Submitted:
08 May 2024
Posted:
10 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
Materials and Methods
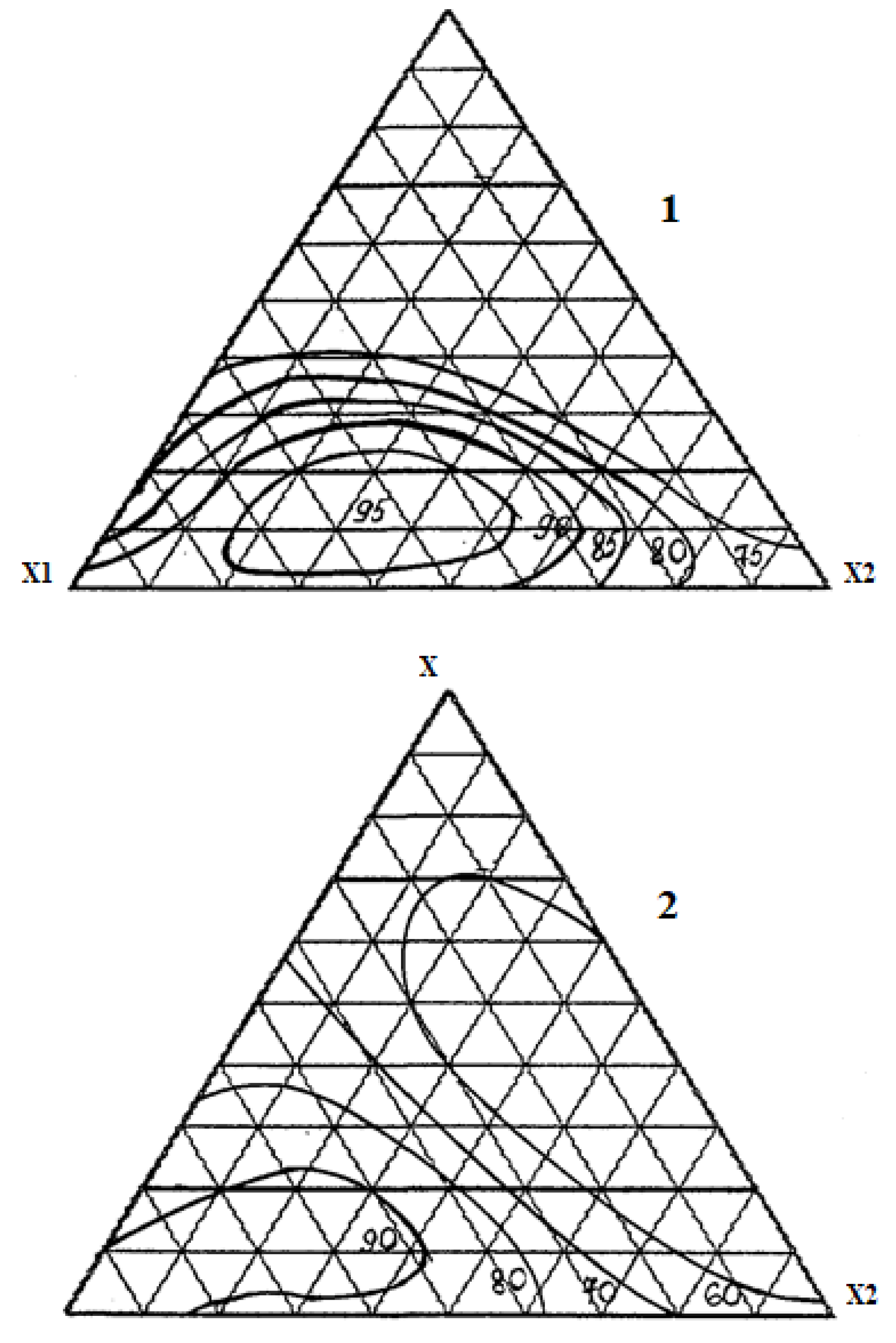
