1. Introduction
Sanchezia nobilis is a shrub belonging to
Sanchezia genus of the Acanthaceae family. Its leaves are light green, with golden yellow veins in the middle. Its leaves are opposite, almost sessile and shiny. It is an upright shrub, with long ovate leaves[
1,
2]. The plants of Acanthaceae prefer warmth in nature and mostly grow in the south of China. According to the Flora of China, there are 68 genera and more than 300 species in China, with various forms. They are distributed in forests, deserts and even mangroves. At the same time, they play an important role in flower mirrors, potted plants, hedges, slope greening and vertical greening. They are important resources for developing new types of cultivated flowers and plants, with fast reproduction strong vitality and few pests and diseases[
3].
S. nobilis blooms in summer and autumn, with small yellow flowers in red bracts; It prefers warm and humid conditions,but is not cold resistant and is suitable for planting in loose, fertile, moisturizing and breathable soil; Its growth temperature range is 15-30 ℃, and the ambient humidity should not be less than 60%; It can grow in the concealed environment with weak illumination or light all year round. It is an important plant resource with high ornamental value and medicinal value[
4,
5,
6]. Originated in tropical America,
S. nobilis was later introduced into China for cultivation and widely used in landscaping. It is a very important landscaping tree species in southern China[
7].
Biotic and abiotic stresses have important effects on plant growth and development.
S. nobilis is one of the most important garden plants. During the cultivation and management of garden plants, the investigation found that
S. nobilis is often subject to biotic and abiotic stresses. For example, the branches and leaves of
S. nobilis are frequently pruned by gardeners, and the leaves are also fed by
Achatina fulica, which seriously hampers the photosynthesis of
S. nobilis and affects its normal growth, seriously reduces its aesthetic value in landscape greening.
A. fulica is an omnivorous mollusk belonging to the family Achatinidae and the genus
Achatina. It likes to be active in humid environments, especially after rain or at night. It originates in eastern Africa and is widely spread around the world with human trade activities[
8].
A. fulica is one of the first 16 alien species to invade China. It is regulated as a Class II quarantine pest entering and exiting in China, and an intermediate host of many human and animal parasites and pathogens, such as tuberculosis and eosinophilic meningitis.
A. fulica was first found in Xiamen, Fujian Province[
9].
In order to improve the resistance and productivity of plants, it is particularly important to study the physiological response mechanisms of plants under biotic and abiotic stresses. Currently, the research on
S. nobilis focuses on rapid propagation, cultivation and management, analysis of ornamental value, and applied research[
2,
4,
6]. However, there have been no reports to date on the physiological responses of
S. nobilis to biotic or abiotic stresses. Additionally,
S. nobilis frequently encounters severe predation by
A. fulica and mechanical pruning stress among other biotic and abiotic stresses during its cultivation and management in gardens. Therefore, this study investigated the phenomenon of biotic and abiotic stresses of
S. nobilis subjected to feeding by
A. fulica and garden pruning, and also measured the temporal changes of malondialdehyde content and activities of catalase and peroxidase of the defenses of
S. nobilis under the conditions of biotic and abiotic stresses, so as to investigate the defense responses of
S. nobilis under the conditions of biotic and abiotic stresses, and aimed at providing the theoretical basis for the breeding of
