4.1. Effects of Sphingomonas on the Histological Structure of Intestine
The gastrointestinal tract (GIT) serves as the primary site for digestion and absorption, and defense against harmful foreign microorganisms. Its function correlates positively with organelle numbers and health status. The villi in the small intestine play a crucial role in nutrient absorption, and the height, integrity, and cell count of these villi significantly affect small intestine digestive and absorption functions [
17]. After adding 4 × 10
5 CFU/mL of
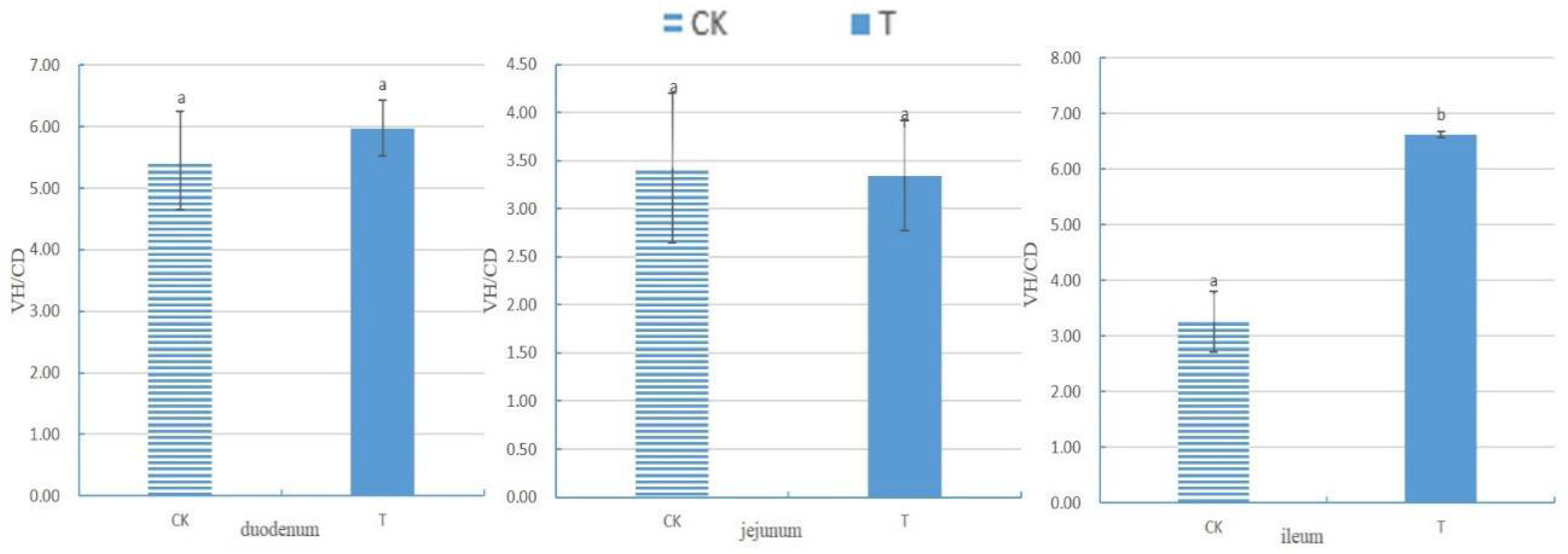
Sphingomonas Z392 to drinking water, broiler chickens exhibited increased integrity of small intestinal villi, an augmented number of goblet cells in small intestinal epithelial cells, enhanced mitochondrial health in the cytoplasm of jejunal villous epithelial cells, and elevated counts of lysosomes in the cytoplasm of goblet cells in the small intestinal epithelium, ileal villous epithelial cells, and mitochondria in the cytoplasm of large intestinal villous epithelial cells. The VH/CD of the ileum significantly increased (
p<0.05).
VH, CD, and VH/CD serve as vital indicators for measuring intestinal nutrient digestion and absorption abilities, as well as its health status [
18]. The villus's edges secrete various digestive enzymes; thus, higher VH indicates stronger enzyme secretion capacity and better digestion and absorption abilities. CD reflects cell generation rates, with shallower crypts indicating increased cell maturation rates and enhanced intestinal secretion function. A higher VH/CD value signifies stronger intestinal digestive and absorption capacities, while a decrease indicates weakened abilities [
19].
