Submitted:
08 May 2024
Posted:
09 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
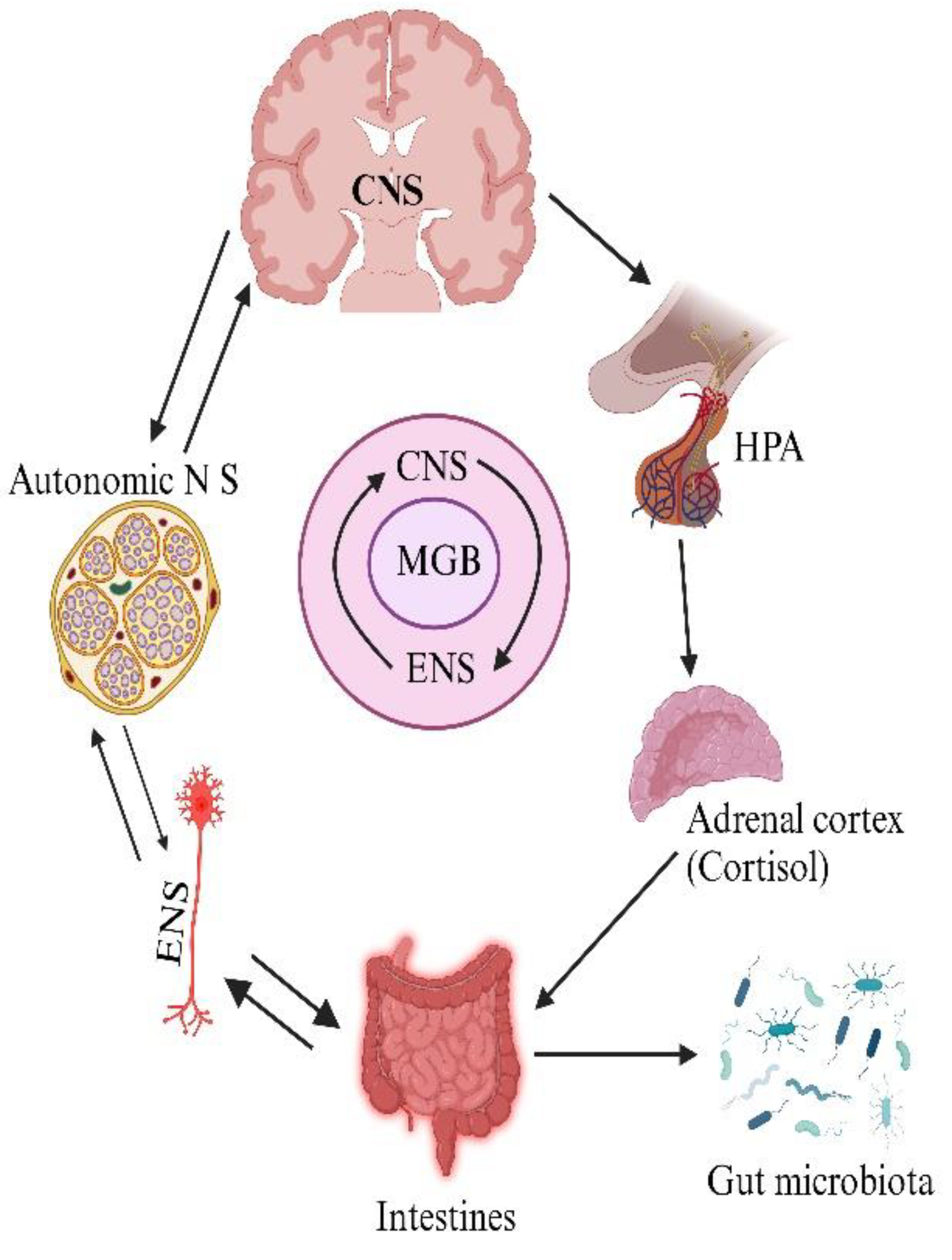
2. Gut-Brain axis Dysbiosis in AD development
2.1. Bridge between Neuroinflammation and Microbiota Dysbiosis
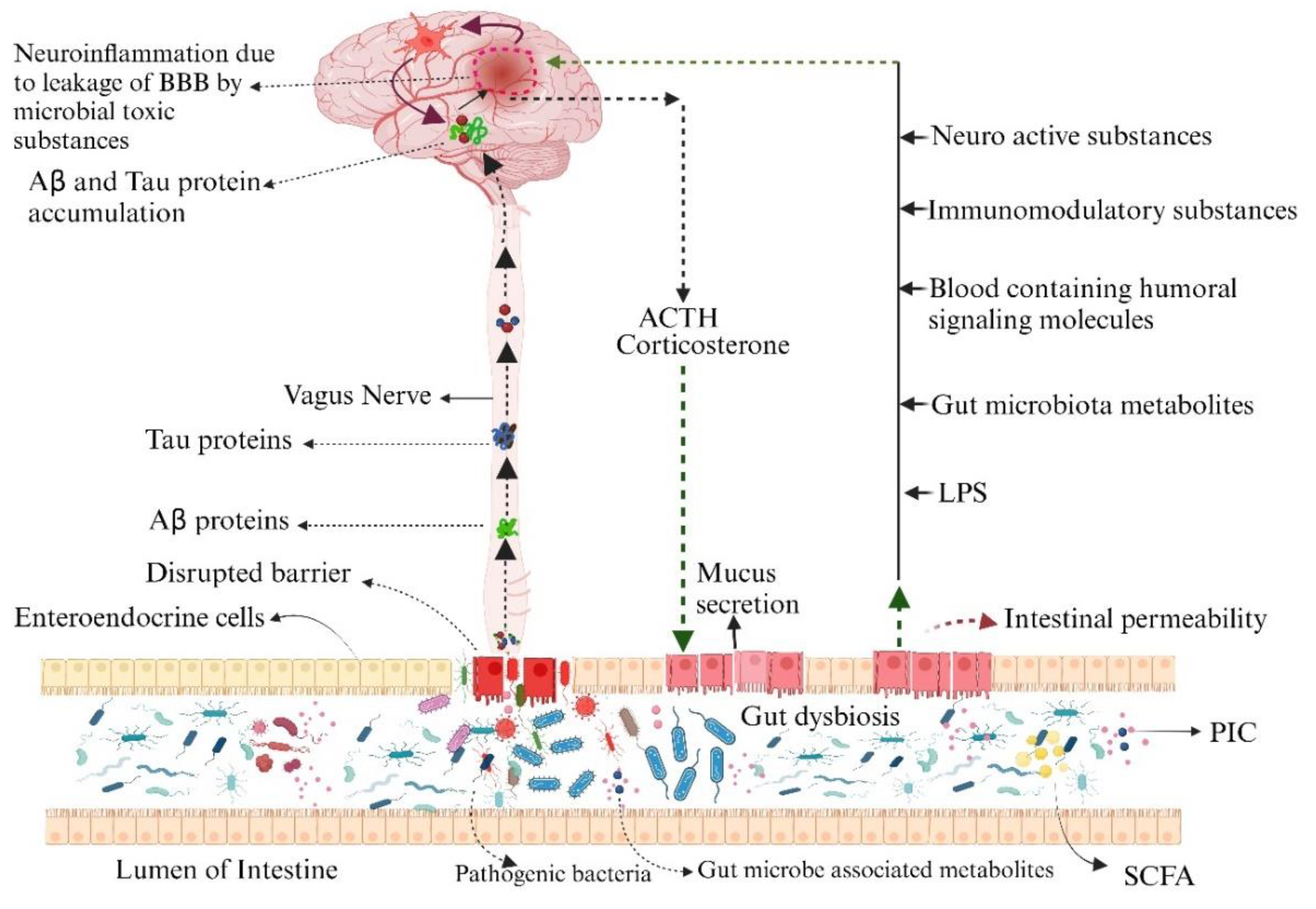
2.2. Blood Brain Axis Alteration by GM
3. Mechanism of Brain and Intestinal Blood Barriers Interaction in Alzheimer's Disease
3.1. The Cerebral Blood Barrier's Involvement in the Brain Microbiota Intestines Axis in Alzheimer's Disease
3.2. Intestinal Disorders
4. Gut Microbiota Models of Animals in Alzheimer’s Disease
5. Biomarkers: A Novel Diagnostic Approach for AD
6. Targeted Therapy for AD: Amelioration of Microbiota
6.1. Probiotics
6.2. Prebiotics
6.3. Fecal Microbiota Transplantation (FMT)
7. Conclusions
Conflicts of Interest
References
- Scheff, S.W.; Price, D.A. Alzheimer’s disease-related alterations in synaptic density: Neocortex and hippocampus. J. Alzheimer’s Dis. 2006, 9, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Beopoulos, A.; Gea, M.; Fasano, A.; Iris, F. Autonomic Nervous System Neuroanatomical Alterations Could Provoke and Maintain Gastrointestinal Dysbiosis in Autism Spectrum Disorder (ASD): A Novel Microbiome-Host Interaction Mechanistic Hypothesis. Nutrients 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9. Available from: https://www.frontiersin.org/articles/10.3389/fpsyt.2018.00044. [CrossRef] [PubMed]
- Thakur, A.K.; Shakya, A.; Husain, G.M.; Emerald, M.; Kumar, V. Gut-Microbiota and Mental Health: Current and Future Perspectives. J. Pharmacol. Clin. Toxicol. 2014, 2, 1016. [Google Scholar]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimers. Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Singh, S.K.; Churruca, M.; Maccioni, R.B. Alzheimer’s Disease and Tau Self-Assembly: In the Search of the Missing Link. Int. J. Mol. Sci. 2022, 23. Available from: https://www.mdpi.com/1422-0067/23/8/. [CrossRef]
- Lloret, A.; Fuchsberger, T.; Giraldo, E.; Viña, J. Molecular mechanisms linking amyloid beta toxicity and Tau hyperphosphorylation in Alzheimer’s Disease. Free Radic. Biol. Med. 2015, 83. [Google Scholar] [CrossRef]
- Rajmohan, R.; Reddy, P.H. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J. Alzheimers. Dis. 2017, 57, 975–999. [Google Scholar] [CrossRef]
- Zlokovic, B. V The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Ballan, R.; Battistini, C.; Xavier-Santos, D.; Saad, S.M.I. Interactions of probiotics and prebiotics with the gut microbiota. Prog. Mol. Biol. Transl. Sci. 2020, 171, 265–300. [Google Scholar] [PubMed]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2020, 13. [Google Scholar] [CrossRef]
- Salminen, S.; Bouley, C.; Boutron-Ruault, M.C.; Cummings, J.H.; Franck, A.; Gibson, G.R.; Isolauri, E.; Moreau, M.C.; Roberfroid, M.; Rowland, I. Functional food science and gastrointestinal physiology and function. Br. J. Nutr. 1998, 80 Suppl 1, S147–71. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.S.B.; Raes, J.; Bork, P. The Human Gut Microbiome: From Association to Modulation. Cell 2018, 172, 1198–1215. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Nandwana, V.; Debbarma, S. Fecal Microbiota Transplantation: A Microbiome Modulation Technique for Alzheimer’s Disease. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Alzheimer’s disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharmacol. Res. 2018, 129, 329–336. [Google Scholar] [CrossRef]
- Luca, M.; Di Mauro, M.; Di Mauro, M.; Luca, A. Gut Microbiota in Alzheimer’s Disease, Depression, and Type 2 Diabetes Mellitus: The Role of Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 4730539. [Google Scholar] [CrossRef]
