1. Introduction
Wine has played a foundational role in contemporary society, transcending its mere function as an alcoholic beverage to become a cultural, social, and economic element of great relevance. In fact, in Spain, a total of 30,745,000 hectoliters were consumed just in 2023 [
1]. This has led to a considerable extension of grape production, with 954,724 hectares registered in Spain in the year 2022 [
1]. However, this situation of splendor for the vineyards has not always been like this. Towards the end of 1870, a plague of vine diseases, such as phylloxera, penetrated the Iberian Peninsula through Oporto [
2]. Phylloxera is a parasitic insect of the
Phylloxeridae family that infects the roots of grapevine plants, where it feeds on the plant’s sap, weakening and eventually killing the roots and hindering vine growth [
3,
4]. This significantly affected France, Italy, Spain, and Germany, leading to the destruction of vast swathes of vineyards and a significant impact on wine production throughout these regions [
5].
During this period, it was discovered that American vines such as
Vitis labrusca and
Vitis riparia were resistant to this plague. This led to these species being used as rootstocks on which many European vines were grafted, thus allowing the recovery of wine at the end of the 19th century [
6,
7]. Once the plague was overcome, most producers eliminated these American varieties, as it was considered that they did not produce wines of sufficient quality. Nevertheless, certain regions, such as Rias Bajas (Pontevedra, Galicia), continued to cultivate this mixture, leading to the emergence of new grape varieties, including “Folla Redonda”. The grapes of this variety are uniform, spherical, dark blue in color, and characterized by an intensely colored pulp and a distinct acid taste [
8].
Barrantes wine (red wine) is produced from this grape variety, and is characterised by a high acidity and an extremely consistent texture, which is thick and dense. Additionally, it has proved to contain a high tannin content. However, the commercialisation of this wine is not permitted due to its hybrid nature. This means that its ancestors are not known, and in Spain, only wines made from grapes of the
Vitis vinifera variety are permitted to be marketed. Recently, a decree that would permit the commercialization of wines produced from a cross between the
Vitis vinifera variety and another of the
Vitis genus has been proposed. The Council of Ribadumia (Galicia) is currently working on this decree to legalize the commercialization of such wines [
9]. Conversely, the low sugar content of this wine, in conjunction with the low alcohol content, precludes malolactic fermentation from occurring in its entirety. Instead, malolactic fermentation often occurs in the bottle itself [
10], contingent upon exposure to varying conditions and time. This phenomenon has the effect of significantly altering the organoleptic properties of the wine. For these two reasons, it is not considered a particularly suitable wine for transportation, which is why it is rarely found outside of Rias Bajas region and, indeed, is virtually unheard of outside of Galicia.
However, the Folla Redonda grape presents curious organoleptic characteristics, including an acid taste and a very characteristic color, which would imply a high content of anthocyanins and other bioactive compounds of interest [
11]. In addition, grapes would not imply any of the problems previously mentioned for their extension and consumption. With this in mind, one would expect a wide knowledge about this characteristic grape, but scientific articles devoted to its chemical composition are almost nonexistent. For this reason, the present study aims to perform a chemical characterization of the grape from the Barrantes region, emphasizing its notable content of compounds of interest and the potential beneficial properties associated with its consumption.
2. Materials and Methods
2.1. Samples
The Folla Redonda grapes were harvested from the Barrantes village (Pontevedra, Spain). The rest of the following Vitis vinifera varieties were collected from Rancho de la Merced (Jerez de la Frontera, Spain): Carbernet Sauvignon, Graciano, Merlot, Tempranillo, Tinta País, Tintilla de Rota, Petit Verdot, Syrah. These grapes were harvested at the time of ripening (between mid-August and the end of September). A total of 5 kilograms of grapes were harvested in a totally heterogeneous manner. The grapes were destemmed and washed with abundant water. Subsequently, half of these grapes were totally crushed in a grinder, then frozen at –80 °C and freeze-dried (Azbil Telstar Technologies, LYOALFA, Terrassa, Spain). The freeze-dried samples were kept at –20 °C until further analysis. The other half were pressed in a manual press to obtain the must, which was then frozen at –20 °C until analysis.
2.2. Chemicals
The ethanol used was purchased from Thermo Fischer Scientific (Loughborough, United Kingdom) while the water used was of Milli-Q purity, obtained from a Millipore water purification system (Bedford, MA, USA). Hydrochloric acid for pH adjustment of the extraction solvent and other reagents related to the analytical methods such as formic acid, methanol, sodium hydroxide, acetic anhydride and sulfuric acid were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA), all in HPLC purity for liquids and analytical grade for solids. The acetone used for the determination of organic acids (HPLC grade) was obtained from VWR International (Radnor, PA, USA). The antioxidant activity was measured using the following standards: 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis-(3- ethylbenzothiazolin-6-sulfonic acid) (ABTS) from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA), and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) from Thermo Fisher Scientific (Loughborough, United Kingdom).
2.3. Extraction
For the determination of some bioactive compounds present in the selected varieties, such as the concentration of anthocyanins or phenolic compounds, it was decided to use ultrasound-assisted extraction (UAE). This extraction system has important characteristics of instrumental green chemistry techniques since the use of ultrasound pulses generates cavitation phenomena that facilitate the diffusion of bioactive compounds from the plant cells to the solvent [
12,
13]. Therefore, the amount of solvent used is much lower, as well as the temperatures required to achieve the same or even better yields than other extraction techniques [
14].
In this case, a UAE extraction system with a Bandelin UW 200 probe and associated thermostatic bath (Berlin, Germany) was used. For the extraction, the method optimized by Carrera et al. [
15] was used, for which 1 g of sample was taken, to which 8 mL of a 50% ethanol solution at pH 2 was added at a temperature of 10 °C and subjected to an amplitude of 66% with a cycle of 0.1 s
–1 for 6 min. Afterwards, the obtained extract was centrifuged for 5 min at 1702g and the supernatant was transferred to a volumetric flask where it was made up to 10 mL. These extracts were frozen at –20 °C until analysis.
2.3. Physicochemical Properties
In order to carry out a complete characterization of the Folla Redonda grapes collected from the Barrantes region, an analysis of the physicochemical properties and their consequent comparison with the rest of the varieties of the Vitis vinifera genus was carried out. The methods used for these properties are described below.
2.3.1. pH and Acidity
The determination of pH and acidity was carried out on the musts obtained during the pressing of the selected grapes. A pH-Matic 23 system (Crison, Barcelona, Spain) was used to perform an automatic acid-base titration to obtain the pH and acidity values (g/L of tartaric acid (TH2)). For the titration, 10 mL of must sample was used as well as a 0.2 N NaOH solution. All analyses were carried out in triplicate.
2.3.1. Density, °Be, °Brix
Density, Baumé and Brix degrees were also determined in the must samples. A DMA HP system (Anton Paar Spain S.L.U., Madrid, Spain) was used for this purpose, using 10 mL of sample. All analyses were carried out in triplicate and the density was obtained in g/cm3.