S. nobilis for resilience or the prevention of harmful organisms, and also has an important significance of guiding the cultivation and management of the plants of the gardens.
2. Materials and Methods
2.1. Plant and Animal
The plant material used in this experiment is S. nobilis, which is grown in the green nursery of Zhaoqing University. The animal used in this experiment is A. fulica, which is with similar size and same vitality.
2.2. Measurement of the Height and Ground Diameter of S. nobilis and the Appetite of A. fulica
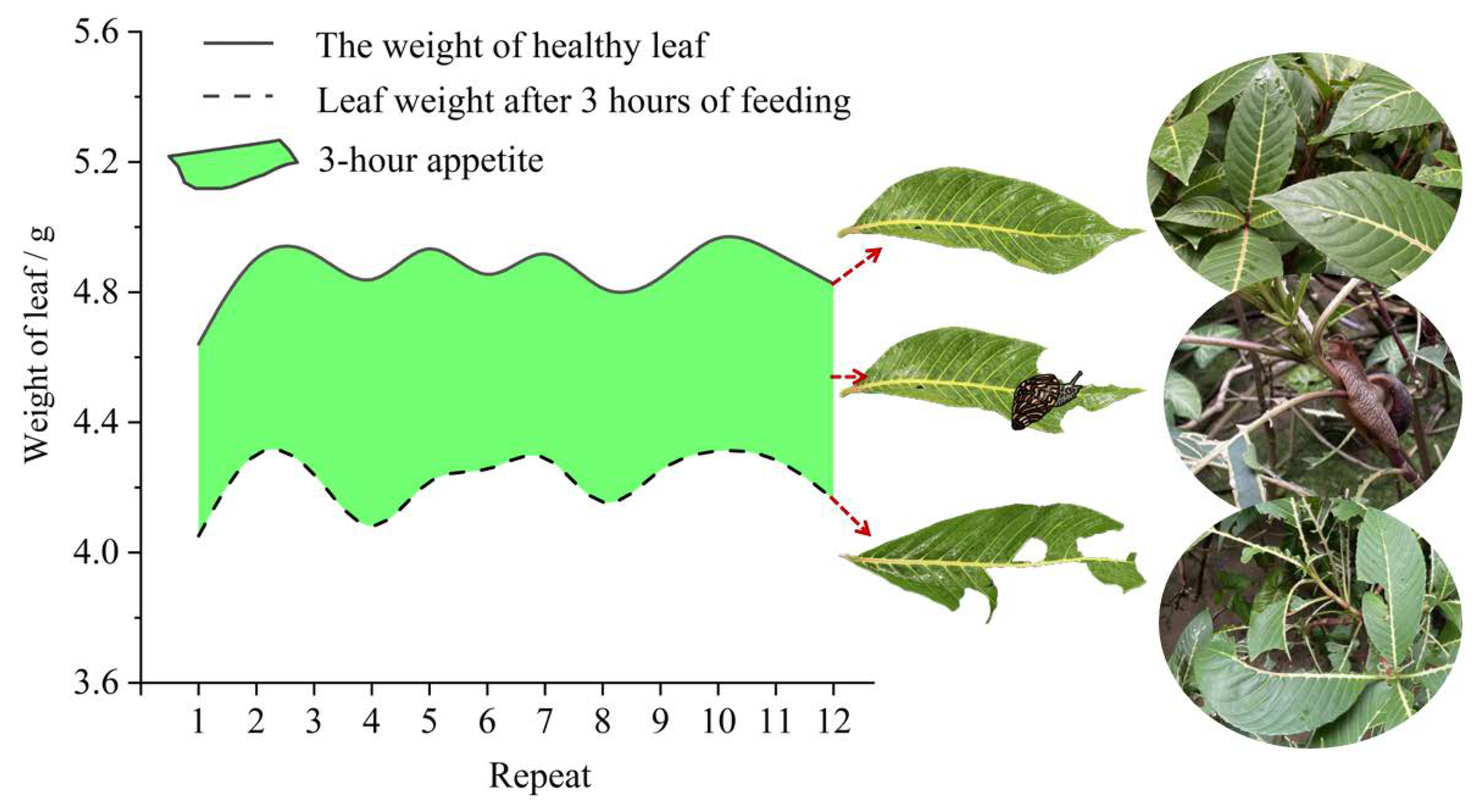
The tree height of S. nobilis was measured with a ruler, and the ground diameter of S. nobilis was measured with vernier calipers. To investigate the damage of feeding by African giant snail in the green nursery on campus, and to measure the quality of feeding leaves of snail for 3 hours.The damage of feeding by A. fulica on S. nobilis was investigated in the green nursery of the campus, and the 3-hour appetite of A. fulica was measured.
2.3. Leaf Clipping Stimulation and Snail Feeding Treatment
Sanchezia nobilis leaves with healthy of about the same size were selected for the test. The time starting point is when snails feed on about one-third of the leaf area. The leaf cutting stimulation group cuts off the similar leaf area and starts timing synchronously. The control group does not do any treatment. After 3 hours, 9 hours, and 15 hours, the leaves of each group are taken for determination of malondialdehyde content, catalase activity, and peroxidase activity. Each test group is set with 3 repetitions, and each 3 blades is regarded as 1 repetition (
Figure 1).
2.4. Determination of Malondialdehyde Content after Stimulated by Leaf Cutting and Snail bite
Determination of malondialdehyde content in plant tissues by thiobarbituric acid method: weigh 1 g of leaves, cut them into pieces and put them into a mortar, add 2 ml of 10% Trichloroacetic Acid (TCA) solution and a small amount of quartz sand, grind them into a homogenate, continue to add 8 ml of 10% TCA solution, fully grind them, centrifuge the homogenate for 10 min, and the supernatant is the sample extraction solution. Suck 2mL of sample extract, add 2mL of 0.6% Thiobarbituric acid (TBA) solution, mix well, put it in a boiling water bath to react for 15min, cool it quickly and then centrifugate again. Take the supernatant and measure the optical density at 450 nm, 532 nm and 600 nm. Calculate the content of Malondialdehyde (MDA) in the sample extract according to the formula in the experimental principle, and substitute into the following formula to calculate the content of MDA in the sample.
2.5. Determination of Peroxidase Activity after Stimulated by Leaf Cutting and Snail Bite
Weigh 0.5 g of sample, add 5.0 mL, extract buffer solution, grind into homogenate under ice bath condition, centrifuge at 4 ℃ at 12000 r/min for 30 min, collect supernatant, which is the enzyme extract. Measure the total volume of the extract and store it at low temperature for standby. The enzymatic reaction system consists of 2.9 mL 20 mmol·L
-1 H
2O
2 Solution and 100 μL enzyme extract composition. With distilled water as the reference blank, start recording the optical density of the reaction system at the wavelength of 240 mm as the initial value (OD
0 ) at 15 s of reaction, then record the optical density value (OD
2 ) at 2 min, and repeat 3 times. Record the optical density value of the reaction system at the wavelength of 240 nm, and calculate the optical density change value ΔOD
2 per minute.
Note: ΔOD240 is the change value of optical density of reaction mixture per minute, OD2 is the optical density value of reaction mixture for 2 minutes, and OD0 is the initial optical density value of reaction mixture, t2 is the end time of reaction, t1 is the initial time of reaction.
