1. Introduction
As a mean to prevent infectious diseases, vaccination has been demonstrated to be the most efficient and cost-effective. Vaccines administration could activate the immune system and generate innated and adaptive immunity against pathogens by delivering antigens. In recently years, the use of nanoparticles in vaccine platform development has gained significant attention due to their unique properties and potential applications. The advent of smart nanocarriers possessing the potential to deliver hydrophobic and hydrophilic pharmaceuticals in a controlled manner has mitigated the diseases treatment scenario [
1,
2]. As a new therapeutic approach, cancer nanovaccine can deliver tumor antigens and immune adjuvants simultaneously. At present there are currently around 50 FDA approved cancer nanomedicines available in the market, illustrating the primary focus of nanomedicines has been on cancer treatment [
3]. Nanoparticles, as a novel drug delivery system, have been demonstrated extensive prospects in the field of medicine. The advantages of nanoparticles for vaccine delivery included enhancing immunogenicity, targeted delivery, and improving stability, providing new solutions for drug and vaccine delivery. Vaccine delivery platform is for delivering antigens and adjuvants to the immune system that can enhance the stability and immunogenicity of vaccines. In the current research status of nanoparticle-based vaccine platform development, there is a growing interest in exploring the potential of nanoparticles as vaccine delivery systems. Nowadays, delivery platforms for vaccines especially mRNA (messenger RNA, mRNA) vaccine are actively investigated in using various types of nanoparticles including lipid-based, peptide-based, polyplexes and polymeric nanoparticles, and inorganic nanoparticles, and so on [
4].
In the past few years, mRNA vaccine technology has drawn increasing research and commercial attention owing to their potential in tackling both infectious disease prevention and cancer therapeutics [
5,
6,
7,
8]. mRNA vaccine administration could inoculate the mRNA to the host, utilize the translation system of the host cell to synthesize encoding protein, induce and activate the host immune response. For example, in the nanoparticle delivery platform, lipid-based nanoparticles are used to encapsulate mRNA and deliver mRNA encoding antigen to immune cells. In particularly, mRNA vaccines have been urgently approved for use in the COVID-19 pandemic. To overcome the barriers to safe and effective RNA delivery, scientists have developed various delivery systems that protect the RNA from degradation, maximize delivery to target cells. The advances of synthetic materials as delivery vehicles that encapsulate RNA such as lipid nanoparticles, polymers, have focused on non-viral-based delivery system [
9]. The therapeutic potency of lipid nanoparticle-based mRNA vaccines for tumor treatment, represented by Pfizer/ BioNTech and Moderna, has been broadly validated in clinical application [
10,
11]. Besides lipid nanoparticle-based delivery platforms for vaccine, the development of other nanoparticles also gained tremendous momentum. For instance, the use of inorganic nanoparticles such as gold, silver and iron oxide nanoparticles for vaccine delivery is also a focus of ongoing research [
12].
Furthermore, development of nanoparticle platforms was also exploring the potential of nanoparticles to enable targeted vaccine delivery to specific immune cells such as dendritic cells or tissues [
13]. Generally, the surface modification of nanoparticles with targeting ligands that can selectively bind to receptors on immune cells, thereby enhancing the uptake of vaccines and the activation of immune responses. In addition to vaccine delivery, current research efforts are also focused on understanding the immunological mechanisms underlying the interaction between nanoparticles and the immune system. This includes investigating the impact of nanoparticle size, shape, surface charge, and surface chemistry on immune cell activation, antigen presentation, and the generation of immune responses [
14]. Overall, the development of nanoparticle-based delivery platform for vaccine is characterized by a diverse range of studies aimed at optimizing the design, formulation, and delivery of nanoparticle-based vaccines. These efforts are driven by the potential of nanoparticles to overcome the limitations of traditional vaccine formulations and to enhance the efficacy and safety of vaccines for infectious diseases, cancer, and other conditions. Here, we summarized the advances in the nanoparticle-based delivery platforms for vaccine development especially mRNA vaccine and their potential prospects in clinical applications.
2. Cancer Vaccines
2.1. Normal Tumor Vaccine Types
Tumor vaccines immunize the body using tumor-specific antigens to stimulate the process of antigen presentation, enhance the immune system's recognition and attack on tumors. The types of tumor vaccines mainly include tumor lysate vaccines, dendritic cell-based vaccines, peptide vaccines, nucleic acid (such as mRNA) vaccines and tumor neoantigen vaccines. Among them, dendritic cell vaccines are the most commonly studied cell-based tumor vaccines [
15]. Dendritic cell vaccines are prepared from dendritic cells collected from patients, which are genetically engineered to express tumor-associated antigens, thereby directly activating T cells to attack tumor cells. Qin et al, designed a dendritic cell-based nanovaccine (TPOP) that regulated lipid metabolism in DCs to restore and improve their ability to cross-presentation [
16]. This vaccine is administered after the tumor cell death caused by DOX, allowing it to capture the tumor-associated antigens (TAAs). By introducing TAAs into DCs and inhibiting lipid accumulation, TPOP restores DCs’ cross-presentation which is crucial for priming T cells. Peptide vaccines are a type of vaccine that utilizes tumor-specific peptide fragments as antigens. These peptide fragments are typically selected from tumor-associated proteins and can be recognized by the immune system [
17]. By injecting these peptide fragments, T cells can be activated, prompting the immune system to mount an immune response against tumor cells, leading to tumor elimination. Tumor neoantigen vaccines are specifically designed vaccines targeting new antigens presented on tumor cells. These new antigens arise from mutations in tumor cells and exhibit high tumor specificity. By activating the immune system to recognize and attack these new antigens, tumor neoantigen vaccines can aid in clearing tumor cells and achieving therapeutic goals.
The design of traditional cancer vaccines aims to activate the immune system to recognize and attack cancer cells that contain those shared antigens. However, due to the high heterogeneity and complexity of tumor, these vaccines often face challenges of limited efficacy and lack of specificity [
18]. Such conventional vaccines have several limitations that may restrict their application in disease prevention and treatment [
19]. For instance, the development of dendritic cell (DC) vaccines requires the preparation of the patient's own cells. Peptide vaccines exhibit MHC restriction, selectively activating monoclonal T cells, thus having a high risk of immune escape [
20].
2.2. mRNA Vaccine
In this decade, nucleic acid vaccines have emerged as innovative vaccines. Nucleic acid vaccines, such as mRNA vaccines, serve as a promising new form of vaccines that rely on using nucleic acids as information carriers. Notably, DNA- or RNA-based vaccines have gradually evolved into a promising form of conventional vaccines [
21]. These vaccines display its prevention and treatment role by delivering exogenous nucleic acids into immune cells. Nucleic acid vaccines transmit the encoding information of nucleic acid to the cells, eliciting the production of encoding antigen. These antigens can be recognized by the immune system, processed, and presented to T cells. With the activation of T cells, vaccine can trigger immune response against tumor-specific antigens and initiate an attack on tumor cells. Compare to mRNA vaccines, DNA vaccine have risk of genomic alternation, long-term expression, and generation of anti-DNA autoantibodies that might impede their utilization in human [
22,
23].
Unlike DNA vaccine, before the COVID-19 pandemic, companies including Moderna and BioNTech had been deeply involved in mRNA vaccines, particularly in the field of tumor vaccines, and had conducted multiple clinical trials [
24,
25]. In current clinical applications that mRNA vaccines have several advantageous features compared to traditional vaccines. mRNA vaccines utilize host cells as natural centers for immune responses. In host cells, mRNA encoding antigen are synthesized and then to trigger immune responses. This method ensures that proteins are correctly folded, assembled, and targeted to the appropriate cellular locations within host cells [
26]. In numerous basic and clinical experimental studies, the use of synthetic mRNA as a tumor vaccine antigen component has shown the ability to induce the synthesis and processing of antigen, activate T cells, and exert efficient anti-tumor effects [
27,
28].
mRNA vaccines, particularly those based on individual-specific neoantigens, represent a new direction in cancer therapy. In comparison to other nucleic acid vaccines, the advantages of mRNA-based cancer vaccine strategy include: (1) mRNA can be translated into protein rapidly in both non-dividing and hard-to-transfect cells such as DCs (dendritic cells, DCs) without the need of nuclear translation and transcription. The rate and magnitude of protein expression of mRNA are typically higher than DNA vaccines. (2) Unlike DNA vaccines, mRNA vaccines will nor influence genetic material or integrate in genome sequence, thus free of insertional mutagenesis [
29,
30]. (3) Other advantages of mRNA vaccines include high potential for rapid development and potency, low-cost manufacture, and safe administration [
18,
19]. Now, mRNA vaccines have shown promise in the development of personalized cancer immunotherapies.
