Submitted:
10 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Frequency and Diversification of HABs
2.1. Major Drivers: Changing Climate and the Global Population
2.2. Roles of Macronutrients in Bloom Dynamics
2.3. Current Efforts for Algal Bloom Detection
3. Trace Metals
3.1. Overview of Trace Metal Distribution and Bioavailability
3.2. Overview of Roles of Trace Metals in Photosynthetic Microorganisms
4. Trace Metals and Harmful Algal Blooms
4.1. Iron (Fe)
4.2. Copper (Cu)
4.3. Zinc (Zn)
4.4. Selenium (Se)
4.5. Other Essentials (Mn, Co, Ni, Cd, Mo, V)
4.5.1. Manganese (Mn)
4.5.2. Cobalt (Co)
4.5.3. Nickel (Ni)
4.5.4. Cadmium (Cd)
4.5.5. Molybdenum (Mo) and Vanadium (V)
4.6. Non-Essentials (Pb, Hg, As, Sn, Ti, Zr)
4.6.1. Lead (Pb)
4.6.2. Mercury (Hg)
4.6.3. Arsenic (As)
4.6.4. Tin (Sn), Titanium (Ti), and Zirconium (Zr)
5. Gaps and Recommendations
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, D. HABs in a Changing World: A Perspective on Harmful Algal Blooms, Their Impacts, and Research and Management in a Dynamic Era of Climactic and Environmental Change. Harmful Algae 2012 Proc. 15th Int. Conf. Harmful Algae Oct. 29 - Novemb. 2 2012 CECO Chang. Gyeongnam Korea Int. Conf. Harmful Algae 15th 2012 Chang. Gyeongnam Kore 2014, 2012, 3–17. [Google Scholar]
- Anderson, C.R.; Moore, S.K.; Tomlinson, M.C.; Silke, J.; Cusack, C.K. Living with Harmful Algal Blooms in a Changing World. In Coastal and Marine Hazards, Risks, and Disasters; Elsevier, 2015; pp. 495–561 ISBN 978-0-12-396483-0.
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.-Y.D.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived Global Increase in Algal Blooms Is Attributable to Intensified Monitoring and Emerging Bloom Impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Anderson, D.M. ; Cembella Manual on Harmful Marine Microalgae; 2nd ed.; UNESCO Publishing: Landais, France, 2004; ISBN 92-3-103948-2. [Google Scholar]
- Anderson, D.M.; Burkholder, J.M.; Cochlan, W.P.; Glibert, P.M.; Gobler, C.J.; Heil, C.A.; Kudela, R.M.; Parsons, M.L.; Rensel, J.E.J.; Townsend, D.W.; et al. Harmful Algal Blooms and Eutrophication: Examining Linkages from Selected Coastal Regions of the United States. Harmful Algae 2008, 8, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Azanza, R.V.; Fukuyo, Y.; Yap, L.G.; Takayama, H. Prorocentrum Minimum Bloom and Its Possible Link to a Massive Fish Kill in Bolinao, Pangasinan, Northern Philippines. Harmful Algae 2005, 4, 519–524. [Google Scholar] [CrossRef]
- Paerl, H.W. Assessing and Managing Nutrient-Enhanced Eutrophication in Estuarine and Coastal Waters: Interactive Effects of Human and Climatic Perturbations. Ecol. Eng. 2006, 26, 40–54. [Google Scholar] [CrossRef]
- Paerl, H.W.; Havens, K.E.; Xu, H.; Zhu, G.; McCarthy, M.J.; Newell, S.E.; Scott, J.T.; Hall, N.S.; Otten, T.G.; Qin, B. Mitigating Eutrophication and Toxic Cyanobacterial Blooms in Large Lakes: The Evolution of a Dual Nutrient (N and P) Reduction Paradigm. Hydrobiologia 2020, 847, 4359–4375. [Google Scholar] [CrossRef]
- Sellner, K.G.; Doucette, G.J.; Kirkpatrick, G.J. Harmful Algal Blooms: Causes, Impacts and Detection. J. Ind. Microbiol. Biotechnol. 2003, 30, 383–406. [Google Scholar] [CrossRef]
- Brown, A.R.; Lilley, M.; Shutler, J.; Lowe, C.; Artioli, Y.; Torres, R.; Berdalet, E.; Tyler, C.R. Assessing Risks and Mitigating Impacts of Harmful Algal Blooms on Mariculture and Marine Fisheries. Rev. Aquac. 2020, 12, 1663–1688. [Google Scholar] [CrossRef]
- Heil, C.A.; Muni-Morgan, A.L. Florida’s Harmful Algal Bloom (HAB) Problem: Escalating Risks to Human, Environmental and Economic Health With Climate Change. Front. Ecol. Evol. 2021, 9, 646080. [Google Scholar] [CrossRef]
- Shirokova, L.S.; Kunhel, L.; Rols, J.-L.; Pokrovsky, O.S. Experimental Modeling of Cyanobacterial Bloom in a Thermokarst Lake: Fate of Organic Carbon, Trace Metal, and Carbon Sequestration Potential. Aquat. Geochem. 2015, 21, 487–511. [Google Scholar] [CrossRef]
- Kim, J.S.; Seo, I.W.; Baek, D. Modeling Spatial Variability of Harmful Algal Bloom in Regulated Rivers Using a Depth-Averaged 2D Numerical Model. J. Hydro-Environ. Res. 2018, 20, 63–76. [Google Scholar] [CrossRef]
- Ralston, D.K.; Moore, S.K. Modeling Harmful Algal Blooms in a Changing Climate. Harmful Algae 2020, 91, 101729. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Processes Regulating Cellular Metal Accumulation and Physiological Effects: Phytoplankton as Model Systems. Sci. Total Environ. 1998, 219, 165–181. [Google Scholar] [CrossRef]
- Gerringa, L.J.A.; De Baar, H.J.W.; Timmermans, K.R. A Comparison of Iron Limitation of Phytoplankton in Natural Oceanic Waters and Laboratory Media Conditioned with EDTA. Mar. Chem. 2000, 68, 335–346. [Google Scholar] [CrossRef]
- Firme, G.F.; Rue, E.L.; Weeks, D.A.; Bruland, K.W.; Hutchins, D.A. Spatial and Temporal Variability in Phytoplankton Iron Limitation along the California Coast and Consequences for Si, N, and C Biogeochemistry. Glob. Biogeochem. Cycles 2003, 17, 2001GB001824. [Google Scholar] [CrossRef]
- de Quiros, P.F. The Voyages of Pedro Fernadez de Quiros 1595 to 1606 (No. 14); Hakluyt Society: London, 1904. [Google Scholar]
- Koenigswald, W.V.; Braun, A.; Pfeiffer, T. Cyanobacteria and Seasonal Death: A New Taphonomic Model for the Eocene Messel Lake. Paläontol. Z. 2004, 78, 417–424. [Google Scholar] [CrossRef]
- Bargu, S.; Silver, M.W.; Ohman, M.D.; Benitez-Nelson, C.R.; Garrison, D.L. Mystery behind Hitchcock’s Birds. Nat. Geosci. 2012, 5, 2–3. [Google Scholar] [CrossRef]
- Smayda, T.J.; Villareal, T.A. The 1985 ‘Brown-Tide’ and the Open Phytoplankton Niche in Narragansett Bay During Summer. In Novel Phytoplankton Blooms; Cosper, E.M., Bricelj, V.M., Carpenter, E.J., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 1989; pp. 159–187. ISBN 978-3-642-75282-7. [Google Scholar]
- Hallegraeff, G.M. A Review of Harmful Algal Blooms and Their Apparent Global Increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L.; et al. Marine Harmful Algal Blooms (HABs) in the United States: History, Current Status and Future Trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef]
- Karlson, B.; Andersen, P.; Arneborg, L.; Cembella, A.; Eikrem, W.; John, U.; West, J.J.; Klemm, K.; Kobos, J.; Lehtinen, S.; et al. Harmful Algal Blooms and Their Effects in Coastal Seas of Northern Europe. Harmful Algae 2021, 102, 101989. [Google Scholar] [CrossRef]
- Yñiguez, A.T.; Lim, P.T.; Leaw, C.P.; Jipanin, S.J.; Iwataki, M.; Benico, G.; Azanza, R.V. Over 30 Years of HABs in the Philippines and Malaysia: What Have We Learned? Harmful Algae 2021, 102, 101776. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Yu, R.-C.; Richardson, A.J.; Sun, C.; Eriksen, R.; Kong, F.-Z.; Zhou, Z.-X.; Geng, H.-X.; Zhang, Q.-C.; Zhou, M.-J. Marked Shifts of Harmful Algal Blooms in the Bohai Sea Linked with Combined Impacts of Environmental Changes. Harmful Algae 2023, 121, 102370. [Google Scholar] [CrossRef]
- Paerl, H.W.; Justić, D. Primary Producers. In Treatise on Estuarine and Coastal Science; Elsevier, 2011; pp. 23–42 ISBN 978-0-08-087885-0.
- Herut, B.; Krom, M.D.; Pan, G.; Mortimer, R. Atmospheric Input of Nitrogen and Phosphorus to the Southeast Mediterranean: Sources, Fluxes, and Possible Impact. Limnol. Oceanogr. 1999, 44, 1683–1692. [Google Scholar] [CrossRef]
- Hamilton, D.P.; Salmaso, N.; Paerl, H.W. Mitigating Harmful Cyanobacterial Blooms: Strategies for Control of Nitrogen and Phosphorus Loads. Aquat. Ecol. 2016, 50, 351–366. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms Like It Hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Gobler, C.J.; Doherty, O.M.; Hattenrath-Lehmann, T.K.; Griffith, A.W.; Kang, Y.; Litaker, R.W. Ocean Warming since 1982 Has Expanded the Niche of Toxic Algal Blooms in the North Atlantic and North Pacific Oceans. Proc. Natl. Acad. Sci. 2017, 114, 4975–4980. [Google Scholar] [CrossRef] [PubMed]
- León-Muñoz, J.; Urbina, M.A.; Garreaud, R.; Iriarte, J.L. Hydroclimatic Conditions Trigger Record Harmful Algal Bloom in Western Patagonia (Summer 2016). Sci. Rep. 2018, 8, 1330. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Michalak, A.M. Exploring Temperature and Precipitation Impacts on Harmful Algal Blooms across Continental U.S. Lakes. Limnol. Oceanogr. 2020, 65, 992–1009. [Google Scholar] [CrossRef]
- Beardall, J.; Stojkovic, S.; Larsen, S. Living in a High CO 2 World: Impacts of Global Climate Change on Marine Phytoplankton. Plant Ecol. Divers. 2009, 2, 191–205. [Google Scholar] [CrossRef]
- Dutkiewicz, S.; Morris, J.J.; Follows, M.J.; Scott, J.; Levitan, O.; Dyhrman, S.T.; Berman-Frank, I. Impact of Ocean Acidification on the Structure of Future Phytoplankton Communities. Nat. Clim. Change 2015, 5, 1002–1006. [Google Scholar] [CrossRef]
- Flynn, K.J.; Clark, D.R.; Mitra, A.; Fabian, H.; Hansen, P.J.; Glibert, P.M.; Wheeler, G.L.; Stoecker, D.K.; Blackford, J.C.; Brownlee, C. Ocean Acidification with (de)Eutrophication Will Alter Future Phytoplankton Growth and Succession. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142604. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, A.H.V.; McManus, M.A.; Cheriton, O.M.; Cowen, R.K.; Greer, A.T.; Kudela, R.M.; Ruttenberg, K.; Sevadjian, J. Hidden Thin Layers of Toxic Diatoms in a Coastal Bay. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 101, 129–140. [Google Scholar] [CrossRef]
- Raine, R.; Berdalet, E.; Yamazaki, H.; Jenkinson, I.; Reguera, B. Key Questions and Recent Research Advances on Harmful Algal Blooms in Stratified Systems. In Global Ecology and Oceanography of Harmful Algal Blooms; Glibert, P.M., Berdalet, E., Burford, M.A., Pitcher, G.C., Zhou, M., Eds.; Ecological Studies; Springer International Publishing: Cham, 2018; Vol. 232, pp. 165–186. ISBN 978-3-319-70068-7. [Google Scholar]
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, J. World Agriculture: Towards 2015/2030 an FAO Perspective; Earthscan publications: London, 2003; ISBN 978-92-5-104835-1. [Google Scholar]
- FAO World Fertilizer Trends and Outlook to 2022; FAO: Rome, 2022.
