1. Introduction
Mosquitoes are considered the most dangerous animals on Earth, because they are vectors of numerous pathogens that cause deadly and debilitating diseases in humans and domestic animals, including malaria, West Nile virus, and heartworm [
1,
2,
3,
4]. The yellow fever mosquito
Aedes aegypti is a vector of several arboviruses of medical importance, including chikungunya, dengue, yellow fever, and Zika To limit transmission of mosquito-borne arboviruses, humans have relied heavily on insecticides to control the larval and/or adult life stages of mosquitoes. Synthetic insecticides, such as pyrethroids, are critical chemical tools used to control
Ae. aegypti; however, the widespread use of insecticides with limited modes of action has led to resistance [
5,
6,
7]. Additionally, the overuse of these insecticides has led to detrimental impacts on both human and environmental health [
8]. Because of this, the discovery of alternatives to synthetic pesticides, like biopesticides, is crucial to improving mosquito management.
Plant secondary metabolites constitute a diverse library of chemicals that mediate a wide array of interactions, including biotic defense, competition, and mutualism [
9]. As such, they provide a rich resource for the discovery of compounds for pharmaceuticals, antibiotics, and biopesticides. [
9,
10,
11,
12].
The hemp plant,
Cannabis sativa, is a rapidly emerging high value specialty crop that can be cultivated for the production of a wide array of products [
13]. For example, hemp seeds, which have numerous health benefits, are used to produce a diversity of goods including milk, dietary supplements, animal feed, and oil [
14,
15,
16,
17,
18]. Furthermore, hemp bark and stems are used for industrial production of textiles, paper and even concrete [
17,
19,
20]. Hemp leaves and inflorescences are also used in many products (e.g., essential oils) because of their high content of secondary metabolites [
21]. Both leaves and inflorescences produce more than 1,000 different secondary metabolites including highly volatile and aromatic terpenes and phenols, as well as semi-volatile phytocannabinoids [
21,
22]. Phytocannabinoids are specialized metabolites that were first discovered in
C. sativa, but have also been found in
Helichrysum umbraculigerum Less [
23,
24],
Amorpha fruticosa L. (Fabaceae),
Glycyrrhiza foetida and several
Rhodondedron species [
24].
In addition to medicinal uses, phytocannabinoids possess insecticidal and/or antifeedant activity against herbivorous insects. For example, as hemp plants produce more phytocannabinoids, lepidopteran larvae (
Trichoplusia ni and
Manduca sexta) have shown to feed less on plant tissue [
25,
26]. Additionally, larval survival and growth of
T. ni is reduced when cannabidiolic acid (CBDA) and cannabigerolic acid (CBGA) were incorporated in larval diets [
25]. Moreover, cannabidiol (CBD) oil (3%) impairs development and lowers survival rates in stored product insect pests (
Plodia interpunctella,
Oryzaephilus surinamensis, and
Tribolium confusum) [
27].
Hemp also produces over 100 terpenes, more than 40 noncannabinoid phenolics, and more than 30 flavonoids [
22,
28]. Notably, the terpenes linalool, eucalyptol, p-cymene, and thymol have shown larvicidal and/or adulticidal activity to various mosquitoes species [
29,
30,
31]. Thus, the hemp plant produces a variety of secondary metabolites with potential for mosquito control.
Consistent with this notion, hemp-based essential oils and extracts are larvicidal and adulticidal against multiple mosquito species [
32,
33,
34,
35,
36,
37]. However, cross resistance of pyrethroid-resistant mosquito strains to hemp extracts and the specific compound(s) responsible for mosquitocidal activities within the extracts are unknown. To address these gaps of knowledge, the objectives of this study were to 1) compare the larvicidal potency of hemp leaf extracts against pyrethroid-susceptible (PS) and pyrethroid-resistant (PR) strains of
Ae. aegypti and 2) identify the principal active larvicidal ingredients in the extracts that elicit mortality.
2. Materials and Methods
2.1. Hemp Plants
The hemp material used to obtain the raw extract was grown under controlled greenhouse conditions (24ºC 14:10 L:D cycle). The cuttings for new plants used for the experiment were obtained from previously grown hemp mother plants (variety Tango Kush). In brief, fresh shoots were cut using a sanitized razor blade, and the cuttings were placed in a 500 ppm DIP ‘N’ Grow Hormonal Rooting Concentrate (Clackamas, OR, USA) for 30 sec. The cuttings were then transferred to a 34 ct double strip OASIS wedge (Grow It Depot, Long Branch NJ, USA) filled with Pro-Mix BX soilless medium (Quebec, Canada) and placed in a mist chamber at controlled conditions. When root growth was adequate, hemp plants were transplanted into 38 L plastic pots and placed in a greenhouse room maintained at 25°C, 16:8 (L:D) by an Argus Control System (Conviron Company, British Columbia, Canada).
2.2. Hemp Extract
Leaves were removed from the hemp plants 7 months after planting and air-dried at 25°C for 7 days. Dried vegetative material was pulverized using a coffee grinder; 150 g of powdered material was extracted in 4 L methanol for 3 weeks at 20°C, shaking the solution for 2-3 min daily. The incubated solution was then filtered through a membrane filter paper on top of a Porcelain Buchner funnel (Fisherbrand, Waltham MA, USA) attached to a Little Giant pressure vacuum pump (Gelman Instrument Company, Ann Arbor, MI, USA). The solution was placed in a Hei-Vap Rotory Evaporator (Heidolph NA, Wood Dale, IL, USA) at 30°C and 65 rpm to remove the methanol. The resulting crude residue (dried extract) was kept at 4°C and aliquots were resuspended in 100% acetone immediately prior to use in bioassays.
2.3. Methanol and Hexane Partitioning
We partitioned 50 mg of dried extract between 20 mL hexane and 20 mL methanol using a 60 mL separatory funnel (Chemglass Life Sciences LLC, Vineland, NJ, USA), with the resulting partitions collected into separate 40 mL glass vials (Thermo Scientific, Rockwood, TN, USA). The solutions were then evaporated to dryness under nitrogen at room temperature, using a Reacti-Vap (Thermo Scientific, Waltham, MA, USA) connected to a Nitrox UHPLCMS12 nitrogen generator (Domnick Hunter, Gateshead, UK). The residues were resuspended in acetone to reach the desired concentration for mosquito bioassays or gas chromatography/nuclear magnetic resonance analysis.
