Submitted:
10 May 2024
Posted:
10 May 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Types of Peripheral Vascular Injuries
Mechanism of Peripheral Vascular Injuries
Primary Survey
Diagnosis of Peripheral Vascular Injuries
Clinical Manifestations
Auxiliary Examination
Ultrasound
MDCTA or CTA
CTA Protocol
CTA Imaging Findings
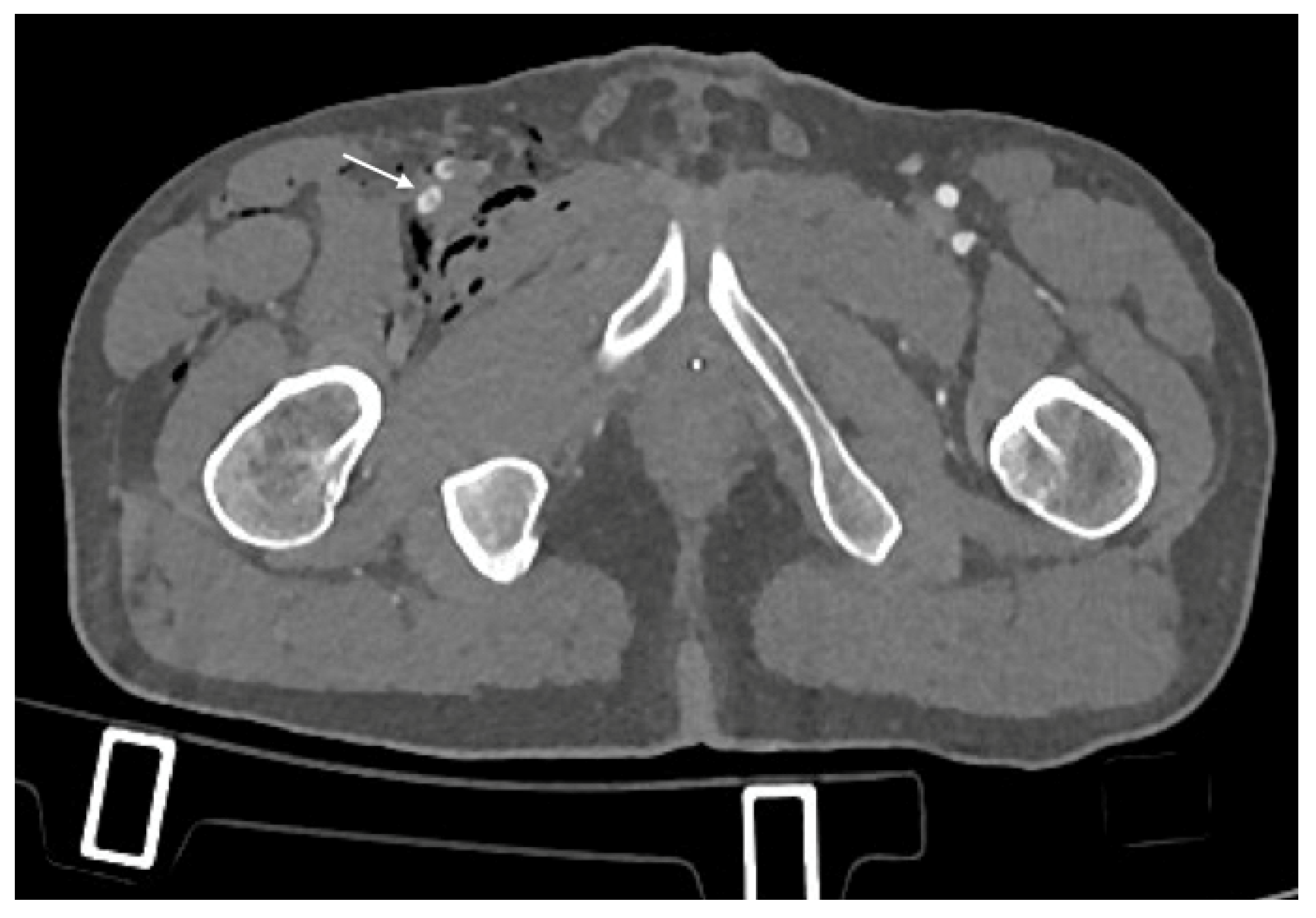
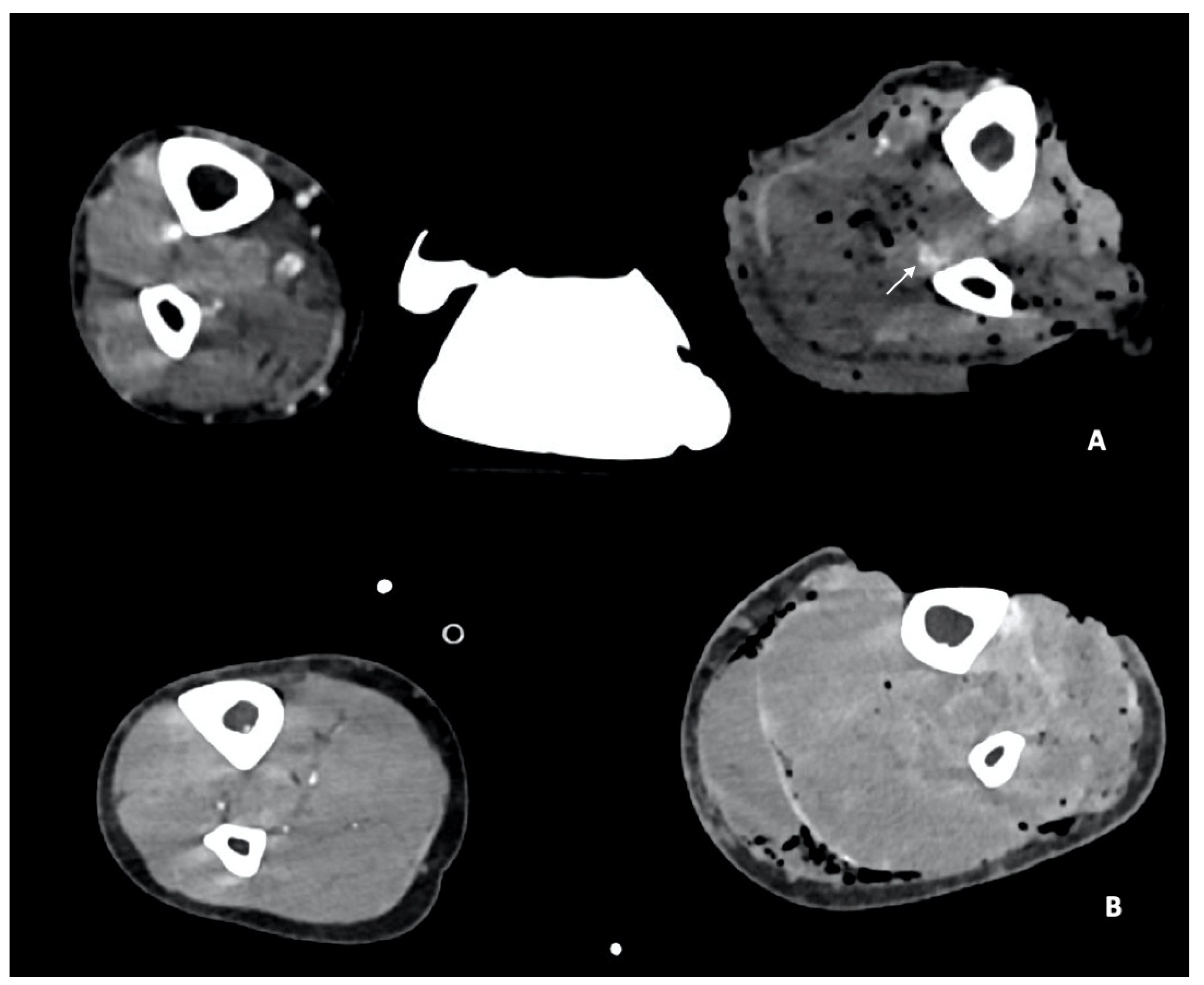
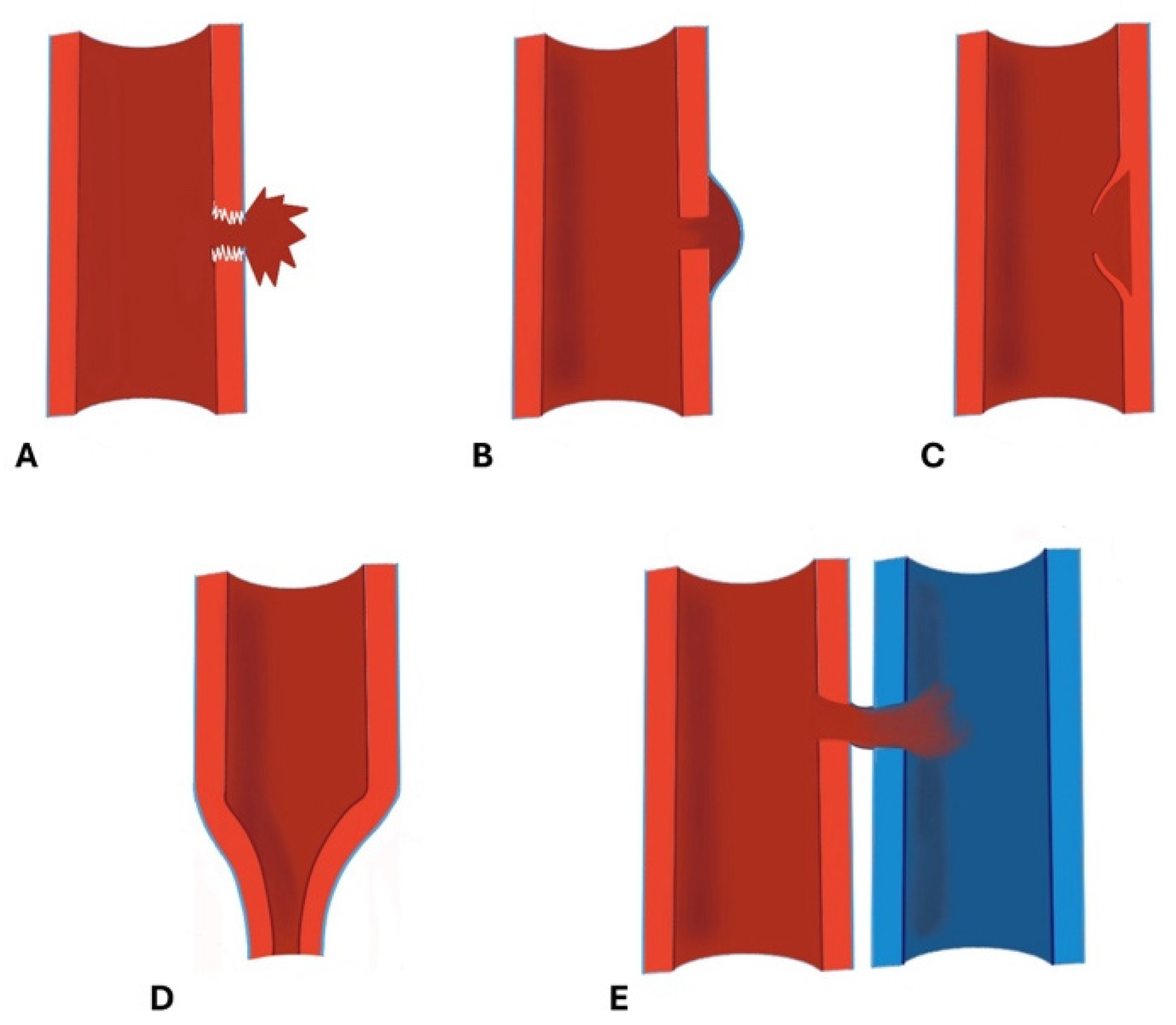
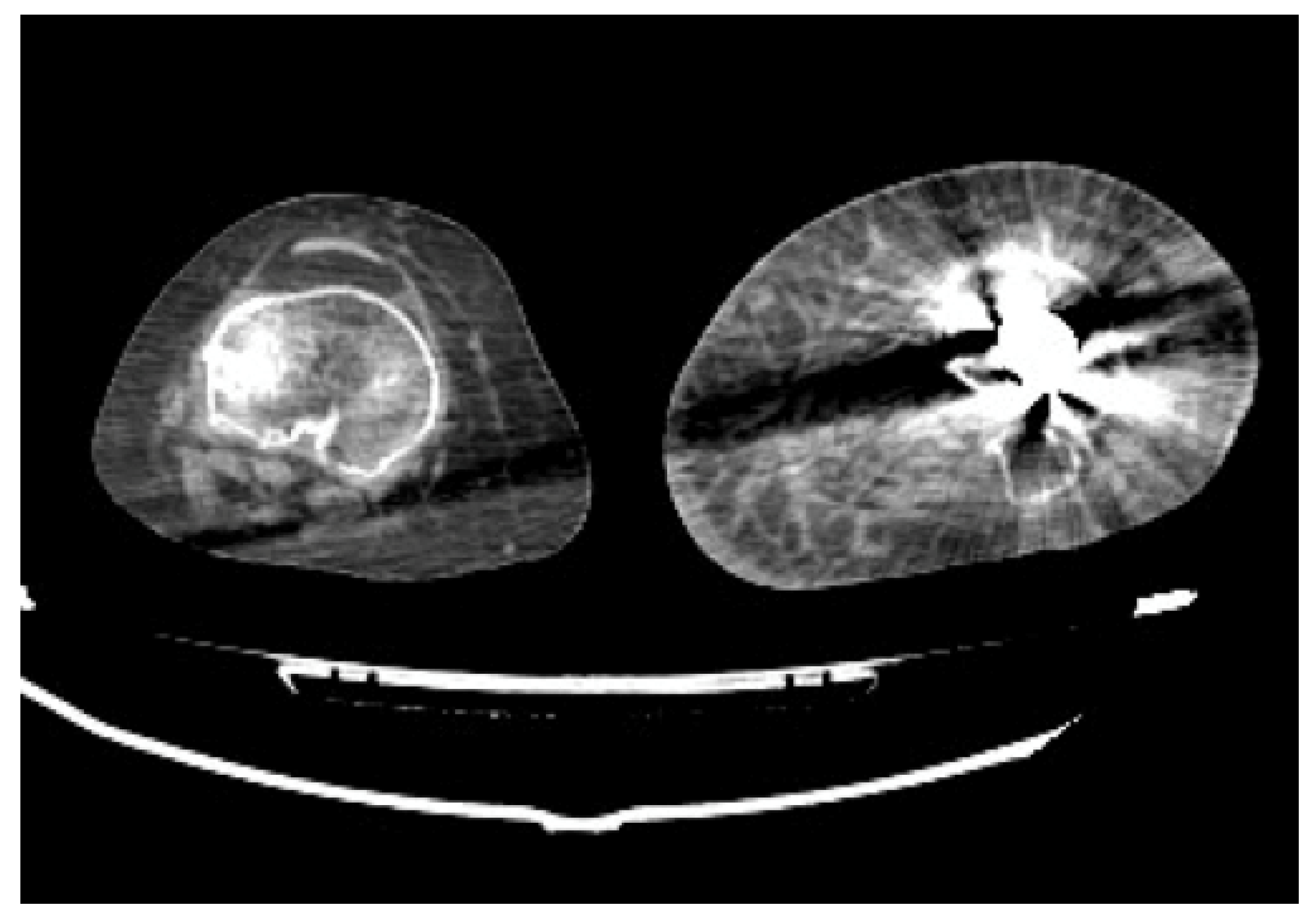
- Arterial Transections represents the complete rupture of the vessel, and determines the loss of distal opacification, a massive hematoma and active bleeding [5] (Figure 3). Active arterial bleeding is visualized as contrast extravasation