1. Introduction
Hazelnuts are a very used nut, consumed either raw or roasted, and that is added as a key ingredient in a wide variety of food and confectionery products. The utilization of hazelnuts as an ingredient in a diversity of sweet and savoury products, besides adding flavour and texture, also contributes to the enhancement of their nutritional value and health benefits [
1]. Hazelnuts provide energy and contain several essential nutrients, making them a highly nutritious food source. Among their constituents are proteins, including essential amino acids, fatty acids, particularly monounsaturated fatty acids like oleic acid, carbohydrates, dietary fiber, dietary minerals and vitamins, including vitamin E. Besides these macro and micronutrients), hazelnuts also contain bioactive compounds, like phytosterols and phenolic antioxidants [
2,
3,
4,
5,
6]. All these components have a role in hazelnuts’ organoleptic appeal, besides nutritional value, turning them into this versatile ingredient widely used in industrial food products, like chocolate, and for culinary applications, such as pastry products [
1].
Hazelnuts are recommended in moderate dosages as a healthy nut, possessing numerous health benefits, the most relevant of which is associated with the prevention of cardiovascular diseases [
7]. The presence of components like monounsaturated fatty acids, fiber and antioxidants contributes to enhancing cardiovascular health by reducing blood cholesterol, improving blood lipid profiles, and fighting oxidative stress [
8,
9]. Moreover, hazelnuts have been recommended for other health properties, such as anti-inflammatory activity, neurodegenerative protection, and anti-cancer effects, as described in various studies [
10,
11,
12,
13,
14,
15,
16].
In Portugal, the dried fruit sector, which includes fruits like walnuts, almonds, and hazelnuts, constitutes a fundamental source of income for rural populations, contributing to both economic sustainability and the mitigation of social desertification. This is an activity still very much influenced by traditional agriculture values and family farming, as seen by a great number of orchars of small dimensions [
17]. Nevertheless, in recent years attention has been given to the sector with scientific research on the improvement of production, and transformation along the hazelnut sector [
18,
19].
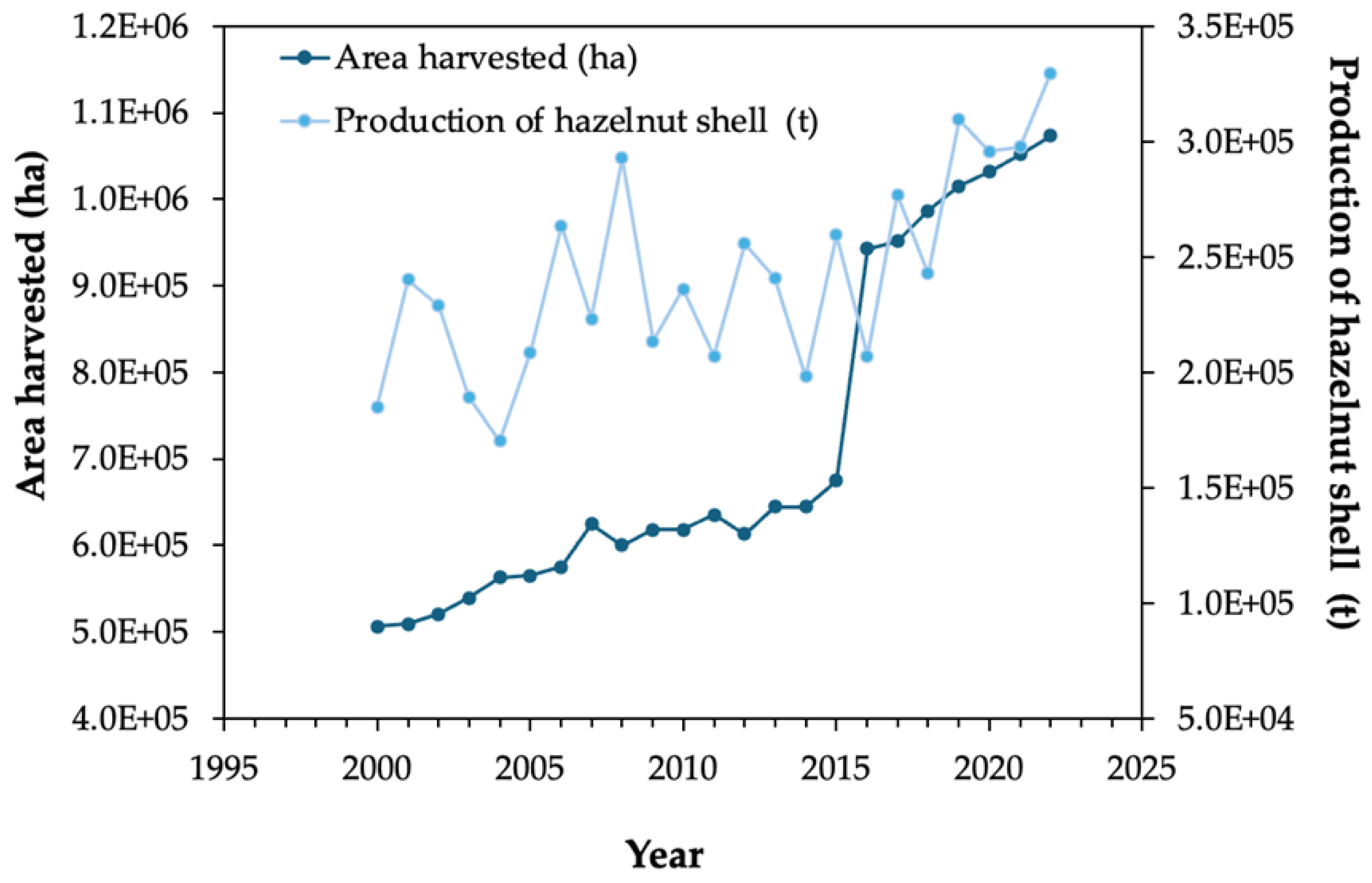
In 2022, in Portugal, 5600 tons were produced [
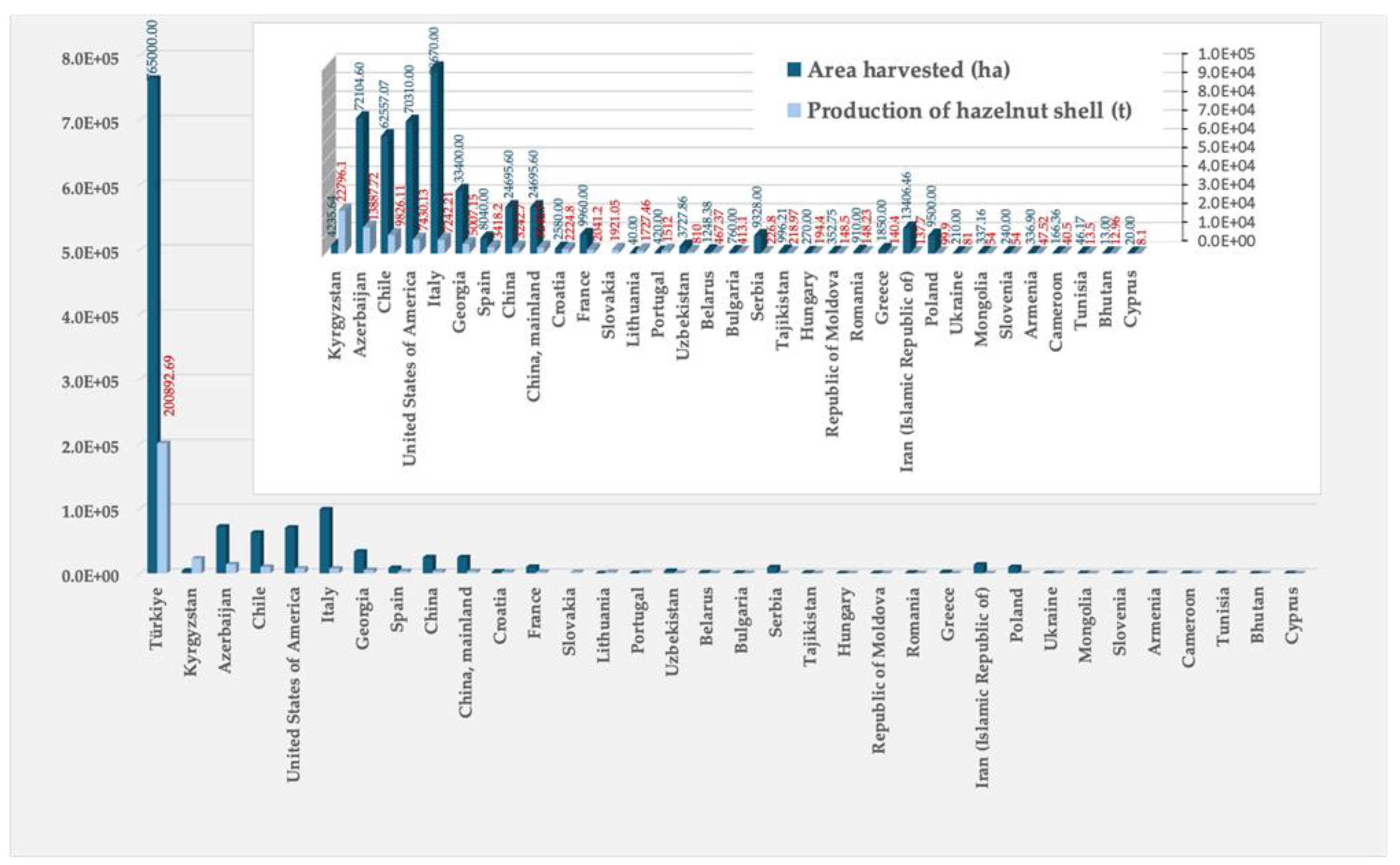
20]. Globally, in the past decades the area of hazelnut cultivation has been increasing worldwide, being Turkey, the United States of America, Azerbaijan and Chile the top producers for this nut. Considering the evolution over time of the hazelnut production, it is observed that in 2022 a maximum world production of 1220.428 thousand tons was achieved, corresponding to a cultivation area of 1073.131 thousand ha.
Hazelnut byproducts include the shells, that are discarded as residues, but which can have alternative utilizations providing added-value products. The production of hazelnut shell was in 2022 of 329.515 thousand tons, as shown in.
Figure 1 gathering data from the Portuguese Institute of Statistics [
20]. The most relevant regions for the hazelnut shell distribution include Turkey, Kyrgyzstan, Azerbaijan, Chile, Italy, Spain, China, Portugal, France, and certain parts of the USA [
20], as depicted in
Figure 2.
Hazelnut shells are composed of structural and non-structural components similarly to most lignocellulosic materials. Non-structural components comprise extractives which are small organic molecules and ashes, which correspond to the inorganic materials that remain after the burning of the material. These compounds are called non-structural since they can be removed from the material without damaging cell walls. Structural compounds are cellulose, hemicelluloses and lignin. Knowing the structure and properties of this material is essential for several applications in many fields, including biomaterials, biofuels, and sustainable materials.
Pre-hydrolysis plays a vital role in biomass processing, serving as preparation for subsequent enzymatic or chemical hydrolysis essential for biofuel or biochemical production. Pre-hydrolysis methods can be done with acid or alkaline catalysts or just with water, also called auto-hydrolysis [
