Submitted:
06 May 2024
Posted:
08 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Statistical Procedures
3. Results
| Thickness (µm) | Axial length (mm) | |
|---|---|---|
| r | p * | |
| Central RT | –0,09 | 0,3496 |
| ST | –0,14 | 0,1523 |
| SN | –0,15 | 0,1042 |
| IN | –0,19 | 0,0491 |
| IT | –0,14 | 0,1408 |
| STp | –0,05 | 0,6152 |
| SNp | –0,13 | 0,1729 |
| INp | 0,05 | 0,5798 |
| ITp | –0,12 | 0,2068 |
| Central CT | –0,06 | 0,5540 |
| ST | –0,08 | 0,4502 |
| SN | –0,01 | 0,9337 |
| IN | –0,03 | 0,7306 |
| IT | –0,04 | 0,6514 |
| STp | 0,03 | 0,7675 |
| SNp | –0,03 | 0,7302 |
| INp | –0,004 | 0,9673 |
| ITp | 0,06 | 0,5115 |
4. Discussion
4.1. Gender-Related Variations in RT and CT
4.2. Age-Related Variations in RT and CT
4.3. Axial Length and the RT and CT
4.4. CT and RT and Ocular Diseases
5. Conclusion
References
- Realini, T.; Zangwill, L.M.; Flanagan, J.G.; Garway-Heath, D.; Patella, V.M.; Johnson, C.A.; Artes, P.H.; Gaddie, I.B.; Fingeret, M. Normative Databases for Imaging Instrumentation. J Glaucoma. 2015, 24, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, L.F.; Zago Ribeiro, L.; de Oliveira, J.A.E. Fairness and generalizability of OCT normative databases: a comparative analysis. Int J Retin Vitr 2023, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Palazon-Cabanes, A.; Palazon-Cabanes, B.; Rubio-Velazquez, E.; Lopez-Bernal, M.D.; Garcia-Medina, J.J.; Villegas-Perez, M.P. Normative Database for All Retinal Layer Thicknesses Using SD-OCT Posterior Pole Algorithm and the Effects of Age, Gender and Axial Lenght. J Clin Med. 2020, 9, 3317. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, A.; Pellegrini, M.; Acquistapace, A.; Benatti, E.; Erba, S.; Cozzi, M.; Cigada, M.; Viola, F.; Gillies, M.; Staurenghi, G. Normative Data for Retinal-Layer Thickness Maps Generated by Spectral-Domain OCT in a White Population. Ophthalmol Retina. 2018, 2, 808–815. [Google Scholar] [CrossRef]
- Cortés, D.A.; Roca, D.; Navarro, P.I.; Rodríguez, F.J. Macular and choroidal thicknesses in a healthy Hispanic population evaluated by high-definition spectral-domain optical coherence tomography (SD-OCT). Int J Retina Vitreous. 2020, 6, 66. [Google Scholar] [CrossRef]
- Entezari, M.; Karimi, S.; Ramezani, A.; Nikkhah, H.; Fekri, Y.; Kheiri, B. Choroidal Thickness in Healthy Subjects. J Ophthalmic Vis Res. 2018, 13, 39–43. [Google Scholar] [PubMed]
