1. Introduction
Cardiac contractility modulation (CCM) is a therapy that delivers electrical stimuli during the cardiac absolute refractory period, increasing the cytosolic calcium level, which increases left ventricular (LV) force production [
1]. The Optimizer® Smart device (Impulse Dynamics, Marlton, NJ, USA) is an implantable, commercially available technology approved by the U.S. Food and Drug Administration that delivers CCM therapy intended for patients with clinical heart failure non-indicated for a cardiac resynchronization therapy (CRT) [
2]. CCM therapy was evaluated against optimal medical therapy in the Evaluate Safety and Efficacy of the Optimizer® System in Subjects with Moderate-to-Severe Heart Failure (FIX-HF-5C) study and led to both reduced heart failure hospitalizations and improvements in exercise tolerance and quality of life. While the initial CCM device required atrioventricular synchrony, the current iteration can deliver therapy in those with rate-controlled atrial fibrillation (AF) [
3]. This interesting case report highlights the possibility of CCM atrium remodeling and restoring Sinus Rhythm (SR) in a patient with a dilated left atrium and a high degree of HF despite optimized medical therapy.
2. Materials and Methods
A 60-year-old male was affected by heart failure (HF) until 2013 when he was diagnosed with Dilated Cardiomyopathy with severe left ventricular dysfunction (HFrEF) with EF 30%, narrow QRS (85ms) and synus rhythm (SR). No evidence of significant coronary lesions. After the first diagnosis of HF, it is decided to implant a dual chamber implantable cardioverter defibrillator (ICD).
The Hospital Heart Failure Ambulatory followed the patient for more than ten years.
In 2021, during a routine visit, persistent atrial fibrillation (PAF) was diagnosed, which was initially well tolerated by the patients. The ICD was programmed in VVI mode at 50 beats per minute.
