Submitted:
10 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
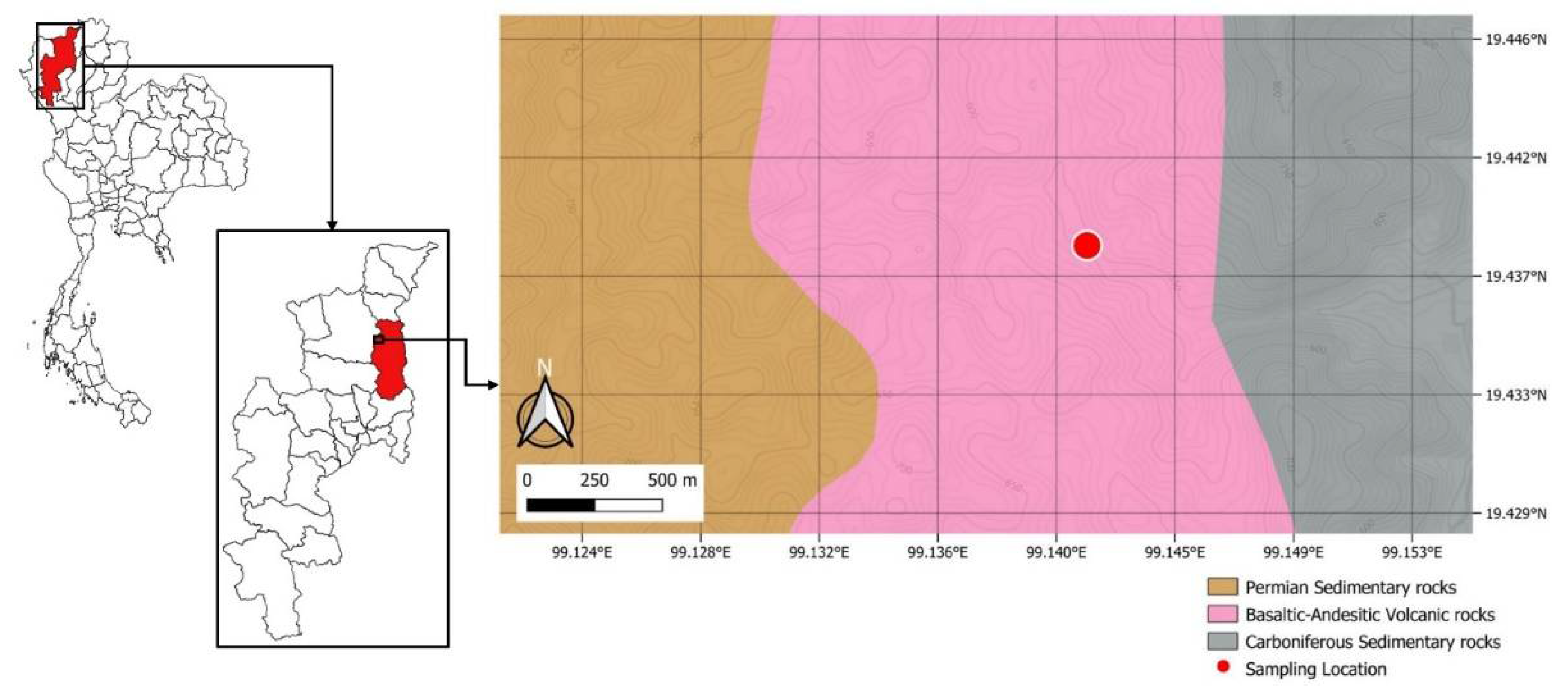
2. Geological Background
3. Materials and Methods