- Zhu, X.; Han, Y.; Du, J.; Liu, R.; Jin, K.; Yi, W. Microbiota-gut-brain axis and the central nervous system. Oncotarget 2017, 8, 53829–53838. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Durgan, D.J.; Lee, J.; McCullough, L.D.; Bryan, R.M.J. Examining the Role of the Microbiota-Gut-Brain Axis in Stroke. Stroke 2019, 50, 2270–2277. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Loreth, D.; Pöttker, B.; Hefti, K.; Innos, J.; Schwald, K.; Hengstler, H.; Menzel, L.; Sommer, C.J.; Radyushkin, K.; et al. Neuronal Growth and Behavioral Alterations in Mice Deficient for the Psychiatric Disease-Associated Negr1 Gene. Front. Mol. Neurosci. 2018, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Webster, S.J.; Bachstetter, A.D.; Nelson, P.T.; Schmitt, F.A.; Van Eldik, L.J. Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front. Genet. 2014, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-P.; Arai, T.; Miklossy, J.; McGeer, P.L. Abeta and tau form soluble complexes that may promote self aggregation of both into the insoluble forms observed in Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Repáraz, J.; Kasper, L.H. The Microbiome and Neurologic Disease: Past and Future of a 2-Way Interaction. Neurother. J. Am. Soc. Exp. Neurother. 2018, 15, 1–4. [Google Scholar] [CrossRef]
- Colpitts, S.L.; Kasper, E.J.; Keever, A.; Liljenberg, C.; Kirby, T.; Magori, K.; Kasper, L.H.; Ochoa-Repáraz, J. A bidirectional association between the gut microbiota and CNS disease in a biphasic murine model of multiple sclerosis. Gut Microbes 2017, 8, 561–573. [Google Scholar] [CrossRef]
- Mehrabadi, S.; Sadr, S.S. Assessment of Probiotics Mixture on Memory Function, Inflammation Markers, and Oxidative Stress in an Alzheimer’s Disease Model of Rats. Iran. Biomed. J. 2020, 24, 220–228. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, C.-H.; Lane, H.-Y. d-glutamate and Gut Microbiota in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Gareau, M.G.; Wine, E.; Rodrigues, D.M.; Cho, J.H.; Whary, M.T.; Philpott, D.J.; MacQueen, G.; Sherman, P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011, 60, 307–317. [Google Scholar] [CrossRef]
- Singhrao, S.K.; Harding, A.; Poole, S.; Kesavalu, L.; Crean, S. Porphyromonas gingivalis Periodontal Infection and Its Putative Links with Alzheimer’s Disease. Mediators Inflamm. 2015, 2015, 137357. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurother. J. Am. Soc. Exp. Neurother. 2018, 15, 5–22. [Google Scholar] [CrossRef]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Jameson, K.G.; Hsiao, E.Y. Linking the Gut Microbiota to a Brain Neurotransmitter. Trends Neurosci. 2018, 41, 413–414. [Google Scholar] [CrossRef]
- Franceschi, F.; Ojetti, V.; Candelli, M.; Covino, M.; Cardone, S.; Potenza, A.; Simeoni, B.; Gabrielli, M.; Sabia, L.; Gasbarrini, G.; et al. Microbes and Alzheimer’ disease: lessons from H. pylori and GUT microbiota. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 426–430. [Google Scholar] [CrossRef]
- Naveed, M.; Mubeen, S.; Khan, A.; Ibrahim, S.; Meer, B. Plasma Biomarkers: Potent Screeners of Alzheimer’s Disease. Am. J. Alzheimers. Dis. Other Demen. 2019, 34, 290–301. [Google Scholar] [CrossRef]
- Sun, Y.; Sommerville, N.R.; Liu, J.Y.H.; Ngan, M.P.; Poon, D.; Ponomarev, E.D.; Lu, Z.; Kung, J.S.C.; Rudd, J.A. Intra-gastrointestinal amyloid-β1-42 oligomers perturb enteric function and induce Alzheimer’s disease pathology. J. Physiol. 2020, 598, 4209–4223. [Google Scholar] [CrossRef]
- Cerovic, M.; Forloni, G.; Balducci, C. Neuroinflammation and the Gut Microbiota: Possible Alternative Therapeutic Targets to Counteract Alzheimer’s Disease? Front. Aging Neurosci. 2019, 11, 284. [Google Scholar] [CrossRef]