in the arterial phase, which enlarges in venous and delayed phases [67]. In partial transection, the arterial tear involves the three layers of the vessel wall, without affecting the entire vessel’s circumference, distal opacification is appreciable although reduced luminal caliber and opacification can be detected [5,55].
- Pseudoaneurysm is caused by focal arterial wall tear involving intimal and medial layers, and represents a collection of blood contained only by the adventitia layer or surrounding tissue [5,56,59]. It appears as an outpouching sac with a round and smooth margin in continuity with the arterial adjacent lumen (Figure 4). Pseudoaneurysm bleeding appears as irregular, lobulated perilesional contrast blush [65,68]. Pre-exiting calcification or pseudoaneurysm should be differentiate form active bleeding; delayed phase acquisition can be useful because in active bleeding the contrast extravasation dissipates along tissue planes instead pseudoaneurysm and calcification remain stable [60,67].
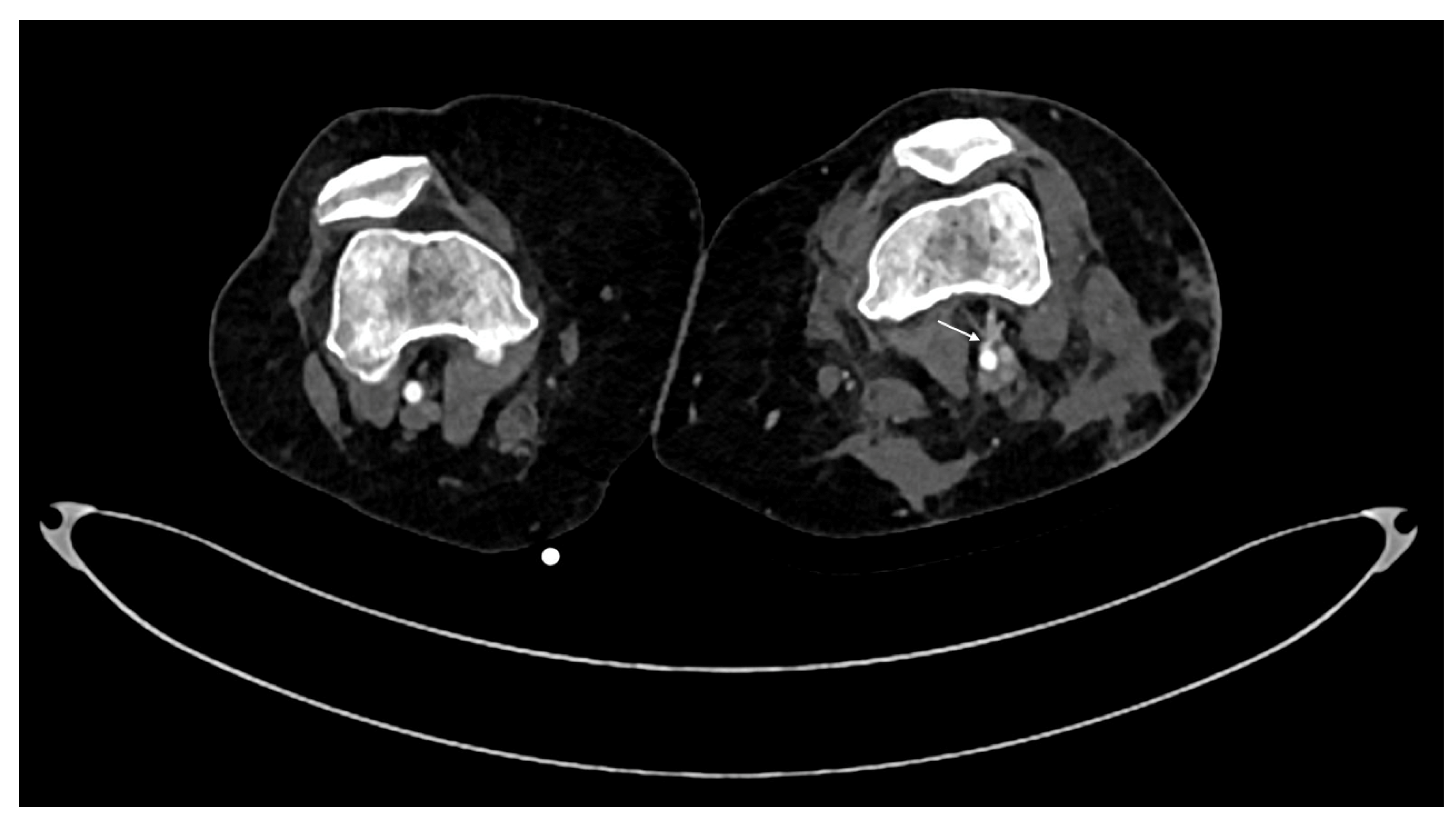
- Dissection is caused by an intimal tear, resulting into an intimal flap, which can float in the vessel lumen or cause occlusion [5]; at CT it appears as a semilunar luminal deformation or eccentric stenosis or complete occlusion. Findings in dissection can be subtle but if evident at CT, the intimal flap can be classically seen as a linear flap within the vessel lumen [5,65,69,70] (Figure 5).
