Submitted:
11 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Detection of SARS-CoV-2 Humoral Immune Response
2.2.1. SARS-CoV-2 Anti-Spike (S) IgG and IgA, and Anti-Nucleocapsid (NCP) IgG Immunoassays
2.2.2. Surrogate SARS-CoV-2 Virus Neutralization Test
2.3. Detection of Cellular Immune Response
2.3.1. ELISpot Test
2.3.2. Flow Cytometry Analysis
2.4. Statistical Analysis
3. Results
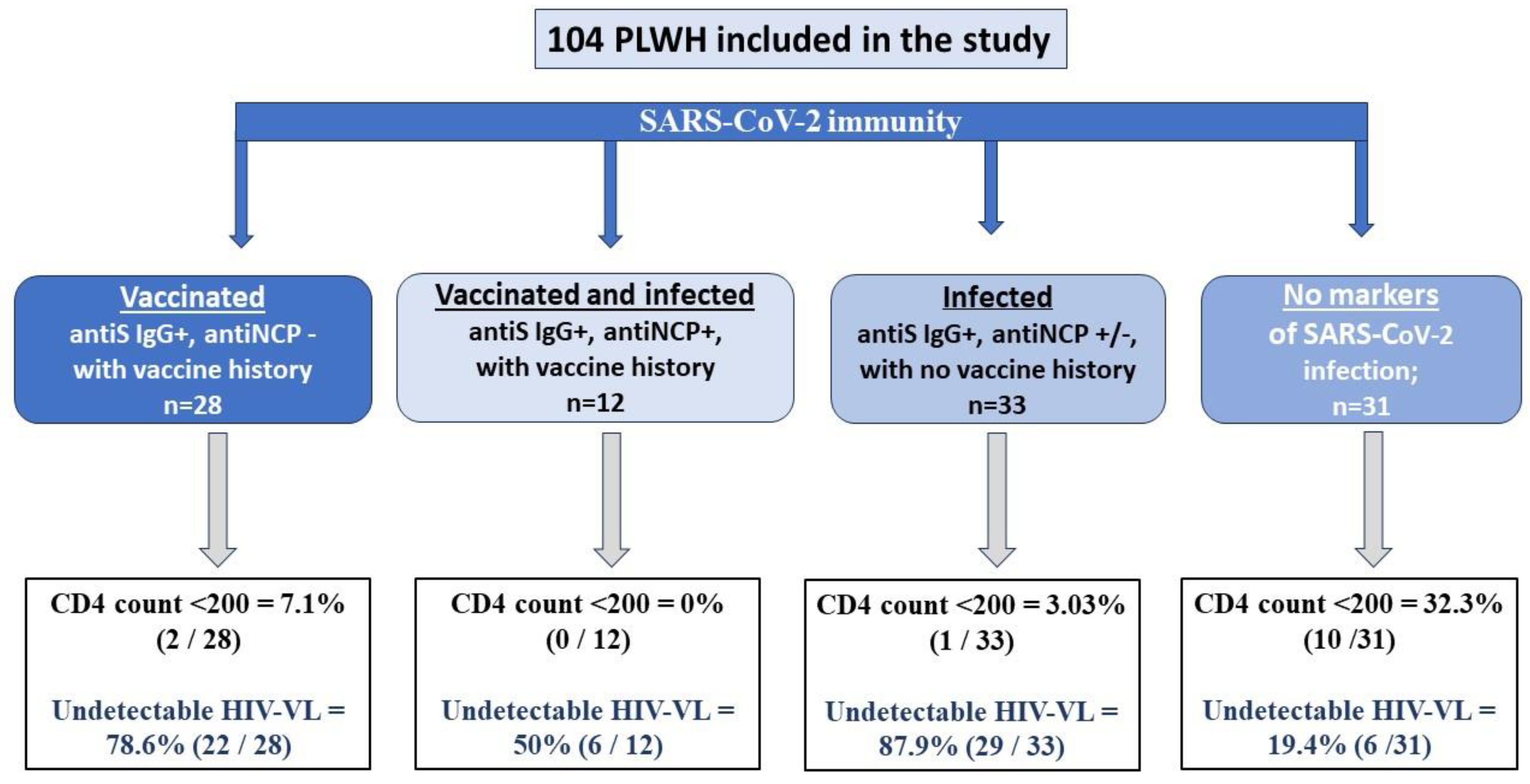
3.1. General Characteristics of the Enrolled Subjects
3.2. SARS-CoV-2 Seroprevalence
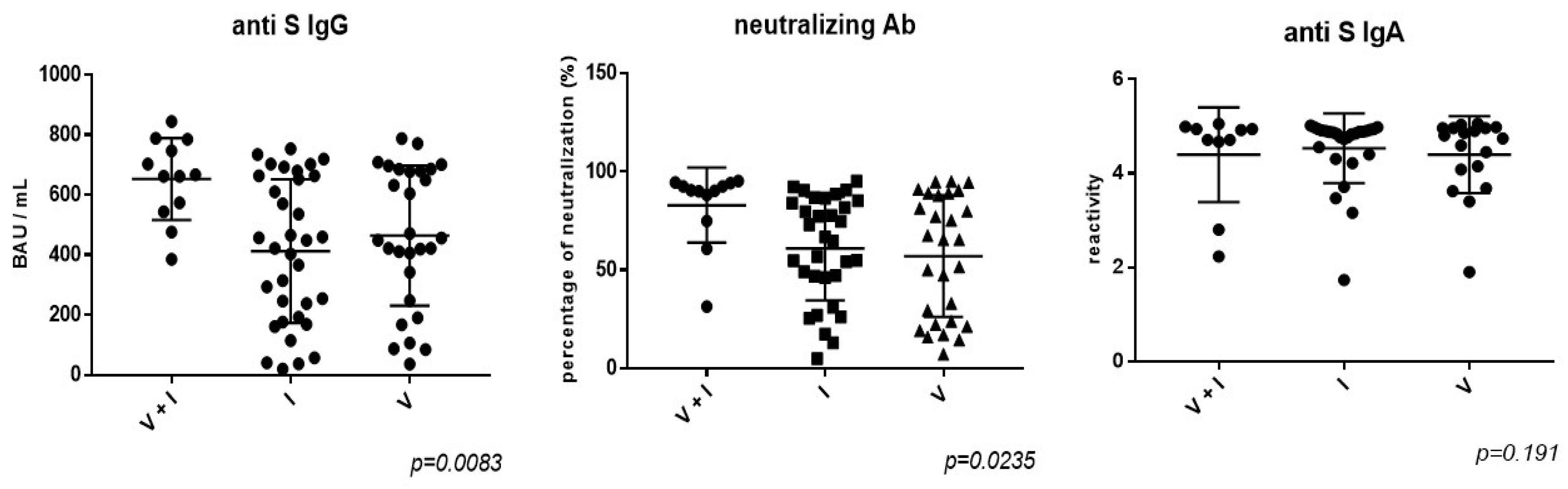
3.3. Antibody Response according to SARS-CoV-2 Vaccination/Infection Status
3.4. SARS-CoV-2 Humoral Immunity in Severe Immunosuppressed PLWH