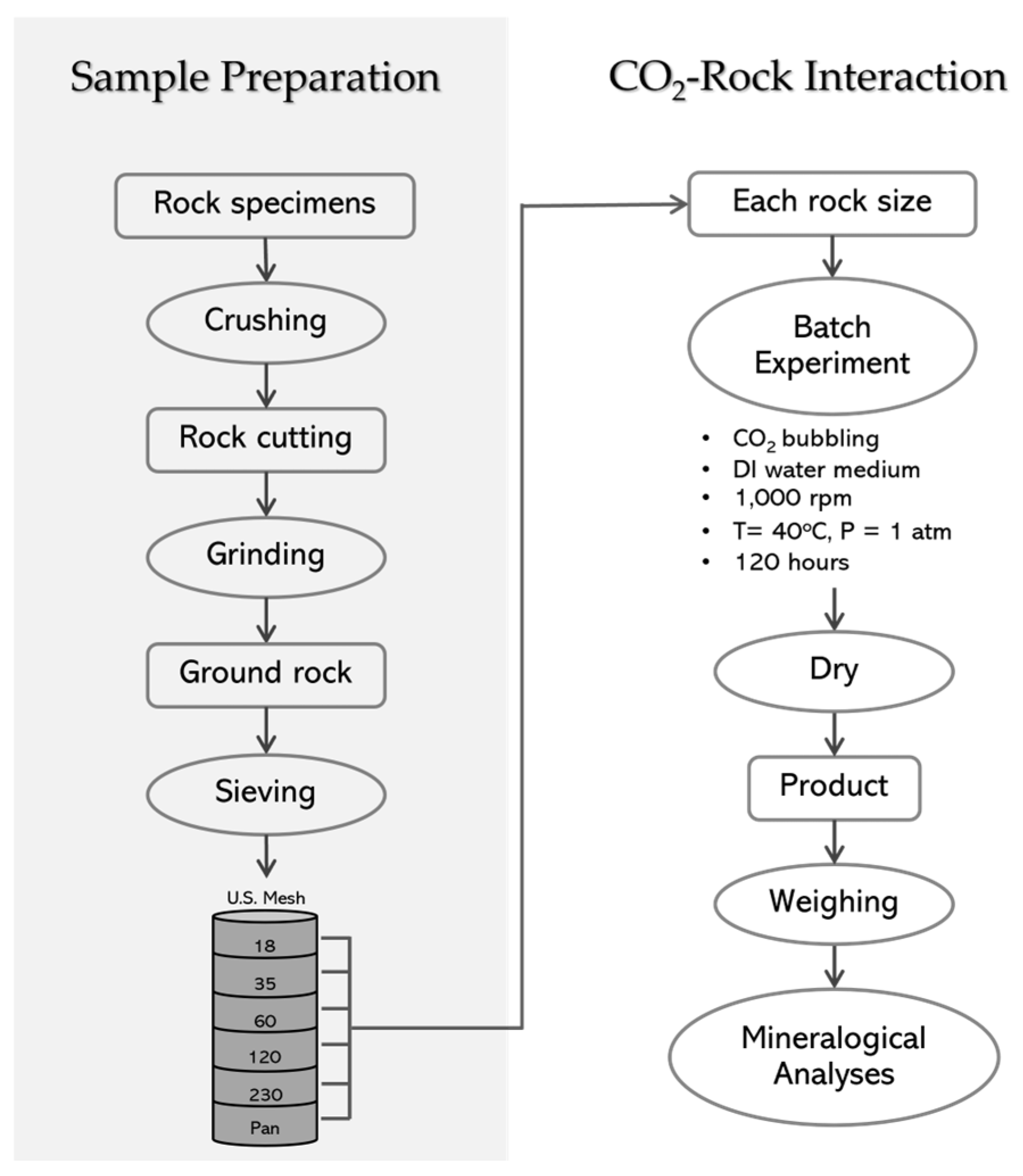
3.1. Rock Sampling and Petrographic Analysis
3.2. Experimental Procedure
3.3. Mineralogical Analysis
4. Results
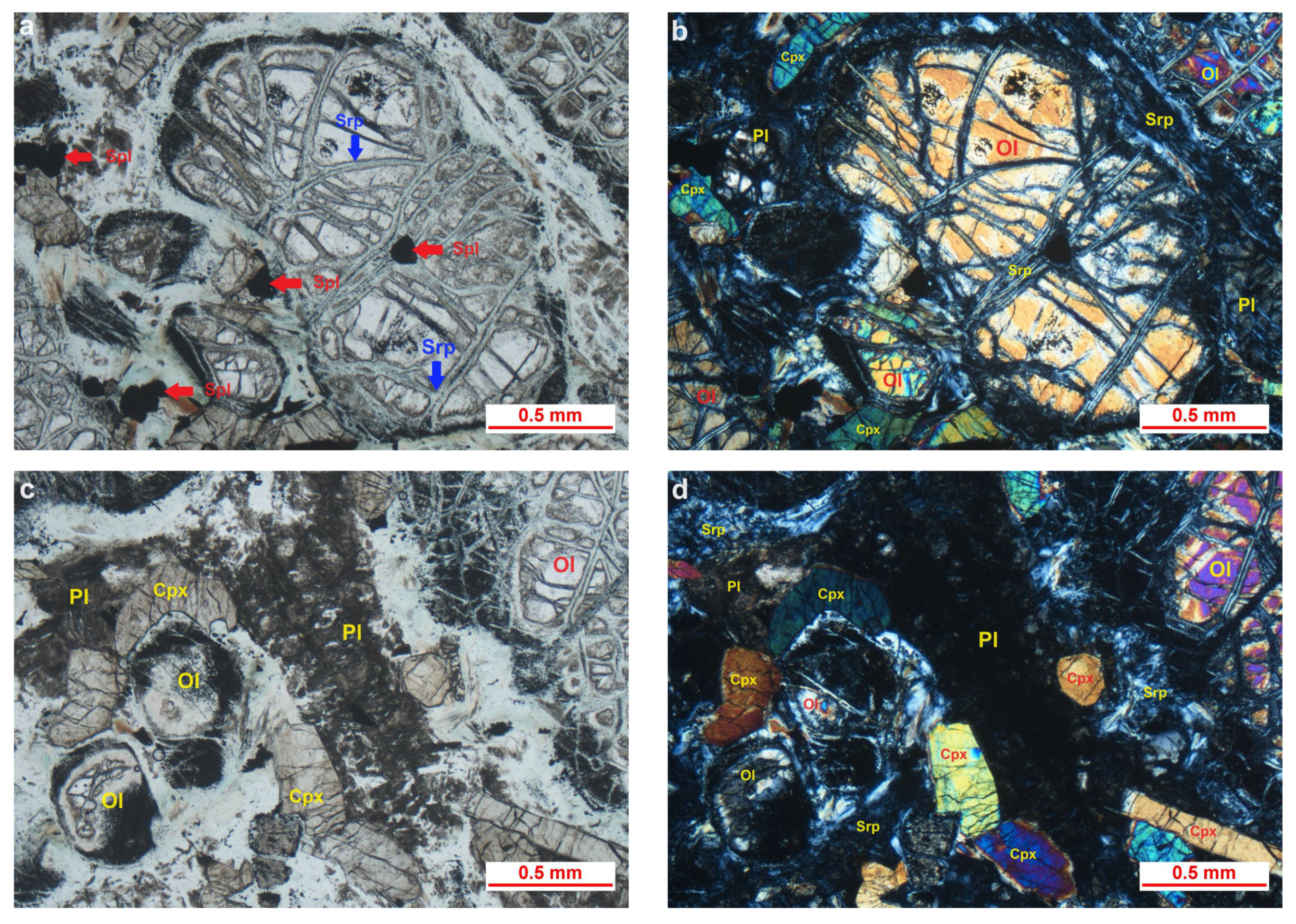