- Silva, P.S.; Cavallerano, J.D.; Haddad, N.M.; Kwak, H.; Dyer, K.H.; Omar, A.F.; Shikari, H.; Aiello, L.M.; Sun, J.K.; Aiello, L.P. Peripheral Lesions Identified on Ultrawide Field Imaging Predict Increased Risk of Diabetic Retinopathy Progression over 4 Years. Ophthalmology 2015, 122, 949–956. [Google Scholar] [CrossRef]
- Ashraf, M.; Cavallerano, J.D.; Sun, J.K.; Silva, P.S.; Aiello, L.P. Ultrawide Field Imaging in Diabetic Retinopathy: Exploring the Role of Quantitative Metrics. J Clin Med. 2021, 10, 3300. [Google Scholar] [CrossRef] [PubMed]
- Orski, M.; Gawęcki, M. Current Management Options in Irvine-Gass Syndrome: A Systemized Review. J Clin Med. 2021, 10, 4375. [Google Scholar] [CrossRef]
- Pongsachareonnont, P.; Somkijrungroj, T.; Assavapongpaiboon, B.; Chitamara, T.; Chuntarapas, M.; Suwajanakorn, D. Foveal and parafoveal choroidal thickness pattern measuring by swept source optical coherence tomography. Eye (Lond). 2019, 33, 1443–1451. [Google Scholar] [CrossRef]
- Hirano, M.; Muraoka, Y.; Kogo, T.; Ishikura, M.; Nishigori, N.; Ueda-Arakawa, N.; Miyata, M.; Hata, M.; Takahashi, A.; Miyake, M.; Tsujikawa, A. Analysis of widefield choroidal thickness maps of healthy eyes using swept source optical coherence tomography. Sci Rep. 2023, 13, 11904. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lim, H.B.; Lee, W.H.; Kim, K.M.; Nam, K.Y.; Kim, J.Y. Wide-Field Swept-Source Optical Coherence Tomography Analysis of Interocular Symmetry of Choroidal Thickness in Healthy Young Individuals. Invest Ophthalmol Vis Sci. 2021, 62, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, X.; Huang, W.; Wang, W.; Chen, S.; Du, S.; Li, X.; Zhang, X. Swept-source optical coherence tomography imaging of macular retinal and choroidal structures in healthy eyes. BMC Ophthalmol. 2015, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.A.; Singh, S.R.; Invernizzi, A.; Cagini, C.; Goud, A.; Sahoo, N.K.; Cozzi, M.; Lupidi, M.; Chhablani, J. Wide-field choroidal thickness profile in healthy eyes. Sci Rep. 2018, 8, 17166. [Google Scholar] [CrossRef] [PubMed]
- Touhami, S.; Philippakis, E.; Mrejen, S.; Couturier, A.; Casteran, C.; Levent, P.; Tadayoni, R.; Gaudric, A. Topographic Variations of Choroidal Thickness in Healthy Eyes on Swept-Source Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 2020, 61, 38. [Google Scholar] [CrossRef]
- Xie, J.; Ye, L.; Chen, Q.; Shi, Y.; Hu, G.; Yin, Y.; Zou, H.; Zhu, J.; Fan, Y.; He, J.; Xu, X. Choroidal Thickness and Its Association With Age, Axial Length, and Refractive Error in Chinese Adults. Invest Ophthalmol Vis Sci. 2022, 63, 34. [Google Scholar] [CrossRef]