21]. Acid pre-hydrolysis, typically employing sulfuric acid as a catalyst, results in the production of oligomeric and monomeric sugars; nevertheless, this method has been described to corrode equipment and induce extensive lignin condensation [
21]. Additionally, there is a significant cellulose hydrolysis, which is not desirable [
22]. Alkaline pre-hydrolysis is commonly conducted using green or white liquor from the kraft process, employing strong alkaline solutions at low temperatures [
23,
24].
Water pre-hydrolysis, commonly referred to as auto-hydrolysis, is the preferred pre-treatment method where biomass is exposed to compressed hot water, facilitating significant solubilization of hemicelluloses [
25]. The primary reaction during this process is the depolymerization of hemicelluloses, which results in the production of sugars and oligosaccharides [
26,
27]. This reaction is driven by hydronium ions formed from the autoionization of water [
28] and the organic acids produced in situ from the cleavage of acetyl groups.
These acids help break down the glycosidic bonds in hemicelluloses, leading to their conversion into lower molecular weight polysaccharides, oligosaccharides, monosaccharides, and byproducts such as furfural and hydroxymethylfurfural. Moreover, pre-hydrolysis enhances the surface area and reduces the crystallinity of cellulose, making it more amenable to subsequent hydrolysis processes. The amount of hemicelluloses removed increases over time, but at the same time, some of the xylose produced is converted into furfural. Therefore, the time and temperature of pre-hydrolysis need to be carefully optimized to maximize sugar recovery while minimizing the conversion to furfural [
29].
2. Materials and Methods
2.1. Materials
The HS used in this work were obtained from Transagri, Lda., a company located in the industrial zone of Mangualde (Portugal). Previous to analysis, the samples were milled using a Retsch SMI mill (Retsch GmbH, Haan, Germany) followed by sieving with a Retsch AS200 (Retsch GmbH, Haan, Germany) for 20 minutes at 50 rpm. Four fractions were obtained: >40 mesh, 40–60 mesh, 60–80 mesh, and <80 mesh. The 40–60 mesh fraction was the one used for hydrolysis and chemical analysis.
2.2. Pre-Hydrolysis
Experiments were carried out using a PARR LKT PED cylindrical glass reactor that holds 600 mL and is double-coated for better thermal insulation. A 20 g sample (40-60 mesh) was mixed with 200 mL of distilled water in the reactor. An automatic stirrer was set to 70 rpm to achieve a uniform mixture. The process involved fine-tuning the temperature (between 160 and 170 °C, regulated by the oil temperature in the reactor’s jacket) and the duration (ranging from 30 to 180 minutes) to find the optimal conditions for maximum solubilization. Following the reaction, the contents were removed from the reactor and filtered through a Buckner funnel with a paper filter to separate the solid residue from the liquid fraction. The solid remains were then oven-dried and weighed to calculate the percentage of material that had been solubilized, equation (1).
The chemical composition of the solid fraction was analyzed using dried samples from the pre-hydrolysis process. This analysis involved determining the levels of Klason lignin, holocellulose, α-cellulose, and hemicelluloses in the 40–60 mesh fraction. It was presumed that most extractives had been removed during the pre-hydrolysis stage.
2.3. Liquefaction
Similarly to pre-hydrolysis, the liquefaction was done in a PARR LKT PED cylindrical glass reactor with a 600 mL capacity. A 10 g of pre-hydrolyzed sample was combined with 200 mL of distilled water within the reactor. The automatic stirrer was then adjusted to 70 rpm to ensure homogeneity. The temperature used was 180 ◦C, for 60 min. After reactor extraction, the resultant mixture was filtered and weighed similarly to pre-hydrolysis, equation (2)
2.4. Chemical Composition
The chemical characterization of hazelnut shells was carried out using standard procedures to measure the contents of ash, extractives (via dichloromethane, ethanol, and hot water), lignin, holocellulose, α-cellulose, and hemicellulose. This analysis was performed to evaluate their potential applications.