Previous research indicates that adding probiotics to the diet can protect the gastrointestinal tract from excessive feeding, increase VH and VH/CD in broiler chickens, and reduce CD [
20]. The results of this experiment demonstrate that adding
Sphingomonas to broiler chickens' drinking water significantly increased the VH/CD ratio of the ileum and enhanced its digestive, absorption, and defense abilities. Moreover, the addition of
Sphingomonas Z392 to drinking water led to a notable increase in intestinal probiotics such as
Lactobacillus, which would activate cell mitosis and induce intestinal epithelial cell proliferation by secreting short-chain fatty acids (SCFAs), resulting in increased intestinal villus length [
21,
22].
4.2. Effects of Sphingomonas on Gut Microbiota
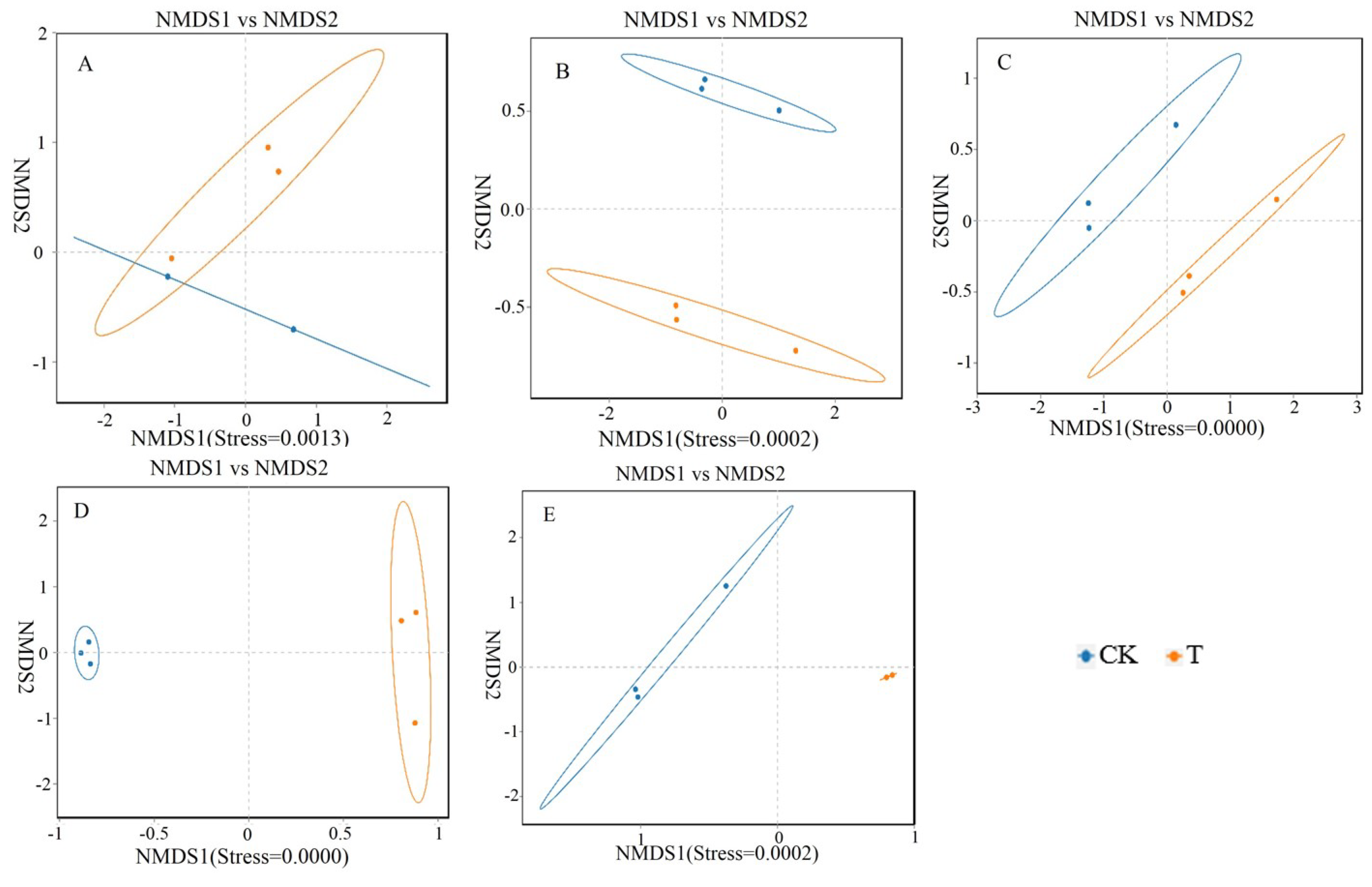
Studying the relative abundance and diversity of the gut microbiota is crucial for understanding the impact of beneficial bacteria [
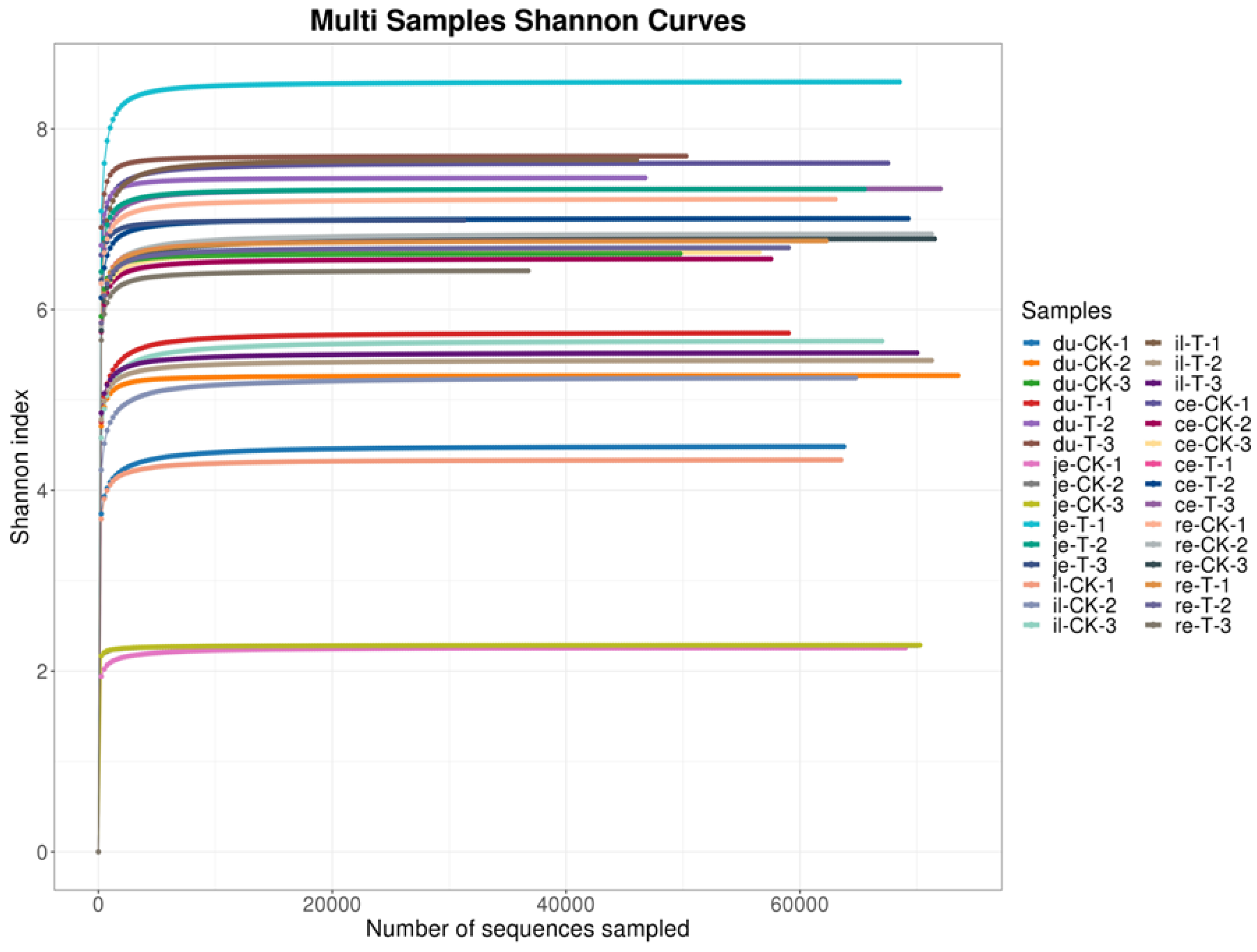
23]. In this experiment, the Shannon curve's horizontal width for multiple samples in each group was relatively large, indicating high species abundance in the samples. Additionally, the curve appeared relatively flat, suggesting a relatively uniform distribution of species across each group of samples. The coverage index for each group was 1, indicating that the different intestinal sequencing results in this experiment accurately reflected the true microbial composition of the samples.