2.4. Acids and Sugars
The acids were determined using a Metrohm ion chromatography system (930 Compact IC Flex, Herisau, Switzerland) with a conductimetric detector and a Metrosep Organic Acids column (250/7.8 column). For the analysis, 10 mL of the must sample diluted 1:50 in the eluent used, a mixture of 0.4 mmol/L sulfuric acid with 12% acetone, was used. The flow rate was 0.4 mL/min for 20 min. A total of five standards were used for acid quantification: tartaric acid (Y = 2.496·10–3X + 0.123; R2 = 0.9997); malic acid (Y = 1.647·10–3X + 6.177·10–3; R2 = 0.9999); succinic acid (Y = 4.060·10–3X – 4.733·10–3; R2 = 0.9998); lactic acid (Y = 1.132·10–3X + 1.433·10–3; R2 = 0.9999); acetic acid (Y = 2.281·10–3X – 1.839·10–3; R2 = 0.9999).
In the case of sugars, the ion chromatography equipment used was the same but with an amperometric detector and a Metrosep Carbs 2 column (150/4 column). The must sample was diluted 1:50 in Milli-Q water. The eluent used was a mixture of 300 mmol/L sodium hydroxide and 1 mmol/L sodium acetate at a constant flow rate of 0.5 mL/min for 20 min. The standard used for the quantification of sugars were glycerol (Y = 6.793X + 244.491; R2 = 0.9992), mannitol (Y = 6.183X + 22.018; R2 = 0.9997), glucose (Y = 4.456X + 153.450; R2 = 0.9993), fructose (Y = 3.652X + 6.944; R2 = 0.9992), and sucrose (Y = 2.287X + 6.628; R2 = 0.9997).
2.5. Phenolic Compounds
2.5.1. Total Phenolic Compounds
First, the determination of total phenolic compounds and anthocyanins present in the extracts obtained by UAE was carried out. For this purpose, a Cary 60 UV-Vis spectrophotometer (Agilent Technologies, Inc., Santa Clara, CA, USA) was used and the total phenolic compounds were determined by their absorbance at 280 nm and the total anthocyanins by their absorbance at 520 nm. In order to determine the concentration, two calibration curves were elaborated. The first one was for gallic acid as a standard for the determination of total polyphenols. The curve obtained was Y = 0.004X – 0.128 with an R2 of 0.9967. On the other hand, cyanidin 3-glucoside was used as a standard for the determination of total anthocyanins, obtaining a calibration curve Y = 0.0018X + 0.0212 with an R2 of 0.9984. Therefore, the total phenolic compounds obtained in the extracts were expressed as mg gallic/L and the total anthocyanins as mg cyanidin 3-glucoside/L.
2.5.2. Anthocyanins
The determination of individual anthocyanins present in the selected grapes was carried out using an Ultra-High Performance Liquid Chromatograph system coupled to a mass spectrometer with a single quadrupole analyzer (UHPLC-QDa) (Waters Acquity UPLC HClass Plus, Waters Corp., Milford, MA, USA). The column used was a reversed-phase C18 with 1.7 µm particle size, and dimensions 2.1 x 50 mm (Acquity UHPLC BEH C18, Waters). Phase A was an acidic solution of Milli-Q water with 0.1% formic acid while phase B was acetonitrile with 0.1% formic acid. The flow rate used was 0.55 mL/min with the following gradient (%B, time): 0%, 0.00 min; 5%, 3.00 min; 10%, 4.00 min; 20%, 7.00 min; 30%, 9.00 min; 100%, 12.00 min. The mass spectrum was obtained with an electrospray source in a negative ionization mode with a desolvation temperature of 600 °C, and a capillary and cone voltages of 0.8 KV and 15 V respectively. The data were collected in full scan mode at (m/z = 50 – 800).
Subsequently, quantification of these anthocyanins was carried out using an UHPLC technique coupled to a photodiode array detector (PDA) (ChromasterUltra Rs, VWR International, Radnor, PA, USA). A reverse–phase C18 analytical column, Kinetex (2.6 µm, EVO 100Å 100 x 2.1 mm) (Phenomenex, Torrance, CA, USA) was selected. The phase A used was Milli-Q water with 5% formic acid while the phase B was MeOH. The flow rate used was 0.7 mL/min with the following gradient (%B, time): 2%, 0.0 min; 15%, 3.30 min; 35%, 4.80 min; 100%, 6 min, with a total analysis time of 9 min. For quantification, cyaniding 3-glucoside was chosen as the reference standard, and a calibration curve (y = 93773.0x – 2925.4) with a coefficient of regression (R
2) of 0.9999 was constructed. Anthocyanins have been measured at a wavelength of 520 nm. The assumption of similar absorbance levels for different anthocyanins, allowed the use of this calibration curve to prepare a calibration curve for each identified anthocyanin, considering their individual molecular weights. To ensure reproducibility and accuracy, all analyses were conducted in duplicate. The calibration curve, R
2 and range of linearity for each anthocyanin can be found in
Table 1.
2.6. Antioxidant Activity
Phenolic compounds are widely known to have high beneficial properties for health among which stand out the antioxidant capacity with important repercussions on ROS species and consequently in the prevention of diseases such as cancer or diabetes [
16,
17]. For this reason, it was decided to evaluate the antioxidant capacity of the extracts obtained from UAE; Specifically, it was decided to use the DPPH and ABTS methods.
In the case of the DPPH method, a 0.06 mM solution of DPPH in MeOH was prepared, and 15 µL of the UAE extract was mixed with 165 µL of this solution. The absorbance of this mixture at 515 nm was measured using a Spectra-Max 190 spectrophotometer (Molecular Devices, San Jose, CA, USA). Trolox in a concentration range of 0 – 100 mg/L was used as a standard for the calibration, obtaining a calibration curve Y = 0.738X + 15.271 with an R2 coefficient of 0.9999, using a 50% EtOH solution as blank. The antioxidant activity of the extracts was expressed as milligrams of Trolox equivalents (TE) per gram of dry weight (mg TE/g dw).
In the case of the ABTS method, a 7 mM aqueous solution of ABTS and a 2.45 mM solution of K2S2O8 were prepared. Both solutions were mixed in the same proportion and left to react in the dark for 16 hours. Subsequently, 8 mL of the above mixture was diluted to 100 mL with 25% EtOH. Finally, 190 µL of this mixture was incubated for 10 min and its absorbance was measured. Then they were mixed with 10 µL of the extracts obtained and the absorbance was measured after 30 min and the percentage of inhibition was calculated. Trolox was also used as a standard in the range 0 – 100 mg/L, obtaining a calibration curve Y = 0.397X – 0.962 and an R2 coefficient of 0.9991.