One catalase activity unit is the change value of optical density per minute decreases by 0.01 per gram of plant tissue sample (fresh weight) min
-1·g
-1. Calculation formula:
Note: U refers to catalase activity, V is the total volume of sample extract (mL), Consider the measured volume of sample extract (mL), m is the sample mass (g).
In addition, with different concentrations of H2O2 as a standard curve is made for the solution. According to the change of optical density value, referring to the standard curve, the catalase activity can be expressed by the consumption of substrate H2O2 solution, and the unit is μmol·min-1·mg-1.
2.6. Determination of Catalase Activity after Stimulated by Leaf Cutting and Snail Bite
Take 1 g of plant material, add 10 mL 20 mmol·L-1 KH2PO4 solution, grind it into homogenate in a mortar, centrifugate it at 4000 r/min for 15 min, collect the supernatant, and store it at low temperature, which is the enzyme solution. Take two cuvettes and add 3 mL of reaction mixture and 1mL of KH2PO4 solution to one cuvette as control. Add 3 mL of reaction mixture and 1 mL of the above enzyme solution to the other cuvette. Turn on the stopwatch immediately for timing, measure the optical density value at 470 nm, read once every 1min, measure for 6min, and repeat three times. POD activity to ΔOD470·min-1·mg-1 (protein) is expressed in units.
2.7. Data Analysis