The degree of intestinal health is determined by indicators such as microbial content and morphology, with the distribution of microorganisms varying among different intestinal segments [
24]. The gut microbiota plays a crucial role in maintaining animal health, immunity, and production performance, and probiotics have been shown to improve the microecological environment of broiler intestines [
23]. The relative research previously indicated that adding
Bacillus amyloliquefaciens to the diet can increase the relative abundance of
Bacteroidetes,
Butyricicoccaceae,
Faecalibacterium,
Heliobacillus, Lactobacillus,
Parabacteroides, and
Ruminococcus in the gut of broiler chickens [
25].
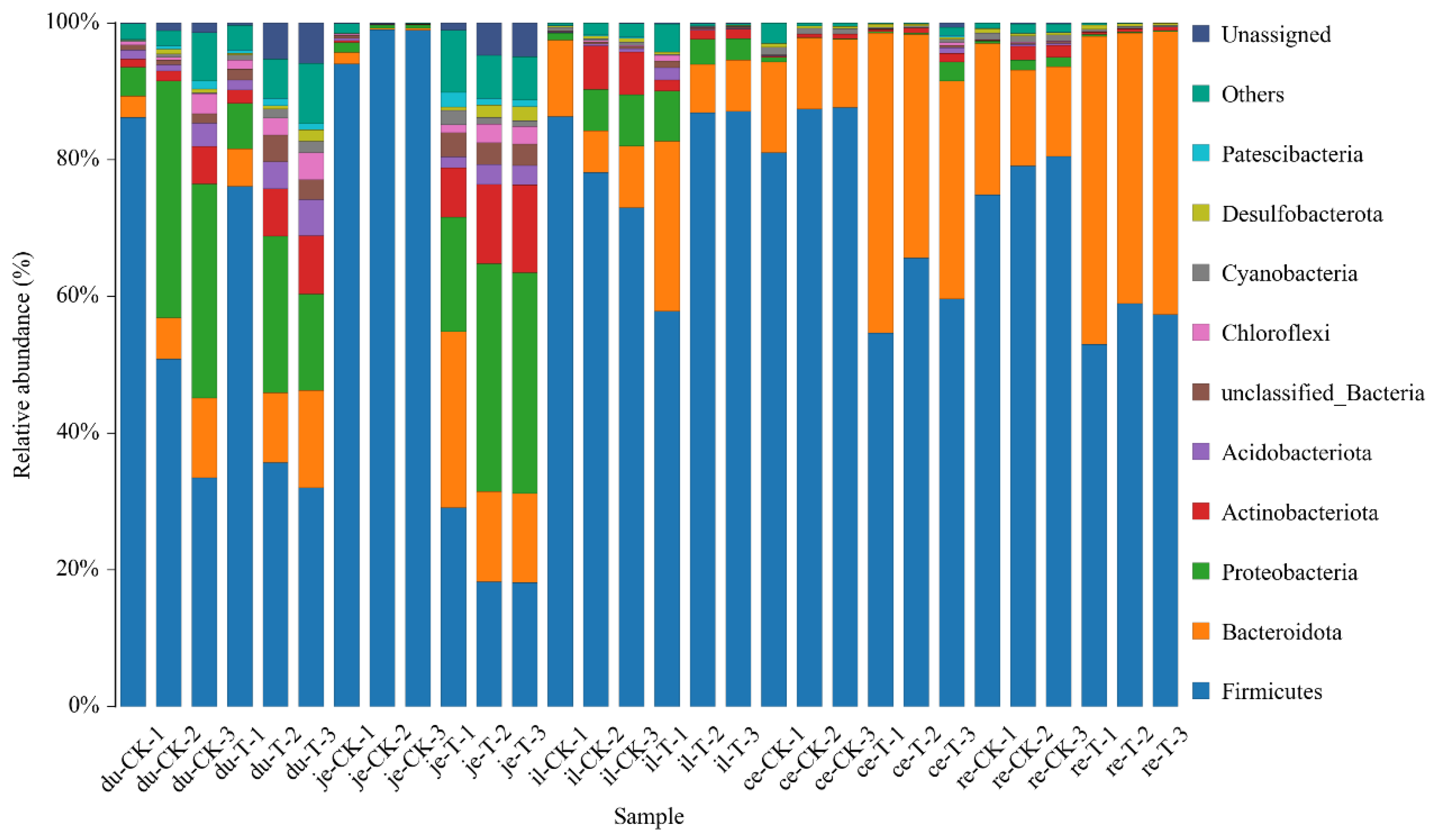
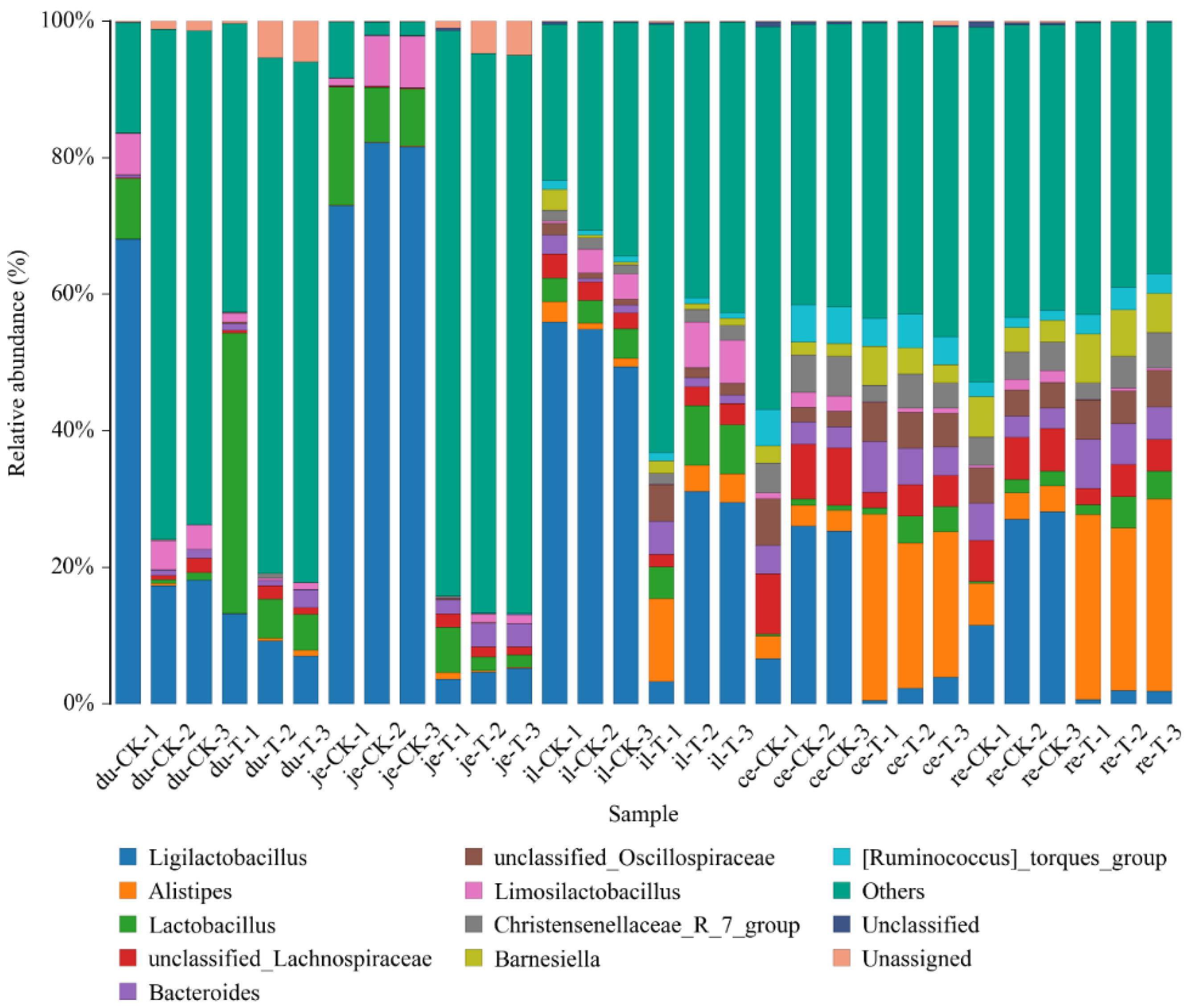
This experiment found that adding 4 × 10
5 CFU/mL
Sphingomonas Z392 to the drinking water of broiler chickens increased the relative abundance of microorganisms, including
actobacillus in the duodenum;
Bacteroides in the duodenum, jejunum, ileum, and rectum;
Lachnospiraceae in the duodenum and jejunum;
Aminobacterium in the duodenum and ileum;
Oribacterium in the jejunum, cecum, and rectum;
Christensenellaceae of bacteria in the jejunum and cecum;
Proteiniphilum in the jejunum and ileum;
Faecalibacterium in the jejunum;
Barnesiella in the jejunum and cecum;
Ruminococcus in the jejunum and rectum;
Phascolarctobacterium in the jejunum;
Butyricicoccaceae in the jejunum;
Caproiciproducens in the cecum. The above microorganisms could promote the digestion and absorption ability of broiler chickens. Furthermore, the microorganisms with reduced relative abundance include
