1. Introduction
Adenoviruses (double-stranded DNA viruses of the family Adenoviridae, with genomes of 25-48 kb) have been intensively studied because of their role as pathogens in humans and domestic animals (Benko et al., 2022; Gallardo, Pérez-Illana, Martín-González, & Martín, 2021; Ntumvi et al., 2021; “Viralzone.Expasy. Org Mastadenovirus (Taxid:10509),” n.d.). In addition, modified versions of adenoviruses are used for the construction of adenovirus-based vaccines and new adenoviruses can be considered as potential for the development of therapeutic vectors (Benko et al., 2022; Kleinberger, 2015; Matsugo et al., 2021; Sallard, Zhang, Aydin, Schröer, & Ehrhardt, 2023) All human adenoviruses belong to the genus Mastadenovirus (MastAdVs), which is one of six genera in the family Adenoviridae (Benko et al., 2022) Humans are infected by seven species (A to G), which are usually subclinical but can cause a wide range of clinical syndromes, especially in immunosuppressed patients, such as acute respiratory syndrome, pneumonia, adenoid-pharyngeal-conjunctival infection, epidemic keratoconjunctivitis, hepatitis, cystitis, gastroenteritis and others (Benko et al., 2022; Doerfler, 1996; Harrach, 2014) To date, >100 different human AdV types (HAdVs) have been discovered (Ntumvi et al., 2021; Wang et al., 2023)
In addition to humans, MastAdVs can infect a variety of mammalian hosts. In general, MastAdVs appear to be host-specific viruses (Ntumvi et al., 2021), but some MastAdVs infect a wide range of hosts. The Canine adenoviruses (CAdVs) could infect a wide range of carnivores such as domestic dogs, wolves, bears, foxes, mink, etc. (Balboni et al., 2019, 2013; Hou et al., 2023; Oleaga, Balseiro, Espí, & Royo, 2022), while some Human adenoviruses (HAdVs) have been detected in monkeys (long-tailed macaques, stump-tailed macaques and great apes) (Kosoltanapiwat et al., 2022; Medkour et al., 2020). The latter supports the view that adenoviruses could be transmitted from primates to humans or vice versa (Chen et al., 2011; Dehghan et al., 2019; Harvey et al., 2023; Kosoltanapiwat et al., 2022; Medkour et al., 2020). It is likely that some known (and unknown) adenoviruses can be transmitted between mammalian species. It is therefore important to study adenoviruses in animals that have become known as reservoirs of viral infections for various mammals, including humans.
Bats (Chiroptera) are known reservoirs of several mammalian viruses, some of which can cause human disease or are ancestors of several human viruses. Mastadenoviruses have been isolated from chiropterans worldwide. To date, bat mastadenoviruses have been classified into ten species (Bat Mastadenovirus A-J) according to ICTV data (Benko et al., 2022), forming three clades on the phylogenetic tree, associated with Vespertilionidae, Rhinolophidae or Miniopteridae/Pteropodidae bats (Katayama et al., 2022; Ogawa et al., 2017). Clade 1 included viruses isolated from Vespertilionidae bats (BatAdVs species A, B, G and J), which are closely related to canine adenoviruses. Clade 2 included viruses from Rhinolophidae bats (BatAdVs species C) and clade 3 included viruses from Miniopteridae and Pteropodidae bats (BatAdVs species D, E, F, H and I) (Katayama et al., 2022). Information on mastadenoviruses in European bats has been published regularly since 2008, with the first surveys in Germany (Sonntag, Mühldorfer, Speck, Wibbelt, & Kurth, 2009). To date, bat mastadenoviruses have been detected in several European countries including France (Dacheux et al., 2014), Germany (Kohl et al., 2012; Sonntag et al., 2009; Vidovszky et al., 2015), Hungary (Jánoska et al., 2011; Vidovszky et al., 2015), Italy (Diakoudi et al., 2019), Spain (Iglesias-Caballero et al., 2018), Sweden (Cholleti, de Jong, Blomström, & Berg, 2022), Switzerland (Hardmeier et al., 2021). However, only one complete genome is available for European isolates: Bat adenovirus 2 strain PPV1 from P. pipistrellus, captured in Germany in 2007 (Kohl et al., 2012). To better understand the evolution of bat adenoviruses in mammals and their distribution across Europe, new data on the genetic diversity of bat adenoviruses may be helpful. Here we report the characterisation of mastadenoviruses obtained from faecal samples of Vespertilionidae inhabiting the Central European part of Russia (Zvenigorodsky district, Moscow region), including the complete genomic characterisation of novel mastadenovirus species obtained from N. noctula.
2. Materials and Methods
2.1. Sample Collection
Bat faecal samples were collected in the summer of 2015 in the Zvenigorodsky district of Moscow Region (Sharapovskoe forestry, coordinates N55.69, E36.70) as described in (Speranskaya et al., 2023). Bats were captured and sampled by professionally trained staff from the Biology Department of Lomonosov Moscow State University and released at the site of capture. Collected swabs were placed in a swab transport and storage medium with mucolytic agent (AmpliSens, Russia) at 4°C during transport to the laboratory and then stored at -80°C prior to processing.
The 12 bats of five species Myotis dasycneme (n = 2), Myotis daubentonii (n = 2), Myotis brandtii (n = 1), Nyctalus noctula (n = 2), Pipistrellus nathusii (n = 5) were analysed.
2.2. RNA Extraction, Reverse Transcription, Library Preparation and High-Throughput Sequencing
RNA was extracted using the QIAamp Viral RNA Mini Kit (Qiagen, Germany). Carrier RNA was dissolved in AVE buffer and added to AVL buffer according to the manufacturer’s recommendations. The 140 ul of resuspended faecal sample was added to the prepared AVL buffer with Carrier RNA-Buffer AVE. Further steps were performed according to the original protocol. RNA was eluted with 60 μL AVE buffer and stored at -70 °C until analysis. 10 ul of RNA was taken for reverse transcription using Reverta-L (AmpliSens, Russia). Second strand cDNA was obtained using the NEBNext Ultra II Non-Directional RNA Second Strand Module (E6111L, New England Biolabs). To increase the input concentration, 24 ul of first strand product was added to 10 ul of H2O (milliQ) for subsequent steps.
Double-stranded cDNA was used for library preparation using the NEBNext Ultra II DNA Library Prep Kit for Illumina (New England Biolabs, England). End prep was performed according to the manufacturer’s protocol. For the adaptor ligation step, Y-shaped adaptors compatible with the Nextera XT Index Kit were used at 4 pM per reaction. PCR amplification with index adaptors at 7.5 pM per reaction was performed using the Nextera XT Index Kit (Illumina) in 25 μL total volume according to the NEBNext Ultra II DNA Library Prep Kit for Illumina protocol with 15 cycles.
High-throughput sequencing was performed on Illumina HiSeq 1500 using HiSeq PE Rapid Cluster Kit v2 and HiSeq Rapid SBS Kit v2 (500 cycles).
2.3. Complete Genome Assembly and Annotation of Novel Virus
Paired reads were filtered using Trimmomatic with the parameters SLIDINGWINDOW:4:25 MINLEN:40. After read trimming, reads were assembled using SPAdes v.3.14 in metaSPAdes mode (Prjibelski, Antipov, Meleshko, Lapidus, & Korobeynikov, 2020). Contigs shorter than 500 bp were filtered out and the remaining contigs were aligned to viral sequences in both the nt database using blastn and nr using blastx. Full genomes were selected by comparing the length of the contig with the median length of the top hits (with a threshold of 90% of the maximum bit score) of the blastn alignment to the nt database or, in the case of the blastx alignment, with the median length of the sequence from which the protein in the nr database was derived. Contigs with a length difference of more than 5% from the median were filtered out, the remaining contigs were considered as full genome candidates and were subsequently checked manually to account for possible errors in the algorithm and the NCBI databases.
