1. Introduction
The relevance of NT assessment as part of the first trimester screening (FTS) is proven and internationally accepted as a sonographic standard in the detection of major fetal aneuploidies. First introduced in 1992 by Nicolaides et al., nuchal translucency was the initial sonographic parameter that enhanced sensitivity in first trimester screening for fetal anomalies [
1]. Prior to this, the risk assessment for fetal trisomy 21 primarily depended on maternal age and hormone levels [
2]. By combining with additional sonographic markers (nasal bone, tricuspid valve flow, ductus venosus flow), the detection rate for fetuses with Trisomy 21 could be increased to over 95%. Due to the fundamental importance for accurate risk calculation, optimal image quality is essential for the precise measurement of the NT [
3,
4].
Given the rapidly advancing assets of modern ultrasound devices, an improvement of image quality can be noticed over the years. Some imaging techniques have the potential to alter line thickness and measurements of NT. This algorithm changes the appearance of B-mode sonographic images. In our estimation, this effect is most noticeable during NT measurements. The aim of this study was to investigate the impact of the ‘Radiant Image Enhancement Technique’ on the measurement of nuchal translucency (NT) and its subsequent clinical relevance.
2. Materials and Methods
2.1. Nuchal Translucency with and without ‘Radiant’ Applied
Images of NT measurements obtained in 2022 (n = 263) on Voluson Expert 22 (GE Healthcare, Solingen, Germany) were reviewed in local archive, and the ‘Radiant’ image enhancement technique was applied post-exam directly on the device. The gestational age for all examinations was 11+0 to 13+6 weeks, as this represents the timespan used for FTS. All examinations were conducted by experienced examiners certified according to the quality criteria of the Fetal Medicine Foundation (FMF) London.
For each examination, if multiple NT images were available, the one that most accurately adhered to FMF standards was selected. Image-enhancing modalities, including ‘Harmonic Imaging,’ ‘Ultra HD,’ Speckle Reduction Imaging (SRI), and Compound Resolution Imaging (CRI), were activated for all cases, as they are standard in our first trimester scans. Unlike ‘Radiant,’ these settings cannot be deactivated after image capture due to their operational method. Maintaining these settings for all cases helps to minimize confounding variations. In the subsequent text, references to ‘Radiant off’ or ‘Native’ indicate that ‘Radiant’ was not used, while ‘Ultra HD,’ SRI, and CRI remained active, as previously mentioned.
For each image, NT measurements were initially taken without the ‘Radiant’ enhancement. Subsequently, the same image underwent repeated measurements with ‘Radiant’ applied at settings ‘min,’ ‘mid,’ and ‘max.’ These measurements were performed using GE’s ‘SonoNT’ tool, which allows the user to define a rectangular region within the image for standardized NT measurement by the device. This tool was selected due to its proven ability to significantly reduce interobserver variability [
5,
6].
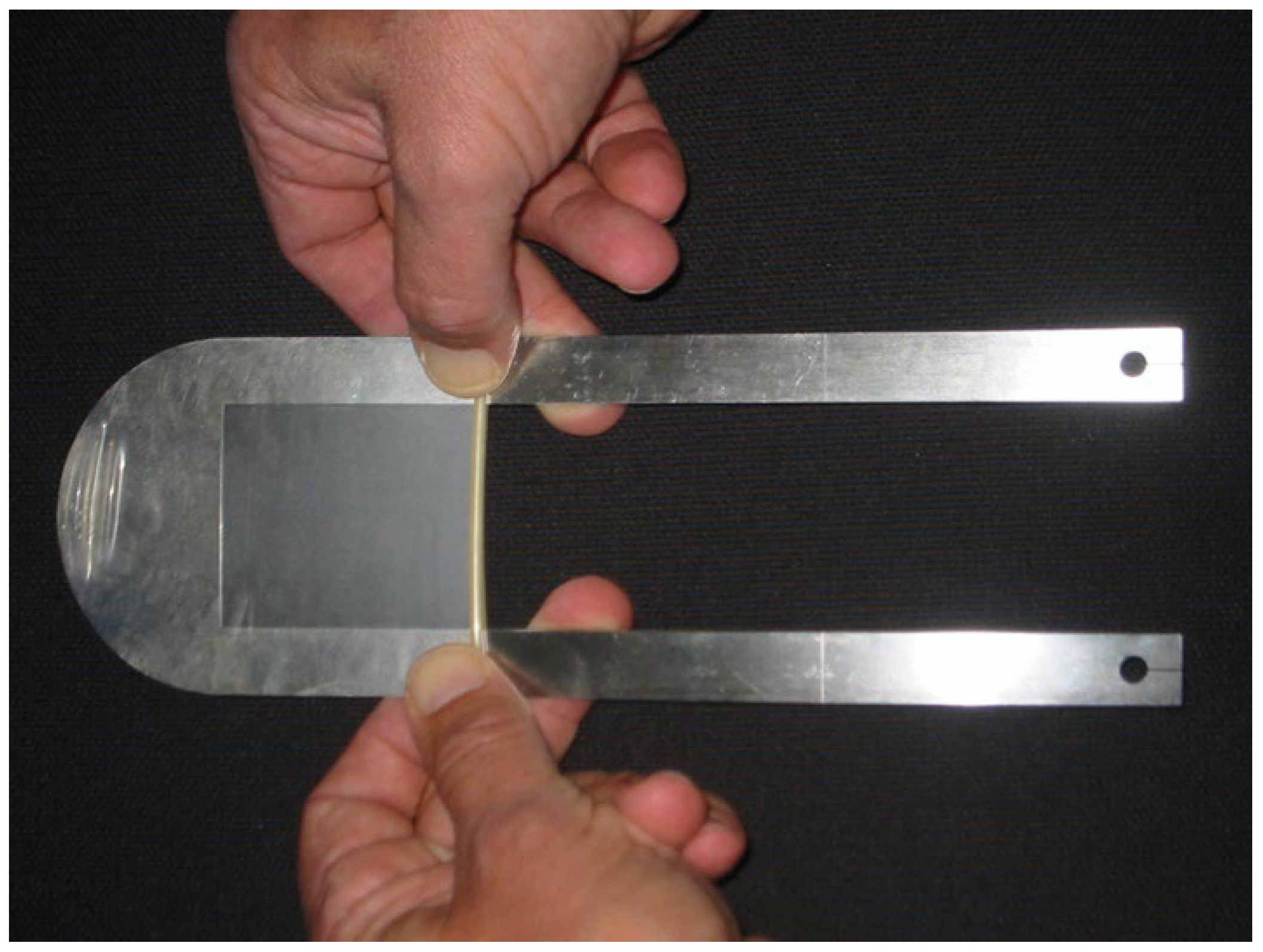
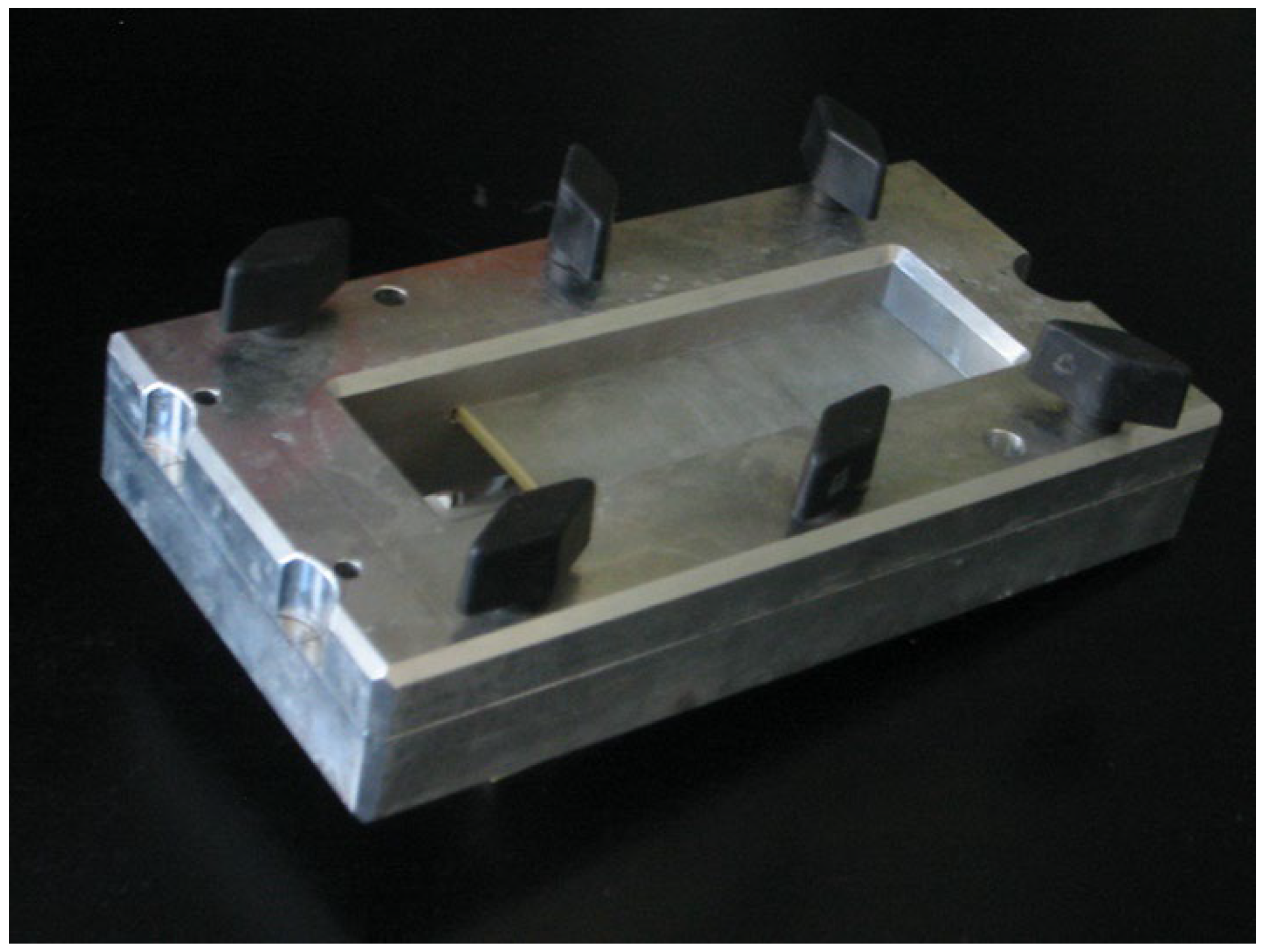
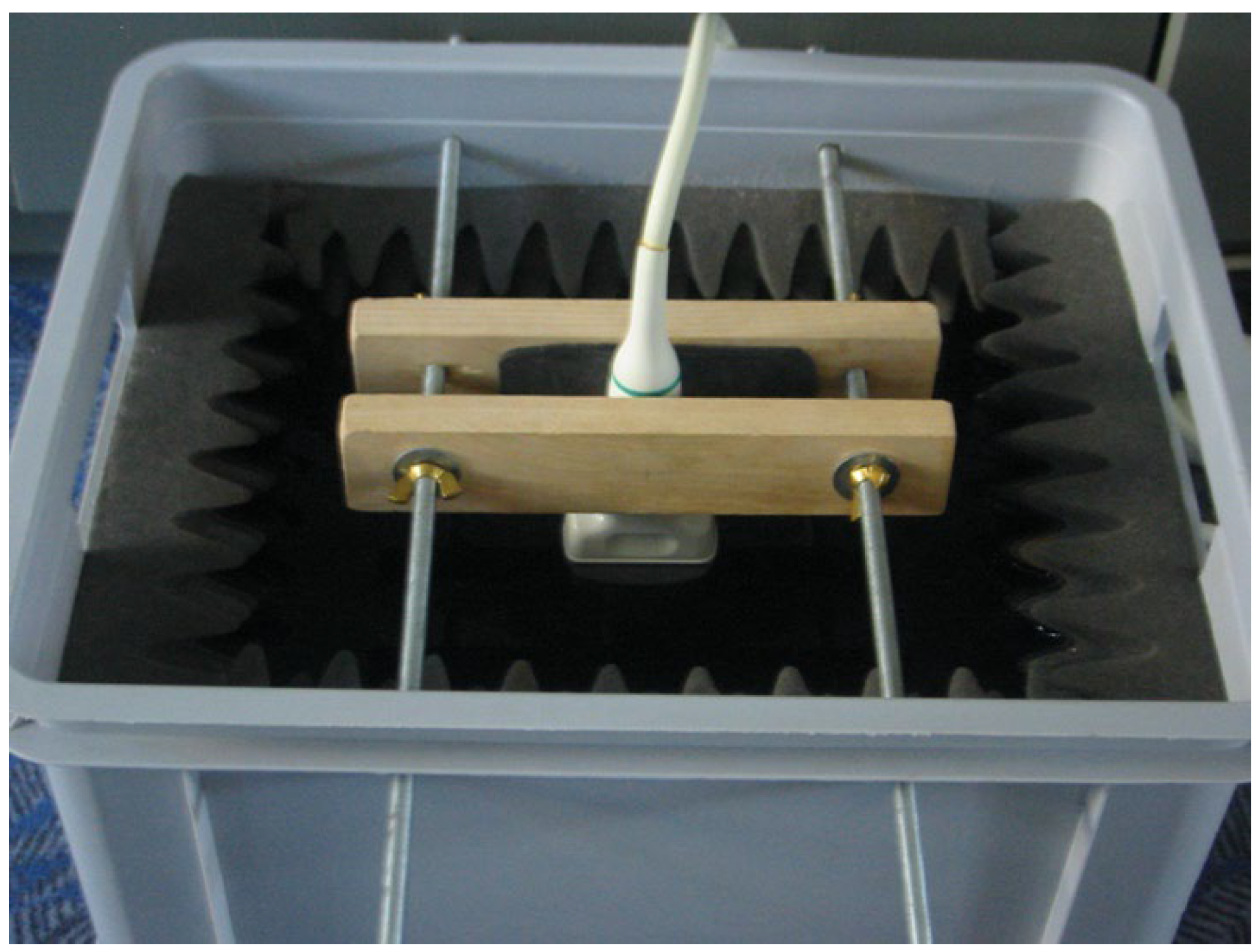
In addition to our patient-based examinations, we conducted an in-vitro study using an inorganic object to objectively assess changes in line thickness relative to the actual size of the measured material. For this, we utilized a mechanical device designed to stretch a condom between its membrane walls at a predefined distance. The stretched condom was submerged in distilled water, and the ultrasound probe was positioned underwater, orthogonal to the condom’s membrane. This method replicates the experimental setup used by Heiko Dudwiesus in his prior research on the effects of Harmonic Imaging [
7]. The setup is illustrated in the figures (
Figure 1,
Figure 2,
Figure 3 and
Figure 4). These measurements were conducted using the same ultrasound machine and probe as in our first trimester screening to minimize variability.
To establish a setting comparable with clinical data, we measured the distance between the condom membranes under various settings, including the factory preset with ‘UltraHD’, SRI, and CRI activated, which we regularly use in first trimester screenings. We also took measurements with ‘Harmonic Imaging’ activated, and with no enhancements (‘Fundamental’), while keeping SRI and CRI activated throughout.
For each setting, we compared ‘Radiant’ with ‘Radiant off’ by measuring the distance between the membranes using the ‘SonoNT’ tool. This examination was conducted with actual distances of 1.0 mm and 2.5 mm between the membranes. Although the operator was aware of the settings being used, the reliance on the automated ‘SonoNT’ tool for measurements obviates the need for operator blinding, as the tool‘s design significantly reduces interobserver variability [
5,
6].
In each examination, details such as gestational age, crown-rump length (CRL), date of the original examination, and the attending physician‘s name were documented.
The measurements obtained under different ‘Radiant’ settings were analyzed using the t-test for dependent variables, employing IBM SPSS (Version 29.0) software.
For this, the acquired NT values for each step of “Radiant” were compared to the group of NT values measured without “Radiant”. The resulting difference in mean NT was named “ΔNT”. The resulting p values of statistical significance are listed in
Table 2.
The study was approved by the ethical committee of RWTH University, Aachen, Germany (No. EK 24-039).
3. Results
Table 1 shows the mean values of gestational age (GA), crown-rump-length (CRL), NT and maternal age by group, along with their relative frequencies.
