1. Introduction
Acute coronary syndrome (ACS) is defined as a condition in which myocardial ischemia or infarction develops due to acute occlusion of coronary blood flow [
1] induced by rupture or erosion of atherosclerotic lesion plaques or coronary artery spasm (CAS) [
2]. Although marked progress has been made in therapeutic procedures for ACS, especially percutaneous coronary intervention (PCI), ACS remains one of the leading causes of mortality worldwide [
3]. However, prompt treatment of ACS, including PCI or coronary artery bypass grafting, can reduce mortality [
4]. Therefore, saving the lives of patients with ACS is highly dependent on a rapid diagnosis being made.
Generally, ACS is diagnosed based on symptoms such as prolonged chest oppression and pain, abnormal electrocardiogram (ECG) findings, and blood biochemical tests, including troponin T and CK-MB. Of these, troponin T reflects ischemia-induced myocardial injury with high specificity, and its concentration in the blood promptly increases (usually within two to four hours) after its onset [
5]. Therefore, the decision to perform coronary intervention for ACS-suspected patients is often based on troponin T testing results [
5,
6]. Indeed, the diagnostic ability of troponin T is well established and highly recommended for the diagnosis of patients suspected of having ACS [
6]. However, a positive result on a troponin T test, even a highly sensitive one, is frequently indefinite or absent in ACS cases [
7] and requires serial measurements to diagnose or rule out ACS in some populations [
