, Raghavendran K, Napolitano LM. Hepcidin and Anemia in Surgical Critical Care: A Prospective Cohort Study. Critical care medicine 2018, 46, e567–e574.
- Lasocki, S.; Lefebvre, T.; Mayeur, C.; Puy, H.; Mebazaa, A.; Gayat, E. Iron deficiency diagnosed using hepcidin on critical care discharge is an independent risk factor for death and poor quality of life at one year: an observational prospective study on 1161 patients. Crit. Care 2018, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Eisenga, M.F. Iron Deficiency Defined by Hepcidin in Critically Ill Patients. Crit. Care 2021, 25, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Nai, A.; Lore, N.I.; Pagani, A.; De Lorenzo, R.; Di Modica, S.; Saliu, F.; Cirillo, D.M.; Rovere-Querini, P.; Manfredi, A.A.; Silvestri, L. Hepcidin levels predict Covid-19 severity and mortality in a cohort of hospitalized Italian patients. Am. J. Hematol. 2021, 96, E32. [Google Scholar] [CrossRef] [PubMed]
- Brandtner, A.; Tymoszuk, P.; Nairz, M.; Lehner, G.F.; Fritsche, G.; Vales, A.; Falkner, A.; Schennach, H.; Theurl, I.; Joannidis, M.; et al. Linkage of alterations in systemic iron homeostasis to patients' outcome in sepsis: a prospective study. J. Intensiv. Care 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Maras, J.S.; Maiwall, R.; Harsha, H.; Das, S.; Hussain, M.; Kumar, C.; Bihari, C.; Rastogi, A.; Kumar, M.; Trehanpati, N.; et al. Dysregulated iron homeostasis is strongly associated with multiorgan failure and early mortality in acute-on-chronic liver failure. Hepatology 2014, 61, 1306–1320. [Google Scholar] [CrossRef]
- Bruns, T.; Nuraldeen, R.; Mai, M.; Stengel, S.; Zimmermann, H.W.; Yagmur, E.; Trautwein, C.; Stallmach, A.; Strnad, P. Low serum transferrin correlates with acute-on-chronic organ failure and indicates short-term mortality in decompensated cirrhosis. Liver Int. 2016, 37, 232–241. [Google Scholar] [CrossRef]
- Maiwall, R.; Kumar, S.; Chaudhary, A.; Maras, J.; Wani, Z.; Kumar, C.; Rastogi, A.; Bihari, C.; Vashisht, C.; Sarin, S. Serum ferritin predicts early mortality in patients with decompensated cirrhosis. J. Hepatol. 2014, 61, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Hippchen, T.; Altamura, S.; Muckenthaler, M.U.; Merle, U. Hypoferremia is Associated With Increased Hospitalization and Oxygen Demand in COVID-19 Patients. HemaSphere 2020, 4, e492. [Google Scholar] [CrossRef] [PubMed]
- Bolondi, G.; Russo, E.; Gamberini, E.; Circelli, A.; Meca, M.C.C.; Brogi, E.; Viola, L.; Bissoni, L.; Poletti, V.; Agnoletti, V. Iron metabolism and lymphocyte characterisation during Covid-19 infection in ICU patients: an observational cohort study. World J. Emerg. Surg. 2020, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lasocki, S.; Piednoir, P.; Couffignal, C.; Rineau, E.; Dufour, G.; Lefebvre, T.; Puy, H.; Duval, X.; Driss, F.; Schilte, C. Does IV Iron Induce Plasma Oxidative Stress in Critically Ill Patients? A Comparison With Healthy Volunteers*. Crit. Care Med. 2016, 44, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Fuchs, D.; Kurz, K.; Weiss, G. Physiology and Inflammation Driven Pathophysiology of Iron Homeostasis—Mechanistic Insights into Anemia of Inflammation and Its Treatment. Nutrients 2021, 13, 3732. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D. Anemia in the ICU: Anemia of chronic disease versus anemia of acute illness. Critical care clinics 2012, 28, 333–343, v. [Google Scholar] [CrossRef]
- van Iperen, C.E.; Gaillard, C.A.J.M.; Kraaijenhagen, R.J.; Braam, B.G.; Marx, J.J.M.; van de Wiel, A. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit. Care Med. 2000, 28, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Litton, E.; French, C.; Herschtal, A.; Stanworth, S.; Pellicano, S.; Palermo, A.M.; Bates, S.; Van Der Laan, S.; Eroglu, E.; Griffith, D.; et al. Iron and erythropoietin to heal and recover after intensive care (ITHRIVE): A pilot randomised clinical trial. Crit. Care Resusc. 2023, 25, 201–206. [Google Scholar] [CrossRef]
- Pieracci, F.M.; Stovall, R.T.; Jaouen, B.; Rodil, M.; Cappa, A.; Burlew, C.C.; Holena, D.N.; Maier, R.; Berry, S.; Jurkovich, J.; et al. A Multicenter, Randomized Clinical Trial of IV Iron Supplementation for Anemia of Traumatic Critical Illness*. Crit. Care Med. 2014, 42, 2048–2057. [Google Scholar] [CrossRef]
