Introduction
Bovine respiratory disease (BRD) continues to be one of the leading causes of beef and dairy calf morbidity and mortality in the US and globally [
1,
2,
3,
4]. BRD is estimated to cost the US cattle industry
$800-900 million annually due to lower weaning weights, reduced average daily gains (ADGs), increased respiratory treatment costs, and increased feeding costs [
5]. This multifactorial disease of cattle is often the result of the interaction between stress, and viral and bacterial infections. Bovine respiratory syncytia virus (BRSV) is frequently associated with BRD and limiting BRSV infections is an important BRD control measure [
6].
One of the most widely accepted disease protection measures is maternal antibody transference at birth. Maternal colostral antibody transfer is a crucial factor in disease prevention and protection in the neonatal animal [
7,
8]. However, maternal antibody is often at odds with, and may inhibit, another commonly used disease management tool: vaccination [
7,
8,
9]. Notwithstanding, a variety of management schemes, herd health statuses, and producer preferences throughout the United States dictate that a calf vaccination program work collectively with colostrum administration. These antibodies protect the calf from potential pathogens but may also neutralize antigens in the vaccine, preventing the calf from developing an effective immune response [
9,
10,
11]. To prevent maternal antibody interference, producers typically vaccinate calves after antibody concentrations decrease [
9,
11]. Maternally derived BRSV antibody titers should be < 1:4 for successful vaccination of most calves [
8,
12,
13]. Depending on the immune status of the dam and the efficiency of colostral immunoglobulin transfer, BRSV maternal antibody titer can decline to this level by 30 to 180 days of age [
8,
12,
13]. Because maternal antibody decays at variable rates in individual calves [
12,
13], standardizing calf vaccination programs to a specific timepoint becomes challenging. This antibody decline can allow for a gap in BRSV protection within the herd depending on maternal antibody decay and induction of active immunity by vaccination when calves are vulnerable to infection. To overcome maternal antibody interference in neonates, traditional management strategies have consisted of administering repeated doses of vaccine or delaying the age of immunization [
9]. Few intranasal (IN) BRSV efficacy studies have been done in the face of maternal antibody [
9,
14,
15] and none at two weeks or less.
Although many vaccines are labeled for use in young calves less than 30 days of age, the licensing studies are normally done in seronegative calves and the effect of maternal antibody (commonly referred to as in the face of maternal antibody-IFOMA) is not determined. In addition, because the immune system matures with age, some young calves <30 days of age may be immunologically immature in addition to the issues of vaccination IFOMA. Most BRD vaccine studies have focused on vaccination around weaning (just before, at, or after) and then observe the effects on BRD morbidity and mortality. Another issue frequently encountered with IN viral vaccines is the use of molecular diagnostics in young calves to detect viral respiratory agents. The use of IN viral vaccines has been fraught with two “diagnostic issues”- the detection of vaccine virus resulting in misdiagnosis or conversely the “lack of detection” of vaccine virus in IN vaccinated calves. There have been few published studies measuring virus levels following IN vaccination. The objective of this study was two-fold: 1) evaluate the development and duration of immunity following vaccination at 9 ±2 days of age in the face of maternal antibody using a BRSV challenge model at ~80 days post-vaccination and 2) measure the kinetics of vaccine virus in vivo following IN vaccination.
Materials and Methods
Personnel who monitored the calves, obtained samples, scored the pulmonary lesions, or performed laboratory procedures were not aware of which Treatment Group a calf was assigned.
Animals, housing, and feeding Fifty (50) beef-dairy crossbred, male, and female calves from a single source were acquired for this study. At birth, calves were separated from their respective dam before suckling. Blood was obtained for serologic determination of exposure to BRSV. An ear notch biopsy was obtained to determine [enzyme linked immunosorbent assay, (ELISA)] persistent infection with bovine virus diarrhea virus (BVDV). Pooled colostrum, characterized for antibody titers to BHV-1 (1:128) and to BRSV (1:128), was then administered by intubation and 6 hours after the first feeding. Within a few hours of birth, the calves were examined and transported to the contract research facility and within 24 hours, received 2 feedings of pooled colostrum (4 liters/feeding). They were identified with individual numbers on ear tags and housed in individual hutches. Calves were observed daily by trained personnel. The animals were weighed at vaccination, 0 day post vaccination (DPV) (9 ±2 days of age), 14 DPV, 80 DPV (days post challenge (DPC) 0) and 88 DPV (DPC 8). Signs of illness or injury were recorded, and care was provided and documented according to protocols.
Fresh water was available ad libitum to each calf throughout the study. The calves were fed milk replacer twice daily and grain supplement was offered beginning at 3 days of age. When the youngest calf was 56 days old, all calves were weaned as a single group. They were consuming 2.5 to 3 lbs. of pelleted feed per head which met nutritional requirements. The animals were weaned using a step-down procedure over 8 days. At the end of the 8-day weaning period, calves were fed only the pelleted feed. Calves were then commingled and had access ad libitum to hay in addition to the grain supplement. Calves that died or were euthanized were submitted to the Animal Disease and Diagnostic Laboratory (ADRDL) at South Dakota State University for diagnostic necropsy.
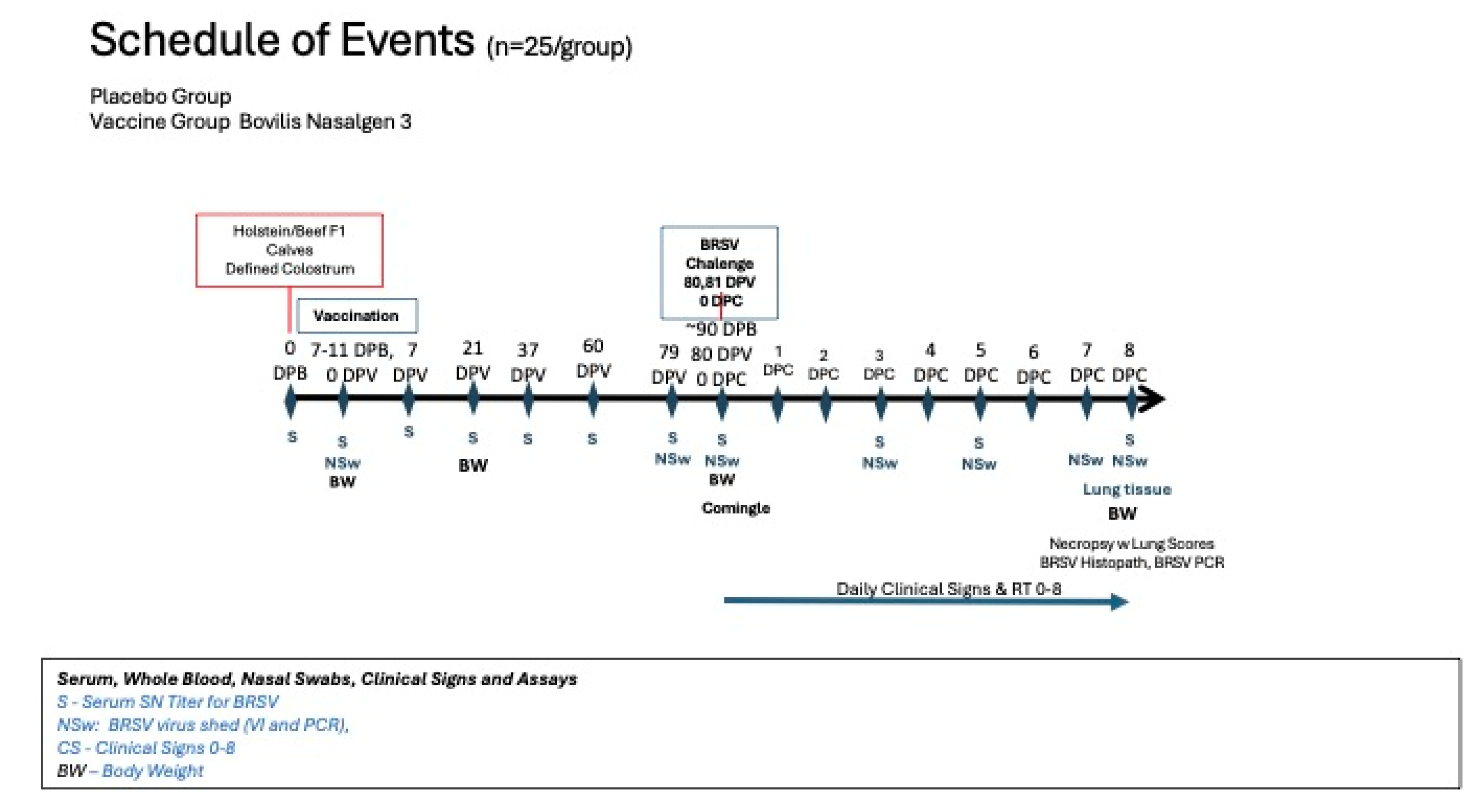
Vaccine and Experimental treatments. Calves were stratified by sex and age to ensure even distribution among two groups and then randomly assigned to treatment group. The vaccine was administered to calves when they were 9±2 days old (
Figure 1).
The tri-valent vaccine [Bovilis Nasalgen 3- bovine herpesvirus 1 (BHV-1), bovine respiratory syncytial virus (BRSV) and bovine parainfluenza 3 (PI3)] was reconstituted, handled, and administered according to label directions. There were 50 animals: 25 in each of the two treatment groups. On day 0, calves in vaccine group (VG) were vaccinated intranasally (IN) with one dose of the tri-valent vaccine (2 mls) instilled in a single naris into the nostril, and one dose (2 ml) of a placebo (sterile water) was administered in a similar manner to calves in placebo group (PG). Mean BRSV SN serum antibody titer was 6.29 log2 for the trivalent vaccinated group and 6.16 log2 for the sham vaccinated placebo group at the time of vaccination. The mean BHV-1 SN serum antibody titer was 5.55 log2 for the trivalent vaccinated group and 5.64 log2 for the sham vaccinated placebo group at the time of vaccination. On day 0 there was no significant difference (P = 0.22) of body weight for calves in VG(mean = 93.36 lb., SEM = 2.68), and for calves in PG mean = 98.04 lb., SEM = 2.68). There was also no significant difference (P = 0.41) of total protein in serum for calves in VG (mean = 6.93 g/100mL, SEM = 0.09), and for calves in PG (mean = 6.83 g/100mL, SEM = 0.09). BRSV and BHV-1 serum neutralization (SN) titers for each calf was < 2 prior to administration of the pooled colostrum. None of the calves was persistently infected with BVDV.
Calves were observed daily by trained personnel for clinical signs of illness or injury that were recorded and treated according to protocols. After enrollment, two (2) calves (one from each treatment group) died or were euthanized before challenge.
Sample Collection
Blood samples Blood samples for serum analysis were obtained from the calves via jugular venipuncture at 7–21 day intervals for approximately 10 weeks prior to challenge, the day before challenge (-1 DPC), and at necropsy (8DPC) (
Figure 1). Briefly, the animal was restrained and 12.5 mL of blood was collected from the jugular vein. Blood samples were used to measure total protein at 24 hrs to determine colostral absorption and for serum neutralization analysis to determine antibody levels against BRSV and BHV-1.
Nasal Swabs Nasal swabs were collected from all calves on days 0, 1, 3, 5, 7, 9 and 14 DPV for both BRSV and BHV-1 for polymerase chain reaction (PCR) assays and 0, 1, 5, 9 and 14 DPV for virus isolation (
Figure 1). Nasal swabs were collected from all calves on days -1, 0, 3, 5, 7 and 8 DPC for BRSV for VI and PCR assays. To collect samples, calves were initially manually restrained in the hutches. Following comingling the calves were physically restrained by head catches. Once their head was gently restrained, a sterile swab (Copan) was inserted gently into one naris, rotated briefly, then removed and deposited into a sterile conical tube, containing 2 mL of BRSV transport medium (Dulbecco’s Minimum Essential Media, Eagle containing 2X antibiotics/antimycotics and 1X L-glutamine).