3. Results
3.1. Physicochemical Properties
In relation to the physicochemical properties of the chosen grapes, the pH, acidity, density, Baumé degree and Brix degrees were selected in order to know in greater depth the nature of these grapes. The results obtained from the methods described above are shown in
Table 2.
In the first place, it was observed that the Folla Redonda grape was the one that presented a significantly higher acidity and a lower pH, being in total agreement with the organoleptic properties described by the consumers of these grapes. In the case of acidity, the parameters observed were between 1.94 ± 0.01 and 3.2 ± 0.02, with the Tempranillo variety having the lowest acidity and the Syrah variety the highest. In general,
Vitis vinifera varieties tend to have acidity levels between 2 and 5 mg/L [
18,
19], which is in line with what was observed. In the case of pH, the
Vitis vinifera varieties are usually higher than 3.4 [
20], which is also in accordance with the results obtained and is significantly lower in the Folla Redonda variety. However, these acidity levels were in line with those observed by other authors in some varieties from the region of Galicia such as Ferrol, Loureira Tinta or Sousón [
21], which could be largely related to climate, terroir or soil characteristics.
In the case of the Baumé grade, Folla Redonda was the one that presented the lowest grade, specifically 10.15 ± 0.05, presenting a lower sugar concentration. It should be noted that due to the cold climate of Galicia, some varieties have low sugar concentrations. Furthermore, if a comparison of the results obtained with another variety typical of the region of Galicia, Caíño Bravo, is performed, the results obtained are very similar [
21]. This fact was also reflected in the Brix degrees, which showed that the Folla Redonda grapes had around 18°. In general, white
Vitis vinifera grapes usually show Brix degrees between 18° and 24° during the harvest, while red grapes show Brix degrees between 20° and 26°, which fits with the results obtained [
22].
In relation to density, the
Vitis vinifera varieties are usually found in a density range of 1.07 – 1.10 g/cm
3 at the time of harvest, a range in which the
Vitis vinifera varieties are found in this study fit [
23]. In relation to the Folla Redonda grape, the density is at the lower end of the range typical of these varieties, showing certain physical properties that would facilitate the harvesting of these grapes.
Subsequently, the sugars present in these grape varieties were evaluated, specifically five sugars (glucose, fructose, glycerol, mannitol and sucrose) whose concentration was evaluated. The results are shown in
Table 2.
It was detected that all grape varieties have a similar concentration of glucose and fructose. This is due to the 1:1 ratio of glucose and fructose that exists in the grapes when they ripen. In this case, the highest concentration of glucose and fructose was achieved by Petit Verdot variety followed by Syrah and Graciano. The ranges of sugars evaluated was on consonance with the detected in the literature for
Vitis vinifera varieties [
24]. In addition, the Folla Redonda variety exhibited the lowest concentration of sugars, expected due to the acidic character associated with this grape.
The concentration of glycerol present in musts was minimal, since it is a sugar that increases during alcoholic fermentation due to the transformation of glucose and it is not naturally present in grapes. Finally, sucrose content was also minimal, since this sugar is the form in which sugars migrate through the plant, but are hydrolyzed in the grape due to the action of enzyme invertase [
25].
In the case of acids, three main acids present in the varieties studied were determined: tartaric acid, malic acid and acetic acid. The average of these concentrations is shown in
Table 2. As expected, the Folla Redonda grape had the highest concentration of both tartaric acid and malic acid (118.60 ± 0.33 and 16.96 ± 0.10 g/L), which is consistent with the properties previously observed. However, it did not show any concentration of acetic acid. In this case, these results cannot be compared with the bibliography since, to the best of the authors’ knowledge, the acidity of this grape has not been measured previously. However, in comparison with other Galician varieties the authors return to the conclusion that the most similar variety to Folla Redonda is Loureira Tinta, with an acidity range of 10 – 11 g/L of tartaric acid [
21].
On the other hand, Merlot and Tinta País were the varieties with the highest acidity among the
Vitis vinifera varieties, while Petit Verdot had the lowest acidity. This data is in agreement with the observed in
Vitis vinifera varieties in the literature [
26,
27].
3.2. Total Anthocyanins and Phenolic Compounds
Subsequently, the extracts obtained through extraction with UAE were analyzed to determine the total phenolic compounds and total anthocyanin content present in these grape varieties. The average of the concentrations obtained is shown in
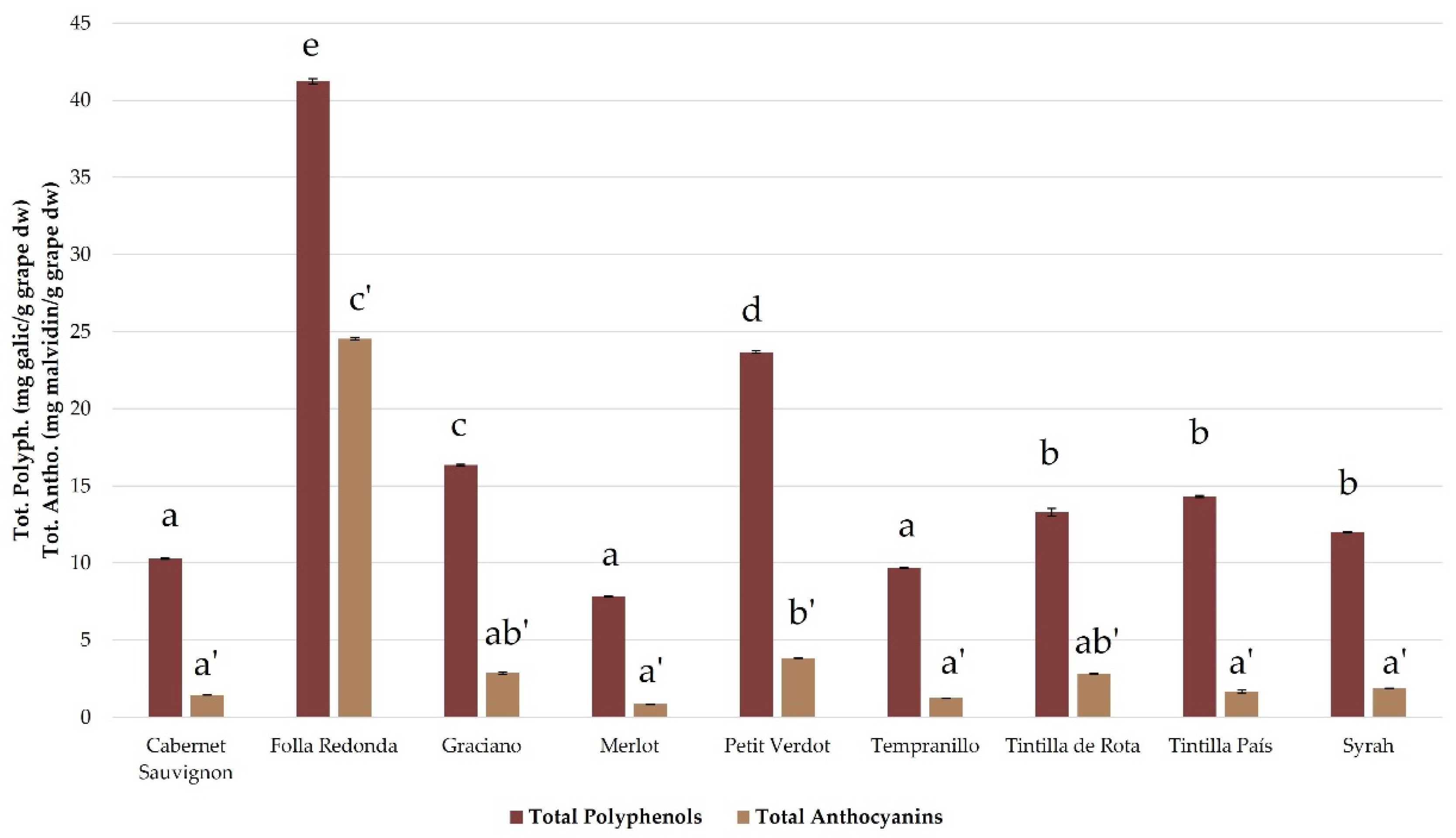
Figure 1, where total phenolic compounds have been expressed as mg of gallic acid per gram of grapes in dry weight, while total anthocyanins have been expressed as mg of malvidin per gram of grapes in dry weight. These two compounds have been used as standards for the calibration curve of these analyses.