3.1. Nuchal Translucency with and without ‘Radiant’ Applied
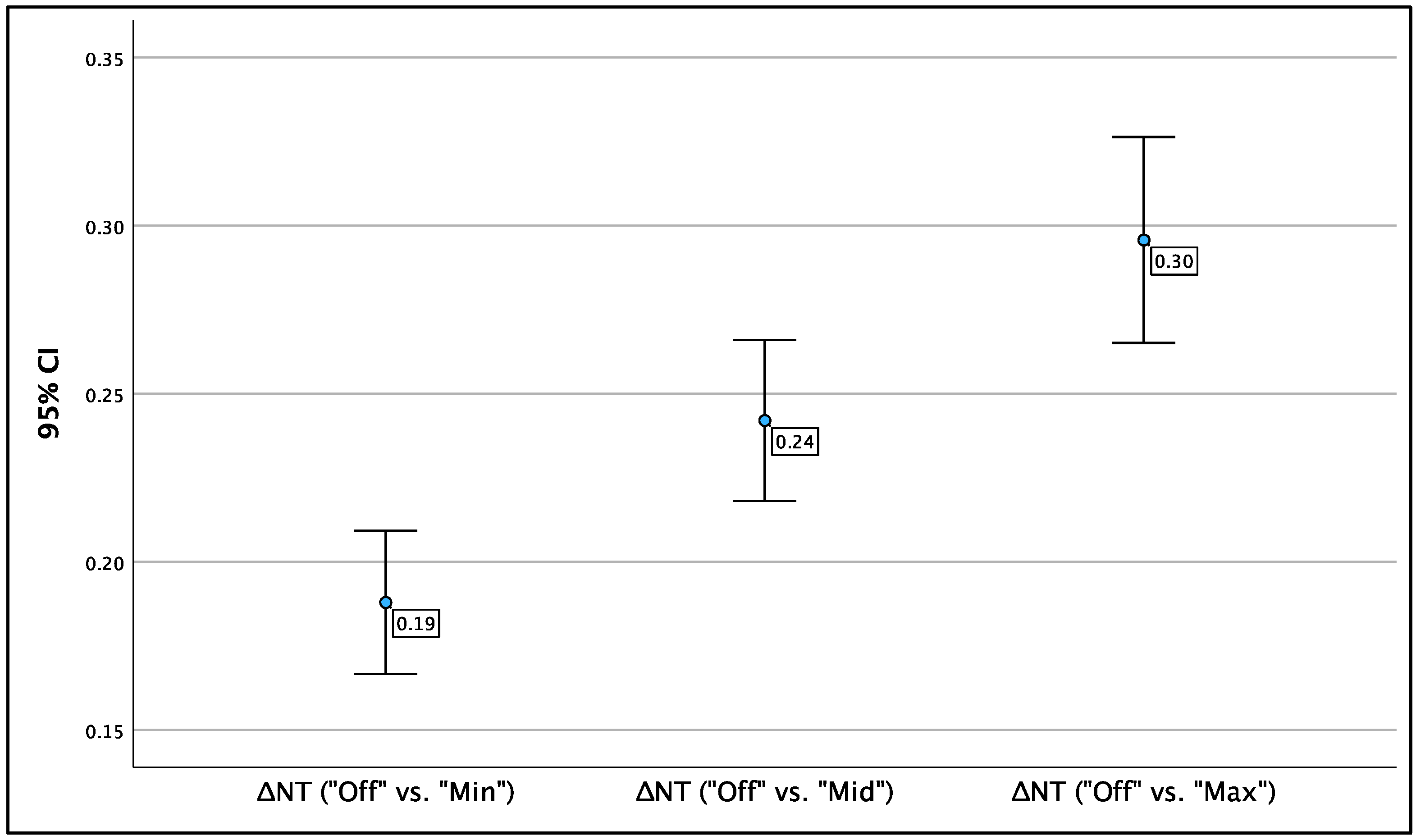
Comparing values without ‘Radiant‘ applied to those with ‘Radiant‘ applied revealed a significant difference in average NT values. For clarity, this difference will be denoted as ‘ΔNT.‘ Significant discrepancies were observed across the three settings of ‘Radiant’—minimum, medium, and maximum—compared to ‘Radiant off.’
Figure 5 illustrates the mean NT values for each ‘Radiant’ method, with brackets indicating the respective 95% confidence intervals (95% CI).
The most notable difference in NT was observed between ‘Radiant off’ and ‘Radiant max’; on average, NT measurements were 0.30 mm greater when ‘Radiant max’ was applied. This difference was statistically significant (p < 0.001; 95% CI: 0.27 – 0.33). The ΔNT between ‘Radiant off’ and ‘Radiant min’ was smaller, averaging 0.19 mm, yet still significant (p < 0.001; 95% CI: 0.17 – 0.21). The average ΔNT between ‘Radiant off’ and ‘Radiant mid’ was 0.24 mm, also statistically significant (p < 0.001; 95% CI: 0.22 – 0.27). The results are presented in
Table 2 and
Figure 5.
Table 2.
Average difference in NT values (ΔNT) between ‘Radiant off’ and each step of ‘Radiant’.
Table 2.