- Glibert, P.M.; Harrison, J.; Heil, C.; Seitzinger, S. Escalating Worldwide Use of Urea – A Global Change Contributing to Coastal Eutrophication. Biogeochemistry 2006, 77, 441–463. [Google Scholar] [CrossRef]
- Yue, F.-J.; Li, S.-L.; Liu, C.-Q.; Zhao, Z.-Q.; Ding, H. Tracing Nitrate Sources with Dual Isotopes and Long Term Monitoring of Nitrogen Species in the Yellow River, China. Sci. Rep. 2017, 7, 8537. [Google Scholar] [CrossRef] [PubMed]
- Tamborski, J.; Brown, C.; Bokuniewicz, H.; Cochran, J.K.; Rasbury, E.T. Investigating Boron Isotopes for Identifying Nitrogen Sources Supplied by Submarine Groundwater Discharge to Coastal Waters. Front. Environ. Sci. 2020, 8, 126. [Google Scholar] [CrossRef]
- Sarma, V.V.S.S.; Krishna, M.S.; Srinivas, T.N.R. Sources of Organic Matter and Tracing of Nutrient Pollution in the Coastal Bay of Bengal. Mar. Pollut. Bull. 2020, 159, 111477. [Google Scholar] [CrossRef] [PubMed]
- Csathó, P.; Sisák, I.; Radimszky, L.; Lushaj, S.; Spiegel, H.; Nikolova, M.T.; Nikolov, N.; ČErmák, P.; Klir, J.; Astover, A.; et al. Agriculture as a Source of Phosphorus Causing Eutrophication in Central and Eastern Europe. Soil Use Manag. 2007, 23, 36–56. [Google Scholar] [CrossRef]
- Ishida, T.; Uehara, Y.; Iwata, T.; Cid-Andres, A.P.; Asano, S.; Ikeya, T.; Osaka, K.; Ide, J.; Privaldos, O.L.A.; Jesus, I.B.B.D.; et al. Identification of Phosphorus Sources in a Watershed Using a Phosphate Oxygen Isoscape Approach. Environ. Sci. Technol. 2019, 53, 4707–4716. [Google Scholar] [CrossRef]
- Oelsner, G.P.; Stets, E.G. Recent Trends in Nutrient and Sediment Loading to Coastal Areas of the Conterminous U.S.: Insights and Global Context. Sci. Total Environ. 2019, 654, 1225–1240. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, H.; Li, H.; Main, C.; Anton, J.; Jaisi, D.P. Tracing the Sources of Phosphorus along the Salinity Gradient in a Coastal Estuary Using Multi-Isotope Proxies. Sci. Total Environ. 2021, 792, 148353. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S. Review: Feed Demand Landscape and Implications of Food-Not Feed Strategy for Food Security and Climate Change. Animal 2018, 12, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Mottet, A.; De Haan, C.; Falcucci, A.; Tempio, G.; Opio, C.; Gerber, P. Livestock: On Our Plates or Eating at Our Table? A New Analysis of the Feed/Food Debate. Glob. Food Secur. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- FAO Food and Agriculture Data Statistics (FAOSTAT); 2022;
- FAO The State of World Fisheries and Aquaculture: Sustainability in Action; 2020;
- Alonso-Rodrı́guez, R.; Páez-Osuna, F. Nutrients, Phytoplankton and Harmful Algal Blooms in Shrimp Ponds: A Review with Special Reference to the Situation in the Gulf of California. Aquaculture 2003, 219, 317–336. [Google Scholar] [CrossRef]
- San Diego-McGlone, M.L.; Azanza, R.V.; Villanoy, C.L.; Jacinto, G.S. Eutrophic Waters, Algal Bloom and Fish Kill in Fish Farming Areas in Bolinao, Pangasinan, Philippines. Mar. Pollut. Bull. 2008, 57, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Yodsuwan, N.; Sawayama, S.; Sirisansaneeyakul, S. Effect of Nitrogen Concentration on Growth, Lipid Production and Fatty Acid Profiles of the Marine Diatom Phaeodactylum Tricornutum. Agric. Nat. Resour. 2017, 51, 190–197. [Google Scholar] [CrossRef]
- Reich, H.G.; Rodriguez, I.B.; LaJeunesse, T.C.; Ho, T.-Y. Endosymbiotic Dinoflagellates Pump Iron: Differences in Iron and Other Trace Metal Needs among the Symbiodiniaceae. Coral Reefs 2020, 39, 915–927. [Google Scholar] [CrossRef]
- Baek, S.H.; Shimode, S.; Han, M.-S.; Kikuchi, T. Growth of Dinoflagellates, Ceratium Furca and Ceratium Fusus in Sagami Bay, Japan: The Role of Nutrients. Harmful Algae 2008, 7, 729–739. [Google Scholar] [CrossRef]
- Longo, S.; Sibat, M.; Darius, H.T.; Hess, P.; Chinain, M. Effects of pH and Nutrients (Nitrogen) on Growth and Toxin Profile of the Ciguatera-Causing Dinoflagellate Gambierdiscus Polynesiensis (Dinophyceae). Toxins 2020, 12, 767. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful Algal Blooms and Eutrophication: Nutrient Sources, Composition, and Consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Lagus, A. Species-Specific Differences in Phytoplankton Responses to N and P Enrichments and the N:P Ratio in the Archipelago Sea, Northern Baltic Sea. J. Plankton Res. 2004, 26, 779–798. [Google Scholar] [CrossRef]
- Li, Q.; Legendre, L.; Jiao, N. Phytoplankton Responses to Nitrogen and Iron Limitation in the Tropical and Subtropical Pacific Ocean. J. Plankton Res. 2015, 37, 306–319. [Google Scholar] [CrossRef]
- Paerl, H.W.; Scott, J.T. Throwing Fuel on the Fire: Synergistic Effects of Excessive Nitrogen Inputs and Global Warming on Harmful Algal Blooms. Environ. Sci. Technol. 2010, 44, 7756–7758. [Google Scholar] [CrossRef] [PubMed]
- Moutin, T.; Karl, D.M.; Duhamel, S.; Rimmelin, P.; Raimbault, P.; Van Mooy, B.A.S.; Claustre, H. Phosphate Availability and the Ultimate Control of New Nitrogen Input by Nitrogen Fixation in the Tropical Pacific Ocean. Biogeosciences 2008, 5, 95–109. [Google Scholar] [CrossRef]
- Wu, H.; Lin, L.; Shen, G.; Li, M. Heavy-Metal Pollution Alters Dissolved Organic Matter Released by Bloom-Forming Microcystis Aeruginosa. RSC Adv. 2017, 7, 18421–18427. [Google Scholar] [CrossRef]
- Moore, C.M.; Mills, M.M.; Langlois, R.; Milne, A.; Achterberg, E.P.; La Roche, J.; Geider, R.J. Relative Influence of Nitrogen and Phosphorous Availability on Phytoplankton Physiology and Productivity in the Oligotrophic Sub-tropical North Atlantic Ocean. Limnol. Oceanogr. 2008, 53, 291–305. [Google Scholar] [CrossRef]
- Piehler, M.F.; Twomey, L.J.; Hall, N.S.; Paerl, H.W. Impacts of Inorganic Nutrient Enrichment on Phytoplankton Community Structure and Function in Pamlico Sound, NC, USA. Estuar. Coast. Shelf Sci. 2004, 61, 197–209. [Google Scholar] [CrossRef]
- Burson, A.; Stomp, M.; Akil, L.; Brussaard, C.P.D.; Huisman, J. Unbalanced Reduction of Nutrient Loads Has Created an Offshore Gradient from Phosphorus to Nitrogen Limitation in the N Orth S Ea. Limnol. Oceanogr. 2016, 61, 869–888. [Google Scholar] [CrossRef]
- Song, Y.; Guo, Y.; Liu, H.; Zhang, G.; Zhang, X.; Thangaraj, S.; Sun, J. Water Quality Shifts the Dominant Phytoplankton Group from Diatoms to Dinoflagellates in the Coastal Ecosystem of the Bohai Bay. Mar. Pollut. Bull. 2022, 183, 114078. [Google Scholar] [CrossRef]
- Hernández-Sandoval, F.E.; Bustillos-Guzmán, J.J.; Band-Schmidt, C.J.; Núñez-Vázquez, E.J.; López-Cortés, D.J.; Fernández-Herrera, L.J.; Poot-Delgado, C.A.; Moreno-Legorreta, M. Effect of Different N:P Ratios on the Growth, Toxicity, and Toxin Profile of Gymnodinium Catenatum (Dinophyceae) Strains from the Gulf of California. Toxins 2022, 14, 501. [Google Scholar] [CrossRef]
- Leong, S.C.Y.; Murata, A.; Nagashima, Y.; Taguchi, S. Variability in Toxicity of the Dinoflagellate Alexandrium Tamarense in Response to Different Nitrogen Sources and Concentrations. Toxicon 2004, 43, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Hattenrath, T.K.; Anderson, D.M.; Gobler, C.J. The Influence of Anthropogenic Nitrogen Loading and Meteorological Conditions on the Dynamics and Toxicity of Alexandrium Fundyense Blooms in a New York (USA) Estuary. Harmful Algae 2010, 9, 402–412. [Google Scholar] [CrossRef]
- Hattenrath-Lehmann, T.K.; Marcoval, M.A.; Mittlesdorf, H.; Goleski, J.A.; Wang, Z.; Haynes, B.; Morton, S.L.; Gobler, C.J. Nitrogenous Nutrients Promote the Growth and Toxicity of Dinophysis Acuminata during Estuarine Bloom Events. PLOS ONE 2015, 10, e0124148. [Google Scholar] [CrossRef] [PubMed]
- 7Zhuang, Y.; Zhang, H.; Hannick, L.; Lin, S. Metatranscriptome Profiling Reveals Versatile N-Nutrient Utilization, CO2 Limitation, Oxidative Stress, and Active Toxin Production in an Alexandrium Fundyense Bloom. Harmful Algae 2015, 42, 60–70. [Google Scholar] [CrossRef]
- Barnard, M.A.; Chaffin, J.D.; Plaas, H.E.; Boyer, G.L.; Wei, B.; Wilhelm, S.W.; Rossignol, K.L.; Braddy, J.S.; Bullerjahn, G.S.; Bridgeman, T.B.; et al. Roles of Nutrient Limitation on Western Lake Erie CyanoHAB Toxin Production. Toxins 2021, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.D.; Quach, E.; Buscho, S.; Ricciardelli, A.; Kannan, A.; Naung, S.W.; Phillip, G.; Sheppard, B.; Ferguson, L.; Allen, A.; et al. Nitrogen Form, Concentration, and Micronutrient Availability Affect Microcystin Production in Cyanobacterial Blooms. Harmful Algae 2021, 103, 102002. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M.; Adams, K.W. The Cell: A Molecular Approach; Ninth edition.; Oxford University Press: New York, NY, 2023; ISBN 978-0-19-758374-6. [Google Scholar]
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis; 2nd ed.; Princeton University Press: Princeton, 2007; ISBN 978-0-691-11550-4.
- Feng, T.-Y.; Yang, Z.-K.; Zheng, J.-W.; Xie, Y.; Li, D.-W.; Murugan, S.B.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Examination of Metabolic Responses to Phosphorus Limitation via Proteomic Analyses in the Marine Diatom Phaeodactylum Tricornutum. Sci. Rep. 2015, 5, 10373. [Google Scholar] [CrossRef]
- Ding, S.; Chen, M.; Gong, M.; Fan, X.; Qin, B.; Xu, H.; Gao, S.; Jin, Z.; Tsang, D.C.W.; Zhang, C. Internal Phosphorus Loading from Sediments Causes Seasonal Nitrogen Limitation for Harmful Algal Blooms. Sci. Total Environ. 2018, 625, 872–884. [Google Scholar] [CrossRef]
- Lin, S.; Litaker, R.W.; Sunda, W.G. Phosphorus Physiological Ecology and Molecular Mechanisms in Marine Phytoplankton. J. Phycol. 2016, 52, 10–36. [Google Scholar] [CrossRef]
- Paerl, H.W.; Scott, J.T.; McCarthy, M.J.; Newell, S.E.; Gardner, W.S.; Havens, K.E.; Hoffman, D.K.; Wilhelm, S.W.; Wurtsbaugh, W.A. It Takes Two to Tango: When and Where Dual Nutrient (N & P) Reductions Are Needed to Protect Lakes and Downstream Ecosystems. Environ. Sci. Technol. 2016, 50, 10805–10813. [Google Scholar] [CrossRef]
- Simpson, T.L.; Volcani, B.E. Silicon and Siliceous Structures in Biological Systems; Springer New York: New York, NY, 1981; ISBN 978-1-4612-5944-2. [Google Scholar]
- Martin-Jézéquel, V.; Hildebrand, M.; Brzezinski, M.A. SILICON METABOLISM IN DIATOMS: IMPLICATIONS FOR GROWTH. J. Phycol. 2000, 36, 821–840. [Google Scholar] [CrossRef]
- Yool, A.; Tyrrell, T. Role of Diatoms in Regulating the Ocean’s Silicon Cycle. Glob. Biogeochem. Cycles 2003, 17, 2002GB002018. [Google Scholar] [CrossRef]
- Ragueneau, O.; Schultes, S.; Bidle, K.; Claquin, P.; Moriceau, B. Si and C Interactions in the World Ocean: Importance of Ecological Processes and Implications for the Role of Diatoms in the Biological Pump. Glob. Biogeochem. Cycles 2006, 20, 2006GB002688. [Google Scholar] [CrossRef]
- Tréguer, P.J.; De La Rocha, C.L. The World Ocean Silica Cycle. Annu. Rev. Mar. Sci. 2013, 5, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Litchman, E. Resource Competition and the Ecological Success of Phytoplankton. In Evolution of Primary Producers in the Sea; Elsevier, 2007; pp. 351–375 ISBN 978-0-12-370518-1.