As can be observed, the Folla Redonda grape has a significantly higher value of total phenolic compounds and total anthocyanins than the rest of the grapes, which is in agreement with the observations of Pomar et al. [
11]. In fact, the anthocyanin/phenolic compounds ratio is much higher in this variety than in the rest, which is consistent with the intense reddish color described by many of its consumers, a color that is usually caused by the anthocyanins accumulation. Specifically, its total anthocyanin content, 24.54 ± 0.09 mg of malvidin per gram of grapes in dry weight, is similar to that observed in the Galician variety Loureira Tinta [
21].
On the other hand, the Petit Verdot, Graciano and Tintilla de Rota varieties were the
Vitis vinifera varieties with the highest content of both total phenolic compounds and total anthocyanins, in the range of 13.41 – 23.59 mg total phenolic compounds per gram of grapes in dry weight. These data agree absolutely with what was observed in the literature for these varieties [
28,
29]. While the Merlot variety presented the lowest content in both total phenolic compounds and total anthocyanins, with a content of about 7.84 ± 0.03 mg/g of total phenolic compounds, which was also in agreement with what was observed in the literature [
30].
3.3. Individual Anthocyanins
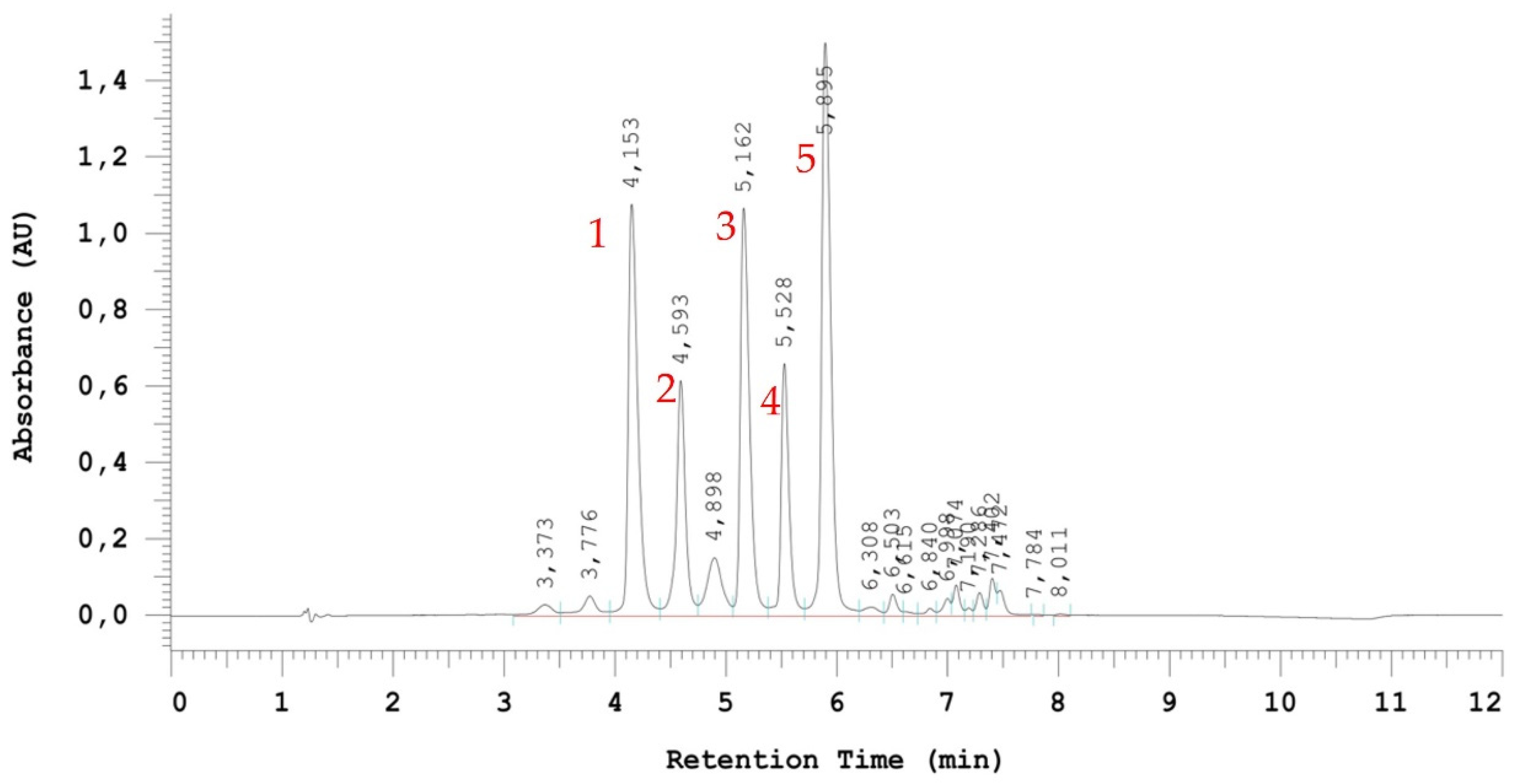
The extracts obtained after UAE extraction of Folla Redonda grapes were analyzed by UHPLC-MS and five main anthocyanins were observed: delphinidin 3-glucoside (
m/z 465.12), cyanidin 3-glucoside (
m/z 449.27), petunidin 3-glucoside (
m/z 479.20), peonidin 3-glucoside (
m/z 463.22), and malvidin 3-glucoside (
m/z 493.21). These extracts were subsequently analyzed by UHPLC-PDA obtaining a chromatogram as shown in
Figure 2.
