1. Introduction
Atlanto-occipital dislocation (AOD) is delineated as a critical and infrequent disorder at the cranio-cervical junction, characterized by the dislocation of the occiput from the spinal column, resulting in a profoundly unstable ligamentous injury [
1,
2,
3]. This condition, while rare in dogs and cats, emerges due to the failure of the inherent stability mechanisms at the cranio-cervical junction, while typically prevent such dislocations [
4]. Since the occipital region of the skull and the first two cervical vertebrae develop together embryologically, AOD frequently manifests concomitantly with other cranio-cervical junction anomaly (CCJA) including atlanto-axial instability (AAI), atlanto-occipital overlapping and Chiari-like malformation [
5,
6,
7]. Traumatic events are the primary cause of AOD in both veterinary and human medicine, though it can also emerge in conjunction with conditions like rheumatoid arthritis, Down syndrome, congenital cervical vertebral fusion, and neoplasia in humans [
1,
4,
8,
9]. The paucity of reported cases, potentially due to the rapid progression towards mortality and diagnostic challenges within the veterinary field [
10,
11].
The clinical manifestation of AOD encompasses a range of neurological deficits, from acute cervical discomfort to pronounced ataxia and tetraparesis, indicative of significant neurological compromise [
12]. The dislocation can also compress the spinal cord and brainstem at the foramen magnum, resulting in acute respiratory failure [
13]. Diagnostic protocols commence with comprehensive neurological evaluations and are augmented by lateral cervical spine radiographs to ascertain the location of the neurological lesion [
14]. Displacement of the atlas with widening of the joint space on lateral view of the cervical spine can be an indication for an advanced image [