After only eight months from PAF diagnosis, the HF symptoms worsened, and the patients came to the emergency room (ER). The ER cardiac visit showed a reduced EF of 20% and worse HF symptoms. The patient underwent optimized guided medical therapy (OGMT) during this hospitalization (sacubitril/valsartan 49/51mg bis in die, metoprolol 100mg bis in die, SGLT2 10mg once daily, diuretics).
Table 1
Despite the OGMT, the HF worsening induces the patient to undergo different rehospitalizations.
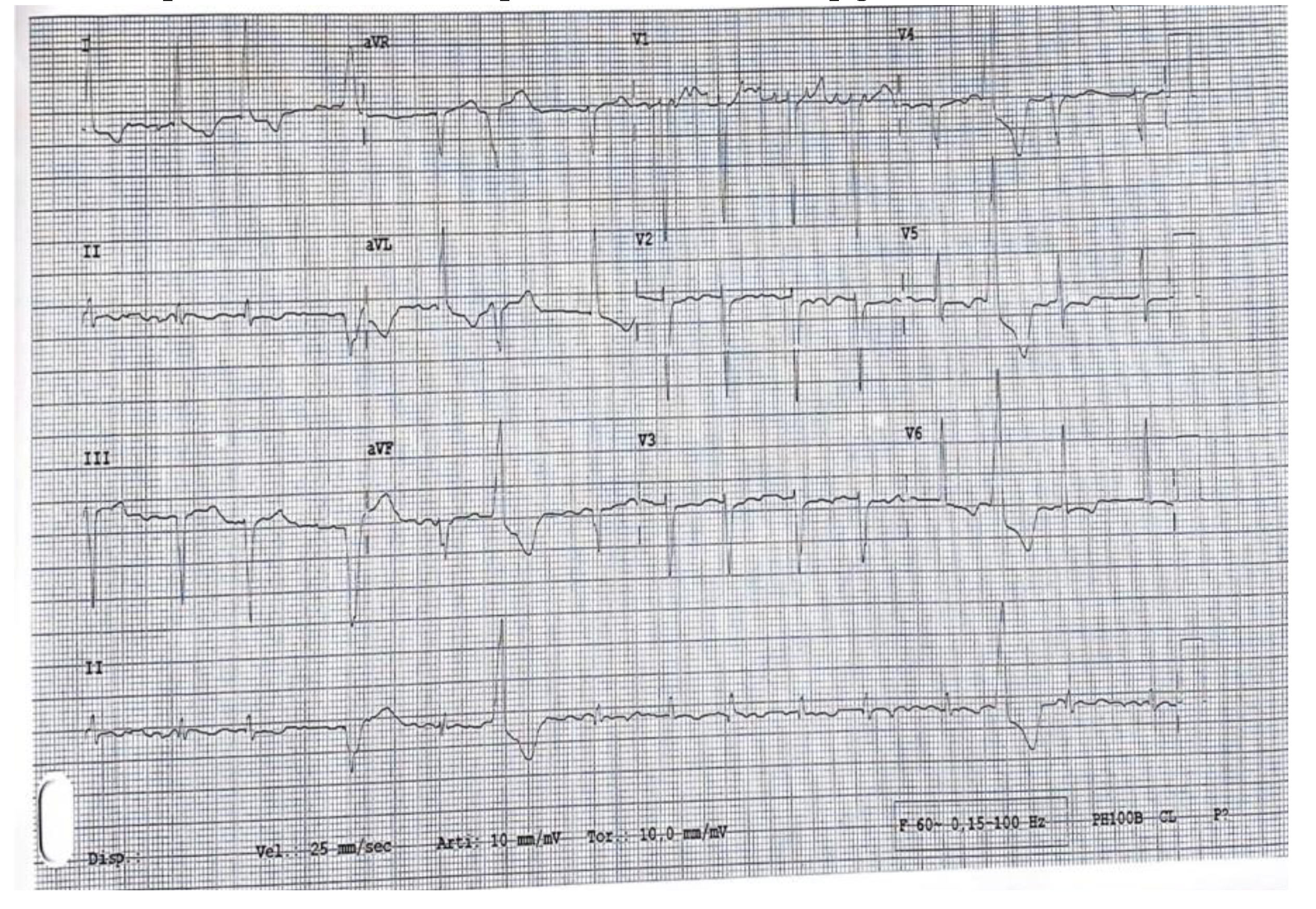
In June 2022, the patient came to the ER with dyspnea, acute pulmonary edema, and AF with high ventricular response (
Figure 1). During the hospital stay, The MLWHFQ was 60 points, the KCCQ was 79.1 points, and the NT-pro-BNP was 2376 pg/ml.
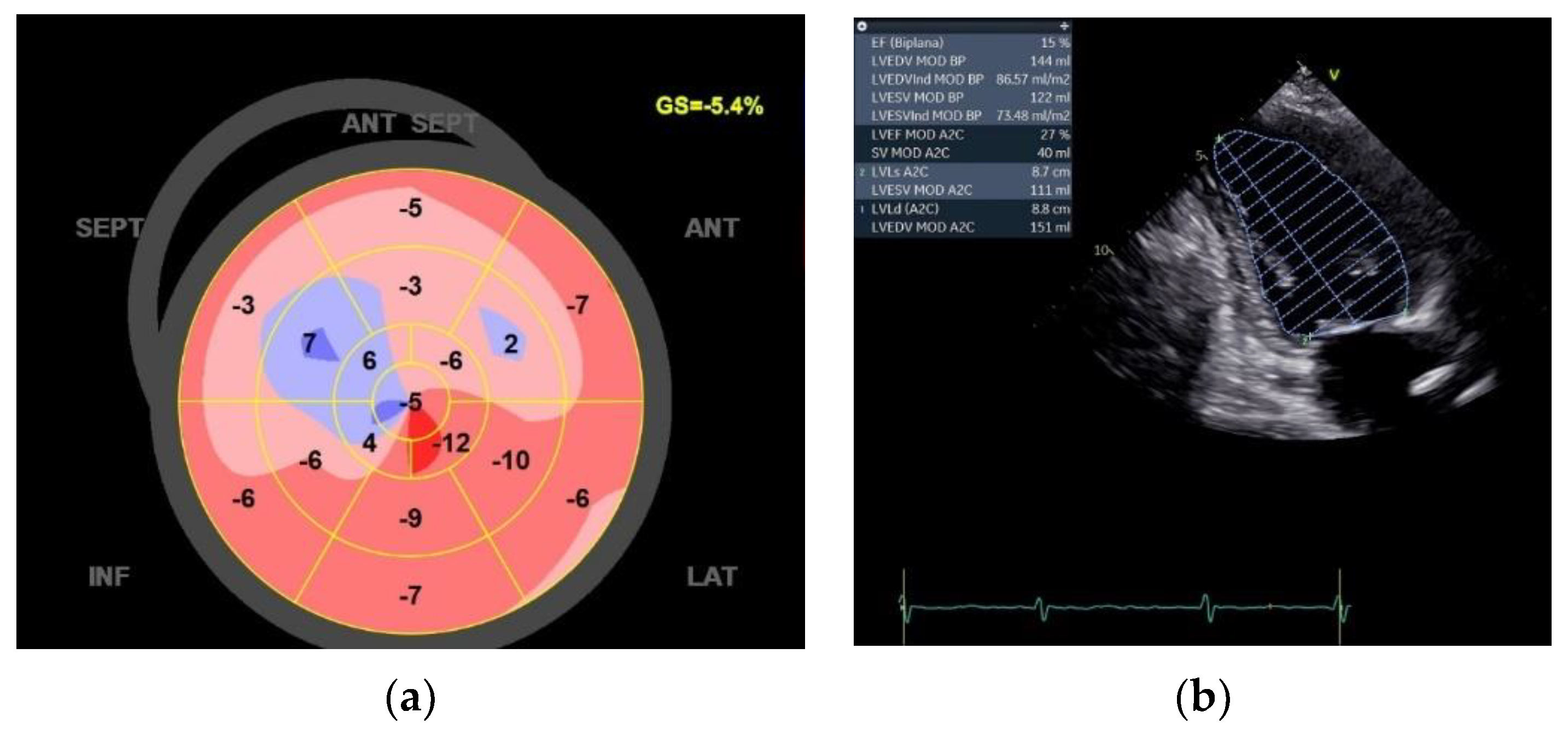