The mean value of tree height and ground diameter was calculated, variance and multiple comparative analysis. The means of malondialdehyde, peroxidase activity, and catalase activity tested were analyzed by analysis of variance (ANOVA) and means were compared by Tukey's HSD (Honestly Significant Difference) test (P = 0.05). All data were analyzed using SPSS 20.0 (IBM SPSS Statistics, Chicago, IL, USA) and plotted using Origin 2018 (OriginLab Inc., Northampton, UK).
4. Discussion
Sanchezia nobilis is one of the most important gardening plants in southern China. In this study, we found that
A. fulica likes to feed on
S. nobilis, and its large feeding volume often leads to large areas of
S. nobilis where the leaves are eaten up, and some studies reported that
A. fulica is characterized by its large individual size, long life span, fast growth and strong reproduction, and in particular, it has a very wide feeding habit, and it can eat more than 500 kinds of plants, which has a serious impact on the development of agriculture and forestry, and often causes serious economic losses[
10]. In addition to the snail feeding biotic stress, in the process of cultivation and management of landscape plants,
S. nobilis is often subjected to pruning mechanical stimulation of abiotic stress, therefore, this thesis discusses the defense response of
S. nobilis to biotic and abiotic stress.
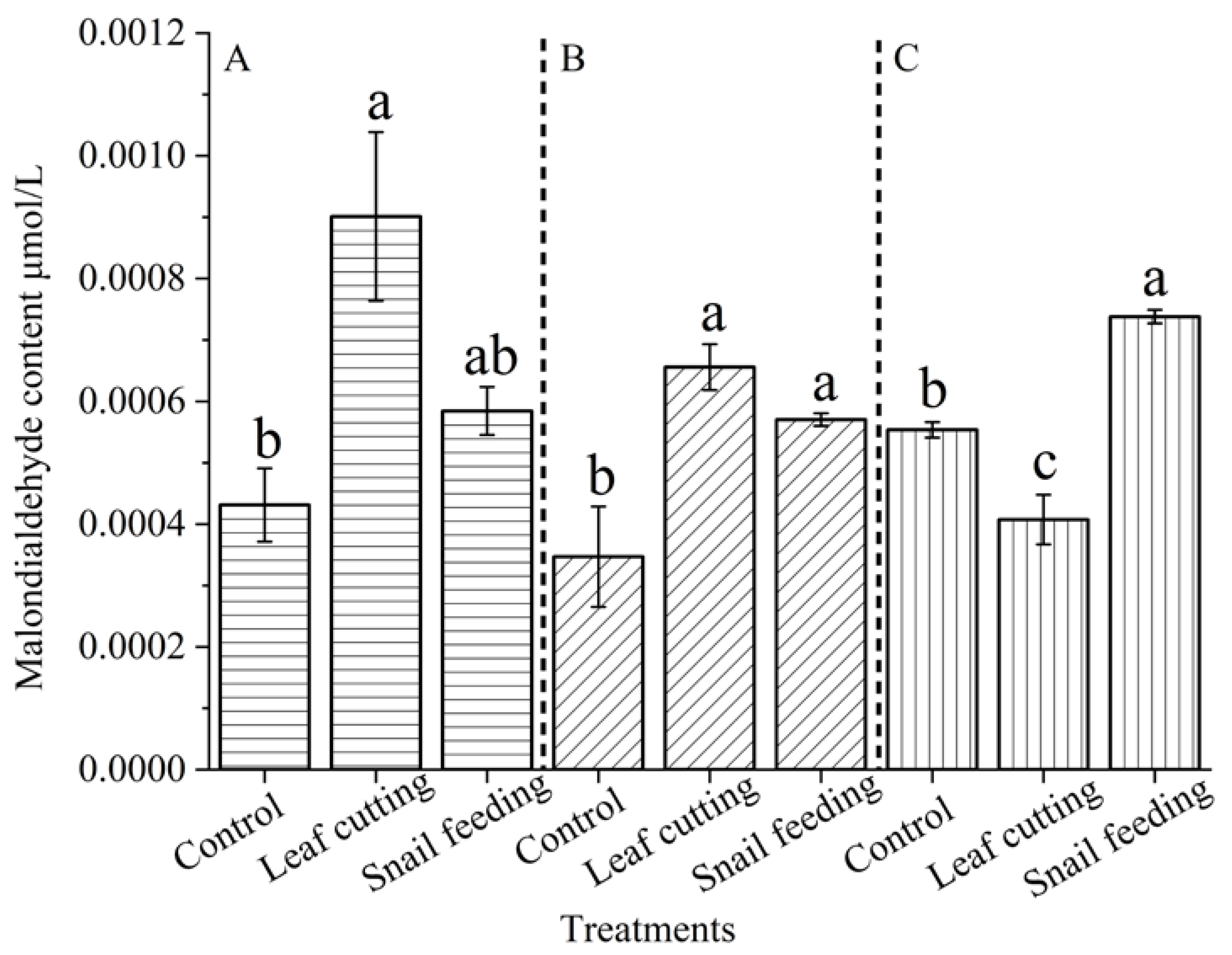
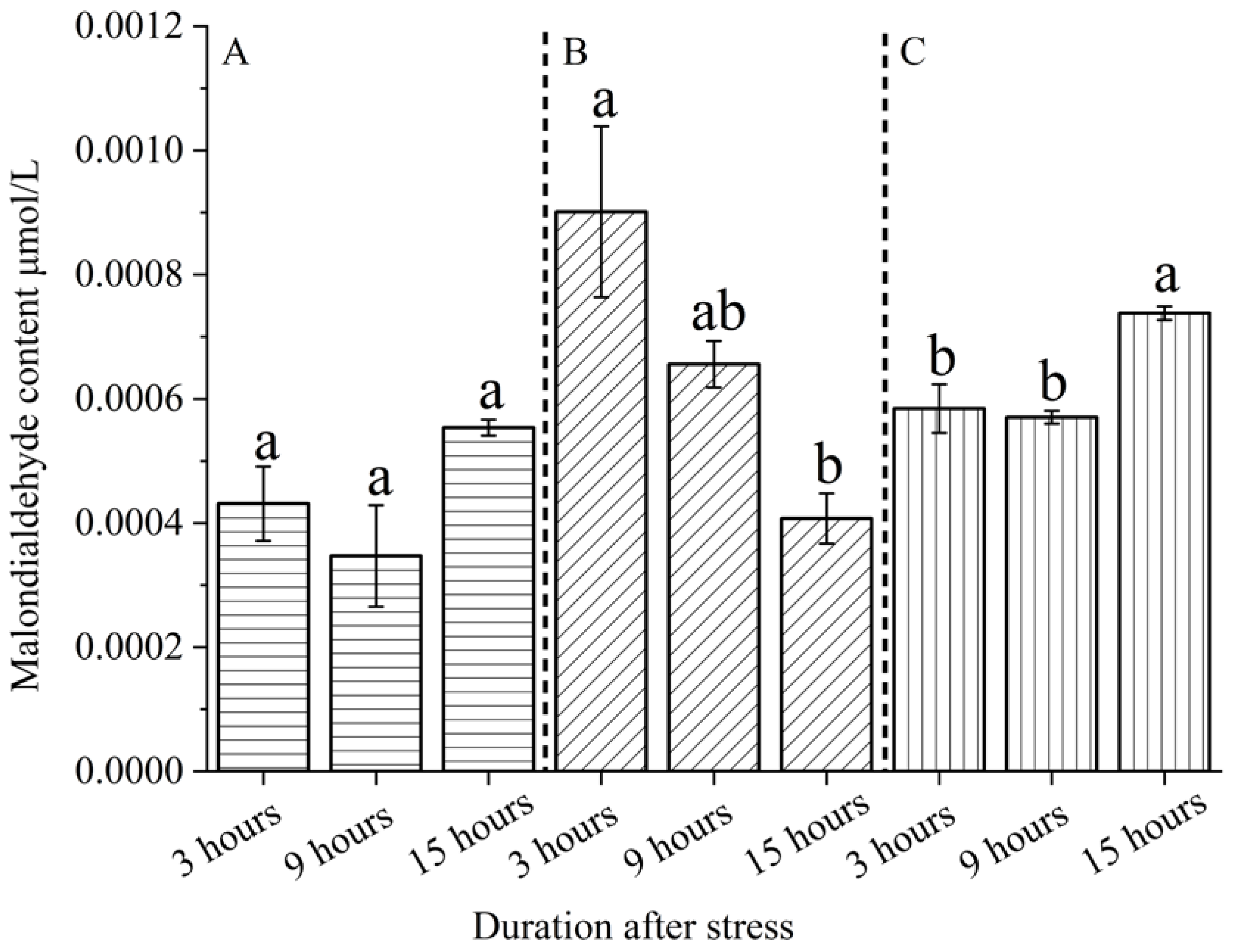