6]. Therefore, the development of novel biomarkers that are more sensitive for ACS is necessary for a more prompt and precise diagnosis of ACS.
The concept of metabolomics was developed in 1998 by Oliver et al. and Tweeddale et al.[
8,
9]. It involves the systematic analysis of the comprehensive metabolites of biological samples, such as biofluids, cells, tissues, or organs, to elucidate their profile features. The chemo-material and quantitative determination of many metabolites in various biological fluid specimens can provide valuable biochemical information on the state of organisms and the interrelations between the various metabolic processes that define the state [
10]. Recent technological advances have enabled high-throughput profiling of many metabolites in biological samples, increasing their application to disease-specific biomarker discovery, including ACS [
11,
12,
13].
Therefore, we first attempted to elucidate the metabolomics profile characteristics of ACS patients’ plasma in this study. We then explored novel biomarkers of ACS with plasma metabolomics by applying a multiple logistic regression (MLR) model that is more sensitive than troponin T and CK-MB.
3. Discussion
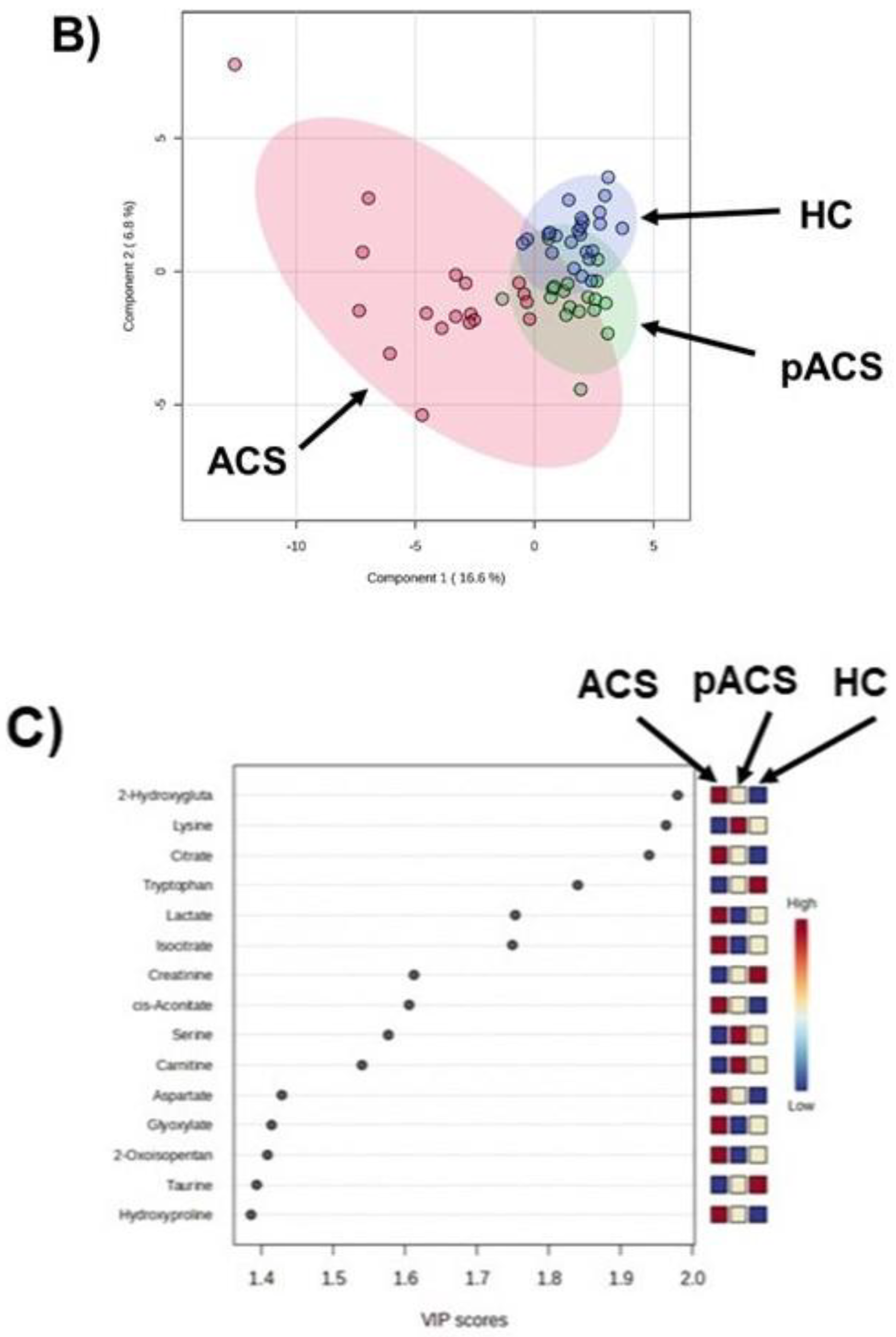
In this study, we first elucidated the dynamics of plasma metabolomic profiles of patients with ACS compared to HCs using LC-TOFMS. The heatmap shows prominent metabolomic profiles of the ACS group with various metabolite concentration changes. The PLS-DA also revealed a distinctive signature of the ACS group metabolomics