- Redfield, A. On the Proportions of Organic Derivatives in Sea Water and Their Relation to Their Planktonic Composition. In James Johnstone Memorial Volume; Woods Hole Oceanographic Institution: Liverpool, 1934; pp. 176–192. [Google Scholar]
- Takahashi, T.; Broecker, W.S.; Langer, S. Redfield Ratio Based on Chemical Data from Isopycnal Surfaces. J. Geophys. Res. Oceans 1985, 90, 6907–6924. [Google Scholar] [CrossRef]
- Geider, R.J.; Roche, J.L. Redfield Revisited: Variability of C[Ratio ]N[Ratio ]P in Marine Microalgae and Its Biochemical Basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- Goldman, J. On Phytoplankton Growth Rates and Particulate C: N: P Ratios at Low Light1. Limnol. Oceanogr. 1986. [Google Scholar] [CrossRef]
- Price, N.M. The Elemental Stoichiometry and Composition of an Iron-Limited Diatom. Limnol. Oceanogr. 2005, 50, 1159–1171. [Google Scholar] [CrossRef]
- Morel, F.; Rueter, J.; Price, N. Iron Nutrition of Phytoplankton and Its Possible Importance in the Ecology of Ocean Regions with High Nutrient and Low Biomass. Oceanography 1991, 4, 56–61. [Google Scholar] [CrossRef]
- Ho, T.; Quigg, A.; Finkel, Z.V.; Milligan, A.J.; Wyman, K.; Falkowski, P.G.; Morel, F.M.M. THE ELEMENTAL COMPOSITION OF SOME MARINE PHYTOPLANKTON 1. J. Phycol. 2003, 39, 1145–1159. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Milligan, A.J.; Saito, M.A. 6.05 Marine Bioinorganic Chemistry: The Role of Trace Metals in the Oceanic Cycles of Major Nutrients. 2003.
- Sunda, W.G. Feedback Interactions between Trace Metal Nutrients and Phytoplankton in the Ocean. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Twining, B.S.; Baines, S.B. The Trace Metal Composition of Marine Phytoplankton. Annu. Rev. Mar. Sci. 2013, 5, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Penta, B.; Dieterle, D.A.; Bissett, W.P. Predictive Ecological Modeling of Harmful Algal Blooms. Hum. Ecol. Risk Assess. Int. J. 2001, 7, 1369–1383. [Google Scholar] [CrossRef]
- Villanoy, C.L.; Azanza, R.V.; Altemerano, A.; Casil, A.L. Attempts to Model the Bloom Dynamics of Pyrodinium, a Tropical Toxic Dinoflagellate. Harmful Algae 2006, 5, 156–183. [Google Scholar] [CrossRef]
- Hill, P.R.; Kumar, A.; Temimi, M.; Bull, D.R. HABNet: Machine Learning, Remote Sensing-Based Detection of Harmful Algal Blooms. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 3229–3239. [Google Scholar] [CrossRef]
- Yñiguez, A.T.; Ottong, Z.J. Predicting Fish Kills and Toxic Blooms in an Intensive Mariculture Site in the Philippines Using a Machine Learning Model. Sci. Total Environ. 2020, 707, 136–173. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Bao, M.; Lou, X.; Zhou, Z.; Yin, K. Monitoring, Modeling and Projection of Harmful Algal Blooms in China. Harmful Algae 2022, 111, 102164. [Google Scholar] [CrossRef] [PubMed]
- Bertani, I.; Obenour, D.R.; Steger, C.E.; Stow, C.A.; Gronewold, A.D.; Scavia, D. Probabilistically Assessing the Role of Nutrient Loading in Harmful Algal Bloom Formation in Western Lake Erie. J. Gt. Lakes Res. 2016, 42, 1184–1192. [Google Scholar] [CrossRef]
- Ai, H.; Zhang, K.; Sun, J.; Zhang, H. Short-Term Lake Erie Algal Bloom Prediction by Classification and Regression Models. Water Res. 2023, 232, 119710. [Google Scholar] [CrossRef]
- Iiames, J.S.; Salls, W.B.; Mehaffey, M.H.; Nash, M.S.; Christensen, J.R.; Schaeffer, B.A. Modeling Anthropogenic and Environmental Influences on Freshwater Harmful Algal Bloom Development Detected by MERIS Over the Central United States. Water Resour. Res. 2021, 57, e2020WR028946. [Google Scholar] [CrossRef]
- Wang, H.; Bouwman, A.F.; Van Gils, J.; Vilmin, L.; Beusen, A.H.W.; Wang, J.; Liu, X.; Yu, Z.; Ran, X. Hindcasting Harmful Algal Bloom Risk Due to Land-Based Nutrient Pollution in the Eastern Chinese Coastal Seas. Water Res. 2023, 231, 119669. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Chen, J.; Sun, X.; Li, D.; Liu, C.; Weng, H. Algae Explosive Growth Mechanism Enabling Weather-like Forecast of Harmful Algal Blooms. Sci. Rep. 2018, 8, 9923. [Google Scholar] [CrossRef] [PubMed]
- Bruland, K.W.; Franks, R.P. Mn, Ni, Cu, Zn and Cd in the Western North Atlantic. In Trace Metals in Sea Water; Wong, C.S., Boyle, E., Bruland, K.W., Burton, J.D., Goldberg, E.D., Eds.; Springer US: Boston, MA, 1983; pp. 395–414. ISBN 978-1-4757-6866-4. [Google Scholar]
- Whitfield, M. Interactions between Phytoplankton and Trace Metals in the Ocean. In Advances in Marine Biology; Elsevier, 2001; Vol. 41, pp. 1–128 ISBN 978-0-12-026141-3.
- John, S.G.; Sunda, W.G. Trace Metal Nutrients. In Encyclopedia of Ocean Sciences; Elsevier, 2019; pp. 208–217 ISBN 978-0-12-813082-7.
- Blain, S.; Quéguiner, B.; Armand, L.; Belviso, S.; Bombled, B.; Bopp, L.; Bowie, A.; Brunet, C.; Brussaard, C.; Carlotti, F.; et al. Effect of Natural Iron Fertilization on Carbon Sequestration in the Southern Ocean. Nature 2007, 446, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Pollard, R.; Sanders, R.; Lucas, M.; Statham, P. The Crozet Natural Iron Bloom and Export Experiment (CROZEX). Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1905–1914. [Google Scholar] [CrossRef]
- Jacques, D.; Šimůnek, J.; Mallants, D.; Van Genuchten, M.Th. Modelling Coupled Water Flow, Solute Transport and Geochemical Reactions Affecting Heavy Metal Migration in a Podzol Soil. Geoderma 2008, 145, 449–461. [Google Scholar] [CrossRef]
- Moore, J.K.; Braucher, O. Sedimentary and Mineral Dust Sources of Dissolved Iron to the World Ocean. Biogeosciences 2008, 5, 631–656. [Google Scholar] [CrossRef]
- Elrod, V.A.; Berelson, W.M.; Coale, K.H.; Johnson, K.S. The Flux of Iron from Continental Shelf Sediments: A Missing Source for Global Budgets. Geophys. Res. Lett. 2004, 31, 2004GL020216. [Google Scholar] [CrossRef]
- Chase, Z.; Johnson, K.S.; Elrod, V.A.; Plant, J.N.; Fitzwater, S.E.; Pickell, L.; Sakamoto, C.M. Manganese and Iron Distributions off Central California Influenced by Upwelling and Shelf Width. Mar. Chem. 2005, 95, 235–254. [Google Scholar] [CrossRef]
- Lohan, M.C.; Bruland, K.W. Elevated Fe(II) and Dissolved Fe in Hypoxic Shelf Waters off Oregon and Washington: An Enhanced Source of Iron to Coastal Upwelling Regimes. Environ. Sci. Technol. 2008, 42, 6462–6468. [Google Scholar] [CrossRef]
- Von Damm, K.L.; Edmond, J.M.; Grant, B.; Measures, C.I.; Walden, B.; Weiss, R.F. Chemistry of Submarine Hydrothermal Solutions at 21 °N, East Pacific Rise. Geochim. Cosmochim. Acta 1985, 49, 2197–2220. [Google Scholar] [CrossRef]
- Fisher, A.T.; Davis, E.E.; Hutnak, M.; Spiess, V.; Zühlsdorff, L.; Cherkaoui, A.; Christiansen, L.; Edwards, K.; Macdonald, R.; Villinger, H.; et al. Hydrothermal Recharge and Discharge across 50 Km Guided by Seamounts on a Young Ridge Flank. Nature 2003, 421, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Middag, R.; De Baar, H.J.W.; Laan, P.; Huhn, O. The Effects of Continental Margins and Water Mass Circulation on the Distribution of Dissolved Aluminum and Manganese in Drake Passage. J. Geophys. Res. Oceans 2012, 117, 2011JC007434. [Google Scholar] [CrossRef]
- Shaw, T.J.; Smith, K.L.; Hexel, C.R.; Dudgeon, R.; Sherman, A.D.; Vernet, M.; Kaufmann, R.S. 234Th-Based Carbon Export around Free-Drifting Icebergs in the Southern Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 1384–1391. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Kang, S.; Sillanpää, M.; Ding, Y.; Han, T.; Wu, Q.; Yu, Z.; Qin, D. Diurnal Dynamics of Minor and Trace Elements in Stream Water Draining Dongkemadi Glacier on the Tibetan Plateau and Its Environmental Implications. J. Hydrol. 2016, 541, 1104–1118. [Google Scholar] [CrossRef]
- Krause, J.; Hopwood, M.J.; Höfer, J.; Krisch, S.; Achterberg, E.P.; Alarcón, E.; Carroll, D.; González, H.E.; Juul-Pedersen, T.; Liu, T.; et al. Trace Element (Fe, Co, Ni and Cu) Dynamics Across the Salinity Gradient in Arctic and Antarctic Glacier Fjords. Front. Earth Sci. 2021, 9, 725279. [Google Scholar] [CrossRef]
- Mahowald, N.; Kohfeld, K.; Hansson, M.; Balkanski, Y.; Harrison, S.P.; Prentice, I.C.; Schulz, M.; Rodhe, H. Dust Sources and Deposition during the Last Glacial Maximum and Current Climate: A Comparison of Model Results with Paleodata from Ice Cores and Marine Sediments. J. Geophys. Res. Atmospheres 1999, 104, 15895–15916. [Google Scholar] [CrossRef]
- 1Chase, Z.; Paytan, A.; Beck, A.; Biller, D.; Bruland, K.; Measures, C.; Sañudo-Wilhelmy, S. Evaluating the Impact of Atmospheric Deposition on Dissolved Trace-Metals in the Gulf of Aqaba, Red Sea. Mar. Chem. 2011, 126, 256–268. [Google Scholar] [CrossRef]
- Liao, W.; Ho, T. Particulate Trace Metal Composition and Sources in the Kuroshio Adjacent to the East China Sea: The Importance of Aerosol Deposition. J. Geophys. Res. Oceans 2018, 123, 6207–6223. [Google Scholar] [CrossRef]
- Millward, G.E.; Morris, A.W.; Tappin, A.D. Trace Metals at Two Sites in the Southern North Sea: Results from a Sediment Resuspension Study. Cont. Shelf Res. 1998, 18, 1381–1400. [Google Scholar] [CrossRef]
- Berger, C.J.M.; Lippiatt, S.M.; Lawrence, M.G.; Bruland, K.W. Application of a Chemical Leach Technique for Estimating Labile Particulate Aluminum, Iron, and Manganese in the Columbia River Plume and Coastal Waters off Oregon and Washington. J. Geophys. Res. Oceans 2008, 113, 2007JC004703. [Google Scholar] [CrossRef]
- Li, T.; Sun, G.; Yang, C.; Liang, K.; Ma, S.; Huang, L.; Luo, W. Source Apportionment and Source-to-Sink Transport of Major and Trace Elements in Coastal Sediments: Combining Positive Matrix Factorization and Sediment Trend Analysis. Sci. Total Environ. 2019, 651, 344–356. [Google Scholar] [CrossRef]
- Sparaventi, E.; Rodríguez-Romero, A.; Barbosa, A.; Ramajo, L.; Tovar-Sánchez, A. Trace Elements in Antarctic Penguins and the Potential Role of Guano as Source of Recycled Metals in the Southern Ocean. Chemosphere 2021, 285, 131423. [Google Scholar] [CrossRef] [PubMed]
- Alba-González, P.; Álvarez-Salgado, X.A.; Cobelo-García, A.; Kaal, J.; Teira, E. Faeces of Marine Birds and Mammals as Substrates for Microbial Plankton Communities. Mar. Environ. Res. 2022, 174, 105560. [Google Scholar] [CrossRef] [PubMed]
- De La Peña-Lastra, S.; Pérez-Alberti, A.; Ferreira, T.O.; Huerta-Díaz, M.Á.; Otero, X.L. Global Deposition of Potentially Toxic Metals via Faecal Material in Seabird Colonies. Sci. Rep. 2022, 12, 22392. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.; Suzuki, Y.; Miyake, Y. The Content of Selenium and Its Chemical Form in Sea Water. J. Oceanogr. 1976, 32, 235–241. [Google Scholar] [CrossRef]
- Duan, L.; Song, J.; Li, X.; Yuan, H.; Xu, S. Distribution of Selenium and Its Relationship to the Eco-Environment in Bohai Bay Seawater. Mar. Chem. 2010, 121, 87–99. [Google Scholar] [CrossRef]
- Nozaki, Y. Rare Earth Elements and Their Isotopes in the Ocean. In Encyclopedia of Ocean Sciences; Steele, J.H., Ed.; Academic Press: Oxford, 2001; pp. 2354–2366. ISBN 978-0-12-227430-5. [Google Scholar]
- Moffett, J.W.; Ho, J. Oxidation of Cobalt and Manganese in Seawater via a Common Microbially Catalyzed Pathway. Geochim. Cosmochim. Acta 1996, 60, 3415–3424. [Google Scholar] [CrossRef]
- Saito, M.A.; Moffett, J.W. Complexation of Cobalt by Natural Organic Ligands in the Sargasso Sea as Determined by a New High-Sensitivity Electrochemical Cobalt Speciation Method Suitable for Open Ocean Work. Mar. Chem. 2001, 75, 49–68. [Google Scholar] [CrossRef]
- Noble, A.E.; Ohnemus, D.C.; Hawco, N.J.; Lam, P.J.; Saito, M.A. Coastal Sources, Sinks and Strong Organic Complexation of Dissolved Cobalt within the US North Atlantic GEOTRACES Transect GA03. Biogeosciences 2017, 14, 2715–2739. [Google Scholar] [CrossRef]
- Landing, W.M.; Bruland, K.W. The Contrasting Biogeochemistry of Iron and Manganese in the Pacific Ocean. Geochim. Cosmochim. Acta 1987, 51, 29–43. [Google Scholar] [CrossRef]
- Ho, P.; Lee, J.-M.; Heller, M.I.; Lam, P.J.; Shiller, A.M. The Distribution of Dissolved and Particulate Mo and V along the U.S. GEOTRACES East Pacific Zonal Transect (GP16): The Roles of Oxides and Biogenic Particles in Their Distributions in the Oxygen Deficient Zone and the Hydrothermal Plume. Mar. Chem. 2018, 201, 242–255. [Google Scholar] [CrossRef]
- Rickli, J.; Janssen, D.J.; Hassler, C.; Ellwood, M.J.; Jaccard, S.L. Chromium Biogeochemistry and Stable Isotope Distribution in the Southern Ocean. Geochim. Cosmochim. Acta 2019, 262, 188–206. [Google Scholar] [CrossRef]
- Chmiel, R.; Lanning, N.; Laubach, A.; Lee, J.-M.; Fitzsimmons, J.; Hatta, M.; Jenkins, W.; Lam, P.; McIlvin, M.; Tagliabue, A.; et al. Major Processes of the Dissolved Cobalt Cycle in the North and Equatorial Pacific Ocean. Biogeosciences 2022, 19, 2365–2395. [Google Scholar] [CrossRef]