2.4. Gas Chromatography-Mass Spectrometry (GC-MS) and Nuclear Magnetic Resonance (NMR)
GC-MS analyses were performed using an Agilent Technologies 7890A GC equipped with a 7683B auto-sampler and interfaced to a 5975C inert mass selective detector (Agilent Technologies, Santa Clara, CA, USA). A 1-µl sample was injected splitless at 280 °C, with a constant He flow of 1.1 ml/min. The GC column was HP-5MS, 30 m x 250 µm diam x 0.25 µm film thickness. The oven was programmed with an initial temperature of 35 °C for 1 min, increased 7 °C/min to 100 °C, then 25 °C/min to 280 °C held for 10 min., with the MS transfer line at 280 °C. The MS was operated in scan mode using m/z 19-450 with source at 230°C and quadrupole at 150 °C. GC peaks were identified by searching their mass spectra against the NIST/EPA/NIH Mass Spectral Library and comparing relative retention times against published values. To quantitate CBD, a calibration curve was prepared from a CBD isolate (99%, Extract Labs, Boulder, CO) dissolved in acetone, and injected into the GC-MS (19.4 - 525 ng, linear R2 = 0.98).
Hemp extracts and methanol/hexane partitions were also analyzed via proton NMR. They were first dissolved in 0.6 mL of deuterated chloroform before analysis with a Bruker AVANCE III 400 MHz NMR (Bruker, Billerica, MA, USA). Resonances were analyzed and compared with previously published data [
38].
2.5. Aedes Aegypti Colony
Larvae of
Ae. aegypti from Liverpool (strain LVP-IB12, MRA-735, contributed by David W. Severson) and Puerto Rico (strain Puerto Rico, NR-48830, contributed by G.G. Clark & J.J. Becnel) strains were reared from eggs using established methods [
39,
40]. The original eggs were provided by the Centers for Disease Control and Prevention for distribution by BEI Resources, NIAID, NIH. Larvae of the Puerto Rico strain used in the present study were over 30-times resistant to the pyrethroid cypermethrin compared to the Liverpool strain (
Supplemental Figure S1). From here, we refer to the Liverpool strain as pyrethroid-susceptible (PS) and the Puerto Rico strain as pyrethroid-resistant (PR). Larvae from both strains were nourished with fish food (Tropical Tablets, Tetramin, Blacksburg, VA, USA). Adult mosquitoes were provided 10% sucrose solution ad libitum. For additional egg production, adult females were fed defibrinated rabbit blood (Hemostat Laboratories, Dixon, CA, USA) using a membrane feeder (Hemotek, Blackburn, UK). All mosquitoes were reared in environmentally controlled chambers held at 28°C and 80% RH, with a 12:12 L:D cycle.
2.6. Larval Bioasay
Larvicidal activities of hemp extracts, methanol/hexane fractions, and CBD isolates against 1
st instar larvae were determined using an established bioassay [
40]. In brief, to each well of a 24-well Falcon® Multiwell plate (Becton Dickinson Labware, Franklin Lakes, NJ, USA) the following was added: 985 µL of deionized H
2O (diH
2O), six 1
st-instar
Ae. aegypti, 5 µL of food solution (13 mg/mL of finely ground fish food flakes; Tetramin, Blacksburg, VA, USA), and 10 µL of a treatment (hemp extract, methanol fraction, hexane fraction, CBD isolate, or suitable solvent control). The hemp extract and the methanol and hexane fractions were serial diluted with acetone to 100, 33, 11, 3.6, and 1.2 ppm. CBD isolate was serial diluted with acetone to 20, 6.0, 2.2, 0.7, and 0.24 ppm. The CBD isolate concentrations were selected to match the corresponding CBD concentrations in the hemp extracts as determined by GC-MS. The plates were held under standard rearing conditions, and larval mortality was assessed at 48 h. Larvae were considered dead if they did not move after gently touching their abdomen with a fine needle or pipette tip. All mortality values were corrected for solvent control mortality using Abbott’s formula [
41]. If solvent control mortality exceeded 20% then the trial was excluded.
2.7. Statistical Analysis
Data analysis and plotting were performed using GraphPad Prism (version 6.07) software (GraphPad, San Diego, CA, USA). Median lethal concentrations (LC50) were determined by plotting percent mortalities against log transformations of the concentration tested. A non-linear regression (‘log(agonist) vs. normalized response’ function) was used to best fit the data and calculate LC50. Statistical comparison of LC50 values was performed through sum-of-squares F-tests (α =0.05).
3. Results
3.1. Hemp Leaf Extract Toxicity against 1st Instar Larvae
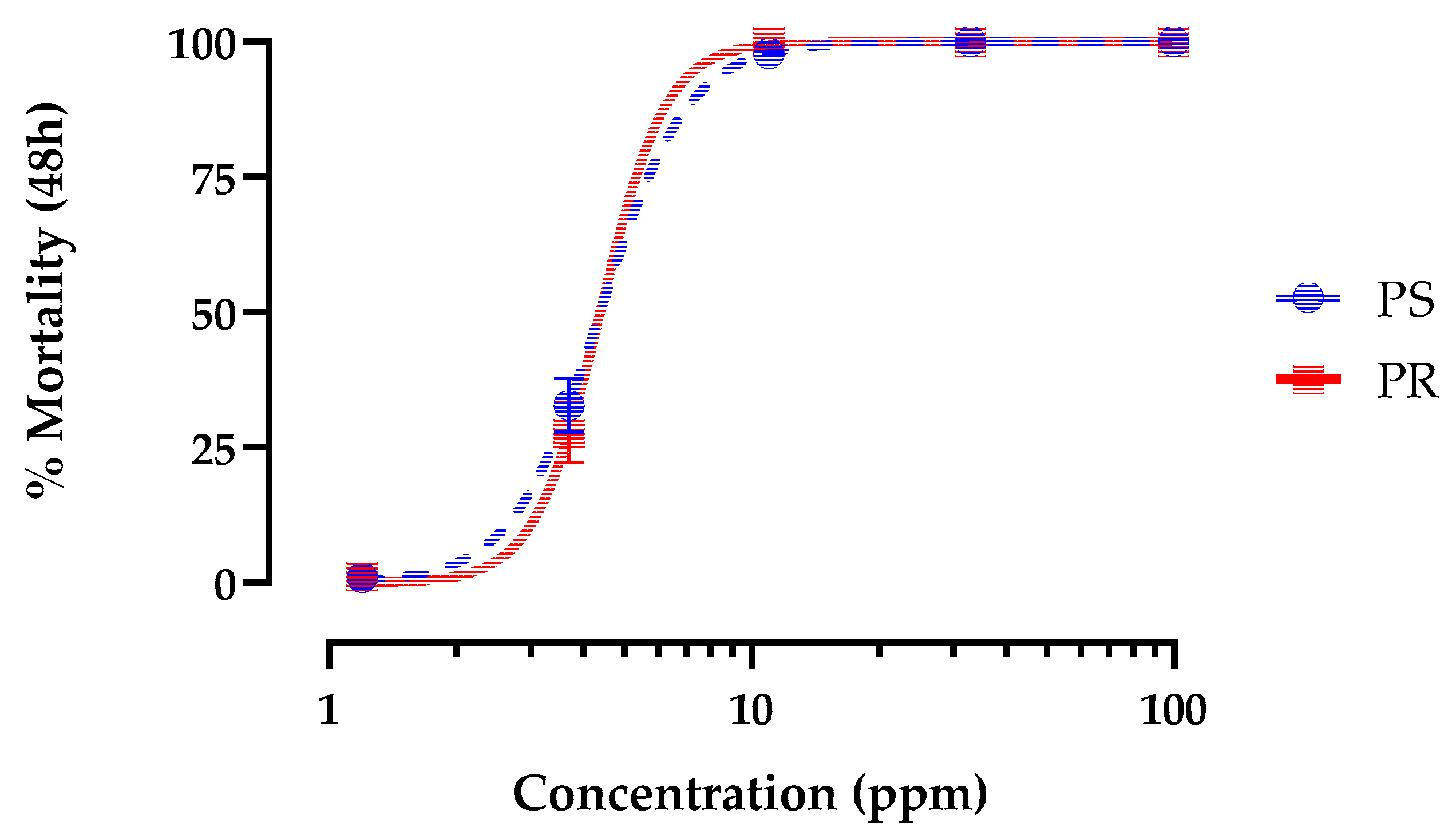
Application of hemp leaf extracts to the rearing water of 1st instar Ae. aegypti caused concentration-dependent mortality in both PS and PR strains within 48 h that reached 100%. The LC50 value of leaf extracts in the PS strain (4.4 ppm; 95% CI = 4.0 - 4.8 ppm) was not different (p = 0.85; F= 0.03) than that of the PR strain (4.3 ppm; 95% CI = 2.37-7.70ppm).