Genome annotation was performed using the following software:
de novo using Prokka software (Seemann, 2014) and
de-reference MK472072.1 using GATU (Tcherepanov, Ehlers, & Upton, 2006) and ViPTree software (Nishimura et al., 2017). The ORFs/features were additionally revised by BLAST-based similarity/homology searches. Coding sequence domains were predicted as open reading frames (ORFs) that shared certain features, such as nucleotide/amino acid sequence similarity, with known viral genes in the GenBank database. Viral ITR sequences were determined by BLAST. The viral map was visualised using the SnapGene Viewer [ “SnapGene software (
www.snapgene.com)”].
2.4. Phylogenetic Analyses
The sequences obtained from our new virus isolate were aligned with homologous sequences of closely related viruses available in the public database — GenBank (153 sequences, see suppl.1). Alignment was performed using the Multiple Sequence Alignment (MSA) tool, MAFFT version 7 (Katoh, Rozewicki, & Yamada, 2018), using default parameters. Phylogenetic analyses were performed using W-IQ-TREE [multicore version 2.2.2.3] with ModelFinder (Kalyaanamoorthy, Minh, Wong, Von Haeseler, & Jermiin, 2017), tree reconstruction (Nguyen, Schmidt, Von Haeseler, & Minh, 2015), and ultrafast bootstrap (1000 replicates) (Minh, Nguyen, & Von Haeseler, 2013). A maximum likelihood (ML) phylogenetic tree was constructed using. The best-fitting nucleotide substitution model was determined using [SYM+I+G4 model]. Bootstrap analysis with 1000 replicates was performed to assess the robustness of the tree topology. The resulting tree topology was visualised and annotated using ITOL version 6.8.1 (Letunic & Bork, 2021).
3. Results
3.1. Mastadenovirus Detection
Total RNA sequencing data from bats captured in 2015, Moscow region, obtained earlier during the analysis of the distribution of coronaviruses (see our previous work) were used to search for viruses fam. Adenoviridae. The 2 out of 12 samples (16%) were found as mastadenovirus positive: Nyctalus noctula (sample №19) and Pipistrellus nathusii (sample №21).
The complete genome of a mastadenovirus was assembled from N. noctula, named “Quixote” (isolate BatAdV/MOW15-Nn19). Novel mastadenovirus was named “Quixote” because the complete genome of an adeno-associated virus (called “Pansa”, not described in this article) was also collected in the faeces of the same animal. The complete nucleotide sequence has been deposited in GenBank under accession number PP297886.
We were also able to assemble the partial genome of a mastadenovirus from P. nathusii: de novo assembly yielded 11 contigs representing mastadenovirus genome fragments with a total of 7112 bp. The isolate was named BatAdV/MOW15-Pn21 and partial genome sequence was deposited in GenBank under accession number PP386572-PP386580.
3.2. Complete Genome of Novel Mastadenovirus BatAdV/MOW15-Nn19
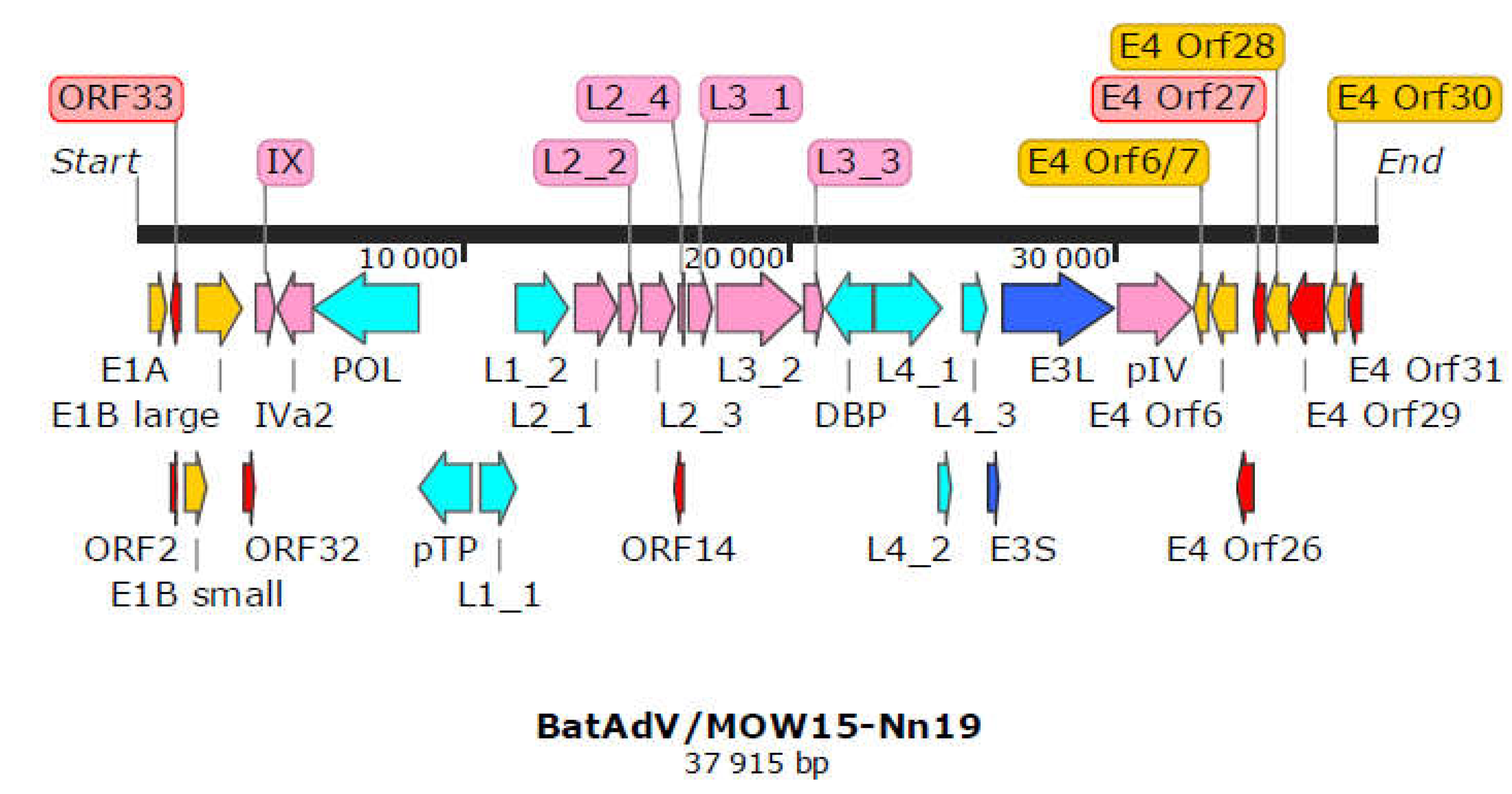
The complete genome of “Quixote” (isolate BatAdV/MOW15-Nn19) contains 35 open reading frames and the typical gene order found in Mastadenoviruses, see
Figure 1. The genome is flanked by inverted terminal repeats (ITR, origins of viral DNA replication) of 59 bp.
All identified genes/ORFs of the “Quixote” (isolate BatAdV/MOW15-Nn19) genome have been accurately annotated according to the ICTV description of the mastadenovirus genome structure (Benko et al., 2022). All genes described as characteristic of mastadenoviruses were identified in the genome of the new virus, see
Table 1. In addition, 8 ORFs encoding polypeptides of unknown function were found. We compared each of the identified ORFs (by na or amino acid sequence) of “Quixote” with Genbank and Uniprot records and found that most of the identified ORFs (27/34) encode proteins that show significant alignments with annotated proteins encoded by genes from the mastadenovirus isolate WA3301 from
Chalinolobus gouldii (Vespertilionidae bat from Australia, genome acc. number MK472072.1), with BLAST homologue identities ranging from 29.21% to 86.52% (see
Table 1). We also found that the re-core protein X encoded by gene L2_4 is most closely related to that of
Rousettus leschenaultii (Pteropodidae bat from China, YP_009388318). The predicted protein encoded by the E3 gene has homology to the product of the E3 gene of guinea pig adenovirus (30.65% identity to YP_010796290). The protein encoded by E4 orf6/7 showed moderate identity (44. 59%) to that of human mastadenovirus D [AGT77890].