- Zhang, C.; Tatham, A.J.; Medeiros, F.A.; Zangwill, L.M.; Yang, Z.; Weinreb, R.N. Assessment of choroidal thickness in healthy and glaucomatous eyes using swept source optical coherence tomography. PLoS One. 2014, 9, e109683. [Google Scholar] [CrossRef]
- Yang, H.; Luo, H.; Gardiner, S.K.; Hardin, C.; Sharpe, G.P.; Caprioli, J.; Demirel, S.; Girkin, C.A.; Liebmann, J.M.; Mardin, C.Y.; Quigley, H.A.; Scheuerle, A.F.; Fortune, B.; Chauhan, B.C.; Burgoyne, C.F. Factors Influencing Optical Coherence Tomography Peripapillary Choroidal Thickness: A Multicenter Study. Invest Ophthalmol Vis Sci. 2019, 60, 795–806. [Google Scholar] [CrossRef]
- Mansoori, T.; Charan, A.S.R.; Nagalla, B. Topography and Choroidal Thickness Measurement in Healthy Asian Indian Subjects using RTVue XR 100 Optical Coherence Tomography. Middle East Afr J Ophthalmol. 2024, 30, 19–23. [Google Scholar] [CrossRef]
- Yao, Y.; Fu, J.; Liu, J.; Li, L.; Chen, W.; Meng, Z. Assessment of macular choroidal and retinal thickness: a cohort study in Tibetan healthy children. Sci Rep. 2024, 14, 1383. [Google Scholar] [CrossRef]
- Abbey, A.M.; Kuriyan, A.E.; Modi, Y.S.; Thorell, M.R.; Nunes, R.P.; Goldhardt, R.; Yehoshua, Z.; Gregori, G.; Feuer, W.; Rosenfeld, P.J. Optical coherence tomography measurements of choroidal thickness in healthy eyes: correlation with age and axial length. Ophthalmic Surg Lasers Imaging Retina. 2015, 46, 18–24. [Google Scholar] [CrossRef]
- Nadeem, S. Macular choroidal thickness and correlations in a healthy pediatric South Asian cohort: A spectral domain optical coherence tomography study. J Biophotonics. 2023, 16, e202300039. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, Y.; Chen, Y.; Ma, B.; Qian, Y.; Zhang, Z.; Zhu, D.; Wang, Z.; Xu, X. Analysis of Changes in Retinal Thickness in Type 2 Diabetes without Diabetic Retinopathy. J Diabetes Res. 2018, 2018, 3082893. [Google Scholar] [CrossRef] [PubMed]
- Boned-Murillo, A.; Fernández-Espinosa, G.; Orduna-Hospital, E.; Díaz-Barreda, M.D.; Sánchez-Cano, A.; Sopeña-Pinilla, M.; Bielsa-Alonso, S.; Pinilla, I. Changes in Inner Retina Thickness and Macular Sensitivity in Patients with Type 2 Diabetes with Moderate Diabetic Retinopathy. Biomedicines 2023, 11, 2972. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Cui, Y.; Jiao, W.; Zhao, B. Potential Prognostic Indicators for Patients With Retinal Vein Occlusion. Front Med (Lausanne). 2022, 9, 839082. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Duan, J.; Zhang, M. Pachychoroid Spectrum Disease: Underlying Pathology, Classification, and Phenotypes. Curr Eye Res. 2021, 46, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Safi, H.; Ahmadieh, H.; Hassanpour, K.; Safi, S. Multimodal imaging in pachychoroid spectrum. Surv Ophthalmol. 2022, 67, 579–590. [Google Scholar] [CrossRef]