Figure 1.
Concentration-response curves for 48 h larvicidal activity of hemp leaf extract against 1st instar pyrethroid-susceptible (blue) and pyrethroid-resistant (red) Ae. aegypti. Values plotted are means ± standard errors of the mean (SEM) based on 28 replicates of 6 larvae per concentration (1.2, 3.7, 11, 33, 100 ppm).
Figure 1.
Concentration-response curves for 48 h larvicidal activity of hemp leaf extract against 1st instar pyrethroid-susceptible (blue) and pyrethroid-resistant (red) Ae. aegypti. Values plotted are means ± standard errors of the mean (SEM) based on 28 replicates of 6 larvae per concentration (1.2, 3.7, 11, 33, 100 ppm).
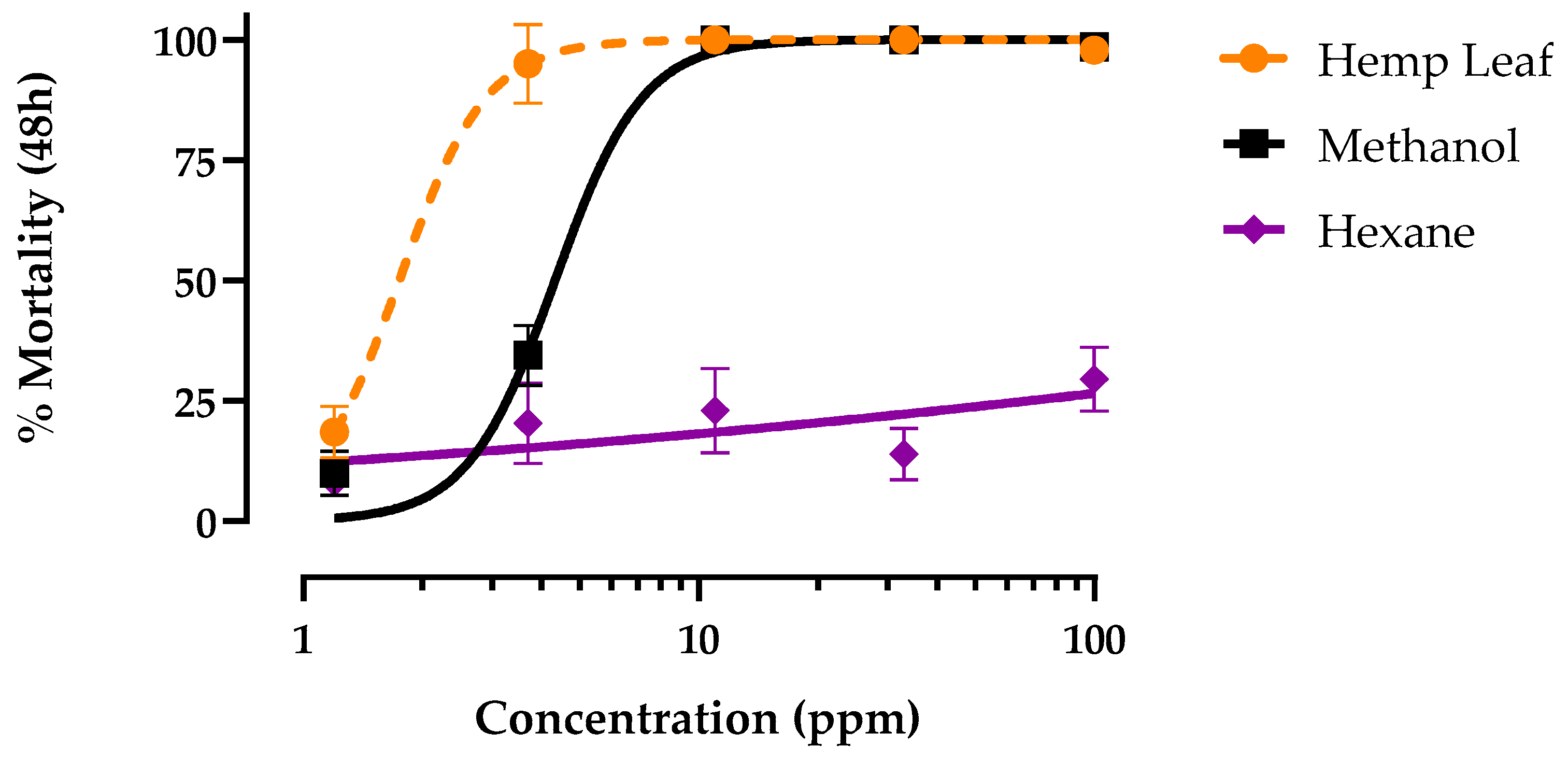
3.2. Methanol and Hexane Partition Toxicity against 1st Instar Larvae
Dried hemp leaf extract was partitioned between methanol and hexane to separate polar and nonpolar constituents, respectively. These partitions were then tested in parallel with the original unpartitioned hemp leaf extract for larvicidal activity against the PS strain. Both the methanol partition and unfractionated leaf extract produced concentration-dependent mortality within 48 h that reached 100% (
Figure 2). However, the LC
50 of the methanol partition (4.3 ppm; 95% CI = 3.8-4.9 ppm) was ~2.5-times less potent (p < 0.001, F = 88.1,) than that of the unfractionated leaf extract (1.7 ppm; 95% CI = 1.4-2.0 ppm). In contrast, the hexane fraction did not elicit concentration-dependent mortality, reaching only 25% mortality at the maximal concentration tested (
Figure 2).