Sample Processing and Analysis
Serum neutralization assay Serum was harvested from whole blood as follows: samples were centrifuged at 1500g for 15 min at 4 °C. Serum was decanted and tested for the presence of neutralizing antibody against BRSV, and BHV-1. Two-fold (2-fold) serial dilutions of sera (1:2 to 1:256) in duplicate were made in 96-well microtiter plates. A constant amount of virus [<500 tissue culture infectious dose-50% (TCID
50)] was added to each serum dilution well of the plate as appropriate. After incubation of the serum/virus mixtures, the mixtures were used to inoculate bovine turbinate cell monolayers contained in 96 well microtiter tissue culture plates. Plates were incubated at approximately 37°C with 5% CO
2 for 3 to 8 days (3 days for BHV-1 and 8 days for BRSV) before being evaluated for virus induced cytopathic effect (CPE) [
16]. The serum titer was determined to be the last dilution that inhibited CPE and was presented as a reciprocal value. Geometric mean values were then calculated using log2 titers.
Nasal virus shedding assay Nasal swabs were processed (vortexed and centrifuged at 600g for 5 min at 4 °C) and aliquoted for BRSV or BHV-1 VI or BRSV or BHV-1 PCR assays. For BRSV VI, dilutions of each sample were made and added in triplicate to BVDV-free bovine turbinate (BT) cell monolayers in microtiter tissue culture plates [
16,
17]. Plates were incubated for 9 days at approximately 37°C in 5% CO2. Plates were stained with BRSV monoclonal antibody (GeneTex, Irvine, CA) and read using a fluorescence microscope (Olympus CK400 fluorescence microscope, Olympus, Melville, NY). For BHV-1 VI, serial 10-fold dilutions of samples were made, and each diluted sample was added in triplicate to MDBK cell monolayers in microtiter tissue culture plates. Samples on culture plates were incubated for 5 days at 37˚C with 5% CO
2 before being evaluated by cytopathic effect and IBR immunofluorescence staining. Samples were considered negative for IBR virus if no cytopathic effect or virus-specific fluorescence was observed in inoculated cells after one blind passage [
16,
18]. TCID
50 was calculated using a Spearman-Karber method [
19]. Results were considered positive if BRSV intracellular staining was seen in inoculated wells. BRSV and BHV-1 PCR nasal sample aliquots and lung were submitted to the Molecular Diagnostics Section at the ADRDL at South Dakota State University Brookings SD for BHV-1 [
20] and BRSV [
21] analysis using real-time Reverse Transcription Polymerase Chain Reaction (RT-PCR). An aliquot of the titrated BRSV challenge virus was diluted in ten-fold dilutions (1:10–1:1,000,000) and assayed by real-time RT-PCR to develop a standard curve to correlate cycle threshold (CT) values to TCID
50/ml in a semiquantiative manner [
22].
Challenge and Clinical Signs. The BRSV challenge material was passaged through colostrum deprived, unvaccinated calves that had been raised in a controlled, isolated environment prior to use as a challenge. The challenge material was generated by infecting 2 calves with BRSV, euthanizing the calves at 8 days post challenge (DPC) and collecting bronchoalveolar fluid that was filtered and titered for BRSV. Prior to use the challenge material was also tested for the presence of BVD by RT-PCR and cultured on MacConkey, blood agar, and Sabouraud plates to detect bacteria and molds. Virulent BRSV was administered (24 calves per treatment group) on two consecutive days (day 80 and day 81) by aerosolization (
Figure 1). The volume administered (4 mL) had an average titer of 1.25x10
5 virus TCID
50/mL using a nebulizer and mask to aerosolize the challenge material for intranasal inhalation by the calves (DriveTM Reusable Nebulizer Kit, Port Washington, NY). Clinical disease parameters including attitude, body temperature, and general respiratory signs were monitored for 8 days following challenge by trained personnel blinded to treatment groups (
Figure 1). Calves were considered to be pyrexic when body temperatures were 103.5°F or greater. Each calf was visually examined and scored for signs of abnormal respiration, nasal and ocular discharge, and depression, using a scale of 0–3 except for nasal discharge which was on a scale of 0–4. Briefly, an abnormal respiration score was given if an animal had short/ rapid breathing (1), mild dyspnea (2) or severe dyspnea (3). Coughing was noted as present (1) or absent (0). Nasal discharge scores ranged from no discharge (0), mucous (1), mucopurulent (2), purulent (3) or blood-tinged (4). Ocular discharge scores ranged from normal slight serous (0), moderate serous (1), moderate mucopurulent (2) or purulent (3). Attitude (Depression) was scored no depression (0), mild (1), moderate (2), or severe depression (3).One animal in the placebo group died on day 87, 7 days following challenge with respiratory distress.
Necropsy – The 2 calves that died before challenge (one from each group) and placebo calf that died at day 87 were necropsied. All calves remaining in the study (47 calves- 24 vaccinates and 23 placebos) were euthanized by barbiturate overdose on day 88 (8 days post-challenge) (
Figure 1). Lungs with trachea attached were removed from each calf. Lesions of the lungs were scored as a percent of lung involved. Each lung lobe was examined in its’ entirety by a board-certified pathologist and the extent of lung involvement was estimated as a percentage of each lobe. Once the lung lesion scores were collected and recorded, their percentages were entered into an Excel spreadsheet and the results were adjusted to reflect the proportion of the lesion in relation to the whole. Specifically, each lung lesion score was multiplied by the percent of the total lung area for each lobe of the bovine lung to determine what proportion of the lungs were damaged by the challenge. A score for both lungs was calculated based on the estimation of the percent of lesions [
23] in each lobe as follows: (Left Cranial x 0.05) + (Left Middle x 0.06) + (Left Caudal x 0.32) + (Right Cranial x 0.06) + (Right Posterior Cranial x 0.05) + (Right Middle x 0.07) + (Right Caudal x 0.35) + (Accessory x 0.04) = Total Lung Score (TLS).Sterile forceps, scissors and disposable scalpels were used to extract pulmonary tissue sections for BRSV RT-PCR, BRSV virus isolation (VI), routine histopathology, and BRSV immunohistochemistry (IHC). Two representative lung samples were collected from each calf for virus isolation (VI) and polymerase chain reaction (PCR) assays. Tissues for viral PCR testing and VI were placed in sterile Whirl Pak® bags (one for PCR and one for VI) and labeled with the study number, date of sample collection, study day, calf ID and lobe ID. A minimum of two pulmonary tissue sections were collected from characteristic BRSV pulmonary lesions for routine histopathology and IHC. Tissue sections for H&E and IHC staining were fixed in 10% neutral buffered formalin (NBF). Formalin containers were labeled with the study number, date of sample collection, study day, calf ID and lobe ID. Formalin-fixed samples were transferred from the study site to the Clinvet-SD laboratory at ambient temperature. Fresh samples of the lung lesions were transported on ice packs to the laboratory. Formalin-fixed tissue sections from characteristic BRSV lesions were trimmed by the investigation pathologist (GMK) and placed in tissue cassettes. Tissues were embedded in paraffin wax, sectioned at 3-5μm, and stained with hematoxylin and eosin (H&E) and by IHC to detect BRSV antigen utilizing protocols employed by histotechnologists at the SDSU Animal Disease Research and Diagnostic Laboratory. For BRSV IHC, appropriate positive and negative control pulmonary tissue sections were included in each run. The investigation pathologist (GMK) analyzed, interpreted, and documented all slides by means of light microscopy without prior knowledge of the animal’s experimental treatment. Interpretation of H&E sections included the extent of consolidation expressed as a percentage of the tissue sections and presence of BRSV-related pathologic alterations including bronchointerstitial pneumonia, necrotizing bronchiolitis, viral syncytia, exudative and proliferative alveolitis, emphysema and bronchus-associated lymphoid tissue (BALT) hyperplasia. The extent and localization of BRSV-antigen in the airway epithelium, exfoliated cellular detritus and alveolar histocytes was evaluated in IHC tissue sections.
Statistical analysis Data were analyzed with the animal as independent experimental units.
General (normal distribution) and generalized (non-normal distributions) linear (mixed) models were used for continuous and non-continuous outcomes respectively with PROC Glimmix (SAS version 9.4, Cary, NC). Data that were not normally distributed were transformed prior to analysis. Repeated measures analyses included fixed effects for treatment, day, and interaction between Treatment x Day, and random effects to account for covariances among observations at different sampling times within animal. Pair-wise comparisons were adjusted for multiple comparisons using Tukey methods. Model-adjusted means, and corresponding standard error of the mean (SEM) are reported and P < 0.05 was considered significant.
Results
Nasal Shedding of BHV-1 or BRSV virus Following Vaccination –
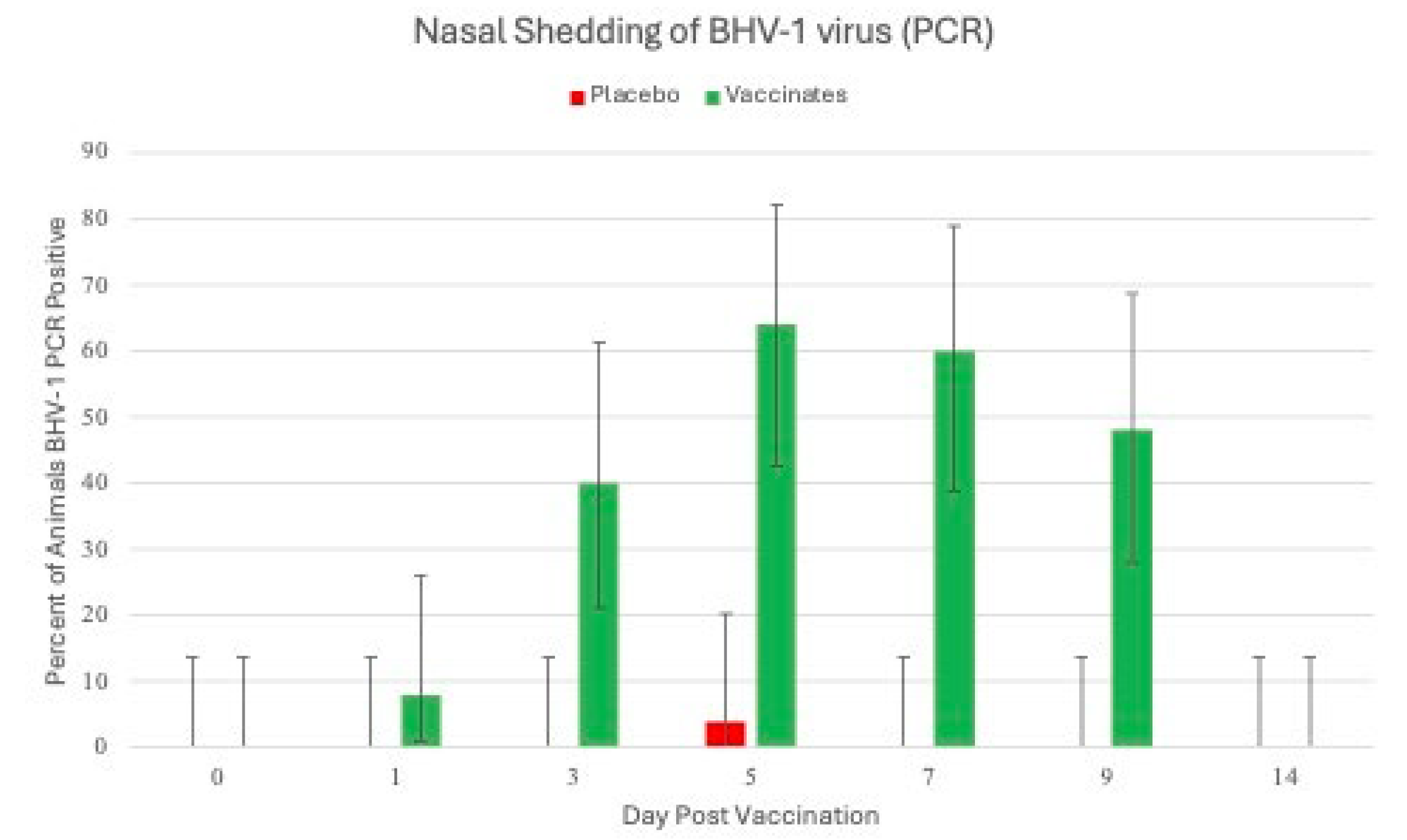
BHV-1. Calves vaccinated with tri-valent MLV vaccine had detectable BHV-1 virus beginning at day 1 post vaccination as detected by PCR in 8% of the calves (2 of 25;
Figure 2). At day 3, 40% of the animals (10 of 25) were PCR positive.