- Wang, W.; Goring-Harford, H.; Kunde, K.; Woodward, E.M.S.; Lohan, M.C.; Connelly, D.P.; James, R.H. Biogeochemical Cycling of Chromium and Chromium Isotopes in the Sub-Tropical North Atlantic Ocean. Front. Mar. Sci. 2023, 10, 1165304. [Google Scholar] [CrossRef]
- Collier, R.W. Molybdenum in the Northeast Pacific Ocean1. Limnol. Oceanogr. 1985, 30, 1351–1354. [Google Scholar] [CrossRef]
- Fan, J.; Duan, L.; Yin, M.; Yuan, H.; Li, X. Nonconservative Behavior of Dissolved Molybdenum and Its Potential Role in Nitrogen Cycling in the Bohai and Yellow Seas. Front. Mar. Sci. 2022, 9, 1094846. [Google Scholar] [CrossRef]
- Whitmore, L.M.; Morton, P.L.; Twining, B.S.; Shiller, A.M. Vanadium Cycling in the Western Arctic Ocean Is Influenced by Shelf-Basin Connectivity. Mar. Chem. 2019, 216, 103701. [Google Scholar] [CrossRef]
- Bormans, M. Spatial and Temporal Variability in Cyanobacterial Populations Controlled by Physical Processes. J. Plankton Res. 2004, 27, 61–70. [Google Scholar] [CrossRef]
- Molot, L.A.; Watson, S.B.; Creed, I.F.; Trick, C.G.; McCabe, S.K.; Verschoor, M.J.; Sorichetti, R.J.; Powe, C.; Venkiteswaran, J.J.; Schiff, S.L. A Novel Model for Cyanobacteria Bloom Formation: The Critical Role of Anoxia and Ferrous Iron. Freshw. Biol. 2014, 59, 1323–1340. [Google Scholar] [CrossRef]
- Müller, S.; Mitrovic, S.M.; Baldwin, D.S. Oxygen and Dissolved Organic Carbon Control Release of N, P and Fe from the Sediments of a Shallow, Polymictic Lake. J. Soils Sediments 2016, 16, 1109–1120. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling Harmful Cyanobacterial Blooms in a World Experiencing Anthropogenic and Climatic-Induced Change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Facey, J.A.; Apte, S.C.; Mitrovic, S.M. A Review of the Effect of Trace Metals on Freshwater Cyanobacterial Growth and Toxin Production. Toxins 2019, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Luoma, S.N.; Van Geen, A.; Lee, B.-G.; Cloern, J.E. Metal Uptake by Phytoplankton during a Bloom in South San Francisco Bay: Implications for Metal Cycling in Estuaries. Limnol. Oceanogr. 1998, 43, 1007–1016. [Google Scholar] [CrossRef]
- Blais, JulesM.; Kalff, J. Atmospheric Loading of Zn, Cu, Ni, Cr, and Pb to Lake Sediments: The Role of Catchment, Lake Morphometry, and Physico-Chemical Properties of the Elements. Biogeochemistry 1993, 23. [Google Scholar] [CrossRef]
- Giles, C.D.; Isles, P.D.F.; Manley, T.; Xu, Y.; Druschel, G.K.; Schroth, A.W. The Mobility of Phosphorus, Iron, and Manganese through the Sediment–Water Continuum of a Shallow Eutrophic Freshwater Lake under Stratified and Mixed Water-Column Conditions. Biogeochemistry 2016, 127, 15–34. [Google Scholar] [CrossRef]
- Saleem, M.; Iqbal, J.; Shah, M.H. Seasonal Variations, Risk Assessment and Multivariate Analysis of Trace Metals in the Freshwater Reservoirs of Pakistan. Chemosphere 2019, 216, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, Q.; Tian, X.; Zhu, X.; Dong, X.; Wu, Z.; Yuan, Y. Spatiotemporal Variation and Ecological Risk Assessment of Sediment Heavy Metals in Two Hydrologically Connected Lakes. Front. Ecol. Evol. 2022, 10, 1005194. [Google Scholar] [CrossRef]
- Sunda, W.G. Trace Metal/Phytoplankton Interactions in the Sea. In Chemistry of Aquatic Systems: Local and Global Perspectives; Bidoglio, G., Stumm, W., Eds.; Springer Netherlands: Dordrecht, 1994; ISBN 978-90-481-4410-5. [Google Scholar]
- Anderson, M.A.; Morel, F.M.M. The Influence of Aqueous Iron Chemistry on the Uptake of Iron by the Coastal Diatom Thalassiosira Weissflogii1. Limnol. Oceanogr. 1982, 27, 789–813. [Google Scholar] [CrossRef]
- Sunda, W.G. Trace Element Nutrients. In Encyclopedia of Ocean Sciences; Elsevier, 2001; pp. 75–86 ISBN 978-0-12-374473-9.
- Maldonado, M.T.; Strzepek, R.F.; Sander, S.; Boyd, P.W. Acquisition of Iron Bound to Strong Organic Complexes, with Different Fe Binding Groups and Photochemical Reactivities, by Plankton Communities in Fe-Limited Subantarctic Waters. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Sunda, W.G. Trace Metals and Harmful Algal Blooms. In Ecology of Harmful Algae; Granéli, E., Turner, J.T., Eds.; Ecological Studies; Springer Berlin Heidelberg, 2006; Vol. 189, pp. 203–214 ISBN 978-3-540-32209-2.
- Wever, R.; De Boer, E.; Plat, H.; E. Krenn, B. Vanadium — an Element Involved in the Biosynthesis of Halogenated Compounds and Nitrogen Fixation. FEBS Lett. 1987, 216, 1–3. [Google Scholar] [CrossRef]
- Kentemich, T.; Danneberg, G.; Hundeshagen, B.; Bothe, H. Evidence for the Occurrence of the Alternative, Vanadium-Containing Nitrogenase in the Cyanobacterium Anabaena Variabilis. FEMS Microbiol. Lett. 1988, 51, 19–24. [Google Scholar] [CrossRef]
- Price, N.M.; Morel, F.M.M. Cadmium and Cobalt Substitution for Zinc in a Marine Diatom. Nature 1990, 344, 658–660. [Google Scholar] [CrossRef]
- Raven, J.A. Predictions of Mn and Fe Use Efficiencies of Phototrophic Growth as a Function of Light Availability for Growth and of C Assimilation Pathway. New Phytol. 1990, 116, 1–18. [Google Scholar] [CrossRef]
- Raven, J.A.; Evans, M.C.W.; Korb, R.E. The Role of Trace Metals in Photosynthetic Electron Transport in O2-Evolving Organisms. Photosynth. Res. 1999, 60, 111–150. [Google Scholar] [CrossRef]
- Lane, T.W.; Morel, F.M.M. A Biological Function for Cadmium in Marine Diatoms. Proc. Natl. Acad. Sci. 2000, 97, 4627–4631. [Google Scholar] [CrossRef] [PubMed]
- Wolfe-Simon, F.; Grzebyk, D.; Schofield, O.; Falkowski, P.G. THE ROLE AND EVOLUTION OF SUPEROXIDE DISMUTASES IN ALGAE 1. J. Phycol. 2005, 41, 453–465. [Google Scholar] [CrossRef]
- Ji, Y.; Sherrell, R.M. Differential Effects of Phosphorus Limitation on Cellular Metals in Chlorella and Microcystis. Limnol. Oceanogr. 2008, 53, 1790–1804. [Google Scholar] [CrossRef]
- Rue, E.L.; Bruland, K.W. Complexation of Iron(II1) by Natural Organic Ligands in the Central North Pacific as Determined by a New Competitive Ligand Equilibration/Adsorptive Cathodic Stripping Voltammetric Method. Mar. Chem. 1995, 50, 117–138. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Lam, P.J.; Saito, M.A. Trace Metal Substitution in Marine Phytoplankton. Annu. Rev. Earth Planet. Sci. 2020, 48, 491–517. [Google Scholar] [CrossRef]
- Rachlin, J.W.; Jensen, T.E.; Warkentine, B. The Growth Response of the Diatom Navicula Incerta to Selected Concentrations of the Metals: Cadmium, Copper, Lead and Zinc. Bull. Torrey Bot. Club 1983, 110, 217. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Reinfelder, J.R.; Roberts, S.B.; Chamberlain, C.P.; Lee, J.G.; Yee, D. Zinc and Carbon Co-Limitation of Marine Phytoplankton. Nature 1994, 369, 740–742. [Google Scholar] [CrossRef]
- Rue, E.; Bruland, K. Domoic Acid Binds Iron and Copper: A Possible Role for the Toxin Produced by the Marine Diatom Pseudo-Nitzschia. Mar. Chem. 2001, 76, 127–134. [Google Scholar] [CrossRef]
- Maldonado, M.T.; Price, N.M. REDUCTION AND TRANSPORT OF ORGANICALLY BOUND IRON BY THALASSIOSIRA OCEANICA (BACILLARIOPHYCEAE). J. Phycol. 2001, 37, 298–310. [Google Scholar] [CrossRef]
- Leblanc, K.; Hare, C.E.; Boyd, P.W.; Bruland, K.W.; Sohst, B.; Pickmere, S.; Lohan, M.C.; Buck, K.; Ellwood, M.; Hutchins, D.A. Fe and Zn Effects on the Si Cycle and Diatom Community Structure in Two Contrasting High and Low-Silicate HNLC Areas. Deep Sea Res. Part Oceanogr. Res. Pap. 2005, 52, 1842–1864. [Google Scholar] [CrossRef]
- Iwade, S.; Kuma, K.; Isoda, Y.; Yoshida, M.; Kudo, I.; Nishioka, J.; Suzuki, K. Effect of High Iron Concentrations on Iron Uptake and Growth of a Coastal Diatom Chaetoceros Sociale. Aquat. Microb. Ecol. 2006, 43, 177–191. [Google Scholar] [CrossRef]
- Guo, J.; Annett, A.L.; Taylor, R.L.; Lapi, S.; Ruth, T.J.; Maldonado, M.T. COPPER-UPTAKE KINETICS OF COASTAL AND OCEANIC DIATOMS 1. J. Phycol. 2010, 46, 1218–1228. [Google Scholar] [CrossRef]
- Varela, D.E.; Willers, V.; Crawford, D.W. EFFECT OF ZINC AVAILABILITY ON GROWTH, MORPHOLOGY, AND NUTRIENT INCORPORATION IN A COASTAL AND AN OCEANIC DIATOM1: ZINC EFFECTS ON MARINE DIATOMS. J. Phycol. 2011, 47, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Twining, B.S.; Baines, S.B.; Vogt, S.; Nelson, D.M. Role of Diatoms in Nickel Biogeochemistry in the Ocean. Glob. Biogeochem. Cycles 2012, 26, 2011GB004233. [Google Scholar] [CrossRef]
- Saito, M.A.; Moffett, J.W.; Chisholm, S.W.; Waterbury, J.B. Cobalt Limitation and Uptake in Prochlorococcus. Limnol. Oceanogr. 2002, 47, 1629–1636. [Google Scholar] [CrossRef]
- Hawco, N.J.; McIlvin, M.M.; Bundy, R.M.; Tagliabue, A.; Goepfert, T.J.; Moran, D.M.; Valentin-Alvarado, L.; DiTullio, G.R.; Saito, M.A. Minimal Cobalt Metabolism in the Marine Cyanobacterium Prochlorococcus. Proc. Natl. Acad. Sci. 2020, 117, 15740–15747. [Google Scholar] [CrossRef]
- Sofen, L.E.; Antipova, O.A.; Ellwood, M.J.; Gilbert, N.E.; LeCleir, G.R.; Lohan, M.C.; Mahaffey, C.; Mann, E.L.; Ohnemus, D.C.; Wilhelm, S.W.; et al. Trace Metal Contents of Autotrophic Flagellates from Contrasting Open-ocean Ecosystems. Limnol. Oceanogr. Lett. 2022, 7, 354–362. [Google Scholar] [CrossRef]
- Cox, Alysia D.; Saito, Mak A., M.A. Proteomic Responses of Oceanic Synechococcus WH8102 to Phosphate and Zinc Scarcity and Cadmium Additions. Front. Microbiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Tuit, C.; Waterbury, J.; Ravizza, G. Diel Variation of Molybdenum and Iron in Marine Diazotrophic Cyanobacteria. Limnol. Oceanogr. 2004, 49, 978–990. [Google Scholar] [CrossRef]
- Yang, N.; Merkel, C.A.; Lin, Y.-A.; Levine, N.M.; Hawco, N.J.; Jiang, H.-B.; Qu, P.-P.; DeMers, M.A.; Webb, E.A.; Fu, F.-X.; et al. Warming Iron-Limited Oceans Enhance Nitrogen Fixation and Drive Biogeographic Specialization of the Globally Important Cyanobacterium Crocosphaera. Front. Mar. Sci. 2021, 8, 628363. [Google Scholar] [CrossRef]
- Tuo, S.; Rodriguez, I.B.; Ho, T. H 2 Accumulation and N 2 Fixation Variation by Ni Limitation in Cyanothece. Limnol. Oceanogr. 2020, 65, 377–386. [Google Scholar] [CrossRef]
- Rodriguez, I.B.; Lin, S.; Ho, J.; Ho, T.-Y. Effects of Trace Metal Concentrations on the Growth of the Coral Endosymbiont Symbiodinium Kawagutii. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Rodriguez, I.B.; Ho, T.-Y. Interactive Effects of Spectral Quality and Trace Metal Availability on the Growth of Trichodesmium and Symbiodinium. PLOS ONE 2017, 12, e0188777. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.B.; Ho, T.-Y. Trace Metal Requirements and Interactions in Symbiodinium Kawagutii. Front. Microbiol. 2018, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Rodriguez, I.B.; Chen, Y.L.; Zehr, J.P.; Chen, Y.; Hsu, S.D.; Yang, S.; Ho, T. Nickel Superoxide Dismutase Protects Nitrogen Fixation in Trichodesmium. Limnol. Oceanogr. Lett. 2022, 7, 363–371. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Iron Uptake and Growth Limitation in Oceanic and Coastal Phytoplankton. Mar. Chem. 1995, 50, 189–206. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Interrelated Influence of Iron, Light and Cell Size on Marine Phytoplankton Growth. Nature 1997, 390, 389–392. [Google Scholar] [CrossRef]
- LaJeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts. Curr. Biol. 2018, 28, 2570–2580.e6. [Google Scholar] [CrossRef]
- Li, T.; Lin, X.; Yu, L.; Lin, S.; Rodriguez, I.B.; Ho, T.-Y. RNA-Seq Profiling of Fugacium Kawagutii Reveals Strong Responses in Metabolic Processes and Symbiosis Potential to Deficiencies of Iron and Other Trace Metals. Sci. Total Environ. 2020, 705, 135767. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.F.; Lu, Y.H. Influence of Environmental and Nutritional Factors on Growth, Toxicity, and Toxin Pro®le of Dino¯agellate Alexandrium Minutum. Toxicon 2000, 38, 1491–1503. [Google Scholar] [CrossRef]
- Wells, M.L.; Trick, C.G.; Cochlan, W.P.; Hughes, M.P.; Trainer, V.L. Domoic Acid: The Synergy of Iron, Copper, and the Toxicity of Diatoms. Limnol. Oceanogr. 2005, 50, 1908–1917. [Google Scholar] [CrossRef]
- Hochmuth, J.D.; Asselman, J.; De Schamphelaere, K.A.C. Are Interactive Effects of Harmful Algal Blooms and Copper Pollution a Concern for Water Quality Management? Water Res. 2014, 60, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, X.; Wu, G.; Qin, B.; Tefsen, B.; Wells, M. Toxins from Harmful Algal Blooms: How Copper and Iron Render Chalkophore a Predictor of Microcystin Production. Water Res. 2023, 244, 120490. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Interactions of Toxic Metals with Algal Toxins Derived from Harmful Algal Blooms. Master of Science Chemistry, Florida International University, 2011.