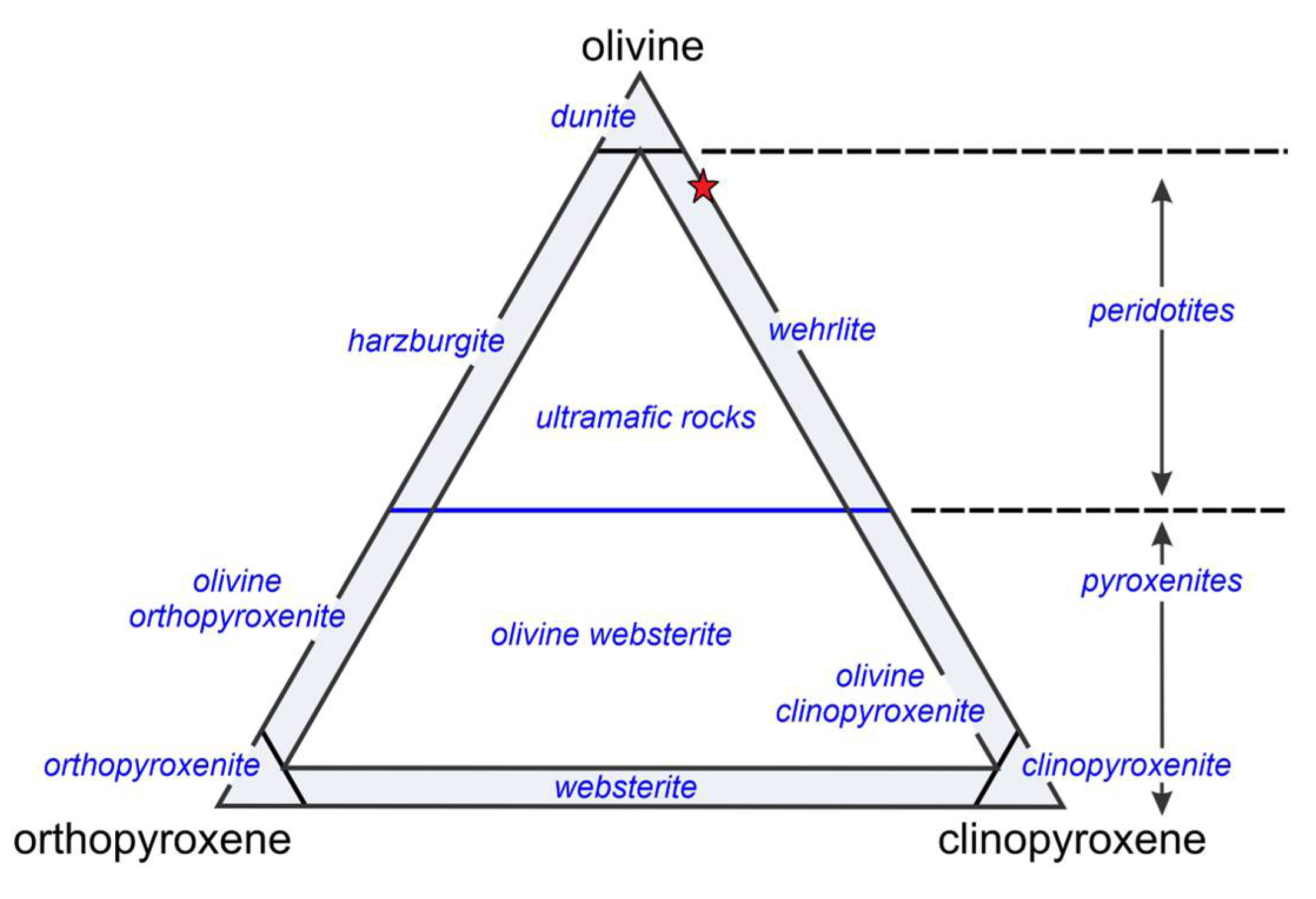
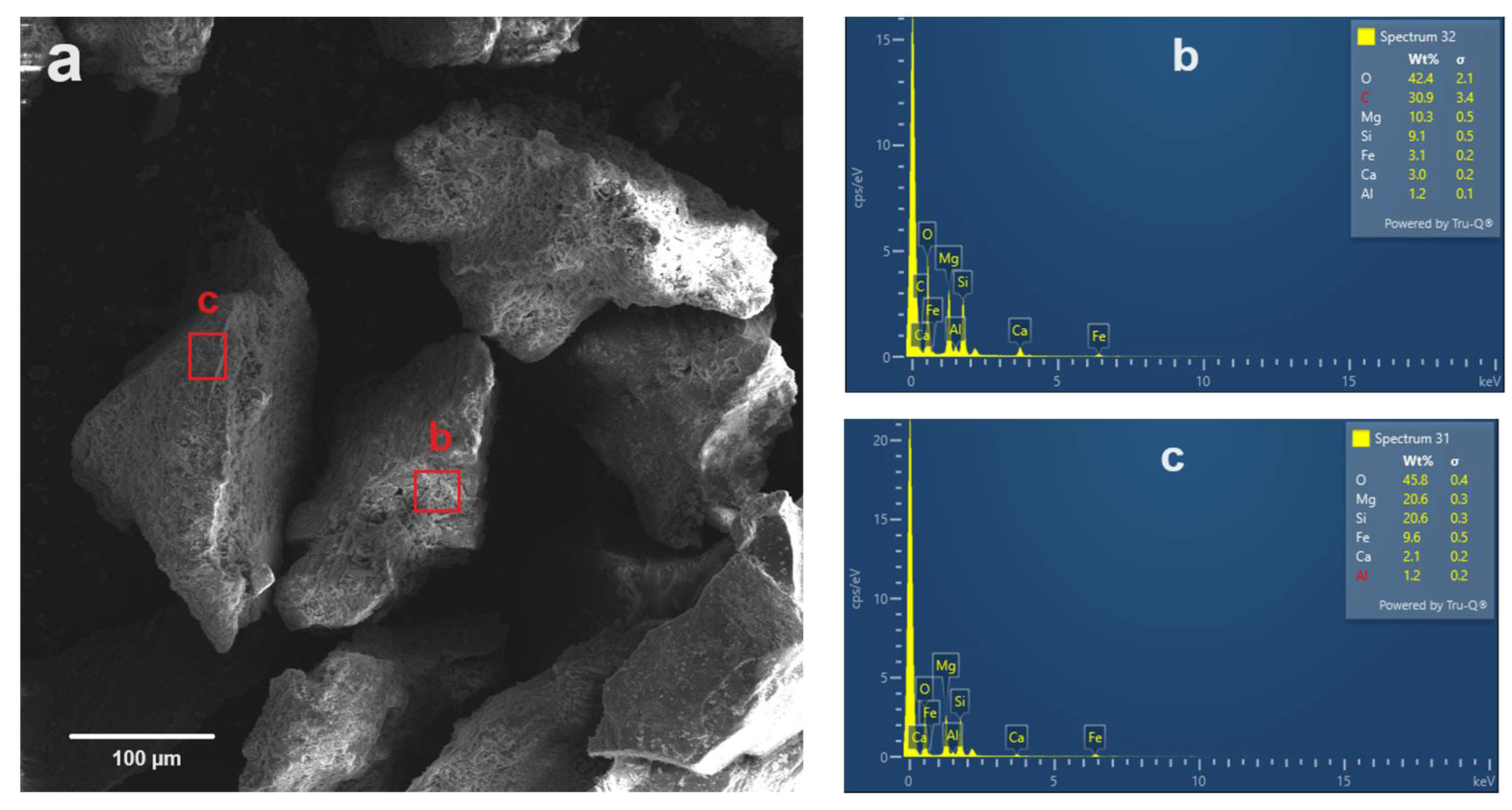
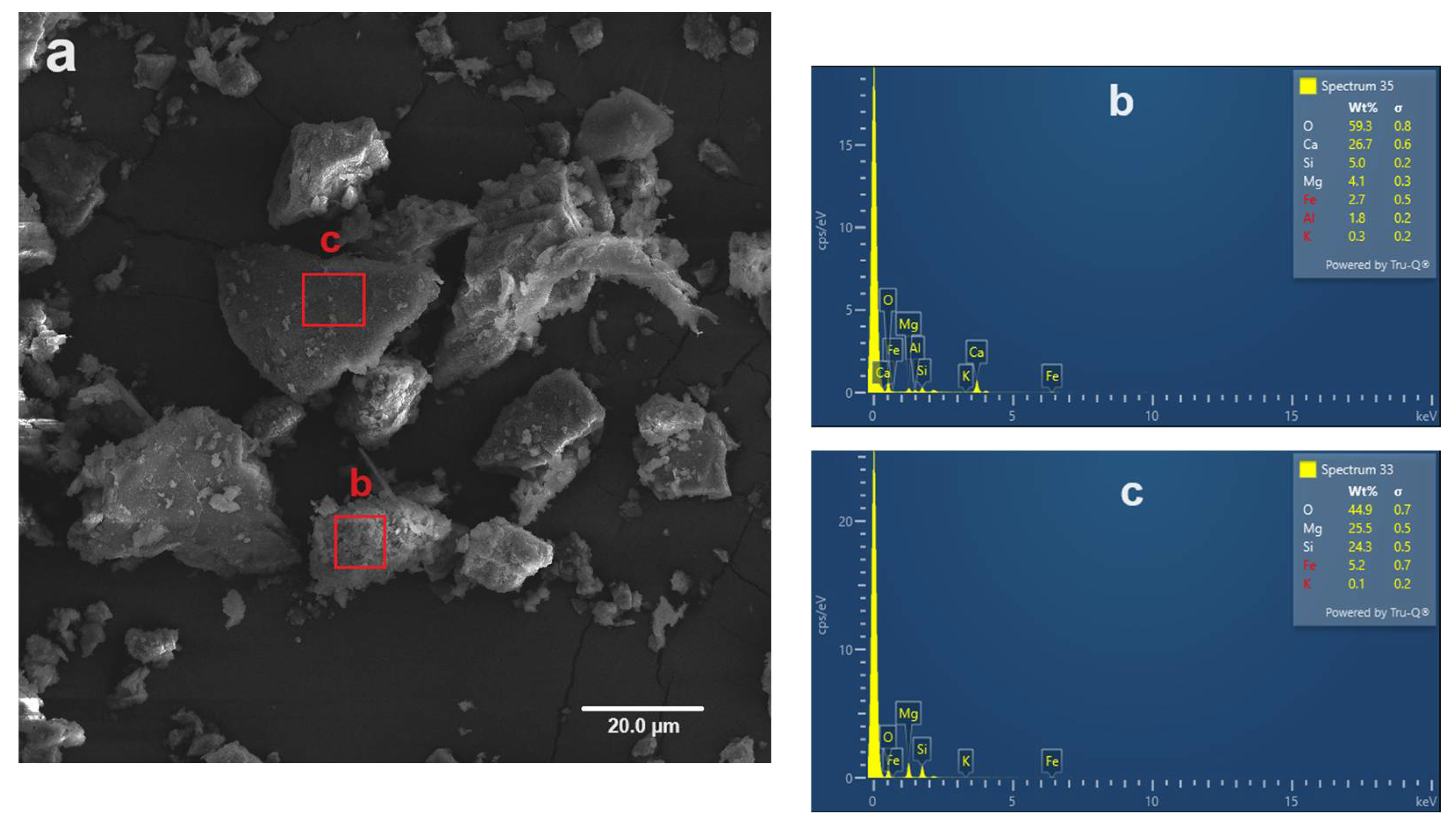
4.1. Mineral Composition and Microtextural Characteristics