12]. Various radiological evaluations using computed tomography (CT), such as the Traynelis classification, Powers’ ratio, the X-line method, and the Harris method, have been utilized in human medicine. However, the absence of established radiological criteria for AOD in veterinary medicine necessitates the use of advanced imaging modalities, such as magnetic resonance imaging (MRI) [
9]. MRI scans precisely delineate the extent of ligamentous damage and assess the severity of associated spinal cord and brainstem injuries [
14].
In human medicine, the treatment of AOD has evolved to include both conservative management and surgical intervention, depending on the severity of the dislocation and the presence of associated injuries. Conservative treatment often entails external coaptation to immobilize the cervical region, while surgical options include decompressive procedures such as dorsal laminectomy and partial occipital bone removal above the foramen magnum [
4,
15,
16]. Internal fixation techniques, employing a variety of materials such as cortical screws and interarticular wires, aim to stabilize the cranio-cervical junction, thus mitigating the risk of further neurological deterioration [
4,
15,
16,
17].
To the best of author’s knowledge, while there are a few reported cases of traumatic AOD in dogs, which have been corrected through ventral fixation, there is no single case of congenital AOD with concurrence of AAI in a dog. This case report aims to describe a novel surgical approach to the treatment of a dog diagnosed with concurrent congenital AOD and AAI. Despite the limited number of documented cases involving both conditions in veterinary literature, this report highlights the successful application of a C1 vertebra facetectomy and ventral fixation of the occipitoatlantoaxial complex.
2. Case Description
An 8-month-old, 3.4kg, castrated male, Toy Poodle was referred to the veterinary referral hospital due to progressive tetraparesis that had been developing over four months, with no history of trauma. Physical examination revealed respiratory distress and cyanosis, along with non-ambulatory paraparesis. The neurological status was assessed to be at modified Frankel score grade 3 [