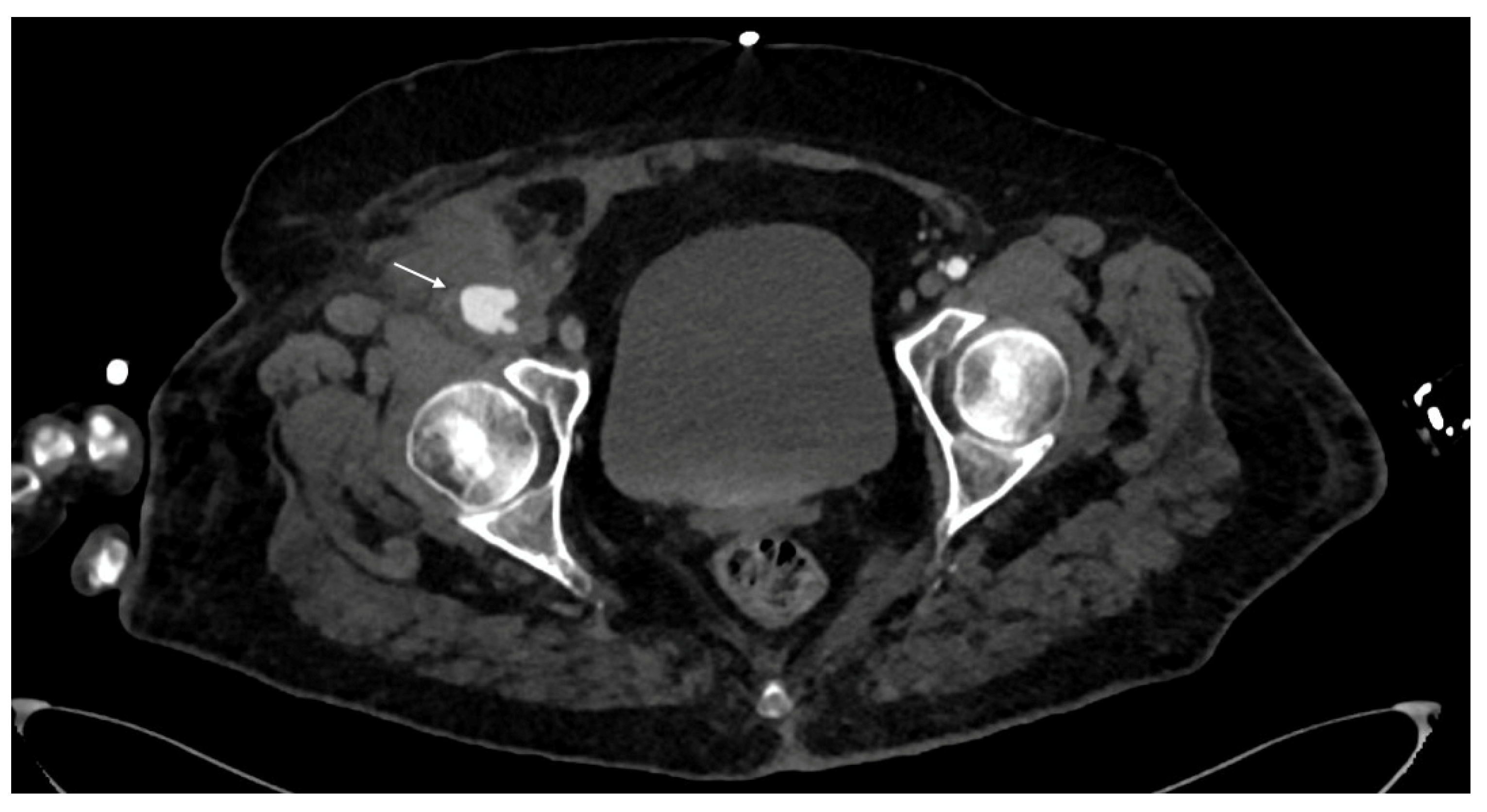
- Luminal narrowing: the vessel wall appears lobulated with eccentric narrowing, it can be the result of extrinsic compression, non-occlusive thrombus, or dissection (Figure 3).
