Submitted:
10 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals, Diet, and Microorganisms
2.2. BCG Vaccination in HFD-Fed Mice
2.3. Bone Marrow Transplantation from BCG Vaccinated Mice
2.4. Intraperitoneal Glucose Tolerance Test and Insulin Tolerance Test
2.5. Measurement of Biochemical Parameters in Serum
2.6. Measurement of Hepatic Lipid Levels
2.7. Histopathological Examination
2.8. Flow Cytometry
2.9. Statistical Analyses
3. Results
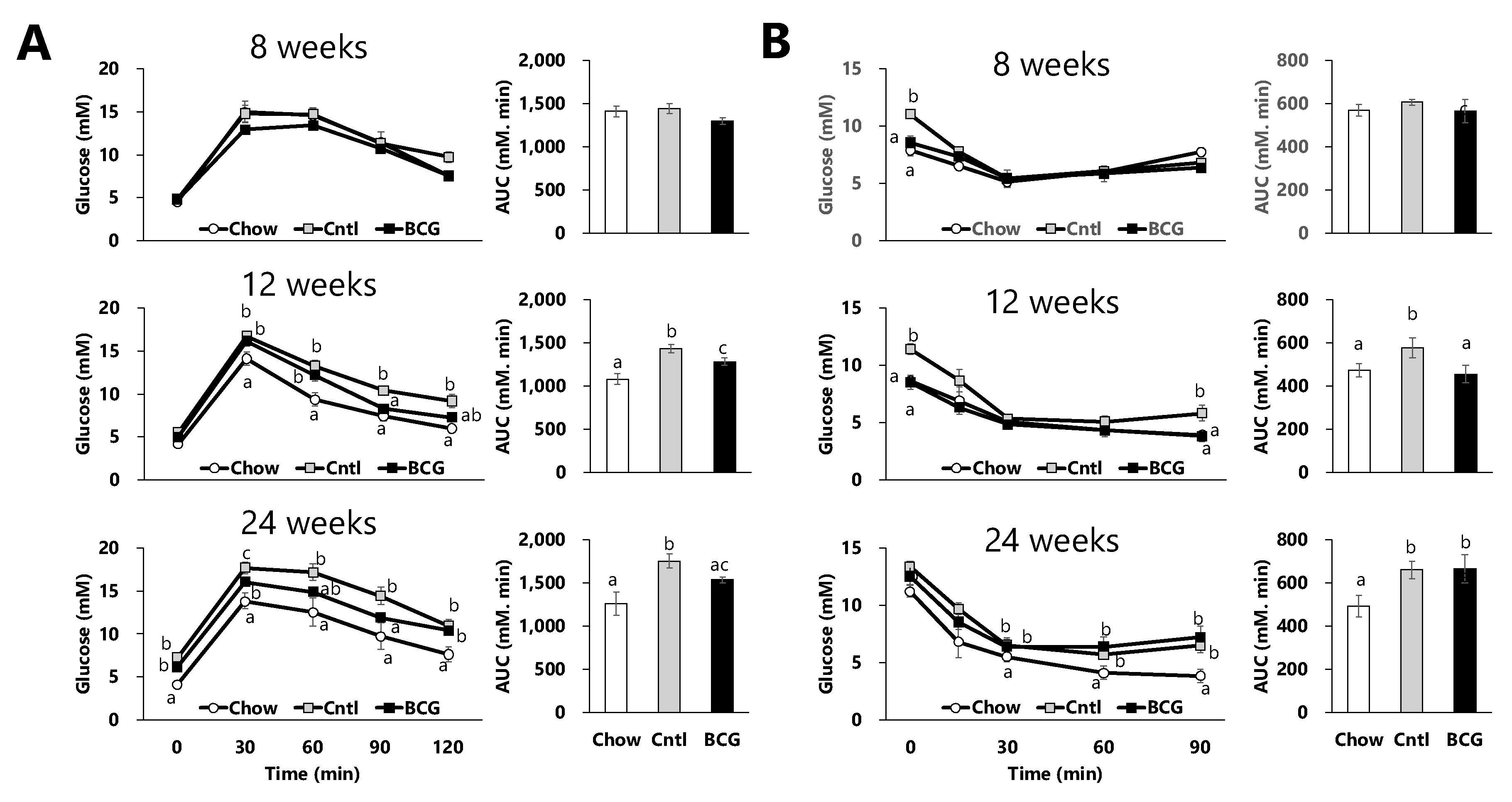
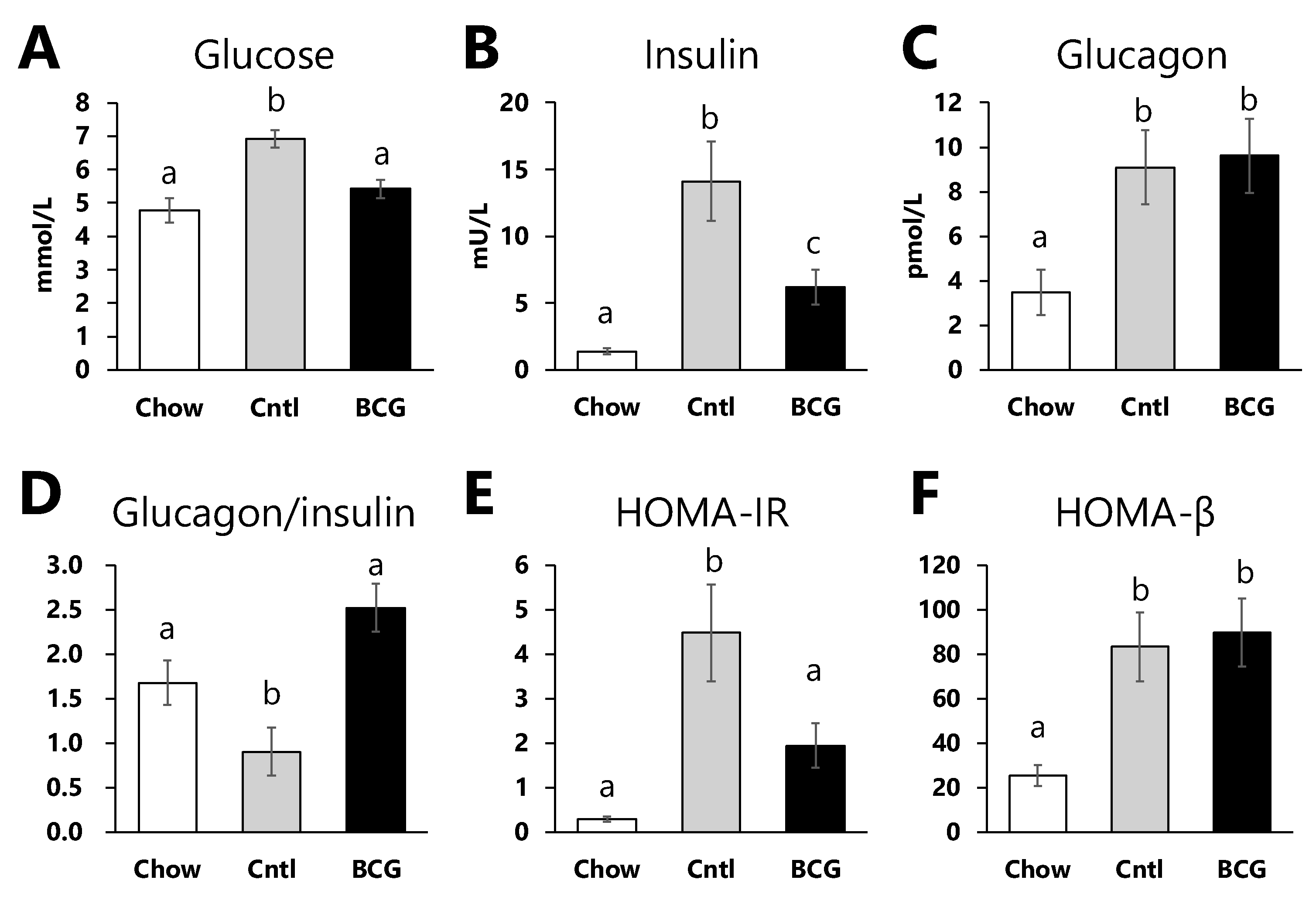
3.1. Effect of BCG Vaccination on Glucose Intolerance in HFD-fed Mice.
| Parameters | Chow | Cntl | BCG |
|---|---|---|---|
| Growth | |||
| Final body weight (g) | 27.5 ± 0.6 a | 39.7 ± 1.8 b | 36.6 ± 1.5 b |
| Liver weight (g) | 0.95 ± 0.03 a | 1.70 ± 0.19 b | 1.30 ± 0.08 ab |
| Serum | |||