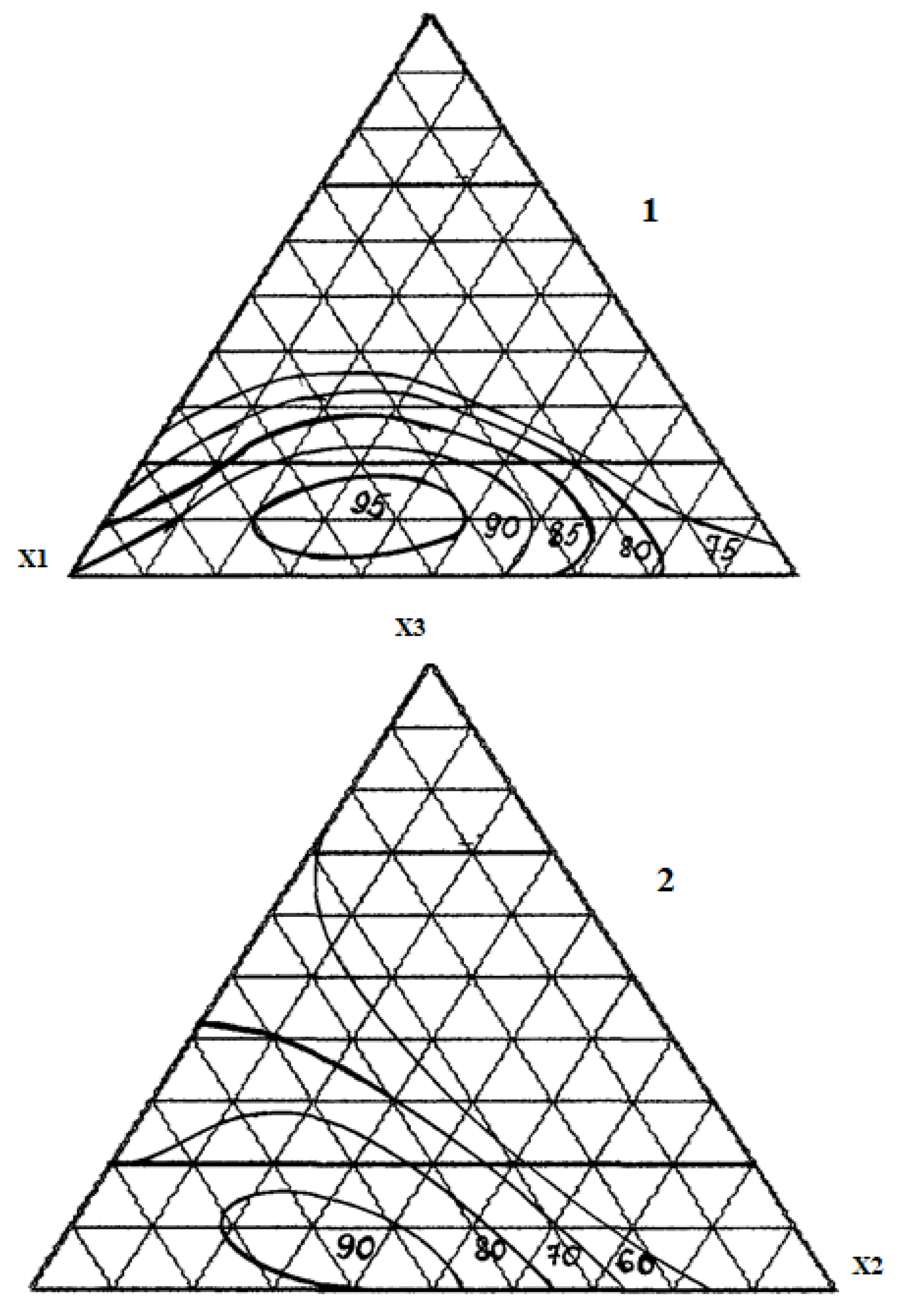
Results and Discussions
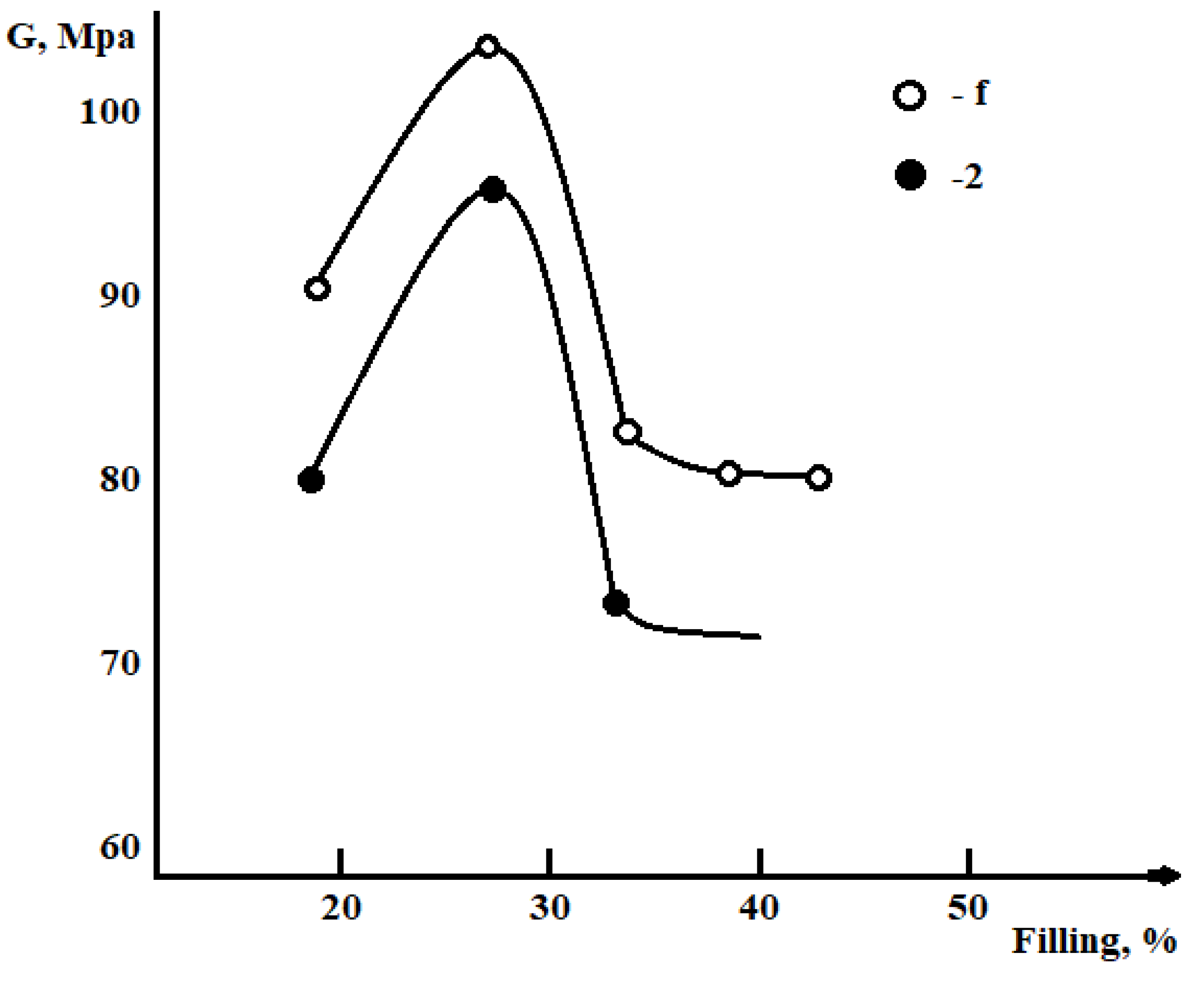
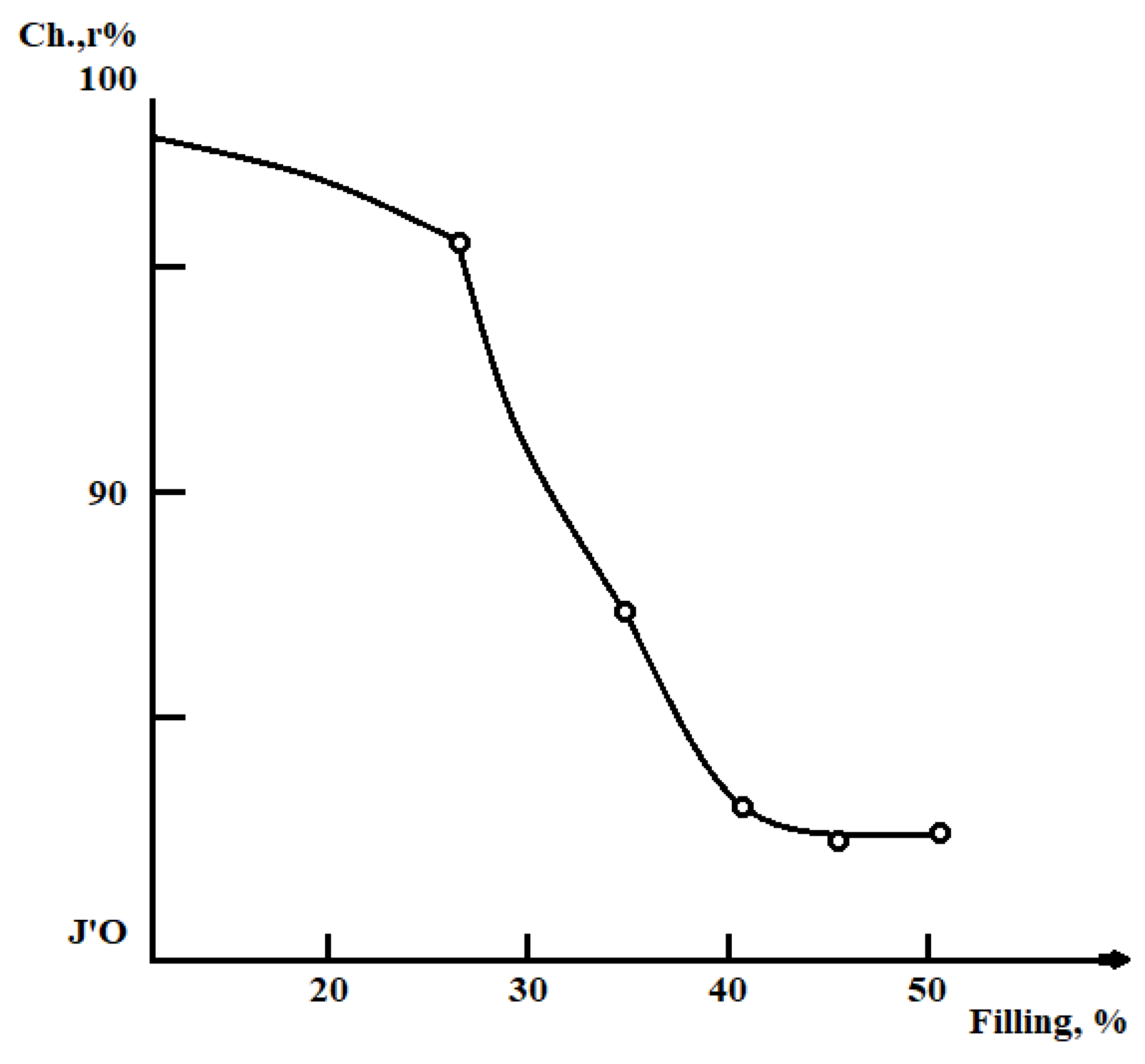
Conclusion
Conflict of Interests
Author Contributions
References
- Mazitova A. K. et al. Fillers for polymer composite materials //Nanotekhnologii v Stroitel'stve. – 2022. – Т. 14. – №. 4. – С. 294-299.