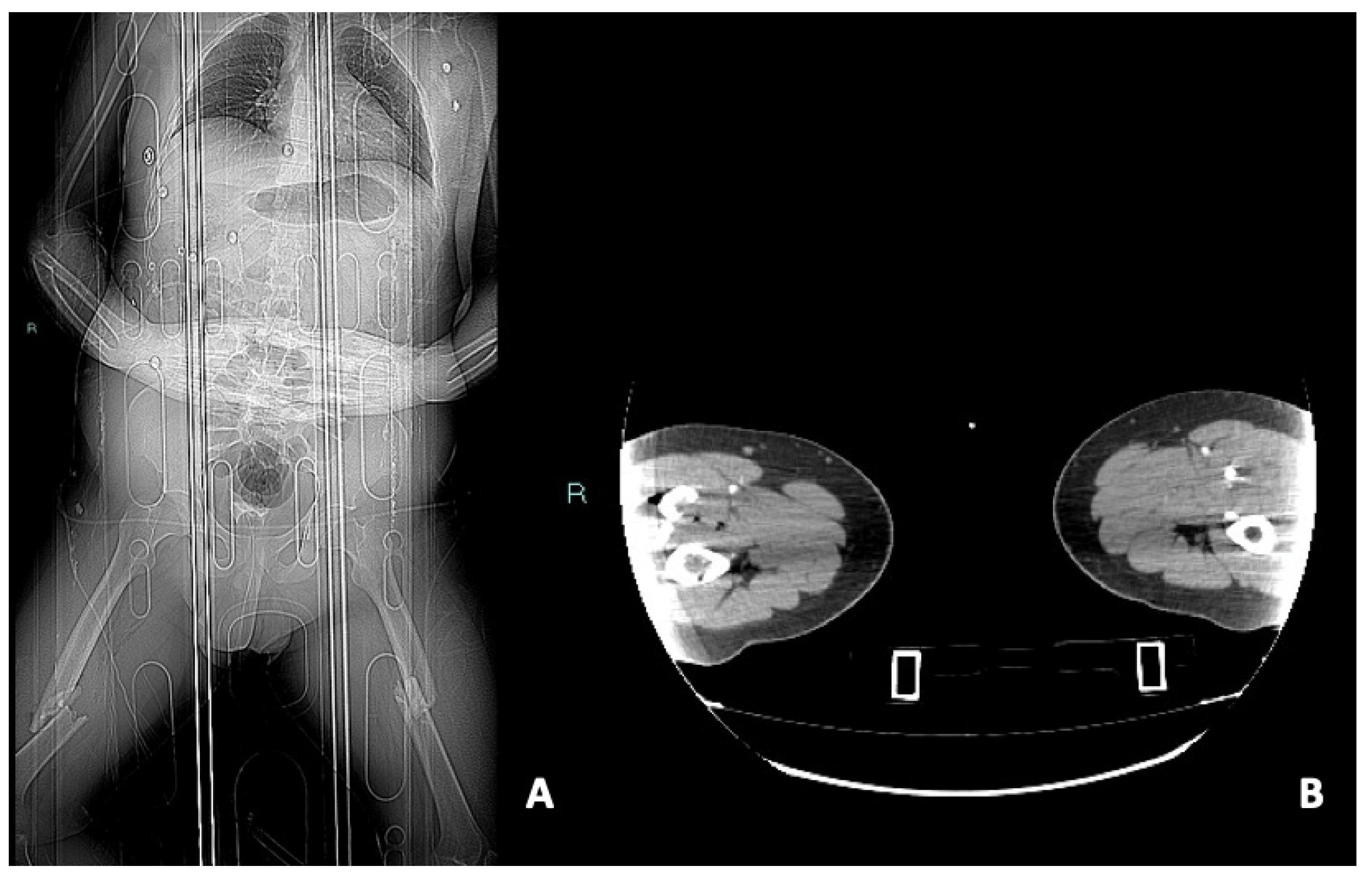
- Vasospasm: it is represented by a concentric, focal and segmental luminal narrowing with smooth margin, caused by the contraction of the arterial wall, as a response to an injury [5].It can be difficult to differentiate from an intimal tear and occlusion in distal small arteries [71,72]. The differential diagnosis between vasospam and dissection often requires DSA for the proper management (Figure 6).

CTA Pitfalls
Peripheral Vascular Injury Grading
CTA Timing in Peripheral Vascular Injury Assessment
Complication of Peripheral Vascular Injuries
Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feliciano DV RT: Evaluation and treatment of vascular injuries. In: Skeletal Trauma Basic Science, Management and Reconstruction. edn. Philadelphia: Elsevier Saunders; 2015: 423-435.
- Mattox, K.L.; Feliciano, D.V.; Burch, J.; Beall, A.C., Jr.; Jordan, G.L., Jr.; De Bakey, M.E. Five thousand seven hundred sixty cardiovascular injuries in 4459 patients. Epidemiologic evolution 1958 to 1987. Ann Surg 1989, 209, 698–705, discussion 706-697. [Google Scholar] [CrossRef] [PubMed]
- Michaels, A.J.; Gerndt, S.J.; Taheri, P.A.; Wang, S.C.; Wahl, W.L.; Simeone, D.M.; Williams, D.M.; Greenfield, L.J.; Rodriguez, J.L. Blunt force injury of the abdominal aorta. J Trauma 1996, 41, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Branco, B.C.; Musonza, T.; Long, M.A.; Chung, J.; Todd, S.R.; Wall, M.J., Jr.; Mills, J.L., Sr.; Gilani, R. Survival trends after inferior vena cava and aortic injuries in the United States. J Vasc Surg 2018, 68, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Dreizin, D.; Smith, E.B.; Champ, K.; Morrison, J.J. Roles of Trauma CT and CTA in Salvaging the Threatened or Mangled Extremity. Radiographics 2022, 42, E50–E67. [Google Scholar] [CrossRef] [PubMed]
- Franz, R.W.; Shah, K.J.; Halaharvi, D.; Franz, E.T.; Hartman, J.F.; Wright, M.L. A 5-year review of management of lower extremity arterial injuries at an urban level I trauma center. J Vasc Surg 2011, 53, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, L.; Coimbra, R.; Goes, A.M.O., Jr.; Reva, V.; Santorelli, J.; Moore, E.E.; Galante, J.; Abu-Zidan, F.; Peitzman, A.B.; Ordonez, C.; et al. American Association for the Surgery of Trauma-World Society of Emergency Surgery guidelines on diagnosis and management of peripheral vascular injuries. J Trauma Acute Care Surg 2020, 89, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Li, J.Y.; Jiang, P.; Jia, W.; Tian, X.; Cheng, Z.Y.; Zhang, Y.X. Literature review of peripheral vascular trauma: Is the era of intervention coming? Chin J Traumatol 2020, 23, 5–9. [Google Scholar] [CrossRef]
- Blaisdell, F.W. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg 2002, 10, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Schueller, G.; Scaglione, M.; Linsenmaier, U.; Schueller-Weidekamm, C.; Andreoli, C.; De Vargas Macciucca, M.; Gualdi, G. The key role of the radiologist in the management of polytrauma patients: indications for MDCT imaging in emergency radiology. Radiol Med 2015, 120, 641–654. [Google Scholar] [CrossRef]
- Ntola, V.C.; Hardcastle, T.C. Diagnostic Approaches to Vascular Injury in Polytrauma-A Literature Review. Diagnostics (Basel), 2023; 13. [Google Scholar]
- Ntola, V.C.; Hardcastle, T.C.; Nkwanyana, N.M. Management of vascular injuries on ICU patients: KZN experience. Injury 2024, 111418. [Google Scholar] [CrossRef]
- Evans, C.; Chaplin, T.; Zelt, D. Management of Major Vascular Injuries: Neck, Extremities, and Other Things that Bleed. Emerg Med Clin North Am 2018, 36, 181–202. [Google Scholar] [CrossRef]
- Iacobellis, F.; Scaglione, M.; Brillantino, A.; Scuderi, M.G.; Giurazza, F.; Grassi, R.; Noschese, G.; Niola, R.; Al Zuhir, N.Y.S.; Romano, L. The additional value of the arterial phase in the CT assessment of liver vascular injuries after high-energy blunt trauma. Emerg Radiol 2019, 26, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Davenport, R.T.N.; Walsh, M. Vascula Trauma. Surgery (Oxford) 2009, 27, 331–336. [Google Scholar] [CrossRef]
- Perkins, Z.B.; De’Ath, H.D.; Aylwin, C.; Brohi, K.; Walsh, M.; Tai, N.R. Epidemiology and outcome of vascular trauma at a British Major Trauma Centre. Eur J Vasc Endovasc Surg 2012, 44, 203–209. [Google Scholar] [CrossRef]
- Frykberg, E.R. Popliteal vascular injuries. Surg Clin North Am 2002, 82, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Rozycki, G.S.; Tremblay, L.N.; Feliciano, D.V.; McClelland, W.B. Blunt vascular trauma in the extremity: diagnosis, management, and outcome. J Trauma 2003, 55, 814–824. [Google Scholar] [CrossRef]