Average difference in NT values (ΔNT) between ‘Radiant off’ and each step of ‘Radiant’.
| ‘Radiant off’ vs.: |
ΔNT |
CI |
SD |
Significance |
| vs. ‘Radiant min’ |
0.19 mm |
0.17 – 0.21 |
0.010 |
p < 0.001 |
| vs. ‘Radiant mid’ |
0.24 mm |
0.22 – 0.27 |
0.012 |
p < 0.001 |
| vs. ‘Radiant max’ |
0.30 mm |
0.27 – 0.33 |
0.016 |
p < 0.001 |
3.2. Relation of Native NT and ΔNT
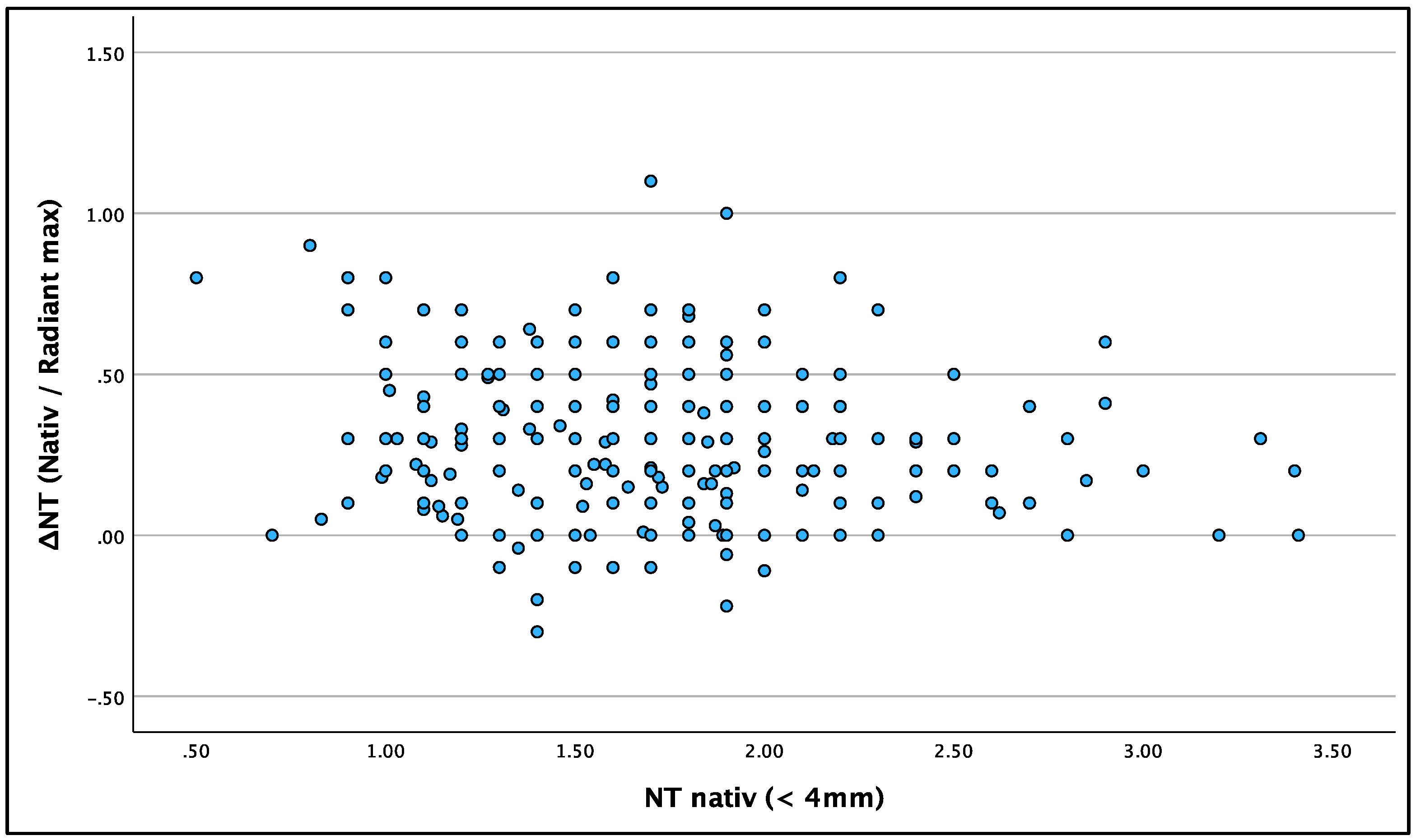
To examine if the radiant effect was more pronounced in large or small NT values, we examined whether ΔNT is dependent on the original NT value. There was no connection between native NT and ΔNT.
Figure 6 shows ΔNT (for ‘Radiant max’) in relation to native NT values. The statistical correlation was poor with Fisher’s correlation at –0.152.