- Baek, J.; Lee, J.H.; Jung, B.J.; Kook, L.; Lee, W.K. Morphologic features of large choroidal vessel layer: age-related macular degeneration, polypoidal choroidal vasculopathy, and central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2018, 256, 2309–2317. [Google Scholar] [CrossRef]
- Sabbaghi, H.; Ahmadieh, H.; Jalili, J.; Behnaz, N.; Fakhri, M.; Suri, F.; Kheiri, B.; Rajabpour, M.; Entezari, M.; Daftarian, N. Choroidal Thickness in Different Types of Inherited Retinal Dystrophies. J Ophthalmic Vis Res. 2020, 15, 351–361. [Google Scholar] [CrossRef]
- Abdolrahimzadeh, S.; Di Pippo, M.; Ciancimino, C.; Di Staso, F.; Lotery, A.J. Choroidal vascularity index and choroidal thickness: potential biomarkers in retinitis pigmentosa. Eye (Lond). 2023, 37, 1766–1773. [Google Scholar] [CrossRef]
- Baltmr, A.; Lightman, S.; Tomkins-Netzer, O. Examining the choroid in ocular inflammation: a focus on enhanced depth imaging. J Ophthalmol. 2014, 2014, 459136. [Google Scholar] [CrossRef] [PubMed]
- Dalvin, L.A.; Shields, C.L.; Ancona-Lezama, D.A.; Yu, M.D.; Di Nicola, M.; Williams, B.K., Jr.; Lucio-Alvarez, J.A.; Ang, S.M.; Maloney, S.M.; Welch, R.J.; Shields, J.A. Combination of multimodal imaging features predictive of choroidal nevus transformation into melanoma. Br J Ophthalmol. 2019, 103, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aggarwal, K.; Pichi, F.; Meng, T.; Munk, M.R.; Bazgain, K.; Bansal, R.; Agrawal, R.; Gupta, V. Clinical and Multimodal Imaging Clues in Differentiating Between Tuberculomas and Sarcoid Choroidal Granulomas. Am J Ophthalmol. 2021, 226, 42–55. [Google Scholar] [CrossRef] [PubMed]
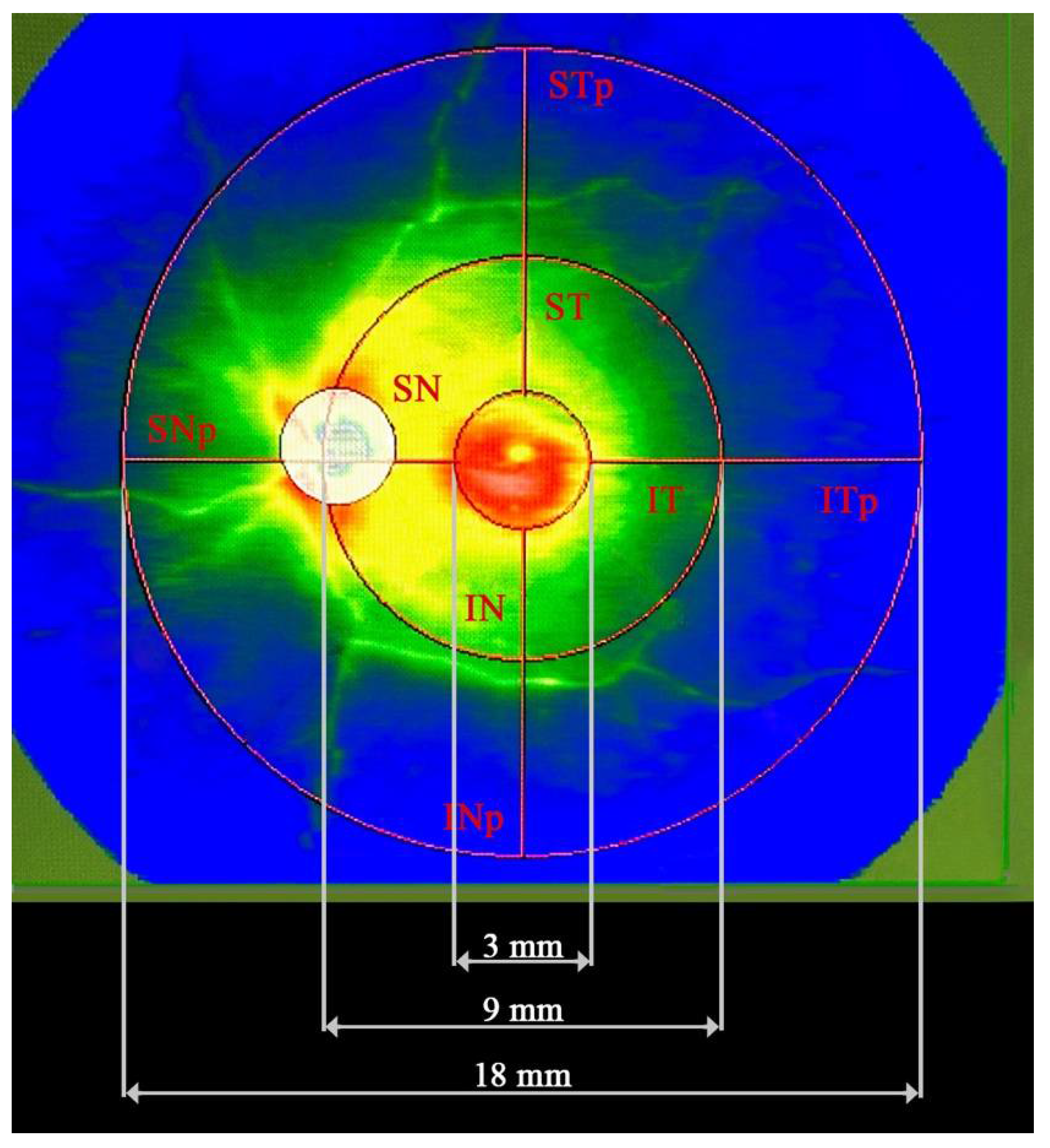
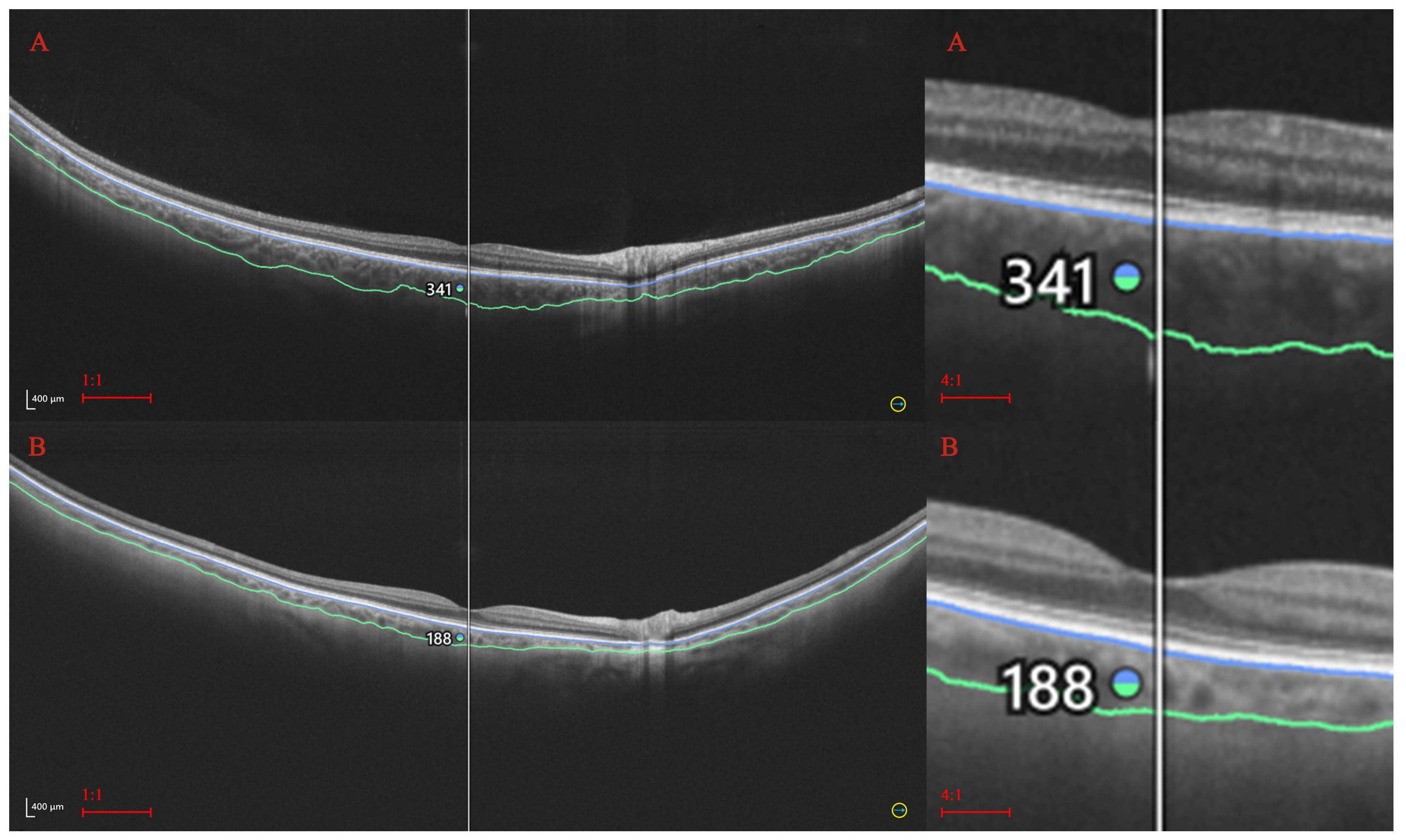
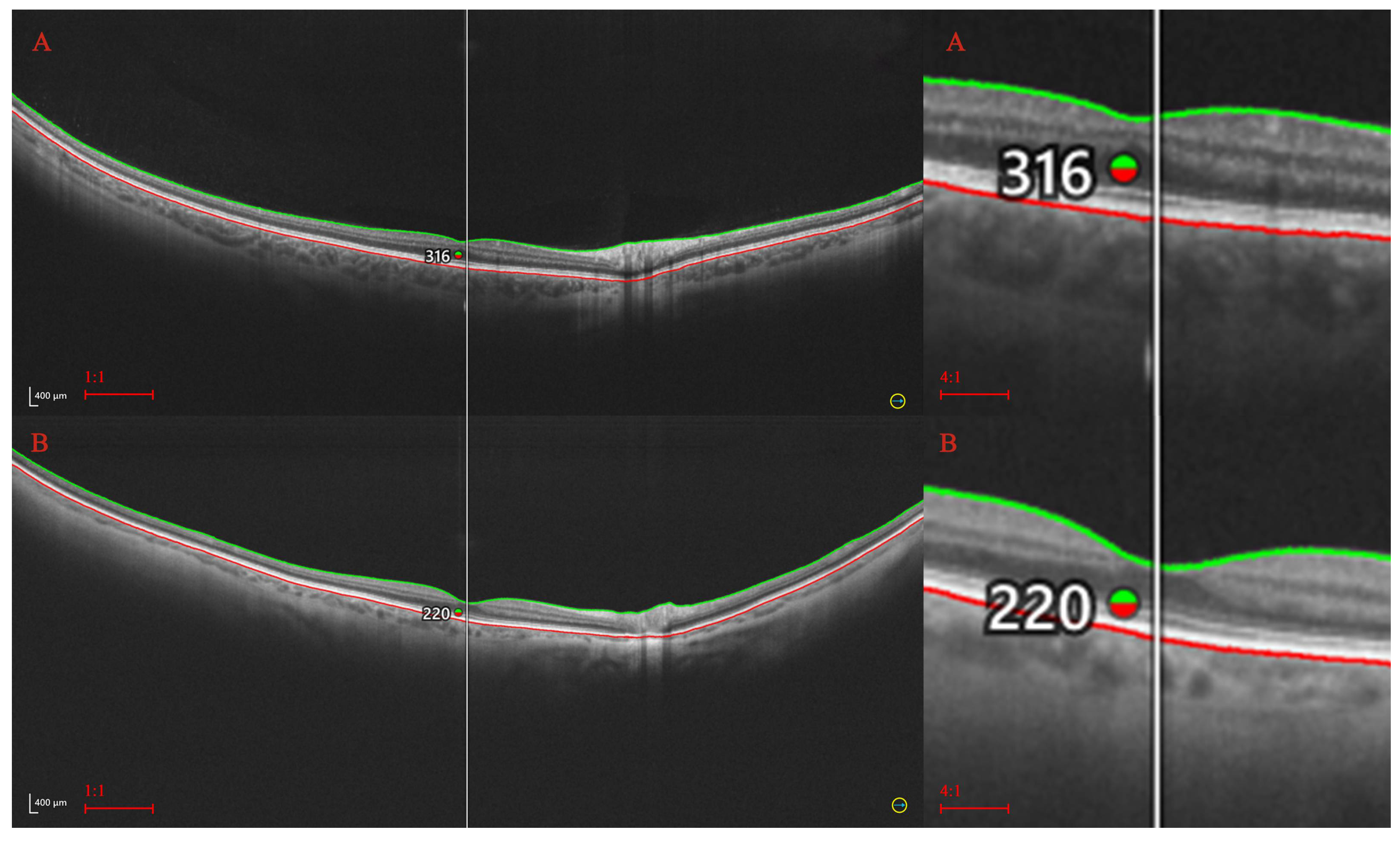
| Analyzed trait | No and (percentage in %.) |
|---|---|
| No. of participants | 75 (50,68) |
| No. of eyes | 125 (43,60) |
| Gender: | |
| Female | 48 (64,00) |
| Male | 27 (36,00) |
| Age group (years): | |
| Up to 40 | 24 (19,20) |
| 41–50 | 12 (9,60) |
| 51–60 | 33 (26,40) |
| 61–70 | 20 (16,00) |
| Over 70 | 36 (28,80) |
| Retinal thickness (µm) |
Statistical parameter | p-value * | |||
|---|---|---|---|---|---|
| M | SD | Me | Q1–Q3 | ||