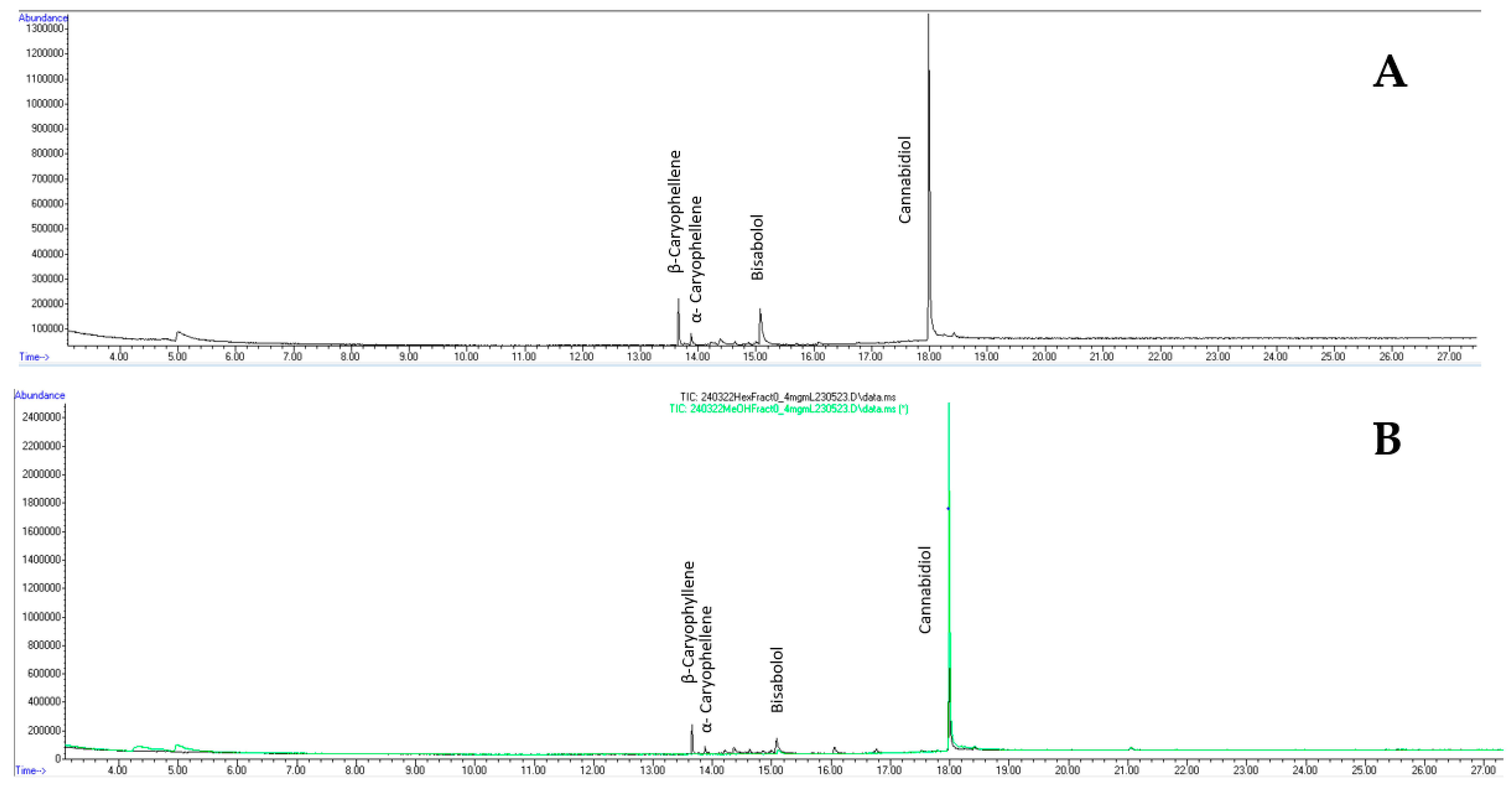
3.3. GC-MS and NMR Analysis
GC-MS identified CBD as the most abundant compound in the original dried leaf extract and both partitions; however, the methanol partition contained ~4x more CBD (80% of CBD found in dried leacf extract) compared to the hexane partition (20%) (
Figure 3B). Additionally, the analysis indicated the prescence of other compounds like α- and β-caryophellene and bisabolol in both the unpartitioned extract and hexane partition, but not the methanol partition (
Supplemental Table S1). The
1H NMR spectra of the leaf extracts measured in deuterated chloroform were superposable with the
1H NMR spectrum reported by Barthlott., et al [
38]. Since CBD was observed as the major compound, quick
13C NMR was collected to confirm its presence in the extract (
Supplemental Figure S2). Moreover, signals arising from proton (
1H) of the active methanol fraction showed CBD as the major compound and a trace of THC analogs (
Supplemenal Figure S3). Resonances arising from the protons of bisabolol were also observed (δ 5.36, brs; δ 5.12, brt; and singlet methyls at δ ~1.6 ppm;
Supplemental Figure S4); they were similar to those reported by Cerceau and co-workers [
42].