Four of the nine ICTV species delimitation criteria for adenoviruses could only be used for viruses identified by NGS methods, namely: phylogenetic distance (>10-15%, based on distance matrix analysis of DNA polymerase amino acid sequence), genome organisation (characteristically in the E3 region), nucleotide composition, host range. According to the ICTV criteria, “Quixote” (isolate BatAdV/MOW15-Nn19) related to a new species of bat mastadenovirus: the complete genome showed 72.41% identity (0.53% total length) with the closest virus, isolate WA3301 [MK472072.1]. DNA polymerase amino acid sequence comparisons showed that “Quixote” (isolate BatAdV/MOW15-Nn19) had 38% identity (0.27 total length) with isolate WA3301 [MK472072.1]. The next closest virus was Nynoc/Switzerland/2019 [MT815933.1], which was found in N. noctula with 5% identity. Bat mastadenoviruses are classified as species A-J according to the ICTV data. Therefore, the new species can be provisionally designated as “K”.
The complete genome of “Quixote” was 37,915 bp and GC=49.9% like the closest isolate WA3301 (37,617 bp and GC=49.1%). At the same time, other BatAdVs species closest to the new virus had a length range of 31629-31806 bp (five thousand shorter) and GC content of 53.5-56.9%. Mastadenovirus genomes range in size from 27,952 (polar bear adenovirus 1) to 37,860 bp (simian adenovirus 31.2 from chimpanzees, species human mastadenovirus C) (Benko et al., 2022; Böszörményi et al., 2020; Roy et al., 2009) and in nucleotide composition (GC) from 43.6% (BAdV-2) to 63.9% GC (PAdV-3) (Harrach, 2014). This means that the genome size of the new adenovirus is within the known range, but the genome size of the new virus is unusual for bat adenoviruses.
The genome organisation of the “Quixote” (isolate BatAdV/MOW15-Nn19) was the same as that of isolate WA3301 in the E3 region. However, “Quixote” differ from WA3301 in the E4 region, as well as both (“Quixote” and WA3301) differ from other bat mastadenoviruses in the E4 region.
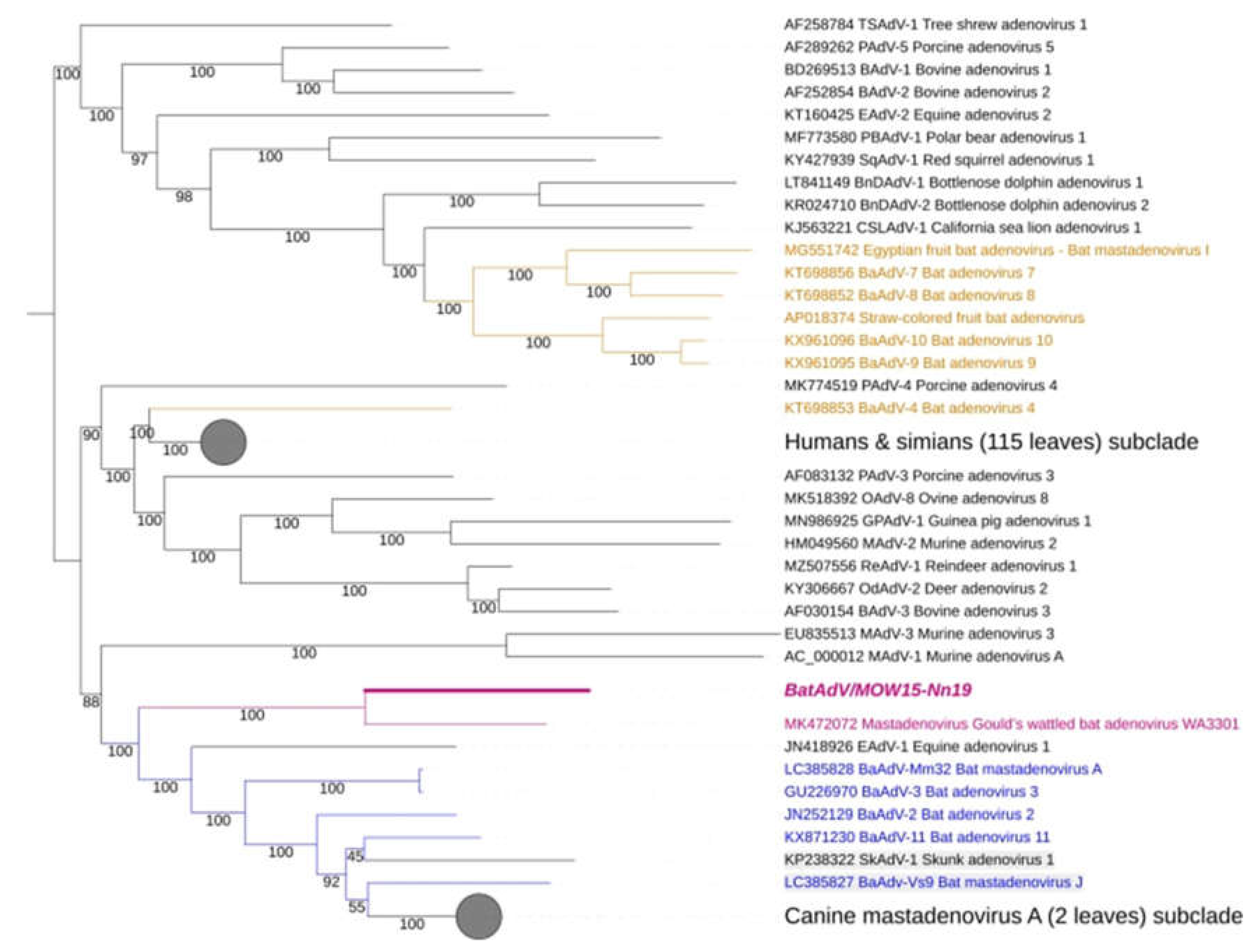
3.2. Phylogenetic Analysis for Bat Mastadenoviruses from Russia
The complete genomic nucleotide sequence of the novel virus was phylogenetically analysed against the full-length genomes of representative members of the genus Mastadenovirus. We found that “Quixote” (isolate BatAdV/MOW15-Nn19) from
N. noctula and isolate WA3301 from
C. gouldii form a subclade that was clearly separated from other Vespertilionidae BatAdVs, see
Figure 2. Both hosts in which the novel virus was found,
N. noctula and
C. gouldii (Gould’s wattled bat), belong to the bat family Vespertilionidae. Interestingly, both viruses were found in geographical regions as far apart as possible: European Russia and Australia.