The 40–60 mesh fraction was dried at 105 °C for a minimum of 24 hours before chemical analyses, adhering to the procedures delineated in Tappi T 264 om-97 [
30]. Chemical composition analysis of each sample was done in triplicate to ensure the strength and reliability of the results.
The ash content of the hazelnut shell was determined using the Tappi T 211 om-93 standard procedure [
31], involving the calcination of the material at 525 °C. This method allows for the quantification of the inorganic constituents present in the biomass. To analyze the inorganic composition, the ash obtained from calcination was subjected to wet digestion in a Leco CHNS-932 Elemental Analyzer, followed by analysis using Inductively Coupled Plasma (ICP) spectroscopy. This approach provides insights into the elemental composition of the ash fraction.
Extractives were quantified via sequential extraction with solvents of ascending polarity (Dichloromethane, ethanol and water), enabling a thorough evaluation of their distribution within the hazelnut shell matrix. The extraction was made through Soxhlet extraction in accordance with Tappi T 204 om-88 [
32]. Approximately 10 g of the dried sample was subjected to Soxhlet extraction, employing 150 mL of each solvent. Dichloromethane extraction was done for 6 hours, while ethanol and water extractions lasted 16 hours, following a sequential extraction with increasing polarity. The extractive content was quantified relative to the dry material.
The lignin content in HS free of extractives was quantified using the Klason method, using 72% H
2SO
4 as per Tappi T 204 om-88 standards [
32]. The procedure consisted of two sequential hydrolysis steps: initially using 72% H
2SO
4 for 1 hour, followed by a second hydrolysis with 3% H
2SO
4 in an autoclave at 120 °C for another hour. The insoluble residue that resulted was gathered through filtration using a G2 glass crucible and dried until it reached a constant weight. Following this, the soluble lignin content was quantified spectrophotometrically by measuring its absorbance at 205 nm.
The holocellulose content was determined using the acid chloride method. This process involved treating the sample in a water bath with a mixture of sodium chlorite solution prepared by dissolving 8.5 g in distilled water and diluting to a final volume of 250 mL and 13.5 g of NaOH dissolved in 50 mL of distilled water, to which 37.5 mL of glacial acetic acid were added, and the final volume adjusted to 250 mL, at 70 °C until it turned white. The sample was then filtered and dried. The insoluble material that remained was also filtered and subsequently dried [
33].
The α-cellulose content was determined by hydrolyzing holocellulose with 2.5 mL of 17.5% NaOH in a thermal bath. After hydrolysis, the insoluble residue was filtered out and then thoroughly washed with 8.3% NaOH and distilled water. A final treatment involved using 3.75 mL of 10% CH3COOH in a G2 glass crucible. The residue was then dried to a constant weight. The hemicellulose content was subsequently calculated as the difference between the original holocellulose and the α-cellulose.
2.5. FTIR Analysis
Fourier transform infrared spectroscopy (FTIR) was used to analyze the functional groups present in untreated hazelnut shell samples, as well as those subjected to pre-hydrolysis, the liquefied material, and the solid residue post-liquefaction. The original dried hazelnut shells, the liquefied fraction, and the remaining solid residue were prepared for FTIR analysis using the attenuated total reflection (ATR) technique. To ensure the complete removal of moisture, the samples were crushed and dried in an oven at 100 °C for one week prior to analysis. The FTIR-ATR spectra were recorded using a Perkin Elmer UATR Spectrum Two instrument (Waltham, MA, USA). The instrument operated at a scan rate of 72 scans per minute and a resolution of 4.0 cm⁻¹, covering a spectral range from 4000 to 400 cm⁻¹. After correcting for background, each sample was placed onto the ATR crystal for analysis. Solid samples were pressed firmly against the crystal surface to ensure optimal contact. The spectral data collected was an average of three individual measurements per sample.
3. Results and Discussion
3.1. Chemical Composition
Table 1 presents the results of the chemical composition analysis of HS. The findings indicate that ashes constitute 3.65% of the composition, while total extractives make up about 3.61%. The majority of extractives are soluble in water (2.97%) and ethanol (0.37%), with a smaller portion soluble in dichloromethane (0.27%). The total lignin content is determined to be 37.56%, predominantly consisting of insoluble lignin (36.70%), with a minor soluble fraction (0.86%). α-Cellulose content is approximately 33.71%, while hemicelluloses contribute 29.59% to the composition. This comprehensive analysis of chemical constituents offers valuable insights into potential applications of hazelnut shells, guiding future utilization strategies. The chemical composition of hazelnut shells seems to differ between provenances. Turkish hazelnut shells’ chemical composition was presented by Demirbas [
34], who obtained 43% lignin, 26% cellulose and 30% hemicelluloses. Similarly, Rivas et al. [
35] reported 40% lignin, 26% Glucose (Glucan) and approximately 32% hemicelluloses for hazelnut shells from Ourense, Spain. Our samples presented a smaller amount of lignin of around 37.6% (38.2% excluding the extractives) a higher amount of cellulose, 33.7%, and lower amount of hemicelluloses (29.6%). Hazelnut shells from Italy (Viterbo, Rome) presented a similar amount of lignin (36.6%), lower amount of hemicelluloses (23.6%), and of cellulose (22.9%), although these authors accounted for one more fraction representing 8% which they called “proteinaceous fraction”[
36]. Hoşgün and Bozan [
37] presented a very different chemical composition of hazelnut from turkey with 51.3% lignin, 16.7% cellulose and 13.3% hemicelluloses, 5.1% extractives and 2.1% ash and with 11% of compounds called others which they did not mention which kind of compounds were and how they were obtained.
Information on the inorganics present in ash is important in several fields, such as when hazelnut shells are used for energy production, since the ash content affects the efficiency and cleanliness of these processes. High ash content can lead to slagging, fouling, and equipment corrosion, reducing efficiency and increasing maintenance costs. High concentrations of Ca, Na, and K have been reported to lower the deformation temperatures of materials, which, according to the ENPlus® standard, must exceed 1200 °C for A1 category wood pellets. Nevertheless, the levels of these elements are significantly lower than those reported before for several shrub species [
38]. More importantly, in order to valorize ash, depending on its composition, ash can have potential applications in agriculture, construction materials, or as a source of valuable minerals. The main inorganic compounds found in HS ash were potassium, with 27.7%, followed by calcium 16.9%, magnesium 1.4% and sodium 0.3%. Ash composition is also very erratic since other authors reported different chemical compositions. For instance, Baran et al. [
39] stated that the main compounds of an HS originating from Turkey were calcium, followed by potassium, sodium and silicon, while Yurt and Bekar [
40] obtained higher amounts of calcium, potassium, silicon followed by iron and magnesium. HS from Serbia had mainly K (26.29%), Ca (11.62%), Mg (6.7%), and P (6.10%) [
41].
The pre-hydrolysis using water as the solvent was performed before liquefaction in order to remove part of the hemicelluloses that can later be converted in usable oligosaccharides. For example, Surek and Buyukkileci [
42] tested several hazelnut residues and concluded that the shells exhibited the highest xylan content at 18.7%, compared to other by-products of hazelnut processing. As a result, they yielded a greater production of xylooligosaccharides (XOS). With a pre-hydrolysis at 190 °C for 15 min Surek et al. [
43] obtained a maximum low-DP-XOS of 22.5 g/L. The optimal temperature for autohydrolysis to produce soluble hemicellulosic oligosaccharides was determined to be 210 °C in accordance with Rivas et al. [
35]. According to Hoşgün and Bozan [
37], who studied different pre-hydrolysis methods for hazelnut shells, the maximum hemicellulose removal was around 15% at 120 °C, 22% at 150 °C and 90% at 200 °C. Although most of the hemicelluloses were removed at 200 °C, a significant amount of cellulose of approximately 15-20% and about 18% of lignin were also removed. Alkaline pre-treatment was the most damaging to lignin, removing almost 80%.