- Feliciano, D.V.; Moore, E.E.; West, M.A.; Moore, F.A.; Davis, J.W.; Cocanour, C.S.; Scalea, T.M.; McIntyre, R.C., Jr. Western Trauma Association critical decisions in trauma: evaluation and management of peripheral vascular injury, part, I.I. J Trauma Acute Care Surg 2013, 75, 391–397. [Google Scholar] [CrossRef]
- Sciarretta, J.D.; Macedo, F.I.; Otero, C.A.; Figueroa, J.N.; Pizano, L.R.; Namias, N. Management of traumatic popliteal vascular injuries in a level I trauma center: A 6-year experience. Int J Surg 2015, 18, 136–141. [Google Scholar] [CrossRef]
- Slama, R.; Villaume, F. Penetrating Vascular Injury: Diagnosis and Management Updates. Emerg Med Clin North Am 2017, 35, 789–801. [Google Scholar] [CrossRef]
- Pinto, A.; Russo, A.; Reginelli, A.; Iacobellis, F.; Di Serafino, M.; Giovine, S.; Romano, L. Gunshot Wounds: Ballistics and Imaging Findings. Semin Ultrasound CT MR 2019, 40, 25–35. [Google Scholar] [CrossRef]
- Kumar, M.K.; Badole, C.; Patond, K. Salvage versus amputation: Utility of mangled extremity severity score in severely injured lower limbs. Indian J Orthop 2007, 41, 183–187. [Google Scholar] [PubMed]
- Meyer, J.P.; Lim, L.T.; Schuler, J.J.; Castronuovo, J.J.; Buchbinder, D.; Woelfel, G.F.; Flanigan, P. Peripheral vascular trauma from close-range shotgun injuries. Arch Surg 1985, 120, 1126–1131. [Google Scholar] [CrossRef]
- Szaniewski, K.B.T.; Sikora, T. Vascular Trauma. In: Emergency Medicine and Trauma. edn. Edited by IntechOpen: 1-18.
- Usman, R.; Jamil, M.; Anwer, M.F. Evaluation, Surgical Management and Outcome of Traumatic Extremity Vascular Injuries: A 5-year Level-1 Trauma Centres Experience. Ann Vasc Dis 2018, 11, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Rich, N.M.; Hobson, R.W., 2nd; Fedde, C.W. Vascular trauma secondary to diagnostic and therapeutic procedures. Am J Surg 1974, 128, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Giswold, M.E.; Landry, G.J.; Taylor, L.M.; Moneta, G.L. Iatrogenic arterial injury is an increasingly important cause of arterial trauma. Am J Surg 2004, 187, 590–592, discussion 592-593. [Google Scholar] [CrossRef] [PubMed]
- Eleshra, A.; Kim, D.; Park, H.S.; Lee, T. Access site pseudoaneurysms after endovascular intervention for peripheral arterial diseases. Ann Surg Treat Res 2019, 96, 305–312. [Google Scholar] [CrossRef]
- Lopera, J.E.; Restrepo, C.S.; Gonzales, A.; Trimmer, C.K.; Arko, F. Aortoiliac vascular injuries after misplacement of fixation screws. J Trauma 2010, 69, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Pulido, L.; Slenker, N.; Macgibeny, M.; Purtill, J.J.; Rothman, R.H. Vascular injuries after total joint arthroplasty. J Arthroplasty 2008, 23, 1115–1121. [Google Scholar] [CrossRef]
- Scalea, J.R.; Crawford, R.; Scurci, S.; Danquah, J.; Sarkar, R.; Kufera, J.; O’Connor, J.; Scalea, T.M. Below-the-knee arterial injury: the type of vessel may be more important than the number of vessels injured. J Trauma Acute Care Surg 2014, 77, 920–925. [Google Scholar] [CrossRef]
- Eccles S HB, Khan U,, McFadyen I N, J, S N: Vascular Injuries. In., edn. Edited by Fractures SftMoO: Oxford Medicine Online; 2020: 93-102.
- Ritondale, J.; Piehl, M.; Caputo, S.; Broome, J.; McLafferty, B.; Anderson, A.; Belding, C.; Tatum, D.; Taghavi, S.; McGrew, P.; et al. The Impact of a Prehospital “x-ABC” Resuscitation Sequence in Patients with Severe Hemorrhage. J Am Coll Surg 2024.
- Prichayudh, S.; Rassamee, P.; Sriussadaporn, S.; Pak-Art, R.; Sriussadaporn, S.; Kritayakirana, K.; Samorn, P.; Narueponjirakul, N.; Uthaipaisanwong, A. Abdominal vascular injuries: Blunt vs. penetrating. Injury 2019, 50, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Davidovic, L.B.; Cinara, I.S.; Ille, T.; Kostic, D.M.; Dragas, M.V.; Markovic, D.M. Civil and war peripheral arterial trauma: review of risk factors associated with limb loss. Vascular 2005, 13, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Gustilo, R.B.; Mendoza, R.M.; Williams, D.N. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma 1984, 24, 742–746. [Google Scholar] [CrossRef]
- Durham, R.M.; Mistry, B.M.; Mazuski, J.E.; Shapiro, M.; Jacobs, D. Outcome and utility of scoring systems in the management of the mangled extremity. Am J Surg 1996, 172, 569–573, discussion 573-564. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.S.; Goldner, R.D.; Urbaniak, J.R.; Nunley, J.A.; Hardaker, W.T., Jr. Management of severe musculoskeletal injuries of the upper extremity. J Orthop Trauma 1990, 4, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Gustilo, R.B.; Anderson, J.T. JSBS classics. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones. Retrospective and prospective analyses. J Bone Joint Surg Am 2002, 84, 682. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Fractures (complex): assessment and management. London: National Institute for Health and Care Excellence (UK). 2016.