profile, showing that the plots of metabolomics were markedly dispersed compared to those of the pACS and HC groups. However, it is noteworthy that the pACS metabolomics plots in the PLS-DA were closer to those of the HC. These data might imply that the treatments performed for ACS subjects, including PCI and anticoagulant administration, played preventive roles in the extension of myocardial injury and the therapeutic effect on myocardial ischemia. We then explored novel ACS biomarkers that can diagnose ACS with higher sensitivity than those of high-sensitivity troponin T and CK-MB using an MLR model with three metabolites: lysine, isocitrate, and tryptophan. To our knowledge, this is the first report to propose a combination of novel ACS biomarkers with higher sensitivity than troponin T and CK-MB.
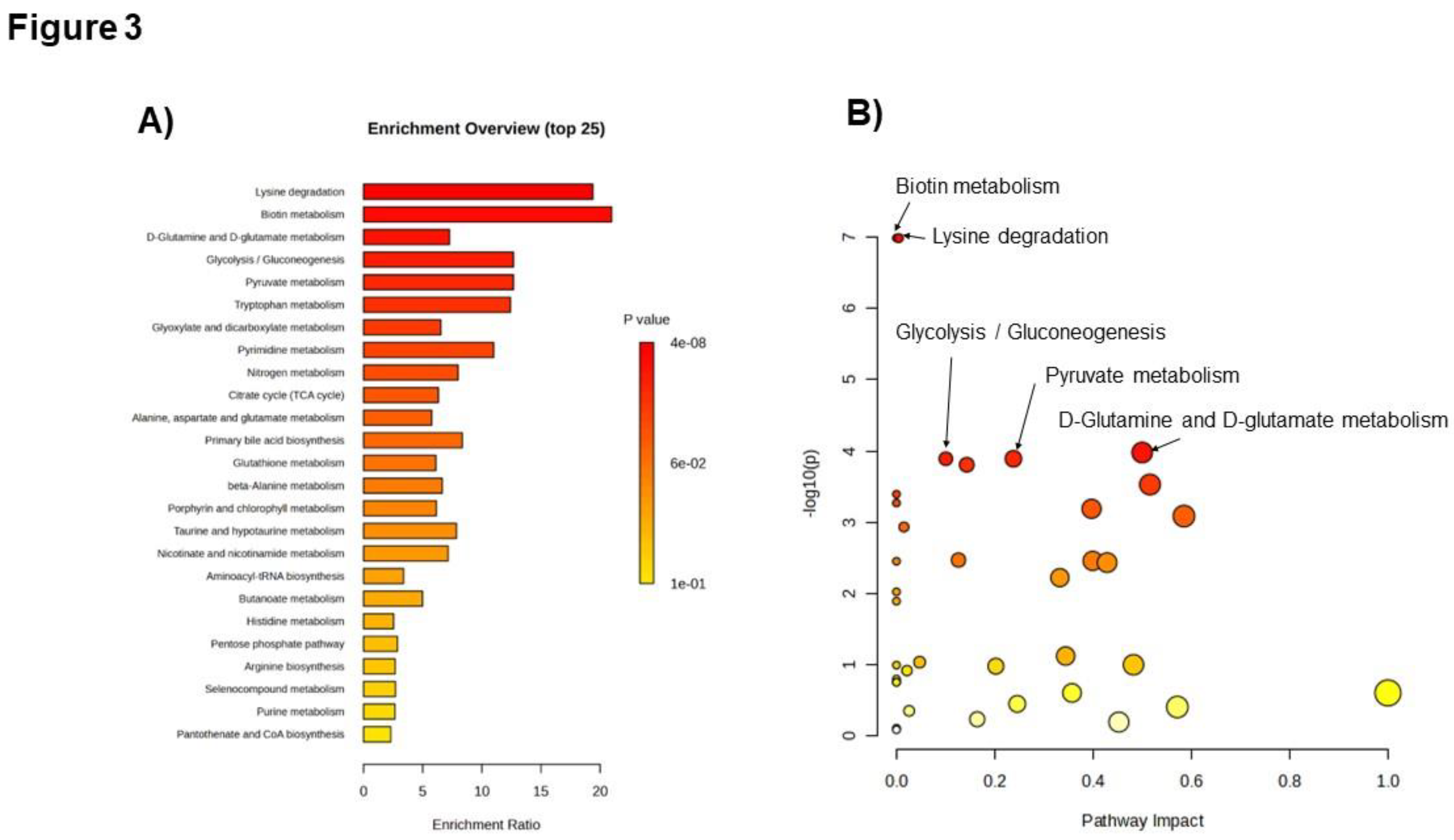
To date, alterations in the metabolomic profiles due to cardiac ischemia, including ACS, have been reported using various tools for metabolomics analyses. Sabatine et al. first suggested that, by comparing human cardiac ischemia to control subjects before and after exercise stress, plasma metabolomics could be applied to determine changes in groups of functionally related metabolites, leading to novel insights into the pathophysiological metabolomic features of human myocardial ischemic injury [
14]. Using LC-MS, they found that 23 metabolites changed due to cardiac ischemia, and 6 were involved in the citric acid pathway (tricarboxylic acid [TCA] cycle). Following this report, TCA disturbances have been observed in patients with myocardial injury and ACS [