This increased to maximum of 64% (16 of 25) at day 5. The number of positive animals began decreasing at day 7 to 60% (15 of 25), 48% (12 of 25) at day 9 and all the animals were negative at day 14. PCR cycle threshold (CT) values (the smaller the CT value, greater amount of virus) had a similar pattern to the number of BHV-1 positive animals with the highest levels of virus at day 5 and the animals negative at day 14. Seventeen (17) of the 25 calves shed BHV-1 at least once during the 9 days following IN vaccination. There were 55 total BHV-1 PCR detections and 15 of the 17 BHV-1 positive animals shed BHV-1 for more than 3 days. Average CT values decreased from day 1 to day 5 (day 1, 29.6; day 3, 26.8; and day 5, 24.0).Average CT values increased from day 5 to day 14 (day 5, 24.0; day 7, 24.5; day 9, 26.3 and day 14 >40).BHV-1 virus isolations (VI) results paralleled the PCR results (results not shown) both in peak percentage of BHV-1 positive animals and in the virus levels on those days that both assays were run. At day 1, 12% (3 of 25) of animals were VI positive with average titer of 2.07 log10 TCID
50/ml, peaking at day 5 at 56% (14 of 25) and average titer of 4.57 log10 TCID
50/ml. VI positive animals decreased at day 9 to 40% (10 of 25) with an average titer of 3.02 log10 TCID
50/ml. In comparing the two virus detection methods, ~90% of the positive animals were positive with both methods.There was a single control animal BHV-1 positive on a single day from all the control animals collected for the 14 days following vaccination (
Figure 2). The animal was BHV-1 PCR positive only on day 5 and negative for PCR and VI for the other collection days.
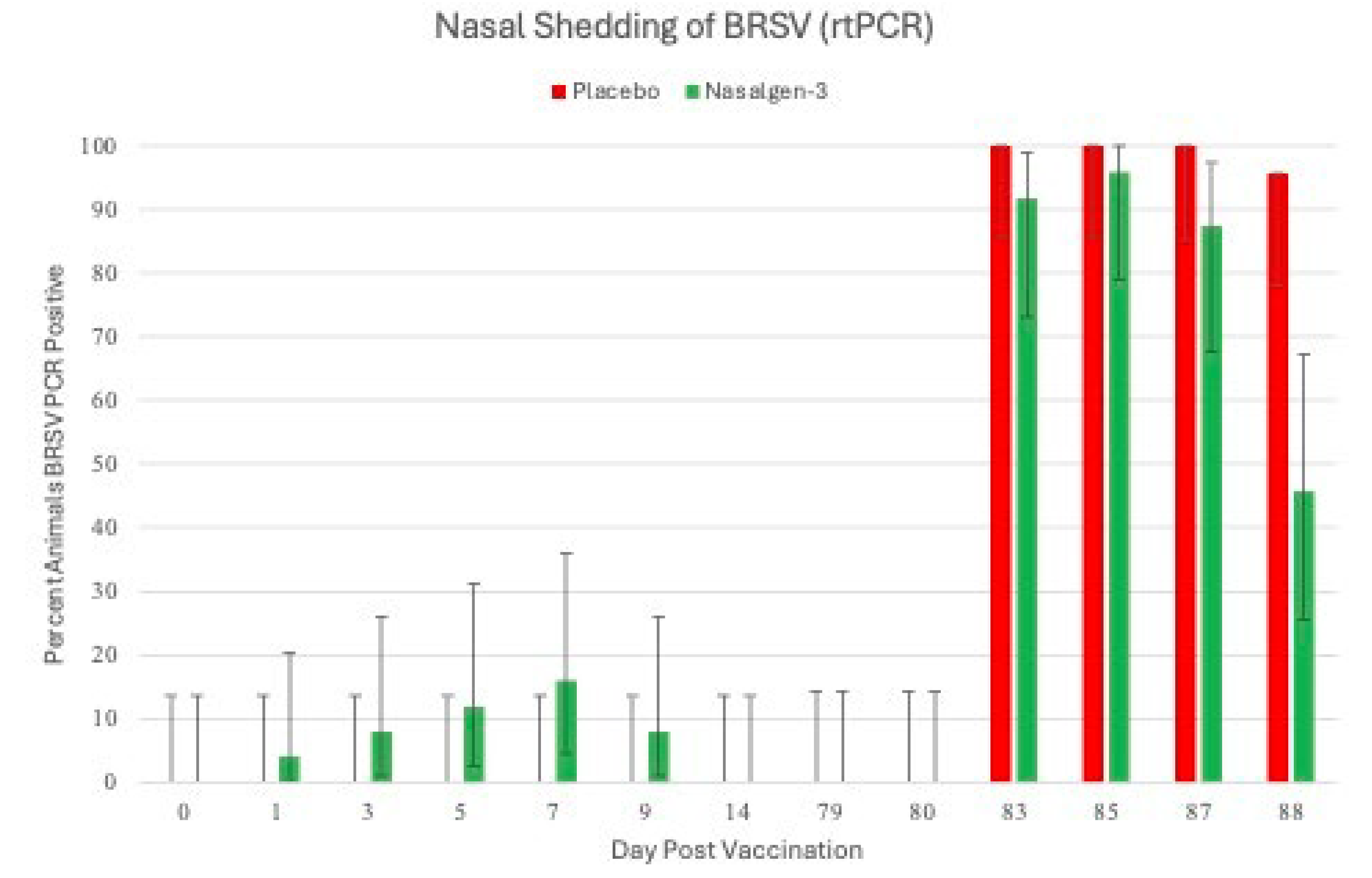
BRSV. Unlike BHV-1, few animals shed BRSV following vaccination (
Figure 3).
At day 1, 4% of the calves (1 of 25) were BRSV PCR positive, at day 3 8% of the animals (2 of 25) were PCR positive and 16% (4 of 25) at day 5. The number of positive animals began decreasing at day 7 to 12% (3 of 25), 8% at day 9 (2 of 25).Seven (7)of the 25 animals were BRSV PCR positive. There were 12 BRSV detections and 7 of the BRSV detections were from 2 animals (3 and 4 positive detections), the remaining5 animals had single day of BRSV detetion. The CT values were 29.8 or greater at all time points with no changes in calculated semiquantitiatve titers over time (data not shown). BRSV VI were also very low with 3 positive BRSV VI detected on day 1 with titers less than 2log10 TCID
50/ml. Calves that were sham-vaccinated with placebo where negative for BRSV throughout the post vaccination period (
Figure 3).
Clinical Signs
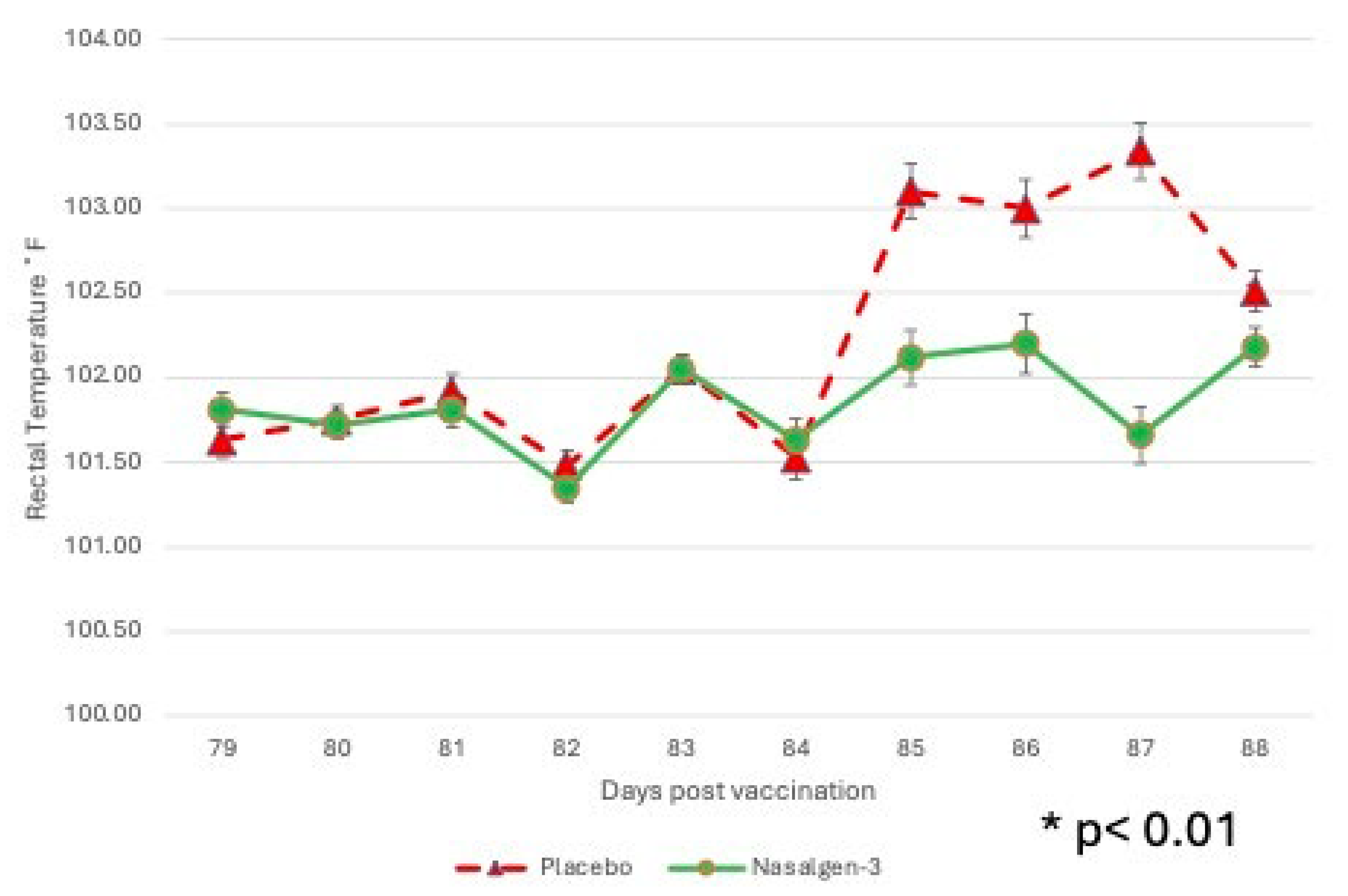
Febrile response There was significant (
P < 0.01) interaction between Treatment x Day, as well as main effect of treatment (
P < 0.01) and of day (
P < 0.01) on rectal temperature. Body temperatures of the two groups were similar for the first 4 days post challenge (DPC)(days 81-84) (
Figure 4).
Mean rectal temperatures in the placebo group on DPC 5-7 (days 85-87) were ~1˚F higher than the vaccinates (
Figure 4). DPC 5-8 (days 85-88) were significantly (
P < 0.01) higher for sham-vaccinated placebo calves as compared to the vaccinated calves with the tri-valent MLV vaccine (
Figure 4).