- Ewing, T.E.H.G.; Perron, A.D.; Strout, T.D. Inter-rater reliability and false positive result rates of ankle brachial index measurements performed by emergency providers. Ann Emerg Med 2010, 56, S132–133. [Google Scholar] [CrossRef]
- Nguyen, T.; Kalish, J.; Woodson, J. Management of civilian and military vascular trauma: lessons learned. Semin Vasc Surg 2010, 23, 235–242. [Google Scholar] [CrossRef]
- Dennis, J.W.; Frykberg, E.R.; Veldenz, H.C.; Huffman, S.; Menawat, S.S. Validation of nonoperative management of occult vascular injuries and accuracy of physical examination alone in penetrating extremity trauma: 5- to 10-year follow-up. J Trauma 1998, 44, 243–252, discussion 242-243. [Google Scholar] [CrossRef]
- Adragao, T.; Pires, A.; Branco, P.; Castro, R.; Oliveira, A.; Nogueira, C.; Bordalo, J.; Curto, J.D.; Prata, M.M. Ankle--brachial index, vascular calcifications and mortality in dialysis patients. Nephrol Dial Transplant 2012, 27, 318–325, discussion 572-563. [Google Scholar] [CrossRef]
- Johansen, K.; Daines, M.; Howey, T.; Helfet, D.; Hansen, S.T., Jr. Objective criteria accurately predict amputation following lower extremity trauma. J Trauma 1990, 30, 568–572, discussion 572-563. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, J.J.; Anz, A.; Langfitt, M.; Deonanan, J.K.; Scott, A.; Teasdall, R.D.; Carroll, E.A. Vascular injury associated with extremity trauma: initial diagnosis and management. J Am Acad Orthop Surg 2011, 19, 495–504. [Google Scholar] [CrossRef]
- Latteri, S.; Malaguarnera, G.; Mannino, M.; Pesce, A.; Curro, G.; Tamburrini, S.; Scuderi, M. Ultrasound as point of care in management of polytrauma and its complication. J Ultrasound 2017, 20, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Tamburrini, S.; Consoli, L.; Garrone, M.; Sfuncia, G.; Lugara, M.; Coppola, M.G.; Piccirillo, M.; Toto, R.; Stella, S.M.; Sofia, S.; et al. The “Black Pattern”, a Simplified Ultrasound Approach to Non-Traumatic Abdominal Emergencies. Tomography 2022, 8, 798–814. [Google Scholar] [CrossRef] [PubMed]
- Stacy, M.R.; Dearth, C.L. Multimodality Imaging Approaches for Evaluating Traumatic Extremity Injuries: Implications for Military Medicine. Adv Wound Care (New Rochelle) 2017, 6, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Tisherman, S.A. Management of Major Vascular Injury: Open. Otolaryngol Clin North Am 2016, 49, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Montorfano, M.A.; Pla, F.; Vera, L.; Cardillo, O.; Nigra, S.G.; Montorfano, L.M. Point-of-care ultrasound and Doppler ultrasound evaluation of vascular injuries in penetrating and blunt trauma. Crit Ultrasound J 2017, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.O.; Holt, P.J.; Cleanthis, M.; Tai, N.; Carrell, T.; Loosemore, T.M.; London Vascular Injuries Working, G. Imaging vascular trauma. Br J Surg 2012, 99, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.; Dennis, J.W.; Vu, J.H.; Frykberg, E.R. Redefining the role of arterial imaging in the management of penetrating zone 3 neck injuries. Vascular 2005, 13, 158–163. [Google Scholar] [CrossRef] [PubMed]
- deSouza, I.S.; Benabbas, R.; McKee, S.; Zangbar, B.; Jain, A.; Paladino, L.; Boudourakis, L.; Sinert, R. Accuracy of Physical Examination, Ankle-Brachial Index, and Ultrasonography in the Diagnosis of Arterial Injury in Patients With Penetrating Extremity Trauma: A Systematic Review and Meta-analysis. Acad Emerg Med 2017, 24, 994–1017. [Google Scholar] [CrossRef]
- Costantini, T.W.; Coimbra, R.; Holcomb, J.B.; Podbielski, J.M.; Catalano, R.; Blackburn, A.; Scalea, T.M.; Stein, D.M.; Williams, L.; Conflitti, J.; et al. Current management of hemorrhage from severe pelvic fractures: Results of an American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg 2016, 80, 717–723, discussion 723-715. [Google Scholar] [CrossRef] [PubMed]
- Dreizin, D.; Munera, F. Blunt polytrauma: evaluation with 64-section whole-body CT angiography. Radiographics 2012, 32, 609–631. [Google Scholar] [CrossRef] [PubMed]
- Monazzam, S.; Goodell, P.B.; Salcedo, E.S.; Nelson, S.H.; Wolinsky, P.R. When are CT angiograms indicated for patients with lower extremity fractures? A review of 275 extremities. J Trauma Acute Care Surg 2017, 82, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.P.; Rambau, G.; Tennent, D.J.; Osborn, P.M. The Role of CT Angiography in Evaluating Lower Extremity Trauma: 157 Patient Case Series at a Military Treatment Facility. Mil Med 2019, 184, e490–e493. [Google Scholar] [CrossRef] [PubMed]
- Bozlar, U.; Ogur, T.; Norton, P.T.; Khaja, M.S.; All, J.; Hagspiel, K.D. CT angiography of the upper extremity arterial system: Part 1-Anatomy, technique, and use in trauma patients. AJR Am J Roentgenol 2013, 201, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Walkoff, L.; Nagpal, P.; Khandelwal, A. Imaging primer for CT angiography in peripheral vascular trauma. Emerg Radiol 2021, 28, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Abu-Omar, A.; Murray, N.; Ali, I.T.; Khosa, F.; Barrett, S.; Sheikh, A.; Nicolaou, S.; Tamburrini, S.; Iacobellis, F.; Sica, G.; et al. Utility of Dual-Energy Computed Tomography in Clinical Conundra. Diagnostics (Basel), 2024; 14. [Google Scholar]