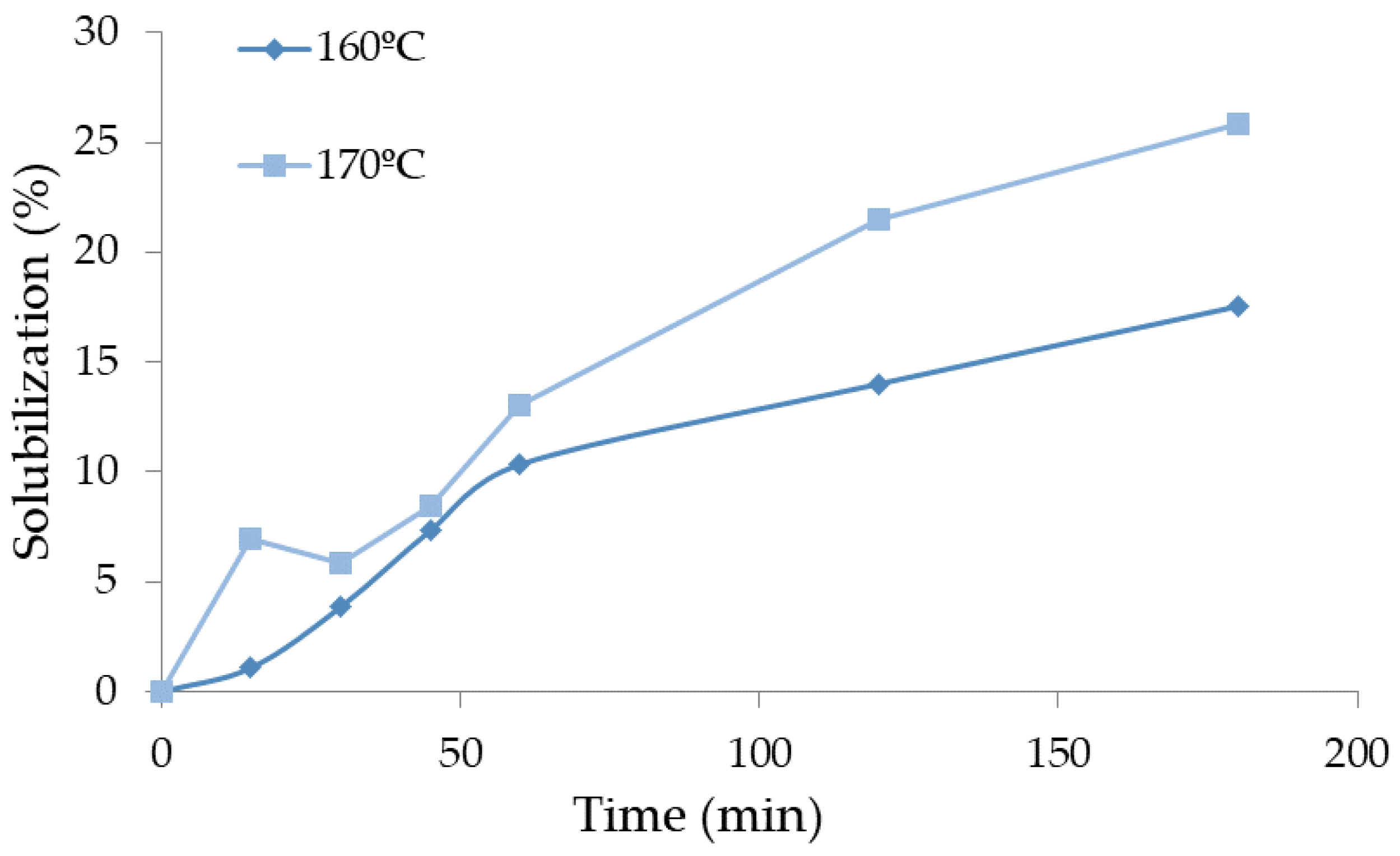
The pre-hydrolysis was made at two different temperatures, 160 °C and 170 °C, and at six times (ranging from 15 to 180 min). The liquefaction yield is presented in
Figure 3 as a function of time for each temperature studied. Liquefaction yield increased both with time and temperature. For instance, at 160 °C, the liquefaction yield was 3.9% after 30 min and 17.5% after 180 min, while at 170 °C, the liquefaction yield was 25.8% for 180 min, higher than the yield obtained at 160 °C. Several authors reported the autohydrolysis of hazelnut shells at different temperatures, 190 °C (15 min) [
43] or 210 °C [
35], but did not mention the solubilization percentage. Similar temperatures were used by Surek et al. [
29] for walnut shells and presented higher percentages of solubility at 170 °C with around 23% (15 min) and 29% (30 min). These percentages of solubility could only be obtained for around 150 min and 180 min for hazelnut shells.
The objective of pre-hydrolisis is the removal of sugars without significantly affecting lignin and cellulose.