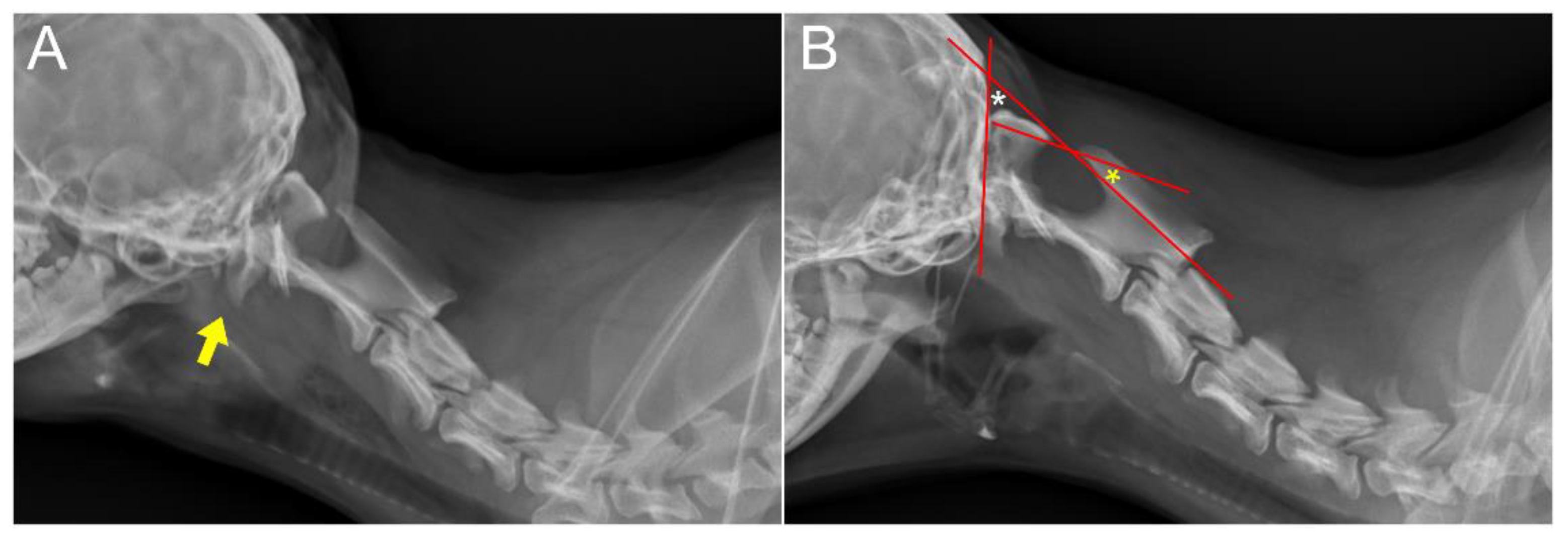
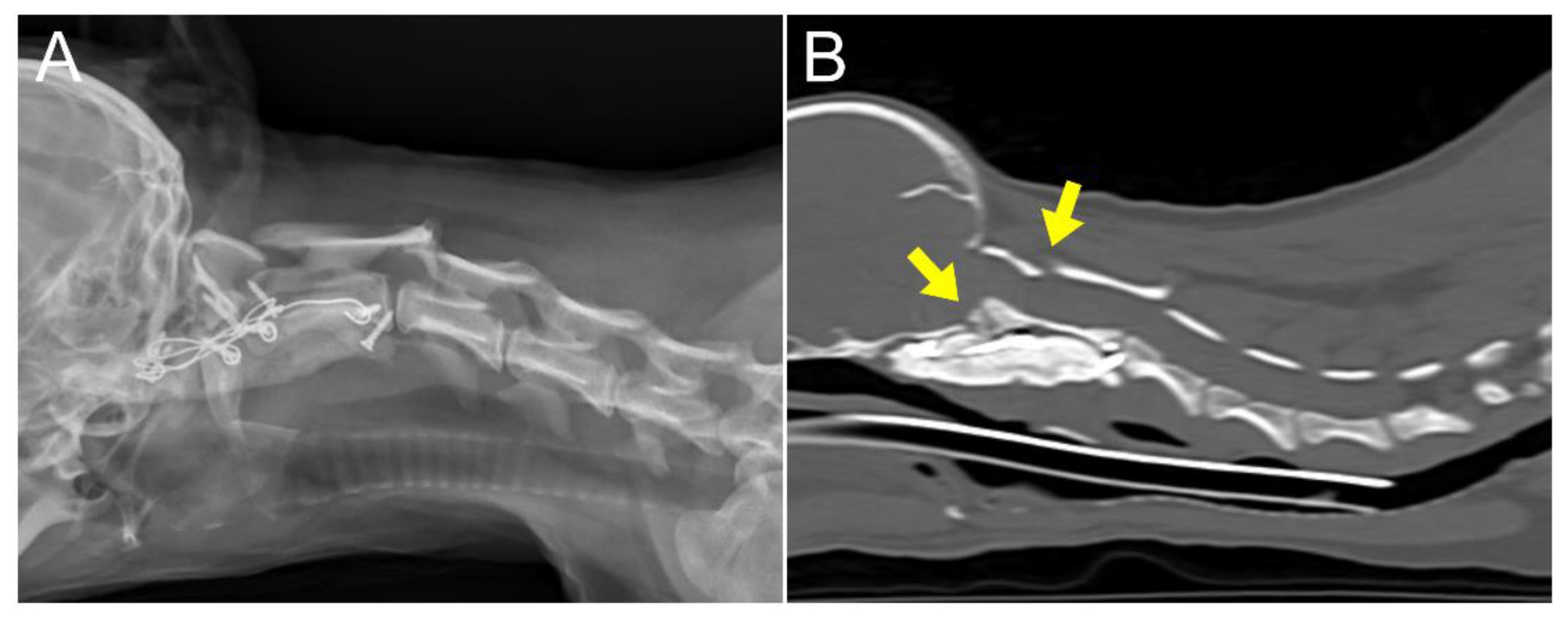
18]. Neurological examination revealed delayed proprioception and postural reactions in all four limbs. Radiographic examination revealed marked luxation between the C1 and C2 vertebra, along with dislocation of the occipital bone and C1 (
Figure 1A and 1B). AAI was objectively diagnosed by applying a 51° flexion cervical radiography [
19] (
Figure 1B). To further assess AAI, both flexion and extension CT views were obtained [
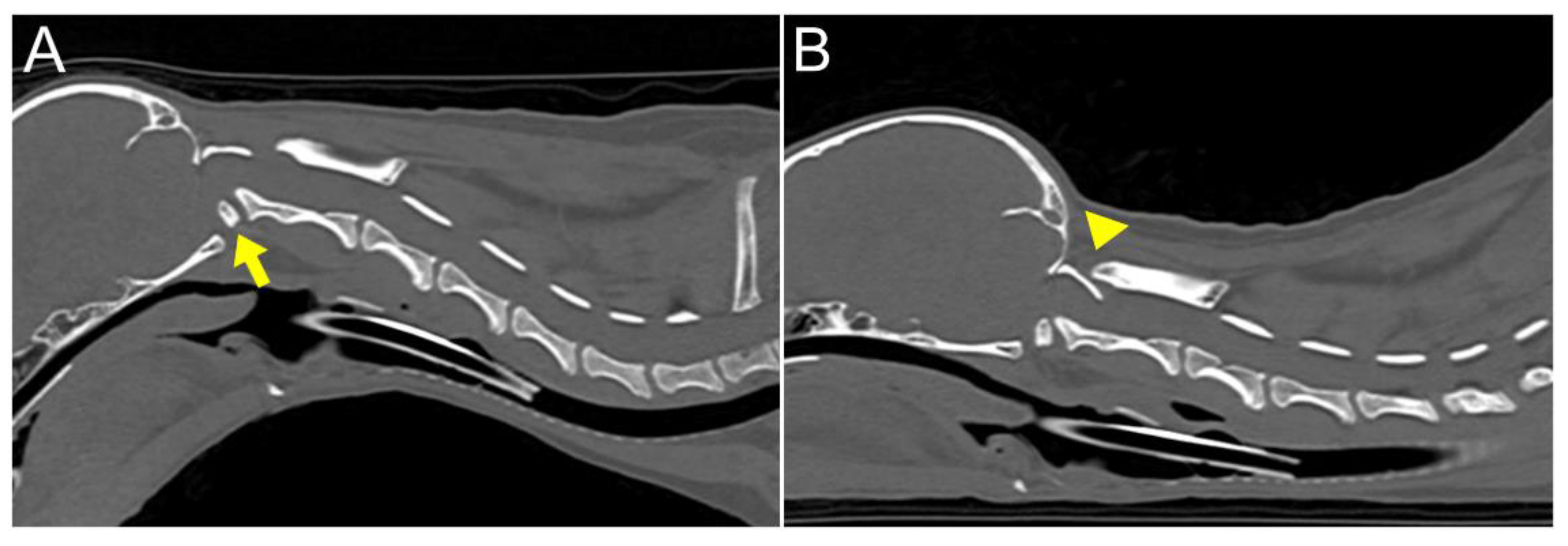
20]. The flexion view was captured in dorsal recumbency, while the extension view was taken in ventral recumbency (
Figure 2). The CT findings revealed dorsal dislocation of the atlas from the occipital bone, along with luxation between C1 and C2 vertebrae, accompanied by hypoplasia of bilateral occipital condyles, wings of atlas, and dens (
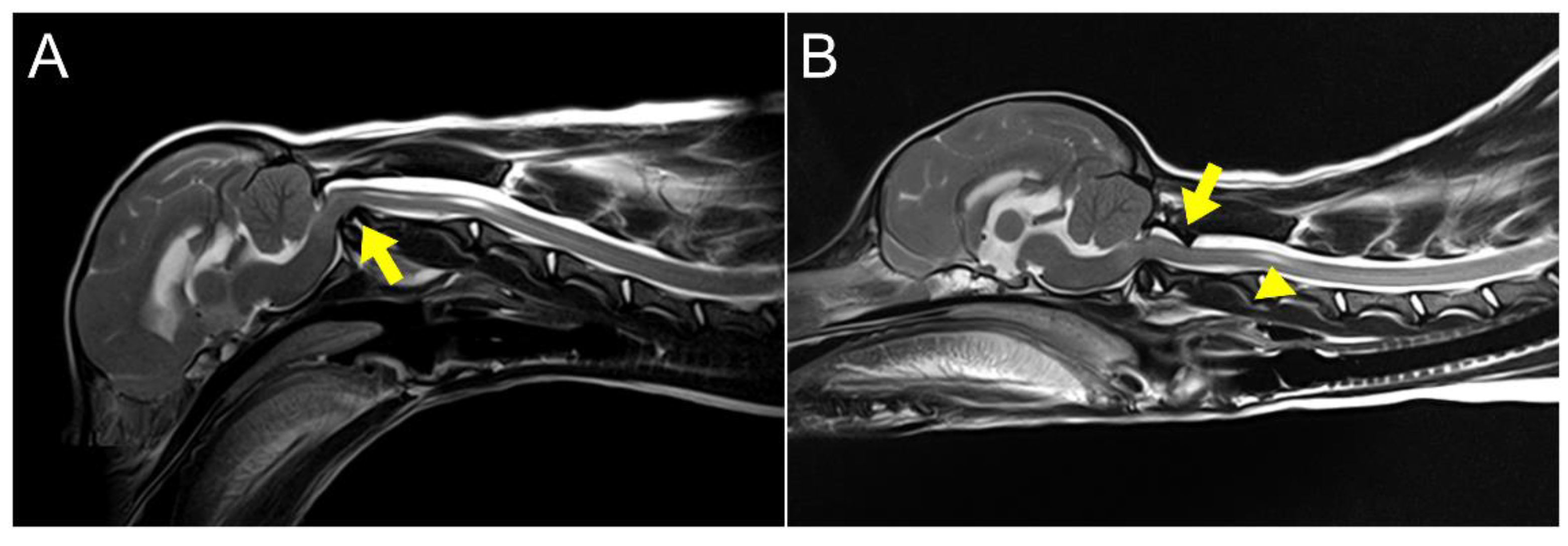
Figure 2). The dislocated atlas, suspected to result from instability in the occipitoatlantoaxial complex, was identified as AOD. A subsequent dynamic MRI, performed in the identical position as the CT scan, showed that disarticulated C1 caused dorsal compression of the spinal cord between C1 and C2 in the sagittal plane during extension, resulting in an enlarged dorsal subarachnoid space. Additionally, AAI, along with mild dens hypoplasia, led to ventral compression of the medulla oblongata and cranial spinal cord in the sagittal plane during flexion. This compression was responsible for the spinal cord injury, characterized by medullary kinking (
Figure 3). The dog was diagnosed with concurrent AAI and AOD after the finding was a 3D reconstructed CT image (Xelis, INFINITT Healthcare Co., Ltd., Korea). The surgical plan involved performing ventral fixation for primary stabilization from the occipital bone to the C2 using screws, wires, and polymethyl methacrylate (PMMA).