| Triglyceride (mg/dL) | 54.4 ± 4.9 a | 69.7 ± 3.3 b | 60.3 ± 6.1 b |
| Total cholesterol (mg/dL) | 160 ± 12 | 188 ± 8.6 | 161 ± 9.5 |
| AST (IU/L) | 29.3 ± 5.2 | 26.9 ± 5.6 | 21.8 ± 5.9 |
| ALT (IU/L) | 4.41 ± 1.24 | 4.02 ± 0.50 | 4.47 ± 0.44 |
| Hepatic | |||
| Triglyceride (mg/g liver) | 31.3 ± 0.6 a | 57.4 ± 6.6 b | 40.6 ± 5.6 b |
3.2. Effect of BCG Vaccination on Growth Parameters, and Blood Parameters, and Hepatic Lipid Contents
3.3. Effect of BCG Vaccination on Glucose Metabolism Parameters
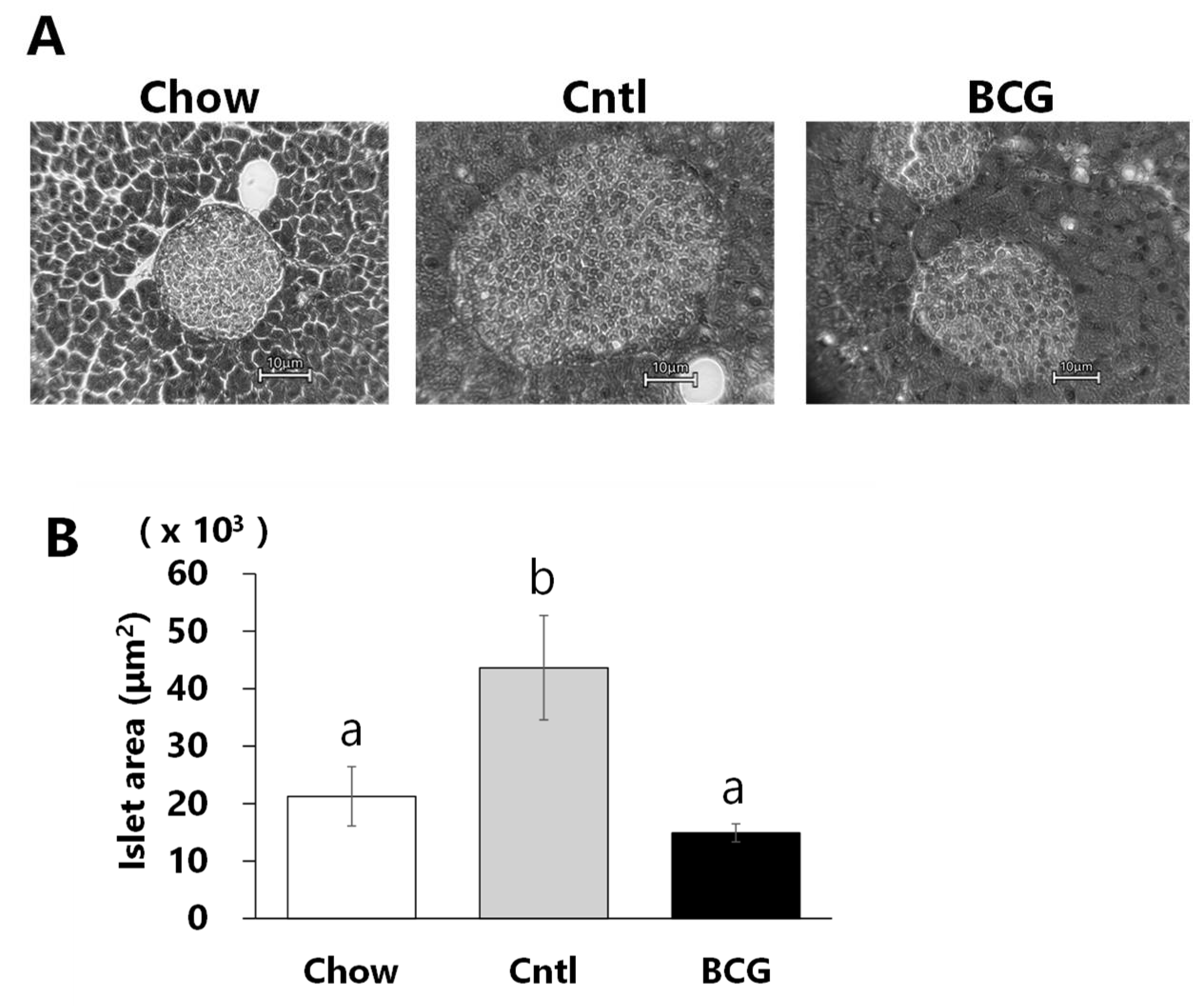
3.4. Effect of BCG Vaccination on Pancreatic Islet Size
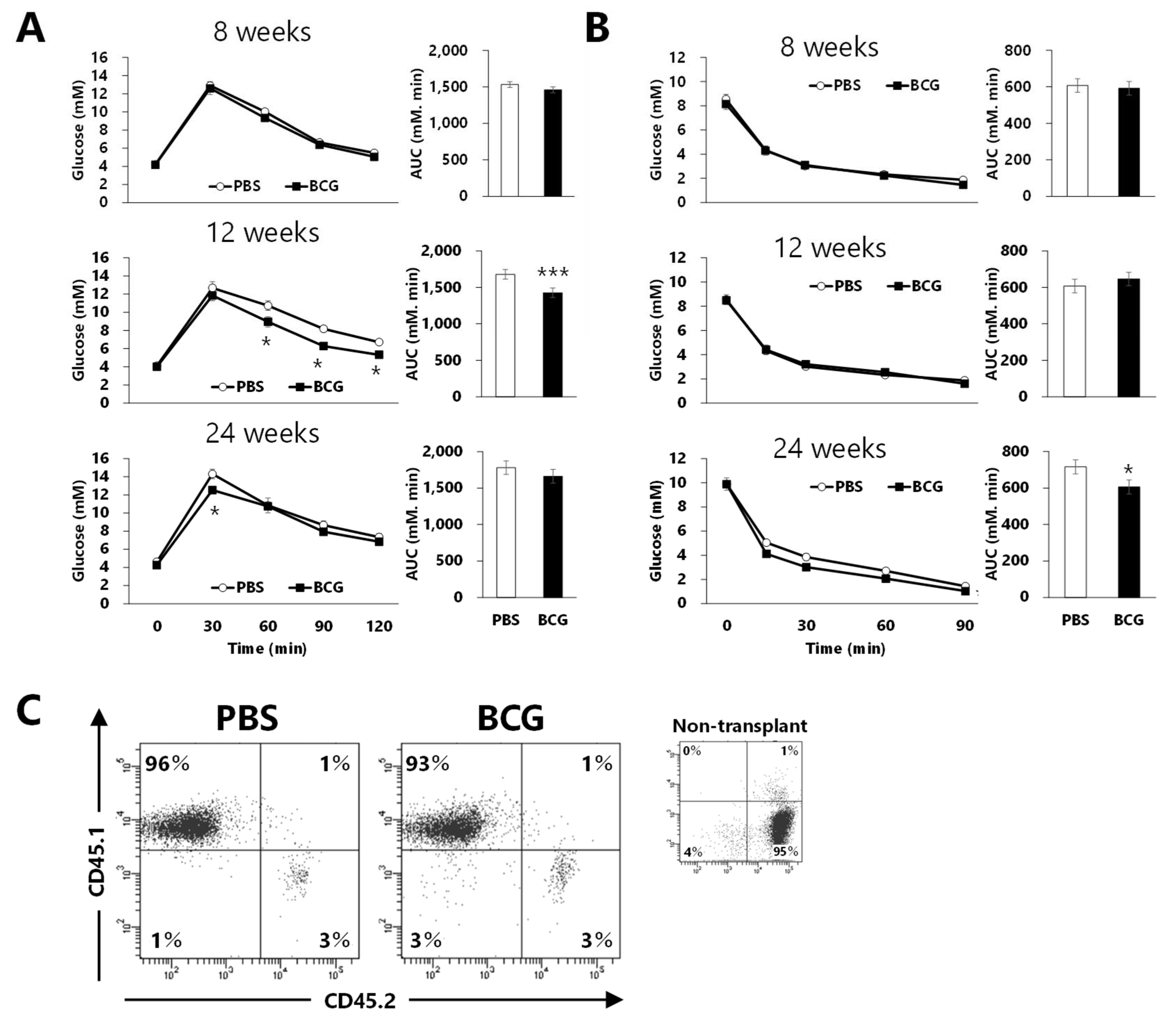
3.5. Effect of Bone Marrow Transplantation from BCG-vaccinated Mice on Glucose Intolerance Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Formiguera, X.; Canton, A. Obesity: epidemiology and clinical aspects. Best Pract Res Clin Gastroenterol 2004, 18, 1125-1146. [CrossRef]
- Patel, P.S.; Buras, E.D.; Balasubramanyam, A. The Role of the Immune System in Obesity and Insulin Resistance. Journal of Obesity 2013, 2013, 1-9. [CrossRef]
- Pedicino, D.; Francesca, A.; Alessandro, V.; Trotta, F.; Liuzzo, G. Type 2 Diabetes, Immunity and Cardiovascular Risk: A Complex Relationship. InTech: 2012.