3. Nanotechnology in mRNA Vaccines Platform
The development of mRNA technology, particularly its successful application in the development of COVID-19 vaccines, also has greatly propelled advancements in the field of cancer vaccines. mRNA vaccines can be rapidly designed and produced, effectively encoding tumor-specific antigens to guide the immune system in recognizing and eliminating cancer cells. This innovative technology provides a more flexible and effective approach for the development of cancer vaccines [
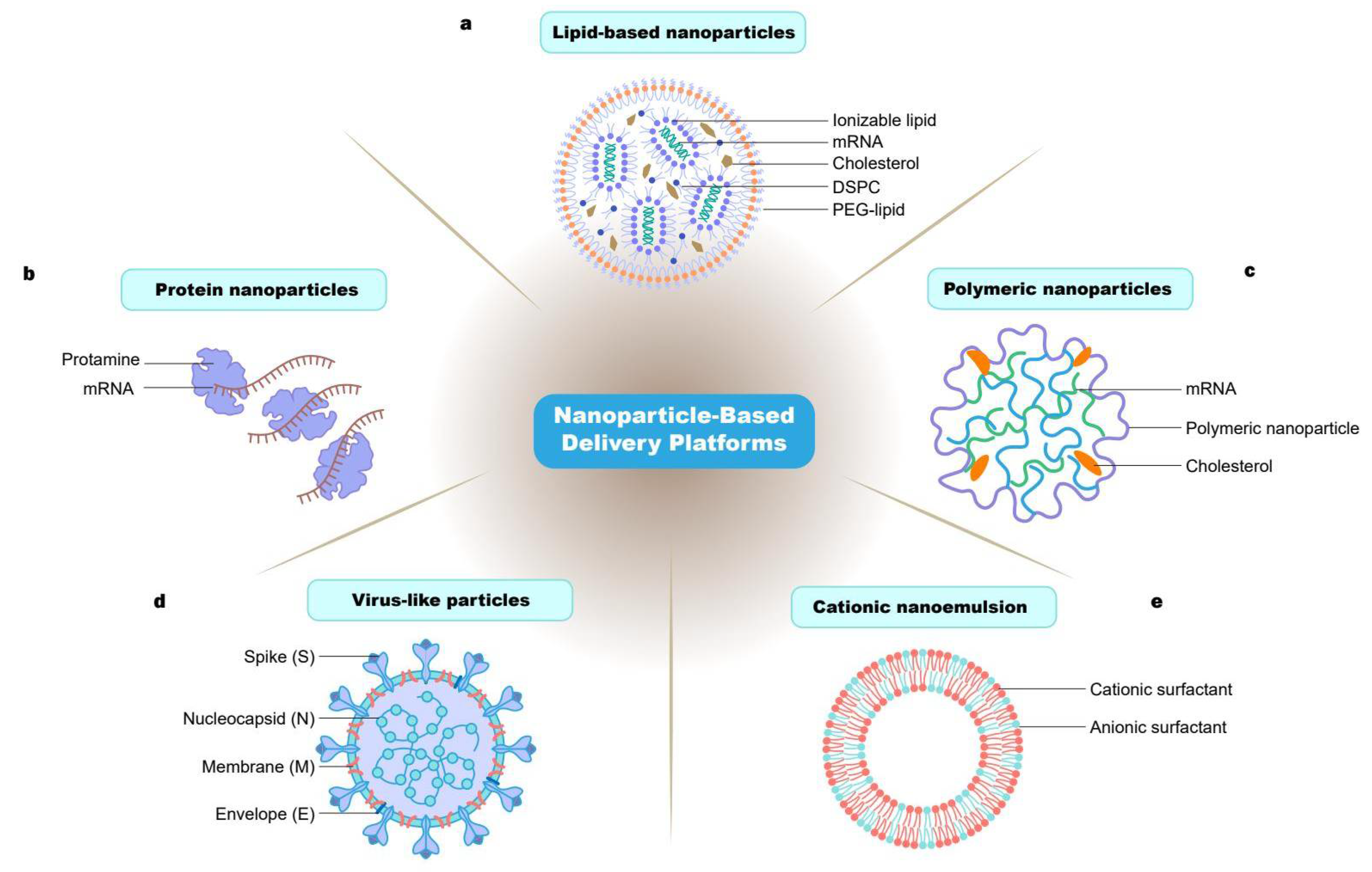
18]. In this review, we mainly focus on the nanoparticle delivery systems of mRNA vaccines. We mainly introduce the nanoparticles delivery platforms for mRNA vaccine such as lipid, polymer, protein, virus-like particles and cationic nanoemulsion- based nanoparticle (
Figure 1). mRNA vaccines involve inoculating the host with mRNA encoding antigenic, utilizing the host cell's expression system to synthesize the corresponding antigens, while inducing and activating the host's immune response to the antigen. Effective in vivo mRNA delivery is crucial for achieving the desired therapeutic effects. However, due to the inherent structural characteristics of mRNA, such as large molecular weight, high negative charge, and susceptibility to degradation [
31]. Therefore, the development and application of mRNA vaccines face numerous challenges, with the main issues being poor mRNA stability and low mRNA delivery efficiency in vivo.
Therefore, efficiently delivering in vitro transcription mRNA encoding antigen into cells is a key challenge in RNA cancer vaccine therapy. With the advancement of nanomedicine, the application of nanomaterials in tumor immunotherapy is becoming increasingly widespread. As antigens and adjuvants need to be co-delivered to antigen-presenting cells for effective antigen presentation, biomimetic nanoparticles with high drug loading capacity and well drug release controllability are ideal carriers for vaccines [
13]. Their degradable shell can help in the sustained release of drugs upon reaching the designated site [
32]. Nanoparticle was classified as a material or substance that is with a dimension between 1 to100 nm or up to 1000 nm. As delivery vehicle, nanoparticles usually were equipped with three major roles: as carriers, adjuvant and presentation platforms [
33].Researches have demonstrated that nanoparticles have remarkable success as nucleic acid delivery carriers and adjuvant [
34]. Here, we will introduce several common nanoparticle delivery platforms with their current research status.
3.1. Lipid-Based Nanoparticles Delivery System
3.1.1. Composition of Lipid-Based Nanoparticles
Lipids are amphiphilic molecules that contain three domains: a polar head group, a hydrophobic tail region and a linker between the two domains [
35,
36]. As a key class of drug delivery system, lipid-based nanoparticles (LNPs) have been approved by the FDA for siRNA and mRNA delivery [
37,
38]. The use of LNPs to encapsulate and deliver mRNA has become an important therapeutic advance. Lipids have been developed for mRNA delivery include cationic lipids, ionizable lipids and other types of lipids such as phospholipids, cholesterol or polyethylene glycol (PEG) [
39]. And LNPs are a nanoscale particle system usually composed of phospholipids, cholesterol, and other components.
This platform is commonly used in vaccine delivery and development. Due to the similarity of lipid components to those found in the body, LNPs exhibit excellent biocompatibility. The surface of LNPs is easily modifiable. Surface modifications could usually improve the targeting property of nanoparticles. This feature allows for precise delivery of antigens to specific cells or tissues, thereby enhancing vaccine efficacy [
40]. Additionally, LNPs demonstrate excellent stability which effectively protect antigens within the carrier from degradation and damage. Those characteristics are crucial for the long-term storage and delivery of vaccines that can help to avoid immune and toxic reactions [
41]. Another key characteristic to be considered when designing LNPs for drug delivery, especially nucleic acid therapy, is the ionizable lipid's ionization state in the system, which is characterized by its apparent pKa (pKa app) [
42]. The degree of ionization significantly influences the surface charge, stability, toxicity, and in vivo efficacy of nanoparticles. Many factors can determine the apparent pKa of ionizable lipids, such as lipid structure, various components in the LNP formulation, nanoparticle size, and pH of the bulk solution [
43]. Previous studies have shown that for effective nucleic acid delivery, the optimal pKa of LNPs should be around pH 6.5. For example, lipid-based nanoparticles have the most important role and are the first type of vector developed for mRNA delivery. They contain amine cations and carry positive charges that can achieve adsorption and complexation of negative mRNA through electrostatic interaction. However, this positivity is potentially cytotoxic. The appearance of ionizable lipids makes up for the deficiency of cationic lipids. Ionizable liposomes are positively charged at acidic pH, electrically neutral under physiological conditions in vivo, which avoid non-specific recruitment with serum proteins, reduce cytotoxicity. These properties determine the high serum stability and long blood circulation time [
44]. After endocytosis into endosomes, ionized lipids could reverse to positive at low pH, and promote endosomal escape of mRNA by "proton sponge" effect, thus improving transfection efficiency [
45].