4.2. Weight Changes
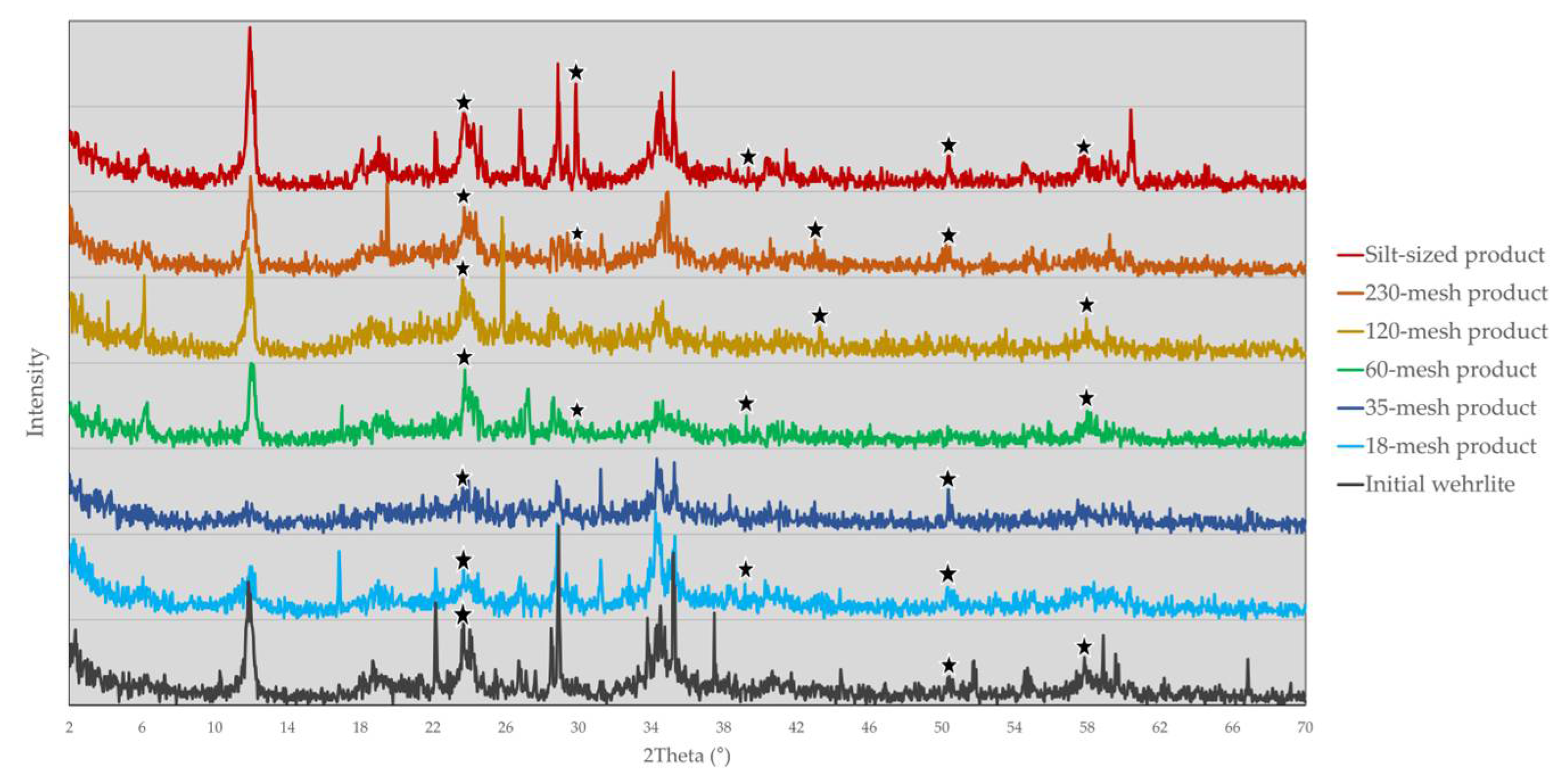
4.3. Mineralogical Changes
5. Discussions
5.1. Evidence for the Occurrence of Carbon Mineralization
5.2. Grain-Size Effects
5.3. Rock Mineralogy versus CO2 Uptake Prediction
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNFCCC. Paris Agreement. 2015.