| Central | 335,08 | 20,69 | 335,00 | 324,00–348,00 | < 0,0001 |
| ST | 271,90 | 14,10 | 273,75 | 262,00–282,00 | |
| SN | 309,65 | 16,69 | 311,50 | 299,00–320,25 | |
| IN | 300,99 | 17,91 | 302,50 | 289,25–311,50 | |
| IT | 267,42 | 16,51 | 268,75 | 259,00–278,00 | |
| STp | 212,32 | 10,60 | 212,00 | 205,00–220,00 | |
| SNp | 242,94 | 16,29 | 243,50 | 232,00–254,50 | |
| INp | 220,38 | 15,42 | 219,50 | 210,00–230,00 | |
| ITp | 205,52 | 11,69 | 206,00 | 199,00–214,00 | |
| Choroidal thickness (µm) |
Statistical parameter | p-value * | |||
|---|---|---|---|---|---|
| M | SD | Me | Q1–Q3 | ||
| Central | 293,90 | 87,10 | 300,00 | 223,00–357,00 | < 0,0001 |
| ST | 283,94 | 77,20 | 285,00 | 223,25–348,00 | |
| SN | 244,60 | 75,52 | 245,25 | 184,00–307,75 | |
| IN | 218,72 | 75,10 | 204,25 | 162,75–289,25 | |
| IT | 256,65 | 79,29 | 246,00 | 192,00–312,75 | |
| STp | 225,16 | 54,80 | 219,00 | 183,50–261,50 | |
| SNp | 192,38 | 57,77 | 189,00 | 151,50–237,50 | |
| INp | 139,98 | 39,78 | 129,50 | 107,50–163,00 | |
| ITp | 188,75 | 48,50 | 179,00 | 150,00–226,00 | |
| RT | Central | ST | SN | IN | IT | STp | SNp | INp |
|---|---|---|---|---|---|---|---|---|
| ST | < 0,0001 | |||||||
| SN | < 0,0001 | < 0,0001 | ||||||
| IN | < 0,0001 | < 0,0001 | < 0,0001 | |||||
| IT | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | ||||
| STp | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | |||
| SNp | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | ||
| INp | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | |
| ITp | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 |
| CT | Central | ST | SN | IN | IT | STp | SNp | INp |
|---|---|---|---|---|---|---|---|---|
| ST | = 0,0148 | |||||||
| SN | < 0,0001 | < 0,0001 | ||||||
| IN | < 0,0001 | < 0,0001 | < 0,0001 | |||||
| IT | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | ||||
| STp | < 0,0001 | < 0,0001 | < 0,0001 | = 0,1744 | < 0,0001 | |||
| SNp | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | ||
| INp | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | |
| ITp | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | < 0,0001 | = 0,2615 | < 0,0001 |
| Analyzed trait | Gender | Statistical parameter * | p-value ** | |||
|---|---|---|---|---|---|---|
| M | SD | Me | Q1–Q3 | |||
|
Central RT (µm) |
Male | 340,43 | 23,86 | 342,50 | 332,00–360,00 | = 0,0191 |
| Female | 332,17 | 18,26 | 334,00 | 323,00–345,00 | ||
| ST | Male | 274,93 | 14,61 | 276,62 | 262,12–283,88 | = 0,0007 |