In addition, different esterified glycosylated forms were analyzed in the current study due to their presence in
Vitis vinifera varieties. Specifically, a total of eleven anthocyanins were quantified (
Table 3).
The extracts obtained from both Folla Redonda and the rest of the
Vitis vinifera varieties were analyzed by UHPLC-PDA and the concentrations of the anthocyanins identified (
Table 3) were calculated based on the calibration curves obtained as explained in the Materials and Methods section. The average of these concentrations were plotted in
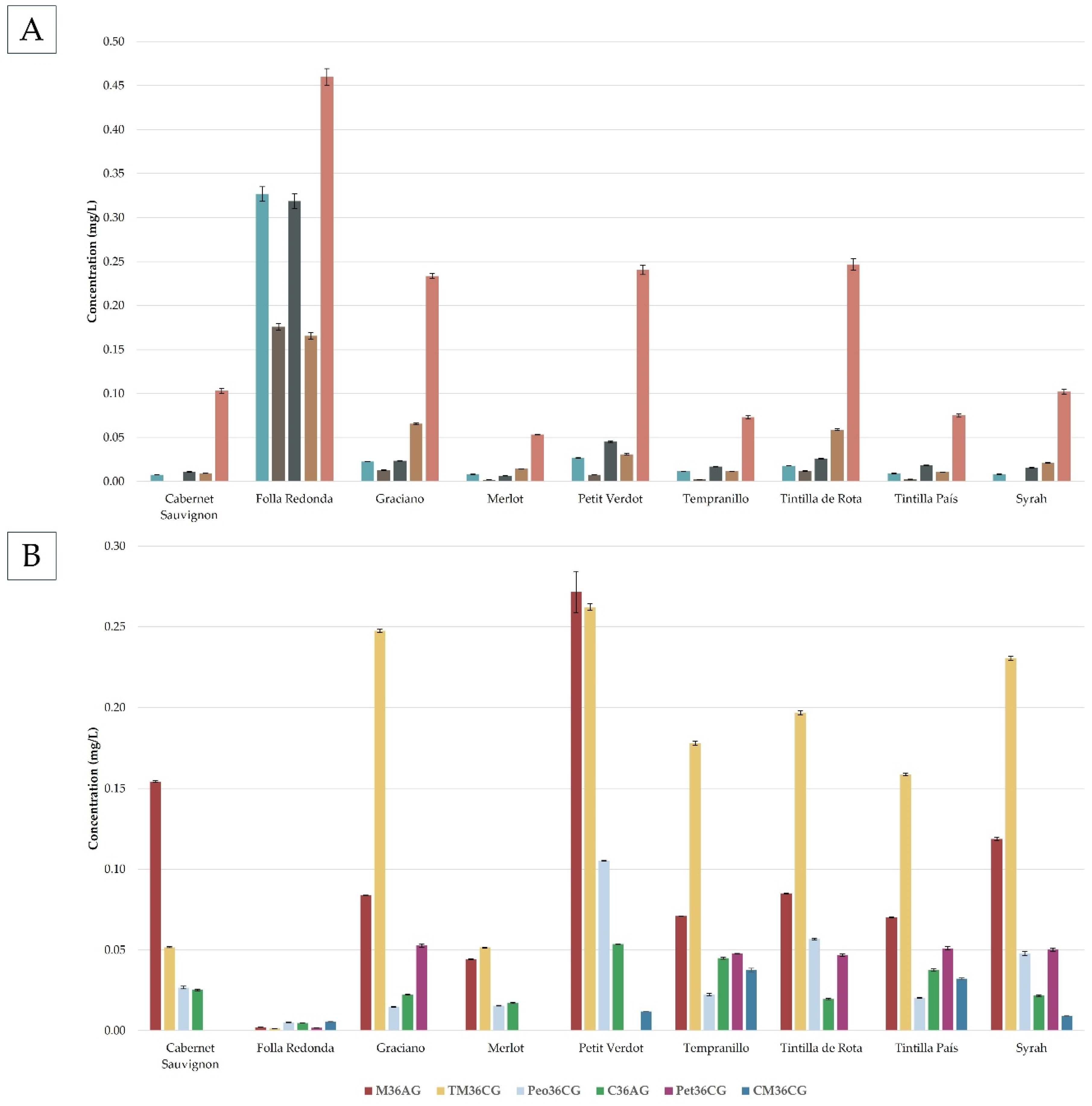
Figure 3A,B.
As can be seen in
Figure 3A, the Folla Redonda variety presented the highest concentration of glycosylated anthocyanins, although it practically did not present esterified glycosylated anthocyanins, showing a higher concentration of anthocyanins with a simpler structure. This is a fact that differentiates the Folla Redonda variety from the
Vitis vinifera varieties, which in addition to having glycosylated anthocyanins, have a fairly high concentration of esterified glycosylated anthocyanins. As it was previously mentioned, anthocyanins are bioactive compounds that have demonstrated important beneficial properties for health as antioxidants, antimicrobial or anti-inflammatory properties, this fact together with the fact that it has been shown that simpler structures of anthocyanins are more easily adsorbed by the human stomach [
31] would be an important advantage in the case of Folla Redonda grapes. In relation to the glycosylated anthocyanins, the majority was malvidin 3-glucoside with a concentration (0.46 ± 0.01 mg/L) more than 20% higher than those varieties of
Vitis vinifera with the highest concentration of this anthocyanin (Graciano, Petit Verdot and Tintilla de Rota). This fact was in agreement with what was observed by Pomar et al. [
11], a study where malvidin 3-glucoside was also the major anthocyanin present in Folla Redonda grapes.
In relation to the varieties of Vitis vinifera evaluated, it was observed that the results obtained were in agreement with those observed in the literature, being the anthocyanin malvidin 3-glucoside the majority in all varieties with ranges of 0.05 – 0.25 mg/L.