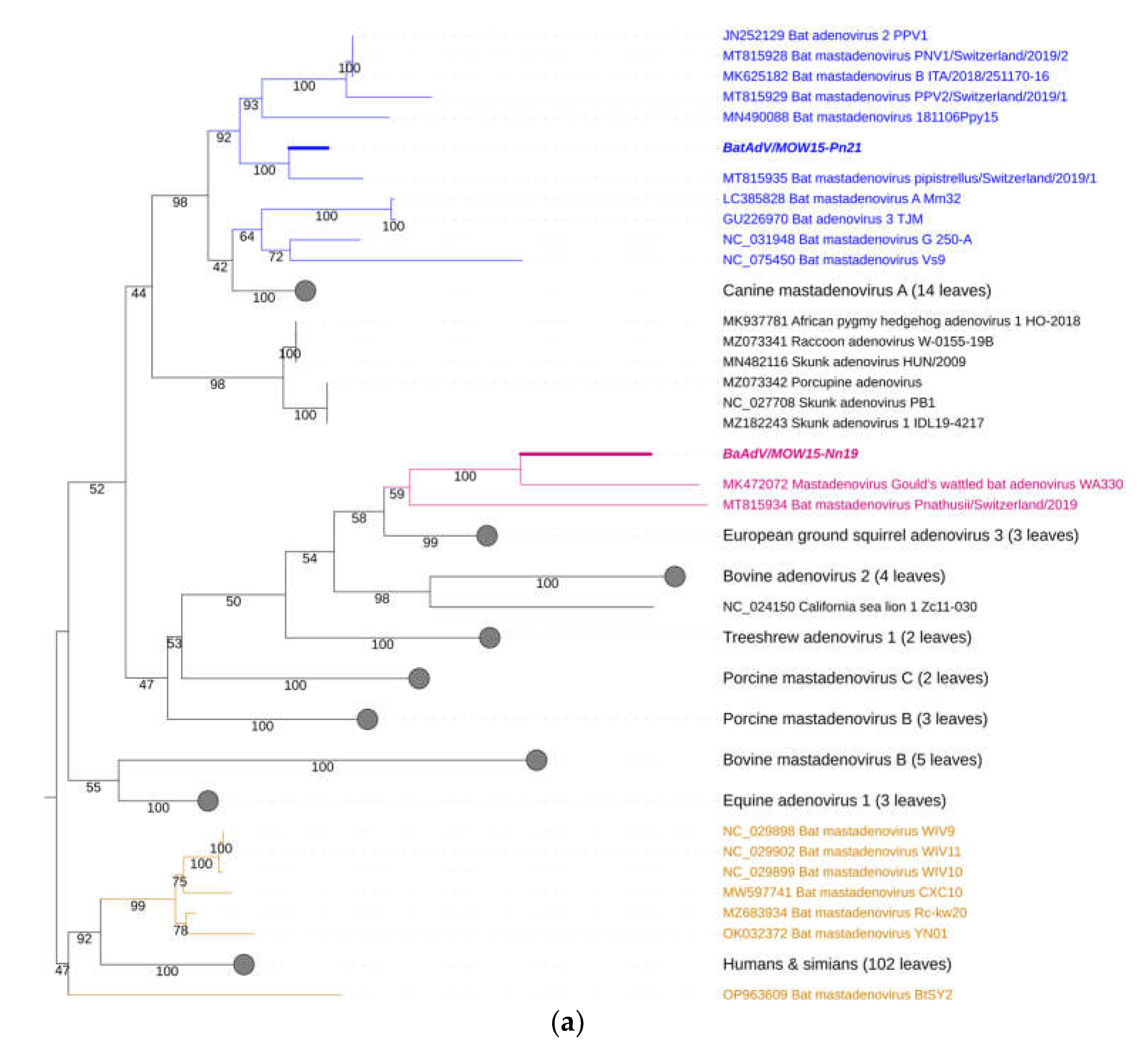
For taxonomy identification of second mastadenovirus identifien in this sudy -BatAdVs/MOW15-Pn21 from P. natusii - a phylogenetic tree was constructed using the partial sequence of the DNA polymerase (DNApol) genes. The two isolates studied here, “Quixote” (isolate BatAdV/MOW15-Nn19) and BatAdVs/MOW15-Pn21 were phylogenetically distant from each other. The isolate BatAdV/MOW15-Pn21 was similar to bat adenovirus 2 which is a typical isolate of species B, according to phylogenetic analysis of the terminal part of DNA polymerase encoding genes, see
Figure 3a. BLAST investigation results the closest nt identity (99%) to the BatAdV from Swiss bats, 2019,
P. pipistrellus [MT815935.1] and nt identity in range 79,8-80,1% to BatAdVs from bats captured during 2017-2019 in Switzerland,
P. pipistrellus [MT815928.1], [MT815929.1], Spain,
P. pipistrellus [MN490088.1], and Italy,
P. kuhlii [MK625182.1].
According to phylogenetic tree constructed using the terminal part of the DNApol, the “Quixote” (isolate BatAdV/MOW15-Nn19) falls into clade together with isolate WA3301 from C. gouldii, Australia and isolate Nynoc/Switzerland/2019 from N. noctula, Europe.
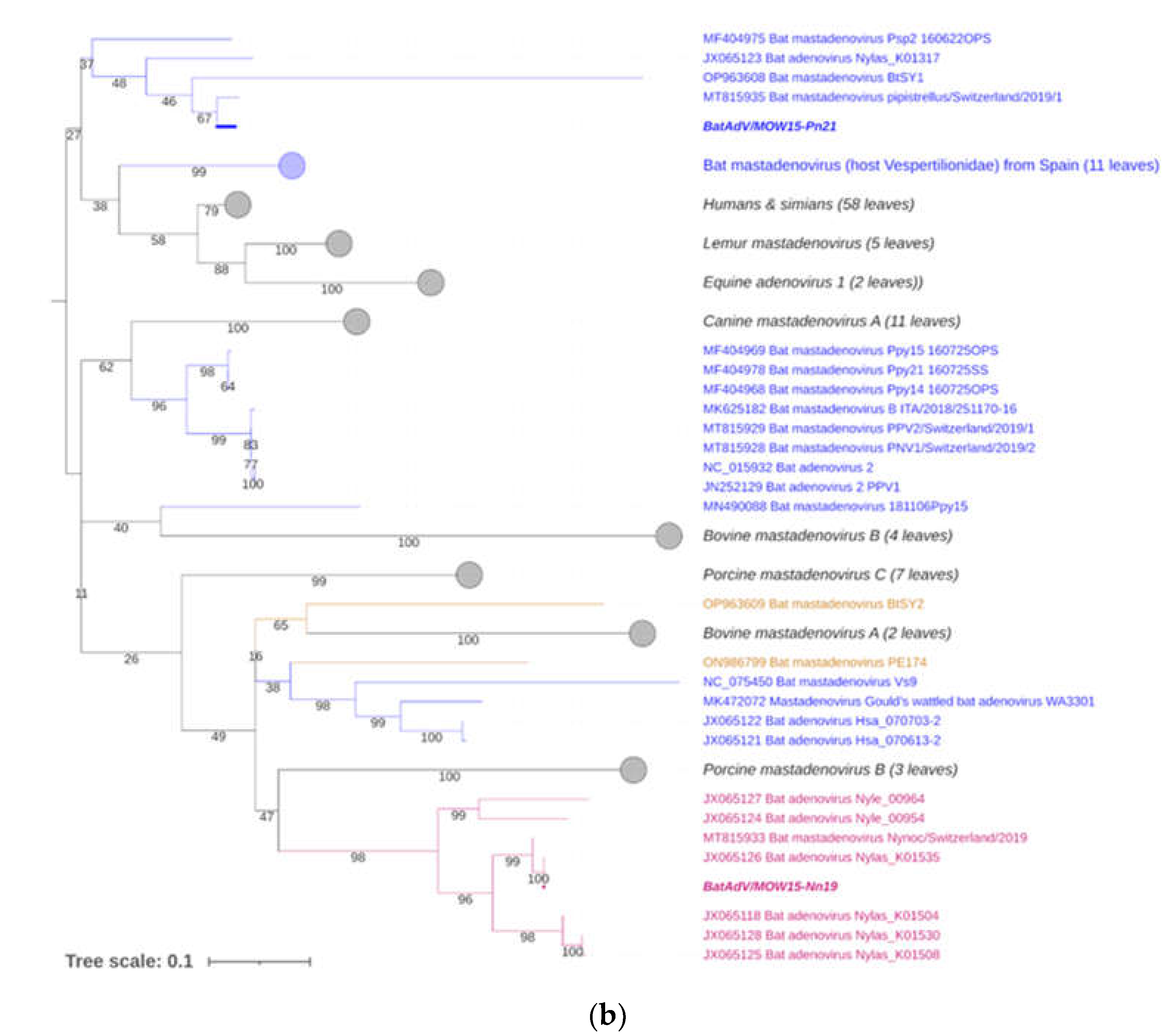
The additional phylogenetic analysis of the central segment of the DNA polymerase gene was carried out in relation to recently studied viruses (it allows increasing sample of viruses analysed), see
Figure 3b. In the context of the additional DNA polymerase gene analysis, the “Quixote” demonstrated similarity with wide spectrum of mastadenoviruses from European bats, including identified in
N. noctula Nynoc/Switzerland/2019 [MT815933.1] and couple of viruses identified in Spanish bats
Nyctalus lasiopterus and
Nyctalus leisleri [JX065118, JX065125, JX065128, JX065124, JX065127]. While the Australian virus - isolate WA3301 obtained from
C. gouldii – fall into different clade, together with viruses from
Hipposideros larvatus, which were captured in China: Yunnan, 2015 [OP963609] and
Phyllostomus discolor captured in Brazil, 2021. This observation suggests a novel mastadenovirus from