| Female | 270,26 | 13,62 | 272,25 | 262,00–278,50 | ||
| SN | Male | 312,35 | 17,05 | 313,87 | 301,87–324,63 | = 0,0013 |
| Female | 308,18 | 16,41 | 311,00 | 297,75–319,75 | ||
| IN | Male | 306,52 | 16,79 | 305,62 | 296,62–317,75 | = 0,0148 |
| Female | 297,98 | 17,87 | 299,00 | 287,75–310,25 | ||
| IT | Male | 271,19 | 16,67 | 273,25 | 260,37–280,38 | = 0,0145 |
| Female | 265,44 | 16,17 | 267,00 | 258,75–275,00 | ||
| STp | Male | 216,30 | 10,23 | 217,00 | 208,00–223,75 | = 0,1218 |
| Female | 210,17 | 10,23 | 210,00 | 203,50–215,50 | ||
| SNp | Male | 249,59 | 15,53 | 250,25 | 238,00–259,75 | = 0,0241 |
| Female | 239,32 | 15,63 | 238,50 | 228,00–247,50 | ||
| INp | Male | 227,05 | 12,61 | 227,25 | 217,75–232,25 | = 0,5537 |
| Female | 216,75 | 15,66 | 216,00 | 205,50–227,50 | ||
| ITp | Male | 209,75 | 10,18 | 211,50 | 201,25–217,00 | = 0,3419 |
| Female | 203,23 | 11,87 | 203,00 | 197,00–212,00 | ||
| Central CT(µm) | Male | 284,70 | 79,44 | 289,00 | 218,00–343,50 | = 0,5435 |
| Female | 298,89 | 91,08 | 300,00 | 227,00–359,00 | ||
| ST | Male | 263,13 | 68,92 | 265,87 | 208,50–318,38 | < 0,0001 |
| Female | 295,25 | 79,48 | 301,50 | 237,25–359,50 | ||
| SN | Male | 239,19 | 73,25 | 232,75 | 175,12–293,38 | = 0,0009 |
| Female | 247,53 | 77,02 | 247,25 | 197,50–309,75 | ||
| IN | Male | 218,36 | 66,08 | 213,87 | 161,12–281,13 | = 0,0005 |
| Female | 218,91 | 79,98 | 200,50 | 162,75–291,25 | ||
| IT | Male | 239,76 | 65,72 | 240,75 | 186,12–281,88 | < 0,0001 |
| Female | 265,82 | 84,74 | 251,75 | 195,25–336,50 | ||
| STp | Male | 211,97 | 44,98 | 209,50 | 180,75–248,25 | < 0,0001 |
| Female | 232,33 | 58,48 | 232,00 | 184,50–269,50 | ||
| SNp | Male | 179,68 | 44,73 | 171,50 | 145,50–202,00 | = 0,0106 |
| Female | 199,28 | 62,94 | 195,00 | 151,50–243,00 | ||
| INp | Male | 133,90 | 27,82 | 127,75 | 112,50–151,00 | = 0,0131 |
| Female | 143,28 | 44,79 | 130,00 | 107,00–175,50 | ||
| ITp | Male | 175,33 | 41,51 | 159,75 | 144,50–217,75 | < 0,0001 |
| Female | 196,04 | 50,67 | 185,00 | 155,50–230,50 | ||
| Analyzed trait | Age group (years) | Statistical parameter * | p-value ** | |||
|---|---|---|---|---|---|---|
| M | SD | Me | Q1–Q3 | |||
|
Central RT (µm) |
Up to 40 | 336,55 | 15,16 | 340,50 | 324,25–348,58 | = 0,0388 |
| 41–50 | 337,58 | 12,00 | 342,50 | 326,42–247,58 | ||
| 51–60 | 344,00 | 17,88 | 344,50 | 336,08–353,58 | ||
| 61–70 | 338,30 | 25,51 | 334,00 | 328,00–352,67 | ||
| Over 70 | 326,67 | 21,97 | 330,50 | 311,42–338,17 | ||