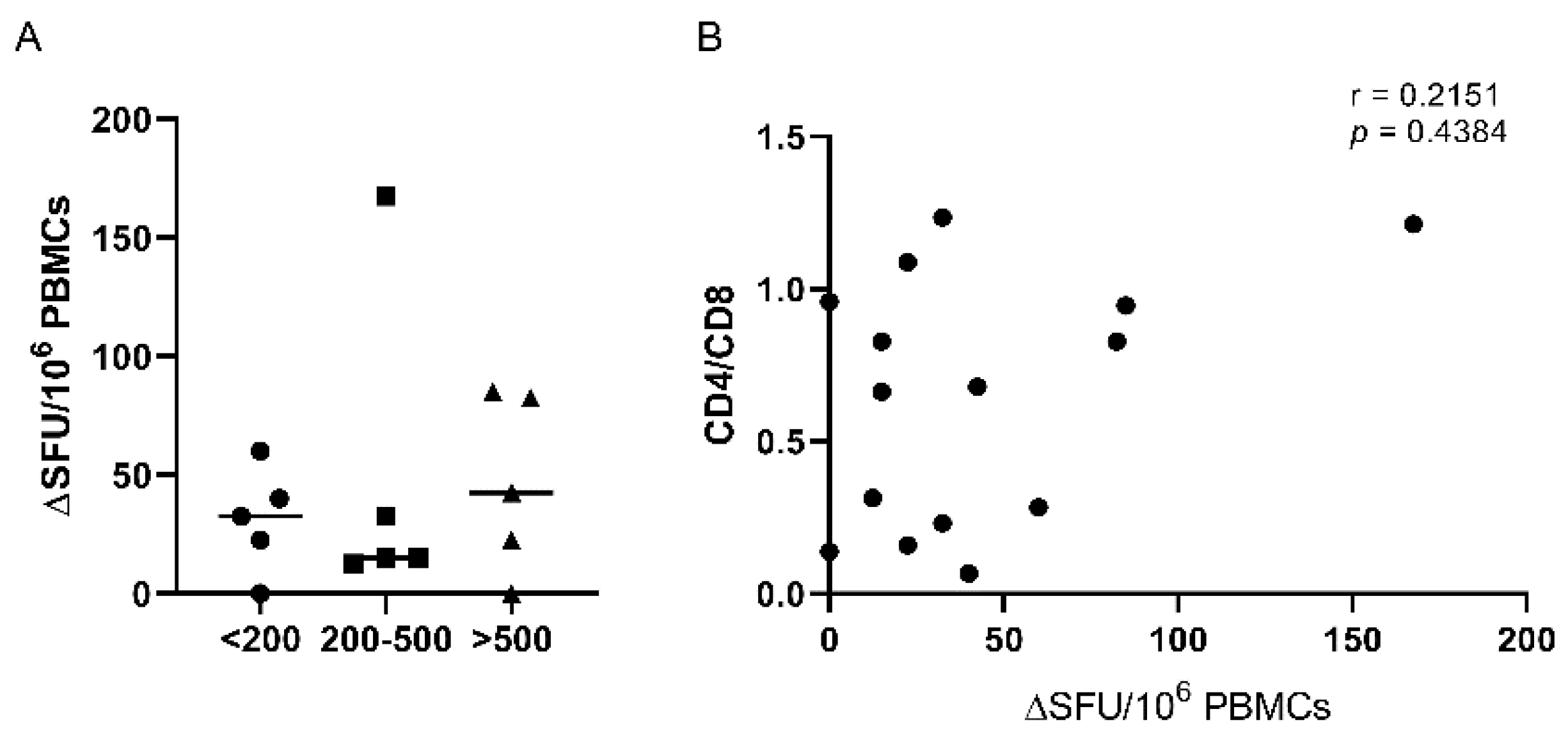
3.5. SARS-CoV-2 Cellular Immunity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubey, A.; Choudhary, S.; Kumar, P.; Tomar, S. Emerging SARS-CoV-2 Variants: Genetic Variability and Clinical Implications. Current Microbiology 2021, 79, 20. [Google Scholar] [CrossRef]
- Bertagnolio, S.; Thwin, S.S.; Silva, R.; Nagarajan, S.; Jassat, W.; Fowler, R.; Haniffa, R.; Reveiz, L.; Ford, N.; Doherty, M.; Diaz, J. Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: Analysis of data from the WHO Global Clinical Platform of COVID-19. The Lancet HIV 2022, 9, e486–e495. [Google Scholar] [CrossRef]
- European AIDS Clinical Society EACS Guidelines Version 12.0, Oct 2023 PART IV 2023; p. 151.