N. noctula represents a distinct evolutionary branch of viruses infecting bats in Europe.
4. Discussion
4.1. Prevalence of Mastadenoviruses in Bat Populations
Adenoviruses have been in evidence in bats across Europe: from Italy to Hungary (Dacheux et al., 2014; Diakoudi et al., 2019; Drexler et al., 2011; Hardmeier et al., 2021; Jánoska et al., 2011; Kohl, Nitsche, & Kurth, 2021; Sonntag et al., 2009; Vidovszky et al., 2015). Here we report MastAdVs from bats captured in central Russia. The 16% of bats examined here were found to be positive for MastAdVs (2 of 12 samples, 5 bat species). Previously, similar results were obtained for bats from Germany and Hungary: 14.73% of bats were found to be MastAdVs positive (51 out of 346 samples, 28 bat species) (Vidovszky et al., 2015). Overall, 17.4% of samples (34/195 bats of 8 different species) collected in northern Italy in 2016-2017 were adenovirus positive (Diakoudi et al., 2019). Some studies give a different estimate, for example an analysis of Swiss bats found adenoviruses in only 2.6% of animals (174 sample pools, 69 feces and 105 organ pools, collected between 2015 and 2020)(Hardmeier et al., 2021). AdV DNA was detected in 3.6-8.3% of bats from Spain, depending on the type of sample examined, oropharyngeal or faecal (Iglesias-Caballero et al., 2018). However, such findings seem to be the exception rather than the rule. It is likely that 15-20% of bats carrying adenovirus are the norm in the wild.
4.2. Diversity and Geographic Distribution of BatAdVs from European Bats
BatAdVs have been isolated several times for European bats. However, the number of described species of bats captured in Europe is quite small. In fact, for most BatAdVs from European bats, only fragments of the genome are known. Phylogenetic analyses are usually based on short fragments of 200-450 bp of DNA polymerase or Hexon genes or short read sequences (70 nt on average) the pool of samples, for example in France (Dacheux et al., 2014). This approach significantly reduces the accuracy of the research and the reliability of the conclusions drawn.
To date, bat mastadenoviruses have been classified into ten species (Bat Mastadenovirus A-J) according to ICTV data (Benko et al., 2022). The majority of mastadenoviruses from European bats belong to species B or an unidentified taxonomic position, which could be explained by the current incompleteness genome sequences. The first case of BatAdV identification in Europe was reported in 2007, from animals captured in Germany. This was the BatAdV-2 isolate PPV1 from P. pipistrellus and its complete genome was determined (Sonntag et al., 2009). This was followed by a number of studies that noted the high diversity of mastadenoviruses from bats in Germany, Hungary, and Italy, but only analysed short genome fragments. Some of investigated sequences showed high nt identity with the BatAdVs species B, but taxonomy position of some viruses were uncleare (Jánoska et al., 2011, Vidovszky et al., 2015) (Diakoudi et al., 2019) (Hardmeier et al., 2021). Some researchers have clearly suggested that mastadenoviruses other than BatAdVs species B circulate in bats in Europe. For example, a total of 1717 samples collected in Spain between 2004 and 2016 from bats representing 27 of the 32 European bat species revealed the multiple BatAdVs.The authors declared that the new groups are different from the previously described BatAdVs species A and B, but without clarification, as they only sequenced partial hexon or DNApol genes (Iglesias-Caballero et al., 2018).
This paper, we report two different BatAdVs from bats captured in Russia (from Central European part of Russia): BatAdV/MOW15-Pn21 from P. nathusii and the “Quixote” (isolate BatAdV/MOW15-Nn19) from N. noctula. According to phylogenetic analysis of DNA polymerase gene BatAdV/MOW15-Pn21 is related to species B, as the first BatAdV identified in a European bat (BtAdV-2 isolate PPV1 from P. pipistrellus, captured in Germany), as the majority of BatAdV strains identified in Italy in 2019 (Diakoudi et al., 2019) and Spain (Iglesias-Caballero et al., 2018). With Russia being the easternmost area of Europe, the results presented here show that members of species B are widespread in bats across Europe. However, the question now arises as to whether the same viruses are present in bats that live on the other side of the Ural Mountains in Siberia.
“Quixote” (isolate BatAdV/MOW15-Nn19) represents a new species (not approved ICTV, yet). This finding is based on both whole genome analysis and phylogenetic analysis of the DNA polymerase gene. We assume that novel species can be designated as a “K” (the next letter after the “J”). Previously, a phylogenetic analysis on a large number of short mastadenovirus sequences obtained from the Spanish bats allows the authors suggested the existence of new members of the genus Mastadenovirus, in addition to the species already described. But the authors only sequenced the short fragments of the DNA polymerase and hexon genes, so the existence of these species remains a guess (Iglesias-Caballero et al., 2018). Here, we found similarity between the “Quixote” and some isolates from European bats captured in Spain when analyzed short (central) segment of the DNA polymerase gene. Most likely, the new members of the genus Mastadenovirus proposed by Iglesias-Caballero et al. (2018) are closely related to “Quixote”, whose complete genome we have now characterised. According to our data, at least one virus from Swiss bat (strain Nynoc/Switzerland/2019) is relatives of novel virus characterised here. Thus, we propose that the novel mastadenovirus “Quixote”, whose complete genome is described here, and its relatives from Spanish and Swiss bats are related to a separate evolutionary branch and represent novel species (tentatively named “K”). Thus, at least two endemic species of mastadenoviruses infect bats in Europe (species B and novel species, provisionally K).
Mastadenoviruses have a tendency to be host specific (Iglesias-Caballero et al., 2018; Ntumvi et al., 2021). Vespertilionidae bats are carriers of species A, B, G and J (Katayama et al., 2022). Besides, mastadenovirus species are specific to animals living in certain geographical regions. The majority of mastadenoviruses from vespertilionid bats captured in Europe belong to species B. Representatives of species A have been described in South Asia, namely China and Japan (see typical genomes of GU226970 and LC385828), while representatives of species G are found in North American bats (see typical genome NC_031948.1) and species J - in Japanese bats (see typical genome LC385827). Novel BatAdVs presented in this work fit this concept well. All bat specimens harbouring viruses similar to “Quixote” relaated to Vespertilionidae. The “Quixote” was identified from N. noctula while similar viruses were identified from different species of the same genus Nyctalis (N. noctula captured in Switzerland, N. lasiopterus and N. leisleri captured in Spain) or other Vespertilionidae (C. gouldii, from Australia).
