Submitted:
08 May 2024
Posted:
10 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Histochemical Analysis of Peduncles
2.2. Hydraulic Conductance
2.3. Gene Expression
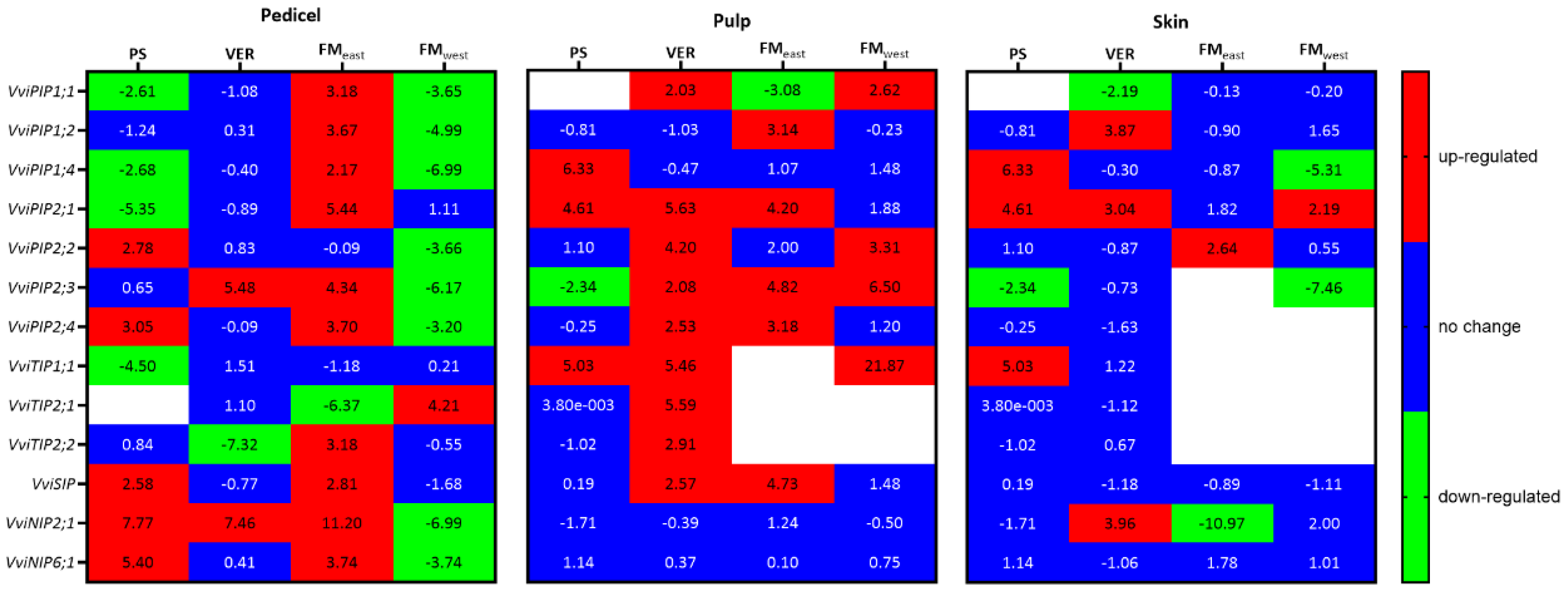
2.3.1. Aquaporins
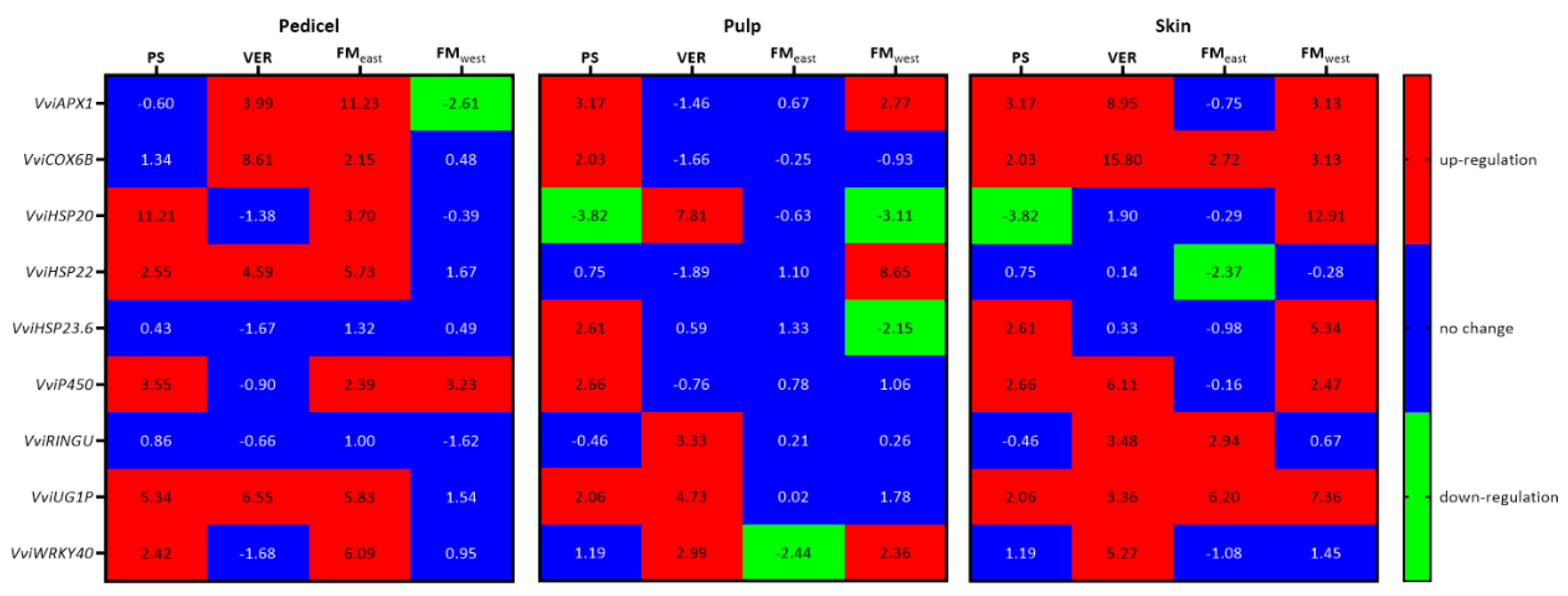
2.3.2. Stress Related Genes
3. Discussion
4. Materials and Methods
4.1. Field Trial and Plant Material
4.2. Histology
4.3. Hydraulic Conductance
4.4. RNA Extraction
4.5. cDNA Synthesis for qPCR
4.6. qPCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change. Climate change. Synthesis report. 2014. 16 pp.