The echocardiographic exam shows an EF of 15%, LVEDV of 144ml, LVESV of 122ml, GLS of -4,0%, left atrium volume of 64ml and area of 22.6cm
2 (
Figure 2).
The blood pressure was shallow, and it was decided to add the patient to the transplant list.
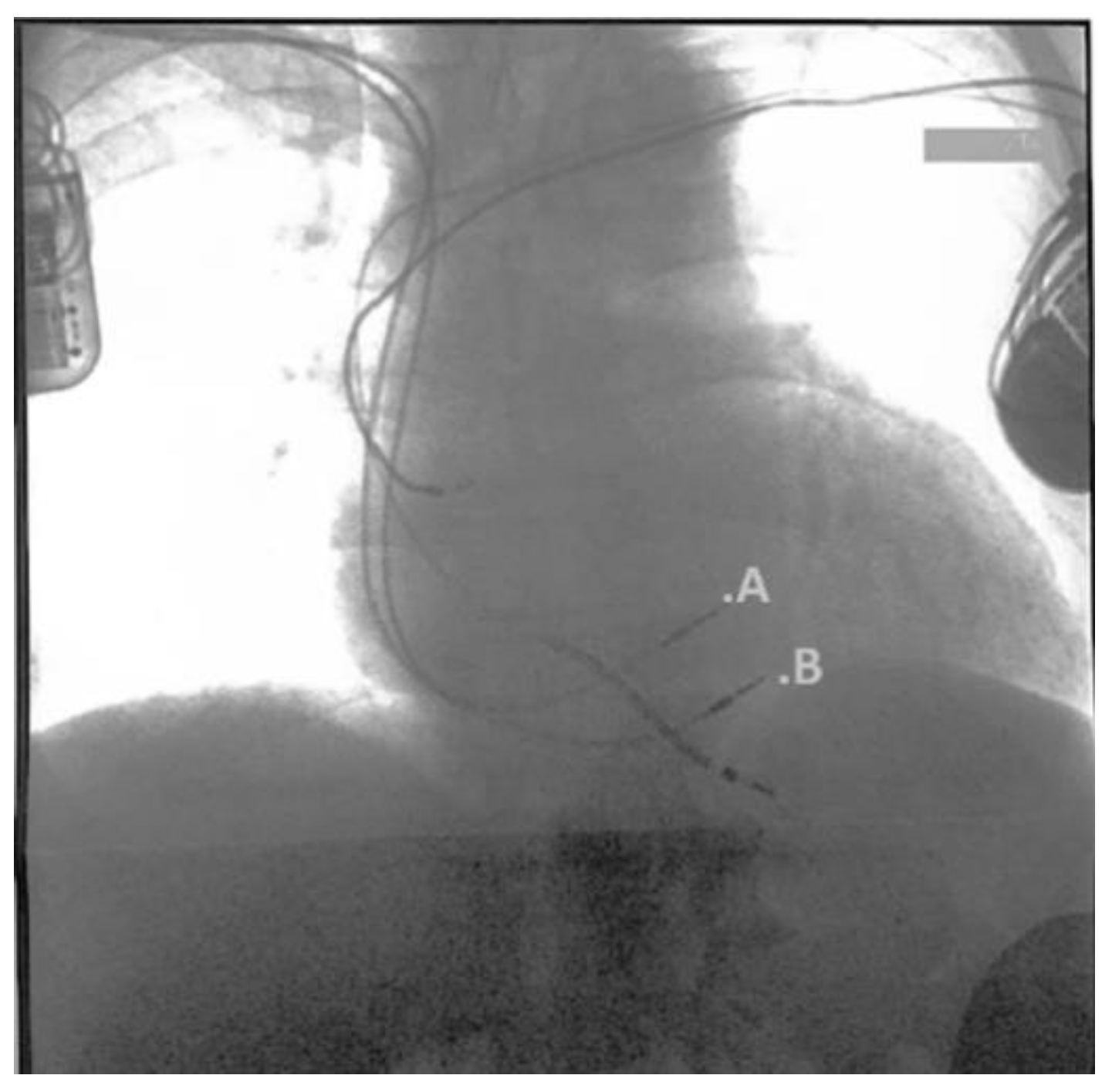
Waiting for the Transplant Center’s response, we showed the patient two different solutions for the HF treatment: the LVAD or the less invasive Cardiac Contractility Modulation (CCM) device, to treat the symptoms and improve his quality of life. The patient refused the LVAD as it was too invasive and accepted a CCM implant (
Figure 3).
The implant was tolerated well by the patient, who was dismissed from the hospital after three days.
3. Results
It’s decided to schedule a follow-up series of 3,6,9,18 months after the implant to collect as much data as possible regarding hemodynamics factors and QoL improvements. (
Table 2).