12,
15,
16]. The TCA cycle in mitochondria releases energy as ATP by oxidizing acetyl-CoA, which is produced from various nutrients, such as carbohydrates, proteins, and lipids. Perturbation of the TCA cycle by a decrease or cessation of oxygen supply due to severe coronary artery stenosis or occlusion induces myocardial dysfunction and cardiomyocyte death. Therefore, alteration of the TCA cycle metabolomic profiles is a critical sign of cardiac ischemia and ACS. In addition to TCA cycle perturbation, reduction of the oxygen supply to the myocardium induces glycogen breakdown and an increase in the glycolysis rate, an ATP anaerobically productive pathway, thereby increasing lactate production [
17,
18]. Thus, alterations in metabolites related to the TCA cycle, such as citrate and isocitrate, and glycolysis, such as lactate, are critical markers of ischemic cardiac injury [
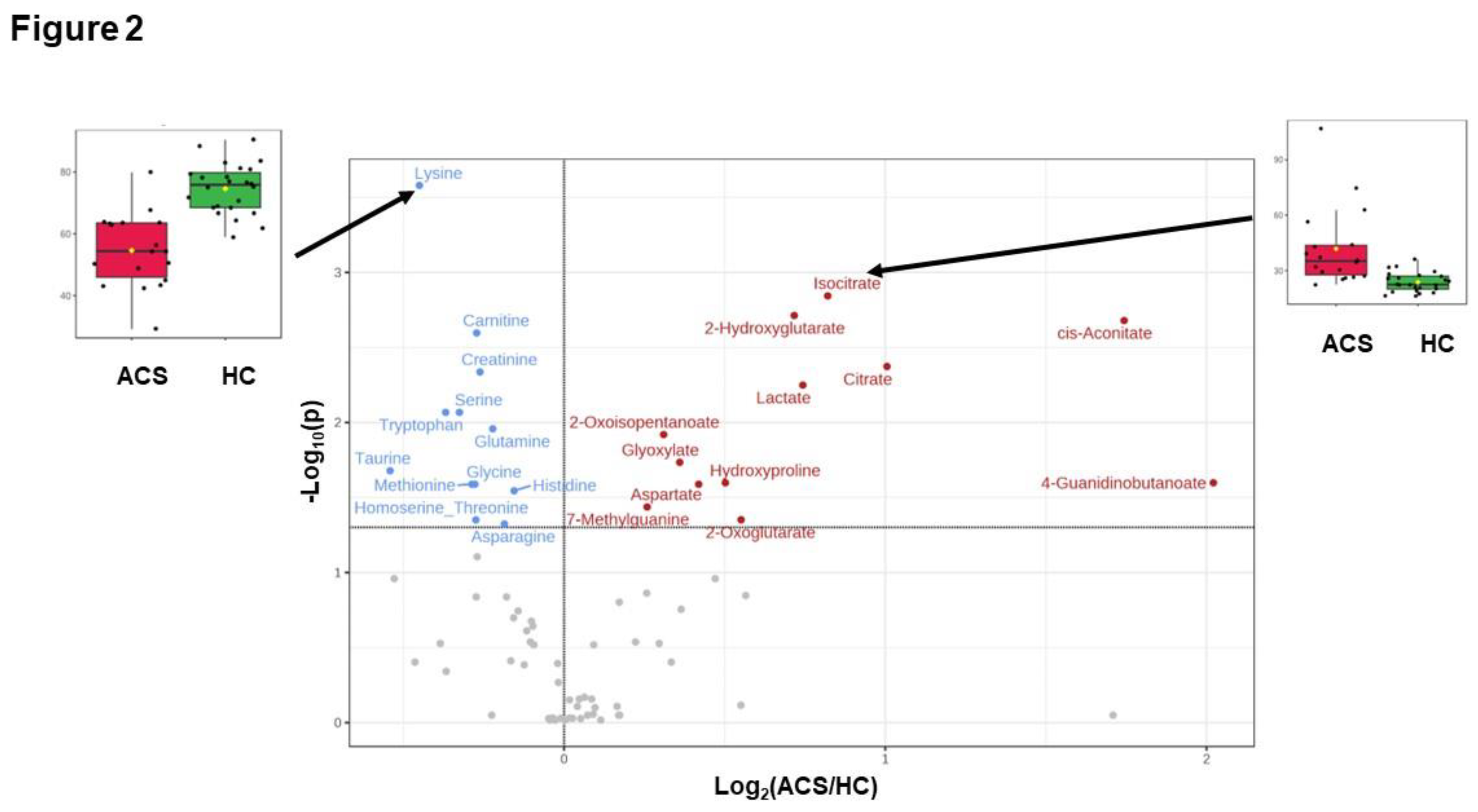
17]. In this respect, our current study observed the changes in these key pathways of glycolysis and the TCA cycle (
Figure 3A) as well as changes in the metabolites involved in these pathways, including citrate, isocitrate, and lactate, in the AGS group (
Figure 2). These data indicate that the current study was performed using adequate and accurate methods and techniques, as in previous reports.
Besides the perturbation of the myocardial energy supply pathway, the increase in oxidative stress is also a major event in cardiomyocytes during ACS. During myocardial ischemia, increased amounts of reactive oxygen species are produced in cardiomyocytes, leading to cell death [