3.1.2. Application of Lipid-Based Nanoparticle Delivery Platform
The key to the success of the currently approved SARS-CoV2 mRNA vaccines and of many mRNA-based prophylactic vaccines in clinical development are efficient delivery vehicles. LNPs as delivery vehicles could mediate efficient mRNA expression in situ and endow the vaccine with intrinsic adjuvant properties[
46]. Multiple studies have revealed that comparative analyses of the immunogenicity and antitumor efficacy of three mRNA-LNP vaccines (U sa-mRNA, U-, or m1Ψ-modified mRNA) encoding the same antigen, gDE7, in a preclinical model of HPV-16–associated tumors [
47]. The potent CD8
+ T cell response induced by gDE7 mRNA-LNP vaccine is associated with increased tumor clearance rates and inhibition of tumor recurrence. Furthermore, in anti-tumor models, it has been shown that CD4
+ T cells contribute to the effective priming of CD8
+ T cells and the development of long-term immune memory [
48]. Chen et al. utilized lipid nanoparticle-mediated lymph node-targeted delivery of mRNA cancer vaccine to elicit robust CD8
+ T cell response. By encapsulating mRNA in lipid nanoparticles, it enhances the immunogenicity and antigen presentation efficiency of the mRNA vaccine, enabling effective targeted delivery to lymph nodes, activating CD8
+ T cells, and thereby enhancing the immune system's antigen-specific response to cancer [
49]. A recent study reported a novel nano lipid polymer system (PIR-Au NPs) based on enhanced penetration and retention (EPR) effects, which can be successfully applied to image-guided passive targeted therapy [
50]. This vaccine may provide a promising tool for the future treatment of cancer. Zhang et al. developed a lipid-like material C1 with 12-carbon tail that could effectively deliver mRNA into DCs, produce efficient mRNA translation and induce robust T cell response [
51]. Overall, mRNA vaccines can enhance the immune response against tumors by stimulating and strengthening the cytotoxic function of CD8
+ T cells, leading to increased tumor clearance rates and potentially preventing tumor recurrence. This highlights the significant role of mRNA vaccines in harnessing the anti-tumor capabilities of CD8
+ T cells.
Lipid-encapsulated mRNA can also be used to promote the production of functional monoclonal antibodies and T-cell receptors in vivo. This property does not require oligomerization but instead involves the independent assembly of heavy and light chains translated from different open reading frames, or the utilization of other polymers to combine with LNPs for co-encapsulation and delivery of mRNA [
52,
53,
54]. Despite these promising clinical applications, their translation efficiency, stability and safety necessitate to be further optimized [
55,
56,
57].
3.1.3. Insufficient and Optimization of Lipid-Based Nanoparticle Delivery Platform
LNPs assist mRNA to cross physiological barriers such as cell membranes and endosomes/lysosomes, promoting the intracellular presentation of mRNA. But the nuclear escape efficiency and biosafety of some commercial LNPs are still unsatisfactory, leading to underutilization of mRNA. Zhang et al. employed a simple fluorination strategy, that fluorinated PEG-lipids were synthesized via efficient condensation reactions to alter the surface properties of LNPs [
58]. This modification can enhance the utilization of mRNA by promoting cellular uptake and endosomal escape. In addition to this, LNPs-based mRNA vaccine platform suffers from increased production of reactive oxygen species during translation, which leads to a decreased translation efficiency and the onset of inflammation and other side effects. In order to address this issue, Yang et al. synthesize a lipid modified poly (guanidine thioctic acid) polymer to fabricate novel LNPs (G-LNPs) for mRNA vaccines. The acquired G-LNPs significantly promote the translation efficiency of loaded mRNA and attenuate inflammation after vaccination through the elimination of reactive oxygen species that are responsible for translational inhibition and inflammatory responses[
59]. In addition, the insufficient accumulation of mRNA vaccines based on LNPs in antigen-presenting cells remains a key barrier to triggering effective anti-tumor immune responses. Lei et al. developed a dendritic cell (DC)-targeting LNPs using mannose receptor-mediated endocytosis [
60]. This sugar-coated LNPs (STLNPs-Man) could deliver mRNA to DCs effectively in vitro and in vivo. Importantly, STLNPs-Man@mRNA possessed the ability to downregulate CTLA4 expression by blocking the CD206/CD45 axis, showing enhanced anti-tumor efficacy through combined immune checkpoint blockade therapy.
To increase mRNA–LNP specificity and reduce off-target expression, there are some other lipid-based nanoparticles delivery platforms. While the LNPs utilized in the COVID-19 vaccines were not specifically targeted and primarily taken up by phagocytic cells (such as dendritic cells and macrophages) after intramuscular injection, for other applications of LNPs, uptake by specific cell types in specific organs in their natural location is required [
61,
62]. To solve this problem, Caitlin et al. proposed a targeted LNPs (tLNPs) strategy that can efficiently deliver RNA to specific cell types based on the expression of cell surface markers [
63]. These tLNPs are encapsulated with targeting antibodies, enabling mRNA cargo delivery to specific cells. The another one, mRNA can be delivered by ionizable lipid nanoparticles (iLNPs) to placental trophoblasts, endothelial cells, and immune cells in clinical practice [
39]. Delivery of this top-tier LNPs formulation containing VEGF-A mRNA can induce placental vascular relaxation, indicating the potential of mRNA LNPs for protein replacement therapy in treating placental diseases during pregnancy [
64]. LNPs deliver VEGFA mRNA into cells to enhance VEGFA expression and function. Treatment with LNP-VEGFA mRNA boosts the effectiveness of cardiac progenitor cell in promoting endothelial cell angiogenesis and modifying extracellular vesicles [
65].
3.1.4. Self-Adjuvant and Adjuvant in Lipid-Based Nanoparticle Delivery Platform
In current research, in addition to lipid nanoparticle vaccines, adjuvant-combined lipid nanoparticle vaccines are being developed. Recently, the development of an mRNA vaccine based on LNPs has been reported, which contains lipid with cyclic amino groups that activate the STING (stimulator of interferon genes) pathway, leading to the stimulation of type I interferons (IFN) and potentially enhancing adaptive immune responses[
66,
67]. Bowen et al. developed a multi-adjuvant mRNA vaccine composed of lipid nanoparticles encapsulated mRNA encoding antigens, optimizing the efficient delivery of mRNA, enhancing activation of innate and adaptive responses, and improving the effectiveness, safety, and usability of mRNA-based immunization. They screened 480 biodegradable and ionizable lipids with cyclic amine adjuvants and fused mRNA encoding antigens with a natural adjuvant derived from C3 complement protein to optimize the vaccine. This multi-adjuvant mRNA vaccine also increased antibody titers against SARS-CoV-2 by 10-fold. Compared to traditional mRNA transcript and LPS co-delivery strategies, the C3d adjuvant approach was more effective in inducing adaptive immune responses. The adjuvant function of C3d-fused mRNA allowed for protective immune responses with a 10-fold lower dose of mRNA vaccine [
29]. This multi-adjuvant mRNA vaccine system has the potential to enhance the safety, effectiveness, and convenience of mRNA vaccines for infectious diseases and other medical indications. A type of LNPs with self-adjuvant property enhance the innate immunity of mRNA-LNP vaccines. By partially replacing ionizable lipids with adjuvant-like lipids, not only the delivery of mRNA is enhanced, but also the LNPs acquire toll-like receptor 7/8 stimulating activity, significantly boosting the innate immunity of SARS-CoV-2 mRNA-LNP vaccines in mice and exhibiting good tolerability [
68]. The formulation of this adjuvant-like lipid combined with SARS-CoV-2 mRNA-LNP vaccine was optimized, and its innate and adaptive immune responses were systematically evaluated, capable of activating DCs to present antigens, expressing co-stimulatory molecules, and producing selective cytokines (such as tumor necrosis factor-alpha (TNF-α)), effectively stimulating the transition from innate immunity to adaptive immunity [
69].
Taken together, LNPs can overcome many challenges, as they have demonstrated efficient cellular uptake and potent mRNA delivery in vivo [
70,
71]. Currently, LNPs are the most clinically advanced nonviral drug delivery platform for nucleic acid therapeutics. Lipid-based vaccine delivery system design helps improve the immunogenicity of the vaccine and antigen presentation efficiency, providing a potential new strategy for cancer immunotherapy.