Odoribacter in the duodenum,
Alistipes in the jejunum,
Parabacteroides in the jejunum, ileum, and rectum, and
Rikenellaceae in the cecum and rectum. These microorganisms could inhibit the occurrence of intestinal diseases [
26,
27].
The microorganisms
Actobacillus,
Lachnospiraceae,
Oribacterium,
Christensenellaceae,
Faecalibacterium,
Ruminococcus, and
Caproiciproducens belong to
Firmicutes, which can hydrolyze starch and other sugars to produce butyrate and other secretes short-chain fatty acids (SCFAs), such as acetate, propionate, butyrate, and lactate [
28,
29]. SCFAs play multiple roles in the intestine. They not only provide energy to the body, but also reduce the types of harmful bacteria, stimulate the proliferation and differentiation of intestinal epithelial villous cells, increase the height of villi, expand the contact area between villi and chyme, and improve the digestion and absorption ability of broilers [
29]. In addition, the increase of beneficial microorganisms such as
Actobacillus in the intestine also helps to maintain the integrity of the intestinal structure, promote metabolism, and thereby plays an important protective role as the first line of defense against pathogenic bacteria [
30].
In addition, Alistipes,
Odoribacter,
Rikenellaceae, Bacteroides, and Parabacteroides belong to
Bacteroidetes. They primarily digest grain feed and secrete mucin to protect and lubricate the intestines, exerting an anti-inflammatory effect [