- Deng, H.; Yang, S.; Zhang, Y.; Qian, K.; Zhang, Z.; Liu, Y.; Wang, Y.; Bai, Y.; Fan, H.; Zhao, X.; et al. Bacteroides fragilis Prevents Clostridium difficile Infection in a Mouse Model by Restoring Gut Barrier and Microbiome Regulation. Front. Microbiol. 2018, 9, 2976. [Google Scholar] [CrossRef]
- Tanida, M.; Yamano, T.; Maeda, K.; Okumura, N.; Fukushima, Y.; Nagai, K. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci. Lett. 2005, 389, 109–114. [Google Scholar] [CrossRef]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef]
- Sigal, M.; Meyer, T.F. Coevolution between the Human Microbiota and the Epithelial Immune System. Dig. Dis. 2016, 34, 190–193. [Google Scholar] [CrossRef]
- Schluter, J.; Peled, J.U.; Taylor, B.P.; Markey, K.A.; Smith, M.; Taur, Y.; Niehus, R.; Staffas, A.; Dai, A.; Fontana, E.; et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 2020, 588, 303–307. [Google Scholar] [CrossRef]
- Khosravi, A.; Yáñez, A.; Price, J.G.; Chow, A.; Merad, M.; Goodridge, H.S.; Mazmanian, S.K. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 2014, 15, 374–381. [Google Scholar] [CrossRef]
- Kierdorf, K.; Prinz, M. Factors regulating microglia activation. Front. Cell. Neurosci. 2013, 7. Available from: https://www.frontiersin.org/articles/. [CrossRef]
- Jouanne, M.; Rault, S.; Voisin-Chiret, A.-S. Tau protein aggregation in Alzheimer’s disease: An attractive target for the development of novel therapeutic agents. Eur. J. Med. Chem. 2017, 139, 153–167. [Google Scholar] [CrossRef]
- Köhler, C.A.; Maes, M.; Slyepchenko, A.; Berk, M.; Solmi, M.; Lanctôt, K.L.; Carvalho, A.F. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Curr. Pharm. Des. 2016, 22, 6152–6166. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Eisenberg, D.S.; Crowther, R.A. Propagation of Tau Aggregates and Neurodegeneration. Annu. Rev. Neurosci. 2017, 40, 189–210. Available from: https://www.frontiersin.org/articles/. [CrossRef]
- Avila, J.; Jiménez, J.S.; Sayas, C.L.; Bolós, M.; Zabala, J.C.; Rivas, G.; Hernández, F. Tau Structures. Front. Aging Neurosci. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Alonso, A. del C.; Grundke-Iqbal, I. Cytosolic abnormally hyperphosphorylated tau but not paired helical filaments sequester normal MAPs and inhibit microtubule assembly. J. Alzheimers. Dis. 2008, 14, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Lott, I.T.; Dierssen, M. Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet. Neurol. 2010, 9, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.-L.; et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Takeda, S.; Uchio-Yamada, K.; Ueda, H.; Fujisawa, T.; Rakugi, H.; Morishita, R. Role of insulin signaling in the interaction between Alzheimer disease and diabetes mellitus: a missing link to therapeutic potential. Curr. Aging Sci. 2011, 4, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Sato, N.; Rakugi, H.; Morishita, R. Molecular mechanisms linking diabetes mellitus and Alzheimer disease: beta-amyloid peptide, insulin signaling, and neuronal function. Mol. Biosyst. 2011, 7, 1822–1827. [Google Scholar] [CrossRef]
- Rayasam, G.V.; Tulasi, V.K.; Sodhi, R.; Davis, J.A.; Ray, A. Glycogen synthase kinase 3: more than a namesake. Br. J. Pharmacol. 2009, 156, 885–898. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Q. Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst. Biol. 2016, 10, 63. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: the role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Morales, I.; Guzmán-Martínez, L.; Cerda-Troncoso, C.; Farías, G.A.; Maccioni, R.B. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 2014, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.; Jiménez, J.S.; Sayas, C.L.; Bolós, M.; Zabala, J.C.; Rivas, G.; Hernández, F. Tau Structures. Front. Aging Neurosci. 2016; 8. Available from: https://www.frontiersin.org/articles/10.3389/fnagi.2016.00262.