3.2. Polyplexes and Polymeric-Based Nanoparticles Delivery System
Polymers also are commonly used for nucleic acid delivery. mRNA vaccines based on polymer are nano-complexes formed by electrostatic interactions between cationic polymers and nucleic acids. Polyethylenimine is the most studied polymer for nucleic acid delivery with excellent efficacy and toxicity owing to high charge density [
72,
73,
74]. Now, several alternative biodegradable polymers with less toxic have been developed including poly(β-amino ester)s, poly(amidoamine)s. Among of these polymers, poly(amidoamine) (PAMAM) is one of the most studied dendrimers, combining a modified PAMAM dendrimer with PEG-lipid, a replicon RNA-based vaccine was developed that provided protective immunity against several pathogens [
75].
The cationic polymer can compress mRNA into nanoparticles with high density, forming polymer micelles that encapsulate mRNA internally, thereby enhancing nucleic acid stability. Although polymer-based mRNA delivery systems are not as advanced in clinical applications as lipid delivery systems they have the potential to provide unique characteristics. And there also have challenges such as relatively lower transfection efficiency and potential toxicity. For example, polymer-based systems can assemble various nanostructures under aqueous conditions with the ability for lyophilization and long-term storage, and exhibit unique pharmacokinetics [
76]. These characteristics may contribute to the development of advanced mRNA therapeutics. In recent study, people established CDN-NPs as a potent innate immune agonist therapy. The conjugation of CDN with NPs ensures that particle-dependent drug delivery exhibits higher potency, drug loading capacity, and stability. CDN-NPs are eventually up-taken by immune cells in the tumor microenvironment (TME) and secondary lymphoid organs, with internalized CDN-NPs transferred to proximal immune cells in the TME. The application of polymer nanoparticles increases vaccine stability and payload, thereby expanding the therapeutic window in multi-gene tumor models and inhibit tumor growth effectively [
77]. This work underscores the importance of nanoparticle structure in modulating the efficacy of immunotherapy. Furthermore, a polymeric acid-based phosphatidyl polymer library has been developed for in vivo mRNA delivery with spleen targeting ability. This polymeric acid-based phosphatidyl polymer library demonstrates the capability of spleen targeting in vivo, enabling effective mRNA delivery and potentially aiding in the development of therapeutic or vaccine delivery systems targeting the spleen [
78]. And Alexandra et al. reported an inhalable polymer carrier for delivering therapeutic mRNA to the lungs. They optimized the biodegradable poly (amino ester) (PACE) complexes using end-capping modifications and polyethylene glycol for mRNA delivery [
79]. These complexes deliver mRNA to lungs with high transfection efficiency, particularly in epithelial cells and antigen-presenting cells. This technology is a mucosal vaccine against severe acute respiratory SARS-CoV-2, and the finding indicated that intranasal administration of mRNA encoding spike protein complexes induced effective cellular and humoral adaptive immunity and protected susceptible mice from lethal virus challenge.
Polymer nanocarriers can easily be modified with targeting ligands on their surface, making them ideal for targeted mRNA delivery through ligand-receptor interactions, ligand-mediated enhanced mRNA delivery, and targeting tumors to improve the precision of immunotherapy [
80].
3.3. Protein-Based mRNA Delivery System
Protein nanoparticles are a type of nanoparticle system assembled from natural protein or synthetic proteins by genetic engineering. Natural proteins such as serum proteins, hemoglobin, etc., are used in vaccine carrier design, while synthetic proteins can be custom-designed using genetic engineering techniques. Protein nanoparticles have a highly ordered and repetitive spatial structure and an optimal size for lymph node transport [
81]. Such nanoparticles possess inherent antigenicity, allowing them to serve directly as vaccine antigens without the need for additional antigenic substances. Arginine-rich protamine is natural cationic peptide mixture mostly known used for cellular delivery of nucleic acid [
82]. Beside to deliver mRNA, protamine can act as an adjuvant by activating Toll-like receptor (TLR7, TLR8) for vaccine or immunotherapy [
83]. Ferritin nanoparticles also have been used to serve as a new vaccine delivery platform that enhances vaccine stability and immunogenicity. Zhang et al. evaluated the safety and immunogenicity of a novel ferritin nanoparticle H2 influenza vaccine [
84]. The research team assessed the safety of this vaccine in humans and its ability to induce immune responses, including antibody and T cell responses. This ferritin nanoparticle vaccine technology represents a novel, safe, and immunogenic platform with potential applications in pandemic preparedness and universal influenza vaccine development. Furthermore, in clinical settings, the structure and composition of proteins can be adjusted as needed to achieve precise therapeutic effects.
The peptide with cationic or amphipathic groups (for instance, argine) can be designed to effectively deliver mRNA molecules to cells. Peptide-based delivery system are with enhancing stability and protection by shielding them from degradation by nucleases and other cellular and extracellular enzymes, thereby effectively improving the efficacy of mRNA vaccines. Importantly, these delivery systems based on peptides, utilizing naturally synthesized peptides, can reduce adverse reactions and enhance safety. A fusogenic cell-penetrating peptide repetitive arginine-alanine-leucin E-alanine (RALA) could deliver mRNA to dendritic cells to trigger T cell medicated immunity response [
85]. In clinical trials, CoVac-1 is a peptide-based T cell activator composed of SARS-CoV-2 epitopes, demonstrating good safety and efficacy in SARS-CoV-2-specific T cell responses. Research data indicates that CoVac-1 induces broad and effective T cell responses in B cell/antibody deficient patients, with good safety profile, ensuring entry into critical Phase III safety and efficacy evaluations [
86]. And, Xu et al. has developed an optimized polyethylene glycolated peptide with enhanced mRNA delivery efficiency and improved safety in mice [
87]. Moreover, cationic peptides as candidate carriers for mRNA have been shown to efficiently deliver nucleic acids to eukaryotic cells. A cationic peptide-based mRNA nanoparticles could induce efficient antigen-specific CD8
+ T cell responses [
88]. Peptide-based mRNA delivery systems show promise in the field of gene therapy, enabling the delivery of therapeutic mRNA to correct genetic disorders. They are also being used for mRNA-based vaccines to induce immune responses against infectious diseases or cancer.
3.4. Other Formulations Used in mRNA Delivery
3.4.1. Virus-Like Particles (VLPs)
Virus-like particles (VLPs) are composed of recombinant proteins expressing viral surface proteins, mimicking the structure of real viruses but lacking viral nucleic acids[
89]. VLPs can accurately replicate the structure of viruses, triggering robust immune responses. They are highly safe, as they do not cause infection or replication, making them suitable for use as vaccine carriers. In addition, due to their highly repetitive and rigid structure, VLPs can display multivalent antigenic epitopes on their surface and therefore can extensively cross-link BCRs, thereby stimulating B cells and inducing a robust and long-lasting antibody response [
90]. Chang et al. have indicated VLPs are able to induce potent antibody responses[
91]. The delivery platform of virus-like nanoparticles has shown relevance to TLR7 signaling, especially in B cells, driving early GC formation and affinity maturation, and being responsible for inducing and maintaining BCR diversity. However, the presentation of viral surface antigens alone on the cell surface may limit the expression of traditional mRNA vaccines. Researchers have proposed a natural infection-mimicking technique that involves encoding self-assembling enveloped virus-like particles (eVLPs). The eVLPs present membrane proteins containing clusters that recruit cytoplasmic ESCRT, combining the features of mRNA-based and protein-based nanovaccines. By using two immune approaches with mRNA-LNP encoding the spike protein, effective CD8
+ T cell responses and superior neutralizing antibody responses against both wild-type and variant SARS-CoV-2 can be induced.
In summary, VLP-based vaccines have achieved success and are used in the market. In the clinical development, VLPs can be designed to carry different types of antigens, including current influenza, HPV, hepatitis B, and so on [
92].