31,
32,
33].
Bacteroidetes can also enhance the disease resistance of broiler chickens by stimulating the immune system, increasing macrophage phagocytosis, and resisting the colonization of pathogenic bacteria [
32]. The lithocholic acid secreted by
Odoribacter promotes fat digestion and absorption, regulates blood lipids, promotes cell proliferation, exhibits anti-inflammatory properties, and protects the gastric mucosa. Additionally, the lithocholic acid produced by
Odoribacter demonstrates excellent anti-inflammatory and antibacterial activities. Even in small amounts, it effectively eliminates pathogenic microorganisms such as
Clostridium and
Enterococcus faecalis, reduces inflammation levels, and regulates the body's immunity [
34].
Bacteroides, a core member of the gut microbiota, regulates the host mucosal immune system, reduces inflammation, participates in carbon metabolism, and secretes SCFAs such as acetate and propionate [
35].
Rikenellaceae in the intestine plays a certain role in protecting the body's health and alleviating diseases [
36].
Aminobacterium Bacteroides, a core member of the gut microbiota, regulates the host mucosal immune system, reduces inflammation, participates in carbon metabolism, and secretes short-chain fatty acids (SCFAs) such as acetate and propionate [
37].
Phascolarctobacterium produces SCFAs, including acetate and propionate, reduces inflammation, and protects the intestinal mucosal barrier by decreasing levels of lipopolysaccharide (LPS)-binding proteins and C-reactive protein (CRP) [
38].