- Zhou, T.; Hu, Z.; Yang, S.; Sun, L.; Yu, Z.; Wang, G. Role of Adaptive and Innate Immunity in Type 2 Diabetes Mellitus. Journal of Diabetes Research 2018, 2018, 1-9. [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice 2022, 183, 109119. [CrossRef]
- Sinclair, A.; Saeedi, P.; Kaundal, A.; Karuranga, S.; Malanda, B.; Williams, R. Diabetes and global ageing among 65-99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2020, 162, 108078. [CrossRef]
- Gao, A.W.; Cantó, C.; Houtkooper, R.H. Mitochondrial response to nutrient availability and its role in metabolic disease. EMBO Molecular Medicine 2014, 6, 580-589. [CrossRef]
- Lachmandas, E.; Van Den Heuvel, C.N.A.M.; Damen, M.S.M.A.; Cleophas, M.C.P.; Netea, M.G.; Van Crevel, R. Diabetes Mellitus and Increased Tuberculosis Susceptibility: The Role of Short-Chain Fatty Acids. Journal of Diabetes Research 2016, 2016, 1-15. [CrossRef]
- Radhakrishnan, R.K.; Thandi, R.S.; Tripathi, D.; Paidipally, P.; McAllister, M.K.; Mulik, S.; Samten, B.; Vankayalapati, R. BCG vaccination reduces the mortality of Mycobacterium tuberculosis–infected type 2 diabetes mellitus mice. JCI Insight 2020, 5. [CrossRef]
- Jiang, Y.; Zhang, W.; Wei, M.; Yin, D.; Tang, Y.; Jia, W.; Wang, C.; Guo, J.; Li, A.; Gong, Y. Associations between type 1 diabetes and pulmonary tuberculosis: a bidirectional mendelian randomization study. Diabetology & Metabolic Syndrome 2024, 16. [CrossRef]
- Sugawara, I.; Mizuno, S. Higher Susceptibility of Type 1 Diabetic Rats to Mycobacterium tuberculosis Infection. The Tohoku Journal of Experimental Medicine 2008, 216, 363-370. [CrossRef]
- Jamshidi, P.; Danaei, B.; Mohammadzadeh, B.; Arbabi, M.; Nayebzade, A.; Sechi, L.A.; Nasiri, M.J. BCG Vaccination and the Risk of Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Pathogens 2023, 12, 581. [CrossRef]
- Arts, R.J.W.; Moorlag, S.; Novakovic, B.; Li, Y.; Wang, S.Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.B.; et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89-100 e105. [CrossRef]
- Blok, B.A.; Arts, R.J.W.; Van Crevel, R.; Benn, C.S.; Netea, M.G. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. Journal of Leukocyte Biology 2015, 98, 347-356. [CrossRef]
- Ijaz, M.U.; Vaziri, F.; Wan, Y.-J.Y. Effects of Bacillus Calmette-Gu erin on immunometabolism, microbiome and liver diseases. Liver Research 2023, 116-123. [CrossRef]
- Angelidou, A.; Pittet, L.F.; Faustman, D.; Curtis, N.; Levy, O. BCG vaccine’s off-target effects on allergic, inflammatory, and autoimmune diseases: Worth another shot? Journal of Allergy and Clinical Immunology 2022, 149, 51-54. [CrossRef]
- Kühtreiber, W.M.; Tran, L.; Kim, T.; Dybala, M.; Nguyen, B.; Plager, S.; Huang, D.; Janes, S.; Defusco, A.; Baum, D.; et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. npj Vaccines 2018, 3. [CrossRef]
- Kühtreiber, W.M.; Faustman, D.L. BCG Therapy for Type 1 Diabetes: Restoration of Balanced Immunity and Metabolism. Trends in Endocrinology & Metabolism 2019, 30, 80-92. [CrossRef]
- Shpilsky, G.F.; Takahashi, H.; Aristarkhova, A.; Weil, M.; Ng, N.; Nelson, K.J.; Lee, A.; Zheng, H.; Kühtreiber, W.M.; Faustman, D.L. Bacillus Calmette-Guerin ‘s beneficial impact on glucose metabolism: Evidence for broad based applications. iScience 2021, 24, 103150. [CrossRef]
- Inafuku, M.; Matsuzaki, G.; Oku, H. Intravenous Mycobacterium Bovis Bacillus Calmette-Guérin Ameliorates Nonalcoholic Fatty Liver Disease in Obese, Diabetic ob/ob Mice. PLOS ONE 2015, 10, e0128676. [CrossRef]
- Akbarian, F.; Rahmani, M.; Tavalaee, M.; Abedpoor, N.; Taki, M.; Ghaedi, K.; Nasr-Esfahani, M.H. Effect of Different High-Fat and Advanced Glycation End-Products Diets in Obesity and Diabetes-Prone C57BL/6 Mice on Sperm Function. Int J Fertil Steril 2021, 15, 226-233. [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of biological chemistry 1957, 226, 497-509.