The dog was premedicated with midazolam (0.2 mg/kg IV) and dexamethasone (0.2 mg/kg IV). Induction was achieved with alfaxalone (3 mg/kg IV) and maintained with isoflurane. Intraoperative analgesia was provided by a constant rate infusion (CRI) of remifentanil (0.1–0.3 μg/kg/min). Amoxicillin and clavulanic acid (AMC) (12.5 mg/kg IV) were administered every 120 minutes, with the first dose given 30 minutes before the incision. The dog was placed in supine position with a vacuum sandbag under the neck. Typically, the neck is immobilized in an extended position during AAI surgery, but in this case, positional reduction with slight flexion of the neck was performed as dorsal spinal cord compression by the C1 lamina was confirmed in the extended position during MRI.
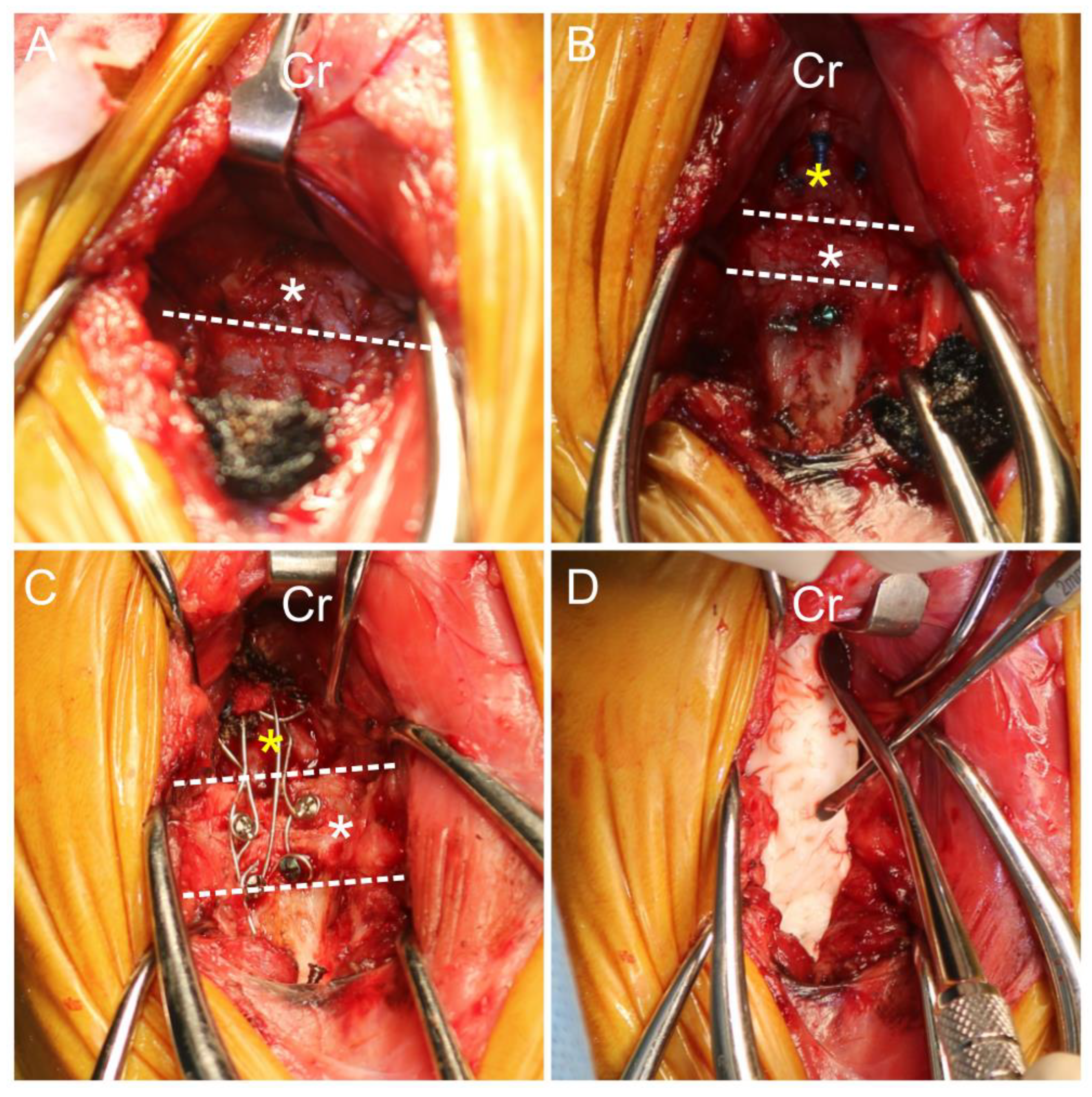
A ventral paramedian incision [
21] was performed to approach the occipital, C1, and C2 vertebrae. Despite the complete removal of fibrous tissue on the ventral side and attempted reduction, realigning the atlanto-occipital joint to its normal position was challenging due to excessive soft tissue fibrosis, especially on the dorsal side. As an alternative, medullary decompression was achieved by partially removing parts of the basioccipital and ventral surface of the articular facet of the atlas using an electric high-speed drill and Kerrison rongeur (
Figure 4). With the aid of the positional reduction, primary screws were inserted in the occipital bone and C2 with 1.4 mm self-drilling screws (LeForte system, Jeil Medical, South Korea). Secondary screws were inserted in C1 with 1.4 mm self-drilling screws. The screws positioned in occipital bone, C1, and C2 were augmented with flexible orthopedic wire. An autogenous cancellous bone graft from the left humeral greater trochanter was applied between the occipitoatlantoaxial joints for bony fusion. The occipitoatlantoaxial joints were then stabilized with PMMA. A gastrostomy tube was placed for dietary management.