4.3. Could the Pipistrellus Bats Distribute Mastadenoviruses around the Old World until Australia?
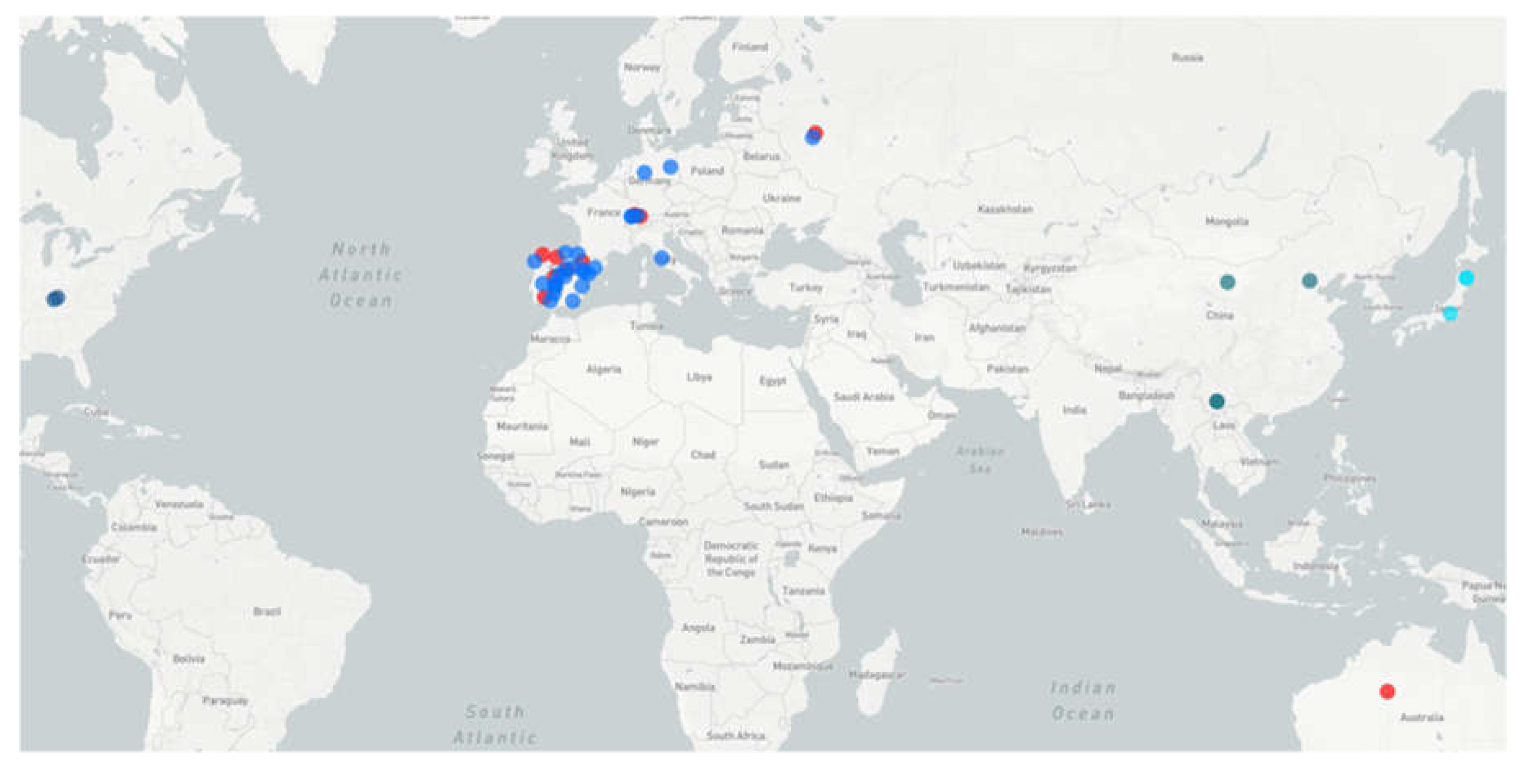
Figure 4 shows the global distribution of bat mastadenoviruses of species B (in European Vespertilionidae bats), as well as other previously described spesies A, G and J (from Asian and Amerycan Vespertilionidae bats) in comparative to a putative new species (tentatively named K), genetically similar to “Quixote” virus described here. The large distance between the geographical locations where Quixote-like viruses have been identified raises the question of how these viruses spread across the globe. There are significant zoological barriers between Europe and Australia.
It is important, the identity of n.a. between complete genomes of BatAdV/MOW15-Nn19 from European N. noctula (Russia) and the closest BatAdV isolate WA3301 from Australian C. gouldii is low and geographically distant from these findings, so it can be assumed that intermediate evolutionary forms will be found in Vespertilionidae bats in the territory of Central or South-East Asia in the future. Not all Australian bat taxa have been genetically characterized, so the timing of divergence and even the status of some Australian bat taxa are only guesses. Four genera are endemic to Australia: Vespadelus, Chalinolobus, Falsistrellus and Scotorepens. They all (presumably) belong to the Hypsugine group, an undescribed taxon (tribe or subtribe) with the greatest diversity in Africa; the time of divergence of Chalinolobus from other genera is estimated to be around 12 million years (Lack, Van Den Busche, 2010), presumably the time of their invasion of Australia via a chain of islands including New Guinea. The genus Chalinolobus (and three other genera) are the “old” Australian vespertilionines, so they are unlikely to be the first hosts of viruses related to European viruses. It is likely that the mastadenovirus BatAdV WA3301, which is related to the European BatAdV/MOW15-Nn19, was acquired by Chalinolobus from some intermediate hosts. These may have been bats of other genera.
Among other vespertilionid genera, occurring in Australia, four have bulk ranges outside Australia across Sunda Islands and tropical Asia (Murina, Phoniscus), the Old World (Pipistrellus) or have a World-wide distribution (Myotis) (Moratelli et al., 2019). These genera almost certainly arrived in Australia in the Pleistocene, during the last major sea regression, when one or two narrow straits remained between Asia and Australia. The Australian myotis, Myotis macrotarsus, has close relatives in Asia (Myotis horsfieldii and others) from which it probably diverged at the end of the Pliocene (and with which it may share related viruses). The bats of the genus Pipistrellus (two species currently living in Australia, namely P. adamsi and P. westralis) presumably belong to the “javanicus” species group and have their possible close relatives in Sunda Islands and tropical Asia (Kitchener et al., 1986; Simmons N.B., 2005). It seems that there is no published genetic data on Australian and New Guinean Pipistrellus species, so their actual phylogenetic relationships are unclear. Morphologically and ecologically almost all Pipistrellus species are more or less similar, including the European species. All European Pipistrellus belong to the ‘western’ clade of the genus (Zhukova et al., 2022). There is the extreme phylogenetic proximity between the ‘western’ clade Pipistrellus and the genus Nyctalus (Lack & Van Den Bussche, 2010; Zhukova et al., 2022), therefore one could expect the presence of related viruses in these two genera.
Both Pipistrellus and Myotis are probably able to cross sea straits and colonize islands. Such possibility is documented for European P. nathusii (Lagerveld, Poerink, & Geelhoed, 2021) and also derived from the fact of wide insular distribution ranges of some Pipistrellus and Myotis species (e.g., P. tenuis and M. ater). Therefore, these two genera provide the most likely bridge for the spread of bat viruses from Asia to Australia. But, the rate of evolution of viral genomes is higher than the rate of migration of bats around the globe. The genetic similarity of the mastadenoviruses found in bats in Europe and Australia suggests that there are likely to be spread by some as yet unknown hosts.
4.4. Structural Features of the Genome of the Novel Mastadenovirus Species
It is closest relative, according to complete genome analysis, is currently a bat mastadenovirus BatAdV WA3301 [MK472072] isolated from C. gouldii captured in Australia in 2018 (Prada, Boyd, Baker, O’Dea, & Jackson, 2019). The two genomes were similar in length (37,915 bp vs. 37,617 bp), GC content (49.9% vs. 49.1%), and genome organisation. Other known BatAdV species were about five thousand bp shorter and varied in GC content (53.5-56.9%). The genome organisation of the new virus BatAdV/MOW15-Nn19 was the same as that of isolate WA3301. However, both MOW15-Nn19 and WA3301 differ from other bat mastadenoviruses in the E4 region. We comprehensively analysed the predicted genes for novel viruses. After performing automatic annotations de novo and against the reference, we found 33 ORFs, 24 of which were similar to previously annotated genes in WA3301. They are homologs of functionally necessary genes in mastadenoviruses, so they are likely to encode proteins. However, the functions of some ORF products could not be reliably determined. The E1 region encodes 3 putative ORFs of unknown function. The E4 region includes putative proteins, none of which have been unambiguously annotated in MOW15-Nn19 (as well as in WA3301). We are of the opinion that further research is necessary in order to determine the functions of the predicted proteins.