- World Health Organization. SAGE updates COVID-19 vaccination guidance. Available online: https://www.who.int/groups/strategic-advisory-group-of-experts-on-immunization/covid-19-materials (accessed on 22 February 2024).
- Miller, K.W.; Gandhi, R.T. The severity of COVID-19 across the spectrum of HIV. Current opinion in HIV and AIDS 2023, 18, 119–125. [Google Scholar] [CrossRef]
- Höft, M.A.; Burgers, W.A.; Riou, C. The immune response to SARS-CoV-2 in people with HIV. Cellular & Molecular Immunology 2024, 21, 184–196. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.; Patel, R.C.; Zhang, J.; Guo, S.; Zheng, Q.; Olex, A.L.; Olatosi, B.; Weissman, S.B.; Islam, J.Y.; Chute, C.G.; Haendel, M.; Kirk, G.D.; Li, X. Associations between HIV infection and clinical spectrum of COVID-19: A population level analysis based on US National COVID Cohort Collaborative (N3C) data. The lancet. HIV 2021, 8, e690–e700. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Liu, Z.; Chen, S.; Olatosi, B.; Poland, G.A.; Weissman, S.; Li, X. COVID-19 breakthrough infections among people living with and without HIV: A statewide cohort analysis. International Journal of Infectious Diseases 2024, 139, 21–27. [Google Scholar] [CrossRef]
- Cele, S.; Karim, F.; Lustig, G.; San, J.E.; Hermanus, T.; Tegally, H.; Snyman, J.; Moyo-Gwete, T.; Wilkinson, E.; Bernstein, M.; Khan, K.; Hwa, S.H.; Tilles, S.W.; Singh, L.; Giandhari, J.; Mthabela, N.; Mazibuko, M.; Ganga, Y.; Gosnell, B.I.; Karim, S.S.A.; Hanekom, W.; Van Voorhis, W.C.; Ndung'u, T.; Lessells, R.J.; Moore, P.L.; Moosa, M.S.; de Oliveira, T.; Sigal, A. SARS-CoV-2 prolonged infection during advanced HIV disease evolves extensive immune escape. Cell host & microbe 2022, 30, 154–162.e155. [Google Scholar] [CrossRef]
- Qu, M.-M.; Song, B.; Yang, B.-P.; Wang, Z.; Yu, M.; Zhang, Y.; Zhang, C.; Song, J.-W.; Fan, X.; Xu, R.; Zhang, J.-Y.; Zhou, C.-B.; Du, F.; Wang, F.-S.; Huang, H.-H.; Jiao, Y.-M. Effect of SARS-CoV-2 Breakthrough Infection on HIV Reservoirs and T-Cell Immune Recovery in 3-Dose Vaccinated People Living with HIV. Viruses 2023, 15, 2427. [Google Scholar] [CrossRef]
- Donadeu, L.; Tiraboschi, J.M.; Scévola, S.; Torija, A.; Meneghini, M.; Jouve, T.; Favà, A.; Calatayud, L.; Ardanuy, C.; Cidraque, I.; Preyer, R.; Strecker, K.; Lozano, J.J.; Podzamczer, D.; Crespo, E.; Bestard, O. Long-lasting adaptive immune memory specific to SARS-CoV-2 in convalescent coronavirus disease 2019 stable people with HIV. AIDS (London, England) 2022, 36, 1373–1382. [Google Scholar] [CrossRef]
- Frater, J.; Ewer, K.J.; Ogbe, A.; Pace, M.; Adele, S.; Adland, E.; Alagaratnam, J.; Aley, P.K.; Ali, M.; Ansari, M.A.; Bara, A.; Bittaye, M.; Broadhead, S.; Brown, A.; Brown, H.; Cappuccini, F.; Cooney, E.; Dejnirattisai, W.; Dold, C.; Fairhead, C.; Fok, H.; Folegatti, P.M.; Fowler, J.; Gibbs, C.; Goodman, A.L.; Jenkin, D.; Jones, M.; Makinson, R.; Marchevsky, N.G.; Mujadidi, Y.F.; Nguyen, H.; Parolini, L.; Petersen, C.; Plested, E.; Pollock, K.M.; Ramasamy, M.N.; Rhead, S.; Robinson, H.; Robinson, N.; Rongkard, P.; Ryan, F.; Serrano, S.; Tipoe, T.; Voysey, M.; Waters, A.; Zacharopoulou, P.; Barnes, E.; Dunachie, S.; Goulder, P.; Klenerman, P.; Screaton, G.R.; Winston, A.; Hill, A.V.S.; Gilbert, S.C.; Pollard, A.J.; Fidler, S.; Fox, J.; Lambe, T. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: A single-arm substudy of a phase 2/3 clinical trial. The lancet. HIV 2021, 8, e474–e485. [Google Scholar] [CrossRef]
- Oyaert, M.; De Scheerder, M.A.; Van Herrewege, S.; Laureys, G.; Van Assche, S.; Cambron, M.; Naesens, L.; Hoste, L.; Claes, K.; Haerynck, F.; Kerre, T.; Van Laecke, S.; Van Biesen, W.; Jacques, P.; Verhasselt, B.; Padalko, E. Evaluation of Humoral and Cellular Responses in SARS-CoV-2 mRNA Vaccinated Immunocompromised Patients. Front Immunol 2022, 13, 858399. [Google Scholar] [CrossRef]