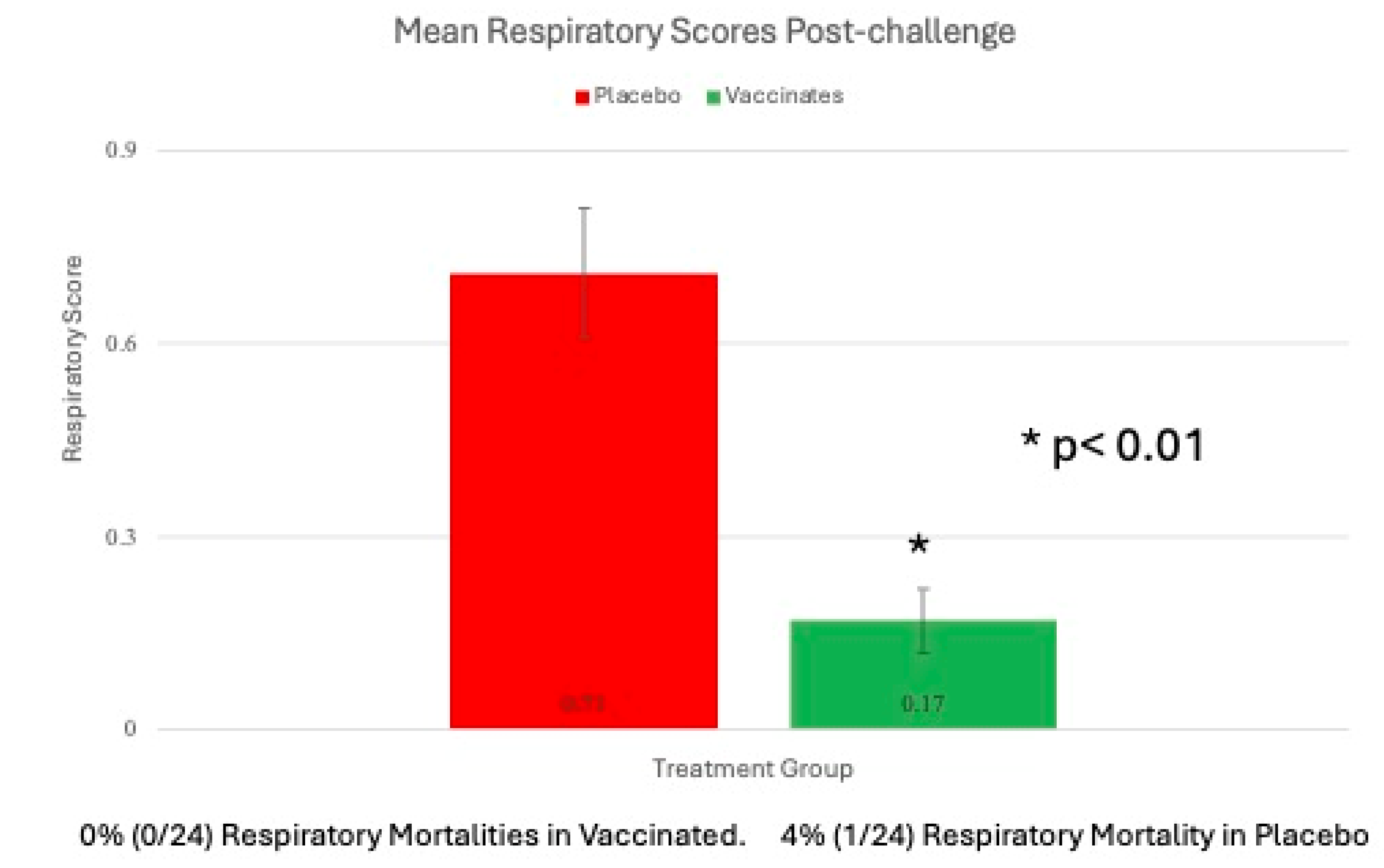
Respiratory signs and Cough Overall effects of Treatment were significant (
P < 0.01) for mean respiratory scores after challenge (
Figure 5).
The mean respiratory scores over the 8-day post BRSV challenge period were 4.0 greater in the placebo animals as compared to vaccinates (
Figure 5). The overall probability of coughing (measured as a binomial: present or absent) was 1.8X times higher. Cough was significantly (
P = 0.01) higher in the placebo group (data not shown).
Mortality After enrollment and before BRSV challenge, one calf in each the vaccinates and placebo group were euthanized for animal welfare consideration due to unresponsive joint infection that resulted in severe lameness. Seven days (day 87) after challenge, one calf sham-vaccinated with the placebo died (
Figure 5). On necropsy of the placebo calf, there was severe lung involvement consistent with BRSV that was confirmed by virus isolation and PCR. No bacteria associated with bovine respiratory disease were cultured (data not shown).
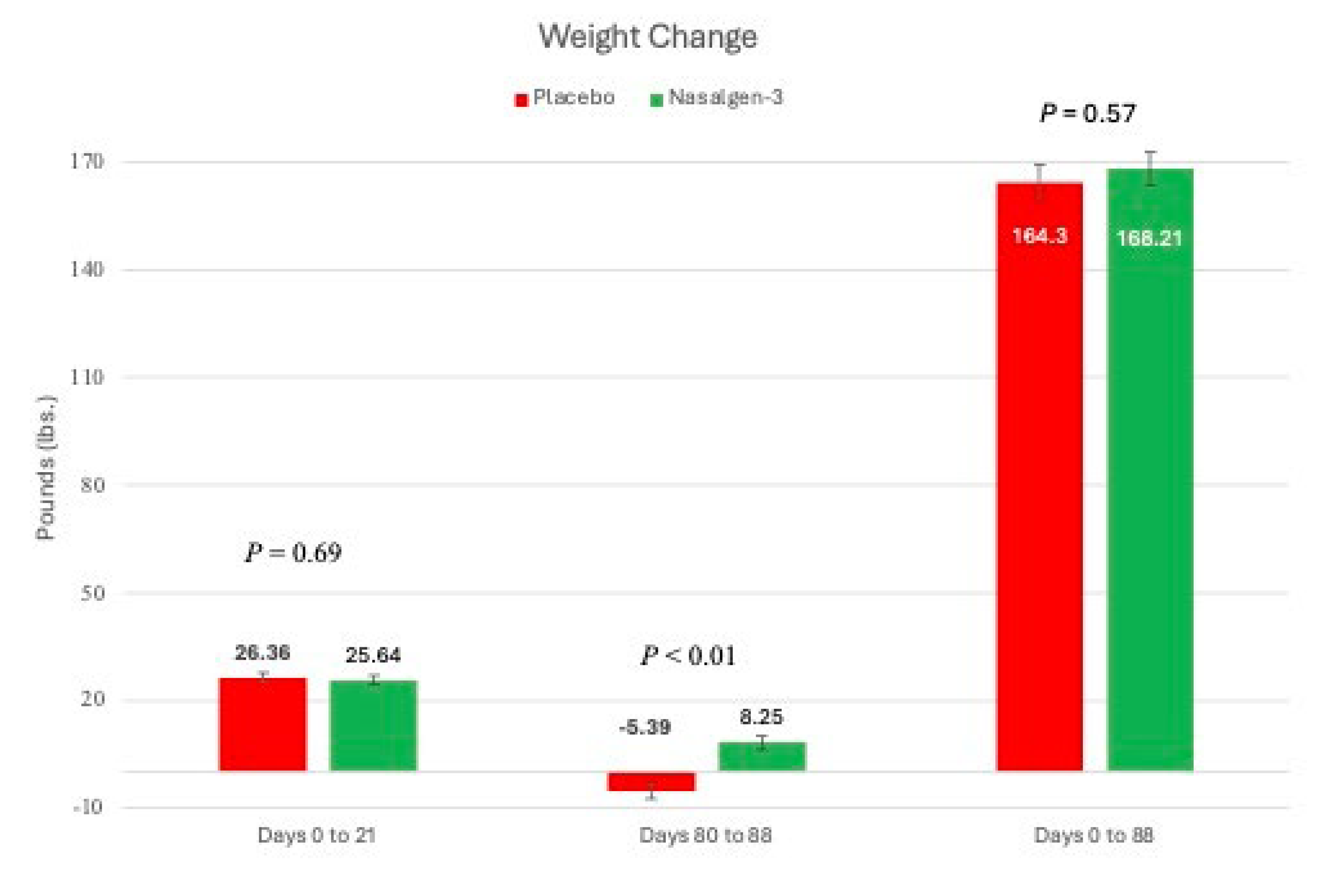
Weight gain following vaccination and challenge Vaccination had no significant effect on weight gain in the first 21 days following vaccination (
Figure 6).
During the post-challenge time, calves vaccinated with the tri-valent MLV vaccine gained 8.25 lb. (SEM = 1.98) body weight while those sham-vaccinated with the placebo lost weight (-5.39 lb; SEM = 2.02) (
P < 0.01) (
Figure 6). There was no overall body weight effect over the 88 day period (
Figure 6).
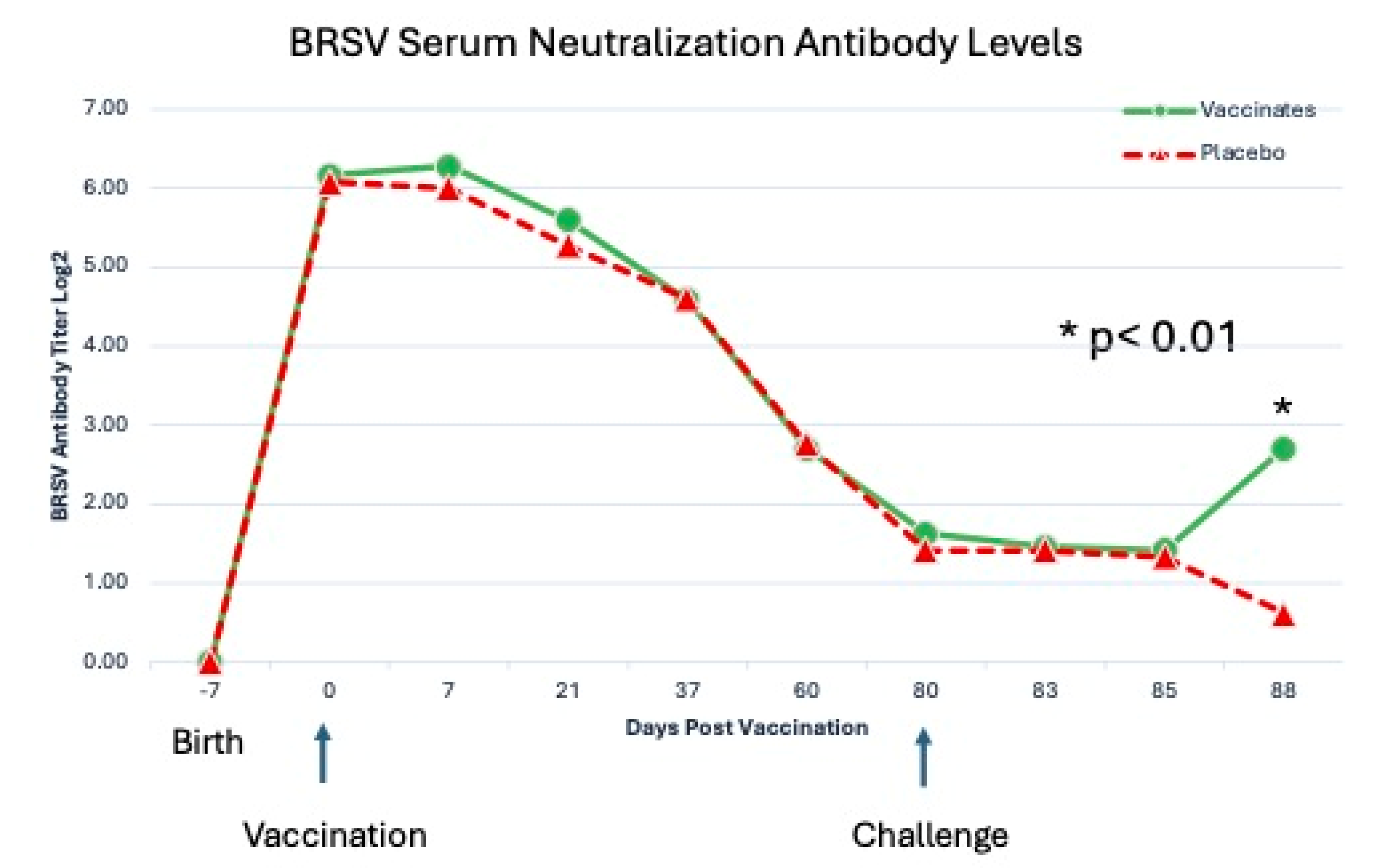
Serum neutralization antibody against BRSV and against BHV-1 Titers of BRSV SN antibodies for all calves were < 2 at the time of enrollment (
Figure 7).