- Wild, M. Decadal changes in radiative fluxes at land and ocean surfaces and their relevance for global warming. Wiley Interdiscip. Rev. Clim. Change 2016, 7, 91–107. [Google Scholar] [CrossRef]
- Climate Impacts in Portugal. 2019 www.climateanalytics.org, accessed on the 10th February 2024.
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; Kartschall, T.; Menz, C.; Molitor, D.; Junk, J.; Beyer, M.; Schultz, H.R. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- INE 2016. (n.d.).
- Silvestre, J.; Damásio, M.; Egipto, R.; Cunha, J.; Brazão, J.; Eiras-Dias, J. Tolerance to sunburn: A variable to consider in the context of climate change. Proceedings of the 21st GiESCO International Meeting, Thessaloniki, Greece 2019, 681–682. [Google Scholar]
- Keller, M. The Science of Grapevines: Anatomy and Physiology. 2015 Elsevier Science.
- Dry, P. Bunch exposure management. Grape And Wine Research And Development Corporation Report 2009 6 p.
- Gambetta, J.M.; Holzapfel, B.P.; Stoll, M.; Friedel, M. Sunburn in Grapes: A Review. Front. Plant Sci. 2021, 11, 604691. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.G.; Wardle, D.A. Evaluation of minimal pruning upon vine performance and berry composition of Chancellor. Am. J. Enol. Vitic. 2001, 52, 45–48. [Google Scholar] [CrossRef]
- Main, G.L.; Morris, J.R. Impact of pruning methods on yield components and juice and wine composition of Cynthiana grapes. Am. J. Enol. Vitic. 2008, 59, 179–187. [Google Scholar] [CrossRef]
- Rustioni, L.; Milani, C.; Parisi, S.; Failla, O. Chlorophyll role in berry sunburn symptoms studied in different grape (Vitis vinifera L.) cultivars. Sci. Hortic. 2015, 185, 145–150. [Google Scholar] [CrossRef]
- Schultz, H.R.; Matthews, M.A. Xylem development and hydraulic conductance in sun and shade shoots of grapevine (Vitis vinifera L.): evidence that low light uncouples water transport capacity from leaf area. Planta 1993, 190, 393–406. [Google Scholar] [CrossRef]
- Tilbrook, J.; Tyerman, S.D. Hydraulic connection of grape berries to the vine: Varietal differences in water conductance into and out of berries, and potential for backflow. Func. Plant Biol. 2009, 36, 541–550. [Google Scholar] [CrossRef]
- Bondada, B.R.; Matthews, M.A.; Shackel, K.A. Functional xylem in the post-veraison grape berry. J. Exp. Bot. 2005, 56, 2949–2957. [Google Scholar] [CrossRef]
- Keller, M.; Smith, J.P.; Bondada, B.R. Ripening grape berries remain hydraulically connected to the shoot. J. Exp. Bot. 2006, 57, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Keller, M. Discharge of surplus phloem water may be required for normal grape ripening. J. Exp. Bot. 2017, 68, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Knoche, M. Water uptake through the surface of fleshy soft fruit: Barriers, mechanism, factors, and potential role in cracking. In Abiotic Stress Biology in Horticultural Plants 2015 Springer Japan, pp. 147–166. [CrossRef]
- Winkler, A.; Peschel, S.; Kohrs, K.; Knoche, M. Rain cracking in sweet cherries is not due to excess water uptake but to localized skin phenomena. J. Am. Soc. Hortic Sci. 2016, 141, 653–660. [Google Scholar] [CrossRef]
- Grimm, E.; Hahn, J.; Pflugfelder, D.; Schmidt, M.J.; van Dusschoten, D.; Knoche, M. Localized bursting of mesocarp cells triggers catastrophic fruit cracking. Hortic. Res. 2019, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- McElrone, A.J.; Manuck, C.M.; Brodersen, C.R.; Patakas, A.; Pearsall, K.R.; Williams, L.E. Functional hydraulic sectoring in grapevines as evidenced by sap flow, dye infusion, leaf removal and micro-computed tomography. AoB PLANTS 2021, 13, plab003. [Google Scholar] [CrossRef] [PubMed]
- Fouquet, R.; Léon, C.; Ollat, N.; Barrieu, F. Identification of grapevine aquaporins and expression analysis in developing berries. Plant Cell Rep. 2008, 27, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Gambetta, G.A.; Shackel, K.A.; Matthews, M.A. Vascular function in grape berries across development and its relevance to apparent hydraulic isolation. Plant Physiol. 2009, 151, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Sabir, F.; Zarrouk, O.; Noronha, H.; Loureiro-Dias, M.C.; Soveral, G.; Gerós, H.; Prista, C. Grapevine aquaporins: Diversity, cellular functions, and ecophysiological perspectives. Biochimie 2021, 188, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Voicu, M.C.; Cooke JE, K.; Zwiazek, J.J. Aquaporin gene expression and apoplastic water flow in bur oak (Quercus macrocarpa) leaves in relation to the light response of leaf hydraulic conductance. J. Exp. Bot. 2009, 60, 4063–4075. [Google Scholar] [CrossRef]
- Gouthu, S.; Deluc, L.G. Timing of ripening initiation in grape berries and its relationship to seed content and pericarp auxin levels. BMC Plant Biol. 2015, 15, 46. [Google Scholar] [CrossRef]
- Hou, X.; Li, H.; Zhang, W.; Yao, Z.; Wang, Y.; Du, T. Water transport in fleshy fruits: Research advances, methodologies, and future directions. Physiol. Plant 2021, 172, 2203–2216. [Google Scholar] [CrossRef] [PubMed]
- Loepfe, L.; Martinez-Vilalta, J.; Piñol, J.; Mencuccini, M. The relevance of xylem network structure for plant hydraulic efficiency and safety. J. Theor. Biol. 2007, 247, 788–803. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef] [PubMed]