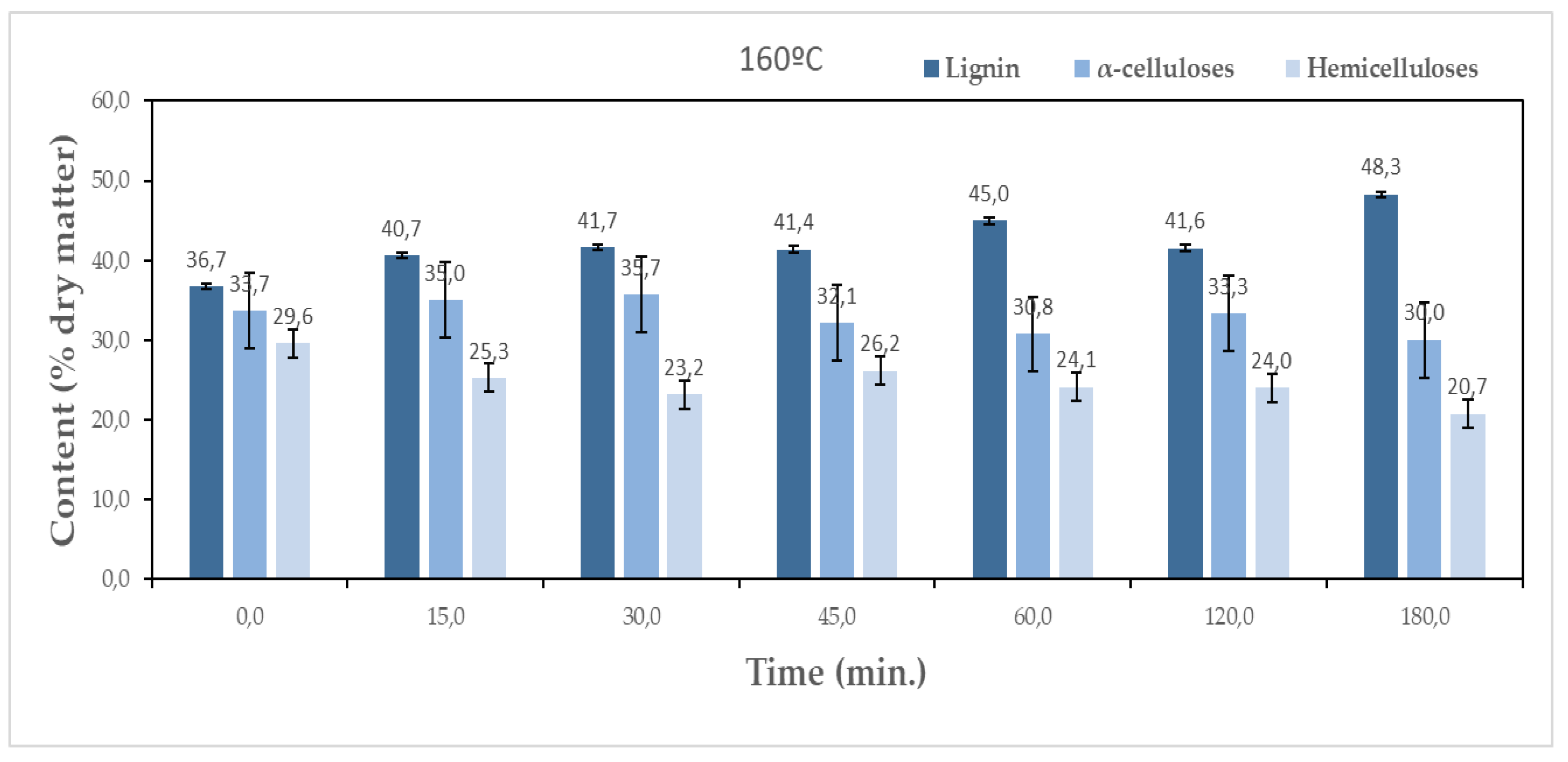
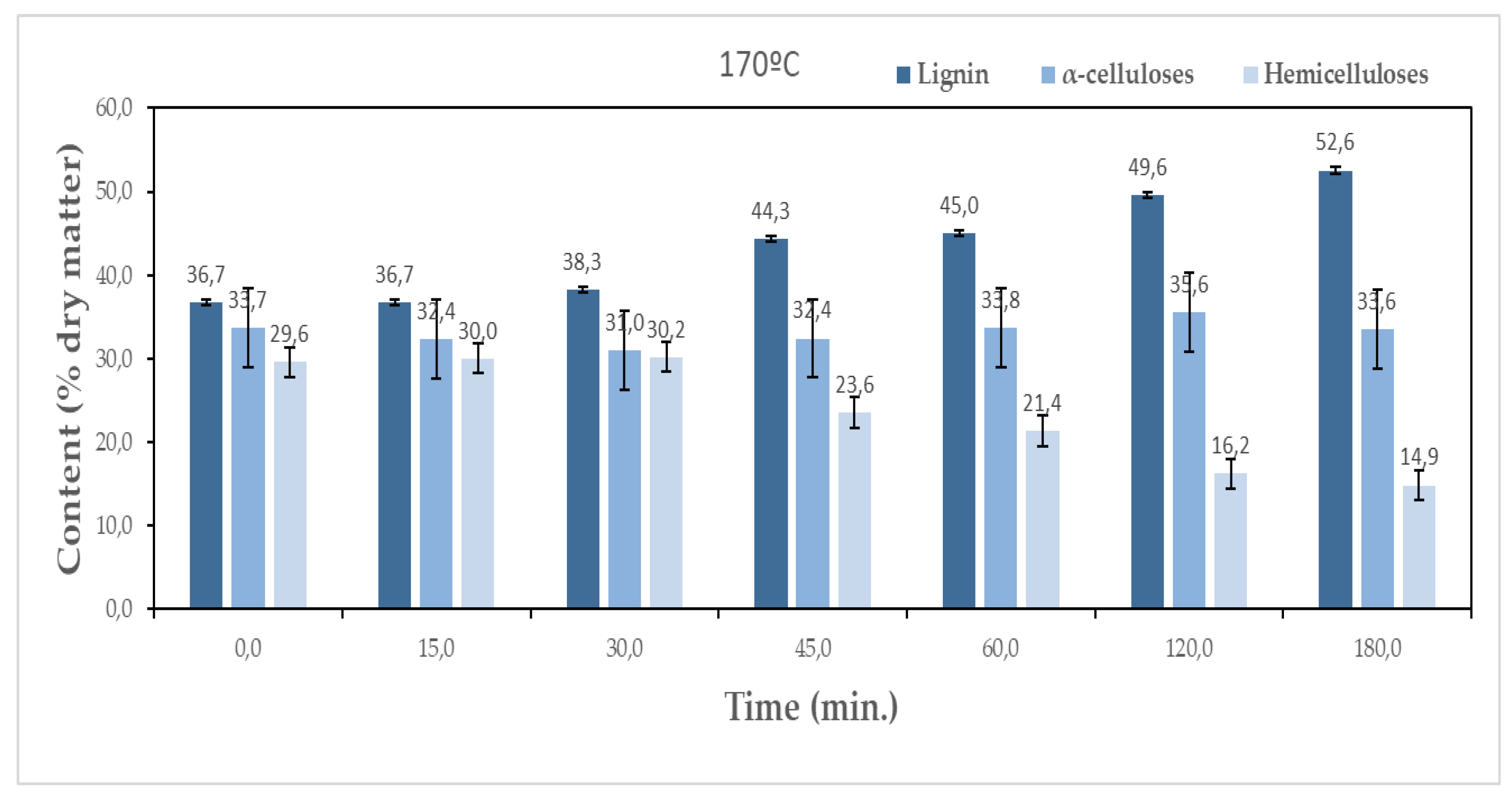
Figure 4 presents the chemical composition variation over the several pre-hydrolysis steps at both temperatures of 160 °C and 170 °C.
An increase in lignin content relative to the initial sample is observed along the pre-hydrolysis for both temperatures. At 160 °C, the initial 36.7% content increases to around 48.3% after 180 min, while for 170 °C it goes up to 52.6%. This does not mean that lignin was produced but rather that lignin resisted more to pre-hydrolysis than the remaining macromolecular compounds. Therefore, the pre-hydrolysis step increases the lignin content on the material, making it better for the production of adhesives or high resistance foams. For α-cellulose, the values obtained at both temperatures are comparable, ranging between 30.0% and 35.7% and 31.0% and 35.6% at 160 °C and 170 °C, respectively. The reduction in holocellulose content relative to the initial sample was higher for longer times and higher temperatures. Since the differences in cellulose are not significant, the main decrease was observed in the hemicelluloses content which decreased from 29.6% to 20.7% and 14.9% for 160 °C and 170 °C, respectively corresponding to a percentual removal rate of 30% and 50% of the initial content.