- R. Taurino, F. Bondioli, M. Messori. Use of different kinds of waste in the construction of new polymer composites: review, Materials Today Sustainability, Volume 21, 2023, 100298, ISSN 2589-2347. [CrossRef]
- Wiener Johannes, Kaineder Hannes, KolednikOtmar, Arbeiter Florian, Optimization of Mechanical Properties and Damage Tolerance inPolymer-Mineral Multilayer Composites, Materials 14(4):725 2021. [CrossRef]
- Savotchenko S., Kovaleva, E. Cherniakov, A. The improvement of mechanical properties of repair and construction compositions based on epoxy resin with mineral fillers. J.Polym. Res 29, 280 (2022). [CrossRef]
- Ismail, H., Sapuan, S.M., & Ilyas, R.A. (Eds.). (2022). Mineral-Filled Polymer Composites Handbook, Two-Volume Set (1st ed.). CRC Press. [CrossRef]
- A. Atiqah, M.A. Maleque, M. Jawaid, M. Iqbal. Development of kenaf-glass reinforced unsaturated polyester hybrid composite for structural applications, Composites Part B: Engineering,Volume 56,2014,Pages 68-73, ISSN 1359-8368,. [CrossRef]
- Burmistr, M.V., Boiko, V.S., Lipko, E.O. et al. Antifriction and Construction Materials Based on Modified Phenol-Formaldehyde Resins Reinforced with Mineral and Synthetic Fibrous Fillers. MechComposMater 50, 213–222 (2014). [CrossRef]
- 2014; 8. Dong Yan, Xiaofeng Li, Yue Jiang, Hao-Bin Zhang, Bing-Bing Jia, Hui-Ling Ma, Zhong-Zhen Yu, Thermally conductive phenol formaldehyde composites filled with carbon fillers, Materials Letters, Volume 118, 2014, Pages 212-216, ISSN 0167-577X. [CrossRef]
- Xing Yang, Charles E. Frazier, Influence of organic fillers on rheological behavior in phenol-formaldehyde adhesives, International Journal of Adhesion and Adhesives, Volume 66, 2016, Pages 93-98, ISSN 0143-7496. [CrossRef]
- L. Ravindran, S. M. S., A. Kumar S., S. Thomas,A comprehensive review on phenol-formaldehyde resin-based composites and foams. Polym. Compos. 2022, 43(12), 8602. [CrossRef]
- Pattanashetty, SureshaBheemappa, Hemanth Rajashekaraiah. Effect of Filler-Filler Interactions on Mechanical Properties of Phenol Formaldehyde Based Hybrid Composites. Int. J. Eng. Technol. 13.24-38. (2017). [CrossRef]
- A. Pizzi. C.C. Ibeh. Chapter 2. Phenol-formaldehyde resins. Handbook of Thermoset Plastics (Fourth Edition) William Andrew Publishing. P13-40. (2022). [CrossRef]
- Rothon, R.N. (1999). Mineral Fillers in Thermoplastics: Filler Manufacture and Characterisation. In: Jancar, J., Fekete, E., Hornsby, P.R., Jancar, J., Pukánszky, B., Rothon, R.N. (eds) Mineral Fillers in Thermoplastics I. Advances in Polymer Science, vol 139. Springer, Berlin, Heidelberg. [CrossRef]
- Burmistr, M.V., Boiko, V.S., Lipko, E.O. et al. Antifriction and Construction Materials Based on Modified Phenol-Formaldehyde Resins Reinforced with Mineral and Synthetic Fibrous Fillers. Mech Compos Mater 50, 213–222 (2014). [CrossRef]
- Smejda-Krzewicka, A.; Irzmańska, E.; Mrozowski, K.; Adamus-Włodarczyk, A.; Litwicka, N.; Strzelec, K.; Szynkowska-Jóźwik, M.I. The New Elastomeric Compounds Made of Butyl Rubber Filled with Phyllosilicates, Characterized by Increased Barrier Properties and Hydrophobicity and Reduced Chemical Degradation. Molecules 2024, 29,1306. [CrossRef]
- AzinAdibi, Dylan Jubinville, Guowei Chen, Tizazu H. Mekonnen, In-situ surface grafting of lignin onto an epoxidized natural rubber matrix: A masterbatch filler for reinforcing rubber composites, Reactive and Functional Polymers, Volume 197, 2024, 105856, ISSN 1381-5148. [CrossRef]
- Sergey Dolmatov, Pavel Kolesnikov, Alexey Kamenchukov, Sergey Voinash, RamilZagidullin, IlgamKiyamov, LinarSabitov; Composite building materials from agricultural waste for house-building in seismic hazardous areas. AIP Conf. Proc. 12 January 2024; 2969 (1): 030020. [CrossRef]
- S. S. Negmatov*, N. S. Abed, S. U. Sultanov and U. K. Kochkarov. Machine-building anti-corrosion composite polymeric materials and coatings based on local raw materials and production waste. Volume 401, 2023. International Scientific Conference “Construction Mechanics, Hydraulics and Water Resources Engineering”.
- Kolosova A.S., Sokolskaya M.K., Vitkalova I.A., Torlova A.S., Pikalov E.S. Fillers for modification of modern polymer composite materials, Journal/Fundamental Research/ No. 10, 2017, pp.459-465[In Russian].
- Torlova, A.S.; Vitkalova, I.A.; Pikalov, E.S. Production technologies, properties and applications of composites based on phenol-formaldehyde resins // Scientific Review. Technical Sciences. -2017.-№2.-С.96-114 [In Russian].
- Melnichenko, M.A. Influence of filler composition on the properties of polymer composite materials / M.A. Melnichenko, O.V. Ershova, L.V. Chuprova. - Text : direct // Young Scientist. - 2015.-No16(96).-С.199-202. [In Russian].
- Khudyakova Lyudmila Ivanovna, Zalutsky Alexey Vyacheslavovich, Paleev Pavel Leonidovich Use of ash and slag waste from thermal power plants // XXI century. Technospheric safety. 2019. No 3 (15). URL:https://cyberleninka.ru/article/n/ispolzovanie-zoloshlakovyh-othodov-teplovyh-elektrostantsiy (date of application: 04.04.2024).[In Russian].
- Adeeva Lyudmila Nikiforovna, Borbat Vladimir Fedorovich. TPP ash promising raw material for industry // VestnikOmSU. 2009. №2. URL: https://cyberleninka.ru/article/n/zola-tets-perspektivnoe-syrie-dlya-promyshlennosti (date of application: 04.04.2024). [In Russian].
- 24. Yue Sun, Chijia Wang, Sicheng Yuan, Bin Liang, Ye Yuan, Xiaoning Li, Renjie Lu, Yanji Zhu, Huaiyuan Wang, Designing multifunctional basalt-CeO2@C3N4/epoxy novolac composite coating with outstanding corrosion resistance and CO2 gas barrier properties, Materials Today Nano, Volume 25, 2024, 100451, ISSN 2588-8420. [CrossRef]
- Belyakova V. S., Demyanova V. S. Practical application of TPP ash in the industry of construction materials // Bulletin of Master's Degree. 2014. №9 (36). URL: https://cyberleninka.ru/article/n/prakticheskoe-primenenie-zol-tets-v-promyshlennosti-stroitelnyh-materialov (date of reference: 04.04.2024).[In Russian].
