Breast carcinoma is the most commonly seen female malignancy in all age groups with significant increased risk of increase age [
1]. Invasive ductal carcinoma (IDC) is the most common histologic subtype of breast cancer (BCs), occupying approximately 90% of all BCs [
2]. Ductal carcinoma in situ (DCIS) is regarded as a direct precursor to IDC, and characterized by a malignant proliferation of ductal epithelial cells within the ductal-lobular system without stromal invasion [
3]. DCIS contributes to approximately 20% of newly diagnosed breast cancer cases, in which approximately 25–60% of untreated DCIS cases have been reported to progress to IDC after a median follow-up of 9–24 years [
4,
5].
IDC shows significant overlapping with DCIS, including epidemiological risk factors such as age and family history, genetic factor such as BRCA1/BRCA2, and molecular status such as ER, PR and HER2 [
6]. IDC can be divided into multiple subtypes, including tubular, mucinous, papillary, cribriform, pleomorphic and solid [
6], which shares a great portion of histomorphological overlapping with DCIS, including papillary, micropapillary, cribriform and comedo-necrosis [
7]. Among these, solid pattern of IDC is one of the most common subtypes that is underestimated and misdiagnosed as DCIS. The aim of this case is to shed light on the big pitfall in diagnosing IDC in a background of extensive DCIS.
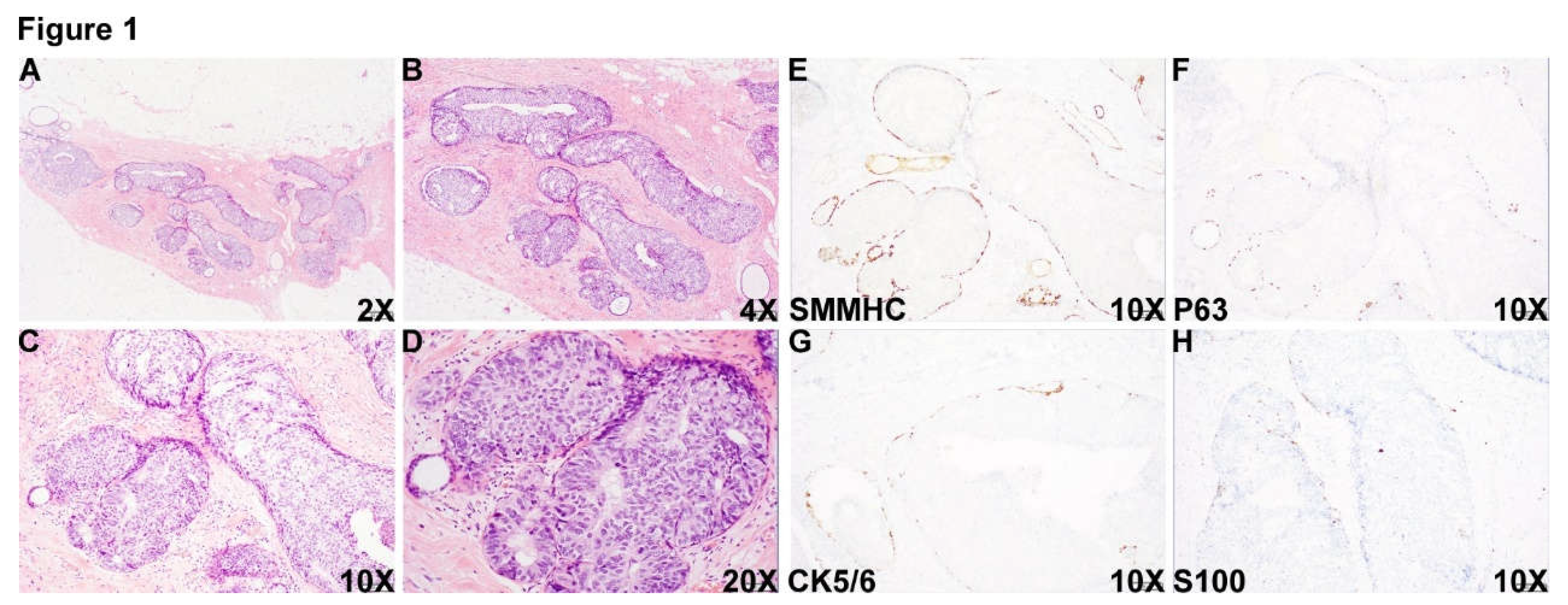
Figure 1.
DCIS area. A 55 year-old female who was noted to have microcalcification at the 11:00 o’clock of the right posterior breast on routine mammographic examination in outside hospital and biopsy of the calcification area showed reported high grade DCIS, ER+ PR- (Pathologic slides were not available to review). The patient was transferred to our institution for mastectomy resection. Histologic examination of the specimen showed an area with multiple densely packed well-border expanded acini composed of monotonous epithelioid cells (Figure 1A-D, 2X (A), 4X (B), 10X (C) and 20X (D)). Immunohistochemical (IHC) staining showed that myoepithelial markers, smooth muscle myosin heavy chain (SMMHC) (Figure 1E), p63 (Figure 1F), CK5/6 (Figure 1G) and S100 (Figure 1H), were retained at the periphery of all the expanded acini, supporting the diagnosis of DCIS in this area.