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Couch, Y.; Stratford, M.; Bannerman, D.M.; Tzortzis, G.; Anthony, D.C.; Burnet, P.W.J. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-β levels in male mice. Brain. Behav. Immun. 2016, 52, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Clinton, L.K.; Blurton-Jones, M.; Myczek, K.; Trojanowski, J.Q.; LaFerla, F.M. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 7281–7289. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lukiw, W.J. Microbiome-generated amyloid and potential impact on amyloidogenesis in Alzheimer’s disease (AD). J. Nat. Sci. 2015, 1. [Google Scholar]
- Dinan, T.G.; Cryan, J.F. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef]
- Mary, A.; Dayan, J.; Leone, G.; Postel, C.; Fraisse, F.; Malle, C.; Vallée, T.; Klein-Peschanski, C.; Viader, F.; De la Sayette, V. Resilience after trauma: The role of memory suppression. Science (80-. ). 2020, 367, eaay8477. [Google Scholar] [CrossRef]
- Ubeda, C.; Djukovic, A.; Isaac, S. Roles of the intestinal microbiota in pathogen protection. Clin. Transl. Immunol. 2017, 6, e128. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.Y.; Cisalpino, D.; Varadarajan, S.; Hellman, J.; Warren, H.S.; Cascalho, M.; Inohara, N.; Núñez, G. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity 2016, 44, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Microbiota-brain-gut axis and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Minter, M.R.; Zhang, C.; Leone, V.; Ringus, D.L.; Zhang, X.; Oyler-Castrillo, P.; Musch, M.W.; Liao, F.; Ward, J.F.; Holtzman, D.M. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Main, B.S.; Minter, M.R. Microbial immuno-communication in neurodegenerative diseases. Front. Neurosci. 2017, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E. Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication. Brain. Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Song, C.; Wang, D.; Cui, S.; Ren, T.; Cao, Z.; Liu, Q.; Chen, Z.; Chen, X.; Zhou, Y. MicroRNA-194 inhibition improves dietary-induced non-alcoholic fatty liver disease in mice through targeting on FXR. Biochim. Biophys. Acta (BBA)-Molecular Basis Dis. 2017, 1863, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-Brain Barrier Breakdown in the Aging Human Hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef]
- Welling, M.M.; Nabuurs, R.J.A.; van der Weerd, L. Potential role of antimicrobial peptides in the early onset of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 51–57. [Google Scholar] [CrossRef]
- Halliday, M.R.; Rege, S. V; Ma, Q.; Zhao, Z.; Miller, C.A.; Winkler, E.A.; Zlokovic, B. V Accelerated pericyte degeneration and blood–brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2016, 36, 216–227. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.-M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N. Stress and gut microbiota: does postnatal microbial colonization program the hypothalamic–pituitary–adrenal system for stress response. Int Congr Ser 2006, 1287, 350–354. [Google Scholar] [CrossRef]
- Bailey, M.T.; Lubach, G.R.; Coe, C.L. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 414–421. [Google Scholar] [PubMed]
- Munyaka, P.M.; Eissa, N.; Bernstein, C.N.; Khafipour, E.; Ghia, J.-E. Antepartum antibiotic treatment increases offspring susceptibility to experimental colitis: a role of the gut microbiota. PLoS One 2015, 10, e0142536. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K. Pericytes regulate the blood–brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Winkler, E.A.; Sagare, A.P.; Singh, I.; LaRue, B.; Deane, R.; Zlokovic, B. V Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010, 68, 409–427. [Google Scholar] [CrossRef]
- Sengillo, J.D.; Winkler, E.A.; Walker, C.T.; Sullivan, J.S.; Johnson, M.; Zlokovic, B. V Deficiency in Mural Vascular Cells Coincides with Blood–Brain Barrier Disruption in A lzheimer’s Disease. Brain Pathol. 2013, 23, 303–310. [Google Scholar] [CrossRef]
- Blennow, K.; Wallin, A.; Fredman, P.; Karlsson, I.; Gottfries, C.G.; Svennerholm, L. Blood-brain barrier disturbance in patients with Alzheimer’s disease is related to vascular factors. Acta Neurol. Scand. 1990, 81, 323–326. [Google Scholar] [CrossRef]
- Fiala, M.; Liu, Q.N.; Sayre, J.; Pop, V.; Brahmandam, V.; Graves, M.C.; Vinters, H. V Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood–brain barrier. Eur. J. Clin. Invest. 2002, 32, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Alata, W.; Ye, Y.; St-Amour, I.; Vandal, M.; Calon, F. Human apolipoprotein e ε4 expression impairs cerebral vascularization and blood—brain barrier function in mice. J. Cereb. Blood Flow Metab. 2015, 35, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Verghese, P.B.; Castellano, J.M.; Holtzman, D.M. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011, 10, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Nishitsuji, K.; Hosono, T.; Nakamura, T.; Bu, G.; Michikawa, M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J. Biol. Chem. 2011, 286, 17536–17542. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Halliday, M.R.; Pomara, N.; Sagare, A.P.; Mack, W.J.; Frangione, B.; Zlokovic, B. V Relationship between cyclophilin a levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood-brain barrier breakdown. JAMA Neurol. 2013, 70, 1198–1200. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. Available from: https://www.sciencedirect.com/science/article/pii/S0092867406001929. [CrossRef] [PubMed]
- Sánchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal Inflammation and Mucosal Barrier Function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Tejpar, Q.Z.; Soo, I.; Madsen, K.L.; Fedorak, R.N. The role of antibiotic and probiotic therapies in current and future management of inflammatory bowel disease. Curr. Gastroenterol. Rep. 2006, 8, 486–498. [Google Scholar] [CrossRef]