- Oyaert, M.; De Scheerder, M.-A.; Van Herrewege, S.; Laureys, G.; Van Assche, S.; Cambron, M.; Naesens, L.; Hoste, L.; Claes, K.; Haerynck, F.; Kerre, T.; Van Laecke, S.; Jacques, P.; Padalko, E. Longevity of the humoral and cellular responses after SARS-CoV-2 booster vaccinations in immunocompromised patients. European Journal of Clinical Microbiology & Infectious Diseases 2024, 43, 177–185. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Karaba, A.H.; Garliss, C.C.; Beck, E.J.; Aytenfisu, T.Y.; Johnston, T.S.; Laeyendecker, O.; Cox, A.L.; Blankson, J.N. Decay of coronavirus disease 2019 mRNA vaccine-induced immunity in people with HIV. AIDS (London, England) 2022, 36, 1315–1317. [Google Scholar] [CrossRef]
- Schmidt, K.G.; Harrer, E.G.; Tascilar, K.; Kübel, S.; El Kenz, B.; Hartmann, F.; Simon, D.; Schett, G.; Nganou-Makamdop, K.; Harrer, T. Characterization of Serum and Mucosal SARS-CoV-2-Antibodies in HIV-1-Infected Subjects after BNT162b2 mRNA Vaccination or SARS-CoV-2 Infection. Viruses 2022, 14, 651. [Google Scholar] [CrossRef] [PubMed]
- Touizer, E.; Alrubayyi, A.; Ford, R.; Hussain, N.; Gerber, P.P.; Shum, H.L.; Rees-Spear, C.; Muir, L.; Gea-Mallorquí, E.; Kopycinski, J.; Jankovic, D.; Jeffery-Smith, A.; Pinder, C.L.; Fox, T.A.; Williams, I.; Mullender, C.; Maan, I.; Waters, L.; Johnson, M.; Madge, S.; Youle, M.; Barber, T.J.; Burns, F.; Kinloch, S.; Rowland-Jones, S.; Gilson, R.; Matheson, N.J.; Morris, E.; Peppa, D.; McCoy, L.E. Attenuated humoral responses in HIV after SARS-CoV-2 vaccination linked to B cell defects and altered immune profiles. iScience 2023, 26, 105862. [Google Scholar] [CrossRef]
- Hensley, K.S.; Jongkees, M.J.; Geers, D.; GeurtsvanKessel, C.H.; Mueller, Y.M.; Dalm, V.; Papageorgiou, G.; Steggink, H.; Gorska, A.; Bogers, S.; den Hollander, J.G.; Bierman, W.F.W.; Gelinck, L.B.S.; Schippers, E.F.; Ammerlaan, H.S.M.; van der Valk, M.; van Vonderen, M.G.A.; Delsing, C.E.; Gisolf, E.H.; Bruns, A.H.W.; Lauw, F.N.; Berrevoets, M.A.H.; Sigaloff, K.C.E.; Soetekouw, R.; Branger, J.; de Mast, Q.; Lammers, A.J.J.; Lowe, S.H.; de Vries, R.D.; Katsikis, P.D.; Rijnders, B.J.A.; Brinkman, K.; Roukens, A.H.E.; Rokx, C. Correction: Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in people living with HIV in the Netherlands: A nationwide prospective cohort study. PLoS medicine 2023, 20, e1004159. [Google Scholar] [CrossRef]
- Vergori, A.; Tavelli, A.; Matusali, G.; Azzini, A.M.; Augello, M.; Mazzotta, V.; Pellicanò, G.F.; Costantini, A.; Cascio, A.; De Vito, A.; Marconi, L.; Righi, E.; Sartor, A.; Pinnetti, C.; Maggi, F.; Bai, F.; Lanini, S.; Piconi, S.; Levy Hara, G.; Marchetti, G.; Giannella, M.; Tacconelli, E.; d'Arminio Monforte, A.; Antinori, A.; Cozzi-Lepri, A.; On Behalf Of The Vax-Icona-Orchestra, S. SARS-CoV-2 mRNA Vaccine Response in People Living with HIV According to CD4 Count and CD4/CD8 Ratio. Vaccines (Basel) 2023, 11. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, Q.; Madhira, V.; Olex, A.L.; Anzalone, A.J.; Vinson, A.; Singh, J.A.; French, E.; Abraham, A.G.; Mathew, J.; Safdar, N.; Agarwal, G.; Fitzgerald, K.C.; Singh, N.; Topaloglu, U.; Chute, C.G.; Mannon, R.B.; Kirk, G.D.; Patel, R.C. Association Between Immune Dysfunction and COVID-19 Breakthrough Infection After SARS-CoV-2 Vaccination in the US. JAMA internal medicine 2022, 182, 153–162. [Google Scholar] [CrossRef]
- Infantino, M.; Pieri, M.; Nuccetelli, M.; Grossi, V.; Lari, B.; Tomassetti, F.; Calugi, G.; Pancani, S.; Benucci, M.; Casprini, P.; Manfredi, M.; Bernardini, S. The WHO International Standard for COVID-19 serological tests: Towards harmonization of anti-spike assays. International Immunopharmacology 2021, 100, 108095. [Google Scholar] [CrossRef]
- Meyer, B.; Reimerink, J.; Torriani, G.; Brouwer, F.; Godeke, G.J.; Yerly, S.; Hoogerwerf, M.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; Reusken, C. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT). Emerging microbes & infections 2020, 9, 2394–2403. [Google Scholar] [CrossRef]