- Wu, H.; Wei, G.; Tan, X.; Li, L.; Li, M. Species-Dependent Variation in Sensitivity of Microcystis Species to Copper Sulfate: Implication in Algal Toxicity of Copper and Controls of Blooms. Sci. Rep. 2017, 7, 40393. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, H.; He, X.; Shi, H.; Stephan, C.; Jiang, H.; Wan, C.; Eichholz, T. Evaluating the Treatment Effectiveness of Copper-Based Algaecides on Toxic Algae Microcystis Aeruginosa Using Single Cell-Inductively Coupled Plasma-Mass Spectrometry. Anal. Bioanal. Chem. 2019, 411, 5531–5543. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Koch, F.; Gobler, C.J. Most Harmful Algal Bloom Species Are Vitamin B 1 and B 12 Auxotrophs. Proc. Natl. Acad. Sci. 2010, 107, 20756–20761. [Google Scholar] [CrossRef]
- Herzi, F.; Jean, N.; Sakka Hlaili, A.; Mounier, S. Three-dimensional (3-D) Fluorescence Spectroscopy Analysis of the Fluorescent Dissolved Organic Matter Released by the Marine Toxic Dinoflagellate A Lexandrium Catenella Exposed to Metal Stress by Zinc or Lead. J. Phycol. 2014, 50, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Couet, D.; Pringault, O.; Bancon-Montigny, C.; Briant, N.; Elbaz Poulichet, F.; Delpoux, S.; Kefi-Daly Yahia, O.; Hela, B.; Charaf, M.; Hervé, F.; et al. Effects of Copper and Butyltin Compounds on the Growth, Photosynthetic Activity and Toxin Production of Two HAB Dinoflagellates: The Planktonic Alexandrium Catenella and the Benthic Ostreopsis Cf. Ovata. Aquat. Toxicol. 2018, 196, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Herzi, F.; Jean, N.; Zhao, H.; Mounier, S.; Mabrouk, H.H.; Hlaili, A.S. Copper and Cadmium Effects on Growth and Extracellular Exudation of the Marine Toxic Dinoflagellate Alexandrium Catenella: 3D-Fluorescence Spectroscopy Approach. Chemosphere 2013, 93, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Jean, N.; Dumont, E.; Herzi, F.; Balliau, T.; Laabir, M.; Masseret, E.; Mounier, S. Modifications of the Soluble Proteome of a Mediterranean Strain of the Invasive Neurotoxic Dinoflagellate Alexandrium Catenella under Metal Stress Conditions. Aquat. Toxicol. Amst. Neth. 2017, 188, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Yarimizu, K.; Mardones, J.I.; Paredes-Mella, J.; Norambuena-Subiabre, L.; Carrano, C.J.; Maruyama, F. The Effect of Iron on Chilean Alexandrium Catenella Growth and Paralytic Shellfish Toxin Production as Related to Algal Blooms. BioMetals 2022, 35, 39–51. [Google Scholar] [CrossRef]
- Doblin, M.; Blackburn, S.; Hallegraeff, G. Comparative Study of Selenium Requirements of Three Phytoplankton Species: Gymnodinium Catenatum, Alexandrium Minutum (Dinophyta) and Chaetoceros Cf. Tenuissimus (Bacillariophyta). J. Plankton Res. 1999, 21, 1153–1169. [Google Scholar] [CrossRef]
- Long, M.; Holland, A.; Planquette, H.; González Santana, D.; Whitby, H.; Soudant, P.; Sarthou, G.; Hégaret, H.; Jolley, D.F. Effects of Copper on the Dinoflagellate Alexandrium Minutum and Its Allelochemical Potency. Aquat. Toxicol. 2019, 210, 251–261. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, F.; Li, H.; Xiang, W.; Li, Y.; Jiang, Y. Effect of Iron on Growth, Biochemical Composition and Paralytic Shellfish Poisoning Toxins Production of Alexandrium Tamarense. Harmful Algae 2010, 9, 98–104. [Google Scholar] [CrossRef]
- Lage, O.M.; Parente, A.M.; Soares, H.M.V.M.; Vasconcelos, M.T.S.D.; Salema, R. Some Effects of Copper on the Dinoflagellates Amphidinium Carterae and Prorocentrum Micans in Batch Culture. Eur. J. Phycol. 1994, 29, 253–260. [Google Scholar] [CrossRef]
- Wang, B.; Axe, L.; Michalopoulou, Z.-H.; Wei, L. Effects of Cd, Cu, Ni, and Zn on Brown Tide Alga Aureococcus Anophagefferens Growth and Metal Accumulation. Environ. Sci. Technol. 2012, 46, 517–524. [Google Scholar] [CrossRef]
- Gobler, C.J.; Lobanov, A.V.; Tang, Y.-Z.; Turanov, A.A.; Zhang, Y.; Doblin, M.; Taylor, G.T.; Sañudo-Wilhelmy, S.A.; Grigoriev, I.V.; Gladyshev, V.N. The Central Role of Selenium in the Biochemistry and Ecology of the Harmful Pelagophyte, Aureococcus Anophagefferens. ISME J. 2013, 7, 1333–1343. [Google Scholar] [CrossRef]
- Gobler, C.J.; Donat, J.R.; Consolvo, J.A.; Sañudo-Wilhelmy, S.A. Physicochemical Speciation of Iron during Coastal Algal Blooms. Mar. Chem. 2002, 77, 71–89. [Google Scholar] [CrossRef]
- Anderson, D.M.; Lively, J.S.; Vaccaro, R.F. Copper Complexation during Spring Phytoplankton Blooms in Coastal Waters. J. Mar. Res. 1984, 42, 677–695. [Google Scholar] [CrossRef]
- Naito, K.; Matsui, M.; Imai, I. Effects of Organic Iron Complexes on the Growth of Red Tide Causative Phytoplankton. In Proceedings of the Oceans ’04 MTS/IEEE Techno-Ocean ’04 (IEEE Cat. No.04CH37600); Vol. 3; IEEE: Kobe, Japan, 2004; pp. 1774–1780. [Google Scholar]
- García-Hernández, J.; García-Rico, L.; Jara-Marini, M.E.; Barraza-Guardado, R.; Hudson Weaver, A. Concentrations of Heavy Metals in Sediment and Organisms during a Harmful Algal Bloom (HAB) at Kun Kaak Bay, Sonora, Mexico. Mar. Pollut. Bull. 2005, 50, 733–739. [Google Scholar] [CrossRef]
- Sanders, J.G.; Riedel, G.F. Trace Element Transformation during the Development of an Estuarine Algal Bloom. Estuaries 1993, 16, 521. [Google Scholar] [CrossRef]
- Ebenezer, V.; Lim, W.A.; Ki, J.-S. Effects of the Algicides CuSO4 and NaOCl on Various Physiological Parameters in the Harmful Dinoflagellate Cochlodinium Polykrikoides. J. Appl. Phycol. 2014, 26, 2357–2365. [Google Scholar] [CrossRef]
- Guo, R.; Wang, H.; Suh, Y.S.; Ki, J.-S. Transcriptomic Profiles Reveal the Genome-Wide Responses of the Harmful Dinoflagellate Cochlodinium Polykrikoides When Exposed to the Algicide Copper Sulfate. BMC Genomics 2016, 17, 29. [Google Scholar] [CrossRef]
- Ebenezer, V.; Ki, J.-S. Evaluation of the Sub-Lethal Toxicity of Cu, Pb, Bisphenol A and Polychlorinated Biphenyl to the Marine Dinoflagellate Cochlodinium Polykrikoides. ALGAE 2012, 27, 63–70. [Google Scholar] [CrossRef]
- Martin, D.F.; Olander, W.K. Effects of Copper, Titanium and Zirconium on the Growth Rates of the Red Tide Organism, Gymnodinium Breve. Environ. Lett. 1971, 2, 135–142. [Google Scholar] [CrossRef]
- Doblin, M.A. Intraspecific Variation in the Selenium Requirement of Different Geographic Strains of the Toxic Dinoflagellate Gymnodinium Catenatum. J. Plankton Res. 2000, 22, 421–432. [Google Scholar] [CrossRef]
- Han, K.H.; Kim, H.J.; Li, Z.; Youn, J.Y.; Kwak, K.Y.; Seo, M.H.; Hwang, J.; Lee, S.D.; Yun, S.M.; Oh, S.J.; et al. Effects of Different Nutrient and Trace Metal Concentrations on Growth of the Toxic Dinoflagellate Gymnodinium Catenatum Isolated from Korean Coastal Waters. Sustainability 2020, 12, 4992. [Google Scholar] [CrossRef]
- Ishimaru, T.; Takeuchi, T.; Fukuyo, Y.; Kodama, M. The Selenium Requirement of Gymnodinium Nagasakiense. In Okaichi, T.Anderson, D.M. and Nemoto, T. In Red tides: Biology, Environmental Science and Toxicology; Elsevier: New York, NY, 1989; pp. 357–360. [Google Scholar]
- Koike, Y.; Nakaguchi, Y.; Hiraki, K.; Takeuchi, T.; Kokubo, T.; Ishimaru, T. Species and Concentrations of Selenium and Nutrients in Tanabe Bay during Red Tide Due toGymnodinium Nagasakiense. J. Oceanogr. 1993, 49, 641–656. [Google Scholar] [CrossRef]
- Doucette, G.; Harrison, P. Some Effects of Iron and Nitrogen Stress on the Red Tide Dinoflagellate Gymnodinium Sanguineum. Mar. Ecol. Prog. Ser. 1990, 62, 293–306. [Google Scholar] [CrossRef]
- Doucette, G.J.; Harrison, P.J. Aspects of Iron and Nitrogen Nutrition in the Red Tide Dinoflagellate Gymnodiniumsanguineum. Mar. Biol. 1991, 110, 165–173. [Google Scholar] [CrossRef]
- Rhodes, L.; Selwood, A.; McNabb, P.; Briggs, L.; Adamson, J.; Van Ginkel, R.; Laczka, O. Trace Metal Effects on the Production of Biotoxins by Microalgae. Afr. J. Mar. Sci. 2006, 28, 393–397. [Google Scholar] [CrossRef]
- Gutierrez-Mejia, E.; Lares, M.L.; Huerta-Diaz, M.A.; Delgadillo-Hinojosa, F. Cadmium and Phosphate Variability during Algal Blooms of the Dinoflagellate Lingulodinium Polyedrum in Todos Santos Bay, Baja California, Mexico. Sci. Total Environ. 2016, 541, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.P.; Hollnagel, H.C.; Glavina, A.B.; Soares, C.O.; Ganini, D.; Dagenais-Bellefeuille, S.; Morse, D.; Colepicolo, P. Molybdate:Sulfate Ratio Affects Redox Metabolism and Viability of the Dinoflagellate Lingulodinium Polyedrum. Aquat. Toxicol. 2013, 142–143, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, S.M.; Fernández Amandi, M.; McKenzie, L.; Furey, A.; James, K.J. Effects of Selenium, Iron and Cobalt Addition to Growth and Yessotoxin Production of the Toxic Marine Dinoflagellate Protoceratium Reticulatum in Culture. J. Exp. Mar. Biol. Ecol. 2004, 313, 337–351. [Google Scholar] [CrossRef]
- Tian, R.; Lin, Q.; Li, D.; Zhang, W.; Zhao, X. Atmospheric Transport of Nutrients during a Harmful Algal Bloom Event. Reg. Stud. Mar. Sci. 2020, 34, 101007. [Google Scholar] [CrossRef]
- Maldonado, M.T.; Hughes, M.P.; Rue, E.L.; Wells, M.L. The Effect of Fe and Cu on Growth and Domoic Acid Production by Pseudo-Nitzschiamultiseries and Pseudo-nitzschia Australis. Limnol. Oceanogr. 2002, 47, 515–526. [Google Scholar] [CrossRef]
- Lelong, A.; Bucciarelli, E.; Hégaret, H.; Soudant, P. Iron and Copper Limitations Differently Affect Growth Rates and Photosynthetic and Physiological Parameters of the Marine Diatom Pseudo-nitzschia Delicatissima. Limnol. Oceanogr. 2013, 58, 613–623. [Google Scholar] [CrossRef]