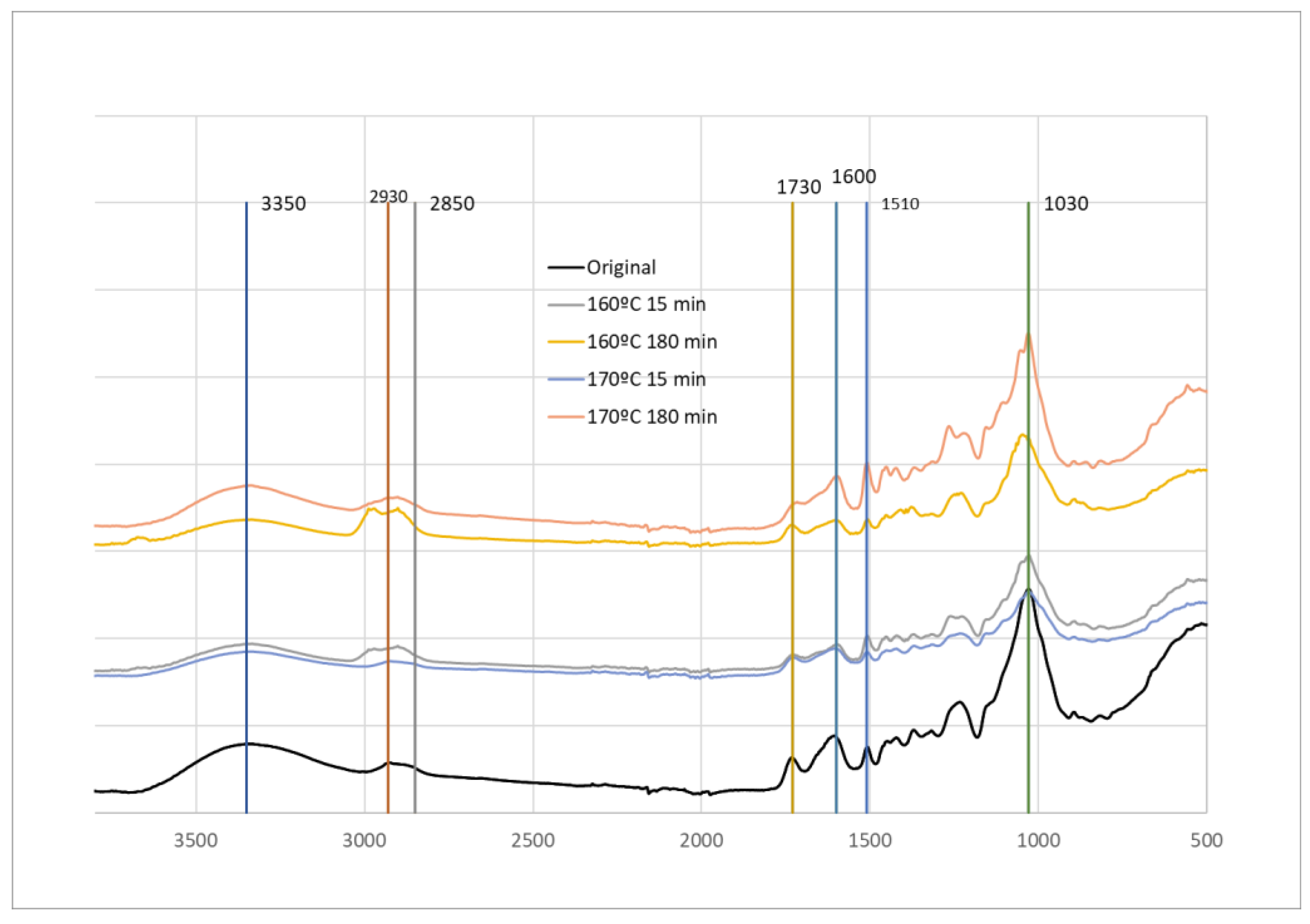
3.2. FTIR Analysis of Pre-Hydrolysis
FTIR-ATR analyses were conducted on HS original material and material after pre-hydrolysis at 160 °C and 170 °C for 15 and 180 min. In accordance with the chemical composition, there is a reduction of carbohydrates leading to a percentual increase in lignin, therefore, an increase in lignin assigned peaks and a decrease of the main carbohydrate peaks was to be expected.
Significant spectral changes were observed upon comparison of the initial material with the material after pre-hydrolysis. In the region around 3350 cm
-1 corresponding to the OH band, there is a slight decrease and narrowing of the band, especially for 160 °C and 180 min of pre-hydrolysis. Changes in the peaks at 2930 cm
-1 and 2850 cm
-1 that correspond to overlapping of -CH
2- (2935-2915 cm
-1) and -CH
3 (2970-2950 cm
-1) stretching asymmetric vibrations and -CH
2- (2865-2845 cm
-1) and -CH
3 (2880-2860 cm
-1) stretching symmetric vibrations [
44,
45,
46] were observed but where not very consistent. There is a slight increase for 160 °C and for 170 °C (180 min) pre-hydrolysis, and there is an increase of the 2850 cm
-1 peak in relation to 2930 cm
-1. A similar change was reported before for heat-treated wood and was stated to be due to the lower frequencies of CH stretching vibrations of methoxyl group in lignin [
46,
47].
In the peaks at 1730 cm-1 and 1600 cm-1, attributed to non-conjugated and conjugated C=O bonds there seems to be a merging of the two peaks, probably due to the increase of the 1600 cm-1 peak where generally lignin exhibits a strong absorption around 1600 cm-1 due to benzene ring stretching vibrations.
The most significant changes are observed, however in the fingerprint region with a significant increase in the 1510 cm
-1 peak, more visible in the samples with 180 min hydrolysis. This peak is associated to benzene ring stretching vibrations [
45]. Moreover the peak is at about 1505 cm
-1 for hardwood lignin (Guaiacyl - G and Syringyl - S) and at about 1510 cm
-1 for softwood lignin (Guaiacyl-G) [
48], which suggests that HS lignin is similar to a G-lignin.
At 170 °C and 180 min an increase of 1260 cm-1 was observed. This increase can be due to the increase in lignin, but this band has several different contributions. Additionally, a decrease of the 1030 cm-1 band, attributed to C-O stretching vibrations of C-O-C in carbohydrates was observed.
Figure 5.
FTIR-ATR of original material and material after pre-hydrolysis at 160 °C and 170 °C.
Figure 5.
FTIR-ATR of original material and material after pre-hydrolysis at 160 °C and 170 °C.
3.3. Liquefaction
After the pre-hydrolysis the remaining solid material was liquefied by polyalcohols with acid catalysis.