The proposed start codons of two genes – E1A and POL (DNA polymerase) - are Val (instead Met). These results were obtained using the “prokka” annotation tool. The standard mammalian genetic code was used as the translation table for the genus Mastadenovirus. Then the coordinates were manually checked with careful consideration using the closest complete genome as a reference (accession number MK472072.1). We suggest possible novel molecular mechanisms leading to the replacement of Val by Met during translation, as some findings suggests that valine tRNA may be involved in the initiation of sgRNA translation (Sanz et al., 2019). These results show that new viral genomes require experimental validation of gene coordinates and function.
5. Conclusion
At least two bat mastadenovirus species are endemic to Europe: the previously described bat adenovirus species B and the novel bat adenovirus species described here (provisionally named “K”). The complete genome of a new species of the genus Mastadenovirus (tentatively named “K”) from bats living in the European part of Russia was determined. Based on the analysis of short fragments of mastadenovirus polymerase genes identified in European bats, we hypothesized that some of them also belong to the described new species. We also found mastadenovirus species B in the European part of Russia, which is widespread in bats captured in Europe. All this suggests that closely related bat mastadenoviruses are circulating in bats throughout Europe (from western to eastern areas).
Author Contributions
Conceptualization and methodology, A.S.S.; experimental works, sequencing, E.V.K., A.S.S.; genome assembling, A.E.S.; genome annotation, I.K.Ch., E.V.K., A.S.S.; phylogenetic analysis, A.S.S., A.D.; review of animal migration and distribution, S.V.K.; original draft preparation, A.S.S., A.D., S.V.K.; editing, E.V.K.; visualisation, A.S.S., A.D.; supervision, project administration, A.S.S.; All authors have read and agreed to the published version of the manuscript.
Funding
The molecular virology, sequencing, bioinformatics and phylogenetic work was funded by the Scientific Research Institute for Systems Biology and Medicine State Task No. 12203090069-4 (A.S.S., A.E.S., E.V.K., I.K.Ch.). The zoology works was funded by the ZMMU State Theme No. 121032300105-0 [20-64-46014] (S.V.K.).
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the biological samples used the same which were evaluated and approved for the study previously published (Speranskaya et al., 2023) by local Ethic Committee of the Pasteur Institute, Saint Petersburg, Russia (protocol code No. 058-06).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data of sequencing are deposited in the GenBank SRA under the accession numbers SRX11823236 and SRX11824039. Genome sequences of BatAdV/MOW15-Nn19 and BatAdV/MOW15-Pn21 have been deposited in the GenBank under accession numbers PP297886 and PP386572-PP386580.
Acknowledgments
The authors are grateful to Artiushin Ilia V. for their help in sampling from bats.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Balboni, A., Verin, R., Morandi, F., Poli, A., Prosperi, S., & Battilani, M. (2013). Molecular epidemiology of canine adenovirus type 1 and type 2 in free-ranging red foxes (Vulpes vulpes) in Italy. Veterinary Microbiology, 162(2–4). [CrossRef] [PubMed]
- Benko, M., Aoki, K., Arnberg, N., Davison, A. J., Echavarria, M., Hess, M., … Harrach, B. (2022). ICTV Virus Taxonomy Profile: Adenoviridae 2022. Journal of General Virology, 103(3). [CrossRef] [PubMed]
- Böszörményi, K. P., Podgorski, I. I., Vidovszky, M. Z., Sós, E., Benkő, M., & Harrach, B. (2020). Full genome sequence analysis of a novel adenovirus from a captive polar bear (Ursus maritimus). Virus Research, 277. [CrossRef] [PubMed]
- Chen, E. C., Yagi, S., Kelly, K. R., Mendoza, S. P., Maninger, N., Rosenthal, A., … Chiu, C. Y. (2011). Cross-species transmission of a novel adenovirus associated with a fulminant pneumonia outbreak in a new world monkey colony. PLoS Pathogens, 7(7). [CrossRef]
- Cholleti, H., de Jong, J., Blomström, A. L., & Berg, M. (2022). Characterization of Pipistrellus pygmaeus Bat Virome from Sweden. Viruses, 14(8). [CrossRef] [PubMed]
- Dacheux, L., Cervantes-Gonzalez, M., Guigon, G., Thiberge, J. M., Vandenbogaert, M., Maufrais, C., … Bourhy, H. (2014). A preliminary study of viral metagenomics of french bat species in contact with humans: Identification of new mammalian viruses. PLoS ONE, 9(1). [PubMed]
- Dehghan, S., Seto, J., Liu, E. B., Ismail, A. M., Madupu, R., Heim, A., … Seto, D. (2019). A Zoonotic Adenoviral Human Pathogen Emerged through Genomic Recombination among Human and Nonhuman Simian Hosts. Journal of Virology, 93(18). [CrossRef] [PubMed]
- Diakoudi, G., Lanave, G., Moreno, A., Chiapponi, C., Sozzi, E., Prosperi, A., … Lelli, D. (2019). Surveillance for adenoviruses in bats in Italy. Viruses, 11(6). [CrossRef] [PubMed]
- 9. Doerfler, W. (1996). Adenoviruses (4th ed., Vol. 67; Walter Doerfler & Samuel Baron, Eds.). Galveston (TX). Retrieved from https://pubmed.ncbi.nlm.nih.gov/21413345/.
- Drexler, J. F., Corman, V. M., Wegner, T., Tateno, A. F., Zerbinati, R. M., Gloza-Rausch, F., … Drosten, C. (2011). Amplification of emerging viruses in a bat colony. Emerging Infectious Diseases, 17(3). [CrossRef]
- Gallardo, J., Pérez-Illana, M., Martín-González, N., & Martín, C. S. (2021). Adenovirus structure: What is new? International Journal of Molecular Sciences, Vol. 22. [CrossRef]
- Hardmeier, I., Aeberhard, N., Qi, W., Schoenbaechler, K., Kraettli, H., Hatt, J. M., … Kubacki, J. (2021). Metagenomic analysis of fecal and tissue samples from 18 endemic bat species in Switzerland revealed a diverse virus composition including potentially zoonotic viruses. PLoS ONE, 16(6 June 2021).
- Harrach, B. (2014). Adenoviruses: General Features☆. In Reference Module in Biomedical Sciences. [CrossRef]
- Harvey, W. , Hutto, E. H., Chilton, J. A., Chamanza, R., Mysore, J. V., Parry, N. M. A., … Bradley, A. E. (2023). Infectious diseases of non-human primates. In Spontaneous Pathology of the Laboratory Non-human Primate. [CrossRef]
- Hou, J., Xu, J., Wang, B., Zhang, H., Yin, B., Li, G., … Wang, L. (2023). First identification of canine adenovirus 1 in mink and bioinformatics analysis of its 100 K protein. Frontiers in Microbiology, 14. [CrossRef] [PubMed]
- Iglesias-Caballero, M., Juste, J., Vázquez-Morón, S., Falcon, A., Aznar-Lopez, C., Ibáñez, C., … Casas, I. (2018). New adenovirus groups in western palaearctic bats. Viruses, 10(8). [CrossRef] [PubMed]
- Jánoska, M., Vidovszky, M., Molnár, V., Liptovszky, M., Harrach, B., & Benko, M. (2011). Novel adenoviruses and herpesviruses detected in bats. Veterinary Journal, 189(1). [CrossRef]
- Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., Von Haeseler, A., & Jermiin, L. S. (2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods, 14(6). [CrossRef] [PubMed]
- Katayama, M., Murakami, S., Matsugo, H., Kamiki, H., Fujii, M., Takenaka-Uema, A., & Horimoto, T. (2022). Complete genome sequence of a novel bat mastadenovirus C strain isolated from Rhinolophus cornutus in Japan. Archives of Virology, 167(3). [CrossRef]
- Katoh, K., Rozewicki, J., & Yamada, K. D. (2018). MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20(4). [CrossRef]
- 21. Kitchener, D.J., Caputi, N., Jones, B. (1986). Revision of Australo-Papuan Pipistrellus and Falsistrellus (Microchiroptera: Vespertilionidae). Records of the Western Australian Museum, 12(4), 435–495.