- Long, M.; Lelong, A.; Bucciarelli, E.; Le Grand, F.; Hégaret, H.; Soudant, P. Physiological Adaptation of the Diatom Pseudo-Nitzschia Delicatissima under Copper Starvation. Mar. Environ. Res. 2023, 188, 105995. [Google Scholar] [CrossRef] [PubMed]
- Sobrinho, B.F.; De Camargo, L.M.; Sandrini-Neto, L.; Kleemann, C.R.; Machado, E.D.C.; Mafra, L.L. Growth, Toxin Production and Allelopathic Effects of Pseudo-Nitzschia Multiseries under Iron-Enriched Conditions. Mar. Drugs 2017, 15, 331. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.S.; Wikfors, G.H. Control of Domoic Acid Toxin Expression in Pseudo-Nitzschia Multiseries by Copper and Silica: Relevance to Mussel Aquaculture in New England (USA). Mar. Environ. Res. 2013, 83, 23–28. [Google Scholar] [CrossRef]
- Ladizinsky, N.L.; Smith J., G. Accumulation of Domoic Acid by the Coastal Diatom Pseudo-Nitzschia Multiseries: A Possible Copper Complexation Strategy. J. Phycol. 2000, 36, 41–41. [Google Scholar] [CrossRef]
- Zhuo-Ping, C.; Wei-Wei, H.; Min, A.; Shun-Shan, D. Coupled Effects of Irradiance and Iron on the Growth of a Harmful Algal Bloom-Causing Microalga Scrippsiella Trochoidea. Acta Ecol. Sin. 2009, 29, 297–301. [Google Scholar] [CrossRef]
- Ho, T.-Y. Nickel Limitation of Nitrogen Fixation in Trichodesmium. Limnol. Oceanogr. 2013, 58, 112–120. [Google Scholar] [CrossRef]
- Greenfield, D.I.; Duquette, A.; Goodson, A.; Keppler, C.J.; Williams, S.H.; Brock, L.M.; Stackley, K.D.; White, D.; Wilde, S.B. The Effects of Three Chemical Algaecides on Cell Numbers and Toxin Content of the Cyanobacteria Microcystis Aeruginosa and Anabaenopsis Sp. Environ. Manage. 2014, 54, 1110–1120. [Google Scholar] [CrossRef]
- Molot, L.A.; Li, G.; Findlay, D.L.; Watson, S.B. Iron-mediated Suppression of Bloom-forming Cyanobacteria by Oxine in a Eutrophic Lake. Freshw. Biol. 2010, 55, 1102–1117. [Google Scholar] [CrossRef]
- Cusick, K.D.; Wetzel, R.K.; Minkin, S.C.; Dodani, S.C.; Wilhelm, S.W.; Sayler, G.S. Paralytic Shellfish Toxins Inhibit Copper Uptake in Chlamydomonas Reinhardtii. Environ. Toxicol. Chem. 2013, 32, 1388–1395. [Google Scholar] [CrossRef]
- Wang, H.; Sathasivam, R.; Ki, J.-S. Physiological Effects of Copper on the Freshwater Alga Closterium Ehrenbergii Meneghini (Conjugatophyceae) and Its Potential Use in Toxicity Assessments. ALGAE 2017, 32, 131–137. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Deng, J.; Cheng, Y.; Chen, Z.; Qin, B.; Tefsen, B.; Wells, M. A New Perspective of Copper-Iron Effects on Bloom-Forming Algae in a Highly Impacted Environment. Water Res. 2021, 195, 116889. [Google Scholar] [CrossRef]
- Bishop, W.M.; Lynch, C.L.; Willis, B.E.; Cope, W.G. Copper-Based Aquatic Algaecide Adsorption and Accumulation Kinetics: Influence of Exposure Concentration and Duration for Controlling the Cyanobacterium Lyngbya Wollei. Bull. Environ. Contam. Toxicol. 2017, 99, 365–371. [Google Scholar] [CrossRef]
- Jin, Z.; Ding, S.; Sun, Q.; Gao, S.; Fu, Z.; Gong, M.; Lin, J.; Wang, D.; Wang, Y. High Resolution Spatiotemporal Sampling as a Tool for Comprehensive Assessment of Zinc Mobility and Pollution in Sediments of a Eutrophic Lake. J. Hazard. Mater. 2019, 364, 182–191. [Google Scholar] [CrossRef]
- Lukač, M.; Aegerter, R. Influence of Trace Metals on Growth and Toxin Production of Microcystis Aeruginosa. Toxicon 1993, 31, 293–305. [Google Scholar] [CrossRef]
- Facey, J.A.; Violi, J.P.; King, J.J.; Sarowar, C.; Apte, S.C.; Mitrovic, S.M. The Influence of Micronutrient Trace Metals on Microcystis Aeruginosa Growth and Toxin Production. Toxins 2022, 14, 812. [Google Scholar] [CrossRef]
- Gu, P.; Li, Q.; Zhang, W.; Zheng, Z.; Luo, X. Effects of Different Metal Ions (Ca, Cu, Pb, Cd) on Formation of Cyanobacterial Blooms. Ecotoxicol. Environ. Saf. 2020, 189, 109976. [Google Scholar] [CrossRef]
- Zeng, L.; Yan, C.; Guo, J.; Zhen, Z.; Zhao, Y.; Wang, D. Influence of Algal Blooms Decay on Arsenic Dynamics at the Sediment-Water Interface of a Shallow Lake. Chemosphere 2019, 219, 1014–1023. [Google Scholar] [CrossRef]
- Yu, S.; Xu, C.; Tang, T.; Zhang, Y.; Effiong, K.; Hu, J.; Bi, Y.; Xiao, X. Down-Regulation of Iron/Zinc Ion Transport and Toxin Synthesis in Microcystis Aeruginosa Exposed to 5,4′-Dihydroxyflavone. J. Hazard. Mater. 2023, 460, 132396. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, S.P.; Boyer, G.L.; Twiss, M.R. Influence of Ultraviolet Radiation, Copper, and Zinc on Microcystin Content in Microcystis Aeruginosa (Cyanobacteria). Harmful Algae 2008, 7, 194–205. [Google Scholar] [CrossRef]
- Deng, J.; Fu, D.; Hu, W.; Lu, X.; Wu, Y.; Bryan, H. Physiological Responses and Accumulation Ability of Microcystis Aeruginosa to Zinc and Cadmium: Implications for Bioremediation of Heavy Metal Pollution. Bioresour. Technol. 2020, 303, 122963. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruiz, E.B.; Martínez-Jerónimo, F. How Do Toxic Metals Affect Harmful Cyanobacteria? An Integrative Study with a Toxigenic Strain of Microcystis Aeruginosa Exposed to Nickel Stress. Ecotoxicol. Environ. Saf. 2016, 133, 36–46. [Google Scholar] [CrossRef]
- Alexova, R.; Fujii, M.; Birch, D.; Cheng, J.; Waite, T.D.; Ferrari, B.C.; Neilan, B.A. Iron Uptake and Toxin Synthesis in the Bloom-Forming Microcystis Aeruginosa under Iron Limitation. Environ. Microbiol. 2011, 13, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Alexova, R.; Dang, T.C.; Fujii, M.; Raftery, M.J.; Waite, T.D.; Ferrari, B.C.; Neilan, B.A. Specific Global Responses to N and Fe Nutrition in Toxic and Non-Toxic Microcystis Aeruginosa. Environ. Microbiol. 2016, 18, 401–413. [Google Scholar] [CrossRef]
- Kosakowska, A.; Nędzi, M.; Pempkowiak, J. Responses of the Toxic Cyanobacterium Microcystis Aeruginosa to Iron and Humic Substances. Plant Physiol. Biochem. 2007, 45, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Bishop, W.M.; Willis, B.E.; Richardson, R.J. Sensitivity of Microcystis Aeruginosa Strains to Copper and Influence of Phosphorus. J Aquat Plant Manage 2019. [Google Scholar]
- Kansole, M.; Lin, T.-F. Impacts of Hydrogen Peroxide and Copper Sulfate on the Control of Microcystis Aeruginosa and MC-LR and the Inhibition of MC-LR Degrading Bacterium Bacillus Sp. Water 2017, 9, 255. [Google Scholar] [CrossRef]
- Liu, H.; Chen, S.; Zhang, H.; Wang, N.; Ma, B.; Liu, X.; Niu, L.; Yang, F.; Xu, Y.; Zhang, X. Effects of Copper Sulfate Algaecide on the Cell Growth, Physiological Characteristics, the Metabolic Activity of Microcystis Aeruginosa and Raw Water Application. J. Hazard. Mater. 2023, 445, 130604. [Google Scholar] [CrossRef]
- Qian, H.; Yu, S.; Sun, Z.; Xie, X.; Liu, W.; Fu, Z. Effects of Copper Sulfate, Hydrogen Peroxide and N-Phenyl-2-Naphthylamine on Oxidative Stress and the Expression of Genes Involved Photosynthesis and Microcystin Disposition in Microcystis Aeruginosa. Aquat. Toxicol. 2010, 99, 405–412. [Google Scholar] [CrossRef]
- Burger, H.; Dickson, S.; Awad, J.; Marzouk, J.; Van Leeuwen, J. Investigation of Cyanobacteria Blooms in Paper Mill Wastewaters and Assessment of Zinc as a Control Agent. Int. J. Environ. Sci. Technol. 2022, 19, 1105–1120. [Google Scholar] [CrossRef]
- Perez, J.L.; Chu, T. Effect of Zinc on Microcystis Aeruginosa UTEX LB 2385 and Its Toxin Production. Toxins 2020, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- ]Gong, Y.; Ao, H.; Liu, B.; Wen, S.; Wang, Z.; Hu, D.; Zhang, X.; Song, L.; Liu, J. Effects of Inorganic Arsenic on Growth and Microcystin Production of a Microcystis Strain Isolated from an Algal Bloom in Dianchi Lake, China. Chin. Sci. Bull. 2011, 56, 2337–2342. [Google Scholar] [CrossRef]
- Morton, S.D.; Lee, T.H. Algal Blooms. Possible Effects of Iron. Environ. Sci. Technol. 1974, 8, 673–674. [Google Scholar] [CrossRef]
- Wells, M.L.; Trick, C.G. Controlling Iron Availability to Phytoplankton in Iron-Replete Coastal Waters. Mar. Chem. 2004, 86, 1–13. [Google Scholar] [CrossRef]
- Dawson, R.M. The Toxicology of Microcystins. Toxicon 1998, 36, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Vasconcelos, V. Molecular Mechanisms of Microcystin Toxicity in Animal Cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef]
- Bates, S.S.; Léger, C.; Satchwell, M.; Boyer, G.L. THE EFFECTS OF IRON ON DOMOIC ACID PRODUCTION BY PSEUDO-NITZSCHIA MULTISERIES. In Proceedings of the Proceeding of the 9th International Conference on Harmful Algal Blooms; Intergov. Oceanogr. Comm., Paris: Tasmania, 2001; pp. 320–323. [Google Scholar]
- Pan, Y.; Subba Rao, D.V.; Mann, K.H. Changes in Domoic Acid Production and Cellular Chemical Composition of the Toxigenic Diatom Pseudo-Nitzschia Multiseries Under Phosphate Limitation1. J. Phycol. 1996, 32, 371–381. [Google Scholar] [CrossRef]
- Fehling, J.; Davidson, K.; Bolch, C.J.; Bates, S.S. Growth and Domoic Acid Production by Pseudo-Nitzschia Seriata (Bacillariophyceae) Under Phosphate and Silicate Limitation1. J. Phycol. 2004, 40, 674–683. [Google Scholar] [CrossRef]
- Sun, J.; Hutchins, D.A.; Feng, Y.; Seubert, E.L.; Caron, D.A.; Fu, F.-X. Effects of Changing pCO2 and Phosphate Availability on Domoic Acid Production and Physiology of the Marine Harmful Bloom Diatom Pseudo-Nitzschia Multiseries. Limnol. Oceanogr. 2011, 56, 829–840. [Google Scholar] [CrossRef]
- Kai, N.; Naito, K.; Mito, S.; Miyahara, K.; Sakamoto, S. Distribution and Behavior of Harmful Algae and Trace Metals in Harima-Nada, Japan.; Japanese Society of Fisheries Science, 2017; Vol. 4006.