The mean BRSV antibody titers decreased after enrollment until challenge (
Figure 7). After vaccination there was no significant (
P = 0.55) effect of treatment. After challenge there was a significant (
P < 0.01) effect of vaccination as on day 88 the mean BRSV SN titer was significantly (
P < 0.05) higher for calves vaccinated with the tri-valent MLV vaccine than for calves sham-vaccinated with placebo (
Figure 7). Fourteen (14) of the 25 vaccinated calves had SN antibody increases from DPC 5 (day 85) to DPC 8 (day 88), 8 stayed the same and 2 decreased. In contrast the sham-vaccinated placebos BRSV antibody levels exhibited classic maternal antibody decay with 0 of the 23 animals having an increase, 12 of 23 staying the same and 11 decreasing. Interestingly, 3 of the tri-valent vaccinated group BRSV titers were <2 (seronegative) at DPC 5 and they had BRSV SN titers ranging from 2-64 three days later on DPC 8, demonstrating memory and anamnestic response IFOMA. For BHV-1, BHV-1 SN titers were measured over the 88 day period and the antibodies decreased at the same level between the two treatments and there was no significant effect (
P = 0.15) of vaccination on SN antibodies against BHV-1 (data not shown).
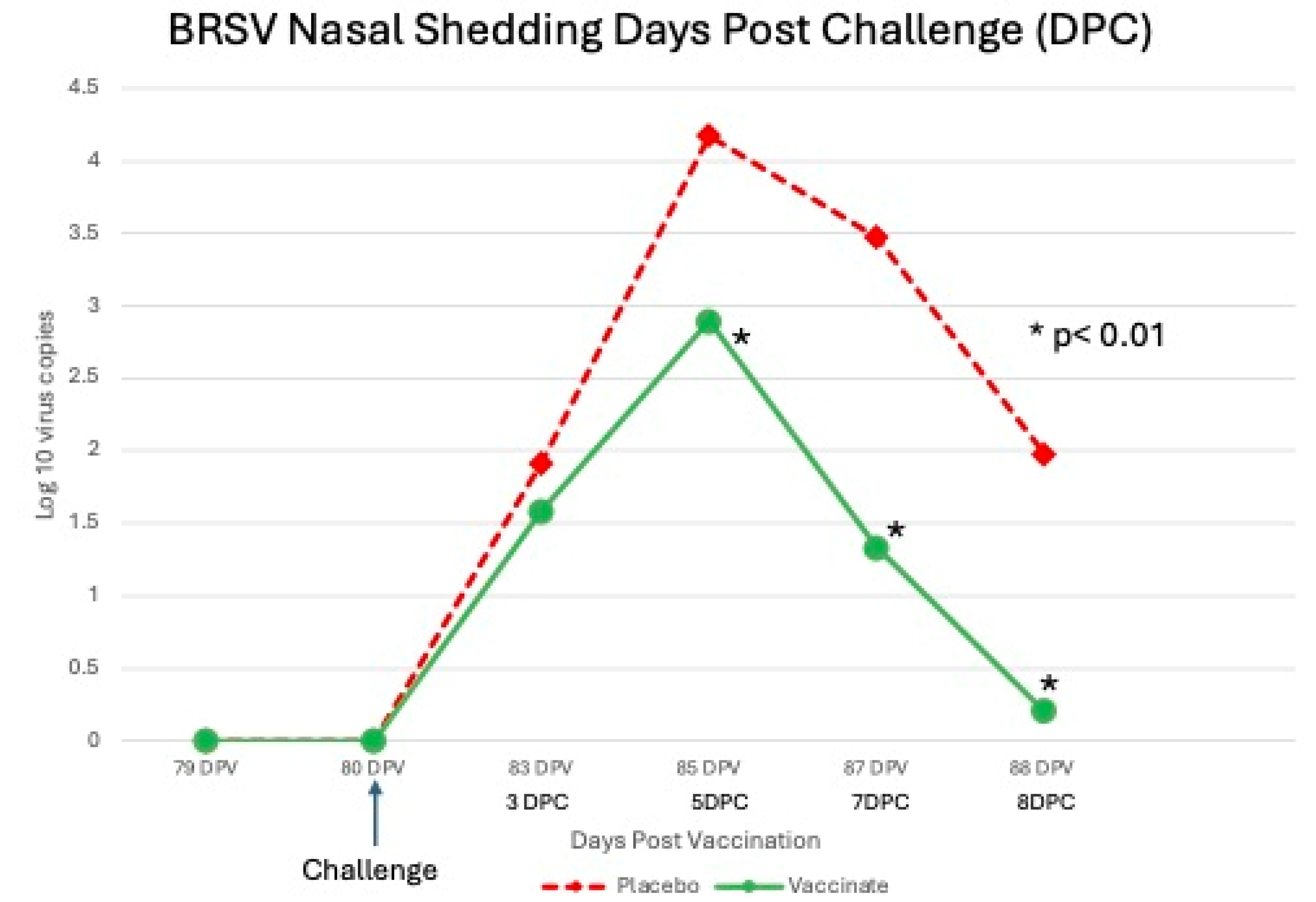
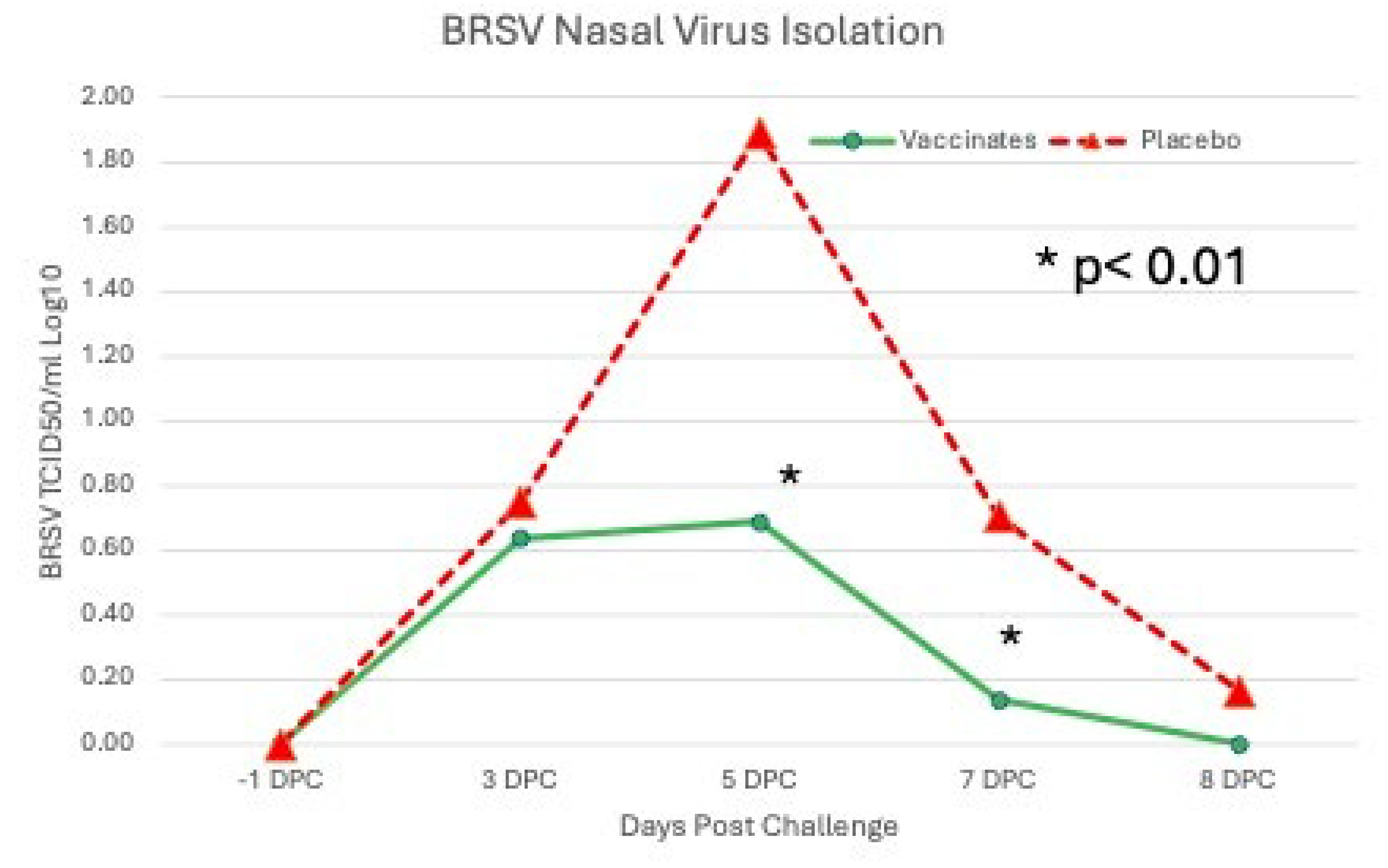
Shedding of BRSV Post Challenge The sham vaccinated placebo calves did not shed BRSV until after challenge with virulent BRSV on day 80. All (100%: 24 of 24 DPC 3-6; 23 of 23 DPC 7) of the placebo calves were positive by RT-PCR BRSV in nasal secretions from DPC 3, 5 & 7 (days 83, 85 & 87) (
Figure 3). On DPC 8, 97% (22 of 23) sham vaccinated placebo calves were positive by RT-PCR (
Figure 3). A higher percentage of the vaccinated animals were positive by RT-PCR BRSV in nasal secretions from DPC 3, 5 & 7 (days 83, 85 & 87; 92%, 96% and 88% respectively). On DPC 8, (day 88) the number of tri-vaccinated animals positive by RT-PCR BRSV dramatically decreased to 46% (11 of 24) (
Figure 3). The number of tri-valent vaccinated animals shedding infectious BRSV virus was much less than the sham vaccinated placebo calves. The sham vaccinated placebo calves shed infectious BRSV in nasal secretions throughout the challenge period. Virus was isolated from 46% (11 of 24) on DPC 3, 92% (22 of 24) on DPC 5, 52% (12 of 23) on DPC 7 and 13% (3 of 23) on DPC 8 of the placebo calves (results not shown).In the vaccinated animals, virus was isolated from 54% (13 of 24) on DPC 3, 50% (12 of 24) on DPC 5, 13% (3 of 24) of DPC 7 and 0% (0 of 24) on DPC 8 (results not shown).
The level of infectious BRSV virus detected or shed was also less in tri-valent vaccinated animals and it was shed for less time.
Levels of BRSV detected by RT-PCR or virus isolation also differed between the sham vaccinated placebo and the tri-valent vaccinated groups throughout the challenge period (
Figure 8 & (
Figure 9).
The levels of BRSV in nasal secretions, as measured by semiquantitative RT-PCR, was higher at all time points in the placebo group (
Figure 8) peaking at DPC 5 (day 85) and was 1-2 log10 significantly higher on DPC 5, 7 and 8 (
P <0.01). Similar results were seen with BRSV infectious virus shed in nasal secretions. Virus was isolated at all time points post challenge in the placebo group (
Figure 9). The placebo group peaked at DPC 5 (day 85) with almost 2 log10 infectious BRSV being shed and was 0.5-1 log10 significantly higher on DPC 5 and 7 (
P <0.01). In contrast the tri-valent vaccinated group shed less than a log10 of infectious virus and was negative on DPC 8 (day 88) (
Figure 9).