- Harada, M.; Kishimoto, Y.; Makino, S. Prevention of overt diabetes and insulitis in NOD mice by a single BCG vaccination. Diabetes Res Clin Pract 1990, 8, 85-89. [CrossRef]
- Shehadeh, N.; Etzioni, A.; Cahana, A.; Teninboum, G.; Gorodetsky, B.; Barzilai, D.; Karnieli, E. Repeated BCG vaccination is more effective than a single dose in preventing diabetes in non-obese diabetic (NOD) mice. Isr J Med Sci 1997, 33, 711-715.
- Doupis, J.; Kolokathis, K.; Markopoulou, E.; Efthymiou, V.; Festas, G.; Papandreopoulou, V.; Kallinikou, C.; Antikidou, D.; Gemistou, G.; Angelopoulos, T. The Role of Pediatric BCG Vaccine in Type 1 Diabetes Onset. Diabetes Therapy 2021, 12, 2971-2976. [CrossRef]
- Faustman, D.; Faustman, D.; ScienceDirect. The value of BCG and TNF in autoimmunity, Second edition. ed.; Academic Press: London, United Kingdom ; San Diego, CA, United States, 2018.
- Lazebnik, T.; Bunimovich-Mendrazitsky, S.; Kiselyov, A. Clinically Relevant Mathematical Model for the BCG-based Treatment Of Type 1 Diabetes. 2021. [CrossRef]
- Chang, Y.-C.; Lin, C.-J.; Hsiao, Y.-H.; Chang, Y.-H.; Liu, S.-J.; Hsu, H.-Y. Therapeutic Effects of BCG Vaccination on Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Diabetes Research 2020, 2020, 1-8. [CrossRef]
- Moghtaderi, M.; Zarei, P.; Shakerian, B.; Babaei, M.; Mostafavi, A.; Modaressi, M. The Non-Significant Benefit of BCG Vaccination for the Treatment of Iranian Patients with Type 1 Diabetes up to 48 Weeks: A Controversial Result. Medical Journal of The Islamic Republic of Iran 2021. [CrossRef]
- Allen, H.F.; Klingensmith, G.J.; Jensen, P.; Simoes, E.; Hayward, A.; Chase, H.P. Effect of Bacillus Calmette-Guerin vaccination on new-onset type 1 diabetes. A randomized clinical study. Diabetes Care 1999, 22, 1703-1707. [CrossRef]
- Faustman, D.L. Benefits of BCG-induced metabolic switch from oxidative phosphorylation to aerobic glycolysis in autoimmune and nervous system diseases. Journal of Internal Medicine 2020, 288, 641-650. [CrossRef]
- Faustman, D.L.; Wang, L.; Okubo, Y.; Burger, D.; Ban, L.; Man, G.; Zheng, H.; Schoenfeld, D.; Pompei, R.; Avruch, J.; et al. Proof-of-Concept, Randomized, Controlled Clinical Trial of Bacillus-Calmette-Guerin for Treatment of Long-Term Type 1 Diabetes. PLoS ONE 2012, 7, e41756. [CrossRef]
- Keefe, R.C.; Takahashi, H.; Tran, L.; Nelson, K.; Ng, N.; Kühtreiber, W.M.; Faustman, D.L. BCG therapy is associated with long-term, durable induction of Treg signature genes by epigenetic modulation. Scientific Reports 2021, 11. [CrossRef]
- Singh, B.; Saxena, A. Surrogate markers of insulin resistance: A review. World J Diabetes 2010, 1, 36-47. [CrossRef]
- Esser, N.; Utzschneider, K.M.; Kahn, S.E. Early beta cell dysfunction vs insulin hypersecretion as the primary event in the pathogenesis of dysglycaemia. Diabetologia 2020, 63, 2007-2021. [CrossRef]
- Tabak, A.G.; Jokela, M.; Akbaraly, T.N.; Brunner, E.J.; Kivimaki, M.; Witte, D.R. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 2009, 373, 2215-2221. [CrossRef]