- IPCC. IPCC special report on Global Warming of 1.5 ◦C; 2018.
- Chalmers, H.; Gibbins, J. Carbon capture and storage: The ten year challenge. Proc. Inst. Mech. Eng. C: J. Mech. Eng. Sci. 2016, 224, 505–518. [Google Scholar] [CrossRef]
- NASEM. Carbon Mineralization of CO2. In Negative emissions technologies and reliable sequestration: a research agenda; The National Academies Press: Washington, DC, 2019. [Google Scholar]
- Wang, P.-T.; Wei, Y.-M.; Yang, B.; Li, J.-Q.; Kang, J.-N.; Liu, L.-C.; Yu, B.-Y.; Hou, Y.-B.; Zhang, X. Carbon capture and storage in China’s power sector: Optimal planning under the 2°C constraint. Appl. Energy 2020, 263. [Google Scholar] [CrossRef]
- Kelemen, P.; Benson, S.M.; Pilorgé, H.; Psarras, P.; Wilcox, J. An overview of the status and challenges of CO2 storage in minerals and geological formations. Front. Clim. 2019, 1. [Google Scholar] [CrossRef]
- 7. Sandalow, D.; Aines, R.; Friedmann, J.; Kelemen, P.; McCormick, C.; Power, I.M.; Schmidt, B.; Wilson, S.A.S. Carbon Mineralization Roadmap; 2021.
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486–486. [Google Scholar] [CrossRef]
- Gerdemann, S.J.; O’Connor, W.K.; Dahlin, D.C.; Penner, L.R.; Rush, H. Ex situ aqueous mineral carbonation. Environ. Sci. Technol. 2007, 41, 2587–2593. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Wilson, S.; Kelemen, P.B.; Hitch, M.; Southam, G. Carbon mineralization: from natural analogues to engineered systems. Rev. Mineral. Geochem. 2013, 77, 305–360. [Google Scholar] [CrossRef]
- Hills, C.D.; Tripathi, N.; Carey, P.J. Mineralization technology for carbon capture, utilization, and storage. Front. Energy Res. 2020, 8. [Google Scholar] [CrossRef]
- Woodall, C.M.; McQueen, N.; Pilorgé, H.; Wilcox, J. Utilization of mineral carbonation products: current state and potential. Greenh. Gases: Sci. Technol. 2019, 9, 1096–1113. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Slotte, M.; Koivisto, E.; Erlund, R. Serpentinite carbonation process routes using ammonium sulfate and integration in industry. Energy Technol. 2017, 5, 945–954. [Google Scholar] [CrossRef]
- Garcia, B.; Beaumont, V.; Perfetti, E.; Rouchon, V.; Blanchet, D.; Oger, P.; Dromart, G.; Huc, A.Y.; Haeseler, F. Experiments and geochemical modelling of CO2 sequestration by olivine: Potential, quantification. Appl. Geochem. 2010, 25, 1383–1396. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Gislason, S.R.; Matter, J. Mineral carbonation of CO2. Elements 2008, 4, 333–337. [Google Scholar] [CrossRef]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Petrol. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Kavaliauskaite, I. Mineral carbonation for long-term CO₂ storage: an exergy analysis. Int. J. Thermodyn. 2004, 7, 23–31. [Google Scholar]
- Hartmann, J.; West, A.J.; Renforth, P.; Köhler, P.; De La Rocha, C.L.; Wolf-Gladrow, D.A.; Dürr, H.H.; Scheffran, J. Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Rev. Geophys. 2013, 51, 113–149. [Google Scholar] [CrossRef]
- Berner, R.A.; Lasaga, A.C.; Garrels, R.M. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am. J. Sci. 1983, 283, 641–683. [Google Scholar] [CrossRef]
- Raymo, M.E.; Ruddiman, W.F. Tectonic forcing of late Cenozoic climate. Nature 1992, 359, 117–122. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allègre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Penman, D.E.; Caves Rugenstein, J.K.; Ibarra, D.E.; Winnick, M.J. Silicate weathering as a feedback and forcing in Earth’s climate and carbon cycle. Earth-Sci. Rev. 2020, 209. [Google Scholar] [CrossRef]
- Bearat, H.; McKelvy, M.J.; Chizmeshya, A.V.; Gormley, D.; Nunez, R.; Carpenter, R.W.; Squires, K.; Wolf, G.H. Carbon sequestration via aqueous olivine mineral carbonation: role of passivating layer formation. Environ. Sci. Technol. 2006, 40, 4802–4808. [Google Scholar] [CrossRef]
- Gadikota, G.; Matter, J.; Kelemen, P.; Park, A.H. Chemical and morphological changes during olivine carbonation for CO2 storage in the presence of NaCl and NaHCO3. Phys. Chem. Chem. Phys. 2014, 16, 4679–4693. [Google Scholar] [CrossRef]
- Hariharan, S.; Mazzotti, M. Kinetics of flue gas CO2 mineralization processes using partially dehydroxylated lizardite. Chem. Eng. J. 2017, 324, 397–413. [Google Scholar] [CrossRef]
- Kashim, M.Z.; Tsegab, H.; Rahmani, O.; Abu Bakar, Z.A.; Aminpour, S.M. Reaction mechanism of wollastonite in situ mineral carbonation for CO2 sequestration: effects of saline conditions, temperature, and pressure. ACS Omega 2020, 5, 28942–28954. [Google Scholar] [CrossRef]
- Larachi, F.; Daldoul, I.; Beaudoin, G. Fixation of CO2 by chrysotile in low-pressure dry and moist carbonation: Ex-situ and in-situ characterizations. Geochim. Cosmochim. Acta. 2010, 74, 3051–3075. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Li, B.; Bai, Z. Electrolysis and heat pretreatment methods to promote CO2 sequestration by mineral carbonation. Chem. Eng. Res. Design 2009, 87, 210–215. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Fauth, D.J.; Kuchta, M.E.; Zhang, Y.; Andrésen, J.M. Activation of magnesium rich minerals as carbonation feedstock materials for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1627–1645. [Google Scholar] [CrossRef]
- Styles, M.T.; Sanna, A.; Lacinska, A.M.; Naden, J.; Maroto-Valer, M. The variation in composition of ultramafic rocks and the effect on their suitability for carbon dioxide sequestration by mineralization following acid leaching. Greenh. Gases: Sci. Technol. 2014, 4, 440–451. [Google Scholar] [CrossRef]
- Kwon, S. Mineralization for CO2 sequestration using olivine sorbent in the presence of water vapor. Ph.D., Georgia Institute of Technology, Georgia, USA, 2011.