| ST | Up to 40 | 276,91 | 18,78 | 279,62 | 276,46–283,79 | = 0,0017 |
| 41–50 | 275,44 | 10,82 | 277,12 | 268,60–284,44 | ||
| 51–60 | 278,27 | 6,82 | 277,75 | 272,35–283,17 | ||
| 61–70 | 271,39 | 13,18 | 267,75 | 260,83–280,58 | ||
| Over 70 | 265,12 | 13,34 | 263,12 | 256,85–272,42 | ||
| SN | Up to 40 | 315,90 | 19,36 | 320,00 | 303,48–328,65 | = 0,0041 |
| 41–50 | 314,85 | 14,70 | 315,50 | 308,87–321,88 | ||
| 51–60 | 314,96 | 9,75 | 315,12 | 306,29–319,75 | ||
| 61–70 | 307,53 | 15,16 | 308,25 | 298,25–317,08 | ||
| Over 70 | 302,87 | 17,29 | 301,00 | 291,56–318,00 | ||
| IN | Up to 40 | 304,12 | 23,71 | 312,12 | 288,60–321,83 | = 0,0072 |
| 41–50 | 306,54 | 13,08 | 309,62 | 303,92–315,44 | ||
| 51–60 | 307,06 | 11,99 | 304,12 | 297,31–315,21 | ||
| 61–70 | 301,45 | 17,19 | 299,50 | 291,67–310,33 | ||
| Over 70 | 293,10 | 17,31 | 294,00 | 282,25–307,42 | ||
| IT | Up to 40 | 270,44 | 22,21 | 274,62 | 269,67–282,31 | = 0,0129 |
| 41–50 | 271,71 | 11,37 | 272,87 | 262,35–279,46 | ||
| 51–60 | 272,56 | 7,98 | 270,37 | 267,00–278,85 | ||
| 61–70 | 269,33 | 15,00 | 267,00 | 258,67–279,25 | ||
| Over 70 | 259,56 | 17,13 | 260,25 | 249,31–268,33 | ||
| STp | Up to 40 | 213,00 | 11,78 | 212,00 | 209,92–220,25 | = 0,1967 |
| 41–50 | 213,73 | 9,33 | 210,75 | 206,42–222,17 | ||
| 51–60 | 214,00 | 7,48 | 214,50 | 206,92–220,79 | ||
| 61–70 | 214,15 | 11,27 | 214,50 | 205,00–220,67 | ||
| Over 70 | 208,78 | 10,64 | 208,25 | 200,00–216,38 | ||
| SNp | Up to 40 | 244,43 | 18,35 | 245,00 | 236,63–250,96 | = 0,0141 |
| 41–50 | 243,81 | 14,66 | 244,50 | 233,13–257,83 | ||
| 51–60 | 247,29 | 12,61 | 250,50 | 236,13–255,67 | ||
| 61–70 | 248,82 | 18,22 | 251,00 | 232,50–259,67 | ||
| Over 70 | 234,68 | 12,33 | 233,50 | 228,00–241,79 | ||
| INp | Up to 40 | 220,88 | 18,49 | 224,00 | 210,45–231,29 | = 0,3508 |
| 41–50 | 218,46 | 11,94 | 218,75 | 210,00–228,29 | ||
| 51–60 | 220,21 | 9,37 | 221,00 | 212,46–228,75 | ||
| 61–70 | 226,53 | 17,96 | 227,50 | 212,17–238,67 | ||
| Over 70 | 215,79 | 13,45 | 215,75 | 206,42–225,75 | ||
| ITp | Up to 40 | 206,23 | 16,14 | 206,50 | 200,71–216,67 | = 0,3770 |
| 41–50 | 206,75 | 9,39 | 204,75 | 200,21–212,29 | ||
| 51–60 | 205,67 | 6,50 | 205,75 | 199,75–210,38 | ||
| 61–70 | 208,11 | 11,17 | 209,50 | 199,33–217,00 | ||
| Over 70 | 201,90 | 11,68 | 202,25 | 194,71–213,29 | ||
|
Central CT (µm) |
Up to 40 | 345,20 | 84,63 | 348,50 | 309,17–395,50 | < 0,0001 |
| 41–50 | 329,00 | 68,41 | 321,50 | 289,17–373,25 | ||
| 51–60 | 288,50 | 111,02 | 259,00 | 196,75–394,42 | ||