After only three months after the implant, the patient showed a very fast improvement in his physical state. This is confirmed by an MLWHFQ of 16 points and a KCCQ of 80,9 points.
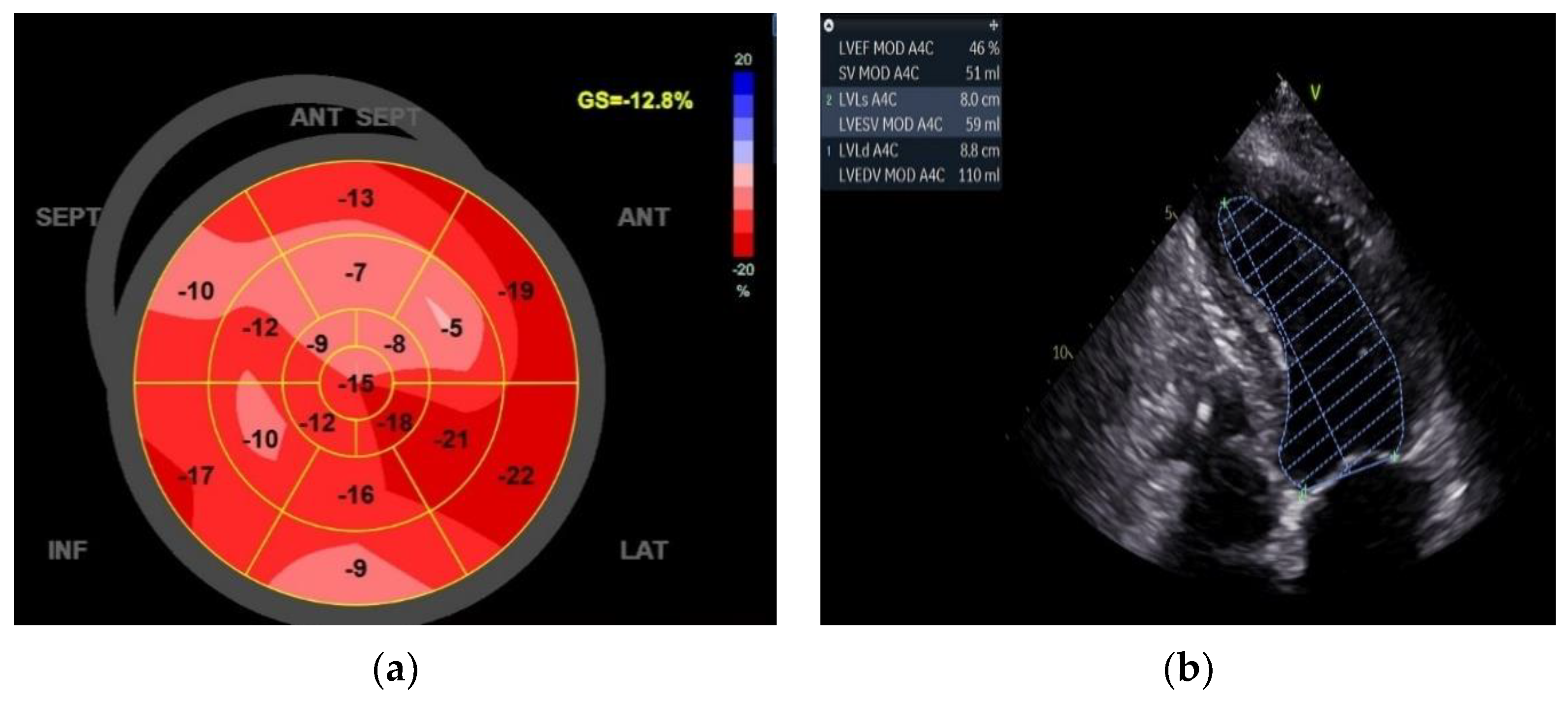
However, the improvement in quality of life (QoL) was followed by the ECO graphic exam, which shows impressive data with an EF of 46% and a GLS of -12.8%. (
Figure 4)
At the 6-month FU, the patient continues improving his health status regarding QoL and cardio Eco graphics parameters.
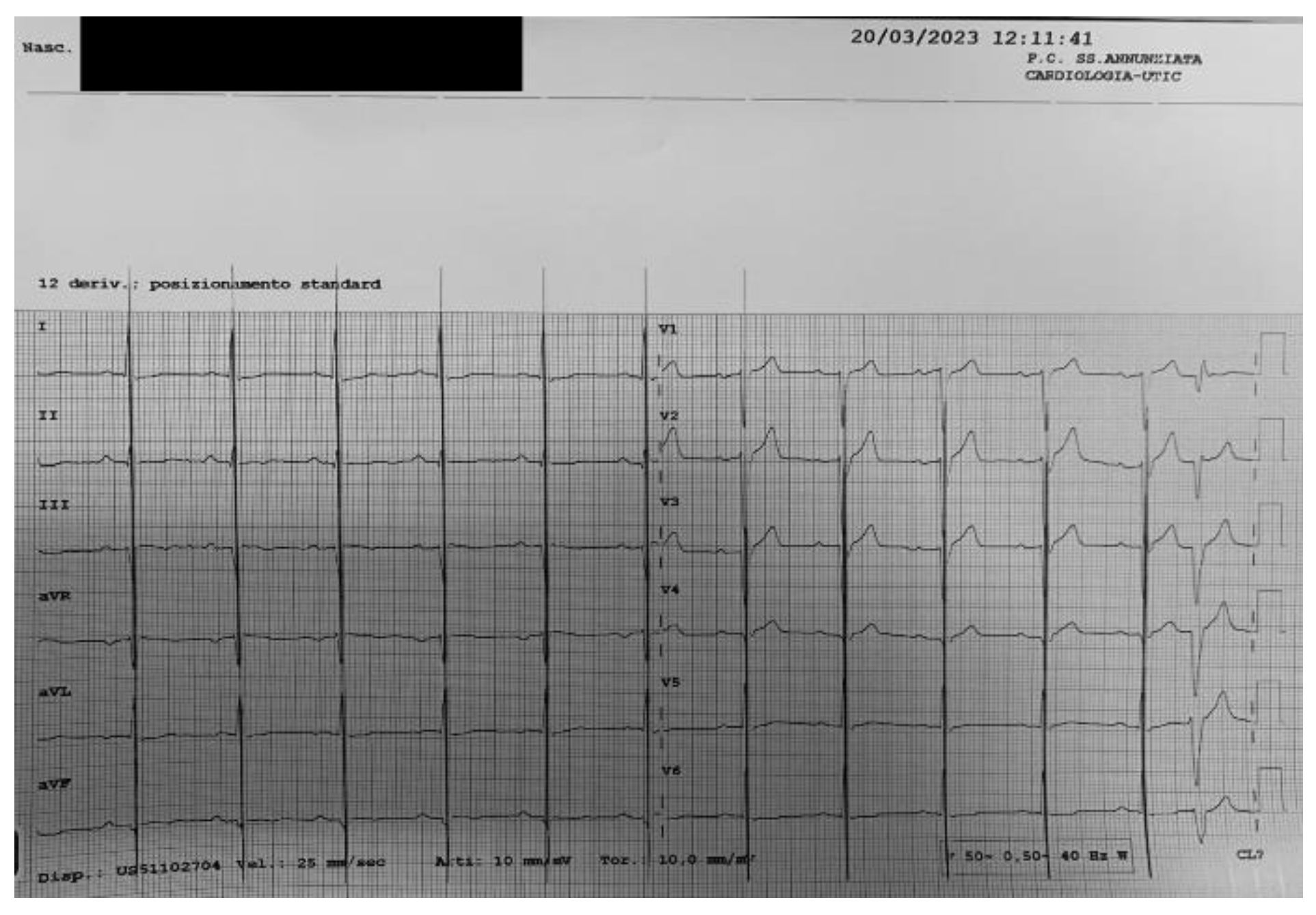
During the 9-month FU, we observe the return of sinus rhythm at the surface ECG (
Figure 5).
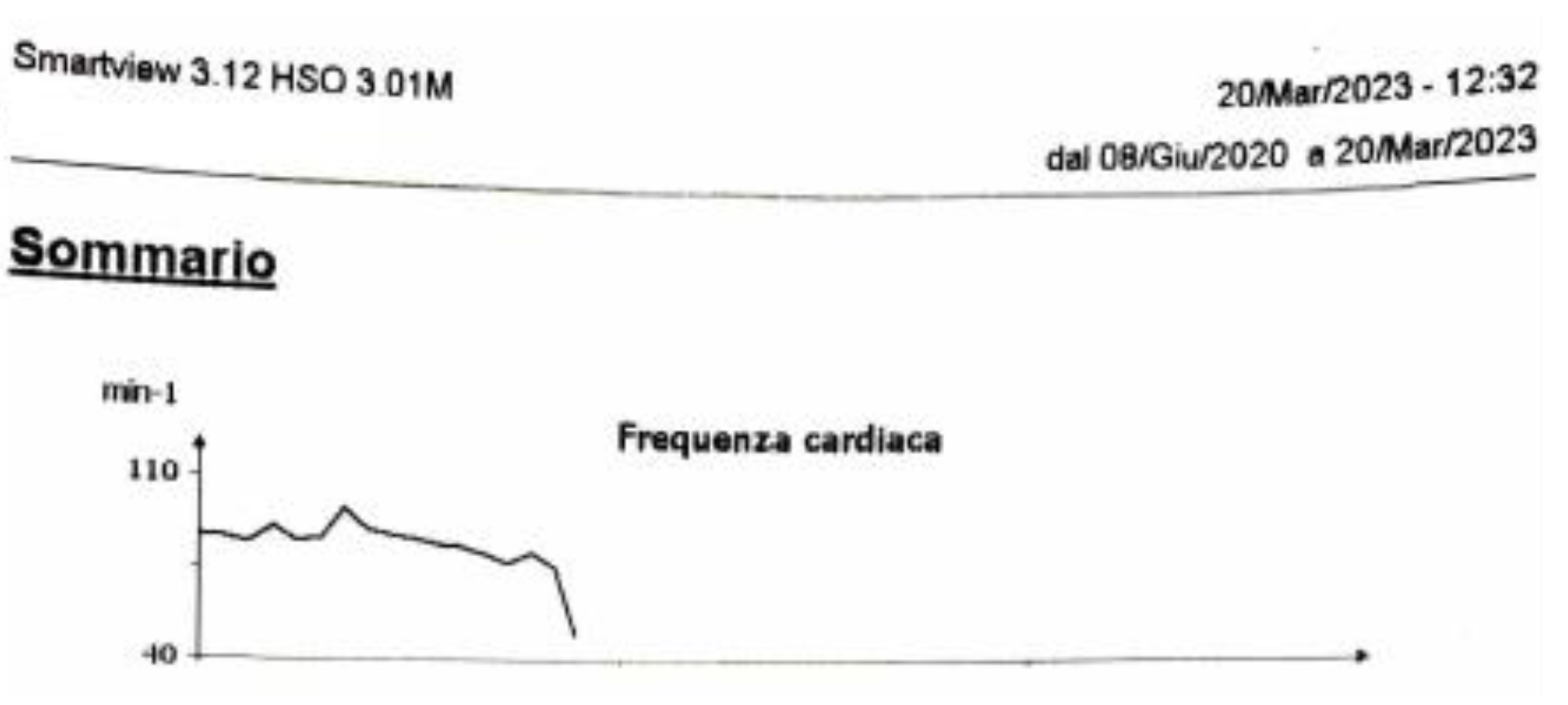
Since we are programming the ICD in VVI mode, we don’t know how often the patient was in SR because. However, the device diagnostic gives the frequency histogram showing how the cardiac frequency decreased and is now stable (