Barnesiella is associated with bile acid production and plays a crucial role in fat metabolism [
39].
Proteiniphilum contributes to the conversion of acetic acid to butyric acid [
40].
Butyricicoccaceae produces digestive enzymes in the intestine that break down starch, protein, and cellulose, promoting the digestion and absorption of these nutrients. Furthermore, butyric acid, the main metabolite of this genus, promotes the regeneration and repair of intestinal epithelial cells. Therefore,
Butyricicoccaceae significantly influences the intestinal microbiota's structure, inhibits pathogenic bacteria, and promotes the growth of beneficial bacteria such as Lactobacillus [
41].
Probiotics not only enhance nutrient digestion and absorption, improve the breeding environment, but also reduce pathogenic microorganisms and increase the relative abundance of anti-inflammatory microorganisms, thereby enhancing the body's resistance to infections [
25]. This study revealed that adding
Sphingomonas Z392 to the drinking water of broiler chickens decreased the relative abundance of microorganisms such as
Campylobacter in the jejunum and ileum,
Shigella Castellani in the ileum, Bilophila in the ileum,
Clostridia in the cecum and rectum, and
Anaerotruncus in the cecum. These microorganisms are positively correlated with intestinal diseases [
26,
27].
But after adding Sphingomonas to the drinking water of broiler chickens, the relative abundance of Staphylococcus was increased in the jejunum, which can easily cause gastrointestinal diseases. Therefore, when using Sphingomonas, it is necessary to use other microorganisms in combination to inhibit the proliferation of potential harmful bacteria, ensuring the health and breeding benefits of broilers.
4.3. Growth Promotion of Sphingomonas spp.
In this study, the relative abundance of microorganisms positively correlated with digestion and absorption and negatively correlated with intestinal diseases in broiler chickens was significantly increased by adding
Sphingomonas spp. to drinking water during the feeding process. These microorganisms promote the digestion and absorption of nutrients in chyme by secreting digestive enzymes such as amylase, protease, glycosidase, and cellulase, or produce SCFAs, which reduce the types of harmful bacteria and stimulate the proliferation and differentiation of intestinal epithelial villous cells. This results in a high increase in villi, expanding the contact area between villi and chyme, and improving the digestion and absorption ability of broiler chickens [
29]. Alternatively, secreted bile acids can promote fat digestion and absorption, regulate blood lipids, promote cell proliferation, exhibit anti-inflammatory properties, and protect the gastric mucosa. Meanwhile, the addition of these strains significantly reduced the degree of inflammation in the small intestine, ensuring its basic digestive and absorption functions, and promoting the digestion of nutrients such as sugars, proteins, fats, and inorganic salts. Additionally, the number of goblet cells in the small intestine villous epithelium increases, playing an important role in maintaining intestinal health and promoting nutrient absorption. At the same time, the degree of mitochondrial swelling in the villous epithelial cells of the jejunum decreases, indicating a decrease in their degree of damage, thereby ensuring the digestive and absorption functions of the jejunum. Importantly, the VH/CD of the ileum significantly increased (
p<0.05), and the number of lysosomes in the villous epithelial cells increased. This significantly improved the digestive and decomposition ability of the ileum, facilitating further absorption of nutrients in the ileum. Lactobacillus and Clostridium butyricum also significantly affect the growth, development, and feed utilization of broiler chickens by regulating small intestinal VH and CD [
6,
42,
43].
Additionally, due to the relatively short intestine of chickens, the digestion of chyme in the small intestine is not complete, so the cecum plays an important role in the chicken's digestive system [
44]. This study found that the number of mitochondria in cecal villous epithelial cells increased under the action of
Sphingomonas, which promoted the absorption of nutrients by the cecum.