3.4.2. Inorganic-Based Nanoparticles
Inorganic nanoparticles also play a crucial role in nucleic acid delivery and imaging. Similar to gold nanoparticles, silica nanoparticles and iron oxide nanoparticles are inorganic nanoparticles. Inorganic nanoparticles have a large surface area, enabling them to carry a large quantity of mRNA molecules, thereby enhancing delivery efficiency and stability [
93]. Not only they can encapsulate mRNA molecules, protecting them from degradation and prolonging their presence in the body to improve delivery efficiency, but also can form rational particle size to facilitate direct transport of antigens to lymph nodes, promoting antigen presentation and enhancing adaptive immune responses. For example, gold nanoparticles were used for DNA and siRNA delivery. And its surface can be modified by anionic nucleic acids and polycations and targeting ligands [
94]. Yang et al. developed a novel amino-modified mesoporous silica manganese nanoparticle (AMMSN) loaded with rF1-V10 (rF1-V10@AMMSN) for the prevention of pneumonic plague [
95]. Subcutaneous immunization with rF1-V10@AMMSN in a prime-boost strategy induced a robust production of rF1-V10-specific IgG antibodies. Additionally, in vivo, rF1-V10@AMMSN was up-taken by dendritic cells (DCs) and promoted DC maturation through the activation of the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway and the production of IFN-I. In the article, they also addressed the limitations of current vaccines for fatal bacterial pulmonary infections, including pneumonic plague, due to insufficient co-delivery of antigens and adjuvants, as well as inadequate immune stimulation. In addition, surface modification allows for the modulation of the interaction between inorganic nanoparticles and cells, facilitating targeted delivery and intracellular release. By adjusting the structure and properties of inorganic nanoparticles, controlled release of mRNA can be achieved, enhancing delivery precision and efficiency.
3.4.3. Cationic Nanoemulsions
Cationic nanoemulsions (CNE) haven been proposed as a potential delivery system for nucleic acids [
96,
97]. They are composed of oil droplets dispersed in an aqueous phase, stabilized by positively charged surfactants. The positive charge of CNEs facilitates interactions with negatively charged cell membranes, promoting cellular uptake of the mRNA cargo and enhancing delivery efficiency. CNEs can efficiently encapsulate mRNA molecules within their oil droplets, protecting the mRNA from degradation and improving its stability during delivery [
97]. Surface modifications of CNE can enable targeted delivery of mRNA vaccines to specific cells or tissues, enhancing therapeutic efficacy and reducing off-target effects. Luis et al. develop a CNEs delivery system to deliver a self-amplify mRNA vaccine based on adjuvant MF59 which elicit potent immune response [
98]. Some CNEs exhibit intrinsic adjuvant properties, capable of activating the immune system and enhancing the immune response to mRNA vaccine antigens. Additionally, Montanide ISA 51 and Montanide ISA 720 are being evaluated in clinical trials as adjuvants for influenza, malaria, melanoma, and other cancer vaccines [
99].
4. Nanovaccines and Anti-Tumor Immunity
Nanoparticle delivery platforms serve as a promising tool in vaccine development, especially in enhancing anti-tumor immunity and improving immune responses against cancer cells. Nanoparticle delivery platforms can effectively deliver tumor-specific antigens to antigen-presenting cells (such as DCs), thereby enhancing antigen presentation and activating CD8
+ T cells. Nucleoside-modified mRNA vaccines for the 2019 coronavirus disease (COVID-19), such as Pfizer/BioNTech's BNT162b2 (Comirnaty) and Moderna's mRNA-1273 (Spikevax), are the first mRNA products to receive approval from the United States Food and Drug Administration (FDA) or the European Medicines Agency (EMA). Nucleoside-modified mRNA-lipid nanoparticle (mRNA-iLNP) vaccines induce robust T follicular helper cell (Tfh) responses and GC formation, which are necessary for sustained and high-affinity antibody production [
100]. Toward enhancing the antigen presentation to DCs, Gokulnath et al. developed a novel shikimoylated mannose receptor targeting lipid nanoparticle (SMART-LNP) system that could effectively deliver mRNAs into DCs [
101]. Importantly, SMART-LNP-TS1 vaccine produced strong Th1-predominant humoral and cellular immune responses. STLNPs-Man successfully downregulated the expression of CTLA-4 on the surface of CD8
+ T cells by targeting CD206 on DCs, further enhancing the cytotoxic capacity of activated CD8
+ T cells. Combined with the immune checkpoint inhibitor αPD-L1, complete eradication of tumors was achieved with relatively low doses of mRNA [
60,
102]. Lyme disease is the most common vector-borne infectious disease in the United States, and currently, there is no effective vaccine directly targeting humans. OspA mRNA-LNP may prove to be a viable method for Lyme disease prevention. The OspA mRNA-LNP vaccine elicits robust innate immune cell infiltration and antigen-specific CD4
+ and CD8
+ T cell responses in mice, as well as inducing high levels of Tfh cells and antigen-specific GC B cells in mice, thereby inducing effective antigen-specific memory B cell and long-lived plasma cell responses, resulting in sustained antibody responses in mice [
103].
Nanoparticles can provide sustained release of antigens, prolonging the exposure of immune cells to antigens to enhance immune cell activation and improve overall anti-tumor immune responses. For instance, the need for the development of better vaccine strategies in the clinical setting to overcome maternal antibody (matAb) suppression of infant immune responses. The nucleoside-modified mRNA-LNP vaccine establishes an extended germinal center response and is able to partially overcome matAb suppression, with the authors speculating that this may be related to the prolonged antigen expression of these vaccines. Some studies suggest that prolonged antigen availability leads to stronger germinal center responses. A recent study indicates that naïve B cells do not enter germinal centers as effectively as B cells preloaded with antigen[
104]. Taken together, activated monocytes, macrophages, and DCs are the key cell types responsible for successful uptake of mRNA-iLNP, protein synthesis, and antigen presentation in lymphoid tissues to drive adaptive immune responses. Nanoparticles can be designed to target specific immune cells or tumor microenvironments, such as form tertiary lymphoid structure that increasing the specificity and efficacy of vaccines. These findings suggest that the materials used in the preparation of the nanovaccine are biocompatible, cost-effective, and FDA-approved, and that the nanovaccine can promote TLS formation, significantly enhance local immune responses, delay tumor growth, indicating good clinical prospects for nanovaccine applications. Furthermore, incorporating CpG and Mn2
+ into the nanovaccine strongly activates T cells and B cells, stimulates DC expression of LT-α and CCL21, and induces peripheral lymph node addressin protein (PNAd) vascular formation [
105,
106].
Nanoparticles can also be designed to modulate immune responses based on clinical needs, such as promoting the activation of cytotoxic T cells, enhancing the production of pro-inflammatory cytokines, and overcoming immune tolerance mechanisms in the tumor microenvironment. Chen et al. found that the clinical use of magnetic resonance imaging contrast agent iron oxide nanoparticles (IONPs) significantly increased the production of interferon gene stimulator (STING) agonist MSA-2, thereby promoting the generation of IFN-I [
107]. The study revealed the potential immunostimulatory role of IONPs in STING cascade activation, providing a scalable and easily translatable strategy for personalized cancer vaccine immunotherapy. The PEIM nano-adjuvant induces a strong immune response by promoting effective antigen uptake and cross-presentation, including inducing iron-induced apoptosis of tumor cells and repolarization of tumor-associated macrophages (TAMs) to activate T cell immunity, ultimately inhibiting tumor growth [
108]. The PEIM nano-adjuvant is the preferred iron nano-adjuvant for loading tumor antigens in cancer vaccine therapy [
108]. Rojas et al. has been demonstrated that the adjuvant autogene cevumeran, a personalized novel antigen vaccine based on uridine mRNA-lipid nanoparticles, is safe and feasible when used in combination with atezolizumab and mFOLFIRINOX, and generates a significant amount of neoantigen-specific T cells in 50% of unresectable pancreatic ductal adenocarcinoma (PDAC) patients [
109].
Overall, nanovaccines hold great potential in leveraging nanotechnology to improve anti-tumor immunity and enhance the effectiveness of cancer immunotherapy [
110,
111]. Current research in this field aims to optimize the design and delivery of nanovaccines to achieve personalized and effective cancer treatment strategies.