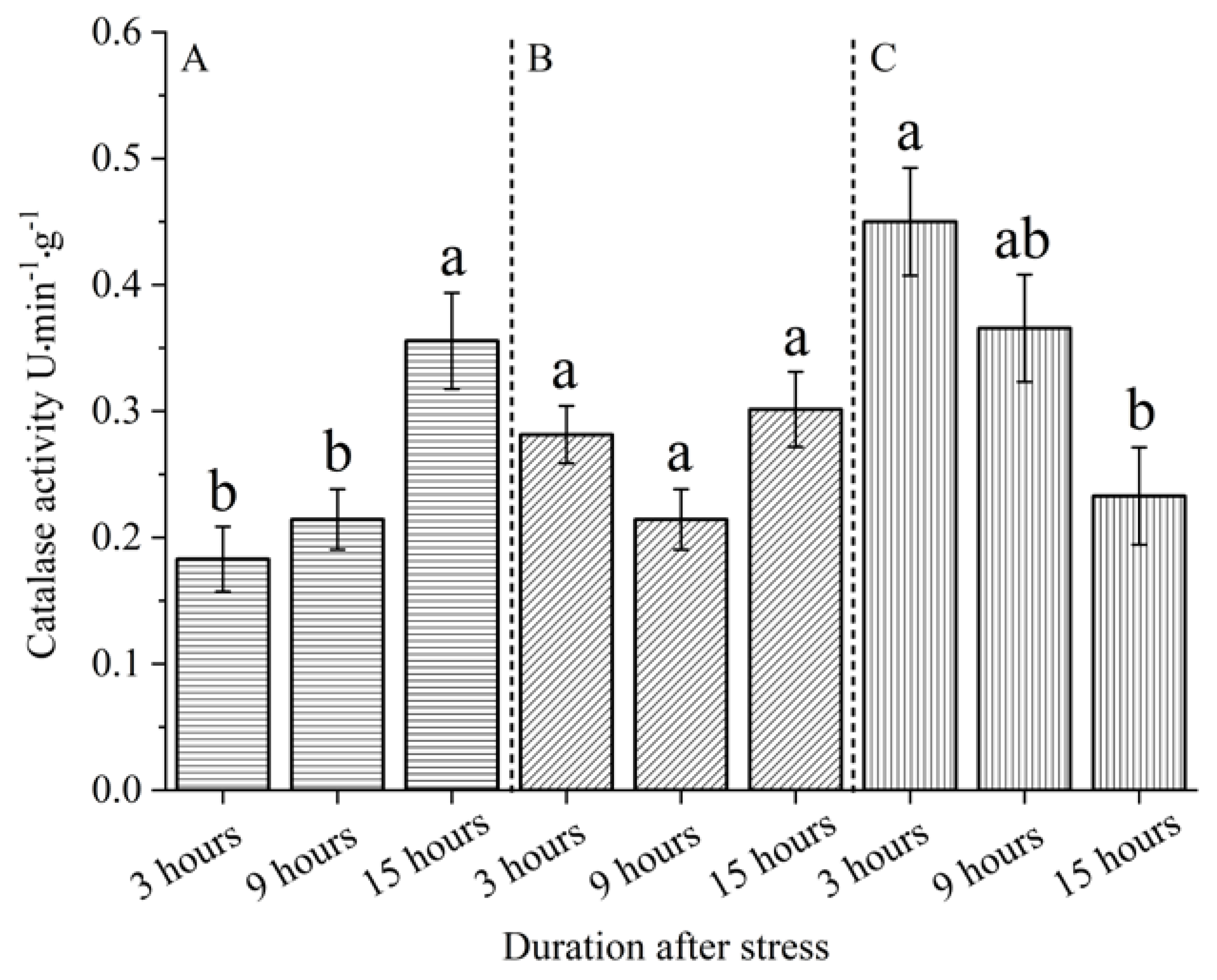
Compared with the control group, biological and abiotic stress modes and different time sequence treatments after stress had significant effects on the content or activity of substances related to the growth physiology of
S. nobilis. The results of this experiment showed that different stresses and different time sequence treatments had different effects on the in vivo response of
S. nobilis leaves; Malondialdehyde content, catalase activity and peroxidase activity changed with the time of insect feeding and mechanical injury; Snail feeding and leaf cutting can induce the defensive response in the body of
S. nobilis, some of which are not timely, and there is a certain lag effect. However, the changes in the content or activity of defense substances at different times after biotic and abiotic stresses are different. Animal feeding stress stimulates the defense response of
S. nobilis more persistently than the mechanical stimulation of leaf cutting. Animal feeding stress will induce the synthesis of more defense substances or improve the activity of defense enzymes, because biotic and abiotic stresses induce plants in different ways, and plants have different defensive responses[
11]. The mechanical stimulation of leaf cutting is a single physical damage to plants, while animal feeding biological stress is not only physically damaged, but also chemically damaged by saliva and other secretions[