- Kleinberger, T. (2015). Mechanisms of cancer cell killing by the adenovirus E4orf4 protein. Viruses, Vol. 7. [CrossRef]
- Kohl, C., Nitsche, A., & Kurth, A. (2021). Update on potentially zoonotic viruses of european bats. Vaccines, Vol. 9. [CrossRef]
- Kohl, C., Vidovszky, M. Z., Mühldorfer, K., Dabrowski, P. W., Radonić, A., Nitsche, A., … Harrach, B. (2012). Genome Analysis of Bat Adenovirus 2: Indications of Interspecies Transmission. Journal of Virology, 86(3). [CrossRef] [PubMed]
- Kosoltanapiwat, N., Tongshoob, J., Ampawong, S., Reamtong, O., Prasittichai, L., Yindee, M., … Boonnak, K. (2022). Simian adenoviruses: Molecular and serological survey in monkeys and humans in Thailand. One Health, 15. [CrossRef] [PubMed]
- Lack, J. B., & Van Den Bussche, R. A. (2010). Identifying the confounding factors in resolving phylogenetic relationships in Vespertilionidae. Journal of Mammalogy, 91(6). [CrossRef]
- Lagerveld, S., Poerink, B. J., & Geelhoed, S. C. V. (2021). Offshore occurrence of a migratory bat, pipistrellus nathusii, depends on seasonality and weather conditions. Animals, 11(12). [CrossRef] [PubMed]
- Letunic, I., & Bork, P. (2021). Interactive tree of life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Research, 49(W1). [CrossRef] [PubMed]
- Matsugo, H., Kitamura-Kobayashi, T., Kamiki, H., Ishida, H., Sekine, W., Takenaka-Uema, A., … Horimoto, T. (2021). A potential bat adenovirus-based oncolytic virus targeting canine cancers. Scientific Reports, 11(1). [CrossRef] [PubMed]
- Medkour, H., Amona, I., Akiana, J., Davoust, B., Bitam, I., Levasseur, A., … Mediannikov, O. (2020). Adenovirus infections in African humans and wild non-human primates: Great diversity and cross-species transmission. Viruses, 12(6). [CrossRef] [PubMed]
- Moratelli R, Burgin C, Cláudio V, Novaes R, López-Baucells A, Haslauer R (2019) Family Vespertilionidae (Vesper Bats): Bats. Lynx Edicions, Barcelona, pp 716-981.
- Minh, B. Q., Nguyen, M. A. T., & Von Haeseler, A. (2013). Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution, 30(5). [CrossRef]
- Nguyen, L. T., Schmidt, H. A., Von Haeseler, A., & Minh, B. Q. (2015). IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution, 32(1). [CrossRef]
- Nishimura, Y., Yoshida, T., Kuronishi, M., Uehara, H., Ogata, H., & Goto, S. (2017). ViPTree: The viral proteomic tree server. Bioinformatics, 33(15). [CrossRef] [PubMed]
- Ntumvi, N. F., Diffo, J. L. D., Tamoufe, U., Ndze, V. N., Takuo, J. M., Mouiche, M. M. M., … Lange, C. E. (2021). Evaluation of bat adenoviruses suggests co-evolution and host roosting behaviour as drivers for diversity. Microbial Genomics, 7(4). [CrossRef]
- Ogawa, H., Kajihara, M., Nao, N., Shigeno, A., Fujikura, D., Hang’Ombe, B. M., … Takada, A. (2017). Characterization of a novel bat adenovirus isolated from straw-colored fruit bat (Eidolon helvum). Viruses, 9(12). [CrossRef]
- Oleaga, A., Balseiro, A., Espí, A., & Royo, L. J. (2022). Wolf (Canis lupus) as canine adenovirus type 1 (CAdV-1) sentinel for the endangered cantabrian brown bear (Ursus arctos arctos). Transboundary and Emerging Diseases, 69(2). [CrossRef] [PubMed]
- Prada, D., Boyd, V., Baker, M. L., O’Dea, M., & Jackson, B. (2019). Viral diversity of microbats within the south west botanical province of western Australia. Viruses, 11(12). [CrossRef]
- Prjibelski, A., Antipov, D., Meleshko, D., Lapidus, A., & Korobeynikov, A. (2020). Using SPAdes De Novo Assembler. Current Protocols in Bioinformatics, 70(1). [CrossRef] [PubMed]
- Roy, S., Vandenberghe, L. H., Kryazhimskiy, S., Grant, R., Calcedo, R., Yuan, X., … Wilson, J. M. (2009). Isolation and characterization of adenoviruses persistently shed from the gastrointestinal tract of non-human primates. PLoS Pathogens, 5(7). [CrossRef]
- Sallard, E., Zhang, W., Aydin, M., Schröer, K., & Ehrhardt, A. (2023). The Adenovirus Vector Platform: Novel Insights into Rational Vector Design and Lessons Learned from the COVID-19 Vaccine. Viruses, Vol. 15. [CrossRef]
- Sanz MA, Almela EG, García-Moreno M, Marina AI, Carrasco L. 2019. A viral RNA motif involved in signaling the initiation of translation on non-AUG codons. RNA 25: 431–452. doi:10.1261/rna.068858.118. [CrossRef]
- Seemann, T. (2014). Prokka: Rapid prokaryotic genome annotation. Bioinformatics, 30(14). [CrossRef]
- Simmons, N.B. (2005). Order Chiroptera. In Wilson, D.E. & Reeder, D.M. (eds.) Mammal Species of the World, (Third edition). The Johns Hopkins University Press, Baltimore.
- Sonntag, M., Mühldorfer, K., Speck, S., Wibbelt, G., & Kurth, A. (2009). New adenovirus in bats, Germany. Emerging Infectious Diseases, 15(12). [CrossRef] [PubMed]
- Speranskaya, A. S., Artiushin, I. V., Samoilov, A. E., Korneenko, E. V., Khabudaev, K. V., Ilina, E. N., … Daszak, P. (2023). Identification and Genetic Characterization of MERS-Related Coronavirus Isolated from Nathusius’ Pipistrelle (Pipistrellus nathusii) near Zvenigorod (Moscow Region, Russia). International Journal of Environmental Research and Public Health, 20(4). [CrossRef] [PubMed]
- Tcherepanov, V., Ehlers, A., & Upton, C. (2006). Genome annotation transfer utility (GATU): Rapid annotation of viral genomes using a closely related reference genome. BMC Genomics, 7. [CrossRef] [PubMed]
- Vidovszky, M. Z., Kohl, C., Boldogh, S., Görföl, T., Wibbelt, G., Kurth, A., & Harrach, B. (2015). Random sampling of the Central European bat fauna reveals the existence of numerous hitherto unknown adenoviruses. Acta Veterinaria Hungarica, 63(4). [CrossRef] [PubMed]
- viralzone.expasy.org Mastadenovirus (taxid:10509). (n.d.). Retrieved January 25, 2024, from Mastadenovirus (taxid:10509) website: https://viralzone.expasy.org/183?outline=all_by_species.
- Wang, F., Zhu, R., Qian, Y., Sun, Y., Chen, D., Wang, F., … Zhao, L. (2023). The changed endemic pattern of human adenovirus from species B to C among pediatric patients under the pressure of non-pharmaceutical interventions against COVID-19 in Beijing, China. Virology Journal, 20(1). [CrossRef]
- Zhukova, S. S., Solovyeva, E. N., Artyushin, I. V., & Kruskop, S. V. (2022). Paraphyly of the Pipistrelles (Pipistrellus; Vespertilionidae) is Confirmed by the Analysis of the Nuclear Gene Markers. Doklady Biochemistry and Biophysics, 507(1). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).