- Iglenkova, M.G. Interrelation of physicochemical and operational parameters of composites based on phosphogypsum / M.G. Iglenkova, A.A. Rodina, V.A. Reshetov, S.B. Romadenkina // Proceedings of Saratov University. New series. Ser.: Chemistry. Biology. Ecology. - 2012. - Vol. 12. - Vol. 3. - С. 45-47.[In Russian].
- Komilov Q.O., Kurbanova A.D., Mukhamedov G.I., Allayev J. Рhosphogyptic compositions to improve meliorative soil properties // Academic research in educational sciences 2021, № 6, 1403-1410.
- Lee C. H., Kim K. M. A Study on Cure Behavior of an Epoxy/Anhydride System and Silica Filler Effects //Journal of Adhesion and Interface. – 2009. – Т. 10. – №. 3. – С. 117-126.
- Parameswaran P. S., Eby Thomas T. Modification of phenol formaldehyde resin for improved mechanical properties :дис. – Cochin University of Science and Technology, 2009.
- Nair CPR Advances in additively curing phenolic resins // Polymer Science Progress. - 2004. - Т. 29. - №. 5. - С. 401-498.[In Russian].
- 2024; 31. Hendrik Kiepfer, Paul Stannek, Marco Grundler, Hans-Jörg Bart, Development and thermal performance of a thermoplastic-graphite-composite based plate heat exchanger for use in corrosive media, Applied Thermal Engineering, Volume 236, Part B, 2024, 121581, ISSN 1359-4311. [CrossRef]