12]. This study found that snails feeding on the leaves of
S. nobilis would produce mucus. Therefore, the chemical stimulators in the secretion of snails feeding on
S. nobilis would have a more rapid impact on the defense response of
S. nobilis.
After stress exposure, animal feeding stress induced the defensive response of
S. nobilis to be more lasting than the mechanical stimulation of leaf cutting, and animal feeding stress induced the synthesis of more malondialdehyde. Malondialdehyde is the final product of membrane lipid peroxidation, and its content can reflect the degree of membrane lipid peroxidation and the strength of plant response to stress conditions[
13]. Leaf cutting treatment will directly cause mechanical damage to plant cells, thus triggering a defense response. In addition to mechanical damage to the feeding wound, insects also have oral secretions, such as saliva, in which a series of effectors target different components of the plant PTI pathway, and promote their own feeding by inhibiting or manipulating the plant's immune response[
14]. For example, NcSP84 of the black tailed leafhopper
Nephotettix bipunctatus and NlSEF1 of the brown planthopper
Nilaparvata lugens prevent screen tube blockage or inhibit calcium signal by combining free calcium ions[
15,
16]. This shows that when snails feed, plant cells are subject to both chemical and physical stimuli, and the ingredients in saliva will stimulate plants to quickly take defensive reactions and synthesize more defensive substances. In the different time sequence treatment of leaf cutting stimulation and snail feeding, the damage of leaf cutting mechanical stimulation is different from that of snail feeding in action time. The induction effect of leaf cutting mechanical stimulation on leaf defense substances is faster than that of snail feeding, that is, in the short term after stress, the leaf cutting mechanical stimulation induces the leaf to synthesize more malondialdehyde. Yuan Hong'e et al proposed that the mechanical damage caused by leaf cutting is different from the mechanical damage caused by insect feeding in the action time[