Postoperatively, remifentanil was administered for analgesia via a CRI of 0.1–0.3 μg/kg/min for three days. Additional analgesia was provided with pregabalin (4 mg/kg q12h) and prednisolone (0.5 mg/kg q12h) orally for a week. AMC (12.5 mg/kg, q12h) was administered as postoperative antibiotics for seven days. A 3D-printed neck brace was applied for external stabilization.
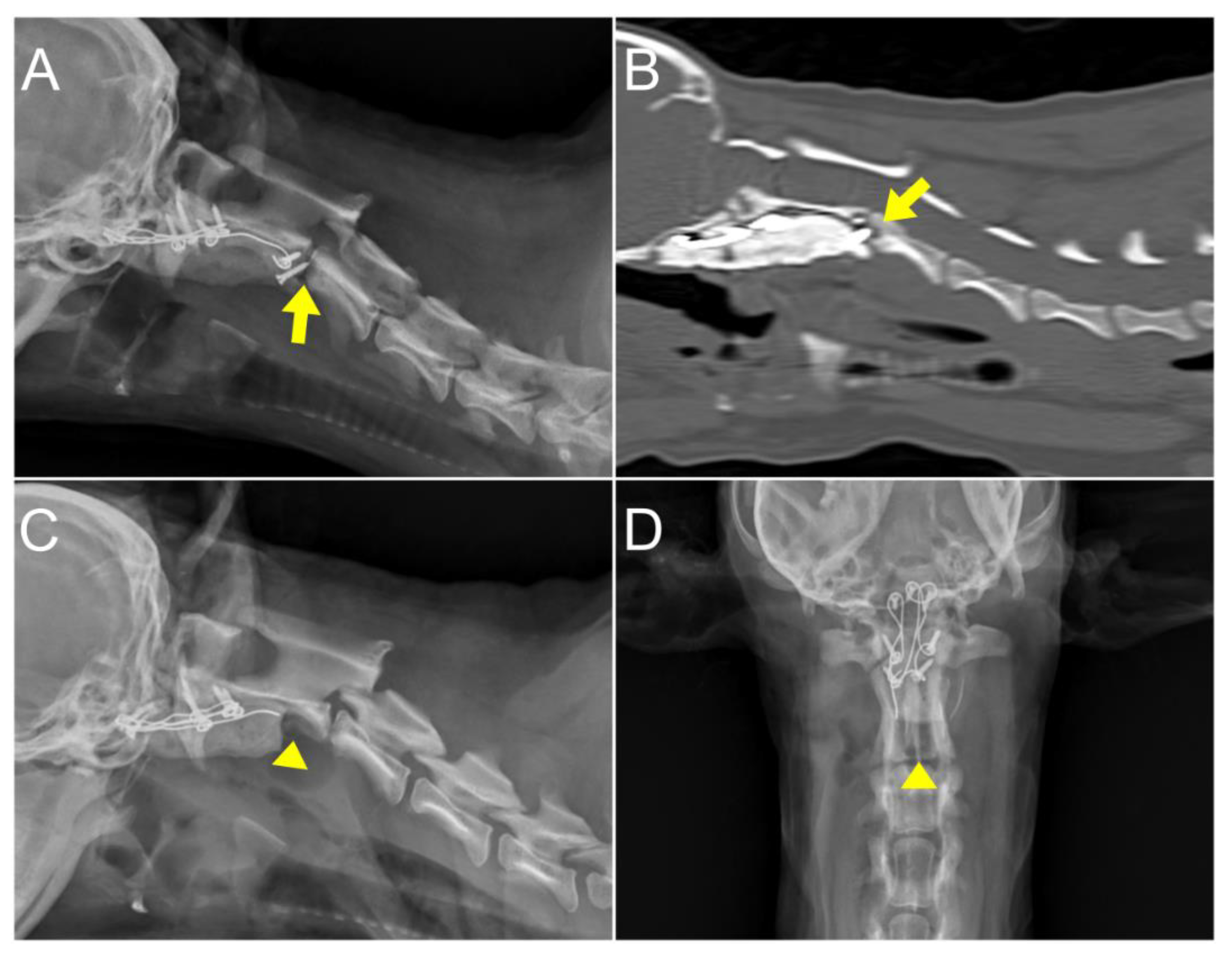
At six days postoperatively, radiographs and CT scan were performed to confirm the decompression of the medulla oblongata and cranial spinal cord. The CT scan with extension view showed improvement; the portion of the C1 lamina compressing the dorsal aspect of the spinal cord was aligned parallel to the C1 dorsal tubercle and the C2 spinous process (
Figure 5). The patient demonstrated gradual improvement in gait with mild hypermetric ataxia in the forelimb without any neurological side effects. The patient was discharged 19 days postoperatively, following owner education, with an improved modified Frankel score from grade 3 to grade 5 [
18]. At two months postoperatively, radiographs revealed no pathological findings, and the patient demonstrated further improvement in gait.
At 4 months postoperatively, the dog exhibited unexplained cervical pain unresponsive to medication and an elevated C-reactive protein (CRP) level, prompting further radiographic and CT examination. These images revealed decreased bone density around the screw located near the caudal endplate of C2. The loosened screws and surrounded PMMA was removed through revision surgery (
Figure 6). The culture from the removed screw indicated septic loosening. Susceptible antibiotics, including AMC (12.5 mg/kg, q12h), clindamycin (11 mg/kg, q24h), and enrofloxacin (5 mg/kg, q24h) were administered. This regimen led to pain relief and a decrease in CRP levels. Subsequent radiography and physical examination revealed no abnormalities until 12 months postoperatively. The patient continued to be monitored until 21months postoperatively; during this time, no signs of pain were noted and there was an improvement in gait, which was close to normal although the hind legs displayed a slightly wide-based stance.