BRSV Lung Evaluation
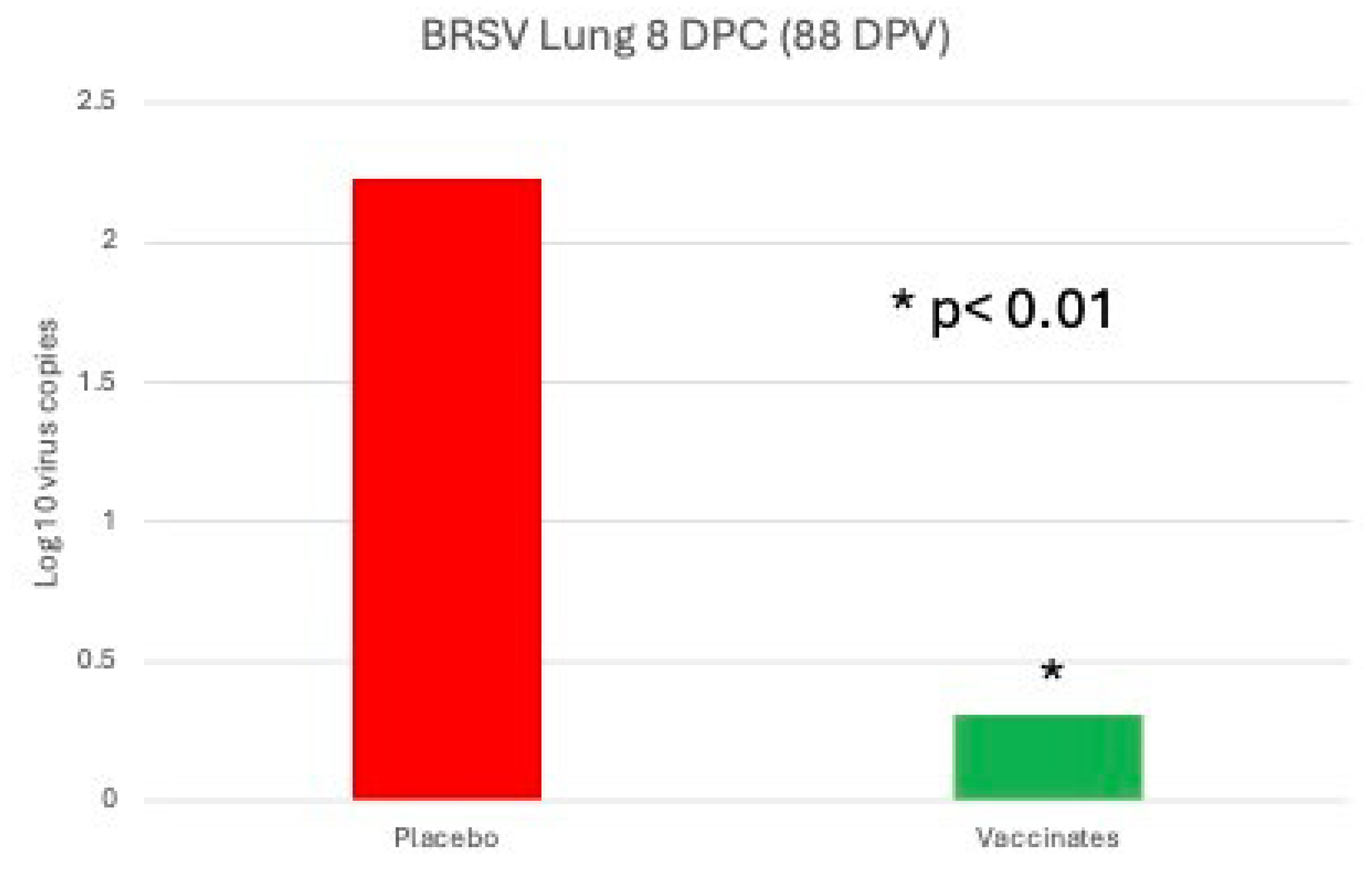
BRSV Virus Detection The lung samples were tested for BRSV following necropsy. Ninety-two percent (92%) (22 of 24) of the sham vaccinated placebo animals were positive as compared to 71% (17 of 24) of the vaccinated animals (results not shown).Similar to the nasal samples, levels of BRSV as detected by RT-PCR were almost 2 log10 significantly lower in the tri-valent vaccinates as compared to the placebo (
Figure 10).
Interestingly the sham vaccinated placebo animal that died on DPC 7 had a CT value of 17.14 that would be 3 log10 higher than the highest nasal sample at DPC 5. BRSV virus isolation was negative on all lung samples (data not shown).
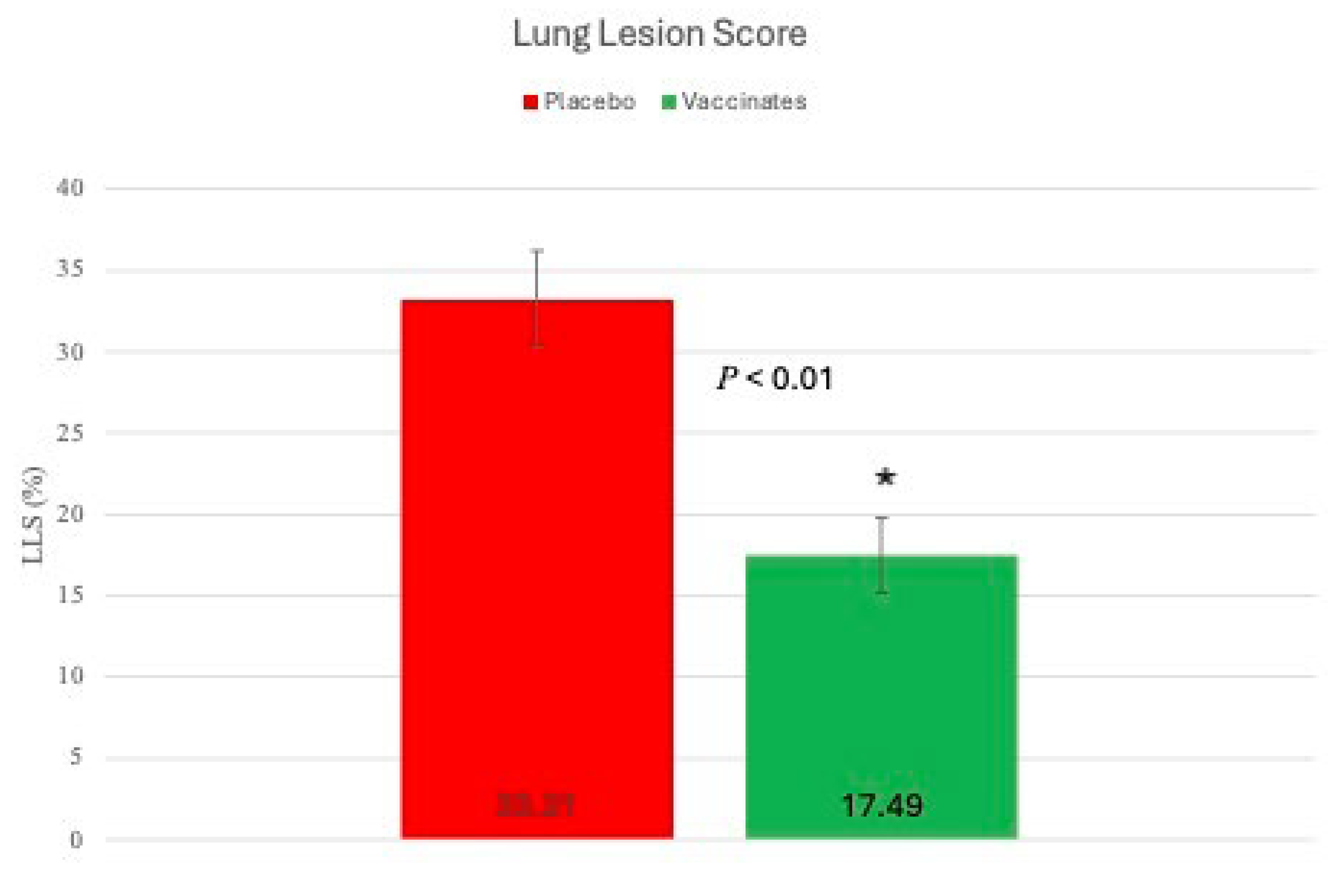
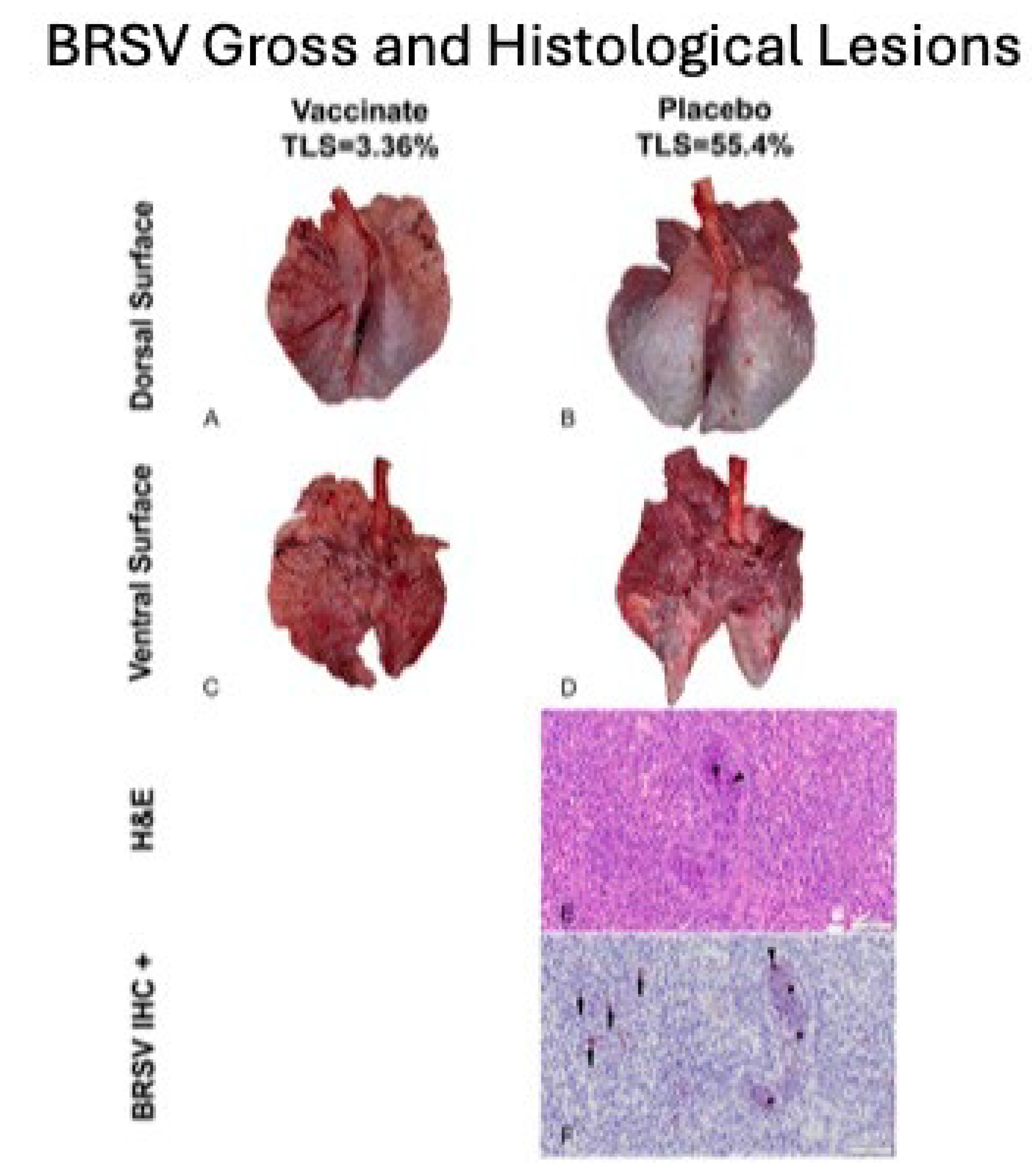
Gross Lung lesion scores Pulmonary lesions were visualized in all experimental animals, irrespective of treatment group. Lung lesions were reduced in the tri-valent vaccinated animals (
Figure 11 &
Figure 12).
Mean LLS (SEM) [17.49 % (2.33)] for calves vaccinated with the tri-valent MLV vaccine were significantly (
P < 0.01) lower than that [33.21% (2.99)] for calves sham-vaccinated with the placebo (
Figure 11). However, distribution and magnitude of pulmonary pathology varied considerably between vaccinates and placebos (
Figure 12, Panels A-D). Affected pulmonary parenchyma was pale red to plum-red, consolidated due to atelectasis and often surrounded or divided pale pink noncollapsing, hyperinflated lung (
Figure 12, Panels B & D). Marked subpleural and interlobular edema fluid and emphysema accentuated individual lobules. Consolidated parenchyma was firm and cut crisp with a knife while hyperinflated lobules were rubbery on cut section and often exuded clear frothy edema fluid.