- De Jager, P.L.; Franchimont, D.; Waliszewska, A.; Bitton, A.; Cohen, A.; Langelier, D.; Belaiche, J.; Vermeire, S.; Farwell, L.; Goris, A. The role of the Toll receptor pathway in susceptibility to inflammatory bowel diseases. Genes Immun. 2007, 8, 387–397. [Google Scholar] [CrossRef]
- Franchimont, D.; Vermeire, S.; El Housni, H.; Pierik, M.; Van Steen, K.; Gustot, T.; Quertinmont, E.; Abramowicz, M.; Van Gossum, A.; Devière, J. Deficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn’s disease and ulcerative colitis. Gut 2004, 53, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Hampe, J.; Franke, A.; Rosenstiel, P.; Till, A.; Teuber, M.; Huse, K.; Albrecht, M.; Mayr, G.; De La Vega, F.M.; Briggs, J. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 2007, 39, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.-G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef]
- Duchmann, R.; Kaiser, I.; Hermann, E.; Mayet, W.; Ewe, K.; BÜSCHENFELDE, K.M.Z.U.M. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin. Exp. Immunol. 1995, 102, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.D.; Mileti, E.; Matteoli, G.; Chieppa, M.; Rescigno, M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009, 2, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, M.; Chieppa, M.; Salucci, V.; Avogadri, F.; Sonzogni, A.; Sampietro, G.M.; Nespoli, A.; Viale, G.; Allavena, P.; Rescigno, M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 2005, 6, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.D.; Spadoni, I.; Mileti, E.; Matteoli, G.; Sonzogni, A.; Sampietro, G.M.; Foschi, D.; Caprioli, F.; Viale, G.; Rescigno, M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 2009, 58, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Smythies, L.E.; Sellers, M.; Clements, R.H.; Mosteller-Barnum, M.; Meng, G.; Benjamin, W.H.; Orenstein, J.M.; Smith, P.D. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 2005, 115, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J. Gastroenterol. 2007, 42, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Wallon, C.; Yang, P.-C.; Keita, Å. V; Ericson, A.-C.; McKay, D.M.; Sherman, P.M.; Perdue, M.H.; Söderholm, J.D. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut 2008, 57, 50–58. [Google Scholar] [CrossRef]
- Lee, K.J.; Kim, Y.B.; Kim, J.H.; Kwon, H.C.; Kim, D.K.; Cho, S.W. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J. Gastroenterol. Hepatol. 2008, 23, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Nguyen, T.T.T.; Fujimura, Y.; Kameya, N.; Nakamura, S.; Arakawa, K.; Morita, H. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer’s disease. Biosci. Biotechnol. Biochem. 2019, 83, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.L. Staddon. The cell biology of the blood-brain barrier. Ann Rev Neurosci 1999, 22, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Bösel, J.; Schiller, P.; Hook, Y.; Andes, M.; Neumann, J.-O.; Poli, S.; Amiri, H.; Schönenberger, S.; Peng, Z.; Unterberg, A. Stroke-related early tracheostomy versus prolonged orotracheal intubation in neurocritical care trial (SETPOINT) a randomized pilot trial. Stroke 2013, 44, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Clearing the brain’s amyloid cobwebs. Neuron 2001, 32, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B. V Cerebrovascular disease in Alzheimer’s disease. Trends Neurosci. 2005, 4, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.M.; Kócsi, Z.; Stone, J. Pericapillary haem-rich deposits: evidence for microhaemorrhages in aging human cerebral cortex. J. Cereb. Blood Flow Metab. 2005, 25, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; McLarnon, J.G. A leaky blood–brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J. Cell. Mol. Med. 2009, 13, 2911–2925. [Google Scholar] [CrossRef] [PubMed]
- Raven, E.P.; Lu, P.H.; Tishler, T.A.; Heydari, P.; Bartzokis, G. Increased iron levels and decreased tissue integrity in hippocampus of Alzheimer’s disease detected in vivo with magnetic resonance imaging. J. Alzheimer’s Dis. 2013, 37, 127–136. [Google Scholar] [CrossRef]
- Singh, N.; Thangaraju, M.; Prasad, P.D.; Martin, P.M.; Lambert, N.A.; Boettger, T.; Offermanns, S.; Ganapathy, V. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J. Biol. Chem. 2010, 285, 27601–27608. [Google Scholar] [CrossRef]
- Wu, J.; Liu, C.; Zhang, L.; Qu, C.; Sui, X.; Zhu, H.; Huang, L.; Xu, Y.; Han, Y.; Qin, C. Histone deacetylase-2 is involved in stress-induced cognitive impairment via histone deacetylation and PI3K/AKT signaling pathway modification. Mol. Med. Rep. 2017, 16, 1846–1854. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cong, L.; Lukiw, W.J. Lipopolysaccharide (LPS) Accumulates in Neocortical Neurons of Alzheimer’s Disease (AD) Brain and Impairs Transcription in Human Neuronal-Glial Primary Co-cultures. Front. Aging Neurosci. 2017, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.; Means, T.K.; Heine, H.; Yoshimura, A.; Kusumoto, S.; Fukase, K.; Fenton, M.J.; Oikawa, M.; Qureshi, N.; Monks, B. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Invest. 2000, 105, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ouanes, S.; Clark, C.; Richiardi, J.; Maréchal, B.; Lewczuk, P.; Kornhuber, J.; Kirschbaum, C.; Popp, J. Cerebrospinal Fluid Cortisol and Dehydroepiandrosterone Sulfate, Alzheimer’s Disease Pathology, and Cognitive Decline. Front. Aging Neurosci. 2022, 14, 892754. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Yang, K.; He, B.; Du, W.; Cai, Y.; Han, Y. Combination of gut microbiota and plasma amyloid-β as a potential index for identifying preclinical Alzheimer’s disease: a cross-sectional analysis from the SILCODE study. Alzheimers. Res. Ther. 2022, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.H.; Hendriksen, H.M.A.; de Leeuw, F.A.; Doorduijn, A.S.; van Leeuwenstijn, M.; Teunissen, C.E.; Barkhof, F.; Scheltens, P.; Kraaij, R.; van Duijn, C.M.; et al. Gut Microbiota Composition Is Related to AD Pathology. Front. Immunol. 2021, 12, 794519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Liu, W.; Wang, T.; Wang, L.; Hao, L.; Ju, M.; Xiao, R. Diet quality, gut microbiota, and microRNAs associated with mild cognitive impairment in middle-aged and elderly Chinese population. Am. J. Clin. Nutr. 2021, 114, 429–440. [Google Scholar] [CrossRef]