3.4. Antioxidant Activity
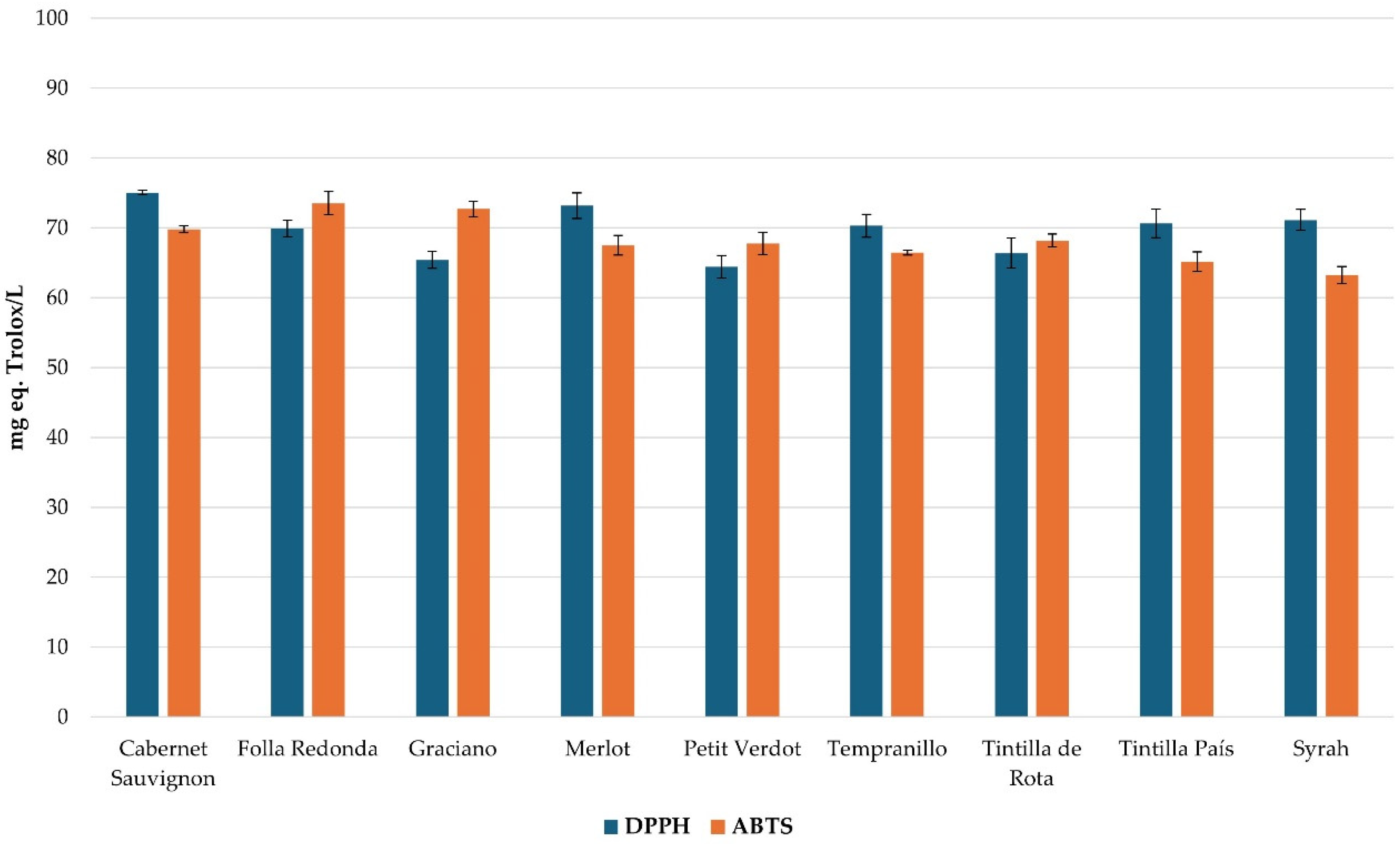
The antioxidant activity of the extracts obtained was evaluated using the DPPH and ABTS methodologies. The results obtained were represented in
Figure 4. It was detected that the Cabernet Sauvignon variety presented the highest antioxidant capacity according to the two methodologies studied with 75.03 ± 0.34 and 69.78 ± 0.47 mg Trolox/L equivalent, while the Petit Verdot variety presented the lowest percentage of inhibition with 64.41 ± 1.57 and 67.49 ± 1.39 mg Trolox/L equivalent. These values of antioxidant capacity are in line with those observed in the literature for
Vitis vinifera varieties (26.4 – 80.0 mg/L) [
32,
33].
On the other hand, the Folla Redonda variety presented 69.90 ± 1.17 and 73.51 ± 1.68 mg Trolox/L equivalent of antioxidant capacity, showing the great potential of this variety in its properties for human health with important applications in the pharmacological and nutritional industries.
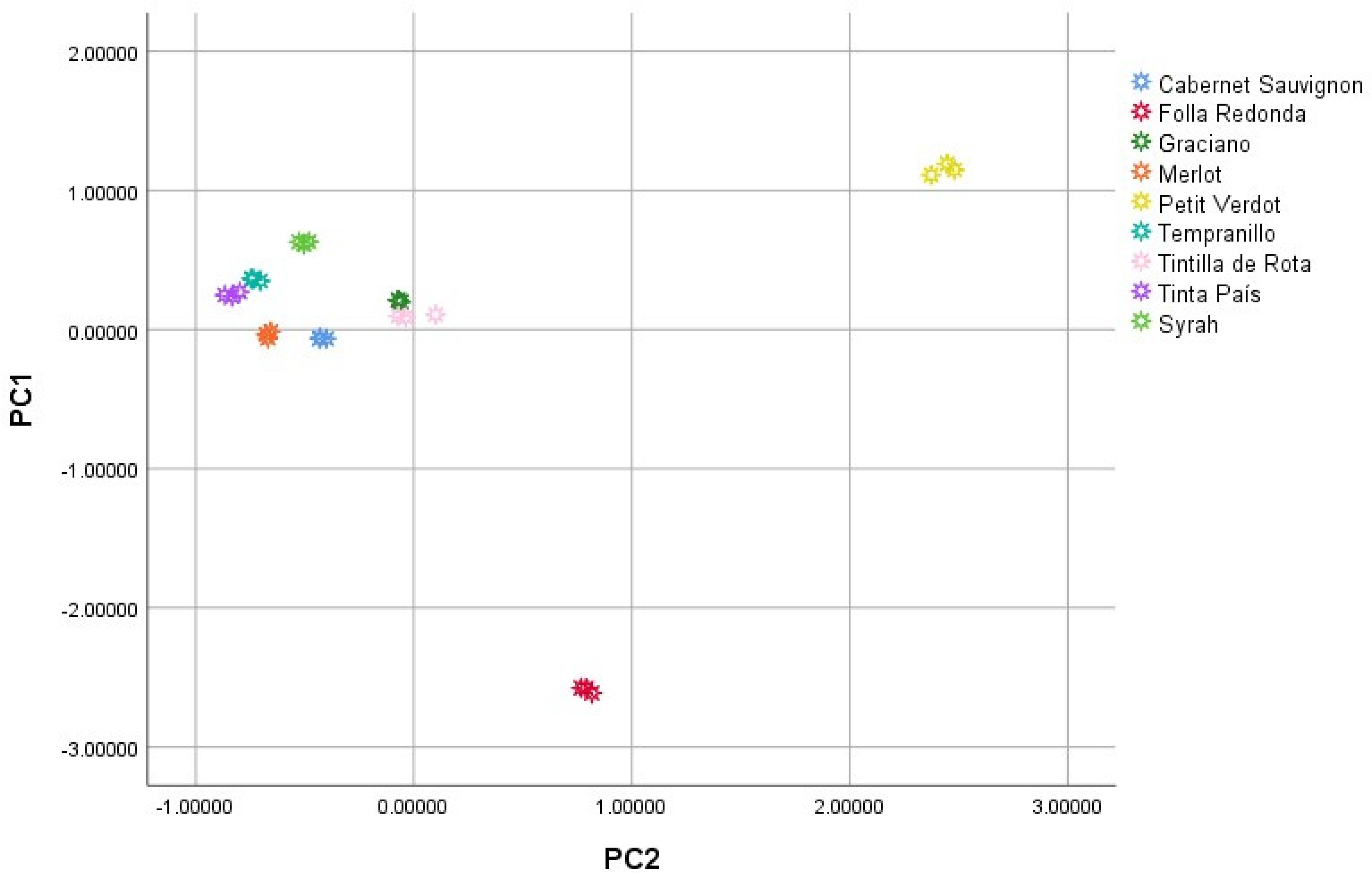
3.5. Grape Distribution
Finally, a principal component analysis (PCA) was carried out with the 28 variables evaluated in the current research (pH, acidity, density, °Bé °Brix, total polyphenols, total anthocyanins, D3G, C3G, Pet3G, Peo3G, M3G, M36AG, TM36CG, Peo36CG, C36AG, Pet36CG, CM36CG, glucose, fructose, glycerol, mannitol, sucrose, tartaric acid, malic acid, acetic acid, DPPH activity and ABTS activity). The samples were plotted according to the scores obtained for the two main components (
Figure 5). As can be seen, the Folla Redonda variety presented negative PC1 values, completely separated from the rest of the samples. On the other hand, among the
Vitis vinifera varieties, all presented positive values or values close to 0 for PC1, while the Petit Verdot variety presented positive values for PC2 while the Petit Verdot variety presented negative values for PC1, demonstrating certain differences between this variety and the others. Finally, within this group, each of the varieties was grouped in a differentiated manner in space, demonstrating their differences. The scores responsible for this distribution were evaluated and it was shown that the concentrations of malic and acetic acid, the density and the concentration of CM36CG were the main responsible for the separation between the Folla Redonda variety and the rest, while the concentration of mannitol, sucrose, C36AG and TM36CG were responsible for the separation between the
Vitis vinifera varieties.
4. Conclusions
In conclusion, this study has revealed significant findings on the physicochemical properties of the Folla Redonda grape, highlighting its richness in anthocyanins and phenolic compounds with potent antioxidant activities. These findings open new opportunities for the use of this grape variety in various applications. The high concentration of antioxidant compounds found in the Folla Redonda grape suggests its potential use in the food industry, in the production of nutritional supplements or even in cosmetics. In addition, its unique nutritional profile may contribute positively to human health by offering antioxidant benefits. This could lead to the revitalization and utilization of a currently untapped variety for the benefit of multiple industries. Furthermore, if this variety were finally accepted for the production of marketable wine, it would offer organoleptic properties completely different from most other varieties of Vitis vinifera and with significant concentrations of anthocyanins and phenolic compounds.
Author Contributions