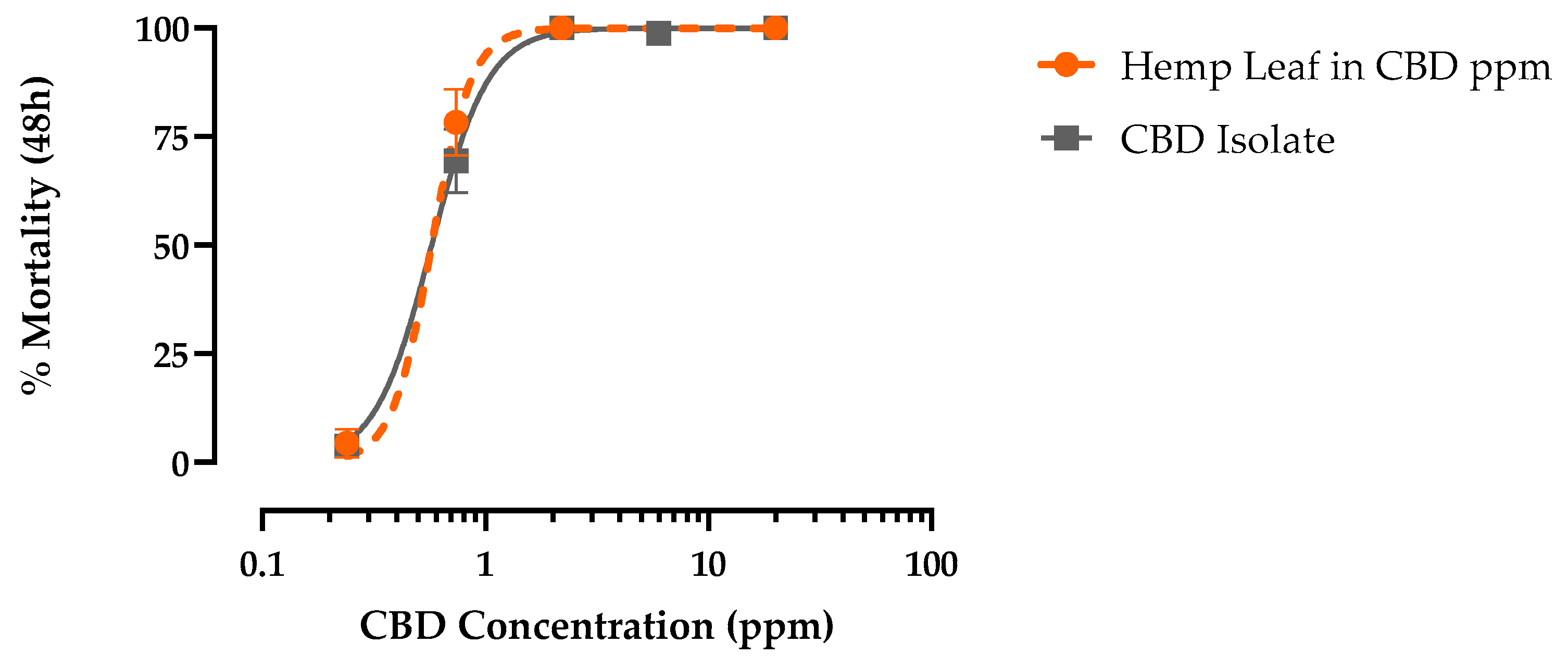
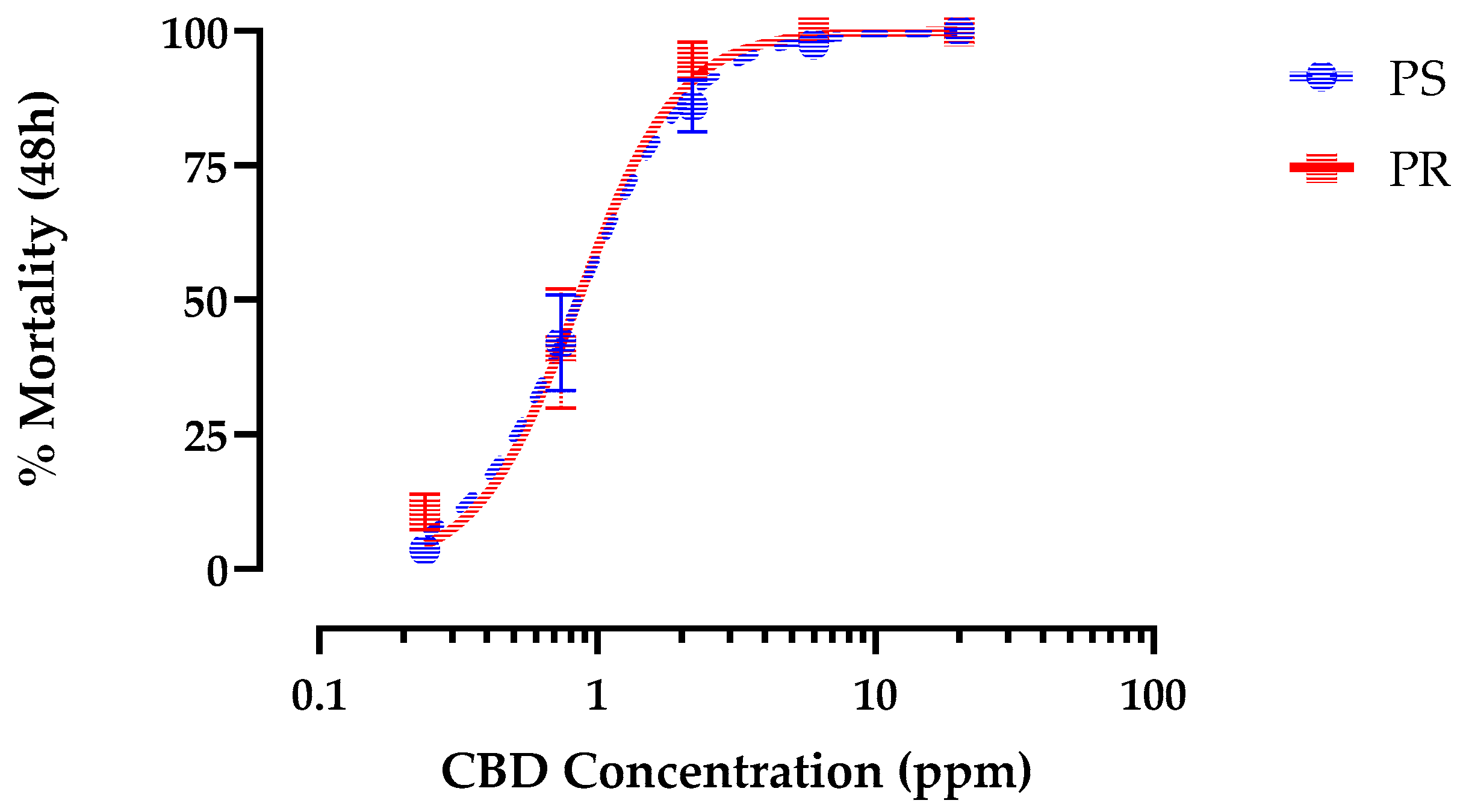
3.4. CBD Toxicity against 1st Instar Larvae
Authentic CBD produced concentration-dependent mortality in PS 1
st instar larvae within 48 h that reached 100% and was indistinguishable from hemp leaf extract when standardized for CBD concentration(
Figure 5). The LC
50 of CBD (0.59 ppm, 95% C.I.=0.52-0.67 ppm) was similar (F = 0.95, p > 0.32), to hemp leaf extract (0.53 ppm, 95% C.I.=0.46-0.62 ppm) in PS 1
st instar Ae. aegypti (
Figure 5). Moreover, like the hemp leaf extract, CBD elicited similar (F = 0.26, p > 0.6) larvicidal potency against PR (LC
50 = 0.83 ppm, 95% C.I.=0.69-0.99 ppm) and PS (LC
50 = 0.88 ppm, 95% C.I. = 0.75-1.04 ppm) strains (
Figure 6).