- Ling, Z.; Zhu, M.; Yan, X.; Cheng, Y.; Shao, L.; Liu, X.; Jiang, R.; Wu, S. Structural and Functional Dysbiosis of Fecal Microbiota in Chinese Patients With Alzheimer’s Disease. Front. cell Dev. Biol. 2020, 8, 634069. [Google Scholar] [CrossRef]
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host-Microbe Interplay. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Marizzoni, M.; Cattaneo, A.; Mirabelli, P.; Festari, C.; Lopizzo, N.; Nicolosi, V.; Mombelli, E.; Mazzelli, M.; Luongo, D.; Naviglio, D.; et al. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J. Alzheimers. Dis. 2020, 78, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Neth, B.J.; Wang, S.; Mishra, S.P.; Craft, S.; Yadav, H. Gut mycobiome and its interaction with diet, gut bacteria and alzheimer’s disease markers in subjects with mild cognitive impairment: A pilot study. EBioMedicine 2020, 59, 102950. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.; Engertsberger, L.; Komarova, I.; Feldbacher, N.; Leber, B.; Pichler, G.; Fink, N.; Scarpatetti, M.; Schippinger, W.; Schmidt, R.; et al. Dysbiosis, gut barrier dysfunction and inflammation in dementia: a pilot study. BMC Geriatr. 2020, 20, 248. [Google Scholar] [CrossRef] [PubMed]
- Plagman, A.; Hoscheidt, S.; McLimans, K.E.; Klinedinst, B.; Pappas, C.; Anantharam, V.; Kanthasamy, A.; Willette, A.A. Cholecystokinin and Alzheimer’s disease: a biomarker of metabolic function, neural integrity, and cognitive performance. Neurobiol. Aging 2019, 76, 201–207. [Google Scholar] [CrossRef]
- Nho, K.; Kueider-Paisley, A.; MahmoudianDehkordi, S.; Arnold, M.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; Kastenmüller, G.; et al. Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: Relationship to neuroimaging and CSF biomarkers. Alzheimers. Dement. 2019, 15, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, Y.; Ma, J.; Huang, P.; Du, J.; Cao, L.; Wang, Y.; Xiao, Q.; Tang, H.; Chen, S. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers. Dement. 2019, 15, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain. Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers. Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Yang, J.-J.; Zhao, D.-M.; Chen, B.; Zhang, G.-Q.; Chen, S.; Cao, R.-F.; Yu, H.; Zhao, C.-Y.; et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol. Res. 2020, 157, 104784. [Google Scholar] [CrossRef]
- Berding, K.; Carbia, C.; Cryan, J.F. Going with the grain: Fiber, cognition, and the microbiota-gut-brain-axis. Exp. Biol. Med. 2021, 246, 796–811. [Google Scholar] [CrossRef]
- Yusufov, M.; Weyandt, L.L.; Piryatinsky, I. Alzheimer’s disease and diet: a systematic review. Int. J. Neurosci. 2017, 127, 161–175. Available from: http://gut.bmj.com/content/69/7/1218.abstract. [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218. [Google Scholar] [CrossRef]
- Hoscheidt, S.; Sanderlin, A.H.; Baker, L.D.; Jung, Y.; Lockhart, S.; Kellar, D.; Whitlow, C.T.; Hanson, A.J.; Friedman, S.; Register, T.; et al. Mediterranean and Western diet effects on Alzheimer’s disease biomarkers, cerebral perfusion, and cognition in mid-life: A randomized trial. Alzheimer’s Dement. 2022, 18, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Dowis, K.; Banga, S. The Potential Health Benefits of the Ketogenic Diet: A Narrative Review. Nutrients 2021, 13, 1654. [Google Scholar] [CrossRef]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (dys)function and insulin resistance: From pathophysiological molecular mechanisms to the impact of diet. Front. Physiol. 2019, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rusek, M.; Pluta, R.; Ułamek-Kozioł, M.; Czuczwar, S.J. Ketogenic Diet in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.X.; Maalouf, M.; Han, P.; Zhao, M.; Gao, M.; Dharshaun, T.; Ryan, C.; Whitelegge, J.; Wu, J.; Eisenberg, D.; et al. Ketones block amyloid entry and improve cognition in an Alzheimer’s model. Neurobiol. Aging 2016, 39, 25–37. [Google Scholar] [CrossRef]
- Maalouf, M.; Rho, J.M.; Mattson, M.P. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res. Rev. 2009, 59, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Ziemer, C.J.; Gibson, G.R. An Overview of Probiotics, Prebiotics and Synbiotics in the Functional Food Concept: Perspectives and Future Strategies. Int. Dairy J. 1998, 8, 473–479. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Q.; Zheng, M.; Hao, S.; Lum, J.S.; Chen, X.; Huang, X.-F.; Yu, Y.; Zheng, K. Supplement of microbiota-accessible carbohydrates prevents neuroinflammation and cognitive decline by improving the gut microbiota-brain axis in diet-induced obese mice. J. Neuroinflammation 2020, 17, 77. [Google Scholar] [CrossRef]
- Wu, S.-C.; Cao, Z.-S.; Chang, K.-M.; Juang, J.-L. Intestinal microbial dysbiosis aggravates the progression of Alzheimer’s disease in Drosophila. Nat. Commun. 2017, 8, 24. Available from: https://www.frontiersin.org/articles/10.3389/fnagi.2016.00262. [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain. Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef]