- Tyerman, S.D.; Tilbrook, J.; Pardo, C.; Kotula, L.; Sullivan, W.; Steudle, E. Direct measurement of hydraulic properties in developing berries of Vitis vinifera L. cv Shiraz and Chardonnay. Aust. J. Grape Wine Res. 2004, 10, 170–181. [Google Scholar] [CrossRef]
- Greenspan, M.D.; Shackel, K.A.; Matthews, M.A. Developmental changes in the diurnal water budget of the grape berry exposed to water deficits. Plant Cell Environ. 1994, 17, 811–820. [Google Scholar] [CrossRef]
- Scharwies, J.D.; Tyerman, S.D. Comparison of isohydric and anisohydric Vitis vinifera L. cultivars reveals a fine balance between hydraulic resistances, driving forces and transpiration in ripening berries. Func. Plant Biol. 2017, 44, 324–338. [Google Scholar] [CrossRef]
- Gisbert, C.; Soler, J. X.; Fos, M.; Intrigliolo, D. S.; Yuste, A.; Picó, B.; Torrent, D.; Peiró, R. Characterization of Local Mediterranean Grapevine Varieties for Their Resilience to Semi-Arid Conditions under a Rain-Fed Regime. Agronomy 2022, 12, 2234. [Google Scholar] [CrossRef]
- Villalobos-González, L.; Muñoz-Araya, M.; Franck, N.; Pastenes, C. Controversies in Midday Water Potential Regulation and Stomatal Behavior Might Result From the Environment, Genotype, and/or Rootstock: Evidence From Carménère and Syrah Grapevine Varieties. Front. Plant Sci. 2019, 10, 1522. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef]
- Vandeleur, R.K.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 2009, 149, 445–460. [Google Scholar] [CrossRef]
- Kuhn, N.; Abello, C.; Godoy, F.; Delrot, S.; Arce-Johnson, P. Differential Behavior within a Grapevine Cluster:Decreased Ethylene-Related Gene Expression Dependenton Auxin Transport Is Correlated with Low Abscission of First Developed Berries. PLoS ONE 2014, 9, e111258. [Google Scholar] [CrossRef]
- Knipfer, T.; Fei, J.; Gambetta, G.A.; McElrone, A.J.; Shackel, K.A.; Matthews, M.A. Water transport properties of the grape pedicel during fruit development: Insights into xylem anatomy and function using microtomography. Plant Physiol. 2015, 168, 1590–1602. [Google Scholar] [CrossRef]
- Keller, M.; Zhang, Y.; Shrestha, P.M.; Biondi, M.; Bondada, B.R. Sugar demand of ripening grape berries leads to recycling of surplus phloem water via the xylem. Plant Cell Environ. 2015, 38, 1048–1059. [Google Scholar] [CrossRef]
- Windt, C.W.; Gerkema, E.; van As, H. Most water in the tomato truss is imported through the xylem, not the phloem: A nuclear magnetic resonance flow imaging study. Plant Physiol. 2009, 151, 830–842. [Google Scholar] [CrossRef]
- Hanssens, J.; De Swaef, T.; Steppe, K. High light decreases xylem contribution to fruit growth in tomato. Plant Cell Environ. 2015, 38, 487–498. [Google Scholar] [CrossRef]
- Simon, J.; Baptiste, C.; Lartaud, M.; Verdeil, J.L.; Brunel, B.; Vercambre, G.; Génard, M.; Cardoso, M.; Alibert, E.; Goze-Bac, C.; Bertin, N. Pedicel anatomy and histology in tomato vary according to genotype and water-deficit environment, affecting fruit mass. Plant Sci. 2022, 321, 111313. [Google Scholar] [CrossRef]
- Pace, M.R.; Alcantara, S.; Lohmann, L.G.; Angyalossy, V. Secondary phloem diversity and evolution in Bignonieae (Bignoniaceae). Ann Bot. 2015, 116, 333–358. [Google Scholar] [CrossRef]
- Aloni, B.; Wyse, R.E.; Griffith, S. Sucrose Transport and Phloem Unloading in Stem of Vicia.faba: Possible Involvement of a Sucrose Carrier and Osmotic Regulation. Plant Physiol. 1986, 81, 482–486. [Google Scholar] [CrossRef]
- Sjolund, R. D. (1997). The Phloem Sieve Element: A River Runs through It. Plant Cell 1997, 9, 1137–1146. [Google Scholar] [CrossRef]
- Van Bel AJ, E. The phloem, a miracle of ingenuity. Plant Cell Environ. 2003, 26, 125–149. [Google Scholar] [CrossRef]
- Sabir, F.; Leandro, M.J.; Martins, A.P.; Loureiro-Dias, M.C.; Moura, T.F.; Soveral, G.; Prista, C. Exploring three PIPs and three TIPs of grapevine for transport of water and atypical substrates through heterologous expression in aqy-null yeast. PLoS ONE 2014, 9, e102087. [Google Scholar] [CrossRef] [PubMed]
- Shelden, M.C.; Howitt, S.M.; Kaiser, B.N.; Tyerman, S.D. Identification and functional characterisation of aquaporins in the grapevine, Vitis vinifera. Func. Plant Biol. 2009, 36, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Noronha, H.; Conde, C.; Delrot, S.; Gerós, H. Identification and functional characterization of grapevine transporters that mediate glucose-6-phosphate uptake into plastids. Planta 2015, 242, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Krasnow, M.; Weis, N.; Smith, R. J.; Benz, M. J.; Mathews, M.; Shackel, K. Inception, progression, and compositional consequences of a berry shrivel disorder. Am. J. Enol. Vitic. 2009, 60, 24–34. [Google Scholar] [CrossRef]
- Pilati, S.; Perazzolli, M.; Malossini, A.; Cestaro, A.; Demattè, L.; Fontana, P.; Dal Ri, A.; Viola, R.; Velasco, R.; Moser, C. Genome-wide transcriptional analysis of grapevine berry ripening reveals a set of genes similarly modulated during three seasons and the occurrence of an oxidative burst at vèraison. BMC Genomics 2007, 8, 428. [Google Scholar] [CrossRef]