- Kelemen, P.B.; Matter, J.; Streit, E.E.; Rudge, J.F.; Curry, W.B.; Blusztajn, J. Rates and mechanisms of mineral carbonation in peridotite: natural processes and recipes for enhanced, in situ CO2 capture and storage. Annu. Rev. Earth Planet. Sci. 2011, 39, 545–576. [Google Scholar] [CrossRef]
- Taylor, L.L.; Quirk, J.; Thorley, R.M.S.; Kharecha, P.A.; Hansen, J.; Ridgwell, A.; Lomas, M.R.; Banwart, S.A.; Beerling, D.J. Enhanced weathering strategies for stabilizing climate and averting ocean acidification. Nat. Clim. Change 2015, 6, 402–406. [Google Scholar] [CrossRef]
- Brady, P.V. The effect of silicate weathering on global temperature and atmospheric CO2. J. Geophys. Res. Solid Earth 2012, 96, 18101–18106. [Google Scholar] [CrossRef]
- Power, I.M.; Paulo, C.; Rausis, K. The mining industry’s role in enhanced weathering and mineralization for CO2 removal. Environ. Sci. Technol. 2024, 58, 43–53. [Google Scholar] [CrossRef]
- Edwards, D.P.; Lim, F.; James, R.H.; Pearce, C.R.; Scholes, J.; Freckleton, R.P.; Beerling, D.J. Climate change mitigation: potential benefits and pitfalls of enhanced rock weathering in tropical agriculture. Biol. Lett. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- Haque, F.; Santos, R.M.; Chiang, Y.W. CO2 sequestration by wollastonite-amended agricultural soils – an Ontario field study. Int. J. Greenh. Gas Control 2020, 97. [Google Scholar] [CrossRef]
- Moosdorf, N.; Renforth, P.; Hartmann, J. Carbon dioxide efficiency of terrestrial enhanced weathering. Environ. Sci. Technol. 2014, 48, 4809–4816. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Bradshaw, P.M.D.; Harrison, A.L. Prospects for CO2 mineralization and enhanced weathering of ultramafic mine tailings from the Baptiste nickel deposit in British Columbia, Canada. Int. J. Greenh. Gas Control. 2020, 94. [Google Scholar] [CrossRef]
- Strefler, J.; Amann, T.; Bauer, N.; Kriegler, E.; Hartmann, J. Potential and costs of carbon dioxide removal by enhanced weathering of rocks. Environ. Res. Lett. 2018, 13. [Google Scholar] [CrossRef]
- Falk, E.S.; Kelemen, P.B. Geochemistry and petrology of listvenite in the Samail ophiolite, Sultanate of Oman: Complete carbonation of peridotite during ophiolite emplacement. Geochim. Cosmochim. Acta. 2015, 160, 70–90. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Hirth, G. Reaction-driven cracking during retrograde metamorphism: olivine hydration and carbonation. Earth Planet. Sci. Lett. 2012, 345-348, 81–89. [Google Scholar] [CrossRef]
- Goff, F.; Lackner, K.S. Carbon dioxide sequestering using ultramafic rocks. Environ. Geosci. 1998, 5, 89–102. [Google Scholar] [CrossRef]
- Mervine, E.M.; Humphris, S.E.; Sims, K.W.W.; Kelemen, P.B.; Jenkins, W.J. Carbonation rates of peridotite in the Samail Ophiolite, Sultanate of Oman, constrained through 14C dating and stable isotopes. Geochim. Cosmochim. Acta. 2014, 126, 371–397. [Google Scholar] [CrossRef]
- Streit, E.; Kelemen, P.; Eiler, J. Coexisting serpentine and quartz from carbonate-bearing serpentinized peridotite in the Samail Ophiolite, Oman. Contribut. Mineral. Petrol. 2012, 164, 821–837. [Google Scholar] [CrossRef]
- Cutts, J.A.; Steinthorsdottir, K.; Turvey, C.; Dipple, G.M.; Enkin, R.J.; Peacock, S.M. Deducing mineralogy of serpentinized and carbonated ultramafic rocks using physical properties with implications for carbon sequestration and subduction zone dynamics. Geochem. Geophys. Geosystems. 2021, 22. [Google Scholar] [CrossRef]
- Lisabeth, H.P.; Zhu, W.; Kelemen, P.B.; Ilgen, A. Experimental evidence for chemo-mechanical coupling during carbon mineralization in ultramafic rocks. Earth Planet. Sci. Lett. 2017, 474, 355–367. [Google Scholar] [CrossRef]