19]. Considering the enrichment (
Figure 3A) and pathway analyses (
Figure 3B), the relatively high impact of the D-glutamine and D-glutamate metabolism pathways on ACS is considerably important in the present data. Since the D-glutamine and D-glutamate metabolism pathways play crucial roles in producing antioxidants that exert cardioprotective effects during myocardial ischemia [
20], the high pathway impact of the D-glutamine and D-glutamate metabolism pathways might imply protective feedback responses in cardiomyocytes against ischemia-induced oxidative stress.
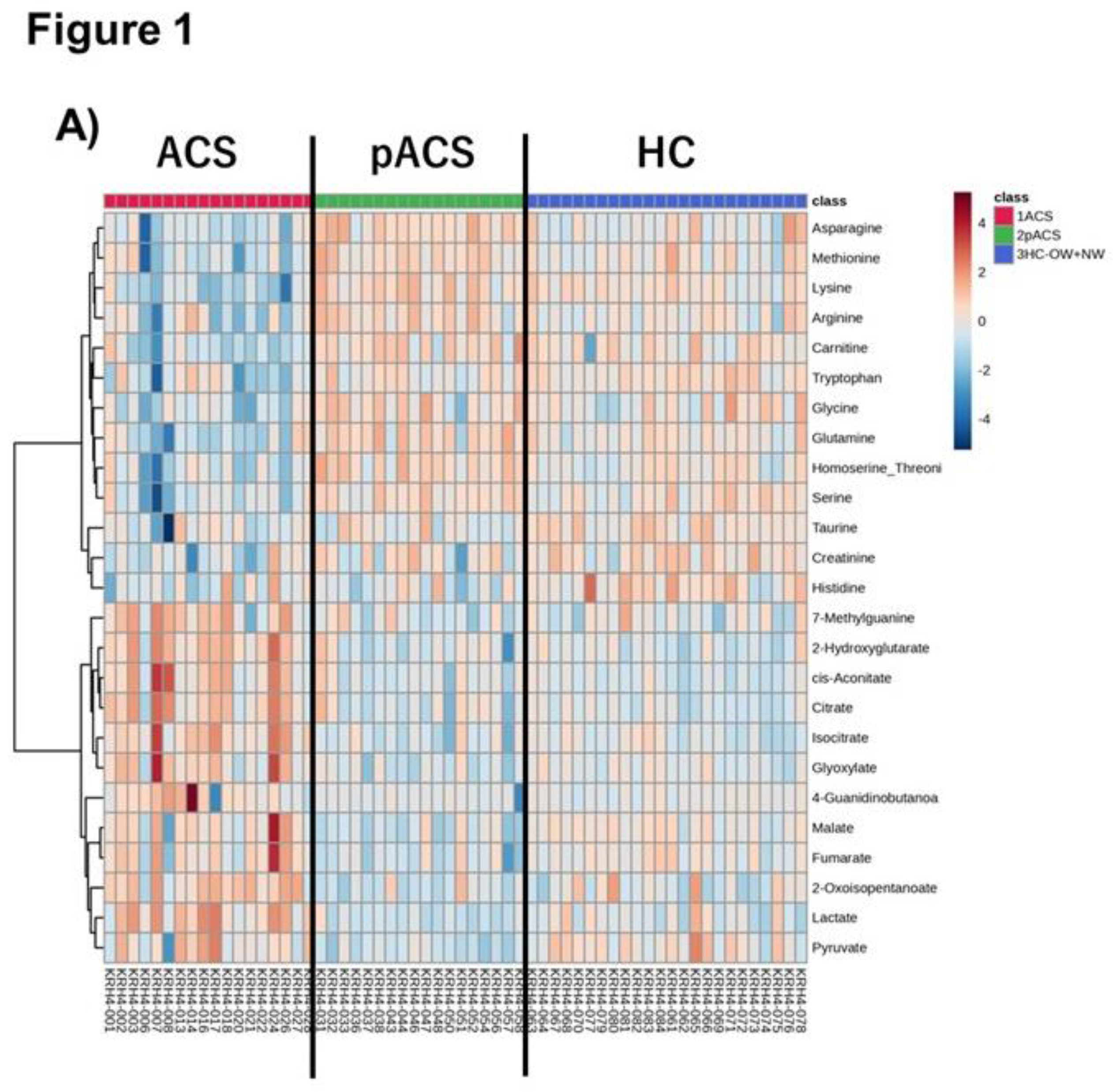
In the present study, we analyzed and compared the metabolomic profiles on the day of discharge from the hospital (pACS group) and at hospital arrival (ACS group). As shown in
Figure 1, the pACS metabolomics profiles differed from those of ACS (
Figure 1A), being closer to those of HC (
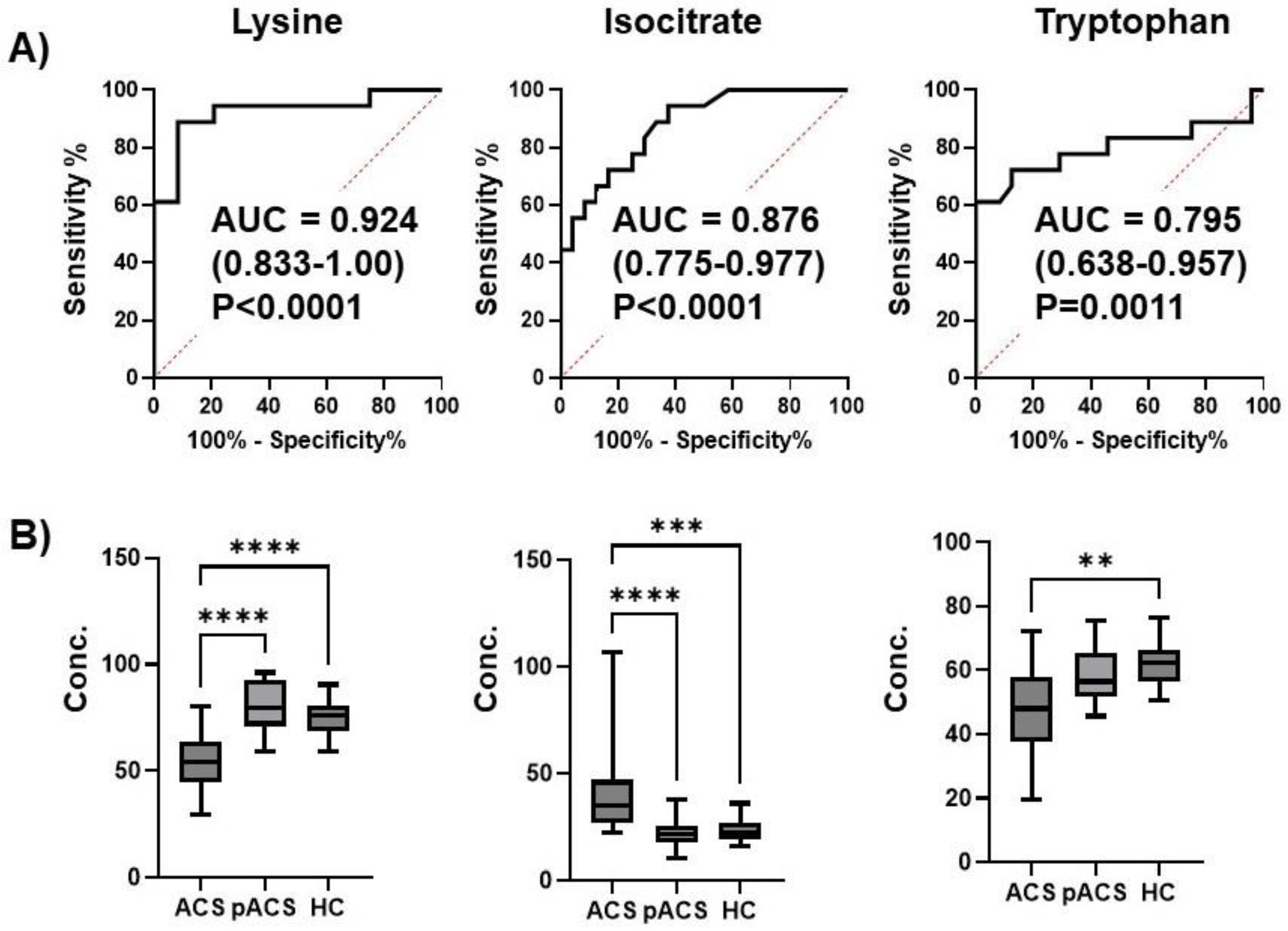
Figure 1B). In particular, lactate, citrate, and isocitrate, metabolites of the key pathways of ischemic cardiac injury, TCA cycle, and glycolysis, were markedly decreased in the pACS group compared to the ACS group. Thus, it is reasonable to speculate that the reduction in the critical metabolites associated with ischemic myocardial injury implies that the treatment of coronary artery stenosis and/or occlusion with PCI and anticoagulants was successful, resulting in all ACS patients enrolled in this study being discharged from the hospital alive.