- Da Silva, F.G.; Iandolino, A.; Al-Kayal, F.; Bohlmann, M.C.; Cushman, M.A.; Lim, H.; Ergul, A.; Figueroa, R.; Kabuloglu, E.K.; Osborne, C.; Rowe, J.; Tattersall, E.; Leslie, A.; Xu, J.; Baek, J.M.; Cramer, G.R.; Cushman, J.C.; Cook, D.R. Characterizing the grape transcriptome. Analysis of expressed sequence tags from multiple vitis species and development of a compendium of gene expression during berry development. Plant Physiol. 2005, 139, 574–597. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Cobb, F.; Tracana, S.; Costa, G.J.; Valente, I.; Goulao, L.F.; Amâncio, S. Relating Water Deficiency to Berry Texture, Skin Cell Wall Composition, and Expression of Remodeling Genes in Two Vitis vinifera L. Varieties. J. Agric. Food Chem. 2015, 63, 3951–3961. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Gambetta, G.A.; Wada, H.; Krasnow, M.N.; Cramer, G.R.; Peterlunger, E.; Shackel, K.A.; Matthews, M.A. Characterization of major ripening events during softening in grape: Turgor, sugar accumulation, abscisic acid metabolism, colour development, and their relationship with growth. J. Exp. Bot 2016, 67, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Xiao, Z.; Rogiers, S.Y.; Sadras, V.O.; Tyerman, S.D. Hypoxia in grape berries: The role of seed respiration and lenticels on the berry pedicel and the possible link to cell death. J. Exp. Bot. 2018, 69, 2071–2083. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Coito, J.L.; Colaço, S.; Sangiogo, M.; Amâncio, S. Heat stress in grapevine: The pros and cons of acclimation. Plant Cell Environ. 2015, 38, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Rocheta, M.; Becker, J.D.; Coito, J.L.; Carvalho, L.; Amâncio, S. Heat and water stress induce unique transcriptional signatures of heat-shock proteins and transcription factors in grapevine. Funct. Integr. Genomics 2014, 14, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, T.; Hu, J.; Zhao, L.; Yu, C.; Ma, F. Research advances in function and regulation mechanisms of plant small heat shock proteins (sHSPs) under environmental stresses. Sci. Total Env. 2022, 825, 154054. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.W.; Shinde, H.; Tesfamicael, K.; Hu, Y.; Fruzangohar, M.; Tricker, P.; Baumann, U.; Edwards, E.J.; Rodríguez López, C.M. Global transcriptome and gene co-expression network analyses reveal regulatory and non-additive effects of drought and heat stress in grapevine. Front. Plant Sci, 2023, 14, 1096225. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K.; Tucker, M.L.; Mattoo, A.K. Ethylene and RIPENING INHIBITOR Modulate Expression of SlHSP17.7A, B Class I Small Heat Shock Protein Genes During Tomato Fruit Ripening. Front. Plant Sci 2020, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.R.; Yu, Y.H.; Ni, P.Y.; Zhang, G.H.; Guo, D.L. Genome-wide identification of small heat-shock protein (HSP20) gene family in grape and expression profile during berry development. BMC Plant Biol. 2019, 19, 433. [Google Scholar] [CrossRef] [PubMed]
- Shafqat, W.; Jaskani, M.J.; Maqbool, R.; Chattha, W.S.; Ali, Z.; Naqvi, S.A.; Haider, M.S.; Khan, I.A.; Vincent, C.I. Heat shock protein and aquaporin expression enhance water conserving behavior of citrus under water deficits and high temperature conditions. Environ. Exp. Bot. 2021, 181, 104270. [Google Scholar] [CrossRef]
- Guo, D.L.; Wang, Z.G.; Pei, M.S.; Guo, L.L.; Yu, Y.H. Transcriptome analysis reveals mechanism of early ripening in Kyoho grape with hydrogen peroxide treatment. BMC Genomics 2020, 21, 784. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Romero, P.; Gohil, H.; Smithyman, R.P.; Riley, W.R.; Casassa, L.F.; Harbertson, J.F. Deficit irrigation alters grapevine growth, physiology, and fruit microclimate. Am. J. Enol. Vitic. 2016, 67, 426–435. [Google Scholar] [CrossRef]
- Xiangyi, L.; Lei, H.; An, X.; Keji, Y.; Meng, N.; Duan, C. Q.; Pan, Q. H. VVIWRKY40, a WRKY transcription factor, regulates glycosylated monoterpenoid production by VviGT14 in grape berry. Genes 2020, 11, 485. [Google Scholar] [CrossRef]
- Teixeira, G.; Monteiro, A.; Santos, C.; Lopes, C.M. Leaf morphoanatomy traits in white grapevine cultivars with distinct geographical origin. Cienc. e Tec. Vitivinic. 2018, 33, 90–101. [Google Scholar] [CrossRef]
- Thorntwaite, C.W. An Approach Toward a Rational Classification of Climate. The Geographical Review, 1948, 38, 55–94. [Google Scholar] [CrossRef]
- Ruzin, S. E. Plant microtechnique and microscopy; Oxford University Press, 1999. [Google Scholar]
- Piermattei, A.; Crivellaro, A.; Carrer, M.; Urbinati, C. The “blue ring”: anatomy and formation hypothesis of a new tree-ring anomaly in conifers. Trees 2015, 29, 613–620. [Google Scholar] [CrossRef]
- Scholz, F.G.; Bucci, S.J.; Meinzer, F.C.; Goldstein, G. Maintenance of Root Function in Tropical Woody Species During Droughts: Hydraulic Redistribution, Refilling of Embolized Vessels, and Facilitation Between Plants. In G. Goldstein, L. Santiago (Eds.), Tropical Tree Physiology: Vol. 6, Tree Physiology, Springer, 2016, pp. 227–241. [CrossRef]
- Sperry, J.S.; Donnelly, J.R.; Tyree, M.T. A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ. 1988, 11, 35–40. [Google Scholar] [CrossRef]
- Coito, J.L.; Rocheta, M.; Carvalho, L.; Amâncio, S. Microarray-based uncovering reference genes for quantitative real time PCR in grapevine under abiotic stress. BMC Res. Notes 2012, 5, 220. [Google Scholar] [CrossRef]
- R Core Team. RStudio: Integrated Development for R; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 1 March 2021).