- Wirth, S.; Hebebrand, J.; Basilico, R.; Berger, F.H.; Blanco, A.; Calli, C.; Dumba, M.; Linsenmaier, U.; Muck, F.; Nieboer, K.H.; et al. European Society of Emergency Radiology: guideline on radiological polytrauma imaging and service (short version). Insights Imaging 2020, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, F.; Ierardi, A.M.; Mazzei, M.A.; Magenta Biasina, A.; Carrafiello, G.; Nicola, R.; Scaglione, M. Dual-phase CT for the assessment of acute vascular injuries in high-energy blunt trauma: the imaging findings and management implications. Br J Radiol 2016, 89, 20150952. [Google Scholar] [CrossRef] [PubMed]
- Gakhal, M.S.; Sartip, K.A. CT angiography signs of lower extremity vascular trauma. AJR Am J Roentgenol 2009, 193, W49–W57. [Google Scholar] [CrossRef]
- Miller-Thomas, M.M.; West, O.C.; Cohen, A.M. Diagnosing traumatic arterial injury in the extremities with CT angiography: pearls and pitfalls. Radiographics 2005, 25 (Suppl. 1), S133–142. [Google Scholar] [CrossRef]
- Madhuripan, N.; Mehta, P.; Smolinski, S.E.; Njuguna, N. Computed Tomography Angiography of the Extremities in Emergencies. Semin Ultrasound CT MR 2017, 38, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Efron, D.T.; Fishman, E.K. Multidetector CT and three-dimensional CT angiography of upper extremity arterial injury. Emerg Radiol 2015, 22, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Jens, S.; Kerstens, M.K.; Legemate, D.A.; Reekers, J.A.; Bipat, S.; Koelemay, M.J. Diagnostic performance of computed tomography angiography in peripheral arterial injury due to trauma: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2013, 46, 329–337. [Google Scholar] [CrossRef]
- Nagpal, K.; Ahmed, K.; Cuschieri, R. Diagnosis and management of acute traumatic arteriovenous fistula. Int J Angiol 2008, 17, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Rieger, M.; Mallouhi, A.; Tauscher, T.; Lutz, M.; Jaschke, W.R. Traumatic arterial injuries of the extremities: initial evaluation with MDCT angiography. AJR Am J Roentgenol 2006, 186, 656–664. [Google Scholar] [CrossRef]
- Uyeda, J.W.; Anderson, S.W.; Sakai, O.; Soto, J.A. CT angiography in trauma. Radiol Clin North Am 2010, 48, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Colip, C.G.; Gorantla, V.; LeBedis, C.A.; Soto, J.A.; Anderson, S.W. Extremity CTA for penetrating trauma: 10-year experience using a 64-detector row CT scanner. Emerg Radiol 2017, 24, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Yaremchuk, M.J.; Brumback, R.J.; Manson, P.N.; Burgess, A.R.; Poka, A.; Weiland, A.J. Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstr Surg 1987, 80, 1–14. [Google Scholar] [CrossRef]
- Pieroni, S.; Foster, B.R.; Anderson, S.W.; Kertesz, J.L.; Rhea, J.T.; Soto, J.A. Use of 64-row multidetector CT angiography in blunt and penetrating trauma of the upper and lower extremities. Radiographics 2009, 29, 863–876. [Google Scholar] [CrossRef]
- Scaglione, M.; Iaselli, F.; Sica, G.; Feragalli, B.; Nicola, R. Errors in imaging of traumatic injuries. Abdom Imaging 2015, 40, 2091–2098. [Google Scholar] [CrossRef]
- Joseph, T.I.; Ratnakanthan, P.J.; Paul, E.; Clements, W. Utility of computed tomography angiography in traumatic lower limb injury: Review of clinical impact in level 1 trauma centre. Injury 2021, 52, 3064–3067. [Google Scholar] [CrossRef] [PubMed]
- le Roux, A.; Du Plessis, A.M.; Pitcher, R. Yield of CT angiography in penetrating lower extremity trauma. Emerg Radiol 2021, 28, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.; Rajani, R.R.; Bokhari, F.; Chiu, W.C.; Kerwin, A.; Seamon, M.J.; Skarupa, D.; Frykberg, E. Eastern Association for the Surgery of, T. Evaluation and management of penetrating lower extremity arterial trauma: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg.
- Moore, E.E.; Malangoni, M.A.; Cogbill, T.H.; Peterson, N.E.; Champion, H.R.; Jurkovich, G.J.; Shackford, S.R. Organ injury scaling VII: cervical vascular, peripheral vascular, adrenal, penis, testis, and scrotum. J Trauma 1996, 41, 523–524. [Google Scholar] [CrossRef] [PubMed]
- Velmahos, G.C.; Toutouzas, K.G.; Vassiliu, P.; Sarkisyan, G.; Chan, L.S.; Hanks, S.H.; Berne, T.V.; Demetriades, D. A prospective study on the safety and efficacy of angiographic embolization for pelvic and visceral injuries. J Trauma 2002, 53, 303–308, discussion 308. [Google Scholar] [CrossRef]
- Goes, A.M.O.; Parreira, J.G.; Kleinsorge, G.H.D.; Dalio, M.B.; Alves, P.H.F.; Gomes, F.; de Araujo, W.J.B.; Joviliano, E.E.; de Oliveira, J.C.P. Brazilian guidelines on diagnosis and management of traumatic vascular injuries. J Vasc Bras 2023, 22, e20230042. [Google Scholar] [CrossRef]
- Percival, T.J.; Rasmussen, T.E. Reperfusion strategies in the management of extremity vascular injury with ischaemia. Br J Surg 2012, 99 (Suppl. 1), 66–74. [Google Scholar] [CrossRef]
- McQueen, M.M.; Gaston, P.; Court-Brown, C.M. Acute compartment syndrome. Who is at risk? J Bone Joint Surg Br 2000, 82, 200–203. [Google Scholar] [CrossRef]
- Lu, C.H.; Tsang, Y.M.; Yu, C.W.; Wu, M.Z.; Hsu, C.Y.; Shih, T.T. Rhabdomyolysis: magnetic resonance imaging and computed tomography findings. J Comput Assist Tomogr 2007, 31, 368–374. [Google Scholar] [CrossRef]
| Grade | Injury |
|---|---|
| I | Digital artery/vein, Palmar artery/vein, deep palmar artery/vein, dorsalis pedis artery, plantar artery/vein, non-named arterial/venous branches. |
| II | Basilic/cephalic vein, saphenous vein, radial artery, ulnar artery. |
| III | Axillary vein, superficial/deep femoral vein, popliteal vein, brachial artery, anterior tibial artery, posterior tibial artery, peroneal artery, tibioperoneal trunk. |
| IV | Superficial/deep femoral artery, popliteal artery. |
| V | Axillary artery, common femoral artery. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