3. Discussion
Atlanto-occipital dislocation is rare in veterinary medicine [
22]. There are some reported cases on traumatic AOD, although the extent of research is limited. Historically, treatments have ranged from conservative approaches like closed reduction to more invasive procedures such as open reduction and internal fixation [
11,
12,
15,
23]. Both ventral and dorsal stabilization techniques have been reported for an acute trauma patient, with some cases also undergoing dorsal laminectomy [
16,
17,
22,
24]. This particular case is significant as it represents the first veterinary instance where ventral facetectomy was combined with ventral stabilization of the O-C1-C2 complex, leading to successful management over a 21-month. This was despite the patient's condition being so severe and chronic that realignment of the atlas and occipital bones was considered unfeasible.
This case represents a unique instance of chronic AOD, which was particularly challenging to realign due to its chronic nature, without any accompanying traumatic events or underlying conditions. The evidence for congenital malformation in this patient is based on the observed morphological alteration in the atlanto-occipital joint interface in CT. The presence of a significant amount of fibrotic scar tissue surrounding the joints and adjacent soft tissues, attributed to chronic factors, impeded the approach to the surgical site and reduction of the dislocation [
22]. During the ventral approach, it was challenging to remove all the fibrotic scar tissue, especially that on the dorsal side, to facilitate atlanto-occipital realignment. Additionally, excessive removal of tissue was avoided to prevent further instability of the joint, as too much removal could exacerbate joint instability. [
17,
22].