5. Conclusion and Future Perspectives
Nanoparticle-based delivery platforms have been demonstrated revolutionary as vaccine vehicles with high deliver efficacy. In the current research status of nanoparticle-based vaccine platform development, there is a growing interest in exploring the potential of nanoparticles as vaccine delivery systems. Researchers are actively investigating the use of various types of nanoparticles, including lipid-based, polymer-based, protein-based and inorganic nanoparticles and so on, for delivering antigens and adjuvants to the immune system. One area of active research is the development of novel nanoparticle formulations that can enhance the stability and immunogenicity of vaccines. Various nanoparticles have been used as new solutions for drug and vaccine delivery. In some clinical studies, nanoparticle delivery platforms are gradually being applied to a wide range of vaccine applications. This includes the use of lipid nanoparticles to encapsulate mRNA vaccines. Lipid nanoparticles have shown potential for efficient delivery of antigens to immune cells in preclinical and clinical studies. Now, lipid nanoparticles and other nanoparticles as nucleic acid especially mRNA delivery systems have shown broad potential for vaccine development. In this review, we focused on the application prospect of the mRNA vaccines especially lipid nanoparticle-based delivery platform, as well as the design of polymeric nanoparticles that can efficiently deliver antigens to immune cells. Additionally, the use of inorganic nanoparticles such as gold and iron oxide nanoparticles for vaccine delivery is also a focus of ongoing research. Furthermore, researchers are also exploring the potential of nanoparticles to enable targeted vaccine delivery to specific immune cells or tissues. This involves the surface modification of nanoparticles with targeting ligands that can selectively bind to receptors on immune cells, thereby enhancing the uptake of vaccines and the activation of immune responses. In addition to vaccine delivery, current research efforts are also focused on understanding the immunological mechanisms underlying the interaction between nanoparticles and the immune system. The nanoparticle delivery platform effectively delivers tumor specific antigens to antigen-presenting cells (such as DCs), thereby enhancing antigen expression and activating CD8
+ T cells. Also, nanoparticles can be designed to target specific immune cells or tumor microenvironments, such as to form TLNs, increasing the specificity and effectiveness of vaccines. Nanoparticle delivery platforms serve as a promising tool in vaccine development, especially in enhancing anti-tumor immunity and improving immune responses against cancer cells. Although T cells have the ability to destroy tumors, cancer cells can gain ways of evading immune attacks. Among these barriers, immunosuppressive cells and intrinsic resistance account for the failure of many therapeutic cancer vaccines [
112]. There are some ways to overcome these barriers for cancer vaccine to achieve their potential. For instance, combining cancer vaccine with immune checkpoint inhibitors that block PD-1 binding to PD-L1 and other treatment regimens could improve the anti-tumor efficacy of vaccine.
Nanoparticle vaccines have a significant effect on genetic diseases, infectious diseases and cancer treatment[
113]. In particular, various types of nanoparticles were used for mRNA (messenger RNA) vaccines for cancer treatment. In recent years, mRNA vaccine technologies have received increasing research and commercial attention because of their potential in the prevention of infectious diseases and cancer treatment. Overall, the current research status in nanoparticle-based vaccine platform development is characterized by a diverse range of studies aimed at optimizing the design, formulation, and delivery of nanoparticle-based vaccines. These efforts are driven by the potential of nanoparticles to overcome the limitations of traditional vaccine formulations and to enhance the efficacy and safety of vaccines. Over the past few years, nanoparticle-based vaccines have emerged as a primary tool in the field of prophylactic vaccines; now, they are being tested in oncology clinical trials. Numerous therapeutic cancer vaccines have shown promising results in clinical trials including melanoma, pancreatic cancer and lymphoma. A personalized mRNA vaccine for pancreatic cancer can trigger about 50% participants developing cancer neoantigens specific T cells [
109]. Although mRNA vaccines are showing encouraging results in trials, there are many obstacles ahead. The future development and clinical trials of therapeutic cancer vaccine may be shaped by several factors including unwieldy clinical trials, immunity monitoring such as T-cell monitoring and streamlining production.
Triggering a tumor-specific cytotoxic T cell response remains an important challenge in cancer immunotherapy. Optimally selecting a delivery system is crucial for vaccine application in infectious diseases or cancer treatment. Nanoparticle-based vaccine platforms have shown potential as delivery vehicles in the prevention of infectious diseases and cancer treatment. In the future, we hope to see an increase in the application of vaccines using various targeting approached or combination therapy, which will allow this versatile and potent platform to gain further new therapeutic applications in oncology.
Author Contributions
The first authors Yang Lin conceived this review and writing the initial draft study concept. The corresponding author Li Liang and HongXia Zhang was predominantly engaged in organizing, designing, and writing the article. The other authors contributed equally to the paper's conception, literature review, writing, and editing of the figures, all authors read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 82102874, 82273358; and the GuangDong Basic and Applied Basic Research Foundation grant number 2023A1515012444; and the Funding by Science and Technology Projects in Guangzhou, grant number 2024A04J5032.
Acknowledgments
Throughout the writing of this review I have received a great deal of support and assistance. I would first like to thank my supervisor, Li Liang and HongXia Zhang whose expertise were invaluable in formulating the research questions and methodology. I would particularly like to acknowledge my team members, for their wonderful collaboration and patient support. Finally: I could not have completed this review without the support of my friends, who provided stimulating discussions as well as happy distractions to rest my mind outside of my research.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Guan, G.; Wu, M.; Han, M. Stimuli-Responsive Hybridized Nanostructures. Adv. Funct. Mater. 2019, 30, 1903439. [Google Scholar] [CrossRef]
- Gabizon, A.; Isacson, R.; Rosengarten, O.; Tzemach, D.; Shmeeda, H.; Sapir, R. An open-label study to evaluate dose and cycle dependence of the pharmacokinetics of pegylated liposomal doxorubicin. Cancer Chemother. Pharmacol. 2007, 61, 695–702. [Google Scholar] [CrossRef]
- Aulic, S. , et al., 16 - Breast cancer nanomedicine market update and other industrial perspectives of nanomedicine, in Nanomedicines for Breast Cancer Theranostics, N.D. Thorat and J. Bauer, Editors. 2020, Elsevier. p. 371-404.
- von Roemeling, C. , et al. , Breaking Down the Barriers to Precision Cancer Nanomedicine. Trends In Biotechnology 2017, 35, 159–171. [Google Scholar] [PubMed]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.-G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
- Guo, M.; Duan, X.; Peng, X.; Jin, Z.; Huang, H.; Xiao, W.; Zheng, Q.; Deng, Y.; Fan, N.; Chen, K.; et al. A lipid-based LMP2-mRNA vaccine to treat nasopharyngeal carcinoma. Nano Res. 2023, 16, 5357–5367. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, C.L.; Haanen, J.B.; Met, O.; Svane, I.M. Clinical advances and ongoing trials of mRNA vaccines for cancer treatment. Lancet Oncol. 2022, 23, E450–E458. [Google Scholar] [CrossRef] [PubMed]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R. , et al. , Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. The New England Journal of Medicine 2021, 384, 403–416. [Google Scholar]
- Mendes, B.B. , et al. , Nanodelivery of nucleic acids. Nature Reviews Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.K.; Moynihan, K.D.; Irvine, D.J. Engineering New Approaches to Cancer Vaccines. Cancer Immunol. Res. 2015, 3, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Bezbaruah, R., et al., Nanoparticle-Based Delivery Systems for Vaccines. LID - 10.3390/vaccines10111946 [doi] LID - 1946. (2076-393X (Print)).
- Garg, A.D.; Coulie, P.G.; Van den Eynde, B.J.; Agostinis, P. Integrating Next-Generation Dendritic Cell Vaccines into the Current Cancer Immunotherapy Landscape. Trends Immunol. 2017, 38, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.-T.; Liu, X.-H.; An, J.-X.; Liang, J.-L.; Li, C.-X.; Jin, X.-K.; Ji, P.; Zhang, X.-Z. Dendritic Cell-Based In Situ Nanovaccine for Reprogramming Lipid Metabolism to Boost Tumor Immunotherapy. ACS Nano 2023, 17, 24947–24960. [Google Scholar] [CrossRef] [PubMed]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2019, 120, 3210–3229. [Google Scholar] [CrossRef] [PubMed]
- Drew, L. Cancer-vaccine trials give reasons for optimism. Nature 2024, 627, S33–S33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 1–30. [Google Scholar] [CrossRef]
- Matsui, H.; Hazama, S.; Shindo, Y.; Nagano, H. Combination treatment of advanced pancreatic cancer using novel vaccine and traditional therapies. Expert Rev. Anticancer. Ther. 2018, 18, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Fiedler, K. , et al., mRNA Cancer Vaccines. Recent Results In Cancer Research. Fortschritte Der Krebsforschung. Progres Dans Les Recherches Sur Le Cancer 2016, 209: p. 61-85.