4. Discussion
We demonstrated that hemp leaf extracts elicit concentration-dependent larvicidal activity against
Ae. aegypti. These results are consistent with previous studies that have found concentration-dependent larvicidal activity of hemp extracts against other mosquitoes, including
Culex quinquefasciatus,
Anopheles stephensi,
An. gambiae,
Ae. albopictus, and
Ae. aegypti [
31,
32,
33,
34,
35,
43]. In addition, we demonstrated that hemp leaf extracts exhibited similar toxic potency against a PR strain of
Ae. aegypti that has both target-site (
kdr) and metabolic resistance to pyrethroids [
5,
7,
44,
45]. This finding suggests that hemp leaf extracts have potential to bypass pyrethroid-resistance in mosquito larvae.
We also identified CBD as the primary active ingredient within the hemp extract responsible for its larvicidal activity. CBD was by far the most abundant compound in our hemp leaf extracts and methanol partitions as detected by GC-MS and proton NMR. Terpenes, which have previously been speculated as the primary larvicidal compounds in hemp extracts [
36,
46,
47,
48,
49,
50] were of nominal abundance. Moreover, the hexane fraction of our dried hemp extract, in which terpenes are expected to partition, was minimally larvicidal. Importantly, the larvicidal potency of CBD isolate matched that of the hemp leaf extract in both the PS and PR strains of
Ae. aegypti. Moreover, the larvicidal potency of the methanol partition of the leaf extract was slightly weaker than that of the unpartitioned dried leaf extract, but far superior to that of the hexane partition of the leaf extract, consistent with the relative abundances of CBD in these samples.
Our findings regarding the toxicity of CBD to mosquitoes align well with prior research suggesting CBD has toxic, antifeedant, and/or growth inhibiting properties against other insects. For example, larvae of three economically important lepidopteran pests, the tobacco hornworm (
Manduca sexta), the corn earworm (
Helicoverpa zea) and the fall army worm (
Spodoptera frugiperda), all showed reduced size, weight loss, and decreased consumption rates when feeding on diets supplemented with CBD [
26,
51,
52]; additionally,
M. sexta larvae that consumed high doses of CBD experienced higher mortality [
26]. Likewise, larvae of the cabbage looper,
T. ni, consumed less leaf area on CBD-dominant
C. sativa genotypes compared to the cannabinoid free genotypes, leading to a decrease in larval mass and increase of mortality [
25]. Furthermore, in larvae of three common stored product insect pests, the meal moth (
P. interpunctella), saw toothed grain beetle (
O. surinamensis), and flour beetle (
T. confusum), mortality significantly increased after exposure to grains that had been sprayed with high doses of CBD oil [
27].
The specific mode of action of CBD toxicity against mosquitoes and other insects is unknown. Intriguingly, insects are one of the few animal groups that do not possess canonical cannabinoid receptors [59,60]. However, at least in mammalian systems, CBD is known to modulate a wide range of biochemical targets, including orthologs of known insecticide targets, such as sodium channels, potassium channels, calcium channels, transient receptor potential channels, G protein-coupled receptors, and acetylcholinesterase [
23,
53,
54,
55,
56] . Thus, it is likely to affect multiple biochemical targets and tissues in insects. Notably, in ventral chain ganglia of
M. sexta larvae, CBD treatment delayed the onset of electrophysiological responses to electrical stimuli, but the magnitude of the responses was enhanced [
30]. Whether these neuromodulatory effects of CBD on the lepidopteran central nervous system contribute to its toxicity remains to be determined.
5. Conclusions
The present research has shown that hemp leaf extracts have larvicidal activity against PS and PR strains of
Ae. aegypti and that CBD is the principal active ingredient in the extract. Altogether, our study provides additional evidence supporting the notion by others that hemp is a valuable resource for developing novel insecticides to control mosquitoes [
33,
43,
57]. Notably, hemp extracts have been shown to be non-toxic to select non-target invertebrates [
43,
58] and may be beneficial to honeybee workers [
59]. Hemp is also an emerging, readily cultivated crop in the U.S. [
13,
16,
19,
60] and its leaves are often discarded [
33,
57]. Thus, availability of raw material would not appear limited as it can be for other sources of biopesticides [
46]. Further research is needed to elucidate the mode of toxic action in larvae and the potential toxic and repellent effects of hemp extracts and CBD on adult female mosquitoes. It is also necessary to examine whether CBD elicits synergistic effects when applied with other established insecticides, as has been found for other natural products [
53,
61].
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, E.J.M.R., P.M.P., P.L.P., L.C., and L.R.; methodology, E.J.M.R., P.M.P., P.L.P., L.R., L.C., and N.A.; validation, E.J.M.R., P.M.P., P.L.P, and L.R.; formal analysis, E.J.M.R., P.M.P. and P.L.P.; investigation, E.J.M.R., P.M.P., P.L.P; resources, E.J.M.R., P.L.P., L.C., L.R., and P.M.P.; data curation, E.J.M.R., and P.M.P.; writing—original draft preparation, E.J.M.R., P.L.P., and P.M.P.; writing—review and editing, E.J.M.R., P.L.P., L.R., L.C., N.A., and P.M.P.; visualization, E.J.M.R., P.M.P. and P.L.P.; supervision, P.M.P.; project administration, P.M.P.; funding acquisition, E.J.M.R. and P.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds provided by the Infectious Diseases Institute (idi.osu.edu) of The Ohio State University and by state and federal funds appropriated to The Ohio State University, College of Food, Agricultural, and Environmental Sciences, Ohio Experiment Station.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
The authors would like to thank Haley Fleetwood and Paola Feliciano for their excellent technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Unlu, I.; Mackay, A.J.; Roy, A.; Yates, M.M.; Foil, L.D. Evidence of Vertical Transmission of West Nile Virus in Field-Collected Mosquitoes. Journal of Vector Ecology 2010, 35, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Karema, C.; Wen, S.; Sidibe, A.; Smith, J.L.; Gosling, R.; Hakizimana, E.; Tanner, M.; Noor, A.M.; Tatarsky, A. History of Malaria Control in Rwanda: Implications for Future Elimination in Rwanda and Other Malaria-Endemic Countries. Malar J 2020, 19, 356. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue Infection. Nat Rev Dis Primers 2016, 2, 16055. [Google Scholar] [CrossRef] [PubMed]
- Spence Beaulieu, M.R.; Federico, J.L.; Reiskind, M.H. Mosquito Diversity and Dog Heartworm Prevalence in Suburban Areas. Parasites Vectors 2020, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Estep, A.S.; Sanscrainte, N.D.; Waits, C.M.; Louton, J.E.; Becnel, J.J. Resistance Status and Resistance Mechanisms in a Strain of Aedes Aegypti (Diptera: Culicidae) From Puerto Rico. Journal of Medical Entomology 2017, 54, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Ranson, H.; Burhani, J.; Lumjuan, N. Insecticide Resistance in Dengue Vectors. 12.