- Nault, L.; Marchitto, L.; Goyette, G.; Tremblay-Sher, D.; Fortin, C.; Martel-Laferrière, V.; Trottier, B.; Richard, J.; Durand, M.; Kaufmann, D.; Finzi, A.; Tremblay, C. Covid-19 vaccine immunogenicity in people living with HIV-1. Vaccine 2022, 40, 3633–3637. [Google Scholar] [CrossRef]
- Antinori, A.; Cicalini, S.; Meschi, S.; Bordoni, V.; Lorenzini, P.; Vergori, A.; Lanini, S.; De Pascale, L.; Matusali, G.; Mariotti, D.; Cozzi Lepri, A.; Gallì, P.; Pinnetti, C.; Gagliardini, R.; Mazzotta, V.; Mastrorosa, I.; Grisetti, S.; Colavita, F.; Cimini, E.; Grilli, E.; Bellagamba, R.; Lapa, D.; Sacchi, A.; Marani, A.; Cerini, C.; Candela, C.; Fusto, M.; Puro, V.; Castilletti, C.; Agrati, C.; Girardi, E.; Vaia, F. Humoral and Cellular Immune Response Elicited by mRNA Vaccination Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in People Living With Human Immunodeficiency Virus Receiving Antiretroviral Therapy Based on Current CD4 T-Lymphocyte Count. Clinical infectious diseases : An official publication of the Infectious Diseases Society of America 2022, 75, e552–e563. [Google Scholar] [CrossRef]
- Corma-Gómez, A.; Fernández-Fuertes, M.; García, E.; Fuentes-López, A.; Gómez-Ayerbe, C.; Rivero-Juárez, A.; Domínguez, C.; Santos, M.; Viñuela, L.; Palacios, R.; Real, L.M.; Rivero, A.; Macías, J.; Pineda, J.A.; García, F. Severe immunosuppression is related to poorer immunogenicity to SARS-CoV-2 vaccines among people living with HIV. Clinical microbiology and infection : The official publication of the European Society of Clinical Microbiology and Infectious Diseases 2022, 28, 1492–1498. [Google Scholar] [CrossRef]
- Benet, S.; Blanch-Lombarte, O.; Ainsua-Enrich, E.; Pedreño-Lopez, N.; Muñoz-Basagoiti, J.; Raïch-Regué, D.; Perez-Zsolt, D.; Peña, R.; Jiménez, E.; de la Concepción, M.L.R.; Ávila, C.; Cedeño, S.; Escribà, T.; Romero-Martín, L.; Alarcón-Soto, Y.; Rodriguez-Lozano, G.F.; Miranda, C.; González, S.; Bailón, L.; Blanco, J.; Massanella, M.; Brander, C.; Clotet, B.; Paredes, R.; Esteve, M.; Izquierdo-Useros, N.; Carrillo, J.; Prado, J.G.; Moltó, J.; Mothe, B. Limited Humoral and Specific T-Cell Responses After SARS-CoV-2 Vaccination in PWH With Poor Immune Reconstitution. The Journal of infectious diseases 2022, 226, 1913–1923. [Google Scholar] [CrossRef]
- Corma-Gómez, A.; Fernández-Fuertes, M.; Viñuela, L.; Domínguez, C.; Santos, M.; Fuentes-López, A.; Rojas, A.; Fernández-Pérez, N.; Martín-Carmona, J.; Serrano-Conde, E.; Real, L.M.; Mendoza, J.; Macías, J.; Pineda, J.A.; García, F. Reduced neutralizing antibody response to SARS-CoV-2 vaccine booster dose in people living with HIV with severe immunosuppression. J Med Virol 2023, 95, e28602. [Google Scholar] [CrossRef]
- Vergori, A.; Cozzi-Lepri, A.; Matusali, G.; Colavita, F.; Cicalini, S.; Gallì, P.; Garbuglia, A.R.; Fusto, M.; Puro, V.; Maggi, F.; Girardi, E.; Vaia, F.; Antinori, A. SARS-CoV-2 Omicron Variant Neutralization after Third Dose Vaccination in PLWH. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Khan, K.; Karim, F.; Ganga, Y.; Bernstein, M.; Jule, Z.; Reedoy, K.; Cele, S.; Lustig, G.; Amoako, D.; Wolter, N.; Samsunder, N.; Sivro, A.; San, J.E.; Giandhari, J.; Tegally, H.; Pillay, S.; Naidoo, Y.; Mazibuko, M.; Miya, Y.; Ngcobo, N.; Manickchund, N.; Magula, N.; Karim, Q.A.; von Gottberg, A.; Abdool Karim, S.S.; Hanekom, W.; Gosnell, B.I.; Lessells, R.J.; de Oliveira, T.; Moosa, M.S.; Sigal, A. Omicron BA.4/BA.5 escape neutralizing immunity elicited by BA.1 infection. Nat Commun 2022, 13, 4686. [Google Scholar] [CrossRef]
- Shoham, S.; Batista, C.; Ben Amor, Y.; Ergonul, O.; Hassanain, M.; Hotez, P.; Kang, G.; Kim, J.H.; Lall, B.; Larson, H.J.; Naniche, D.; Sheahan, T.; Strub-Wourgaft, N.; Sow, S.O.; Wilder-Smith, A.; Yadav, P.; Bottazzi, M.E. Vaccines and therapeutics for immunocompromised patients with COVID-19. EClinicalMedicine 2023, 59, 101965. [Google Scholar] [CrossRef]