- 32. Gizem Ariturk, Cagla Girisken, Kaan Bilge, Ceren Yargici Kovanci, Yusuf Ziya Menceloglu, Senem Avaz Seven, Hybrid green composites of PLA incorporated with upcycled waste cellulose and vermiculite, European Polymer Journal, Volume 203, 2024, 112667, ISSN 0014-3057. [CrossRef]
- 0400; 33. Gaukhar Zahievna Turebekova, Saule Ospandiyarovna Akhmetova and Zarina Ilesovna Bagova, Ways of the lead-bearing slag waste utilization, E3S Web of Conferences 262, 04003 (2021) . [CrossRef]
- Yang Q. et al. Properties and microstructure of CO2 activated binder produced by recycling phosphorous slag //Construction and Building Materials. – 2021. – Т. 282. – С. 122698.
- Ionescu AC et al. Effect of cure time on the microbiological behavior of bulk-filled nanohybrid resin composites //Polymers. - 2021. - Т. 13. - №. 17. - С. 2948.[InRussian].
- Tonge L. et al. Influence of initial phenol formaldehyde (PF) reaction products on the vulcanization properties of PF resin // Journal of Thermal Analysis and Calorimetry. - 2001. - T. 64. - №. 2. - P. 721-730.[In Russian].
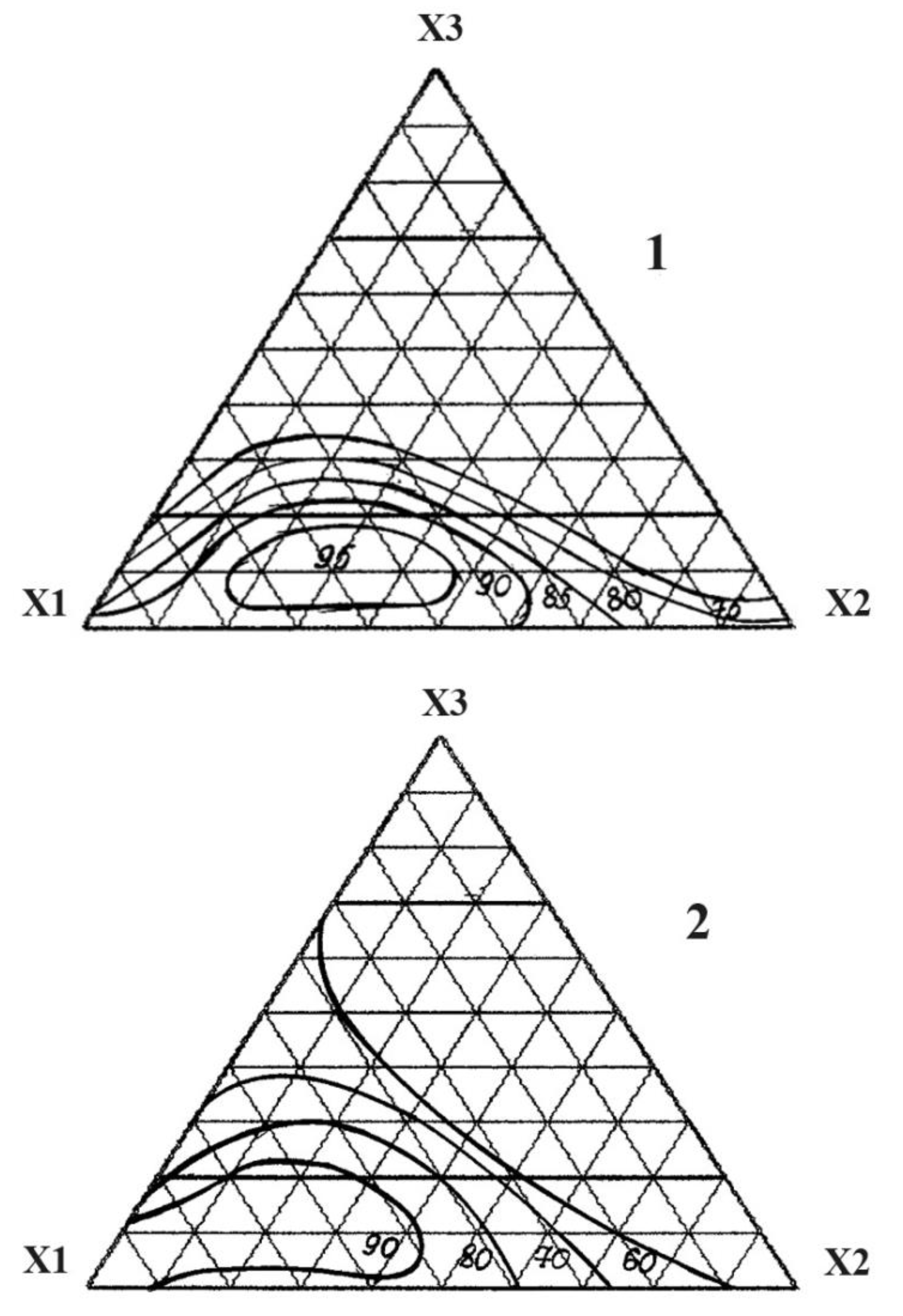
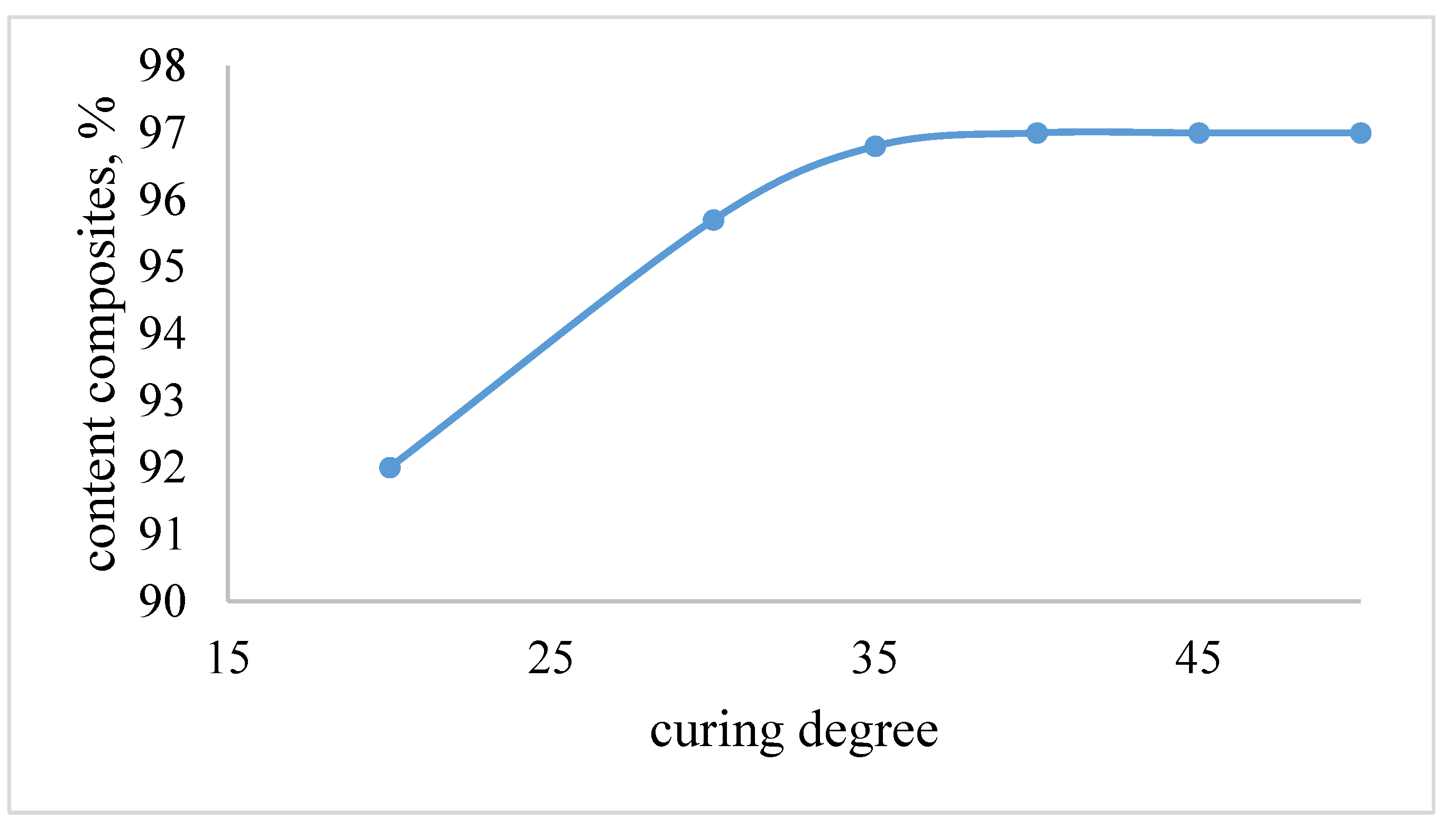
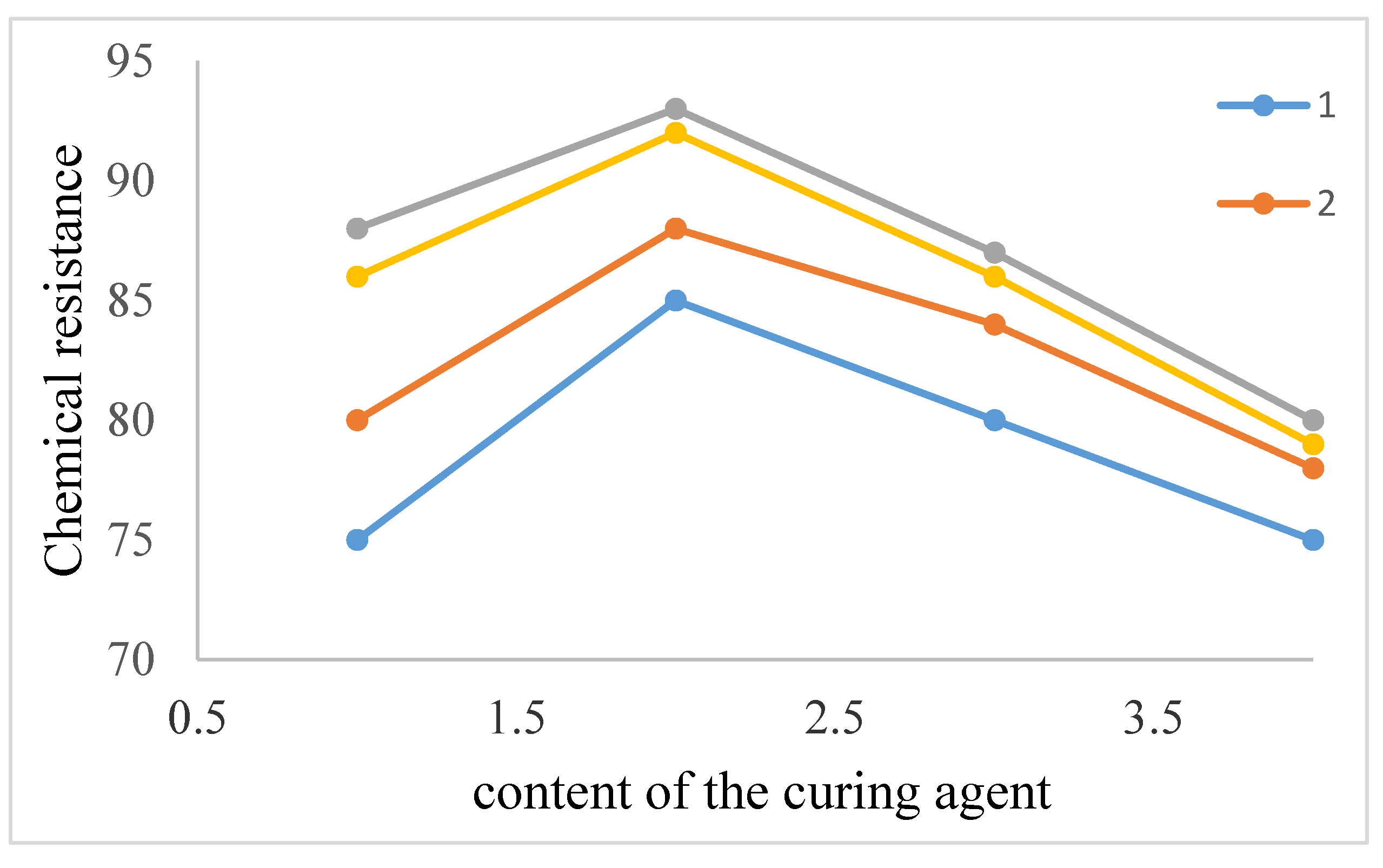