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. mRNA vaccine: a potential therapeutic strategy. Mol. Cancer 2021, 20, 1–23. [Google Scholar] [CrossRef]
- Schlake, T. , et al. , Developing mRNA-vaccine technologies. RNA Biology 2012, 9, 1319–1330. [Google Scholar] [PubMed]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The promise of mRNA vaccines: a biotech and industrial perspective. npj Vaccines 2020, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U., K. Karikó, and Ö. Türeci, mRNA-based therapeutics--developing a new class of drugs. Nature Reviews. Drug Discovery 2014, 13, 759–780. [Google Scholar]
- Li, B.; Jiang, A.Y.; Raji, I.; Atyeo, C.; Raimondo, T.M.; Gordon, A.G.R.; Rhym, L.H.; Samad, T.; MacIsaac, C.; Witten, J.; et al. Enhancing the immunogenicity of lipid-nanoparticle mRNA vaccines by adjuvanting the ionizable lipid and the mRNA. Nat. Biomed. Eng. 2023, 1–18. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, Y.; He, Y.; Boucetta, H.; Wu, J.; Chen, Z.; He, W. Lipid carriers for mRNA delivery. Acta Pharm. Sin. B 2023, 13, 4105–4126. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Zhao, L. , et al., Nanoparticle vaccines. (1873-2518 (Electronic)).
- Huete-Carrasco, J.A.-O. , et al., Rational design of polymer-based particulate vaccine adjuvants. (1521-4141 (Electronic)).
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv. Mater. 2023, 35, e2303261. [Google Scholar] [CrossRef]
- Hou, X. , et al., Lipid nanoparticles for mRNA delivery. (2058-8437 (Print)).
- Adams, D. , et al., Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. (1533-4406 (Electronic)).
- Dobrowolski, C.; Paunovska, K.; Hatit, M.Z.C.; Lokugamage, M.P.; Dahlman, J.E. Therapeutic RNA Delivery for COVID and Other Diseases. Adv. Heal. Mater. 2021, 10, 2002022. [Google Scholar] [CrossRef] [PubMed]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2. [Google Scholar] [CrossRef]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Accounts Chem. Res. 2021, 55, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Geetha, K.M. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J. Drug Deliv. Sci. Technol. 2022, 74, 103553–103553. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Ibrahim, N.M.; Cheng, K. The Importance of Apparent pKa in the Development of Nanoparticles Encapsulating siRNA and mRNA. Trends Pharmacol. Sci. 2021, 42, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Yu, H. , et al., pH-Dependent Lyotropic Liquid Crystalline Mesophase and Ionization Behavior of Phytantriol-Based Ionizable Lipid Nanoparticles. Small (Weinheim an Der Bergstrasse, Germany), 2024: p. e2309200.
- Kim, M.; Jeong, M.; Hur, S.; Cho, Y.; Park, J.; Jung, H.; Seo, Y.; Woo, H.A.; Nam, K.T.; Lee, K.; et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci. Adv. 2021, 7, eabf4398. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, Y.; Han, X. Ionizable Lipid Nanoparticles for mRNA Delivery. Adv. NanoBiomed Res. 2023, 3. [Google Scholar] [CrossRef]
- Alameh, M.-G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2022, 55, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Ramos da Silva, J.A.-O. , et al., Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. (1946-6242 (Electronic)).
- Ramos da Silva, J. , et al. , Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Science Translational Medicine 2023, 15, eabn3464. [Google Scholar]
- Chen, J.; Ye, Z.; Huang, C.; Qiu, M.; Song, D.; Li, Y.; Xu, Q. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8(+) T cell response. Proc. Natl. Acad. Sci. 2022, 119. [Google Scholar] [CrossRef]
- Appidi, T.; Sivasankaran, R.P.; Chinchulkar, S.A.; Patra, P.; Murugaiyan, K.; Veeresh, B.; Rengan, A.K. A lipo-polymeric hybrid nanosystem with metal enhanced fluorescence for targeted imaging of metastatic breast cancer. Nanotheranostics 2024, 8, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; You, X.; Wang, X.; Cui, L.; Wang, Z.; Xu, F.; Li, M.; Yang, Z.; Liu, J.; Huang, P.; et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl. Acad. Sci. 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C. , et al. , Local delivery of mRNA-encoded cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Science Translational Medicine 2021, 13, eabc7804. [Google Scholar] [PubMed]
- Mukherjee, J.; Ondeck, C.A.; Tremblay, J.M.; Archer, J.; Debatis, M.; Foss, A.; Awata, J.; Erasmus, J.H.; McNutt, P.M.; Shoemaker, C.B. Intramuscular delivery of formulated RNA encoding six linked nanobodies is highly protective for exposures to three Botulinum neurotoxin serotypes. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Barrett, D.M.; Jiang, S.; Fang, C.; Kalos, M.; A Grupp, S.; June, C.H.; Zhao, Y. Improved anti-leukemia activities of adoptively transferred T cells expressing bispecific T-cell engager in mice. Blood Cancer J. 2016, 6, e430–e430. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Boettler, T. , et al. , SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. Journal of Hepatology 2022, 77, 653–659. [Google Scholar] [PubMed]
- Szebeni, J. , et al. , Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nature Nanotechnology 2022, 17, 337–346. [Google Scholar] [PubMed]
- Zhang, H.; Meng, C.; Yi, X.; Han, J.; Wang, J.; Liu, F.; Ling, Q.; Li, H.; Gu, Z. Fluorinated Lipid Nanoparticles for Enhancing mRNA Delivery Efficiency. ACS Nano 2024, 18, 7825–7836. [Google Scholar] [CrossRef]
- Yang, K.; Bai, B.; Lei, J.; Yu, X.; Qi, S.; Wang, Y.; Huang, F.; Tong, Z.; Yu, G. Biodegradable Lipid-Modified Poly(Guanidine Thioctic Acid)s: A Fortifier of Lipid Nanoparticles to Promote the Efficacy and Safety of mRNA Cancer Vaccines. J. Am. Chem. Soc. 2024. [Google Scholar] [CrossRef]
- Lei, J.; Qi, S.; Yu, X.; Gao, X.; Yang, K.; Zhang, X.; Cheng, M.; Bai, B.; Feng, Y.; Lu, M.; et al. Development of Mannosylated Lipid Nanoparticles for mRNA Cancer Vaccine with High Antigen Presentation Efficiency and Immunomodulatory Capability. Angew. Chem. Int. Ed. 2024, 63, e202318515. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Garg, A.; Varshney, V. Recent Updates on Applications of Lipid-Based Nanoparticles for Site- Specific Drug Delivery. Pharm. Nanotechnol. 2022, 10, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Tombácz, I. , et al. , Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Molecular Therapy : the Journal of the American Society of Gene Therapy 2021, 29, 3293–3304. [Google Scholar]
- Tilsed, C.M.; Sadiq, B.A.; Papp, T.E.; Areesawangkit, P.; Kimura, K.; Noguera-Ortega, E.; Scholler, J.; Cerda, N.; Aghajanian, H.; Bot, A.; et al. IL7 increases targeted lipid nanoparticle–mediated mRNA expression in T cells in vitro and in vivo by enhancing T cell protein translation. Proc. Natl. Acad. Sci. 2024, 121. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Swingle, K.L.; Safford, H.C.; Geisler, H.C.; Hamilton, A.G.; Thatte, A.S.; Billingsley, M.M.; Joseph, R.A.; Mrksich, K.; Padilla, M.S.; et al. Ionizable Lipid Nanoparticles for In Vivo mRNA Delivery to the Placenta during Pregnancy. J. Am. Chem. Soc. 2023, 145, 4691–4706. [Google Scholar] [CrossRef]
- Nawaz, M.; Heydarkhan-Hagvall, S.; Tangruksa, B.; Garibotti, H.G.; Jing, Y.; Maugeri, M.; Kohl, F.; Hultin, L.; Reyahi, A.; Camponeschi, A.; et al. Lipid Nanoparticles Deliver the Therapeutic VEGFA mRNA In Vitro and In Vivo and Transform Extracellular Vesicles for Their Functional Extensions. Adv. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Barber, G.N. STING signaling and host defense against microbial infection. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Han, X. , et al. , Adjuvant lipidoid-substituted lipid nanoparticles augment the immunogenicity of SARS-CoV-2 mRNA vaccines. Nature Nanotechnology 2023, 18, 1105–1114. [Google Scholar]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Tao, W.; Peppas, N.A. Robotic pills for gastrointestinal-tract-targeted oral mRNA delivery. Matter 2022, 5, 775–777. [Google Scholar] [CrossRef]
- Xiao, Y.; Tang, Z.; Huang, X.; Chen, W.; Zhou, J.; Liu, H.; Liu, C.; Kong, N.; Tao, W. Emerging mRNA technologies: delivery strategies and biomedical applications. Chem. Soc. Rev. 2022, 51, 3828–3845. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.; Debska, G.; Szewczyk, A.C. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Chahal, J.S. , et al. , Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proceedings of the National Academy of Sciences of the United States of America 2016, 113, E4133–E4142. [Google Scholar]
- Guerrero-Cázares, H.; Tzeng, S.Y.; Young, N.P.; Abutaleb, A.O.; Quiñones-Hinojosa, A.; Green, J.J. Biodegradable Polymeric Nanoparticles Show High Efficacy and Specificity at DNA Delivery to Human Glioblastoma in Vitro and in Vivo. ACS Nano 2014, 8, 5141–5153. [Google Scholar] [CrossRef] [PubMed]
- Dosta, P.; Cryer, A.M.; Dion, M.Z.; Shiraishi, T.; Langston, S.P.; Lok, D.; Wang, J.; Harrison, S.; Hatten, T.; Ganno, M.L.; et al. Investigation of the enhanced antitumour potency of STING agonist after conjugation to polymer nanoparticles. Nat. Nanotechnol. 2023, 18, 1351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, S.; Qi, Y.; Gao, Y.; Zhang, Y.; Li, M.; Chen, J.; Song, W.; Chen, X. A polyamino acid-based phosphatidyl polymer library for in vivo mRNA delivery with spleen targeting ability. Mater. Horizons 2024. [Google Scholar] [CrossRef]
- Suberi, A. , et al. , Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination. Sci Transl Med 2023, 15, eabq0603. [Google Scholar]
- Yang, W.; Mixich, L.; Boonstra, E.; Cabral, H. Polymer-Based mRNA Delivery Strategies for Advanced Therapies. Adv. Heal. Mater. 2023, 12, e2202688. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T. , et al., Vaccine adjuvants: mechanisms and platforms. Signal Transduction and Targeted Therapy 2023, 8(1).