ACS is generally diagnosed based on symptoms of chest pain and/or oppression, abnormal ECG findings, and blood biochemical tests, such as troponin T and CK-MB. Serum troponin T and CK-MB are biomarkers specific to myocardial injury, and the elevation of their serum concentration is the major indicator for deciding to perform emergent catheter coronary angiography to check the indication for PCI. However, the sensitivities of troponin T and CK-MB were not 100%. For instance, it takes two to four hours after the onset of ACS until the troponin T serum concentration reaches a positive diagnostic value [
5]. Recently, Ali et al. reported that, in 30 patients with acute myocardial infarction, no elevation in serum troponin concentration was observed within 2 h after the onset of chest pain. In contrast, the serum metabolomic profiles of the patients at the same time point were quite different from those of HCs, suggesting that some blood metabolites can be novel ACS biomarkers able to diagnose ACS earlier than established biomarkers, including troponin T and CK-MB [
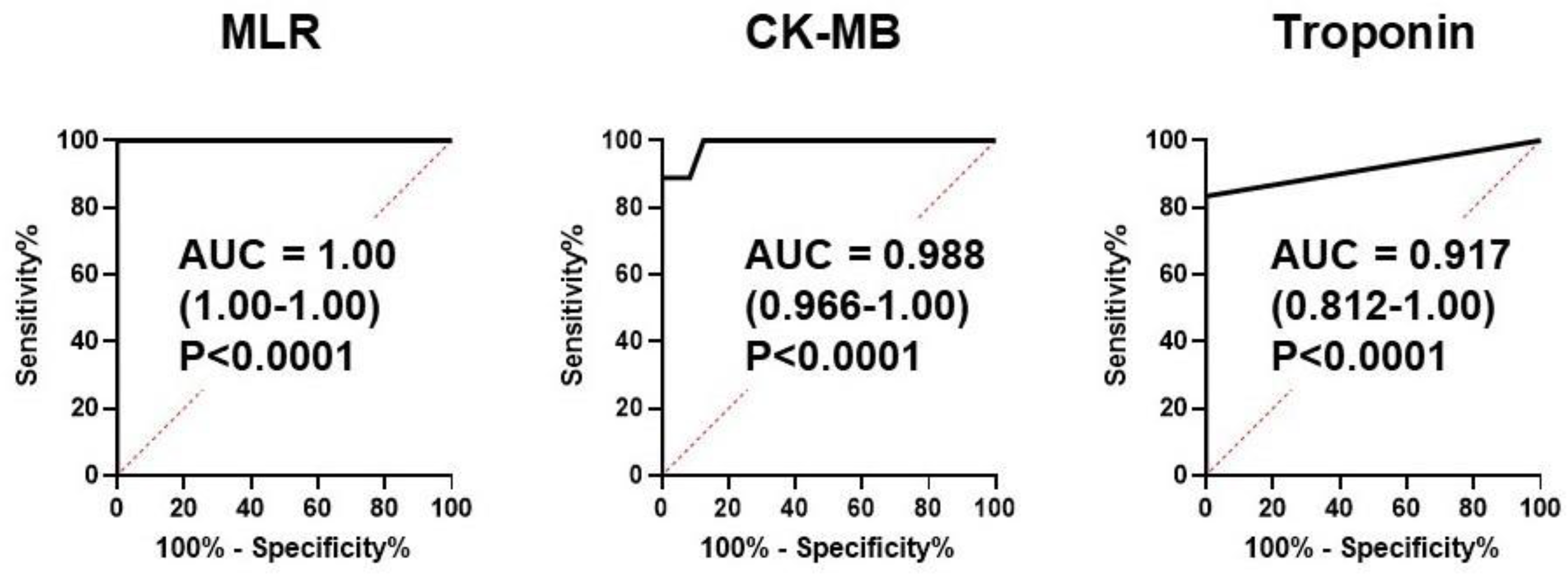
21]. We developed an MLR model to combine multiple metabolites as novel ACS biomarker. Consistent with previous reports [5 ,21], both serum troponin T and CK-MB levels in ACS patients at hospital arrival showed high sensitivities, with AUCs of 0.917 and 0.988, respectively (
Figure 4). However, surprisingly, an MLR model composed of the 3 plasma metabolites lysin, isocitrate, and tryptophan showed 100% sensitivity with an AUC of 1.0 (
Figure 4). These data indicate that this MLR model with three plasma metabolites can be an ACS biomarker that can diagnose ACS earlier than troponin T and CK-MB with extremely high sensitivity. The three metabolites were thus selected as MLR model features, constituting an independent and minimum set. These metabolites also showed a high VIP score (VIP >1.7) (
Figure 1C), suggesting the excellent reliability of this model.