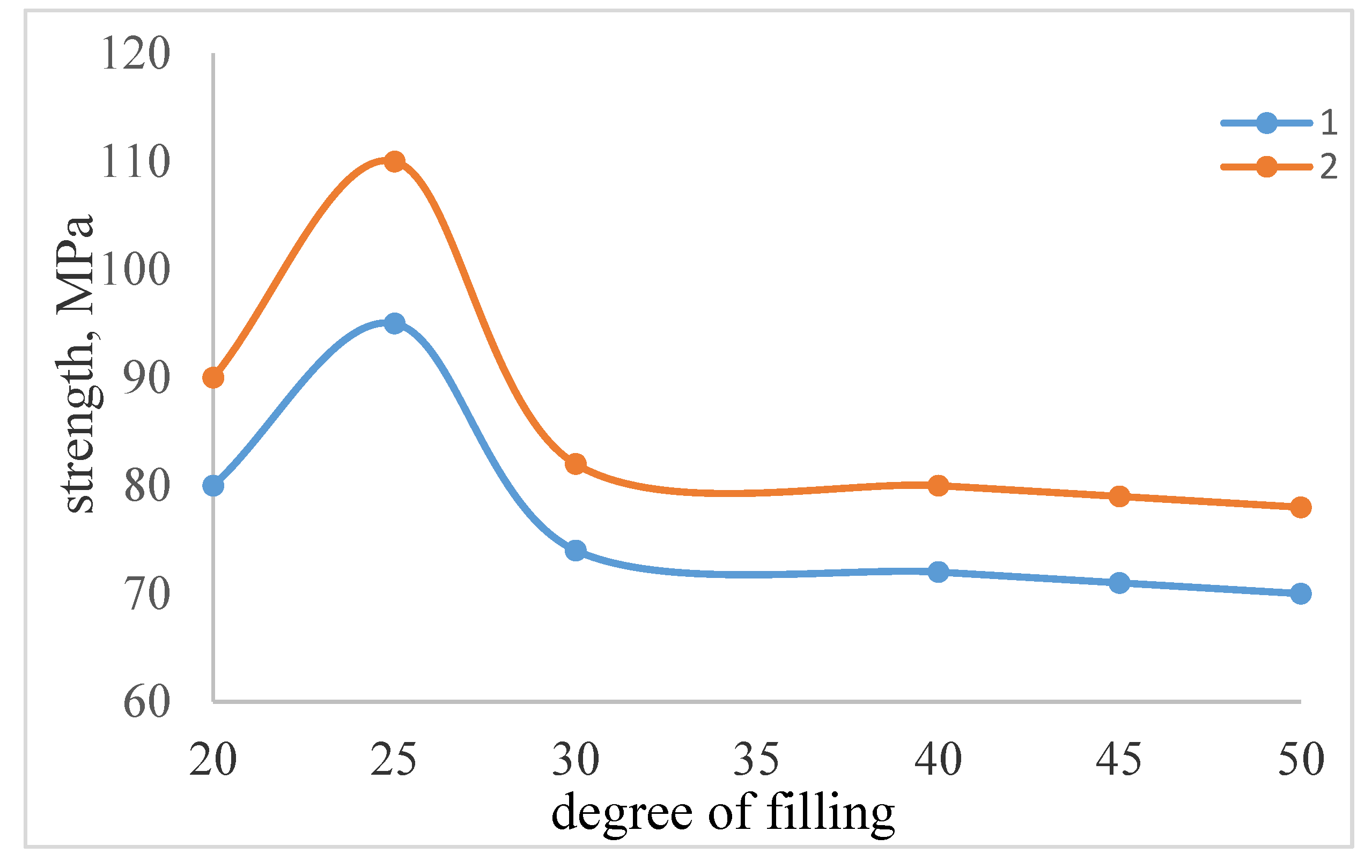
| The name of material | Composition,mass% | |||||||
| SiO2 | Al2 O3 | CaO | MgO | Fetotal | SO3 | P2 O5 | ∑ | |
|
Phosphogypsum
Phosphorus resins Anhydrite Ash of TPP |
11.0 43.4 - 61.3 |
7.5 1.2 - 29.3 |
32.4 47.6 41.2 0.9 |
- 3.7 - 0.7 |
- 0.3 - 3.2 |
42.5 0.6 58.0 C lim 3.6 |
6.0 1.5 - - |
99.4 98.3 99.2 99.0 |
| No. | Component, fractionofunits | Chemical Persistencein 30% HCl,% |
Tensile strength under compression, MPa |
||
| X1 | X2 | X3 | |||
| 1 | 1 | 0 | 0 | 98.0 | 95.0 |
| 2 | 0 | 1 | 0 | 85.4 | 50.2 |
| 3 | 0 | 0 | 1 | 52.2 | 42.4 |
| 4 | ½ | 1/2 | 0 | 90.6 | 75.1 |
| 5 | ½ | 0 | 1/2 | 71.0 | 54.8 |
| 6 | 0 | 1/2 | 1/2 | 48.8 | 40.5 |
| 7 | ¾ | 1/4 | 0 | 93.2 | 68.8 |
| 8 | ¼ | 3/4 | 0 | 80.3 | 60.1 |
| 9 | ¾ | 0 | 1/4 | 75.1 | 75.4 |
| 10 | ¼ | 0 | 3/4 | 52.4 | 54.9 |
| 11 | 0 | 3/4 | 1/4 | 60.7 | 45.1 |
| 12 | 0 | 1/4 | 3/4 | 50.1 | 50.5 |
| 13 | ½ | 1/4 | 1/4 | 95.3 | 70.6 |
| 14 | ¼ | 1/2 | 1/4 | 84.6 | 55.4 |
| 15 | ¼ | 1/4 | 1/2 | 65.0 | 50.2 |
| Components | The contents of the composites, mass% | ||||
| 1 | 2 | 3 | 4 | 5 | |
| Phosphogypsum | 15.0 | ||||
| Phosphorusresins | 15.0 | ||||
| Anhydrite | 15.0 | 15.0 | 15.0 | ||
| Ash of TPP | 20.0 | 20.0 | 20.0 | 25.0 | 30.0 |