Conceptualization, C.C. and G.F.B.; methodology, C.C., G.F.B. and N.C.; software, M.J.A.-G. and C.C.; validation, C.C., G.F.B. and N.C.; formal analysis, A.G.-L., A.V.G.-d.-P. and M.V.-E.; investigation, A.V.G.-d.-P. and M.V.-E.; resources, C.C., M.P. and G.F.B.; data curation, A.G.-L., M.J.A.-G., and C.C.; writing—original draft preparation, M.J.A.-G. and A.G.-L.; writing—review and editing, M.V.-E., G.F.B., and C.C.; visualization, G.F.B., C.C., and N.C.; project administration, G.F.B. and M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the project “EQC2018-005135-P” (Equipment for liquid chromatography by means of mass spectrometry and ion chromatography) of the State Sub-program of Research Infrastructures and Technical Scientific Equipment.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented are contained within the article.
Acknowledgments
The authors are grateful to the “Instituto de Investigación Vitivinícola y Agroalimentaria” (IVAGRO) for providing the necessary facilities to carry out the research. Special acknowledgment is extended to the Mass Spectrometry Division from the Central Research Ser-vices for Science and Technology (SC-ICYT) of the University of Cadiz for their collaboration throughout the analysis of the samples. María José Aliaño-González would like to thank the University of Cádiz for the grant in the modality “Margarita Salas”, of the call for the Requalification of the Spanish University System for 2021–2023 (Resolution of the Rector of the University of Cadiz UCA/R155REC/2021, of 2 July), financed by the Ministry of Universities of the Government of Spain through the European Recovery Instrument “Next Generation EU”, of the European Union (Order UNI/551/2021, of 26 May).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Base de Datos | OIV. Available online: https://www.oiv.int/es/what-we-do/data-discovery-report?oiv (accessed on 16 April 2024).
- Vasylyk, I.; Gorislavets, S.; Matveikina, E.; Lushchay, E.; Lytkin, K.; Grigoreva, E.; Karzhaev, D.; Volkov, V.; Volodin, V.; Spotar, G.; et al. SNPs Associated with Foliar Phylloxera Tolerance in Hybrid Grape Populations Carrying Introgression from Muscadinia. Horticulturae 2022, 8, 16. [Google Scholar] [CrossRef]
- Wilmink, J.; Breuer, M.; Forneck, A. Grape Phylloxera Genetic Structure Reveals Root–Leaf Migration within Commercial Vineyards. Insects 2021, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Savi, T.; Herrera, J.C.+L.; Forneck, A. Leaf vs. Whole-Plant Biotic Attack: Does Vine Physiological Response Change? Water 2021, 13, 1429. [Google Scholar] [CrossRef]
- Charters, S. Chapter 2 - The History of Wine. In Wine and Society; Charters, S., Ed.; Butterworth-Heinemann: Oxford, 2006; pp. 10–45. ISBN 978-0-7506-6635-0. [Google Scholar]
- Lund, K.T.; Riaz, S.; Walker, M.A. Population Structure, Diversity and Reproductive Mode of the Grape Phylloxera (Daktulosphaira vitifoliae) across Its Native Range. PLOS ONE 2017, 12, e0170678. [Google Scholar] [CrossRef]
- Hajdu, E. 6 - Grapevine Breeding in Hungary. In Grapevine Breeding Programs for the Wine Industry; Reynolds, A., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Oxford, 2015; pp. 103–134. ISBN 978-1-78242-075-0. [Google Scholar]
- Folla Redonda. VIOR - Misión Biológica de Galicia. Available online: https://vior.mbg.csic.es/variedad-vid/folla-redonda/ (accessed on 16 April 2024).
- El tinto se reivindica: «Barrantes á Denominación de Orixe». Available online: https://www.lavozdegalicia.es/noticia/arousa/ribadumia/2022/06/05/tinto-reivindica-barrantes-a-do/0003_202206A5C4992.htm (accessed on 20 April 2024).