- Bergman, P.; Blennow, O.; Hansson, L.; Mielke, S.; Nowak, P.; Chen, P.; Söderdahl, G.; Österborg, A.; Smith, C.I.E.; Wullimann, D.; Vesterbacka, J.; Lindgren, G.; Blixt, L.; Friman, G.; Wahren-Borgström, E.; Nordlander, A.; Gomez, A.C.; Akber, M.; Valentini, D.; Norlin, A.C.; Thalme, A.; Bogdanovic, G.; Muschiol, S.; Nilsson, P.; Hober, S.; Loré, K.; Chen, M.S.; Buggert, M.; Ljunggren, H.G.; Ljungman, P.; Aleman, S. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine 2021, 74, 103705. [Google Scholar] [CrossRef]
- Rabinowich, L.; Grupper, A.; Baruch, R.; Ben-Yehoyada, M.; Halperin, T.; Turner, D.; Katchman, E.; Levi, S.; Houri, I.; Lubezky, N.; Shibolet, O.; Katchman, H. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. Journal of hepatology 2021, 75, 435–438. [Google Scholar] [CrossRef]
- Pourcher, V.; Belin, L.; Soulie, C.; Rosenzwajg, M.; Marot, S.; Lacombe, K.; Valin, N.; Pialoux, G.; Calin, R.; Palacios, C.; Malet, I.; Zafilaza, K.; Tubiana, R.; Valantin, M.A.; Klatzmann, D.; Calvez, V.; Simon-Tillaux, N.; Marcelin, A.G. High seroconversion rate and SARS-CoV-2 Delta neutralization in people with HIV vaccinated with BNT162b2. AIDS (London, England) 2022, 36, 1545–1552. [Google Scholar] [CrossRef]
- Fernandes, M.d.C.R.; Vasconcelos, G.S.; de Melo, A.C.L.; Matsui, T.C.; Caetano, L.F.; de Carvalho Araújo, F.M.; Fonseca, M.H.G. Influence of age, gender, previous SARS-CoV-2 infection, and pre-existing diseases in antibody response after COVID-19 vaccination: A review. Molecular Immunology 2023, 156, 148–155. [Google Scholar] [CrossRef]
- Augello, M.; Bono, V.; Rovito, R.; Tincati, C.; d'Arminio Monforte, A.; Marchetti, G. Six-month immune responses to mRNA-1273 vaccine in combination antiretroviral therapy treated late presenter people with HIV according to previous SARS-CoV-2 infection. AIDS (London, England) 2023, 37, 1503–1517. [Google Scholar] [CrossRef]
- Fidler, S.; Fox, J.; Tipoe, T.; Longet, S.; Tipton, T.; Abeywickrema, M.; Adele, S.; Alagaratnam, J.; Ali, M.; Aley, P.K.; Aslam, S.; Balasubramanian, A.; Bara, A.; Bawa, T.; Brown, A.; Brown, H.; Cappuccini, F.; Davies, S.; Fowler, J.; Godfrey, L.; Goodman, A.L.; Hilario, K.; Hackstein, C.-P.; Mathew, M.; Mujadidi, Y.F.; Packham, A.; Petersen, C.; Plested, E.; Pollock, K.M.; Ramasamy, M.N.; Robinson, H.; Robinson, N.; Rongkard, P.; Sanders, H.; Serafimova, T.; Spence, N.; Waters, A.; Woods, D.; Zacharopoulou, P.; Barnes, E.; Dunachie, S.; Goulder, P.; Klenerman, P.; Winston, A.; Hill, A.V.S.; Gilbert, S.C.; Carroll, M.; Pollard, A.J.; Lambe, T.; Ogbe, A.; Frater, J. Booster Vaccination Against SARS-CoV-2 Induces Potent Immune Responses in People With Human Immunodeficiency Virus. Clinical Infectious Diseases 2022, 76, 201–209. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Q.; Gu, X.; Ren, L.; Huang, T.; Li, Y.; Zhang, H.; Liu, Y.; Zhong, J.; Wang, X.; Chen, L.; Zhang, Y.; Li, D.; Fang, M.; Xu, L.; Li, H.; Wang, Z.; Li, H.; Bai, T.; Liu, W.; Peng, Y.; Dong, T.; Cao, B.; Wang, J. Durability and cross-reactive immune memory to SARS-CoV-2 in individuals 2 years after recovery from COVID-19: A longitudinal cohort study. The Lancet Microbe 2024, 5, e24–e33. [Google Scholar] [CrossRef]
- Ogbe, A.; Pace, M.; Bittaye, M.; Tipoe, T.; Adele, S.; Alagaratnam, J.; Aley, P.K.; Ansari, M.A.; Bara, A.; Broadhead, S.; Brown, A.; Brown, H.; Cappuccini, F.; Cinardo, P.; Dejnirattisai, W.; Ewer, K.J.; Fok, H.; Folegatti, P.M.; Fowler, J.; Godfrey, L.; Goodman, A.L.; Jackson, B.; Jenkin, D.; Jones, M.; Longet, S.; Makinson, R.A.; Marchevsky, N.G.; Mathew, M.; Mazzella, A.; Mujadidi, Y.F.; Parolini, L.; Petersen, C.; Plested, E.; Pollock, K.M.; Rajeswaran, T.; Ramasamy, M.N.; Rhead, S.; Robinson, H.; Robinson, N.; Sanders, H.; Serrano, S.; Tipton, T.; Waters, A.; Zacharopoulou, P.; Barnes, E.; Dunachie, S.; Goulder, P.; Klenerman, P.; Screaton, G.R.; Winston, A.; Hill, A.V.; Gilbert, S.C.; Carroll, M.; Pollard, A.J.; Fidler, S.; Fox, J.; Lambe, T.; Frater, J. Durability of ChAdOx1 nCoV-19 vaccination in people living with HIV. JCI insight 2022, 7. [Google Scholar] [CrossRef]