- Burnet, P.W.J.; Cowen, P.J. Psychobiotics highlight the pathways to happiness. Biol. Psychiatry 2013, 74, 708–709. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods (Basel, Switzerland) 2019, 8. [Google Scholar] [CrossRef]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl). 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, S.; Ling, Z.; Wang, F.; Ling, Y.; Gong, T.; Fang, N.; Ye, S.; Si, J.; Liu, J. Fructooligosaccharides Ameliorating Cognitive Deficits and Neurodegeneration in APP/PS1 Transgenic Mice through Modulating Gut Microbiota. J. Agric. Food Chem. 2019, 67, 3006–3017. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lai, D.-M.; Huang, H.-J.; Lee-Chen, G.-J.; Chang, C.-H.; Hsieh-Li, H.M.; Lee, G.-C. Prebiotic Lactulose Ameliorates the Cognitive Deficit in Alzheimer’s Disease Mouse Model through Macroautophagy and Chaperone-Mediated Autophagy Pathways. J. Agric. Food Chem. 2021, 69, 2422–2437. [Google Scholar] [CrossRef]
- Wang, T.; Kuang, W.; Chen, W.; Xu, W.; Zhang, L.; Li, Y.; Li, H.; Peng, Y.; Chen, Y.; Wang, B.; et al. A phase II randomized trial of sodium oligomannate in Alzheimer’s dementia. Alzheimers. Res. Ther. 2020, 12, 110. [Google Scholar] [CrossRef]
- Hazan, S. Rapid improvement in Alzheimer’s disease symptoms following fecal microbiota transplantation: a case report. J. Int. Med. Res. 2020, 48, 300060520925930. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Yang, N.; Li, S.; Huang, N.; Fang, X.; Zhang, J.; Zhu, B.; Yang, L.; Yang, C.; Luo, A. Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging (Albany. NY). 2018, 10, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, H.B.; Kuntz, T.; Shaik, S.M.; Baufeld, C.; Leibowitz, J.; Zhang, X.; Gottel, N.; Zhang, X.; Butovsky, O.; Gilbert, J.A.; et al. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J. Exp. Med. 2019, 216, 1542–1560. [Google Scholar] [CrossRef]
| Reference | Subject | Bacteria/organism | Effects(+/-) | Role |
|---|---|---|---|---|
| Cerovic et al., 2019 | AD patients | Bacteroides fragilis | + | Protection against CNS demyelination diseases. [42] |
| Deng, H et al., 2018 | C57BL/6 mice | + | In pregnant mice showed an immediate significant diminished autistic behavior [43] | |
| Cattaneo et al 2017 | AD patients | Eubacterium rectale | - | Leads to amyloidosis [35] |
| Tanida et al., 2005 | BB-DR rats Healthy humans |
Lactobacillus johnsonii |
+ | Improved gastric vagus nerve activity. [44] |
| Singhrao et al., 2015 | AD patients |
Porphyromonas gingivalis |
- | Responsible for cirrhosis, which leads to neurodegeneration [34] |
| Yanget al., 2020 | SAMP8 mice |
Lactobacillus acidophilus |
+ | Enhanced the injury in neural proteolysis.[45] |
| Gareau et al., 2005 | C57BL/6 mice |
Campylobacter rodentium |
- | Led to stress and contributed to behavioral abnormalities. [33] |
| Mehrabadi and Sadr,2020 | Wistar rats |
Lactobacillus rhamnosus |
+ | Improved the inflammation level in the brain. [31] |
| First author journal year | Animal Model | Exposure | Outcome measured | Main results | Proposed mechanism of intestinal microbiota involvement in dementia physiopathology |
|---|---|---|---|---|---|
| [75]Harach,Sci Rep,2017 | APPPSI transgenic mice | Mice that are germ-free thanks to IVF and are raised in a sterile environment | Cerebral amyloid deposition | Germ-free mice have less cerebral amyloid deposition than controls, whereas germ-free mice who received fecal microbiota transplants from controls have more amyloid deposition. | Bacteria in the gastrointestinal tract may cause subclinical brain inflammation that facilitates the deposition of amyloid. |
| [76] Minter,Sci Rep,2016 | APPSWE/PSIΔE9Transgenic mice | Long term broad-Spectrum combinatorial antibiotic therapy | Aβamyloid deposition soluble Aβlevels cerebral glial reactivity Circulating cytokine and chemokine level | Mice given antibiotics had higher levels of circulating cytokines and chemokine’s, less cerebral A-plaque formation, and higher levels of glial activity and circulating A. | Gut dysbiosis may have beneficial effects during the acute phase (reduced A plaque deposition), but negative effects during the long term. |
| [77] Minter, Sci Rep,2017 | APPSWE/PSIΔE9Transgenic mice | Postnatal antibiotic treatment | makeup of the gut microbiome Brain deterioration Foxp3+ Inflammation caused by T-reg cells in the blood, brain serum, and cerebrospinal fluid | Post-antibiotic therapy led to stable changes in GM diversity, including a decrease in species richness and an increase in Lachnospiraceae representation, an up regulation of circulating and brain Foxp3+Treg cells, and a decrease in the breakdown of brain A-amyloid. | Through the synthesis of butyrate and the differentiation of T-cells into Foxp3+Treg cells, the GM may mediate neuroinflammation. |
| [78]Frohlich, Brain Behav Immun, 2016 | Adult mice | Intragastric treatment with multiple antibiotics | colon's metabolic profile circulation microbial metabolites expression of signaling molecules in neurons Cognitive Conduct | Enhanced cytokine representation in the amygdala and hippocampus, decreased neuronal signaling-related chemicals in the brain, decreased colonic and circulating microbial metabolites, and worse novel object recognition memory are all indicators of GM dysbiosis. | Depletion of microbial metabolites in the gut may function as endocrine messengers, affecting the synthesis of neutral signaling molecules, particularly in the amygdala and hippocampus (BDNF, NMDA receptor, serotonin transporter, and NPY). |