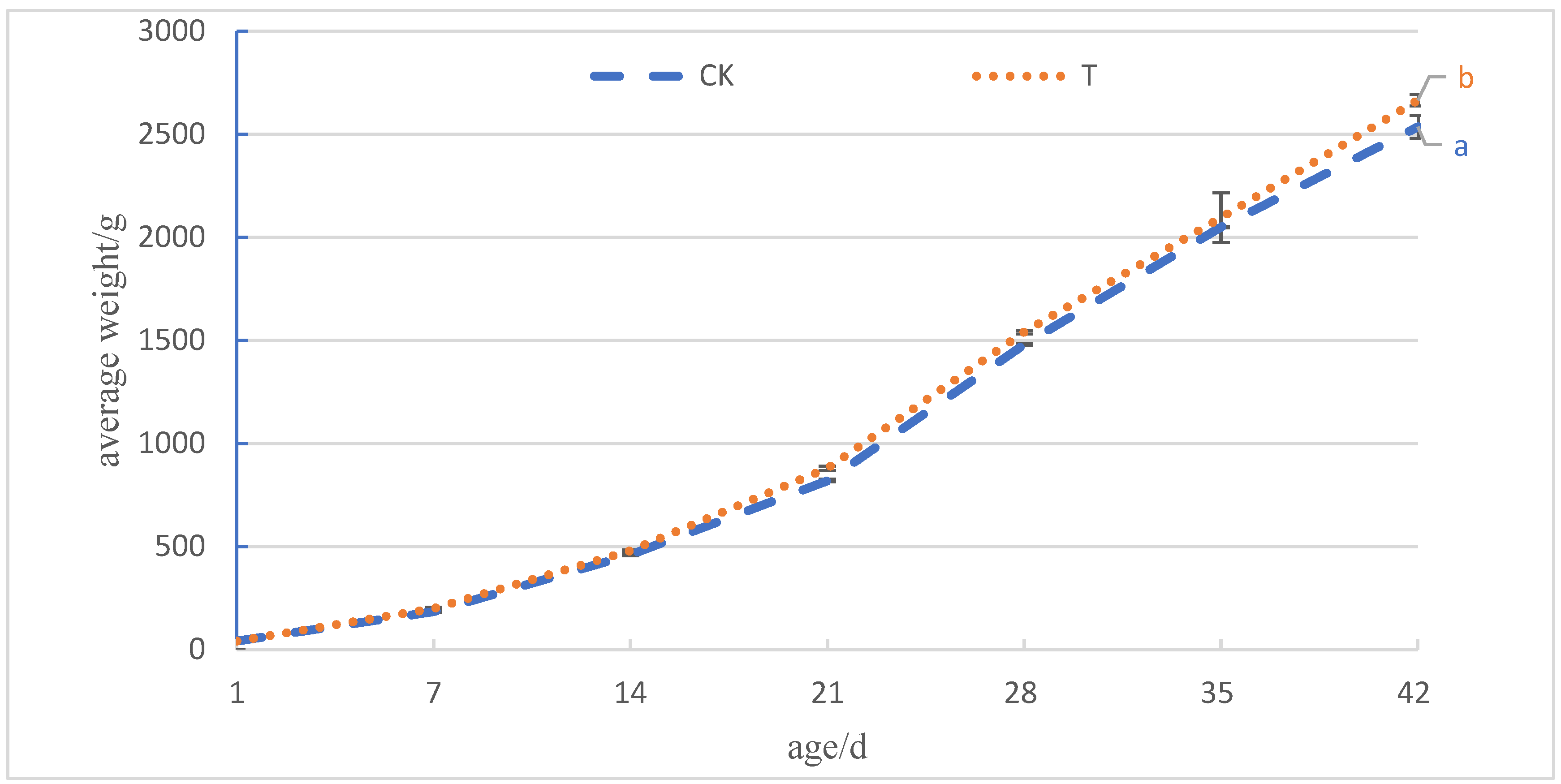
After adding 4.0 × 105 CFU/mL of Sphingomonas Z392 to the drinking water of broiler chickens, the improvement of intestinal histology and microbial community structure not only enhanced the absorption function of the overall digestive tract of broiler chickens, but also improved the utilization rate of feed and the growth performance of broiler chickens, resulting in an increase of 4.33% in slaughter weight and 10.10% in the EPI, resulting in better economic benefits for broiler chickens.
In practical applications, adding an appropriate amount of Sphingomonas Z392 to the drinking water of broiler chickens could significantly increase their final weight, increase the EPI, and thus bring significant economic benefits. These results indicate that Sphingomonas Z392 has broad application prospects in broiler feeding and is expected to provide new strategies for the sustainable development of the broiler industry.