| PFR + BSА | 65.0 | 65.0 | 65.0 | 60.0 | 55.0 |
| Cureconditions | |||||
| Temperature, o С | 120 | 120 | 100 | 110 | 120 |
| Time,min | 240 | 200 | 120 | 160 | 180 |
| Chemicalresistance,% | |||||
| 30% H2SO 4 | 96.6 | 98.8 | 97.0 | 98.5 | 99.3 |
| 30% HCl | 96.2 | 97.4 | 96.0 | 97.5 | 98.0 |
| 20% NaOH | 96.0 | 96.7 | 96.0 | 97.1 | 97.5 |
| Tensilestrength,MPa | |||||
| Atcompression | 45.3 | 53.0 | 50.2 | 57.0 | 60.2 |
| Atbending | 18.7 | 23.4 | 21.1 | 25.3 | 28.0 |
| No. | Indicator name | Number of composites | ||||
| 1 | 2 | 3 | 4 | 5 | ||
| 1 | Density, g/cm3 | 1.37 | 1.38 | 1.38 | 1.37 | 1.39 |
| 2 | Heat resistance according to Martens, oС | 159 | 142 | 156 | 151 | 164 |
| 3 | Linea rexpansion coefficient 107 | 40.0 | 44.1 | 48.0 | 50.7 | 48.0 |
| 4 | Temperature of destruction, oC | 250 | 255 | 250 | 260 | 250 |
| 5 | Water absorption, mg | 37 | - | 34 | 39 | 38 |
| 6 | Shrinkage, % | 0.47 | 0.46 | 0.41 | 0.47 | 0.42 |
| 7 | Beats volumetric Electronresistance, Om | 3.6 | 7.4 | 8.8 | 9.1 | 1.3 |
| 8 | Beats on top Electron Resist, Om/cm |
7.4 | 9.5 | 6.2 | 8.7 | 7.6 |
| 9 | Electric strength, MV/m |
20 | 17.7 | 17.8 | 18.3 | 18.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