- Pandharipande, P.; Girard, T.; Jackson, J.; Morandi, A.; Thompson, J.; Pun, B.; Brummel, N.; Hughes, C.; Vasilevskis, E.; Shintani, A.; et al. Long-Term Cognitive Impairment after Critical Illness. New Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Peri, V.; Devlin, P.; Perry, L.; Richards, T.; Miles, L.F. Associations Between Nonanemic Iron Deficiency and Postoperative Outcomes in Cardiac Surgery: A Systematic Review and Meta-Analysis. Obstet. Anesthesia Dig. 2024. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.E.; Bhawnani, N.; Ethirajulu, A.; Alkasabera, A.; Onyali, C.B.; Anim-Koranteng, C.; Mostafa, J.A. Iron Deficiency-Induced Changes in the Hippocampus, Corpus Striatum, and Monoamines Levels That Lead to Anxiety, Depression, Sleep Disorders, and Psychotic Disorders. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Wu, Q.; Ren, Q.; Meng, J.; Gao, W.-J.; Chang, Y.-Z. Brain Iron Homeostasis and Mental Disorders. Antioxidants 2023, 12, 1997. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Yehuda, S. The neurochemical basis of cognitive deficits induced by brain iron deficiency: involvement of dopamine-opiate system. Cellular and molecular biology 2000, 46, 491–500. [Google Scholar] [PubMed]
- Lozoff, B. Early Iron Deficiency Has Brain and Behavior Effects Consistent with Dopaminergic Dysfunction. J. Nutr. 2011, 141, 740S–746S. [Google Scholar] [CrossRef]
- Geense WW, Zegers M, Peters MAA; et al. New Physical, Mental, and Cognitive Problems 1 Year after ICU Admission: A Prospective Multicenter Study. American journal of respiratory and critical care medicine 2021, 203, 1512–1521.
- Guarneri, B.; Bertolini, G.; Latronico, N. Long-term outcome in patients with critical illness myopathy or neuropathy: the Italian multicentre CRIMYNE study. J. Neurol. Neurosurg. Psychiatry 2008, 79, 838–841. [Google Scholar] [CrossRef]
- Lasocki, S.; Chudeau, N.; Papet, T.; Tartiere, D.; Roquilly, A.; Carlier, L.; Mimoz, O.; Seguin, P.; Malledant, Y.; Asehnoune, K. Prevalence of iron deficiency on ICU discharge and its relation with fatigue: a multicenter prospective study. Crit. Care 2014, 18, 542. [Google Scholar] [CrossRef] [PubMed]
- Piednoir P, Allou N, Driss F; et al. Preoperative iron deficiency increases transfusion requirements and fatigue in cardiac surgery patients: A prospective observational study. European journal of anaesthesiology 2011, 28, 796–801.
- Finch, C.A.; Miller, L.R.; Inamdar, A.R.; Person, R.; Seiler, K.; Mackler, B. Iron deficiency in the rat. Physiological and biochemical studies of muscle dysfunction. J. Clin. Investig. 1976, 58, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Willis, W.T.; Gohil, K.; Brooks, G.A.; Dallman, P.R. Iron Deficiency: Improved Exercise Performance within 15 Hours of Iron Treatment in Rats. J. Nutr. 1990, 120, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Krayenbuehl, P.-A.; Battegay, E.; Breymann, C.; Furrer, J.; Schulthess, G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 2011, 118, 3222–3227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Mozaffaritabar, S.; Kolonics, A.; Kawamura, T.; Koike, A.; Kéringer, J.; Gu, Y.; Karabanov, R.; Radák, Z. Long-term iron supplementation combined with vitamin B6 enhances maximal oxygen uptake and promotes skeletal muscle-specific mitochondrial biogenesis in rats. Front. Nutr. 2024, 10, 1335187. [Google Scholar] [CrossRef] [PubMed]
- Alnuwaysir, R.I.S.; Hoes, M.F.; van Veldhuisen, D.J.; van der Meer, P.; Beverborg, N.G. Iron Deficiency in Heart Failure: Mechanisms and Pathophysiology. J. Clin. Med. 2021, 11, 125. [Google Scholar] [CrossRef]
- Jankowska, E.A.; Kasztura, M.; Sokolski, M.; Bronisz, M.; Nawrocka, S.; Kowska-Florek, W.O.; Ski, R.Z.; Biegus, J.; Owski, P.S.; Banasiak, W.; et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur. Hear. J. 2014, 35, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Mordi IR, Tee A, Lang CC. Iron Therapy in Heart Failure: Ready for Primetime? Cardiac failure review 2018, 4, 28–32.