- Paulo, C.; Power, I.M.; Zeyen, N.; Wang, B.; Wilson, S. Geochemical modeling of CO2 sequestration in ultramafic mine wastes from Australia, Canada, and South Africa: implications for carbon accounting and monitoring. Appl. Geochem. 2023, 152. [Google Scholar] [CrossRef]
- DMR. Geology of Thailand, 1st ed.; Department of Mineral Resources, Ministry of Natural Resources and Environment: Bangkok, Thailand, 2014. [Google Scholar]
- Phajuy, B.; Panjasawatwong, Y.; Osataporn, P. Preliminary geochemical study of volcanic rocks in the Pang Mayao area, Phrao, Chiang Mai, northern Thailand: tectonic setting of formation. J. Asian Earth Sci. 2005, 24, 765–776. [Google Scholar] [CrossRef]
- Barr, S.M.; Tantisukrit, C.; Yaowanoiyothin, W.; Macdonald, A.S. Petrology and tectonic implications of Upper Paleozoic volcanic rocks of the Chiang Mai belt, northern Thailand. J. Southeast Asian Earth Sci. 1990, 4, 37–47. [Google Scholar] [CrossRef]
- Metcalfe, I. Permian tectonic framework and palaeogeography of SE Asia. J. Asian Earth Sci. 2002, 20, 551–566. [Google Scholar] [CrossRef]
- DMR. Geological map of Thailand on 1:50,000 scale. 2007.
- Le Maitre, R.W.; Streckeisen, A.; Zanettin, B.; Le Bas, M.J.; Bonin, B.; Bateman, P. , (Eds.) Igneous rocks: a classification and glossary of terms: recommendations of the international union of geological sciences subcommission on the systematics of igneous rocks. 2 ed.; Cambridge University Press: Cambridge, England, 2002. [Google Scholar]
- Feniak, M.W. Grain sizes and shapes of various minerals in igneous rocks. American Mineralogist 1944, 29, 415–421. [Google Scholar]
- Liu, L.; Liu, Y.; Tian, X.; Chen, X. Superior CO2 uptake and enhanced compressive strength for carbonation curing of cement-based materials via flue gas. Constr. Build. Mater. 2022, 346. [Google Scholar] [CrossRef]
- Nielsen, P.; Quaghebeur, M. Determination of the CO2 uptake of construction products manufactured by mineral carbonation. Minerals 2023, 13. [Google Scholar] [CrossRef]
- Yang, K.-H.; Seo, E.-A.; Tae, S.-H. Carbonation and CO2 uptake of concrete. Environ. Impact Assess. Rev. 2014, 46, 43–52. [Google Scholar] [CrossRef]
- Raza, A.; Glatz, G.; Gholami, R.; Mahmoud, M.; Alafnan, S. Carbon mineralization and geological storage of CO2 in basalt: mechanisms and technical challenges. Earth-Sci. Rev. 2022, 229. [Google Scholar] [CrossRef]

| Sample ID | Mesh No. | Particle Size (mm) | Weight (g) | Difference (g) | % Change | |
|---|---|---|---|---|---|---|
| Pre-test | Post-test | |||||
| PR-18 | 18 | ≥ 1.0 | 10.000 | 10.004 | + 0.004 | 0.04 |
| PR-35 | 35 | < 1.0 – 0.5 | 10.000 | 10.006 | + 0.006 | 0.06 |
| PR-60 | 60 | < 0.5 – 0.25 | 10.000 | 10.010 | + 0.010 | 0.10 |
| PR-120 | 120 | < 0.25 – 0.125 | 5.000 | 5.016 | + 0.016 | 0.32 |
| PR-230 | 230 | < 0.125 – 0.0625 | 5.000 | 5.015 | + 0.015 | 0.30 |
| PR-silt | Pan | < 0.0625 | 5.000 | 5.018 | + 0.018 | 0.36 |
| Sample Material | Major phase [>30%] |
Moderate phase [10-30%] |
Minor phase [2-10%] |
Trace phase [<2%] |
|---|---|---|---|---|
| Wehrlite | Antigorite | Augite Olivine |
Calcite Albite Chlorite |
Illite |
| 18-mesh product | n/d | Antigorite Augite Olivine Albite |
Chlorite Mg-bearing calcite |
Illite |
| 35-mesh product | Olivine | Albite Calcite Augite |
Antigorite | Chlorite Illite |
| 60-mesh product | Antigorite | Ca-bearing albite Calcite |
Chlorite Illite Olivine |
Augite |
| 120-mesh product | Albite | Antigorite Illite |
Calcite Olivine Augite Cr-bearing chlorite |
n/d |
| 230-mesh product | n/d | Antigorite Olivine Augite Illite Calcite |
Chlorite Albite |
n/d |
| Silt-sized product | Antigorite | Augite Calcite Albite Olivine |
Chlorite | Illite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