17]. Insect feeding takes a certain time, and the damage to plants is a gradual process, while the mechanical stimulation of leaf cutting is short and there is no secretion accompanying the process of insect feeding.
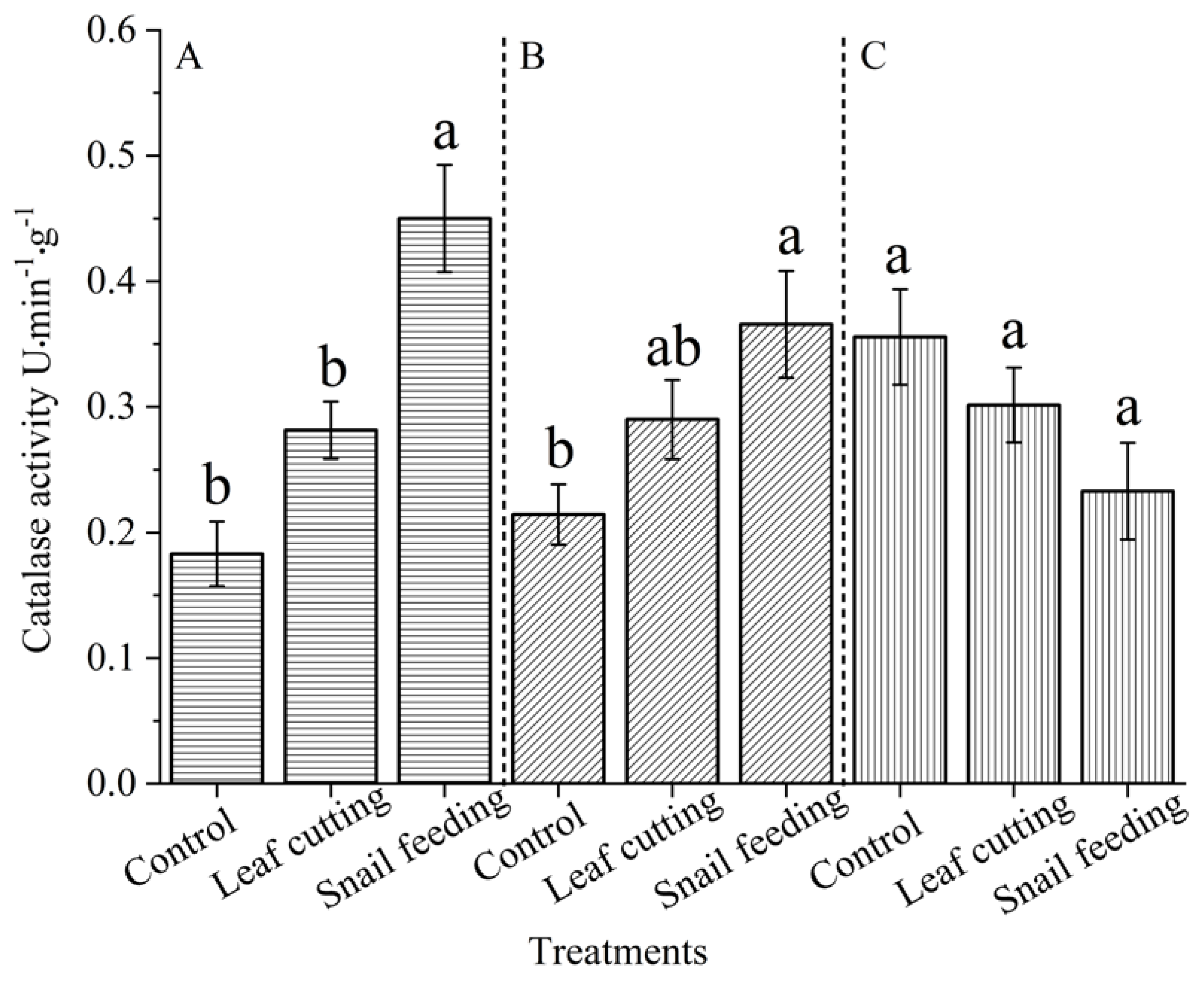
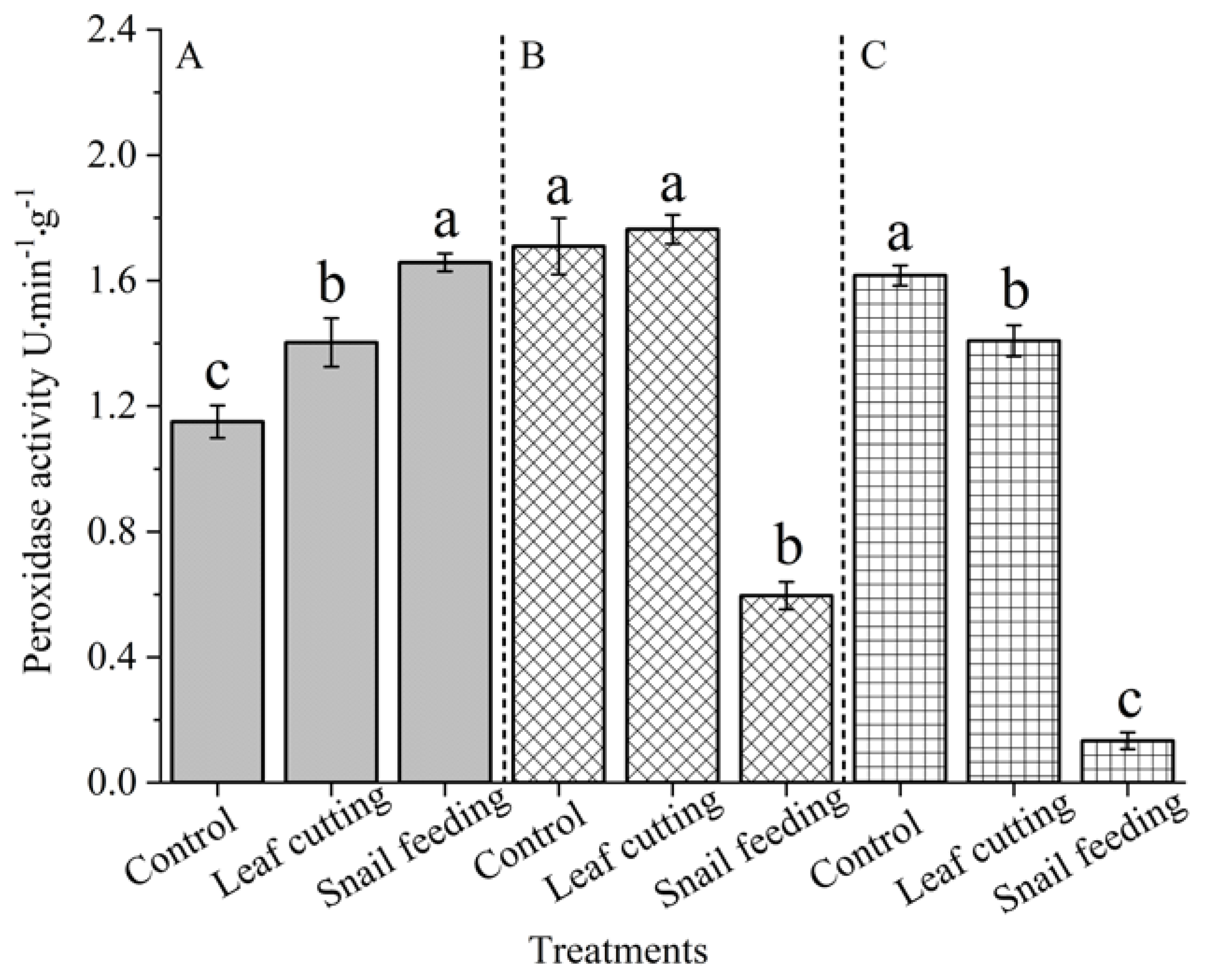
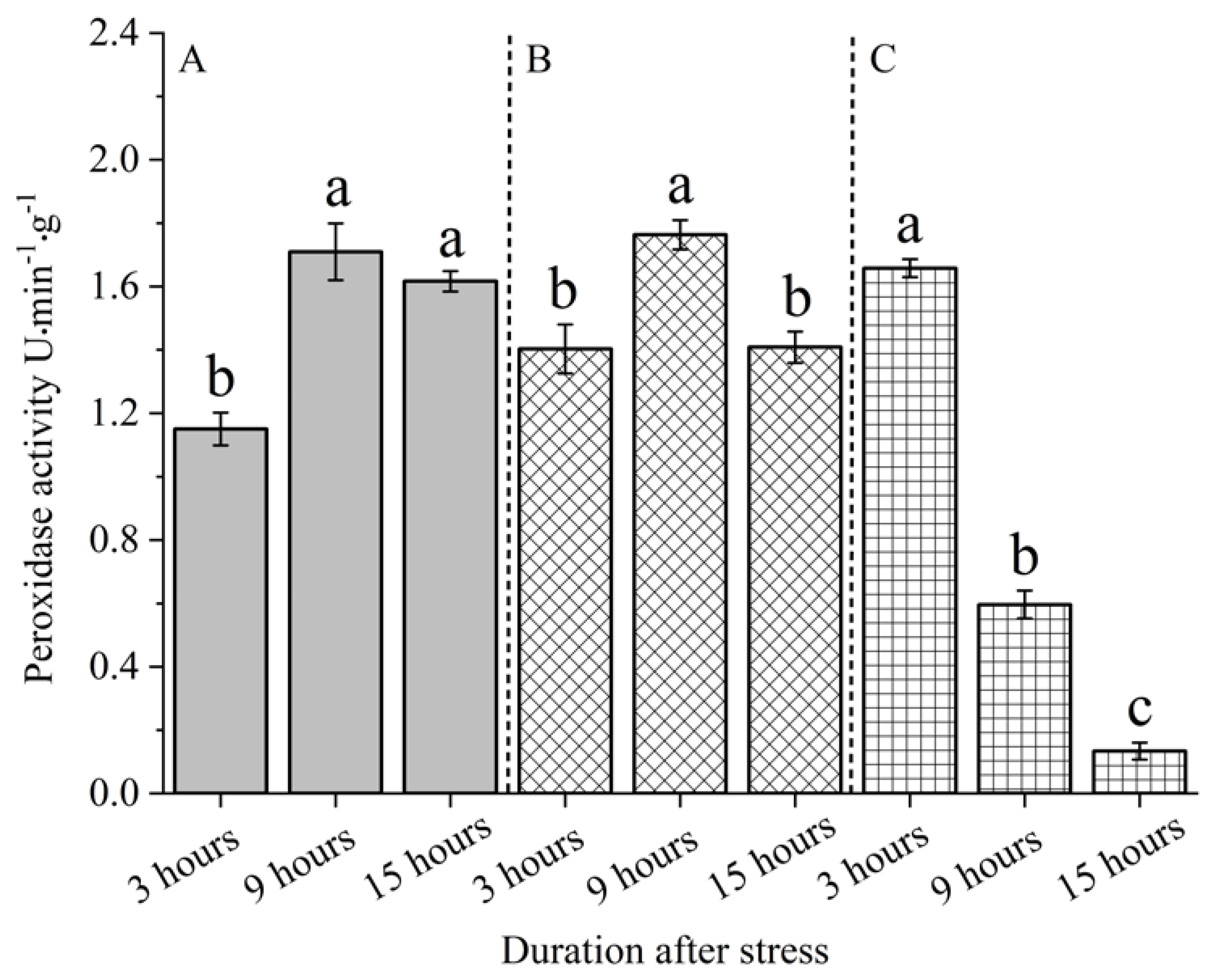
Catalase and peroxidase play an important role in the resistance and adaptation of plants to stress, and have an important positive impact on the growth and development of plants. Their activities can be used as a physiological indicator to judge the ability of plants to resist stress[
18]. In the process of normal metabolism of plant cells, both biotic and abiotic stresses increase the level of reactive oxygen species and the content of peroxides in cells. CAT and POD are important protective enzymes for cells to resist the damage of reactive oxygen species. They play an important role in scavenging O
-2, H
2O
2 and peroxides, preventing or reducing the formation of hydroxyl radicals, and thus maintaining the membrane system from damage[
18]. POD is involved in lignin biosynthesis, pathogen defense and cell wall polysaccharide cross-linking, and plays an important role in plant resistance to stress[
19].
The activities of catalase and peroxidase were enhanced by snail feeding and leaf cutting stimulation. After snails feeding and mechanical stimulation stress, the activities of the above defense enzymes were significantly higher than those of the control group. Both biotic and abiotic stresses resulted in mechanical damage and stimulation to the leaves. Cell responses to damage included increasing the activity of antioxidant enzymes to cope with the generated oxidative stress, thus leading to increased defense enzyme activities. In addition to mechanical damage to leaves caused by animal feeding biological stress, there is also chemical stimulation, and its induction effect is more complex. Dong Yumei et al showed that insect oral secretions, oviposition fluid, deposited feces or secreted honeydew all contain substances that can be recognized by PRRs on plant cytoplasmic membrane, activate defense signal pathways, and activate plant defense responses to enhance their own resistance[