To overcome the chronic AOD induced incomplete anatomical reduction, we modified the surgical plan to remove the portion where the basioccipital and ventral surface of the articular facet of the atlas were compressing the spinal cord. However, with the removal of joint structures, the instability of the cranio-cervical junction increased. To alleviate this, we planned to reinforced stabilization through OAA arthrodesis, applying screws, wires, and PMMA on the ventral side. While ventral compression was resolved by addressing the area compressing the spinal cord, the resolution of dorsal compression was still required. For the resolution of dorsal compression, we opted for positional reduction based on the dynamic MRI. We initiated the OAA multiple joint arthrodesis with the patient in a neutral position with a slight flexion, where compression would potentially be less severe.
Dynamic CT and MRI have been pivotal in confirming the simultaneous compression on both the dorsal and ventral aspects of the cranial cervical spinal cord. These imaging modalities have proven essential in understanding the dynamic instability of AAI and AOD. Through dynamic CT and MRI, we can conduct comprehensive evaluations of the cranio-cervical junction by obtaining images with the head and neck in various positions, such as mild flexion and mild extension, as recommended in references [
5,
6,
25]. These imaging studies have shown that in the flexion view, ventral compression of the spinal cord is more distinctly observed, whereas in the extension view, dorsal compression becomes more evident. The positional differences in spinal cord compression, as revealed by these dynamic advanced imaging techniques, are crucial for guiding diagnosis and selecting appropriate surgical techniques. Furthermore, the feasibility of positional reduction has been effectively assessed through dynamic CT and MRI, enabling the evaluation of the degree of spinal cord compression in different postures. It's important to recognize that the degree of compression might be relatively lower in a flexed position compared to an extended state. Therefore, the author advocates for the utilization of dynamic advanced imaging techniques in the evaluation and formulation of treatment plans for cases exhibiting multiple joint instability and subluxation in the craniocervical region, particularly when accurate reduction may be difficult due to the chronicity of the lesion.
This case report has several limitations. First, it is based on a single patient. Additionally, due to the single-case nature, we were unable to compare various surgical techniques introduced in diverse literature, thereby limiting the evaluation to the application of a singular surgical method. Moreover, the inability to evaluate the degree of spinal cord parenchymal compression using dynamic advanced imaging from multiple angles restricted the evaluation. The comparative analysis of the examination of various internal fixation systems and the investigation of flexion angles in dynamic CT and MRI, may contribute to a comprehensive understanding and improved diagnosis of AOD in veterinary medicine. Finally, this report does not assess whether a dorsal approach could have allowed for more accurate reduction and stabilization than the ventral approach used, adding to the limitations in evaluating the best surgical orientation for cases like this.
4. Conclusion
In concurrence of AOD and AAI, dynamic CT and MRI serve as the pivotal roles in diagnosing and establishing a surgical plan. This plan involves surgical decompression using ventral placement of screws and PMMA, which has been effective for addressing these conditions. In instances where reduction poses challenges, implementing ventral facetectomy can significantly aid decompression by alleviating areas of cord compression and stabilizing the occipitoatlantoaxial joint.
Author Contributions
Conceptualization, K.-B.K. and H.-B.L.; methodology, D.-H.K.; software, K.-B.K. and Y.-J.J.; validation, D.-H.K. and S.-M.J.; formal analysis, S.-M.J.; investigation, K.-B.K., J.-M.J. and H.-B.L; resources, K.-B.K. and J.-M.J.; data curation, K.-B.K., Y.-J.J., D.-H.K., S.-M.J., J.-M.J. and H.-B.L.; writing—original draft preparation, K.-B.K. and J.-M.J.; writing—review and editing, K.-B.K., Y.-J.J., D.-H.K., S.-M.J., J.-M.J. and H.-B.L.; visualization, K.-B.K. and Y.-J.J.; supervision, J.-M.J. and H.-B.L.; project administration, H.-B.L.; funding acquisition, H.-B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Acknowledgments
This study was supported by the research fund of Chungnam National University.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Joaquim, A.F., G.D. Schroeder, and A.R. Vaccaro, Traumatic atlanto-occipital dislocation—A comprehensive analysis of all case series found in the spinal trauma literature. Int J Spine Surg, 2021. 15(4): p. 724-739. [CrossRef]
- Blackwood, N., III. Atlo-occipital dislocation: a case of fracture of the atlas and axis, and forward dislocation of the occiput on the spinal column, life being maintained for thirty-four hours and forty minutes by artificial respiration, during which a laminectomy was performed upon the third cervical vertebra. Annals of Surgery, 1908. 47(5): p. 654.