- Xu, W.; Barrientos, T.; Mao, L.; Rockman, H.A.; Sauve, A.A.; Andrews, N.C. Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart. Cell Rep. 2015, 13, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Khammy, O.; dos Remedios, C.; Kaye, D.M. Myocardial and Systemic Iron Depletion in Heart Failure: Implications for Anemia Accompanying Heart Failure. Circ. 2011, 58, 474–480. [Google Scholar] [CrossRef]
- Jankowska, E.A.; Tkaczyszyn, M.; Suchocki, T.; Drozd, M.; von Haehling, S.; Doehner, W.; Banasiak, W.; Filippatos, G.; Anker, S.D.; Ponikowski, P. Effects of intravenous iron therapy in iron-deficient patients with systolic heart failure: a meta-analysis of randomized controlled trials. Eur. J. Hear. Fail. 2016, 18, 786–795. [Google Scholar] [CrossRef]
- Lewis GD, Malhotra R, Hernandez AF; et al. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF Randomized Clinical Trial. Jama 2017, 317, 1958–1966.
- Lan M, Wu S, Fernandes TM. Iron deficiency and pulmonary arterial hypertension. Nutrition in clinical practice: Official publication of the American Society for Parenteral and Enteral Nutrition 2022, 37, 1059–1073.
- Zochios, V.; Parhar, K.; Tunnicliffe, W.; Roscoe, A.; Gao, F. The Right Ventricle in ARDS. Chest 2017, 152, 181–193. [Google Scholar] [CrossRef]
- Kramer, T.; Wissmüller, M.; Natsina, K.; Gerhardt, F.; Freyhaus, H.T.; Dumitrescu, D.; Viethen, T.; Hellmich, M.; Baldus, S.; Rosenkranz, S. Ferric carboxymaltose in patients with pulmonary arterial hypertension and iron deficiency: a long-term study. J. Cachex- Sarcopenia Muscle 2021, 12, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, C.; Venturini, S.; Meregaglia, M.; Marmifero, M.; Tarricone, R. Efficacy and Safety of Ferric Carboxymaltose and Other Formulations in Iron-Deficient Patients: A Systematic Review and Network Meta-analysis of Randomised Controlled Trials. Clin. Drug Investig. 2015, 36, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, A.; Lubas, A.; Fiedor, P.; Fiedor, M.; Niemczyk, S. SAFETY AND EFFICACY OF INTRAVENOUS ADMINISTRATION OF IRON PREPARATIONS. . 2017, 74, 13–24. [Google Scholar] [PubMed]
- Boots, J.M.M.; Quax, R.A.M. High-Dose Intravenous Iron with Either Ferric Carboxymaltose or Ferric Derisomaltose: A Benefit-Risk Assessment. Drug Saf. 2022, 45, 1019–1036. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef]
- Blumenstein I, Shanbhag S, Langguth P, Kalra PA, Zoller H, Lim W. Newer formulations of intravenous iron: A review of their chemistry and key safety aspects - hypersensitivity, hypophosphatemia, and cardiovascular safety. Expert opinion on drug safety 2021, 20, 757–769.