Regarding the biochemical characteristics of the three metabolites, isocitrate was involved in the TCA cycle described above. Blood tryptophan levels are decreased in patients with ACS [
22]. During ischemic myocardial injury, tryptophan is metabolized in endothelial cells, and its metabolites affect several cardiac functions, including contractility [
23]. Therefore, the decrease in tryptophan levels in the plasma of patients with ACS might reflect activated tryptophan degradation in endothelial cells due to ischemic myocardial injuries.
Although the precise role of lysine in myocardial ischemic injury is still unclear, lysine is well known to be decreased in the blood of ACS patients
22, 24. Lysine inhibition modulates nitric oxide synthesis [
24,
25], and nitric oxide controls vascular tone as a vasodilator [
26]. Therefore, the decreased lysine levels in ACS patients’ plasma might be a phenomenon that protects against ischemic myocardial injury by dilating arteries to increase the supply of oxygen to the injured myocardium via coronary arterial stenosis and occlusion.
This study has several limitations. Although our study was prospective, the number of patients in each group was relatively small, and the study was performed at one institution. Further research is warranted to validate the MLR model by enrolling more patients at multiple institutions.
In conclusion, we have developed an MLR model with higher sensitivity and earlier diagnostic ability than the currently available ACS biomarkers, high-sensitivity troponin T, and CK-MB. These data provide novel insights and strategies for the diagnosis and treatment of ACS.
4. Materials and Methods
4.1. Subjects
Since the incidence of ACS has consistently increased in patients under 60 years old in Japan [
27,
28], we focused on laborers who were productive workers and under 60 years old. Therefore, patients with ACS under 60 years old who were admitted to our hospital were enrolled in this study (ACS group). All patients underwent an ECG and blood biochemical tests, including high-sensitivity troponin T and creatinine kinase-MB tests, and plasma sampling for metabolome analyses soon after arrival at the hospital. ACS was diagnosed based on the symptoms and ECG findings with or without a positive serum high-sensitivity troponin T test. Emergent coronary angiography catheters were used for all subjects, and ad hoc PCI was performed for all subjects, except for two CAS subjects. Before emergent coronary angiography, 3000 IU of heparin was intravenously administered, and 7000 IU of heparin was added before PCI. All patients who underwent PCI were treated with dual antiplatelet therapy and statins for plaque stabilization.
For the healthy control (HC) group, age-matched males who visited our Health Administration Center for medical checkups were enrolled. As all subjects in the ACS group were male, only males were enrolled in the HC group. Plasma samples obtained from 9 ACS and 11 HC subjects were analyzed using a different analytical method in another preliminary study with other research purposes [
29], while the remaining samples were used in the present study. However, preprocessing, measurement, and data analyses for metabolomics were conducted simultaneously with other samples to eliminate unexpected bias.
4.2. Metabolomic Analyses
Venous samples were collected in blood collection tubes containing EDTA-2Na (Venoject II; Terumo, Tokyo, Japan) through a 22-gauge needle set (Safetouch PSV set; Nipro, Osaka, Japan). After centrifugation for 15 min at 1,500×g, the plasma was stored at −80 °C until it was used for metabolomic analyses. Venous blood samples of the ACS group were collected soon after arrival at the hospital and on the morning of discharge after overnight fasting (pACS group samples). Venous blood samples of the HC group were collected in the morning after overnight fasting, and plasma was stored as described above.
The metabolomic analysis was performed as previously described [
30], with modifications. First, 10 μL of the plasma sample was mixed with 90 μL of methanol containing 1.5 μM of each standard compound (d8-spermine, d8-spermidine, d6-
N1-acetylspermidine, d3-
N1-acetylspermine, d6-
N1,
N8-diacetylspermidine, d6-
N1,
N12-diacetylspermine, hypoxanthine-
13C
2,
15N, and 1,6-diaminohexane) and 1μM camphor-10-sulfonic acid. After centrifugation at 20,380×
g for 10 min at 4 °C , 90 μL of the supernatant was transferred to a fresh tube and vacuum-dried. Third, the sample was mixed with 10 μL of 90% methanol and 190 μL of water and then mixed with a vortex mixer and centrifuged at 20,380×
g for 10 min at 4 °C. Finally, 1 μL of each supernatant was subjected to liquid chromatography-time-of-flight mass spectrometry (LC-TOFMS).