- Matveyenko, A.V.; Gurlo, T.; Daval, M.; Butler, A.E.; Butler, P.C. Successful Versus Failed Adaptation to High-Fat Diet–Induced Insulin Resistance. Diabetes 2009, 58, 906-916. [CrossRef]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102-110. [CrossRef]
- Yang, H.-W.; Son, M.; Choi, J.; Oh, S.; Jeon, Y.-J.; Byun, K.; Ryu, B.M. Ishige okamurae reduces blood glucose levels in high-fat diet mice and improves glucose metabolism in the skeletal muscle and pancreas. Fisheries and Aquatic Sciences 2020, 23. [CrossRef]
- Hull, R.L.; Kodama, K.; Utzschneider, K.M.; Carr, D.B.; Prigeon, R.L.; Kahn, S.E. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: evidence for specificity of impaired beta cell adaptation. Diabetologia 2005, 48, 1350-1358. [CrossRef]
- Moh Moh, M.A.; Jung, C.H.; Lee, B.; Choi, D.; Kim, B.Y.; Kim, C.H.; Kang, S.K.; Mok, J.O. Association of glucagon-to-insulin ratio and nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Diab Vasc Dis Res 2019, 16, 186-195. [CrossRef]
- Bang, J.; Lee, S.A.; Koh, G.; Yoo, S. Association of Glucagon to Insulin Ratio and Metabolic Syndrome in Patients with Type 2 Diabetes. Journal of Clinical Medicine 2023, 12, 5806. [CrossRef]
- Lee, E.E.; Sears, D.D.; Liu, J.; Jin, H.; Tu, X.M.; Eyler, L.T.; Jeste, D.V. A novel biomarker of cardiometabolic pathology in schizophrenia? J Psychiatr Res 2019, 117, 31-37. [CrossRef]
- Kurtz, J.; Franz, K. Innate defence: evidence for memory in invertebrate immunity. Nature 2003, 425, 37-38. [CrossRef]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; Van Loenhout, J.; De Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proceedings of the National Academy of Sciences 2012, 109, 17537-17542. [CrossRef]
- Cirovic, B.; de Bree, L.C.J.; Groh, L.; Blok, B.A.; Chan, J.; van der Velden, W.; Bremmers, M.E.J.; van Crevel, R.; Handler, K.; Picelli, S.; et al. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe 2020, 28, 322-334 e325. [CrossRef]
- Wu, Y.; Zhang, X.; Zhou, L.; Lu, J.; Zhu, F.; Li, J. Research progress in the off-target effects of Bacille Calmette-Guerin vaccine. Chin Med J (Engl) 2023. [CrossRef]
- van Puffelen, J.H.; Keating, S.T.; Oosterwijk, E.; van der Heijden, A.G.; Netea, M.G.; Joosten, L.A.B.; Vermeulen, S.H. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat Rev Urol 2020, 17, 513-525. [CrossRef]
- Atallah, A.; Grossman, A.; Nauman, R.W.; Paré, J.F.; Khan, A.; Siemens, D.R.; Cotechini, T.; Graham, C.H. Systemic versus localized <scp>Bacillus Calmette Guérin</scp> immunotherapy of bladder cancer promotes an anti-tumoral microenvironment: Novel role of trained immunity. International Journal of Cancer 2024. [CrossRef]
- Kühtreiber, W.M.; Takahashi, H.; Keefe, R.C.; Song, Y.; Tran, L.; Luck, T.G.; Shpilsky, G.; Moore, L.; Sinton, S.M.; Graham, J.C.; et al. BCG Vaccinations Upregulate Myc, a Central Switch for Improved Glucose Metabolism in Diabetes. iScience 2020, 23, 101085. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