- Vontas, J.; Kioulos, E.; Pavlidi, N.; Morou, E.; della Torre, A.; Ranson, H. Insecticide Resistance in the Major Dengue Vectors Aedes Albopictus and Aedes Aegypti. Pesticide Biochemistry and Physiology 2012, 104, 126–131. [Google Scholar] [CrossRef]
-
Environmental Deterioration and Human Health: Natural and Anthropogenic Determinants; Malik, A., Grohmann, E., Akhtar, R., Eds.; Springer Netherlands: Dordrecht, 2014; ISBN 978-94-007-7889-4.
- Rattan, R.S. Mechanism of Action of Insecticidal Secondary Metabolites of Plant Origin. Crop Protection 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental Stress and Secondary Metabolites in Plants. In Plant Metabolites and Regulation Under Environmental Stress; Elsevier, 2018; pp. 153–167 ISBN 978-0-12-812689-9.
- Górski, R.; Sobieralski, K.; Siwulski, M. The Effect of Hemp Essential Oil on Mortality Aulacorthum solani Kalt. And Tetranychus Urticae Koch. Ecological Chemistry and Engineering S 2016, 23, 505–511. [Google Scholar] [CrossRef]
- Fang, X.; Yang, C.; Wei, Y.; Ma, Q.; Yang, L.; Chen, X. Genomics Grand for Diversified Plant Secondary Metabolites. 13.
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A Review on the Current State of Knowledge of Growing Conditions, Agronomic Soil Health Practices and Utilities of Hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A Review of Hemp as Food and Nutritional Supplement. Cannabis and Cannabinoid Research 2021, 6, 19–27. [Google Scholar] [CrossRef]
- Vahanvaty, U.S. Hemp Seed and Hemp Milk: The New Super Foods? ICAN: Infant, Child, & Adolescent Nutrition 2009, 1, 232–234. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Cheng, C.; Lv, J.; Lambo, M.T.; Zhang, G.; Li, Y.; Zhang, Y. Nutritional Values of Industrial Hemp Byproducts for Dairy Cattle. Animals 2022, 12, 3488. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.T.M.F.; Islam, M.Z.; Mahmud, M.S.; Sarker, M.E.; Islam, M.R. Hemp as a Potential Raw Material toward a Sustainable World: A Review. Heliyon 2022, 8, e08753. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.A.; Andres, M.; Cole, M.; Cowley, J.M.; Augustin, M.A. Industrial Hemp Seed: From the Field to Value-Added Food Ingredients. J Cannabis Res 2022, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, P.; Venturi, G. Hemp as a Raw Material for Industrial Applications. Euphytica 2004, 140, 1–6. [Google Scholar] [CrossRef]
- Jami, T.; Karade, S.R.; Singh, L.P. A Review of the Properties of Hemp Concrete for Green Building Applications. Journal of Cleaner Production 2019, 239, 117852. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci Rep 2020, 10, 3309. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis Sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Berman, P.; de Haro, L.A.; Jozwiak, A.; Panda, S.; Pinkas, Z.; Dong, Y.; Cveticanin, J.; Barbole, R.; Livne, R.; Scherf, T.; et al. Parallel Evolution of Cannabinoid Biosynthesis. Nat. Plants 2023, 9, 817–831. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Stack, G.M.; Snyder, S.I.; Toth, J.A.; Quade, M.A.; Crawford, J.L.; McKay, J.K.; Jackowetz, J.N.; Wang, P.; Philippe, G.; Hansen, J.L.; et al. Cannabinoids Function in Defense against Chewing Herbivores in Cannabis Sativa L. Horticulture Research 2023, 10, uhad207. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Staples, S.K.; Gostin, E.L.; Smith, J.P.; Vigil, J.J.; Seifried, D.; Kinney, C.; Pauli, C.S.; Heuvel, B.D.V. Contrasting Roles of Cannabidiol as an Insecticide and Rescuing Agent for Ethanol–Induced Death in the Tobacco Hornworm Manduca Sexta. Sci Rep 2019, 9, 10481. [Google Scholar] [CrossRef] [PubMed]
- Mantzoukas, S.; Ntoukas, A.; Lagogiannis, I.; Kalyvas, N.; Eliopoulos, P.; Poulas, K. Larvicidal Action of Cannabidiol Oil and Neem Oil against Three Stored Product Insect Pests: Effect on Survival Time and in Progeny. Biology 2020, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Flores Guillermo, A.; Nahuel, F.; María, Td.; Andrés, Mv.; Sara, Mp. Adulticidal Effect of Seven Terpenes and a Binary Combination against Aedes Aegypti. J Vector Borne Dis 2020, 57, 356. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-H.; Kim, M.-K.; Noh, D.-J.; Yoon, C.; Kim, G.-H. Spray Adulticidal Effects of Plant Oils against House Mosquito, Culex Pipiens Pallens (Diptera: Culicidae). J. Pestic. Sci. 2009, 34, 100–106. [Google Scholar] [CrossRef]
- Ramzi, A.; El Ouali Lalami, A.; Annemer, S.; Ez zoubi, Y.; Assouguem, A.; Almutairi, M.H.; Kamel, M.; Peluso, I.; Ercisli, S.; Farah, A. Synergistic Effect of Bioactive Monoterpenes against the Mosquito, Culex Pipiens (Diptera: Culicidae). Molecules 2022, 27, 4182. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Larvicidal Property of Essential Oils against Culex Quinquefasciatus Say (Diptera: Culicidae). Industrial Crops and Products 2009, 30, 311–315. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Lupidi, G.; Nabissi, M.; Petrelli, R.; Ngahang Kamte, S.L.; Cappellacci, L.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; et al. The Crop-Residue of Fiber Hemp Cv. Futura 75: From a Waste Product to a Source of Botanical Insecticides. Environ Sci Pollut Res 2018, 25, 10515–10525. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Benelli, G.; Conti, B. Cannabis Sativa and Humulus Lupulus Essential Oils as Novel Control Tools against the Invasive Mosquito Aedes Albopictus and Fresh Water Snail Physella acuta. Industrial Crops and Products 2016, 85, 318–323. [Google Scholar] [CrossRef]
- Maurya, P.; Mohan, L.; Sharma, P.; Srivastava, C.N. Larval Susceptibility of Aloe barbadensis and Cannabis sativa against Culex Quinquefasciatus, the Filariasis Vector. 2008.