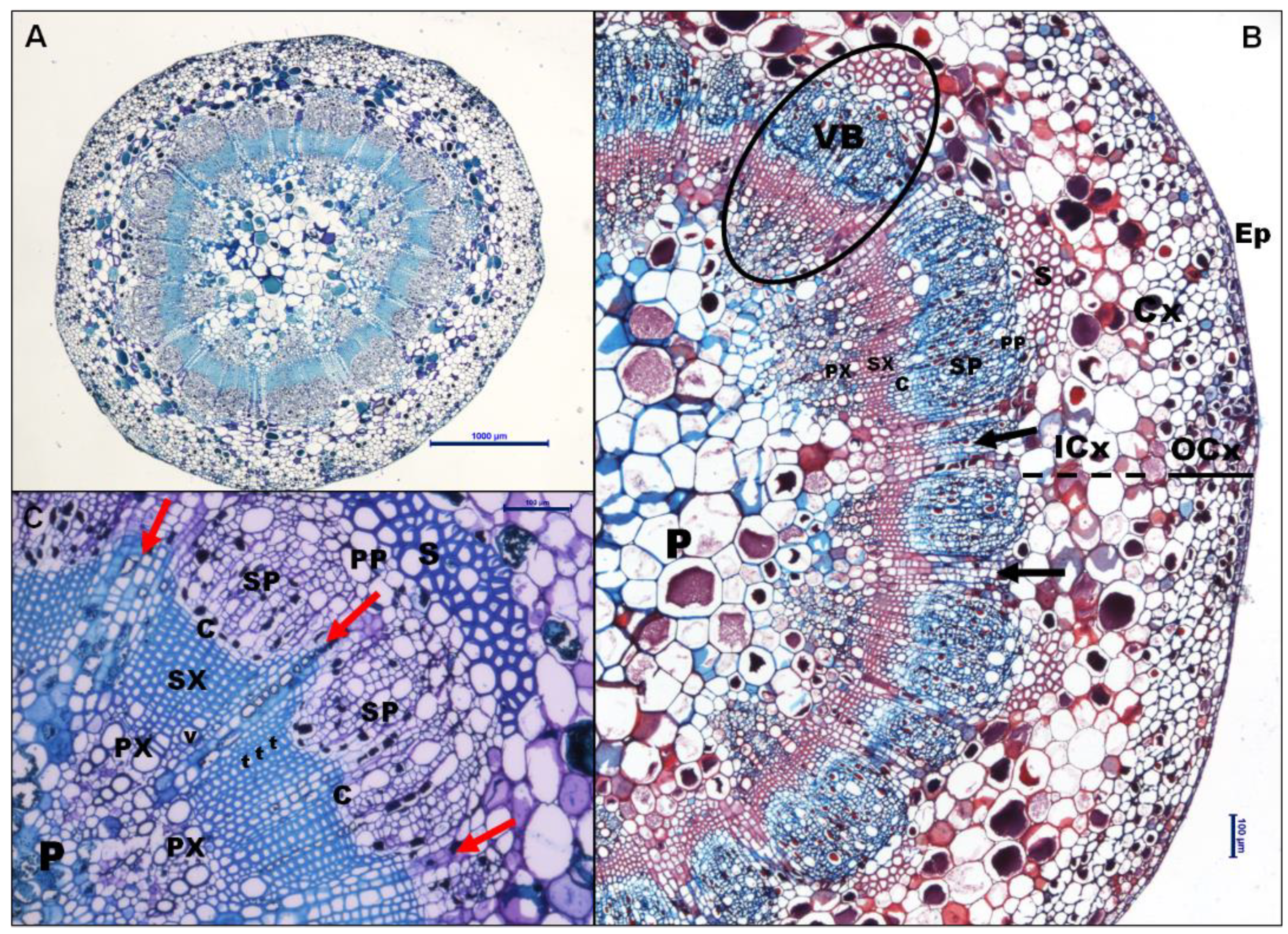
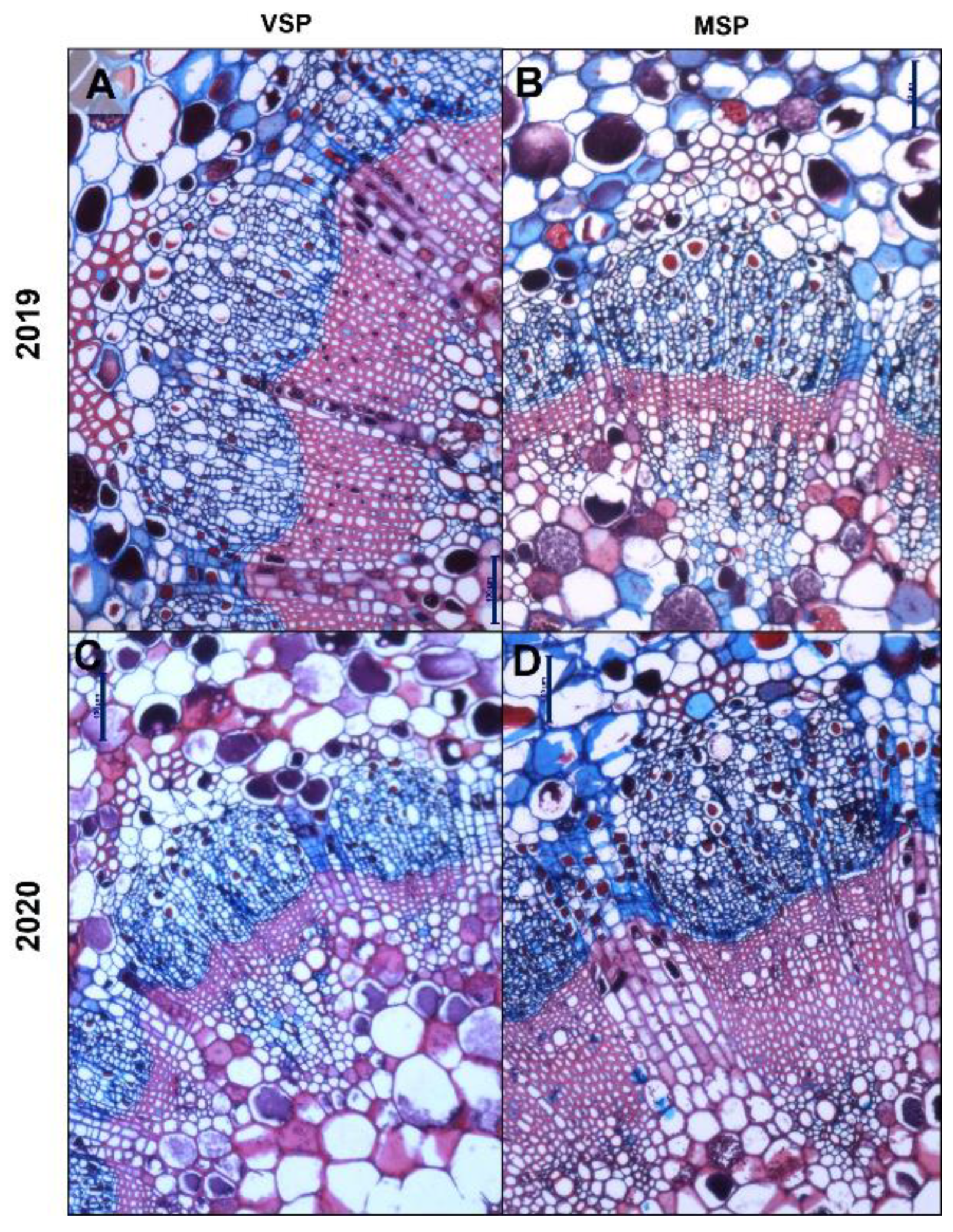
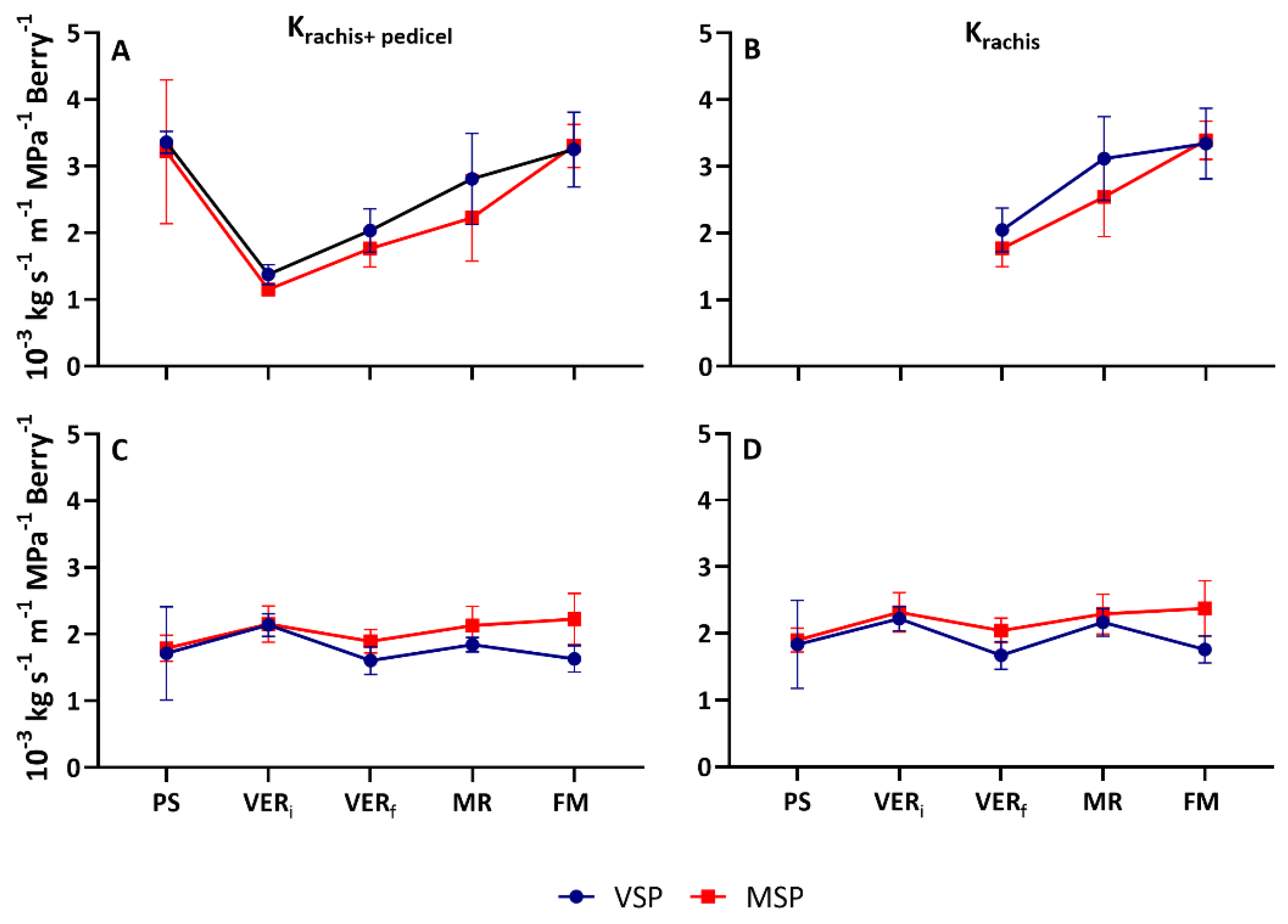
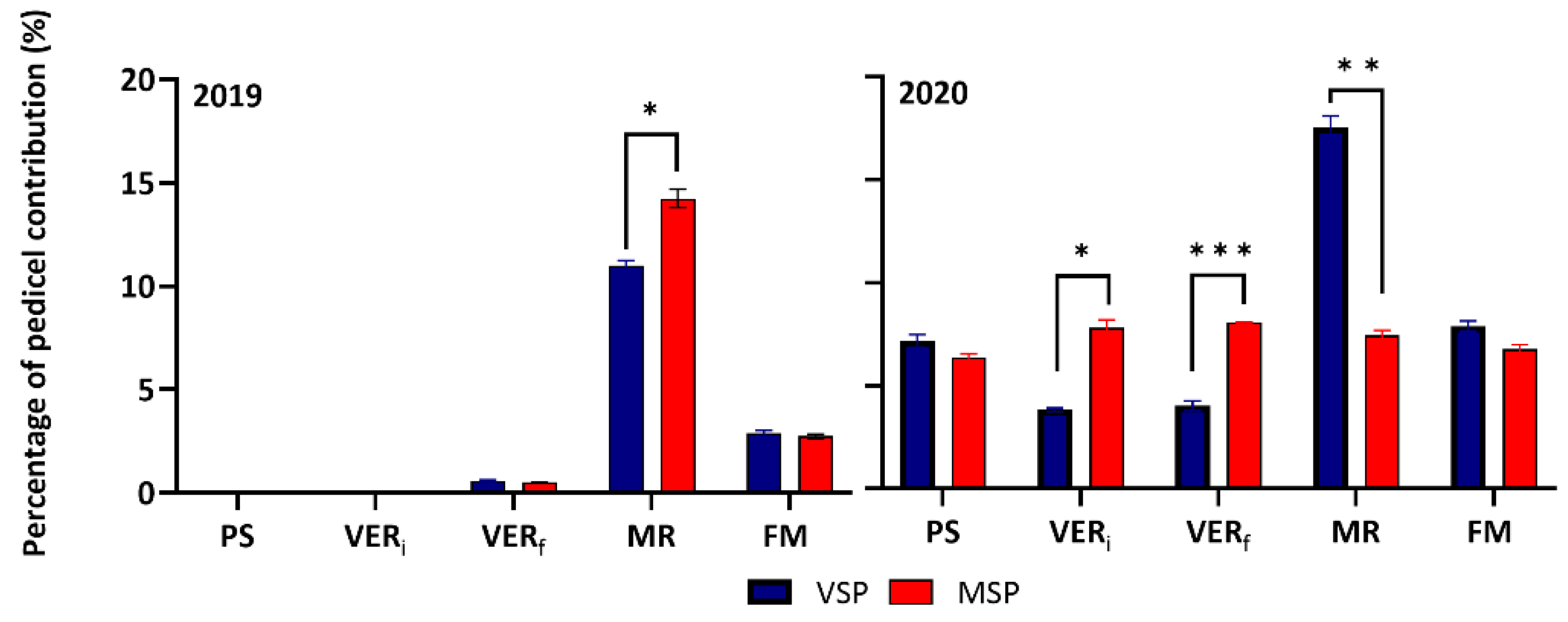
| 2019 Season | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Pea size | Veraison | Full Maturation | ||||||
| VSP | MSP | Sig. | VSP | MSP | Sig. | VSP | MSP | Sig. | |
| Peduncle section area (mm2) | 12.71±0.55 | 12.86±0.41 | ns | 11.49±0.55 | 12.46±0.47 | ns | 12.25±0.50 | 11.57±0.43 | ns |
| Cortex area (mm2) | 7.20±0.26 | 7.18±0.23 | ns | 6.55±0.27 | 7.11±0.25 | ns | 6.94±0.25 | 6.64±0.23 | ns |
| Vascular cylinder area (mm2) | 5.51±0.31 | 5.68±0.21 B | ns | 4.94±0.29 | 5.34±0.22 AB | ns | 5.32±0.26 | 4.93±0.21 A | ns |
| Xylem area (mm2) | 1.54±0.09 ab | 1.43±0.11 | ns | 1.28±0.07 a | 1.44±0.12 | ns | 1.61±0.07 b | 1.11±0.09 | * |
| Phloem area (mm2) | 1.66±0.08 | 1.84±0.12 B | ns | 1.47±0.08 | 1.50±0.07 A | ns | 1.52±0.06 | 1.32±0.07 A | * |
| Pith area (mm2) | 2.32±0.16 | 2.41±0.05 | ns | 2.20±0.17 | 2.40±0.17 | ns | 2.19±0.16 | 2.51±0.06 | ns |
| Ratio Phloem/Xylem | 1.10±0.03 b | 1.31±0.03 B | *** | 1.15±0.02 b | 1.11±0.04 A | ns | 0.97±0.04 a | 1.24±0.03 B | *** |
| Vascular bundles (nº) | 24.35±0.92 | 24.75±0.85 A | ns | 24.05±0.95 | 27.95±0.83 B | * | 23.45±0.71 | 24.80±0.60 A | ns |