- Jarzebska, N.T.; Mellett, M.; Frei, J.; Kündig, T.M.; Pascolo, S. Protamine-Based Strategies for RNA Transfection. Pharmaceutics 2021, 13, 877. [Google Scholar] [CrossRef] [PubMed]
- Kallen, K.J. , et al., A novel, disruptive vaccination technology: self-adjuvanted RNActive(®) vaccines. (2164-554X (Electronic)).
- Houser, K.; Chen, G.L.; Carter, C.; Crank, M.C.; Nguyen, T.A.; Florez, M.C.B.; Berkowitz, N.M.; Mendoza, F.; Hendel, C.S.; Gordon, I.J.; et al. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: a phase 1 trial. Nat. Med. 2022, 28, 383. [Google Scholar] [CrossRef] [PubMed]
- Udhayakumar, V.K.; De Beuckelaer, A.; McCaffrey, J.; McCrudden, C.M.; Kirschman, J.L.; Vanover, D.; Van Hoecke, L.; Roose, K.; Deswarte, K.; De Geest, B.G.; et al. Arginine-Rich Peptide-Based mRNA Nanocomplexes Efficiently Instigate Cytotoxic T Cell Immunity Dependent on the Amphipathic Organization of the Peptide. Adv. Heal. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, J.S.; Tandler, C.; Marconato, M.; Nelde, A.; Habibzada, T.; Rittig, S.M.; Tegeler, C.M.; Maringer, Y.; Jaeger, S.U.; Denk, M.; et al. Phase I/II trial of a peptide-based COVID-19 T-cell activator in patients with B-cell deficiency. Nat. Commun. 2023, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. , et al. , PEGylated pH-responsive peptide-mRNA nano self-assemblies enhance the pulmonary delivery efficiency and safety of aerosolized mRNA. Drug Deliv 2023, 30, 2219870. [Google Scholar] [PubMed]
- D’haese, S.; Laeremans, T.; Roover, S.D.; Allard, S.D.; Vanham, G.; Aerts, J.L. Efficient Induction of Antigen-Specific CD8+ T-Cell Responses by Cationic Peptide-Based mRNA Nanoparticles. Pharmaceutics 2022, 14, 1387. [Google Scholar] [CrossRef]
- Frietze, K.M.; Peabody, D.S.; Chackerian, B. Engineering virus-like particles as vaccine platforms. Curr. Opin. Virol. 2016, 18, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus–like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Chang, X.; Krenger, P.; Krueger, C.C.; Zha, L.; Han, J.; Yermanos, A.; Roongta, S.; Mohsen, M.O.; Oxenius, A.; Vogel, M.; et al. TLR7 Signaling Shapes and Maintains Antibody Diversity Upon Virus-Like Particle Immunization. Front. Immunol. 2022, 12, 827256. [Google Scholar] [CrossRef]
- Hoffmann, M.A.G. , et al. , ESCRT recruitment to SARS-CoV-2 spike induces virus-like particles that improve mRNA vaccines. Cell 2023, 186, 2380–2391e9. [Google Scholar] [PubMed]
- Li, S.; Xu, X.; Xu, L.; Lin, H.; Kuang, H.; Xu, C. Emerging trends in chiral inorganic nanomaterials for enantioselective catalysis. Nat. Commun. 2024, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef] [PubMed]
- OuYang, X.; Xu, X.; Qin, Q.; Dai, C.; Wang, H.; Liu, S.; Hu, L.; Xiong, X.; Liu, H.; Zhou, D. Manganese-Based Nanoparticle Vaccine for Combating Fatal Bacterial Pneumonia. Adv. Mater. 2023, 35, e2304514. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, H.F.; Bruxel, F.; Fraga, M.; Schuh, R.S.; Zorzi, G.K.; Matte, U.; Fattal, E. Cationic nanoemulsions as nucleic acids delivery systems. Int. J. Pharm. 2017, 534, 356–367. [Google Scholar] [CrossRef]
- Shi, Y. , et al., Structural and biochemical characteristics of mRNA nanoparticles determine anti–SARS-CoV-2 humoral and cellular immune responses. Science Advances. 8, eabo1827.
- Brito, L.A.; Chan, M.; Shaw, C.A.; Hekele, A.; Carsillo, T.; Schaefer, M.; Archer, J.; Seubert, A.; Otten, G.R.; Beard, C.W.; et al. A Cationic Nanoemulsion for the Delivery of Next-generation RNA Vaccines. Mol. Ther. 2014, 22, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Aucouturier, J.; Dupuis, L.; Deville, S.; Ascarateil, S.; Ganne, V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev. Vaccines 2002, 1, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, R.; Hogan, M.J.; Lore, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef]
- Mahalingam, G.; Rachamalla, H.K.; Arjunan, P.; Karuppusamy, K.V.; Periyasami, Y.; Mohan, A.; Subramaniyam, K.; M, S.; Rajendran, V.; Moorthy, M.; et al. SMART-lipid nanoparticles enabled mRNA vaccine elicits cross-reactive humoral responses against the omicron sub-variants. Mol. Ther. 2024. [Google Scholar] [CrossRef]
- Kon, E.; Ad-El, N.; Hazan-Halevy, I.; Stotsky-Oterin, L.; Peer, D. Targeting cancer with mRNA–lipid nanoparticles: key considerations and future prospects. Nat. Rev. Clin. Oncol. 2023, 20, 739–754. [Google Scholar] [CrossRef]
- Pine, M. , et al. , Development of an mRNA-lipid nanoparticle vaccine against Lyme disease. Molecular Therapy 2023, 31, 2702–2714. [Google Scholar] [PubMed]
- Willis, E.; Pardi, N.; Parkhouse, K.; Mui, B.L.; Tam, Y.K.; Weissman, D.; Hensley, S.E. Nucleoside-modified mRNA vaccination partially overcomes maternal antibody inhibition of de novo immune responses in mice. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Gong, L.; Liang, Z.; Zhu, C.; Ren, C.; Zheng, N.; Zhang, Q.; Liu, H.; Liu, W.; et al. Manganese nanodepot augments host immune response against coronavirus. Nano Res. 2021, 14, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Chelvanambi, M.; Fecek, R.J.; Taylor, J.L.; Storkus, W.J. STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment. J. Immunother. Cancer 2021, 9, e001906. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013, 4, 2266. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, T.; Zhang, H.; Saeed, M.; Liu, X.; Huang, L.; Wang, X.; Gao, J.; Hou, B.; Lai, Y.; et al. Acid-Ionizable Iron Nanoadjuvant Augments STING Activation for Personalized Vaccination Immunotherapy of Cancer. Adv. Mater. 2023, 35, e2209910. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv. Mater. 2023, 35, e2303261. [Google Scholar] [CrossRef]
- Saxena, M. , et al. , Therapeutic cancer vaccines. Nature Reviews Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Dong, Y. Lipid Nanoparticle–mRNA Formulations for Therapeutic Applications. Accounts Chem. Res. 2021, 54, 4283–4293. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).