- Chivu-Economescu, M.; Bleotu, C.; Grancea, C.; Chiriac, D.; Botezatu, A.; Iancu, I.V.; Pitica, I.; Necula, L.G.; Neagu, A.; Matei, L.; Dragu, D.; Sultana, C.; Radu, E.L.; Nastasie, A.; Voicu, O.; Ataman, M.; Nedeianu, S.; Mambet, C.; Diaconu, C.C.; Ruta, S.M. Kinetics and persistence of cellular and humoral immune responses to SARS-CoV-2 vaccine in healthcare workers with or without prior COVID-19. Journal of cellular and molecular medicine 2022, 26, 1293–1305. [Google Scholar] [CrossRef]
- Ekström, N.; Leino, T.M.; Juutinen, A.; Lehtonen, T.; Haveri, A.; Liedes, O.; Vara, S.; Salo, H.; Palmu, A.A.; Nohynek, H.; Martelius, T.; Melin, M. Hybrid Immunity Improves the Immune Response after the Fourth COVID-19 Vaccine Dose in Individuals with Medical Conditions Predisposing to Severe COVID-19. Vaccines 2024, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, H.R.; Mwimanzi, F.; Cheung, P.K.; Sang, Y.; Yaseen, F.; Speckmaier, S.; Barad, E.; Moran-Garcia, N.; Datwani, S.; Duncan, M.C.; Kalikawe, R.; Ennis, S.; Young, L.; Ganase, B.; Omondi, F.H.; Umviligihozo, G.; Dong, W.; Toy, J.; Sereda, P.; Burns, L.; Costiniuk, C.T.; Cooper, C.; Anis, A.H.; Leung, V.; Holmes, D.; DeMarco, M.L.; Simons, J.; Hedgcock, M.; Prystajecky, N.; Lowe, C.F.; Romney, M.G.; Barrios, R.; Guillemi, S.; Brumme, C.J.; Montaner, J.S.G.; Hull, M.; Harris, M.; Niikura, M.; Brockman, M.A.; Brumme, Z.L. Antibody response durability following three-dose coronavirus disease 2019 vaccination in people with HIV receiving suppressive antiretroviral therapy. AIDS (London, England) 2023, 37, 709–721. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, T.; Chen, L.; Jiang, G.; Geng, Y.; Li, W.; Yin, S.; Tong, X.; Tao, Y.; Ni, J.; Lu, Q.; Ning, M.; Wu, C. SARS-CoV-2 Omicron infection augments the magnitude and durability of systemic and mucosal immunity in triple-dose CoronaVac recipients. mBio 2024, 15, e0240723. [Google Scholar] [CrossRef]
- Koutsakos, M.; Reynaldi, A.; Lee, W.S.; Nguyen, J.; Amarasena, T.; Taiaroa, G.; Kinsella, P.; Liew, K.C.; Tran, T.; Kent, H.E.; Tan, H.X.; Rowntree, L.C.; Nguyen, T.H.O.; Thomas, P.G.; Kedzierska, K.; Petersen, J.; Rossjohn, J.; Williamson, D.A.; Khoury, D.; Davenport, M.P.; Kent, S.J.; Wheatley, A.K.; Juno, J.A. SARS-CoV-2 breakthrough infection induces rapid memory and de novo T cell responses. Immunity 2023, 56, 879–892. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; Gunn, C.; Hockett, R.; Mudumba, S.; Guihot, A.; Luyt, C.E.; Mayaux, J.; Beurton, A.; Fourati, S.; Bruel, T.; Schwartz, O.; Lacorte, J.M.; Yssel, H.; Parizot, C.; Dorgham, K.; Charneau, P.; Amoura, Z.; Gorochov, G. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Science translational medicine 2021, 13. [Google Scholar] [CrossRef]
- Denis, J.; Garnier, A.; Cheutin, L.; Ferrier, A.; Timera, H.; Jarjaval, F.; Hejl, C.; Billon-Denis, E.; , P.I.g.; Ricard, D.; Tournier, J.-N.; Trignol, A.; Mura, M. Long-term systemic and mucosal SARS-CoV-2 IgA response and its association with persistent smell and taste disorders. Frontiers in Immunology 2023, 14. [CrossRef]
- Yin, J.; Chen, Y.; Li, Y.; Wang, C.; Zhang, X. Immunogenicity and efficacy of COVID-19 vaccines in people living with HIV: A systematic review and meta-analysis. International Journal of Infectious Diseases 2022, 124, 212–223. [Google Scholar] [CrossRef]
- Zhou, Q.; Zeng, F.; Meng, Y.; Liu, Y.; Liu, H.; Deng, G. Serological response following COVID-19 vaccines in patients living with HIV: A dose-response meta-analysis. Scientific reports 2023, 13, 9893. [Google Scholar] [CrossRef]
- INSP. Raportare Vaccinari Impotriva COVID-19 Si RAPI Luna Noiembrie 06-30.11.2023 [in Romanian]. Available online: https://insp.gov.ro/wpfb-file/raportare-vaccinari-impotriva-covid-19-si-rapi_luna-noiembrie-06-30-11-2023-pdf/.