Figure 1.
DCIS area. A 55 year-old female who was noted to have microcalcification at the 11:00 o’clock of the right posterior breast on routine mammographic examination in outside hospital and biopsy of the calcification area showed reported high grade DCIS, ER+ PR- (Pathologic slides were not available to review). The patient was transferred to our institution for mastectomy resection. Histologic examination of the specimen showed an area with multiple densely packed well-border expanded acini composed of monotonous epithelioid cells (Figure 1A-D, 2X (A), 4X (B), 10X (C) and 20X (D)). Immunohistochemical (IHC) staining showed that myoepithelial markers, smooth muscle myosin heavy chain (SMMHC) (Figure 1E), p63 (Figure 1F), CK5/6 (Figure 1G) and S100 (Figure 1H), were retained at the periphery of all the expanded acini, supporting the diagnosis of DCIS in this area.
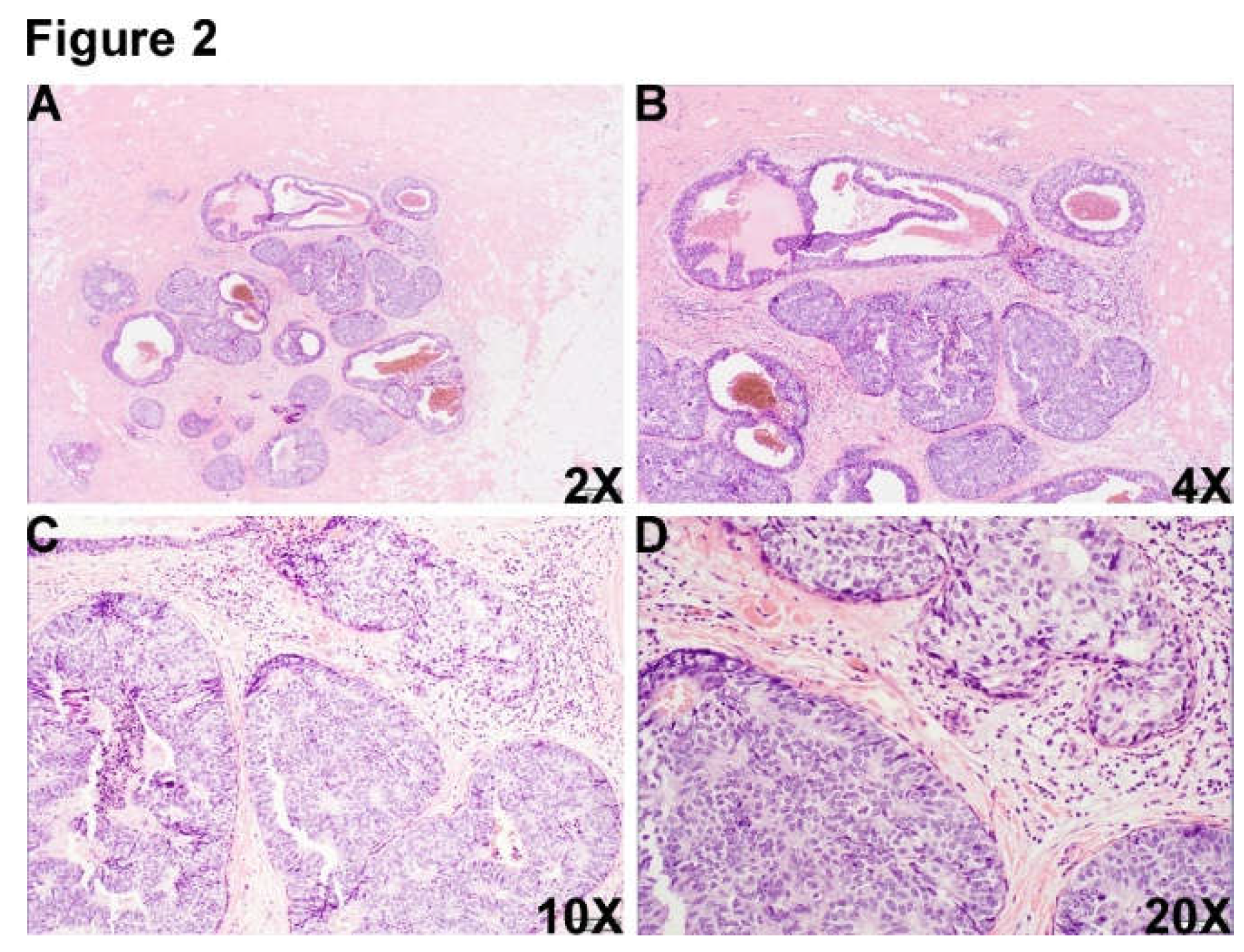
Figure 2.