The parameters used for the measurement instruments, Agilent Technologies 1290 Infinity LC system and G6230B TOFMS (Agilent Technologies, Santa Clara, CA, USA), and the processing of raw data using Agilent MassHunter Qualitative Analysis software (version B.08.00; Agilent Technologies), have been described elsewhere [
31].
The peak areas were integrated and divided by those of the internal standards to eliminate fluctuations in spectrometer sensitivity. All integrated peaks were visually checked, and reintegration was conducted if necessary. Most of the peak areas in the linear range were confirmed. Peaks smaller than the lower linearity limit were treated as non-detected peaks. All samples were processed and measured for six consecutive days.
4.3. Data Analyses
The differences between the metabolite concentrations of the ACS and HC groups were evaluated using the Mann-Whitney test. The false discovery rate (FDR) was used to consider multiple independent tests. The discrimination ability of each metabolite was evaluated using the receiver operating characteristic curve (ROC) and the area under the ROC curve (AUC). The Kruskal–Wallis test and Dunn’s post-test were used to compare the ACS, pACS, and HC groups.
The MetaboAnalyst (ver. 5.0) [
32] was used for the following analyses. A heatmap with clustering, partial least squares discriminant analysis (PLS-DA), and volcano plots were used to visualize the overall metabolomic profile. The accuracy of the PLS-DA was evaluated by R
2 value, and its generalization ability was assessed by Q
2 value using five-fold cross-validation (CV). For heatmap visualization, the log
10 of the metabolite concentration was transformed to a Z-score. Euclidean distance and Ward method were used for the clustering of metabolomic profiles. Metabolites showing large variations within the top 25 based on a non-parametric analysis of variance were visualized. For the PLS-DA, the metabolite concentration was transferred to the Z-score. Volcano plots were generated using the log
10 metabolite concentration.
Enrichment and pathway analyses were conducted to evaluate pathway-level differences between the ACS and HC groups. The data were mapped into Kyoto Encyclopedia and Genes and Genomes (KEGG) pathway maps. No data normalization or scaling was performed for this analysis.
An MLR model was developed to evaluate the combination of multiple metabolites to discriminate between ACS and HC. Stepwise feature selection (feedforward band backward with a cutoff of p=0.05) was used to select the independent minimum metabolites. The generalization ability of the developed MLR model was determined using k-fold CV (k=10, 5, and 2) with 200 random values (
Figure S1).
4.4. Statistical Analyses
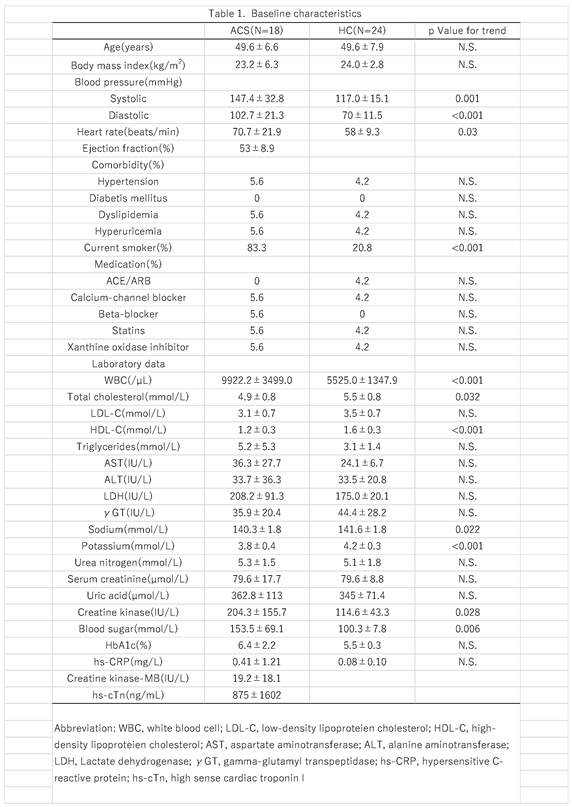
Statistical analyses were performed using the SPSS Statistics software program, version 23 (IBM Corp., Armonk, NY, USA). Normally distributed continuous variables are expressed as the mean ± standard deviation (SD). Non-normally distributed continuous variables were expressed as medians and ranges. Categorical variables were expressed as numbers and percentages. Fisher’s exact test or unpaired t-test was used to compare the ACS and HC groups.
These analyses were conducted using the JMP (Pro 16.0.0; SAS Institute Inc., Cary, NC, USA), GraphPad Prism (v.8.4.3; GraphPad Software, San Diego, CA, USA), and Weka (ver. 3.6.1.4, University of Waikato, Hamilton, New Zealand) software programs.