- Vergori, A.; Cozzi Lepri, A.; Cicalini, S.; Matusali, G.; Bordoni, V.; Lanini, S.; Meschi, S.; Iannazzo, R.; Mazzotta, V.; Colavita, F.; Mastrorosa, I.; Cimini, E.; Mariotti, D.; De Pascale, L.; Marani, A.; Gallì, P.; Garbuglia, A.; Castilletti, C.; Puro, V.; Agrati, C.; Girardi, E.; Vaia, F.; Antinori, A.; Amendola, A.; Baldini, F.; Bellagamba, R.; Bettini, A.; Bordi, L.; Camici, M.; Casetti, R.; Costantini, S.; Cristofanelli, F.; D’Alessio, C.; D’Aquila, V.; De Angelis, A.; De Zottis, F.; de Pascale, L.; Francalancia, M.; Fusto, M.; Gagliardini, R.; Gramigna, G.; Grassi, G.; Grilli, E.; Grisetti, S.; Iafrate, D.; Lapa, D.; Lorenzini, P.; Marani, A.; Masone, E.; Marongiu, S.; Mondi, A.; Notari, S.; Ottou, S.; Paulicelli, J.; Pellegrino, L.; Pinnetti, C.; Plazzi, M.M.; Possi, A.; Sacchi, A.; Tartaglia, E.; group, H.-V.s. Immunogenicity to COVID-19 mRNA vaccine third dose in people living with HIV. Nature Communications 2022, 13, 4922. [Google Scholar] [CrossRef]
- Lin, J.; Law, R.; Korosec, C.S.; Zhou, C.; Koh, W.H.; Ghaemi, M.S.; Samaan, P.; Ooi, H.K.; Matveev, V.; Yue, F.; Gingras, A.-C.; Estacio, A.; Buchholz, M.; Cheatley, P.L.; Mohammadi, A.; Kaul, R.; Pavinski, K.; Mubareka, S.; McGeer, A.J.; Leis, J.A.; Heffernan, J.M.; Ostrowski, M. Longitudinal Assessment of SARS-CoV-2-Specific T Cell Cytokine-Producing Responses for 1 Year Reveals Persistence of Multicytokine Proliferative Responses, with Greater Immunity Associated with Disease Severity. Journal of Virology 2022, 96, e00509–e00522. [Google Scholar] [CrossRef]
- Nkosi, T.; Chasara, C.; Papadopoulos, A.O.; Nguni, T.L.; Karim, F.; Moosa, M.-Y.S.; Gazy, I.; Jambo, K.; Team, C.K.; Hanekom, W.; Sigal, A.; Ndhlovu, Z.M. Unsuppressed HIV infection impairs T cell responses to SARS-CoV-2 infection and abrogates T cell cross-recognition. eLife 2022, 11, e78374. [Google Scholar] [CrossRef]
| Patients’ immunological status | CD4 count> 500 n=62 |
CD4 count 200-500 n=29 |
CD4 count< 200 n=13 |
p |
|---|---|---|---|---|
| Male (%) | 41 (66.1%) | 20 (68.9%) | 9 (69.2%) | 0.9009 |
|
Age (years) mean ± SD |
38.3 ± 9.7 | 38.8 ± 12.3 | 38.3 ± 4.6 | 0.9736 |
| AIDS C3 (%) | 27.4 | 34.5 | 35 | 0.7107 |
|
HIV infection duration (months), median [IQR] |
132 [56-240] |
78.5 [23.5-144] |
72.5 [1-122] |
0.0376 |
| Age at HIV diagnosis (years), median [IQR] | 27 [14-35] |
26 [21-36] |
33.5 [24-37.5] |
0.5185 |
| cART treatment duration (month), mean ± SD | 108 [60-228] |
72 [36-204] |
60 [2-120] |
0.1343 |
| CD4 nadir (cells/mm3), mean ± SD | 328.9 ± 267.4 | 175.2 ± 120.5 | 86.8 ± 71.9 | 0.0003 |
|
CD4/CD8 ratio, mean ± SD |
1.16 ± 0.7 | 0.63 ± 0.3 | 0.13 ± 0.08 | <0.0001 |
|
HIV viral load (log10 HIV-RNA copies/mL), mean ± SD |
4.23 ± 3.9 | 5.1 ± 4.5 | 5.38 ± 4.6 | 0.005 |
|
Zenith HIV viral load (log10 copies/mL), mean ± SD |
5.65 ± 4.8 | 5.72 ± 4.9 | 6.1 ± 5.7 | 0.1023 |
|
Undetectable HIV RNA, n (%) |
45 (72.6%) | 16 (55.2%) | 2 (15.4%) | 0.0005 |
| Number of cART regimens, mean ± SD | 2.9 ± 2.3 | 2.96 ± 2.6 | 2.3 ± 1.6 | 0.6708 |
| Patients’ immunological status | CD4 count >500 n=62 | CD4 count 200-500; n=29 |
CD4 count <200 n=13 |
p |
|---|---|---|---|---|
| Positive for anti-S IgG antibodies, n (%) | 49 (79.1%) | 21 (72.4%) | 3 (23.1%) | 0.0003 |
| Anti-S IgG titer (BAU/ml) mean ± SD | 377.6 ± 276.1 | 354.5 ± 301.1 | 106.8 ± 228.1 | 0.005 |
| Positive for anti-S IgA antibodies n (%) | 56 (90.3%) | 21 (72.4%) | 4 (30.8%) | <0.0001 |
| Anti-S IgA antibidies reactivity, mean ±SD | 3.6 ± 1.7 | 3.2 ± 1.9 | 1.4 ± 1.1 | 0.002 |
| Positive for neutralizing activity (>30%), n (%) | 51 (82.3%) | 23 (79.3%) | 2 (15.4%) | <0.0001 |
| SARS-CoV-2 neutralizing capacity, mean ± SD | 63.8 ± 27.6 | 64.2 ± 30.1 | 45.5 ± 29.7 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