20]. Mccloud and Baldwin studies show that plant recognition of herbivorous insects starts from the specific elicitors in insect saliva. The specific elicitors induce specific signal transmission in plants, induce the expression of defense genes, and cause the accumulation of toxic metabolites and defense proteins in plants[
21]. In this study, the defense response of snails to feed induced leaves was lagging behind that of mechanical stimulation of leaf cutting, and significantly enhanced the activities of catalase and peroxidase in the later stage.Leaf cutting mechanical stimulation is a physical stimulation caused by simple mechanical damage, which is a rapid short-term induction.That is to say, it can induce the synthesis of more defense substances or enhance the activity of defense enzymes in a relatively short time, while animal feeding includes physical stimulation of mechanical injury and chemical stimulation of saliva, which is a comprehensive induction and a continuous process[
17]. Shi Yuanyuan et al found a similar change law when studying the leaf cutting treatment and the feeding stress of
Dendrolimus tabulaeformis[
22]. The response of plants to insect feeding is an "on-off effect", that is, when the external environmental stimulus reaches a certain degree, the defense is turned on, and when the environmental stimulus exceeds a certain range, the defense system is turned off[
22].
Achatina fulica is a terrestrial snail, which has been listed as one of the 100 invasive species on the IUCN blacklist[
10]. As an invasive pest, effective control of
A. fulica is a key task in the cultivation and management of landscaping plants[
23]. So far, there are not many research reports on the control of
A. fulica, such as through quarantine, pesticide control or manual trapping[
23]. In addition, Wang Xinfeng et al. reported that a new type of
A. fulica baiting agent was made from the extracts of two invasive plants, namely,
Ipomoea cairica and
Mikania micrantha, and snail pheromone, and the baiting effect was good[
24]. Therefore, based on the phenomenon of
A. fulica feeding on
S. nobilis, we can explore plant-derived attractants based on the volatiles of gold-veined juglone and apply them to the control of
A. fulica in the future.