Microscopic Findings Fulminant bronchointerstitial pneumonia was visualized in pulmonary tissue sections from 48/48 animals and necrotizing bronchiolitis was detected in 15/48 animals (
Figure 12, Panel E) with all but one of 15 belonging to the placebos. Caseonecrotic pneumonia superimposed upon bronchointerstitial pneumonia in pulmonary tissue sections from one placebo animal inferred mycoplasma co-infection owing to impaired lung defenses and permissive bacterial infection resulting from viral injury. Viral syncytial cells were observed in 2/48 animals, both placebos (
Figure 12, Panel E).Varying degrees of BALT hyperplasia were visualized in 47/48 animals. Often tissue sections depicted reparative efforts following lymphocyte-mediated lysis of virus-infected cells. These changes included hyperplastic bronchiolar epithelium, disappearance of syncytial cells with clearance of viral antigen, and alveolar tombstoning-that is, cuboidal epithelialization of the alveoli often labeled Type II pneumocyte hypertrophy and hyperplasia.
Immuohistochemistry BRSV immunosignaling was restricted to a limited number of animals (7/48) all of whom were placebos. Antigen expression was visualized in the airway epithelium, exfoliated cellular detritus occluding airway lumina, and alveolar histiocytes (
Figure 11, Panel F). Limited immunolabeling of pulmonary tissues indicates clearance of viral antigen.
Discussion
Managing BRSV and the resulting secondary bacterial continues to be an enormous problem for beef and dairy cattle producers nationwide.The complications associated with both disease prevention IFOMA and the economics of food production create a strong demand for better vaccination strategies and vaccines.The inhibition of BRSV vaccines administered both parenterally and intranasally by BRSV maternal antibody has been well documented [
11,
24,
25].BRSV infection frequently occurs in young animals and vaccination needs to occur IFOMA to minimize disease risk in production systems. The primary purpose of this study was to evaluate the efficacy of a tri-valent MLV IN vaccine to stimulate the immune response and protect against virulent BRSV challenge with BRSV in the face of maternal antibodies (IFOMA) acquired from colostrum. This trivalent MLV IN vaccine reduced BRSV associated disease in two studies in non-suckled, BRSV seronegative calves administered vaccine at ~5-7 days of age when challenged at 30 [
26] or 78 [
27] days postvaccination. By controlling and defining the maternal transfer in the calves involved in the study, we hoped to evaluate the specific interactions between IN vaccination and passive acquired immune systems in young calves. To evaluate vaccine efficacy, maternal antibody transfer was standardized to provide a consistent baseline level to compare vaccine group to placebo group. The pooled colostrum utilized in the study contained BRSV titers of approximately 1:128, and the average antibody titer in the calves at 36 hours was found to be half that (1:64), supporting the common paradigm that complete maternal antibody transference does not occur [
24]. This level of antibody was similar to levels we had seen in a previous study [
17] using a parenteral vaccine and also in a BRSV intranasal vaccine study where calves had been fed undiluted colostrum [
14]. In that study, calves from a large colostrum-fed cohort were screened and 22 calves having similar BRSV maternal antibody titers were enrolled in the study. The establishment and documentation of the calves’ BRSV antibody levels from birth was critical because the goal was to evaluate the animal’s response to MLV IN mucosal exposure when maternal antibody was at low levels, thus elucidating the actual effect of a IN tri-valent MLV vaccine administered to calves IFOMA. This is the second study where the maternal antibody was characterized and controlled to “normalize” the effect vaccine response IFOMA on BRSV viral challenge. While previous studies [
12,
28] have helped to define the half-life and decay of maternal BRSV antibody in colostrum and calves, the animals in this study were monitored and the BRSV SN titer was (<1:8) at challenge. The clinical, virological, immunological and pathological differences following BRSV between the tri-valent IN vaccinated calves and the placebo indicated that there is a distinct advantage to IN MLV vaccination IFOMA. Tri-valent vaccinated calves had a reduction in the duration and severity of disease that was statistically significant and was similar to that seen with another IN BRSV vaccination IFOMA [
14].The decrease in the number of animals shedding virus (
Figure 3), viral burden (
Figure 8 &
Figure 9), reduction of clinical signs (
Figure 4 &
Figure 5), and decreased lung viral burden and pathology (
Figure 10,
Figure 11 and
Figure 12) indicate that protective response was mounted by the vaccinated calves.
The administration of mucosal based vaccines such as the tri-valent IN MLV have been traditional thought to result in primarily a mucosal response with little systemic response [
29,
31]. What was surprising was the significantly higher BRSV SN titer for calves vaccinated with the tri-valent MLV vaccine that occurred on day 88, 8 days post challenge indicating an anamnestic response after “systemic priming” with a mucosal delivered vaccination (
Figure 7). The original publications that were done with Nasalgen IP, which contained the BHV-1 and bovine parainfluenza virus 3 (these are the same viruses in Nasalgen 3) demonstrated a BHV-1 SN response which was boosted following a BHV-1 challenge [
30]. This systemic BRSV boost was not seen following challenge in our previous study using an adjuvanted parenteral MLV BRSV vaccine [
17]. It is possible that the more active immune activation that occurs with the “normal” nonmutated BHV-1 in mucosal replication and immune interaction provides an “enhancement” for the development of BRSV systemic immunity. To further support this hypothesis, a recent publication using the same BRSV and BHV-1 strains in the tri-valent vaccine as monovalent vaccines, demonstrated higher BRSV systemic antibody response in animals given a concurrent IN BRSV and IN BHV-1 than the animals that received a monovalent IN BRSV vaccine [
10]. In contrast, animals vaccinated IN with combination a temperature sensitive mutant BHV-1 vaccine and BRSV did not stimulate any BRSV SN response [
29].
A second objective was to detect IN administered vaccine viruses to provide insight on duration of detection and levels of viral replication in nasal samples IFOMA as nasal samples are frequently used for the diagnosis of calf respiratory disease. One of the areas of great confusion with IN nasal vaccines are two “diagnostic issues”- the detection of vaccine virus resulting in misdiagnosis or conversely “the lack of detection” of vaccine virus in IN vaccinated calves (did they even get the vaccine).In this study, we found that BHV-1 from the tri-valent IN MLV vaccine was detected by PCR as late as 9 days post vaccination (
Figure 2) from nasal swabs. PCR virus detection peaked at DPV 5 and was negative at DPV 14 (
Figure 2).Virus was detected in a small percentage of animals at DPV 1.The highest level of BHV-1 was also detected at DPV 5. Virus isolation had a similar temporal pattern and was a little less sensitive than PCR (i.e. on DPC 5, 16 animals were detected by PCR vs. 14 with VI) (data not shown). On DPV 1, 3 of 25 (12%) vaccinated animals were BHV-1 VI positive and peaked at 56% (14 of 25) at DPC 5. The number of BHV-1 VI positive declined to 40% (10 of 25) at DPV 9 and were negative at DPV 14.The highest viral titers were at DPC 5 when average titer was 4.57 log10 TCID
50/ml. These results agree with original work done with Nasalgen IP (aka Bovilis Nasalgen IP) that demonstrated a similar temporal and peak VI detection [
30].The detection of BRSV following vaccination was much less, peaking at 16% at DPV 7 (
Figure 3) and BRSV was only detected by PCR in 12 samples out of the 125 samples from trivalent IN vaccinated animals. Alternately, BHV-1 was detected by PCR in 55 samples out of the 125 samples from trivalent IN vaccinated animals (data not shown). In another study, 30 beef calves were vaccinated intranasally within 24 hours of birth with a BHV-1, BRSV and PI3 vaccine (Inforce 3, Zoetis Animal Health) and sampled once between 1-7 days post vaccination for BRSV [
31]. Ten of these animals were revaccinated 14 days later and again sampled once between 1-7 days post vaccination for BRSV. At both timepoints, all the animals were negative for BRSV using the same BRSV RT-PCR assay and laboratory used in this study [
31].This second study further supports the low level of detection of IN administered BRSV vaccine virus seen in our study.
To summarize, results of this study support immunogenic and protective findings of other studies with the tri-valent MLV vaccine used in this study [
26,
27]. An important finding was that colostral antibodies did not interfere with the immune response and there was protective effect of one dose of the tri-valent MLV vaccine, when calves were challenged 80 days after vaccination. Virological, immunological and pathological findings all supported a significant advantage for vaccinated calves. Another new finding was the weight gained by calves vaccinated with the tri-valent MLV vaccine contrasted to the weight lost by calves sham-vaccinated with placebo after challenge with virulent BRSV. The presence of ‘vaccine virus” is always a diagnostic dilemma and this study provided evidence that BHV-1 in the tri-valent vaccine is highly likely to be present in samples up to 9 days and likely will be negative after 14 days. BRSV is less likely than BHV-1 to be detected by PCR on nasal swabs but similar to BHV-1 can be present in samples up to 9 days and likely will be negative after 14 days. Results of this and previous tri-valent IN MLV efficacy studies collectively support the claim that this tri-valent MLV vaccine is safe and effective for intranasal vaccination of calves at one week of age or older against respiratory disease caused by BRSV,a nd there is a duration of immunity of at least 80 days.
Virological, immunological and pathological findings also supported a significant advantage for vaccinated calves. While the protection conferred is not complete, up to a 50% reduction of severity of disease was noted in the vaccinated calves. Vaccinated calves mounted an immune response sufficient to reduce disease severity when challenged 3 months after vaccination in the face of maternal antibody.
Author Contributions
Conceptualization, BM and CCLC. Methodology, SP-O, NDS, GMK, KA, and DR; Formal Analysis DR; Investigation, SP-O, NDS, BM, GMK and KA; Writing Original-Draft, CCLC SP-O; Writing-Review & Editing, SP-O, NDS, BM, GMK and DR; Project Administration, SP-O, and NDS; Supervision, BM and CCLC; Funding Acquisition, BM.
Funding
for this study was provided by Merck Animal Health
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approval 19-08 was issued by the Institutional Laboratory Animal Care and Use Committee of RTI, LLC (now ClinVet-SD) in May 2020 in compliance with all federal, state, and local regulations prior to initiation of the study.
Acknowledgements
The authors would like to acknowledge the critical assistance of the animal care personnel and the laboratory technical staff of ClinVet-SD and RTI Laboratories for the successful completion of this study.
Conflicts of Interest
Dr. Meyer is an employee of Merck Animal Health.Drs. Chase and Renter have received research funding and/or compensation for continuing education speaking events from Merck Animal Health.Dr. Chase is also the Chief Scientific Officer for Clinvet-SD.
References
- Short, D. M.; Lombard, J. E. The National Animal Health Monitoring System’s Perspective on Respiratory Disease in Dairy Cattle. Anim. Heal. Res. Rev. 2020, 21 (2), 135–138. [CrossRef]
- White, B. J.; Larson, B. L. Impact of Bovine Respiratory Disease in U.S. Beef Cattle. Anim. Heal. Res. Rev. 2020, 21 (2), 132–134. [CrossRef]
- Preview: Economic Effects of Bovine Respiratory Disease. J Anim Sci, 2020;98. [CrossRef]
- 4. Blakebrough-Hall C, McMeniman JP, González LA. An evaluation of the economic effects of bovine respiratory disease on animal performance, carcass traits, and economic outcomes in feedlot cattle defined using four BRD diagnosis methods. J Anim Sci 2020;98. [CrossRef]
- Peel, D. S. Economic Considerations of Enhanced BRD Control. Anim Heal Res Rev 2020, 21 (2), 139–142. [CrossRef]
- Makoschey, B. , Berge, A.C.Review on bovine respiratory syncytial virus and bovine parainfluenza – usual suspects in bovine respiratory disease – a narrative review. Bmc Vet Res 2021, 17, 261. [CrossRef]
- Chase C, Hurley DJ, Reber AJ. Neonatal Immune Development in the Calf and Its Impact on Vaccine Response. Vet Clin North Am Food Animal Pract 2008, 24, 87–104. [CrossRef]
- Chase CCL, Parreno V. In the Beginning: Development and Maximizing the Neonate Immune System. In Bovine Immunity: Making Immunology and Vaccinology Come Alive ed. CCL Chase. Amer Spain: Hipra; 2022. Pp. 25-28. https://bovineimmunity.hipra.com/index-en.html.
- Windeyer, M. C.; Gamsjäger, L. Vaccinating Calves in the Face of Maternal Antibodies. Vet Clin North Am Food Animal Pract 2019, 35 (3), 557–573. [CrossRef]
- Flynn, A.; McAloon, C. et al. Investigation into the Safety, and Serological Responses Elicited by Delivery of Live Intranasal Vaccines for Bovine Herpes Virus Type 1, Bovine Respiratory Syncytial Virus, and Parainfluenza Type 3 in Pre-Weaned Calves. Front. Vet. Sci. 2024, 11, 1283013. [CrossRef]
- Ellis, JA. How efficacious are vaccines against bovine respiratory syncytial virus in cattle??. Vet Microbiol 2017, 206:59–68.