| Primary xylem vessels | |||||||||
| Area (µm2) | 247.23±18.96 b | 309.69±25.69 B | * | 158.33±7.90 a | 244.26±15.76 A | *** | 215.87±11.43 b | 198.41±10.11 A | ns |
| Perimeter (µm) | 53.94±2.19 b | 59.79±2.50 B | ns | 43.22±1.13 a | 53.85±1.79 AB | *** | 50.75±1.42 b | 48.36±1.31 A | ns |
| Diameter (µm) | 17.17±0.70 b | 19.03±0.80 B | ns | 13.76±0.36 a | 17.14±0.57 AB | *** | 16.15±0.45 a | 15.39±0.42 A | ns |
| Secondary xylem vessels | |||||||||
| Area (µm2) | 527.44±91.52 b | 432.35±102.06 A | ns | 175.83±14.80 a | 175.30±28.83 A | ns | 216.91±30.84 a | 841.67±217.17 B | *** |
| Perimeter (µm) | 76.96±6.56 b | 67.04±7.59 AB | ns | 46.22±2.18 a | 44.03±3.80 A | ns | 50.65±3.21 a | 94.13±13.63 B | *** |
| Diameter (µm) | 24.50±2.09 b | 21.34±2.41 AB | ns | 14.71±0.69 a | 14.01±1.21 A | ns | 16.12±1.02 a | 29.96±4.34 B | *** |
| % Phenolic compounds in Phloem | 22.40±2.48 | 22.32±1.78 | ns | 19.23±1.11 | 21.01±2.21 | ns | 16.83±1.87 | 15.39±2.31 | ns |
| Different letters in the same row indicate significant differences between sampling times for VSP (lower-case) or MSP (upper-case) (ANOVA and Tukey’s HSD. P<0.05). Comparison between treatments at the same sampling time were performed by Student's t-test (*: P<0.05; **: P<0.01; ***: P<0.001. ns: No significant). | |||||||||