3.3. In-Vitro-Examination with and without ‘Radiant’ Applied
To corroborate our findings from live patient examinations, we established an in-vitro setup with constant, predefined line distances of 1.0 and 2.5 mm, as previously described. The in-vitro results align with those observed during live NT measurements. The ‘Radiant’ setting resulted in thinner, more defined line thicknesses. In the ‘Fundamental’ mode— the native B-image without enhancements—the lines were slightly thicker, with distances between lines increasing by 0.1 to 0.2 mm for both measured distances. ‘Harmonic Imaging’ did not affect smaller distances but had a more noticeable impact on larger ones, increasing line distance by up to 0.5 mm. With ‘Harmonic Imaging,’ deviations from the actual distances were significant, showing a reduction of –0.3 mm for 1.0 mm of actual distance, and ranging from –0.4 to –0.9 mm for 2.5 mm of actual distance. This effect is visible in B-mode images as blurring and increased line thickness, which reduces the measured distance. The comparison of line thickness using only ‘Harmonic Imaging’ versus ‘Harmonic Imaging’ combined with ‘Radiant’ is depicted in
Figure 7.
Using ‘Ultra HD,’ measurements were generally more accurate, with a maximum deviation of –0.2 mm for both 1.0 mm and 2.5 mm of actual distances. ‘Ultra HD’ appears to enhance precision by reducing line thickness. Although the effect of combining ‘Radiant’ with ‘Ultra HD’ was observable, it was less dramatic than when combined with ‘Harmonic Imaging’. All in-vitro measurement values are listed in
Table 3 and
Table 4.
Additionally, we observed that at smaller distances, measurements tended to increase up to ‘Radiant mid’ but decreased again at ‘Radiant max’. This pattern is documented in
Table 3 for both ‘Fundamental’ and ‘Ultra HD’ settings. This trend was also evident during data acquisition, where smaller NT values initially increased with ‘Radiant mid’ but decreased with ‘Radiant max’.
4. Discussion
This retrospective single-center cohort study was conducted to validate clinical observations and assess the impact of a novel image enhancement technology called ‘Radiant.’ By applying ‘Radiant’ to in-device images post-examination, we simulated real patient scenarios, thereby creating a consistent experimental setting. To minimize confounding variables, each image was processed through all stages of ‘Radiant.’ Additionally, we employed the ‘SonoNT’ tool to further enhance inter-observer reliability.
Further, the ‘Radiant’ image enhancement technology significantly improves image quality by sharpening the visual output. This is evidenced by our controlled experiments, where the most accurate measurements of horizontal line distances were obtained using ‘Radiant mid’. Consequently, it appears that without the use of ‘Radiant’, NT measurements are consistently underestimated. In general, the measurement of horizontal line distances seems to be most precise when combining ‘Ultra HD’ and ‘Radiant’, because this combination created the least deviation from the actual distance presented (greyed in
Table 3 and
Table 4).
When introducing a new diagnostic method that yields significantly different values, it is crucial to assess the clinical implications. Over a three-month period, our cohort of 263 cases demonstrated that the number of cases with nuchal translucency (NT) measurements exceeding 3.5 mm increased incrementally with each level of ‘Radiant’ enhancement applied. Specifically, without ‘Radiant’, four cases exceeded this threshold and were subjected to invasive testing (CVS or AC), confirming two cases of Trisomy 21, one of Trisomy 18, and one of Monosomy X. With ‘Radiant’ enhancements—‘Radiant min’, ‘Radiant mid’, and ‘Radiant max’—the numbers increased to five, six, and seven cases, respectively. Notably, the additional cases identified with ‘Radiant’ showed no further signs of aneuploidy in second trimester screenings and did not require invasive procedures.
This results in a marked increase in the false-positive rate (FPR) from 0% with ‘Radiant off’ to 43% with ‘Radiant max’. However, drawing definitive conclusions from these rates is challenging due to the small sample size. Nonetheless, our in-vitro results suggest that ‘Radiant’ may enhance the precision of NT measurements. The apparent contradiction between higher precision and increased FPR may indicate that the traditional reference values are outdated. Given that ‘Radiant’ tends to produce systematically larger, yet potentially more accurate NT values, this discrepancy could be due to historically smaller reference values.
Recent technological advancements in ultrasound imaging underscore the need to revisit the NT reference tables established by Nicolaides et al. in 1992, which have been periodically updated in response to technological progress [
1,
8,
9]. The ongoing debate about NT cutoff margins, including proposed thresholds of 3.0 mm, 3.5 mm, and the 99th percentile, should be informed by these advancements. These cutoffs are crucial as they align closely with current reference tables used in first trimester screening [
10].