- Leung, T.; Wilkinson, G.M.; Swanner, E.D. Iron Availability Allows Sustained Cyanobacterial Blooms: A Dual-Lake Case Study. Inland Waters 2021, 11, 417–429. [Google Scholar] [CrossRef]
- Wang, S.; Diao, X.; He, L. Effects of Algal Bloom Formation, Outbreak, and Extinction on Heavy Metal Fractionation in the Surficial Sediments of Chaohu Lake. Environ. Sci. Pollut. Res. 2015, 22, 14269–14279. [Google Scholar] [CrossRef]
- Thamdrup, B.; Fossing, H.; Jørgensen, B.B. Manganese, Iron and Sulfur Cycling in a Coastal Marine Sediment, Aarhus Bay, Denmark. Geochim. Cosmochim. Acta 1994, 58, 5115–5129. [Google Scholar] [CrossRef]
- Schoemann, V.; De Baar, H.J.W.; De Jong, J.T.M.; Lancelot, C. Effects of Phytoplankton Blooms on the Cycling of Manganese and Iron in Coastal Waters. Limnol. Oceanogr. 1998, 43, 1427–1441. [Google Scholar] [CrossRef]
- Sun, Q.; Lin, J.; Ding, S.; Gao, S.; Gao, M.; Wang, Y.; Zhang, C. A Comprehensive Understanding of Enhanced Pb Mobilization in Sediments Caused by Algal Blooms. Sci. Total Environ. 2019, 691, 969–980. [Google Scholar] [CrossRef]
- Sedmak, B.; Kosi, G. The Role of Microcystins in Heavy Cyanobacterial Bloom Formation. J. Plankton Res. 1998, 20, 691–708. [Google Scholar] [CrossRef]
- Kemp, A.; John, J. Microcystins Associated with Microcystis Dominated Blooms in the Southwest Wetlands, Western Australia. Environ. Toxicol. 2006, 21, 125–130. [Google Scholar] [CrossRef]
- Steiner, K.; Wood, S.; Puddick, J.; Hawes, I.; Dietrich, D.; Hamilton, D. A Comparison of Bacterial Community Structure, Activity and Microcystins Associated with Formation and Breakdown of a Cyanobacterial Scum. Aquat. Microb. Ecol. 2017, 80, 243–256. [Google Scholar] [CrossRef]
- Brand, L.E. Minimum Iron Requirements of Marine Phytoplankton and the Implications for the Biogeochemical Control of New Production. Limnol. Oceanogr. 1991, 36, 1756–1771. [Google Scholar] [CrossRef]
- Berman-Frank, I.; Quigg, A.; Finkel, Z.V.; Irwin, A.J.; Haramaty, L. Nitrogen-Fixation Strategies and Fe Requirements in Cyanobacteria. Limnol. Oceanogr. 2007, 52, 2260–2269. [Google Scholar] [CrossRef]
- Chen, M.; Ding, S.; Chen, X.; Sun, Q.; Fan, X.; Lin, J.; Ren, M.; Yang, L.; Zhang, C. Mechanisms Driving Phosphorus Release during Algal Blooms Based on Hourly Changes in Iron and Phosphorus Concentrations in Sediments. Water Res. 2018, 133, 153–164. [Google Scholar] [CrossRef]
- Yao, Y.; Han, X.; Chen, Y.; Li, D. The Variations of Labile Arsenic Diffusion Driven by Algal Bloom Decomposition in Eutrophic Lake Ecosystems. Sci. Total Environ. 2022, 842, 156703. [Google Scholar] [CrossRef]
- Orihel, D.M.; Schindler, D.W.; Ballard, N.C.; Wilson, L.R.; Vinebrooke, R.D. Experimental Iron Amendment Suppresses Toxic Cyanobacteria in a Hypereutrophic Lake. Ecol. Appl. 2016, 26, 1517–1534. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Xu, H.; Wells, M.; Tefsen, B.; Qin, B. Effect of Micronutrients on Algae in Different Regions of Taihu, a Large, Spatially Diverse, Hypereutrophic Lake. Water Res. 2019, 151, 500–514. [Google Scholar] [CrossRef]
- Lelong, A.; Jolley, D.F.; Soudant, P.; Hégaret, H. Impact of Copper Exposure on Pseudo-Nitzschia Spp. Physiology and Domoic Acid Production. Aquat. Toxicol. 2012, 118–119, 37–47. [Google Scholar] [CrossRef]
- Castruita, M.; Casero, D.; Karpowicz, S.J.; Kropat, J.; Vieler, A.; Hsieh, S.I.; Yan, W.; Cokus, S.; Loo, J.A.; Benning, C.; et al. Systems Biology Approach in Chlamydomonas Reveals Connections between Copper Nutrition and Multiple Metabolic Steps. Plant Cell 2011, 23, 1273–1292. [Google Scholar] [CrossRef]
- Humble, A.V.; Gadd, G.M.; Codd, G.A. Binding of Copper and Zinc to Three Cyanobacterial Microcystins Quantified by Differential Pulse Polarography. Water Res. 1997, 31, 1679–1686. [Google Scholar] [CrossRef]
- Yan, F.; Ozsoz, M.; Sadik, O.A. Electrochemical and Conformational Studies of Microcystin–LR. Anal. Chim. Acta 2000, 409, 247–255. [Google Scholar] [CrossRef]
- Saito, K.; Sei, Y.; Miki, S.; Yamaguchi, K. Detection of Microcystin–Metal Complexes by Using Cryospray Ionization-Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Toxicon 2008, 51, 1496–1498. [Google Scholar] [CrossRef]
- Le Faucheur, S.; Schildknecht, F.; Behra, R.; Sigg, L. Thiols in Scenedesmus Vacuolatus upon Exposure to Metals and Metalloids. Aquat. Toxicol. 2006, 80, 355–361. [Google Scholar] [CrossRef]
- Yang, F.; Hu, Y.; Qiu, G.; Li, Q.; Wang, G. Complexation of Copper Algaecide and Algal Organic Matter in Algae-Laden Water: Insights into Complex Metal–Organic Interactions. Environ. Pollut. 2023, 333, 122032. [Google Scholar] [CrossRef]
- Zwolsman, J.J.G.; van Eck, G.T.M. Geochemistry of Major Elements and Trace Metals in Suspended Matter of the Scheldt Estuary, Southwest Netherlands. Mar. Chem. 1999, 66, 91–111. [Google Scholar] [CrossRef]
- Luengen, A.C.; Raimondi, P.T.; Flegal, A.R. Contrasting Biogeochemistry of Six Trace Metals during the Rise and Decay of a Spring Phytoplankton Bloom in San Francisco Bay. Limnol. Oceanogr. 2007, 52, 1112–1130. [Google Scholar] [CrossRef]
- Kuwabara, J.S.; Chang, C.C.Y.; Cloern, J.E.; Fries, T.L.; Davis, J.A.; Luoma, S.N. Trace Metal Associations in the Water Column of South San Francisco Bay, California. Estuar. Coast. Shelf Sci. 1989, 28, 307–325. [Google Scholar] [CrossRef]
- Araie, H.; Shiraiwa, Y. Selenium Utilization Strategy by Microalgae. Molecules 2009, 14, 4880–4891. [Google Scholar] [CrossRef]
- Boyer, G.L.; Brand, L.E. Trace Elements and Harmful Algal Blooms. In Physiological Ecology of Harmful Algal Blooms; Springer Verlag: Heidelberg, 1989; Vol. 41, pp. 498–508. [Google Scholar]
- Chang, Y.; Wu, Y.; Zhang, J.; Wang, X.; Jiang, S.; Cao, W.; Wang, X.; Qu, J.; Zhang, Z.; Jin, J.; et al. Effects of Algal Blooms on Selenium Species Dynamics: A Case Study in the Changjiang Estuary, China. Sci. Total Environ. 2021, 768, 144235. [Google Scholar] [CrossRef]
- Conde, J.E.; Sanz Alaejos, M. Selenium Concentrations in Natural and Environmental Waters. Chem. Rev. 1997, 97, 1979–2004. [Google Scholar] [CrossRef]
- Danbara, A.; Shiraiwa, Y. The Requirement of Selenium for the Growth of Marine Coccolithophorids, Emiliania Huxleyi, Gephyrocapsa Oceanica and Helladosphaera Sp. (Prymnesiophyceae). Plant Cell Physiol. 1999, 40, 762–766. [Google Scholar] [CrossRef]
- Cutter, G.A.; Bruland, K.W. The Marine Biogeochemistry of Selenium: A Re-evaluation1. Limnol. Oceanogr. 1984, 29, 1179–1192. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, M. Uptake and Transformation of Selenium by Marine Phytoplankton. J. Oceanogr. Taiwan Strait 1996, 15, 225–231. [Google Scholar]
- Ivanenko, N.V. The Role of Microorganisms in Transformation of Selenium in Marine Waters. Russ. J. Mar. Biol. 2018, 44, 87–93. [Google Scholar] [CrossRef]
- Chang, Y.; Müller, M.; Wu, Y.; Jiang, S.; Cao, W.W.; Qu, J.G.; Ren, J.L.; Wang, X.N.; Rao, E.M.; Wang, X.L.; et al. Distribution and Behaviour of Dissolved Selenium in Tropical Peatland-Draining Rivers and Estuaries of Malaysia. Biogeosciences 2020, 17, 1133–1145. [Google Scholar] [CrossRef]
- Peers, G.; Price, N.M. A Role for Manganese in Superoxide Dismutases and Growth of Iron-deficient Diatoms. Limnol. Oceanogr. 2004, 49, 1774–1783. [Google Scholar] [CrossRef]
- Graneli, E.; Haraldsson, C. Can Increased Leaching of Trace Metals from Acidified Areas Influence Phytoplankton Growth in Coastal Waters? 1994.
- Granéli, E.; Risinger, L. Effects of Cobalt and Vitamin B12 on the Growth of Chrysochromulina Polylepis (Prymnesiophyceae). Mar. Ecol. Prog. Ser. 1994, 113, 177–183. [Google Scholar] [CrossRef]
- Saito, M.A.; Rocap, G.; Moffett, J.W. Production of Cobalt Binding Ligands in a Synechococcus Feature at the Costa Rica Upwelling Dome. Limnol. Oceanogr. 2005, 50, 279–290. [Google Scholar] [CrossRef]
- Sañudo-Wilhelmy, S.A.; Gómez-Consarnau, L.; Suffridge, C.; Webb, E.A. The Role of B Vitamins in Marine Biogeochemistry. Annu. Rev. Mar. Sci. 2014, 6, 339–367. [Google Scholar] [CrossRef]
- Roth, J.; Lawrence, J.; Bobik, T. COBALAMIN (COENZYME B 12 ): Synthesis and Biological Significance. Annu. Rev. Microbiol. 1996, 50, 137–181. [Google Scholar] [CrossRef]
- Förstner, U.; Wittmann, G.T.; Prosi, F.; Lierde, J.H. van Metal Pollution in the Aquatic Environment; Springer study edition; 2., rev. ed., 2. print.; Springer: Berlin Heidelberg New York Tokyo, 1983; ISBN 978-3-540-12856-4.
- Tovar-Sanchez, A.; Sañudo-Wilhelmy, S.A. Influence of the Amazon River on Dissolved and Intra-Cellular Metal Concentrations in <I>Trichodesmium</I> Colonies along the Western Boundary of the Sub-Tropical North Atlantic Ocean. Biogeosciences 2011, 8, 217–225. [Google Scholar] [CrossRef]
- Price, N.M.; Morel, F.M.M. Colimitation of Phytoplankton Growth by Nickel and Nitrogen. Limnol. Oceanogr. 1991, 36, 1071–1077. [Google Scholar] [CrossRef]
- Dupont, C.L.; Buck, K.N.; Palenik, B.; Barbeau, K. Nickel Utilization in Phytoplankton Assemblages from Contrasting Oceanic Regimes. Deep Sea Res. Part Oceanogr. Res. Pap. 2010, 57, 553–566. [Google Scholar] [CrossRef]
- Gobler, C.J.; Berry, D.L.; Dyhrman, S.T.; Wilhelm, S.W.; Salamov, A.; Lobanov, A.V.; Zhang, Y.; Collier, J.L.; Wurch, L.L.; Kustka, A.B.; et al. Niche of Harmful Alga Aureococcus Anophagefferens Revealed through Ecogenomics. Proc. Natl. Acad. Sci. 2011, 108, 4352–4357. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Effect of Zn, Mn, and Fe on Cd Accumulation in Phytoplankton: Implications for Oceanic Cd Cycling. Limnol. Oceanogr. 2000, 45, 1501–1516. [Google Scholar] [CrossRef]
- Xu, Y.; Morel, F.M.M. Cadmium in Marine Phytoplankton. In Cadmium: From Toxicity to Essentiality; Sigel, A., Sigel, H., Sigel, R.K., Eds.; Metal Ions in Life Sciences; Springer Netherlands: Dordrecht, 2013; Vol. 11, pp. 509–528. ISBN 978-94-007-5178-1. [Google Scholar]
- Granéli, E.; Persson, H.; Edler, L. Connection between Trace Metals, Chelators and Red Tide Blooms in the Laholm Bay, SE Kattegat—an Experimental Approach. Mar. Environ. Res. 1986, 18, 61–78. [Google Scholar] [CrossRef]
- Kaplan, D. Absorption and Adsorption of Heavy Metals by Microalgae. In Handbook of Microalgal Culture; Richmond, A., Hu, Q., Eds.; Wiley, 2013; pp. 602–611 ISBN 978-0-470-67389-8.