| 61–70 | 286,33 | 66,65 | 281,00 | 260,00–338,33 | ||
| Over 70 | 250,72 | 87,46 | 229,50 | 181,75–325,83 | ||
| ST | Up to 40 | 339,04 | 70,66 | 345,37 | 297,31–380,81 | = 0,0002 |
| 41–50 | 311,45 | 73,91 | 306,50 | 254,85–388,38 | ||
| 51–60 | 278,58 | 80,97 | 262,75 | 196,27–359,00 | ||
| 61–70 | 269,54 | 53,54 | 266,25 | 235,25–314,33 | ||
| Over 70 | 249,98 | 80,54 | 238,37 | 190,44–310,13 | ||
| SN | Up to 40 | 274,55 | 74,53 | 293,37 | 226,90–340,08 | = 0,0809 |
| 41–50 | 260,87 | 63,83 | 239,12 | 212,60–321,88 | ||
| 51–60 | 251,52 | 84,97 | 246,37 | 175,87–329,33 | ||
| 61–70 | 241,94 | 65,57 | 247,50 | 206,00–274,67 | ||
| Over 70 | 217,24 | 82,63 | 204,62 | 144,60–286,25 | ||
| IN | Up to 40 | 252,46 | 63,59 | 270,00 | 218,17–299,73 | = 0,0217 |
| 41–50 | 239,48 | 69,80 | 211,75 | 174,79–309,83 | ||
| 51–60 | 223,12 | 89,06 | 194,12 | 146,98–284,35 | ||
| 61–70 | 212,36 | 92,93 | 204,50 | 183,67–263,50 | ||
| Over 70 | 190,48 | 81,63 | 160,75 | 128,77–227,73 | ||
| IT | Up to 40 | 299,30 | 72,50 | 292,62 | 257,08–342,94 | = 0,0021 |
| 41–50 | 289,04 | 77,39 | 272,75 | 222,00–355,63 | ||
| 51–60 | 258,87 | 107,23 | 218,50 | 182,62–653,60 | ||
| 61–70 | 242,96 | 62,02 | 240,25 | 201,67–274,67 | ||
| Over 70 | 223,15 | 72,54 | 193,87 | 155,35–257,23 | ||
| STp | Up to 40 | 274,23 | 40,18 | 259,25 | 240,08–313,71 | < 0,0001 |
| 41–50 | 240,98 | 54,80 | 237,75 | 199,42–271,88 | ||
| 51–60 | 224,79 | 46,28 | 234,75 | 180,12–267,21 | ||
| 61–70 | 209,86 | 36,25 | 210,50 | 196,00–222,50 | ||
| Over 70 | 201,50 | 59,76 | 190,75 | 154,24–242,13 | ||
| SNp | Up to 40 | 217,75 | 53,90 | 234,50 | 176,58–256,21 | = 0,2324 |
| 41–50 | 200,06 | 52,46 | 186,00 | 159,00–256,42 | ||
| 51–60 | 199,96 | 70,33 | 162,00 | 141,37–243,67 | ||
| 61–70 | 179,82 | 38,77 | 174,50 | 157,83–198,17 | ||
| Over 70 | 183,17 | 69,71 | 168,00 | 129,50–211,29 | ||
| INp | Up to 40 | 161,10 | 36,41 | 161,25 | 134,79–191,08 | = 0,1168 |
| 41–50 | 142,52 | 38,28 | 129,00 | 109,67–165,04 | ||
| 51–60 | 141,88 | 38,64 | 128,00 | 109,5–172,83 | ||
| 61–70 | 131,64 | 24,76 | 130,00 | 116,50–148,00 | ||
| Over 70 | 133,57 | 50,27 | 111,00 | 98,71–150,25 | ||
| ITp | Up to 40 | 219,10 | 31,22 | 224,00 | 196,46–237,92 | = 0,0029 |
| 41–50 | 202,63 | 49,94 | 182,25 | 163,42–248,67 | ||
| 51–60 | 195,83 | 62,17 | 167,00 | 149,96–242,67 | ||
| 61–70 | 177,62 | 39,24 | 170,50 | 151,67–198,83 | ||
| Over 70 | 170,40 | 49,09 | 152,50 | 133,67–194,97 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