| 2020 Season | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Pea size | Veraison | Full Maturation | ||||||
| VSP | MSP | Sig. | VSP | MSP | Sig. | VSP | MSP | Sig. | |
| Peduncle section area (mm2) | 15.17±0.80 b | 14.51±0.95 B | ns | 11.80±0.67 a | 11.39±0.90 A | ns | 13.41±1.00 ab | 13.00±0.64 AB | ns |
| Cortex area (mm2) | 8.74±0.47 b | 7.95±0.60 | ns | 6.709±0.44 a | 6.76±0.72 | ns | 7.59±0.57 ab | 7.44±0.42 | ns |
| Vascular cylinder area (mm2) | 6.44±0.34 b | 6.56±0.45 B | ns | 5.01±0.24 a | 4.64±0.21 A | ns | 5.81±0.43 ab | 5.57±0.23 AB | ns |
| Xylem area (mm2) | 1.55±0.09 b | 1.78±0.22 | ns | 1.23±0.02 a | 1.36±0.09 | ns | 1.18±0.10 a | 1.81±0.10 | *** |
| Phloem area (mm2) | 1.75±0.12 | 1.98±0.17 B | ns | 1.43±0.08 | 1.35±0.06 A | ns | 1.48±0.13 | 1.69±0.07 AB | ns |
| Pith area (mm2) | 3.14±0.21 b | 2.80±0.16 B | ns | 2.35±0.16 a | 1.93±0.13 A | * | 3.15±0.27 b | 2.07±0.07 A | *** |
| Ratio Phloem/Xylem | 1.13±0.02 a | 1.20±0.06 B | ns | 1.16±0.06 ab | 1.05±0.06 AB | ns | 1.27±0.04 b | 0.95±0.02 A | *** |
| Vascular bundles (nº) | 24.65±0.36 | 25.30±1.04 B | ns | 22.87±0.56 | 22.10±0.54 A | ns | 24.40±0.91 | 22.90±0.68 AB | ns |
| Primary xylem vessels | |||||||||