- Kirkpatrick J, Fulton RW, et al. Passively transferred immunity in newborn calves, rate of antibody decay, and effect on subsequent vaccination with modified live virus vaccine. The Bovine Practitioner 2001, 35:47–55.
- Fulton RW, Briggs RE, et al. Maternally derived humoral immunity to bovine viral diarrhea virus (BVDV) 1a, BVDV1b, BVDV2, bovine herpesvirus- 1, parainfluenza-3 virus bovine respiratory syncytial virus, Mannheimia haemolytica and Pasteurella multocida in beef calves, antibody decline by half-life studies and effect on response to vaccination. Vaccine 2004, 22, 643–9. [CrossRef]
- Vangeel I., Antonis AFG, et al. Efficacy of a Modified Live Intranasal Bovine Respiratory Syncytial Virus Vaccine in 3-Week-Old Calves Experimentally Challenged with BRSV. Vet J 2007, 174 (3), 627–635. [CrossRef]
- Woolums, AR, Brown, CC, et al. Effects of a Single Intranasal Dose of Modified-Live Bovine Respiratory Syncytial Virus Vaccine on Resistance to Subsequent Viral Challenge in Calves. American Journal of Veterinary Research 2004, 65 (3), 363–372.
- Manual of standards for diagnostic tests and vaccines. Office International des Epizooties, Paris, France; 2008.
- Kolb EA, Buterbaugh RE, et al. Protection against bovine respiratory syncytial virus in calves vaccinated with adjuvanted modified live vaccine administered in the face of maternal antibody. Vaccine 2020, 38, 298-308. [CrossRef]
- Fairbanks KF, Campbell, J, et al.Rapid Onset of Protection against Infectious Bovine Rhinotracheitis with a Modified-Live Virus Multivalent Vaccine. Vet Therap 2004, 5 (1), 17–25.
- Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Archiv f experiment Pathol u Pharmakol 1931,162,480–3.
- Zimmerman, A. D.; Klein, AL, et al. Protection against Bovine Herpesvirus Type 1 (BHV-1) Abortion Following Challenge 8 Months or Approximately 1 Year after Vaccination. The Bovine Practitioner 2013, 47 (2), 73–81.
- 21. Timsit E, Maingourd C, Le Dréan E, et al. Evaluation of a commercial real-time reverse transcription polymerase chain reaction kit for the diagnosis of bovine respiratory syncytial virus infection. J Vet Diag Invest 2010 22,238–41.
- Achenbach JE, Topliff CL, Vassilev VB, et al. Detection and quantitation of bovine respiratory syncytial virus using real-time quantitative RT-PCR and quantitative competitive RT-PCR assays. J Virol Methods 2004 121,1–6.
- Jericho, K. W.; Langford, E. V. Aerosol Vaccination of Calves with Pasteurella Haemolytica against Experimental Respiratory Disease. Can J Comp Med 1982, 46 (3), 287–292.
- Ellis JA, Gow SP, et al. Response to Experimentally Induced Infection with Bovine Respiratory Syncytial Virus Following Intranasal Vaccination of Seropositive and Seronegative Calves. J Am Vet Med Assoc 2010, 236 (9), 991–999. [CrossRef]
- Kimman TG,Westenbrink F, et al. Priming for Local and Systemic Antibody Memory Responses to Bovine Respiratory Syncytial Virus: Effect of Amount of Virus, Virus Replication, Route of Administration and Maternal Antibodies. Veterinary Immunology and Immunopathology 1989, 22 (2), 145–160.
- Merck Animal Health. Efficacy of the Bovine Respiratory Syncytial Virus Fraction of Nasalgen® 3-PMH in Calves 4 to 7 Days Old. Nasalgen 3-PMH Tech Bulletin Series; Merck Animal Health, 2020, pp 1–7.
- Merck Animal Health. Duration of Immunity of the Bovine Respiratory Syncytial Virus Fraction of Nasalgen® 3-PMH Administered to Calves 5 to 7 Days of Age.Nasalgen 3-PHM Tech Bulletin Series; Merck Animal Health, 2020, pp 1–10.
- Chamorro MF, Walz PH, et al. Comparison of levels and duration of detection of antibodies to bovine viral diarrhea virus 1, bovine viral diarrhea virus 2, bovine respiratory syncytial virus, bovine herpesvirus 1, and bovine parainfluenza virus 3 in calves fed maternal colostrum or a colostrum-replacement product. Can. J. Vet. Res. 2012, 78, 81–8.
- Cortese VS, Woolums A, et al. Comparison of Interferon and Bovine Herpesvirus-1-Specific IgA Levels in Nasal Secretions of Dairy Cattle Administered an Intranasal Modified Live Viral Vaccine Prior to Calving or on the Day of Calving. Vet Immunol Immunopath 2017, 187, 35–41. [CrossRef]
- Todd JD, Volenec FJ, et al. Interferon in Nasal Secretions and Sera of Calves after Intranasal Administration of Avirulent Infectious Bovine Rhinotracheitis Virus: Association of Interferon in Nasal Secretions with Early Resistance to Challenge with Virulent Virus. InfectImmun 1972, 5 (5), 699–706.
- Daly R,Rozeboom C et al. Assessing Passive Transfer and Respiratory Pathogen Colonization of Neonatal Beef Calves in a Confinement Operation. SDSU Beef Day 2020 Proceedings 2020, 44-49. https://openprairie.sdstate.edu/sd_beefday_2020/1/.
Figure 1.
Schedule of Events. Calves were collected at birth from the dairy and fed colostrum withing 24 hours of birth. The calves were vaccinated at 9±2 days age. The animals were then sampled and challenged as indicated in the schedule of events.
Figure 1.
Schedule of Events. Calves were collected at birth from the dairy and fed colostrum withing 24 hours of birth. The calves were vaccinated at 9±2 days age. The animals were then sampled and challenged as indicated in the schedule of events.
Figure 2.
Percentage of Animals Positive for Bovine Herpesvirus Virus Nasal as detected by PCR. Nasal swabs were collected from all calves on days 0, 1, 3, 5, 7, 9 and 14 days post vaccination (DPV) BHV-1 for detection of BHV-1 using the polymerase chain reaction (PCR) assay.
Figure 2.
Percentage of Animals Positive for Bovine Herpesvirus Virus Nasal as detected by PCR. Nasal swabs were collected from all calves on days 0, 1, 3, 5, 7, 9 and 14 days post vaccination (DPV) BHV-1 for detection of BHV-1 using the polymerase chain reaction (PCR) assay.
Figure 3.
Percentage of Animals Positive for Bovine Respiratory Syncytial Virus as detected by RT-PCR. Nasal swabs were collected from all calves on days 0, 1, 3, 5, 7, 9 and 14 days post vaccination (DPV) and on days -1, 0, 3, 5, 7 and 8 days post challenge (DPC) for detection of BRSV using the reverse transcriptase-polymerase chain reaction (RT-PCR) assay.
Figure 3.
Percentage of Animals Positive for Bovine Respiratory Syncytial Virus as detected by RT-PCR. Nasal swabs were collected from all calves on days 0, 1, 3, 5, 7, 9 and 14 days post vaccination (DPV) and on days -1, 0, 3, 5, 7 and 8 days post challenge (DPC) for detection of BRSV using the reverse transcriptase-polymerase chain reaction (RT-PCR) assay.
Figure 4.
Mean Rectal Temperature. Body temperature was monitored for 2 days prior to challenge and 8 days following challenge. *p<0.01.
Figure 4.
Mean Rectal Temperature. Body temperature was monitored for 2 days prior to challenge and 8 days following challenge. *p<0.01.
Figure 5.
Mean Respiratory Scores. Clinical disease parameters including general respiratory signs were monitored for 2 days prior to challenge and 8 days following challenge. There was one placebo animal that succumbed to the BRSV challenge. *p<0.01.
Figure 5.
Mean Respiratory Scores. Clinical disease parameters including general respiratory signs were monitored for 2 days prior to challenge and 8 days following challenge. There was one placebo animal that succumbed to the BRSV challenge. *p<0.01.
Figure 6.
Weight Change following Intranasal Vaccination and BRSV Challenge. Change of body weight for calves in each Treatment Group during the first 21 days, during the post-challenge phase, and throughout the duration of the study. *p<0.01.
Figure 6.
Weight Change following Intranasal Vaccination and BRSV Challenge. Change of body weight for calves in each Treatment Group during the first 21 days, during the post-challenge phase, and throughout the duration of the study. *p<0.01.
Figure 7.
BRSV Serum Neutralization Titers. Blood samples for BRSV serum antibody analysis were obtained from the calves via jugular venipuncture at 7–21 day intervals for approximately 10 weeks prior to challenge, the day before challenge (-1 DPC), and at necropsy (8DPC). *p<0.01.
Figure 7.
BRSV Serum Neutralization Titers. Blood samples for BRSV serum antibody analysis were obtained from the calves via jugular venipuncture at 7–21 day intervals for approximately 10 weeks prior to challenge, the day before challenge (-1 DPC), and at necropsy (8DPC). *p<0.01.
Figure 8.
Levels of BRSV detected from Nasal Swabs following BRSV Challenge using RT-PCR. Nasal swabs were collected from all calves on days -1, 0, 3, 5, 7 and 8 days post challenge (DPC) for BRSV RT-PCR assay. *p<0.01.
Figure 8.
Levels of BRSV detected from Nasal Swabs following BRSV Challenge using RT-PCR. Nasal swabs were collected from all calves on days -1, 0, 3, 5, 7 and 8 days post challenge (DPC) for BRSV RT-PCR assay. *p<0.01.
Figure 9.
Levels of BRSV Shed as detected by Virus Isolation following BRSV Challenge. Nasal swabs were collected from all calves on days -1, 0, 3, 5, 7 and 8 days post challenge (DPC) for BRSV for VI assay. *p<0.01.
Figure 9.
Levels of BRSV Shed as detected by Virus Isolation following BRSV Challenge. Nasal swabs were collected from all calves on days -1, 0, 3, 5, 7 and 8 days post challenge (DPC) for BRSV for VI assay. *p<0.01.
Figure 10.
Levels of BRSV in the Lung following BRSV Challenge using RT-PCR. Following euthanasia, two representative areas of the lung were collected. Five grams of lung was processed with Media with antibiotics and 1 ml of supernatant was then submitted for BRSV RT-PCR. *p<0.01.
Figure 10.
Levels of BRSV in the Lung following BRSV Challenge using RT-PCR. Following euthanasia, two representative areas of the lung were collected. Five grams of lung was processed with Media with antibiotics and 1 ml of supernatant was then submitted for BRSV RT-PCR. *p<0.01.
Figure 11.
Mean lung lesion scores (LLS) on Day 88 (8 days post-challenge). Lesions of the lungs were scored as a percent of lung involved. Each lung lobe was examined in its’ entirety and the extent of lung involvement was estimated as a percentage of each lobe. *p<0.01.
Figure 11.
Mean lung lesion scores (LLS) on Day 88 (8 days post-challenge). Lesions of the lungs were scored as a percent of lung involved. Each lung lobe was examined in its’ entirety and the extent of lung involvement was estimated as a percentage of each lobe. *p<0.01.
Figure 12.
Lung Gross Lesions and Histopathology. Panel a) Vaccinate gross lesions dorsal surface; b) Placebo/Control gross lesions dorsal surface; c) Vaccinate gross lesions ventral surface; d) Placebo/Control gross lesions ventral surface; e) H & E Placebo/Control histological lesions; e) Placebo/Control BRSV IHC staining.
Figure 12.
Lung Gross Lesions and Histopathology. Panel a) Vaccinate gross lesions dorsal surface; b) Placebo/Control gross lesions dorsal surface; c) Vaccinate gross lesions ventral surface; d) Placebo/Control gross lesions ventral surface; e) H & E Placebo/Control histological lesions; e) Placebo/Control BRSV IHC staining.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).