To conclusively validate the findings reported, further investigations involving larger cohorts are essential. In practical terms, the use of ‘Radiant’ has subjectively enhanced the visual perception of anatomical landmarks in everyday clinical practice. This improvement not only supports the objective effects observed but also enhances the utility of this technology in routine first trimester screening
In our study, we observed that smaller NT values, around 1 mm, often decreased when ‘Radiant max’ was applied, rather than the expected increase seen with ‘Radiant mid’. This phenomenon was also replicated in our in-vitro setup. However, the current dataset does not provide sufficient information to determine the cause of this effect.
Despite measures to minimize interobserver-variations and creating a consistent experimental setting, the generalizability of the study is limited due to its small sample size and the singular model of ultrasound device used, restricting the applicability of our findings to other settings or devices. At the time of manuscript preparation, no other device or brand offers a technology comparable to ‘Radiant’.
For historical context, a similar enhancement in clinical practice was observed with the introduction of ‘Harmonic Imaging’ (HI) in the late 1990s. This technology markedly improved texture assessment and overall image quality, leading to its rapid integration as a standard feature in sonographic evaluations [
11].
5. Conclusions
Given the observed increase in the precision of NT measurements and the enhanced overall image quality provided by ‘Radiant,’ we recommend its adoption for first trimester sonographic examinations. However, for clinical decision-making, we advise using ‘Radiant off’ mode until more comprehensive data on false-positive rates and updated NT reference values become available.
Author Contributions
Conceptualization, A.B. and J.R.; formal analysis, A.B.; investigation, A.B. and J.R.; data curation, A.B. and J.R.; resources, H.D.; writing—original draft preparation, A.B.; writing—review and editing, A.B., J.R., J.D. and O.G.; visualization, A.B., J.R. and J.D.; supervision, J.R., R.S. and O.G.; All authors have read and agreed to the published version of the manuscript.’
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of RWTH Aachen University (protocol code EK 24-039, date of approval: 22.03.2024).
Data Availability Statement
Research data from this study can be requested from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nicolaides, K.H.; Azar, G.; Byrne, D.; Mansur, C.; Marks, K. Fetal nuchal translucency: ultrasound screening for chromosomal defects in first trimester of pregnancy. Bmj. 1992, 304, 867–9. [Google Scholar] [CrossRef]
- Russo, M.L.; Blakemore, K.J. A historical and practical review of first trimester aneuploidy screening. Semin Fetal Neonatal Med. 2014, 19, 183–7. [Google Scholar] [CrossRef] [PubMed]
- Chaoui, R.; Nicolaides, K.H. From nuchal translucency to intracranial translucency: towards the early detection of spina bifida. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2010, 35, 133–8. [Google Scholar]
- Kagan, K.O.; Wright, D.; Nicolaides, K.H. First-trimester contingent screening for trisomies 21, 18 and 13 by fetal nuchal translucency and ductus venosus flow and maternal blood cell-free DNA testing. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2015, 45, 42–7. [Google Scholar]
- Moratalla, J.; Pintoffl, K.; Minekawa, R.; Lachmann, R.; Wright, D.; Nicolaides, K.H. Semi-automated system for measurement of nuchal translucency thickness. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2010, 36, 412–6. [Google Scholar]
- Karl, K.; Kagan, K.O.; Chaoui, R. Intra- and interoperator reliability of manual and semi-automated measurements of intracranial translucency. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2012, 39, 164–8. [Google Scholar]
- Wunsch, R.; Dudwiesus, H.; Reinehr, T. [Prospective comparison of different ultrasound modalities to measure thicknesses less than 1 mm]. Rofo. 2007, 179, 65–71. [Google Scholar] [CrossRef]
- Snijders, R.J.; Noble, P.; Sebire, N.; Souka, A.; Nicolaides, K.H. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal-translucency thickness at 10-14 weeks of gestation. Fetal Medicine Foundation First Trimester Screening Group. Lancet. 1998, 352, 343–6. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.H. Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am J Obstet Gynecol. 2004, 191, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Kagan, K.O.; Sonek, J.; Kozlowski, P. Antenatal screening for chromosomal abnormalities. Arch Gynecol Obstet. 2022, 305, 825–35. [Google Scholar] [CrossRef]
- Mesurolle, B.; Helou, T.; El-Khoury, M.; Edwardes, M.; Sutton, E.J.; Kao, E. Tissue harmonic imaging, frequency compound imaging, and conventional imaging: use and benefit in breast sonography. J Ultrasound Med. 2007, 26, 1041–51. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).