| [79]Cui, BBA Mol Basis Dis, 2017 | C57BL/6Jmice | Total abdominal irradiation | circulating and intestinal miRNAs Expression of BDNF in the brain and gut microbiome | Cognitive impairment brought on by radiation, dysbiosis of the gut microbiome, overexpression of the circulating miR-34a-5p gene, and decreased expression of the brain protein BDNF GM eubiosis and BDNF synthesis were restored by injection of miR-34a-5p antagonists. | GM may influence the expression of miRNAs, specifically miR-34a-5p, which may mediate a bidirectional gut-brain interaction. |
| S.No | Biomarker(s) proposed | Samples and methods | Results | Refrences |
|---|---|---|---|---|
| 1. | Cortisol and DHEAS | ELISA, CLIA | 1.↑ CSF cortisol was associated with↓global cognitive performance and↑disease severity at baseline 2.↑ Cortisol and cortisol/DHEAS ratio were associated with↑tau and p-tau CSF levels and↓ amygdala and insula volumes at baseline. |
[126] |
| 2. | Plasma Aβ + gut microbiota | 16SrRNA Miseq sequencing of fecal microbiome and MSD quantifying the plasma Aβ 40 , Aβ42 , and Aβ 42/Aβ40 | 1.↑↑ The relative abundance of phylum Bacteroidetes in CN+ compared to CN 2.↓↓ Phylum Firmicutes and class Delta proteobacteria in CN+compared to CN 3. The relative abundance of phylum Firmicutes and its corresponding SCFA-producing bacteria in CN−>CN+>CI |
[127] |
| 3. | SCFA-producing microorganisms | 16SrRNA Miseq sequencing of fecal microbiome and CSF Aβ, p-tau | 1.↑ Clostridium leptum,↓ Eubacterium ventriosum group spp., Lachnospiraceae spp., Marvinbryantia spp.,Monoglobus spp., Ruminococcus torques group spp.,Roseburia hominis, and Christensenellaceae R-7 spp. with higher odds of amyloid positivity 2.↓ Lachnospiraceae spp., Lachnoclostridium spp.,Roseburia hominis, and Bilophila wadsworthia with higher odds of positive p-tau status |
[128] |
| 4. | Gut microbiota composition + diet quality scores + serum miRNA | 16SrDNA gene sequencing of fecal microbiome and serum miRNA expression | 1.↓ Microbial diversity, Faecalibacterium, Ruminococcaceae, and Alipstes in MCI compared to controls 2.↑ Proteobacteria and Gammaproteobacteria in MCI compared to controls |
[129] |
| 5. | Faecalibacterium, Bifidobacterium | 16SrRNA Miseq sequencing of fecal microbiome | 1.↓ Microbial diversity in AD compared to controls 2.↓ Butyrate-producing bacteria (Faecalibacterium) in AD 3.↑ Lactate-producing bacteria (Bifidobacterium) in AD |
[130] |
| 6. | Indole-3-pyruvic acid five SCFAs | LC/GC/MS metabolomics profiling of fecal microbiome | 1.↓ Tryptophan metabolites in MCI and, more pronounced, in AD compared to controls 2.↓↓ SCFAs (formic acid, acetic acid, propanoic acid, 2-methylbutyric acid, and isovaleric acid) in AD compared to controls;↓ in MCI compared to controls |
[131] |
| 7. | SCFAs and LPS | ELISA measured blood levels of LPS, and SCFAs by mass spectrometry | 1.↑ Blood LPS, acetate and valerate, pro-inflammatory cytokines, and biomarkers of endothelial dysfunction in dementia 2.↓ Butyrate and the anti-inflammatory cytokine IL10 in dementia |
[132] |
| 8. | Mycobiome signatures | 16SrRNA sequencing and fungal rRNA ITS1 sequencing of fecal bacterial and mycobiome | 1.↑ Genera Botrytis, Kazachstania, Phaeoacremonium, Cladosporium, and families Sclerotiniaceae, Phaffomyceteceae, Trichocomaceae, Cystofilobasidiaceae, and Togniniaceae in MCI compared to CN 2. Meyerozyma in MCI< CN 3. Different correlation patterns with AD markers and GM are displayed by specific fungal species (Debariomyce, Sarocladium, Filobasidium, Candida, and Cladosporium) |
[133] |
| 9. | Lachnospiraceae NK4A136 group, DAO, sCD14 | GM composition, gut barrier dysfunction, bacterial translocation, and inflammation were assessed from stool and serum samples by 16SrRNA sequencing, QIIME 2, Calypso 7.14 tools, and ELISA | 1.↓↓ Lachnospiraceae, genus Lachnospiraceae NK4A136 group in dementia. 2.↓ Eubacterium rectale in dementia. 3.↑ DAO and sCD14 in dementia. 4. Faecalibacterium prausnitzii was associated with mild dementia; Lactobacillus amylovorus was associated with moderate dementia; Clostridium clostridiforme and Streptococcus salivarius were associated with Severe dementia |
[134] |
| 10. | CCK | Targeted quantitation | 1.↑ CCK was a strong relationship associated with↑tau levels 2.↑ CCK was related to↑global and memory scores3.↑ CCK was related to↑gray matter volume |
[135] |
| 11. | BA | targeted metabolomics profiling of the Serum levels of 20 primary and secondary BA metabolite | 1. Three BA (↑ GDCA: CA, TDCA: CA, and GLCA: CDCA)signatures were associated with↓ CSF Aβ1-42 (“A”) 2. Three BA (↑ GCDCA、GLCA and TLCA) with↑ CSFp-tau181 (“T”) 3. The CSF t-tau, glucose metabolism, and atrophy (“N”) were connected to three, twelve, and fourteen BA signatures, respectively |
[136] |
| 12. | Microbial diversity | Analysis of microbiome community in the feces and blood via 16SrRNA sequencing | 1.↓ Microbial diversity in AD and MCI compared to controls 2.11 genera in the feces (↑ Dorea, Lactobacillus, Streptococcus, Bifidobacterium, Blautia, and Escherichia;↓ Alistipes, Bacteroides, Parabacteroides, Sutterella, and Paraprevotella) and in the blood (↑ Propionibacterium, Pseudomonas, Glutamicibacter, Escherichia, and Acidovora;↓ Acinetobacter, Aliihoeflea, Halomonas, Leucobacter, Pannonibacter, and Ochrobactrum) between AD, MCI and controls |
[137] |
| 13. | Enterobacteriaceae | Phylogenetic analysis of communities using 16S rRNA MiSeq sequencing and reconstruction of the feces | 1.↓ Microbial diversity in AD compared to MCI and controls 2.↓ Firmicutes in AD compared to controls 3.↑ Proteobacteria in AD compared to controls 4.↑ Gammaproteobacteria, Enterobacteriales, and Enterobacteriaceae in AD > MCI > controls |
[138] |
| 14. | TMAO | Cerebrospinal TMAO levels measurement and relationships between CSF TMAO and CSF biomarkers of AD | 1.↑ TMAO in AD and MCI compared to controls 2. Elevated CSF TMAO is associated with biomarkers of AD pathology |
[139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