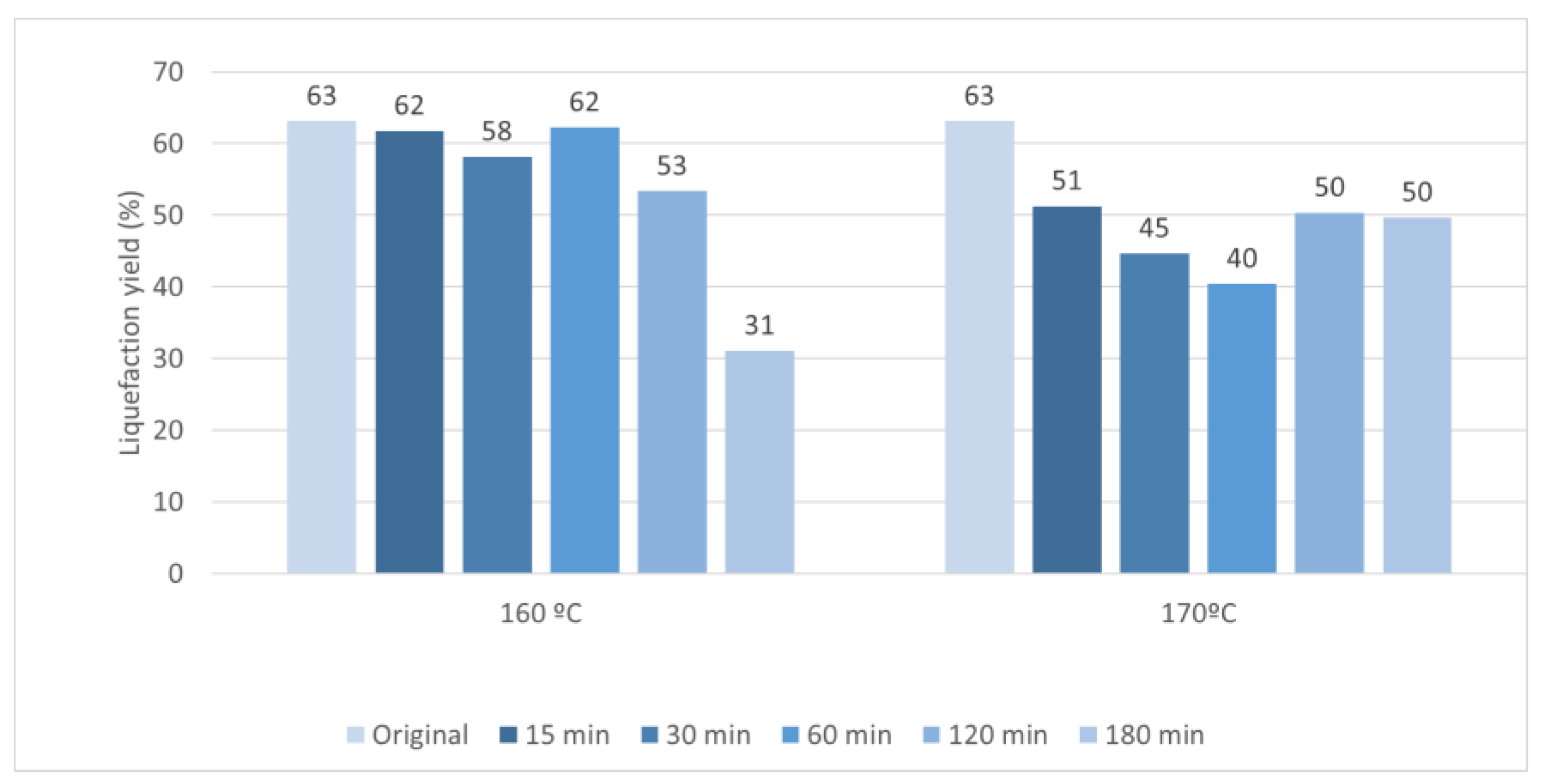
Figure 6 presents the liquefaction yield for the original material (without pre-hydrolysis) and of the solid residues obtained after the pre-hydrolysis at 160 °C and 170 °C. Generally, the liquefaction yield of HS pre-hydrolysed at 160 °C decreased with the time of the pre-hydrolysis, starting at around 63% for the original material and ending at 31% for the 180 min pre-hydrolysed samples. This is probably due to the lower amount of soluble compounds in the samples, since most of the hemicelluloses were already removed. Similarly, for 170 °C, there is a reduction of the liquefaction yield for the pre-hydrolysed materials during 15-60 min, but there is a posterior increase for the 120 min and 180 min pre-hydrolysed samples.
HS liquefaction yield is slightly lower compared to the liquefaction yield of other shells at the same conditions. For instance the liquefaction yield of walnut shells at 180ºC and 60 min and similar polyalcohol lead to a liquefaction yield of around 80% [
49]. Nevertheless the liquefaction yield is higher than the obtained before for a liquefaction with glycerol catalysed by sodium carbonate that needed a temperature of around 270ºC to obtain similar liquefaction yield [
50]. The liquefaction is also higher than the obtained before using water and other supercritical solvents that obtained similar percentages only at 300ºC [
51].
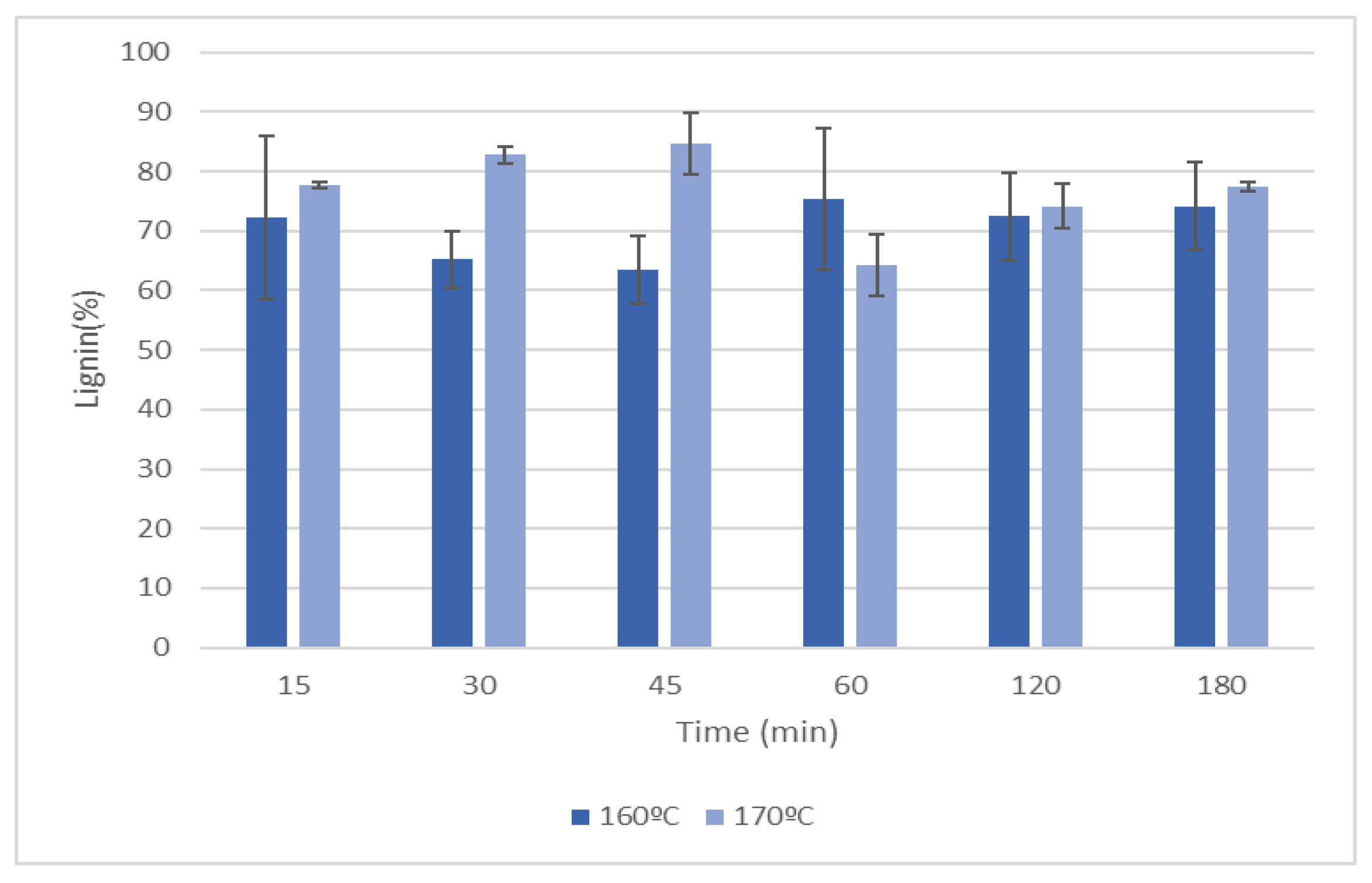
Figure 7 presents the klason lignin of the solid residues after liquefaction by polyalcohols with acid catalysis.