| Area (µm2) | 286.26±22.67 b | 289.94±17.31 B | ns | 242.76±16.79 ab | 212.40±16.17 A | ns | 204.65±12.50 a | 224.80±12.94 A | ns |
| Perimeter (µm) | 55.86±2.34 b | 58.41±1.88 B | ns | 52.51±1.85 ab | 48.94±2.07 A | ns | 48.71±1.50 a | 51.08±1.48 A | ns |
| Diameter (µm) | 17.78±0.75 b | 18.59±0.60 B | ns | 16.71±0.59 ab | 15.58±0.66 A | ns | 15.50±0.48 a | 16.26±0.47 A | ns |
| Secondary xylem vessels | |||||||||
| Area (µm2) | 380.26±44.62 | 602.15±100.28 B | * | 236.45±24.50 | 223.15±31.26 A | ns | 423.28±50.10 | 198.12±12.72 A | * |
| Perimeter (µm) | 66.16±4.82 ab | 86.87±8.30 B | ns | 53.11±2.78 a | 50.54±3.54 A | ns | 82.31±6.59 b | 48.72±1.66 A | * |
| Diameter (µm) | 22.01±1.54 ab | 27.65±2.64 B | ** | 16.91±0.89 a | 16.09±1.13 A | ns | 26.20±2.10 b | 15.51±0.53 A | ** |
| % Phenolic compounds in Phloem | 20.58±2.42 | 18.84±3.53 | ns | 14.06±1.31 | 13.77±1.17 | ns | 18.23±1.02 | 14.61±1.52 | ns |
| Different letters in the same row indicate significant differences between sampling times for VSP (lower-case) or MSP (upper-case) (ANOVA and Tukey’s HSD. P<0.05). Comparison between treatments at the same sampling time were performed by Student's t-test (*: P<0.05; **: P<0.01; ***: P<0.001. ns: No significant). | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