Figure 6). This had an excellent impact on the CCM function because, in that way, the device can deliver the maximum percentage of the therapy associated with the stability of the cardiac frequency and the heart rate under 110 bpm (the maximum CCM two leads only tracking frequency).
4. Discussion
This Case Report showed how, in complex patients, CCM therapy could be effective and quickly improve quality of life and cardiac function. This patient doesn’t have another chance over the transplant, but CCM gives him a new life and a new perspective in life. Another important observation is the reduced volume and area of the left atrium. This could be caused by the reverse modelling of atrial myocytes, which decreases left atrial pressure, prevents left atrial remodelling and precipitates AF [
4]. This case shows that additional investigation into the association between AF and CCM therapy is needed.
5. Conclusions
In this case report, we described for the first time a case of a cardiac transplant candidate patient with advanced HFrEF treated with CCM therapy with an 18-month follow-up and sinus rhythm restoration. Based on our case, we believe CCM could be used as a bridge to transplant strategy in selected patients with advanced HFrEF.
References
- Lyon AR, Samara MA, Feldman DS. Cardiac contractility modulation therapy in advanced systolic heart failure. Nat Rev Cardiol. 2013;10(10):584–598.
- Abraham WT, Kuck KH, Goldsmith RL, et al. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC Heart Fail. 2018;6(10):874–883.
- Wiegn P, Chan R, Jost C, et al. Safety, performance, and efficacy of cardiac contractility modulation delivered by the 2-lead optimizer smart system the FIX-HF-5C2 study. Circ Heart Fail. 2020;13(4):e006512.
- Shin JW, Atoot R, Heyer M, Jamal S. Does Cardiac Contractility Modulation Therapy Reduce Atrial Fibrillation Burden? J Innov Card Rhythm Manag. 2022 Oct 15;13(10):5202-5204.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).