There was another morphologically similar area adjacent to DCIS area, which was composed of multiple well-border expanded acini composed of monotonous epithelioid cells and dilated cystic change (Figure 2A-D, 2X (A), 4X (B), 10X (C) and 20X (D).).
Figure 2.
There was another morphologically similar area adjacent to DCIS area, which was composed of multiple well-border expanded acini composed of monotonous epithelioid cells and dilated cystic change (Figure 2A-D, 2X (A), 4X (B), 10X (C) and 20X (D).).
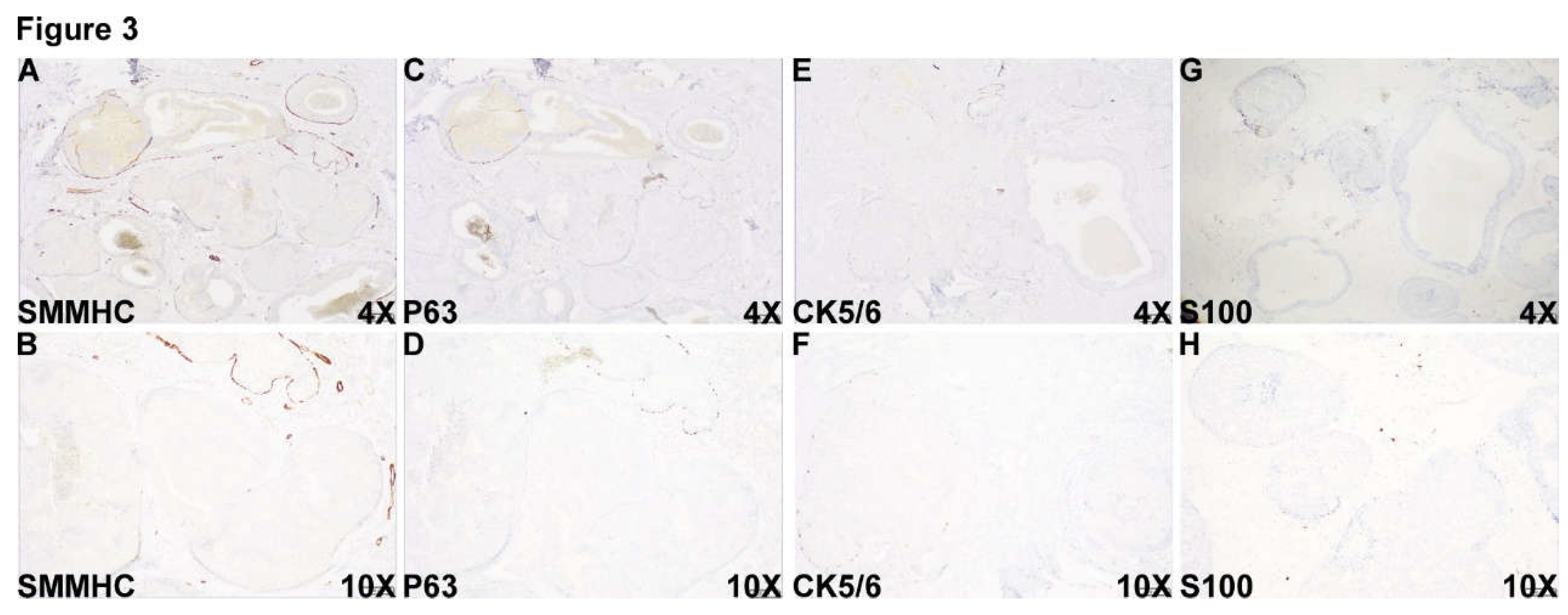
Figure 3.
Unexpectedly and surprisingly, myoepithelial markers, including SMMHC (Figure 3A and B), p63(Figure 3C and D), CK5/6 (Figure 3E and F) and S100 (Figure 3G and H) were completely lost at the periphery of part of the DCIS-looking acini in this area, immunohistochemically compatible with the diagnosis of invasive ductal carcinoma admixed with DCIS.
Figure 3.
Unexpectedly and surprisingly, myoepithelial markers, including SMMHC (Figure 3A and B), p63(Figure 3C and D), CK5/6 (Figure 3E and F) and S100 (Figure 3G and H) were completely lost at the periphery of part of the DCIS-looking acini in this area, immunohistochemically compatible with the diagnosis of invasive ductal carcinoma admixed with DCIS.
In this case, the diagnosis of DCIS is easily made without the awareness of DCIS-looking IDC morphology. This case will definitely increase recognition and awareness of pathologists to always consider the possibility of IDC in expansile DCIS-looking area.