- ¿Qué es el vino Barrantes? Available online: https://parrayvino.com/vino-gallego/vino-barrantes/ (accessed on 20 April 2024).
- Pomar, F.; Novo, M.; Masa, A. Varietal Differences among the Anthocyanin Profiles of 50 Red Table Grape Cultivars Studied by High Performance Liquid Chromatography. J. Chromatogr. A 2005, 1094, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-Assisted Extraction of Natural Products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Guerrero, R.F.; Fernández-Marin, M.I.; Cantos-Villar, E.; Palma, M. Ultrasound-Assisted Extraction of Stilbenoids from Grape Stems. J. Agric. Food Chem. 2013, 61, 12549–12556. [Google Scholar] [CrossRef]
- Palma, M.; Barbero, G.F.; Piñeiro, Z.; Liazid, A.; Barroso, C.G.; Rostagno, M.A.; Prado, J.M.; Meireles, M.A.A. Extraction of Natural Products: Principles and Fundamental Aspects. 2013. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound Assisted Extraction of Phenolic Compounds from Grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- Alavarsa-Cascales, D.; Aliaño-González, M.J.; Palma, M.; Barbero, G.F.; Carrera, C. Optimization of an Enzyme-Assisted Extraction Method for the Anthocyanins Present in Açai (Euterpe oleracea Mart.). Agronomy 2022, 12, 2327. [Google Scholar] [CrossRef]
- Aliaño González, M.J.; Carrera, C.; Barbero, G.F.; Palma, M. A Comparison Study between Ultrasound–Assisted and Enzyme–Assisted Extraction of Anthocyanins from Blackcurrant (Ribes nigrum L.). Food Chem. X 2022, 13, 100192. [Google Scholar] [CrossRef]
- Acevedo-Opazo, C.; Ortega-Farias, S.; Fuentes, S. Effects of Grapevine (Vitis vinifera L.) Water Status on Water Consumption, Vegetative Growth and Grape Quality: An Irrigation Scheduling Application to Achieve Regulated Deficit Irrigation. Agric. Water Manag. 2010, 97, 956–964. [Google Scholar] [CrossRef]
- Ebrahimi, I.; de Castro, R.; Ehsani, R.; Brillante, L.; Feng, S. Advancing Grape Chemical Analysis through Machine Learning and Multi-Sensor Spectroscopy. J. Agric. Food Res. 2024, 16, 101085. [Google Scholar] [CrossRef]
- Gamboa, M.J.; Ortega-Farias, S.; de la Fuente, D.; Fuentes-Peñailillo, F.; Vargas, S.; Laurie, V.F. Grape Ripening and Phenolic Content Monitoring in Cabernet Sauvignon under Regulated Deficit Irrigation Using Spectral Reflectance Indices. Sci. Hort. 2024, 328, 112920. [Google Scholar] [CrossRef]
- Segade, S.R.; Orriols, I.; Gerbi, V.; Rolle, L. Phenolic Characterization of Thirteen Red Grape Cultivars from Galicia by Anthocyanin Profile and Flavanol Composition. OENO One 2009, 43, 189–198. [Google Scholar] [CrossRef]
- Gil, M.; Kontoudakis, N.; González, E.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Influence of Grape Maturity and Maceration Length on Color, Polyphenolic Composition, and Polysaccharide Content of Cabernet Sauvignon and Tempranillo Wines. J. Agric. Food Chem. 2012, 60, 7988–8001. [Google Scholar] [CrossRef] [PubMed]
- González-Neves, G.; Gil, G.; Favre, G.; Ferrer, M. Influence of Grape Composition and Winemaking on the Anthocyanin Composition of Red Wines of Tannat. Int. J. Food Sci. Technol. 2012, 47, 900–909. [Google Scholar] [CrossRef]
- Scettri, A.; Baroldi, I.; Allari, L.; Bolognini, L.; Guardini, K.; Schievano, E. NMR Sugar-Profile in Genuine Grape Must. Food Chem. 2024, 451, 139374. [Google Scholar] [CrossRef] [PubMed]
- Amores, R.A.P.; Vigara, J.J.M. Química Enológica; Madrid, 2010; ISBN 978-84-8476-390-1.
- de Andrade Kaltbach, S.B.; Kaltbach, P.; Santos, C.G.; Cunha, W.; Giacomini, M.; Domingues, F.; Malgarim, M.; Herter, F.G.; Costa, V.B.; Couto, J.A. Influence of Manual and Mechanical Grape Harvest on Merlot Wine Composition. J. Food Compos. Anal. 2022, 110, 104548. [Google Scholar] [CrossRef]
- Azuara, M.; González, M.R.; Rodríguez-Nogales, J.M.; Martín, P. Impact of Pre-Veraison Cluster Treatments with Nordihydroguaiaretic Acid on the Composition of Verdejo Grapes and Wines. Food Biosci. 2024, 59, 104139. [Google Scholar] [CrossRef]
- López-Giral, N.; López, R.; Santamaría, P.; González-Arenzana, L.; Garde-Cerdán, T. Phenolic and Colour Characteristics of Must and Wine Obtained from Red Grapes Treated by Pulsed Electric Fields. Efficacy of PEF to Reduce Maceration Time in Elaboration of Red Wines. Eur. Food Res. Technol. 2023, 249, 273–282. [Google Scholar] [CrossRef]
- Somkuwar, R.G.; Bhange, M.A.; Oulkar, D.P.; Sharma, A.K.; Ahammed Shabeer, T.P. Estimation of Polyphenols by Using HPLC–DAD in Red and White Wine Grape Varieties Grown under Tropical Conditions of India. J. Food Sci. Technol. 2018, 55, 4994–5002. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.Y.; Treadwell, M.; Liu, T.; Hochberg, M.; Sack, M.; Mueller, G.; Sigler, J.; Silcock, P.; Oey, I. Influence of Pulsed Electric Fields Processing at High-Intensity Electric Field Strength on the Relationship between Anthocyanins Composition and Colour Intensity of Merlot (Vitis Vinifera L.) Musts during Cold Maceration. Innov. Food Sci. Emerg. Technol. 2020, 59, 102243. [Google Scholar] [CrossRef]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Ağçam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants 2022, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Marianne, L.-C.; Lucía, A.-G.; de Jesús, M.-S.M.; Eric Leonardo, H.-M.; Mendoza-Sánchez, M. Optimization of the Green Extraction Process of Antioxidants Derived from Grape Pomace. Sustain. Chem. Pharm. 2024, 37, 101396. [Google Scholar] [CrossRef]
- Hofmann, T.; Visi-Rajczi, E.; Vaculciakova, S.; Guran, R.; Voberkova, S.; Vrsanska, M.; Zitka, O.; Albert, L. Direct Microwave Treatment Enhances Antioxidant and Antibacterial Properties of the Seed Extracts of Kékfrankos Grapes. Heliyon 2023, 9, e21497. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).