The results indicate that the solid residues after liquefaction are mainly composed of lignin with values ranging between 60-80%. However, generally lignin and hemicelluloses are considered to be the most susceptible materials to liquefaction [
52]. The high content of klason lignin can be due to the highly condensed structure that might not allow the determination of lignin by this method. The higher amount of lignin in the solid residues of samples pre-hydrolized at 170ºC seems correct since these materials had higher lignin content after pre-hydrolysis.
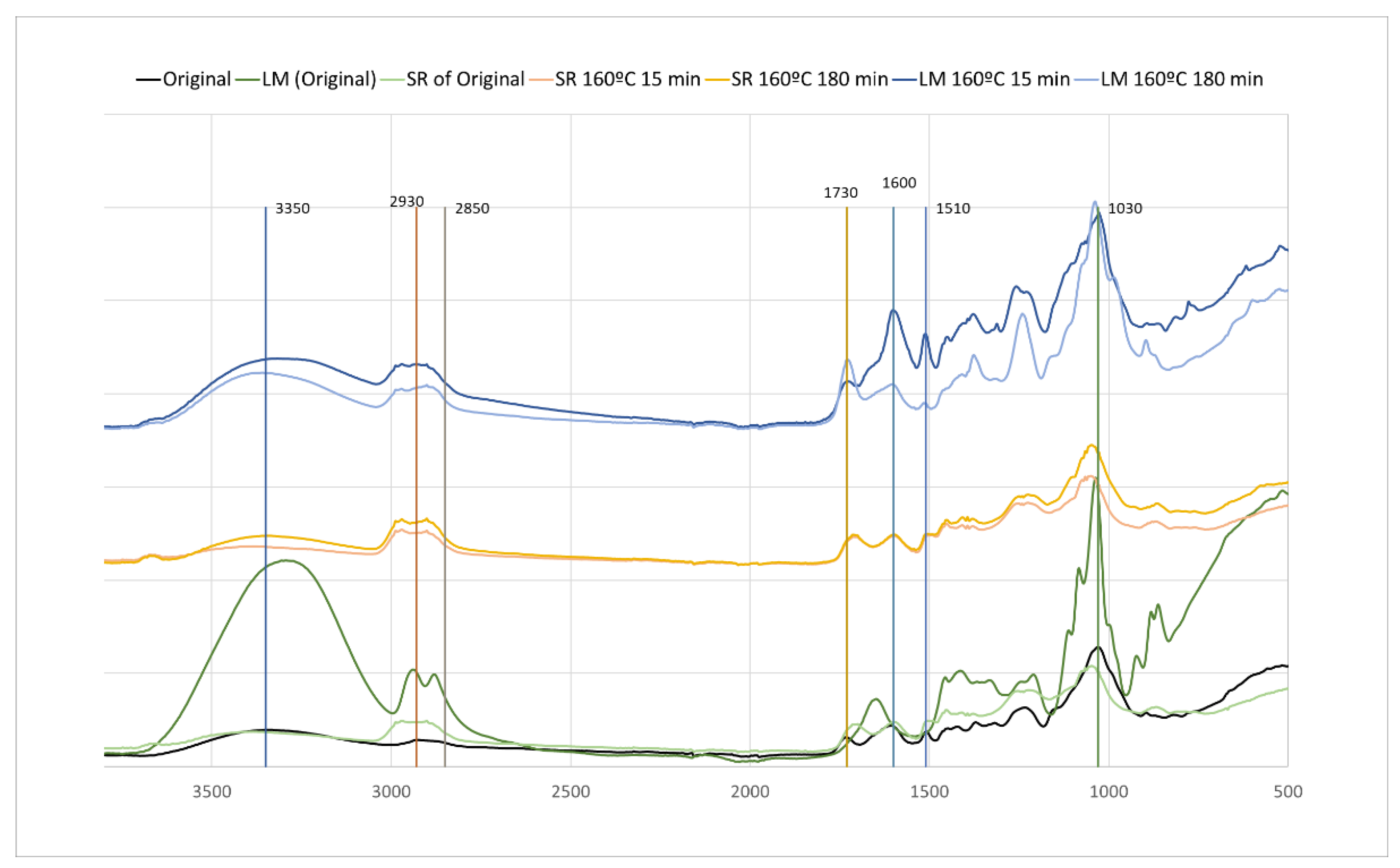
3.4. FTIR-ATR Analysis after Liquefaction
FTIR analysis was performed in the liquefied material (LM) and in the solid residue after liquefaction (SR) (
Figure 8). The temperatures (160 °C and 170 °C) and times (15 min, 180 min) refer to the pre-hydrolysis step. All the liquefied materials have a higher and broader OH stretching peak at around 3400 cm
-1. This peak results not only from the liquefied material but also from the polyalcohols (glycerol and ethylene glycol) used in the liquefaction procedure. This peak is not much different between the original material and the liquefaction solid residues. Both peaks at 2930 cm
-1 and 2850 cm
-1 stretching asymmetric and symmetric vibrations of CH, as mentioned before, are significantly different from the original HS. These peaks in LM and SR are wider and higher, with their maximum shifting for 2980 cm
-1 and 2900 cm
-1. The main difference is that in the liquefied material, the highest peak is at 2900 cm
-1 and for the liquefied residue is 2980 cm
-1. The bands at around 1730 cm
-1, corresponding to non-conjugated C=O linkages, and 1600 cm
-1, corresponding to conjugated C=O linkages, present relevant differences between the original, LM and SR materials. The liquefied material with pre-hydrolysis at 160 °C, initially (15 min) presents a high absorption band at 1600 cm
-1 in relation to the 1730 cm
-1 peak. This fact and the high absorption at 1510 cm
-1 can be due to lignin liquefaction at an early stage. The spectrum for 160 °C (180 min) has, however, a higher absorption at 1730 cm
-1 in relation to 1600 cm
-1. No significant changes are seen for the solid residue with 15 min or 180 min pre-hydrolysis, and similarly to the solid residue of the original material without pre-hydrolysis. There are two peaks that increase significantly in the liquefied material, which are around 1235 cm
-1 and 1370 cm
-1. The band around 1235 cm
-1 can have several contributions, from skeletal vibrations in lignin, but the band around 1370 cm
-1 has been associated with C-H bending vibrations in CH
3 groups of acyl fragments [
53].
In the spectrum of the solid residues there are weak absorptions at 1600 cm
-1, 1460 cm
-1, and 1320 cm
-1 which seem to indicate that the residues do not have high amounts of lignin as the wet chemistry showed which can be due to the unsuitability of klason method for lignin determination in these residues as discussed before. Similar results were presented before by Silva et al. [
54] that studied the liquefaction of Kraft lignin using polyhydric alcohols and organic acids as catalysts for sustainable polyols production.
The spectrum of the liquefied original material is very different from the liquefied materials with pre-hydrolisis, presenting higher peaks of OH (3350 cm-1), CHs (2930 cm-1 and 2859 cm-1) and C-O-C (1030 cm-1) mostly assigned to carbohydrates. The band around 1235 cm-1 is lower than for liquefied pre-hydrolised materials which can be due to the lower percentage of phenolic compounds in the liquefied original material in comparison with the pre-hydrolised materials.
Author Contributions
Conceptualization, B.E. and L.P.C.-L.; investigation, J.D., Y.D. and L.P.C.-L.; resources, B.E. and L.P.C.-L; writing—original draft preparation, B.E. and L.P.C.-L.; writing—review and editing, B.E., R.P.F.G., and L.P.C.-L. project administration, B.E., R.P.F.G., and L.P.C.-L; funding acquisition, B.E., R.P.F.G., and L.P.C.-L. All authors have read and agreed to the published version of the manuscript.