- Lasocki, S.; Asfar, P.; Jaber, S.; Ferrandiere, M.; Kerforne, T.; Asehnoune, K.; Montravers, P.; Seguin, P.; Peoc’h, K.; Gergaud, S.; et al. Impact of treating iron deficiency, diagnosed according to hepcidin quantification, on outcomes after a prolonged ICU stay compared to standard care: a multicenter, randomized, single-blinded trial. Crit. Care 2021, 25, 1–10. [Google Scholar] [CrossRef]
- Litton, E.; on behalf of the IRONMAN Study investigators; Baker, S. ; Erber, W.; Farmer, S.; Ferrier, J.; French, C.; Gummer, J.; Hawkins, D.; Higgins, A.; et al. Hepcidin predicts response to IV iron therapy in patients admitted to the intensive care unit: a nested cohort study. J. Intensiv. Care 2018, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Butragueño-Laiseca L, de la Mata Navazo S, Sánchez Galindo AC, Santiago Lozano MJ. Intravenous iron for critically ill children. Comparison of three dose regimens. Pediatric blood & cancer 2024, 71, e30734.
- Muñoz, M.; Breymann, C.; García-Erce, J.A.; Gómez-Ramírez, S.; Comin, J.; Bisbe, E. Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox Sang. 2007, 94, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; Lombraña, A.; Duarte, P.; Di Gennaro, F. Intravenous Iron Reduces NT-Pro-Brain Natriuretic Peptide in Anemic Patients With Chronic Heart Failure and Renal Insufficiency. Circ. 2007, 50, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Chester-Jones, M.; Dutton, S.J.; Marian, I.R.; Barber, V.S.; Griffith, D.M.; Singleton, J.; Wray, K.; James, T.; Drakesmith, H.; et al. Intravenous iron to treat anaemia following critical care: a multicentre feasibility randomised trial. Br. J. Anaesth. 2021, 128, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Litton E, Baker S, Erber WN; et al. Intravenous iron or placebo for anaemia in intensive care: The IRONMAN multicentre randomized blinded trial: A randomized trial of IV iron in critical illness. Intensive care medicine 2016, 42, 1715–1722.
- Georgopoulos, D.; Matamis, D.; Routsi, C.; Michalopoulos, A.; Maggina, N.; Dimopoulos, G.; Zakynthinos, E.; Nakos, G.; Thomopoulos, G.; Mandragos, K.; et al. Recombinant human erythropoietin therapy in critically ill patients: a dose-response study [ISRCTN48523317]. Crit. Care 2005, 9, R508–R515. [Google Scholar] [CrossRef]
- Sheashaa, H.; El-Husseini, A.; Sabry, A.; Hassan, N.; Salem, A.; Khalil, A.; El-Agroudy, A.; Sobh, M. Parenteral Iron Therapy in Treatment of Anemia in End-Stage Renal Disease Patients: A Comparative Study between Iron Saccharate and Gluconate. Nephron Clin. Pr. 2005, 99, c97–c101. [Google Scholar] [CrossRef] [PubMed]
- Kalra, P.R.; Cleland, J.G.F.; Petrie, M.C.; A Thomson, E.; A Kalra, P.; Squire, I.B.; Ahmed, F.Z.; Al-Mohammad, A.; Cowburn, P.J.; Foley, P.W.X.; et al. Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): an investigator-initiated, prospective, randomised, open-label, blinded-endpoint trial. Lancet 2022, 400, 2199–2209. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; von Haehling, S.; Kalra, P.R.; Court, E.; Bhandari, S.; McDonagh, T.; Cleland, J.G. Safety and Efficacy of Intravenous Ferric Derisomaltose Compared to Iron Sucrose for Iron Deficiency Anemia in Patients with Chronic Kidney Disease With and Without Heart Failure. Am. J. Cardiol. 2021, 152, 138–145. [Google Scholar] [CrossRef]
- Song JW, Soh S, Shim JK; et al. Effect of Perioperative Intravenous Iron Supplementation for Complex Cardiac Surgery on Transfusion Requirements: A Randomized, Double-blinded Placebo-controlled Trial. Annals of surgery 2022, 275, 232–239.
- Kong, R.; Hutchinson, N.; Hill, A.; Ingoldby, F.; Skipper, N.; Jones, C.; Bremner, S.; Bruce, C.; Wright, J.; Lewis, M.; et al. Randomised open-label trial comparing intravenous iron and an erythropoiesis-stimulating agent versus oral iron to treat preoperative anaemia in cardiac surgery (INITIATE trial). Br. J. Anaesth. 2022, 128, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Marcusohn E, Borreda I, Hellman Y; et al. IV Sodium Ferric Gluconate Complex in Patients With Iron Deficiency Hospitalized due to Acute Heart Failure-Investigator Initiated, Randomized Controlled Trial. Journal of cardiovascular pharmacology 2022, 80, 194–196.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).