- Ehlinger, M., et al., Survivor of a traumatic atlanto-occipital dislocation. Orthop Traumatol Surg Res, 2011. 97(3): p. 335-340. [CrossRef]
- Dolera, M., et al., Zygomatic arch-atlas wing stabilization in 5 dogs with atlanto-occipital dislocation. Journal of Veterinary Medical Science, 2016. 78(6): p. 963-970. [CrossRef]
- Marino, D.J., et al., Morphometric features of the craniocervical junction region in dogs with suspected Chiari-like malformation determined by combined use of magnetic resonance imaging and computed tomography. Am J Vet Res, 2012. 73(1): p. 105-111. [CrossRef]
- Dewey, C.W.; Da Costa, R.C. Practical Guide to Canine and Feline Neurology, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 157-167.
- Rotter, C., C. Rusbridge, and N. Fitzpatrick, Occipitoatlantoaxial malformation in a dog treated with a custom-made implant. VCOT Open, 2020. 3(02): p. e170-e176. [CrossRef]
- Onodera, R., et al., Congenital atlanto-occipital dislocation in a patient with Down syndrome: a case report. Skeletal Radiol, 2023: p. 1-5. [CrossRef]
- Hall, G.C., et al., Atlanto-occipital dislocation. World J Orthop, 2015. 6(2): p. 236. [CrossRef]
- Traynelis, V.C., et al., Traumatic atlanto-occipital dislocation: case report. J Neurosurg, 1986. 65(6): p. 863-870. [CrossRef]
- Lye, G., et al., Computed Tomographic Diagnosis of Traumatic Atlanto-occipital Rotatory Luxation and Successful Closed Reduction in a Dog. VCOT Open, 2020. 3(02): p. e164-e169. [CrossRef]
- Steffen, F., M. Flueckiger, and P.M. Montavon, Traumatic atlanto-occipital luxation in a dog: associated hypoglossal nerve deficits and use of 3-dimensional computed tomography. Vet Surg, 2003. 32(5): p. 411-415. [CrossRef]
- Shamoun, J.M., L. Riddick, and R.W. Powell, Atlanto-occipital subluxation/dislocation: a “survivable” injury in children. Am Surg, 1999. 65(4): p. 317-320. [CrossRef]
- Pang, D., W.R. Nemzek, and J. Zovickian, Atlanto-occipital dislocation: Part 1 normal occipital condyle C: 1: Interval in 89 children. Neurosurg, 2007. 61(3): p. 514-521. [CrossRef]
- Crane, S.W., Surgical management of traumatic atlanto-occipital instability in a dog. Vet Surg, 1978. 7(2): p. 39-42; DOI: 10.1111/j.1532-950X.1978.tb00577.x.
- Greenwood, K. and J. Oliver Jr, Traumatic atlanto-occipital dislocation in two dogs. J Am Vet Med Assoc, 1978. 173(10): p. 1324-1327; DOI: 173(10):1324-1327.
- Buks, Y., S. Snelling, and G. Yates, Ventral fixation of chronic atlanto-occipital luxation in a dog. J Small Anim Pract, 2011. 52(9): p. 497-500. [CrossRef]
- Levine, J.M., et al., Matrix metalloproteinase-9 activity in the cerebrospinal fluid and serum of dogs with acute spinal cord trauma from intervertebral disk disease. Am J Vet Res, 2006. 67(2): p. 283-287. [CrossRef]
- White, D.A., et al., Flexed radiographic angles for determination of atlantoaxial instability in dogs. Vet Surg, 2019. 48(8): p. 1406-1415. [CrossRef]
- Gupta, V., et al., Dynamic magnetic resonance imaging evaluation of craniovertebral junction abnormalities. J Comput Assist Tomogr, 2007. 31(3): p. 354-359. [CrossRef]
- Shores, A. and L.C. Tepper, A modified ventral approach to the atlantoaxial junction in the dog. Vet Surg, 2007. 36(8): p. 765-770. [CrossRef]
- Rylander, H. and J.C. Robles, Diagnosis and treatment of a chronic atlanto-occipital subluxation in a dog. J Am Anim Hosp Assoc, 2007. 43(3): p. 173-178. [CrossRef]
- Lappin, M.R. and S. Dow, Traumatic atlanto-occipital luxation in a cat. Vet Surg, 1983. 12(1): p. 30-32. [CrossRef]
- Sorjonen, D., et al., Ventral Surgical Fixation and Fusion for Atlanto-occipital Subluxation in a Goat. Vet Surg, 1983. 12(3): p. 127-129. [CrossRef]
- Cerda-Gonzalez, S., et al., Imaging features of atlanto-occipital overlapping in dogs. Vet Radiol Ultrasound, 2009. 50(3): p. 264-268. [CrossRef]
- Song, J.-H., et al., Successful management of and recovery from multiple cranial nerve palsies following surgical ventral stabilization in a dog with atlantoaxial subluxation. Vet Sci, 2022. 9(7): p. 322. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).