- McPartland, J.M.; Sheikh, Z. A Review of Cannabis Sativa-Based Insecticides, Miticides, and Repellents. Journal of Entomology and Zoology Studies, 13.
- Shaalan, E.A.-S.; Canyon, D.; Younes, M.W.F.; Abdel-Wahab, H.; Mansour, A.-H. A Review of Botanical Phytochemicals with Mosquitocidal Potential. Environment International 2005, 31, 1149–1166. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, I.; Scharinger, A.; Golombek, P.; Kuballa, T.; Lachenmeier, D. A Quantitative 1H NMR Method for Screening Cannabinoids in CBD Oils. Toxics 2021, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Inocente, E.A.; Shaya, M.; Acosta, N.; Rakotondraibe, L.H.; Piermarini, P.M. A Natural Agonist of Mosquito TRPA1 from the Medicinal Plant Cinnamosma fragrans That Is Toxic, Antifeedant, and Repellent to the Yellow Fever Mosquito Aedes Aegypti. PLoS Negl Trop Dis 2018, 12, e0006265. [Google Scholar] [CrossRef]
- Martínez Rodríguez, E.J.; Evans, P.; Kalsi, M.; Rosenblatt, N.; Stanley, M.; Piermarini, P.M. Larvicidal Activity of Carbon Black against the Yellow Fever Mosquito Aedes Aegypti. Insects 2022, 13, 307. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. Journal of Economic Entomology 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Cerceau, C.I.; Barbosa, L.C.A.; Alvarenga, E.S.; Ferreira, A.G.; Thomasi, S.S. A Validated 1H NMR Method for Quantitative Analysis of α-Bisabolol in Essential Oils of Eremanthus erythropappus. Talanta 2016, 161, 71–79. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The Essential Oil from Industrial Hemp (Cannabis sativa L.) by-Products as an Effective Tool for Insect Pest Management in Organic Crops. Industrial Crops and Products 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Şengül Demirak, M.Ş.; Canpolat, E. Plant-Based Bioinsecticides for Mosquito Control: Impact on Insecticide Resistance and Disease Transmission. Insects 2022, 13, 162. [Google Scholar] [CrossRef]
- Reid, W.R.; Thornton, A.; Pridgeon, J.W.; Becnel, J.J.; Tang, F.; Estep, A.; Clark, G.G.; Allan, S.; Liu, N. Transcriptional Analysis of Four Family 4 P450s in a Puerto Rico Strain of Aedes Aegypti (Diptera: Culicidae) Compared With an Orlando Strain and Their Possible Functional Roles in Permethrin Resistance. J Med Entomol 2014, 51, 605–615. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends in Plant Science 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and Prospects of Botanical Biopesticides in Europe and Mediterranean Countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Kleinhenz, M.D.; Magnin, G.; Ensley, S.M.; Griffin, J.J.; Goeser, J.; Lynch, E.; Coetzee, J.F. Nutrient Concentrations, Digestibility, and Cannabinoid Concentrations of Industrial Hemp Plant Components. Applied Animal Science 2020, 36, 489–494. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are They Important? Med Cannabis Cannabinoids 2020, 3, 25–60. [Google Scholar] [CrossRef]
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the Compositions of Cannabinoid and Terpenoids in Cannabis Sativa Derived from Inflorescence Position along the Stem and Extraction Methods. Industrial Crops and Products 2018, 113, 376–382. [Google Scholar] [CrossRef]
- Jackson, B.; Gilbert, L.; Tolosa, T.; Henry, S.; Volkis, V.; Zebelo, S. The Impact of Insect Herbivory in the Level of Cannabinoids in CBD Hemp Varieties; In Review, 2021.
- Abendroth, J.A.; Gondhalekar, A.D.; Scharf, M.E.; Couture, J.J. Cannabidiol Reduces Fall Armyworm (Spodoptera Frugiperda) Growth by Reducing Consumption and Altering Detoxification and Nutritional Enzyme Activity in a Dose-Dependent Manner. Arthropod-Plant Interactions 2023, 17, 195–204. [Google Scholar] [CrossRef]
- Norris, E.J.; Gross, A.D.; Bartholomay, L.C.; Coats, J.R. Plant Essential Oils Synergize Various Pyrethroid Insecticides and Antagonize Malathion in Aedes aegypti. Medical Vet Entomology 2019, 33, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.-R.; Ruben, P.C. Cannabidiol and Sodium Channel Pharmacology: General Overview, Mechanism, and Clinical Implications. Neuroscientist 2022, 28, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Puopolo, T.; Liu, C.; Ma, H.; Seeram, N.P. Inhibitory Effects of Cannabinoids on Acetylcholinesterase and Butyrylcholinesterase Enzyme Activities. Med Cannabis Cannabinoids 2022, 5, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.-R.; Shuart, N.G.; Mezeyova, J.; Dean, R.A.; Ruben, P.C.; Goodchild, S.J. Inhibitory Effects of Cannabidiol on Voltage-Dependent Sodium Currents. Journal of Biological Chemistry 2018, 293, 16546–16558. [Google Scholar] [CrossRef]
- Fiorini, D.; Molle, A.; Nabissi, M.; Santini, G.; Benelli, G.; Maggi, F. Valorizing Industrial Hemp (Cannabis Sativa L.) by-Products: Cannabidiol Enrichment in the Inflorescence Essential Oil Optimizing Sample Pre-Treatment Prior to Distillation. Industrial Crops and Products 2019, 128, 581–589. [Google Scholar] [CrossRef]
- Mazzara, E.; Spinozzi, E.; Maggi, F.; Petrelli, R.; Fiorini, D.; Scortichini, S.; Perinelli, D.R.; Bonacucina, G.; Ricciardi, R.; Pavela, R.; et al. Hemp (Cannabis Sativa Cv. Kompolti) Essential Oil and Its Nanoemulsion: Prospects for Insecticide Development and Impact on Non-Target Microcrustaceans. Industrial Crops and Products 2023, 203, 117161. [Google Scholar] [CrossRef]
- Skowronek, P.; Strachecka, A. Cannabidiol (CBD) Supports the Honeybee Worker Organism by Activating the Antioxidant System. Antioxidants 2023, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Schluttenhofer, C.; Yuan, L. Challenges towards Revitalizing Hemp: A Multifaceted Crop. Trends in Plant Science 2017, 22, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Norris, E.J.; Bloomquist, J.R. Co-Toxicity Factor Analysis Reveals Numerous Plant Essential Oils Are Synergists of Natural Pyrethrins against Aedes Aegypti Mosquitoes. Insects 2021, 12, 154. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).