- McIntyre, A.M.; Guéguen, C. Binding Interactions of Algal-Derived Dissolved Organic Matter with Metal Ions. Chemosphere 2013, 90, 620–626. [Google Scholar] [CrossRef]
- Mühlenbruch, M.; Grossart, H.; Eigemann, F.; Voss, M. Mini-review: Phytoplankton-derived Polysaccharides in the Marine Environment and Their Interactions with Heterotrophic Bacteria. Environ. Microbiol. 2018, 20, 2671–2685. [Google Scholar] [CrossRef] [PubMed]
- Tulcan, R.X.S.; Ouyang, W.; Lin, C.; He, M.; Wang, B. Vanadium Pollution and Health Risks in Marine Ecosystems: Anthropogenic Sources over Natural Contributions. Water Res. 2021, 207, 117838. [Google Scholar] [CrossRef] [PubMed]
- Yeh, G.; Lin, C.; Nguyen, D.-H.; Hoang, H.-G.; Shern, J.-C.; Hsiao, P.-J. A Five-Year Investigation of Water Quality and Heavy Metal Mass Flux of an Industrially Affected River. Environ. Sci. Pollut. Res. 2022, 29, 12465–12472. [Google Scholar] [CrossRef] [PubMed]
- Tulcan, R.X.S.; Ouyang, W.; Guo, Z.; Lin, C.; Gu, X.; Wang, A.; Wang, B. Watershed Seasonality Regulating Vanadium Concentrations and Ecological Risks in the Coastal Aquatic Habitats of the Northwest Pacific. Environ. Pollut. 2023, 322, 121145. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. The Iron and Molybdenum Use Efficiencies of Plant Growth with Different Energy, Carbon and Nitrogen Sources. New Phytol. 1988, 109, 279–287. [Google Scholar] [CrossRef]
- Wilhelm, C.; Wild, A. The Variability of the Photosynthetic Unit in Chlorella II. The Effect of Light Intensity and Cell Development on Photosynthesis, P-700 and Cytochrome f in Homocontinuous and Synchronous Cultures of Chlorella. J. Plant Physiol. 1984, 115, 125–135. [Google Scholar] [CrossRef]
- Gilmour, D.J.; Kaaden, R.; Gimmler, H. Vanadate Inhibition of ATPases of Dunaliella Parva in Vitro and in Vivo. J. Plant Physiol. 1985, 118, 111–126. [Google Scholar] [CrossRef]
- Nalewajko, C.; Lee, K.; Jack, T.R. Effects of Vanadium on Freshwater Phytoplankton Photosynthesis. Water. Air. Soil Pollut. 1995, 81, 93–105. [Google Scholar] [CrossRef]
- Meisch, H.-U.; Benzschawel, H. The Role of Vanadium in Green Plants: III. Influence on Cell Division of Chlorella. Arch. Microbiol. 1978, 116, 91–95. [Google Scholar] [CrossRef]
- Vaishampayan, A. VANADIUM AS A TRACE ELEMENT IN THE BLUE-GREEN ALGA, NOSTOC MUSCORUM : INFLUENCE ON NITROGENASE AND NITRATE REDUCTASE. New Phytol. 1983, 95, 55–60. [Google Scholar] [CrossRef]
- Arnon, D.I.; Wessel, G. Vanadium as an Essential Element for Green Plants. Nature 1953, 172, 1039–1040. [Google Scholar] [CrossRef]
- Patrick, R. Effects of Trace Minerals in the Aquatic Ecosystem. Am. Sci. 1978, 66, 185–191. [Google Scholar]
- Howarth, R.W.; Cole, J.J. Molybdenum Availability, Nitrogen Limitation, and Phytoplankton Growth in Natural Waters. Science 1985, 229, 653–655. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, D.; Li, C.; You, X.; Zhou, L.; Zhang, L.; Xiao, J.; Chen, M.; Ding, S.; Hang, X. Cyanobacterial Blooms Increase the Release of Vanadium through Iron Reduction and Dissolved Organic Matter Complexation in the Sediment of Eutrophic Lakes. Water Res. 2023, 243, 120377. [Google Scholar] [CrossRef]
- Schaule, B.K.; Patterson, C.C. Lead Concentrations in the Northeast Pacific: Evidence for Global Anthropogenic Perturbations. Earth Planet. Sci. Lett. 1981, 54, 97–116. [Google Scholar] [CrossRef]
- Reuer, M.K.; Weiss, D.J. Anthropogenic Lead Dynamics in the Terrestrial and Marine Environment. Philos. Trans. R. Soc. Lond. Ser. Math. Phys. Eng. Sci. 2002, 360, 2889–2904. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chang-Chien, G.-P.; Chiang, P.-C.; Chen, W.-H.; Lin, Y.-C. Multivariate Analysis of Heavy Metal Contaminations in Seawater and Sediments from a Heavily Industrialized Harbor in Southern Taiwan. Mar. Pollut. Bull. 2013, 76, 266–275. [Google Scholar] [CrossRef]
- Mu, W.; Chen, Y.; Liu, Y.; Pan, X.; Fan, Y. Toxicological Effects of Cadmium and Lead on Two Freshwater Diatoms. Environ. Toxicol. Pharmacol. 2018, 59, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Poniedzialek, B.; Niedzielski, P.; Tabaczewski, P.; Wiktorowicz, K. Cadmium and Lead Toxicity and Bioaccumulation in Microcystis Aeruginosa. Front. Environ. Sci. Eng. 2014, 8, 427–432. [Google Scholar] [CrossRef]
- Deb, S.C.; Fukushima, T. Metals in Aquatic Ecosystems: Mechanisms of Uptake, Accumulation and release-Ecotoxicological Perspectives. Int. J. Environ. Stud. 1999, 56, 385–417. [Google Scholar] [CrossRef]
- Kamp-Nielsen, L. The Effect of Deleterious Concentrations of Mercury on the Photosynthesis and Growth of Chlorella Pyrenoidosa. Physiol. Plant. 1971, 24, 556–561. [Google Scholar] [CrossRef]
- Le Faucheur, S.; Campbell, P.G.C.; Fortin, C.; Slaveykova, V.I. Interactions between Mercury and Phytoplankton: Speciation, Bioavailability, and Internal Handling. Environ. Toxicol. Chem. 2014, 33, 1211–1224. [Google Scholar] [CrossRef]
- Protopopov, F.F.; Todorenko, D.A.; Nikolaev, I.N.; Alekseev, A.A.; Bratkovskaya, L.B.; Matorin, D.N. The Fluorescence of Phytoplankton Chlorophyll from the Moskva River in the Presence of Mercury Ions. Biophysics 2021, 66, 779–785. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, W.-X. Thiol Compounds Induction Kinetics in Marine Phytoplankton during and after Mercury Exposure. J. Hazard. Mater. 2012, 217–218, 271–278. [Google Scholar] [CrossRef]
- Satoh, M.; Matsumoto, Y. Mercury-Induced Oxidative Stress in Marine Phytoplankton Tetreselmis Tetrahele (Prasinophyceae). Nat. Sci. Res. 2008, 22, 57–63. [Google Scholar]
- Mason, R.P.; Morel, F.M.M.; Hemond, H.F. The Role of Microorganisms in Elemental Mercury Formation in Natural Waters. Water. Air. Soil Pollut. 1995, 80, 775–787. [Google Scholar] [CrossRef]
- Achá, D.; Guédron, S.; Amouroux, D.; Point, D.; Lazzaro, X.; Fernandez, P.E.; Sarret, G. Algal Bloom Exacerbates Hydrogen Sulfide and Methylmercury Contamination in the Emblematic High-Altitude Lake Titicaca. Geosciences 2018, 8, 438. [Google Scholar] [CrossRef]
- Pickhardt, P.C.; Folt, C.L.; Chen, C.Y.; Klaue, B.; Blum, J.D. Algal Blooms Reduce the Uptake of Toxic Methylmercury in Freshwater Food Webs. Proc. Natl. Acad. Sci. 2002, 99, 4419–4423. [Google Scholar] [CrossRef]
- Fendorf, S.; Michael, H.A.; van Geen, A. Spatial and Temporal Variations of Groundwater Arsenic in South and Southeast Asia. Science 2010, 328, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Healey, F.P. Effects of Arsenate on Growth and Phosphorus Metabolism of Phytoplankton1,2. J. Phycol. 1978, 14, 337–341. [Google Scholar] [CrossRef]
- Sabath, E. 18 - Arsenic, Kidney, and Urinary Bladder Disorders. In Handbook of Arsenic Toxicology (Second Edition); Flora, S.J.S., Ed.; Academic Press: Oxford, 2023; pp. 485–500. ISBN 978-0-323-89847-8. [Google Scholar]
- Donohue, J. Some Comparisons among Ring Compounds of Phosphorus and Arsenic. Acta Crystallogr. 1962, 15, 708–713. [Google Scholar] [CrossRef]
- Nyulászi, L.; Veszprémi, T. Photoelectron Spectroscopic Study of the Aromaticity of Phosphorus and Arsenic Compounds. J. Mol. Struct. 1995, 347, 57–71. [Google Scholar] [CrossRef]
- Nava, P.; Ahlrichs, R. Theoretical Investigation of Clusters of Phosphorus and Arsenic: Fascination and Temptation of High Symmetries. Chem. – Eur. J. 2008, 14, 4039–4045. [Google Scholar] [CrossRef] [PubMed]
- Andreae, M. Arsenic Speciation in Seawater and Interstitial Waters: The Influence of Biological-chemical Interactions on the Chemistry of a Trace Element. Limnol. Oceanogr. 1979, 24, 440–452. [Google Scholar] [CrossRef]
- Hellweger, F.L.; Farley, K.J.; Lall, U.; Di Toro, D.M. Greedy Algae Reduce Arsenate. Limnol. Oceanogr. 2003, 48, 2275–2288. [Google Scholar] [CrossRef]
- Duan, L.; Song, J.; Zhang, Y.; Yuan, H.; Li, X.; Sun, L. Role of Marine Algal Blooms in the Release of Arsenic at the Sediment-Seawater Interface: Evidence from Microcosm Experiments. Water Res. 2023, 244, 120508. [Google Scholar] [CrossRef]
- Safonova, T.A.; Annenkov, V.V.; Chebykin, E.P.; Danilovtseva, E.N.; Likhoshway, Y.V.; Grachev, M.A. Aberration of Morphogenesis of Siliceous Frustule Elements of the Diatom Synedra Acus in the Presence of Germanic Acid. Biochem. Biokhimiia 2007, 72, 1261–1269. [Google Scholar] [CrossRef]
- Basharina, T.N.; Danilovtseva, E.N.; Zelinskiy, S.N.; Klimenkov, I.V.; Likhoshway, Y.V.; Annenkov, V.V. The Effect of Titanium, Zirconium and Tin on the Growth of Diatom Synedra Acus and Morphology of Its Silica Valves. Silicon 2012, 4, 239–249. [Google Scholar] [CrossRef]
- Sanville, W.D.; Powers, C.F.; Schuytema, G.S.; Stay, F.S.; Lauer, W.L. Phosphorus Inactivation by Zirconium in a Eutrophic Pond. J. Water Pollut. Control Fed. 1982, 54, 434–443. [Google Scholar]
- Couture, P.; Blaise, C.; Cluis, D.; Bastien, C. Zirconium Toxicity Assessment Using Bacteria, Algae and Fish Assays. Water. Air. Soil Pollut. 1989, 47, 87–100. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Kaczorowska, R.; Skowroński, T. The Impact of Inorganic Tin on the Planktonic Cyanobacterium Synechocystis Aquatilis: The Effect of pH and Humic Acid. Environ. Pollut. 1997, 97, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Yallop, M.; Wang, Y.; Masuda, S.; Daniels, J.; Ockenden, A.; Masani, H.; Scott, T.B.; Xie, F.; Ryan, M.; Jones, C.; et al. Quantifying Impacts of Titanium Dioxide Nanoparticles on Natural Assemblages of Riverine Phytobenthos and Phytoplankton in an Outdoor Setting. Sci. Total Environ. 2022, 831, 154616. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Juneau, P.; Lian, Y.; Zhang, W.; Wang, S.; Wang, C.; Shu, L.; Yan, Q.; He, Z.; Xu, K. Effects of Titanium Dioxide Nanoparticles on Photosynthetic and Antioxidative Processes of Scenedesmus Obliquus. Plants 2020, 9, 1748. [Google Scholar] [CrossRef]
- Kulacki, K.J.; Cardinale, B.J. Effects of Nano-Titanium Dioxide on Freshwater Algal Population Dynamics. PLOS ONE 2012, 7, e47130. [Google Scholar] [CrossRef]
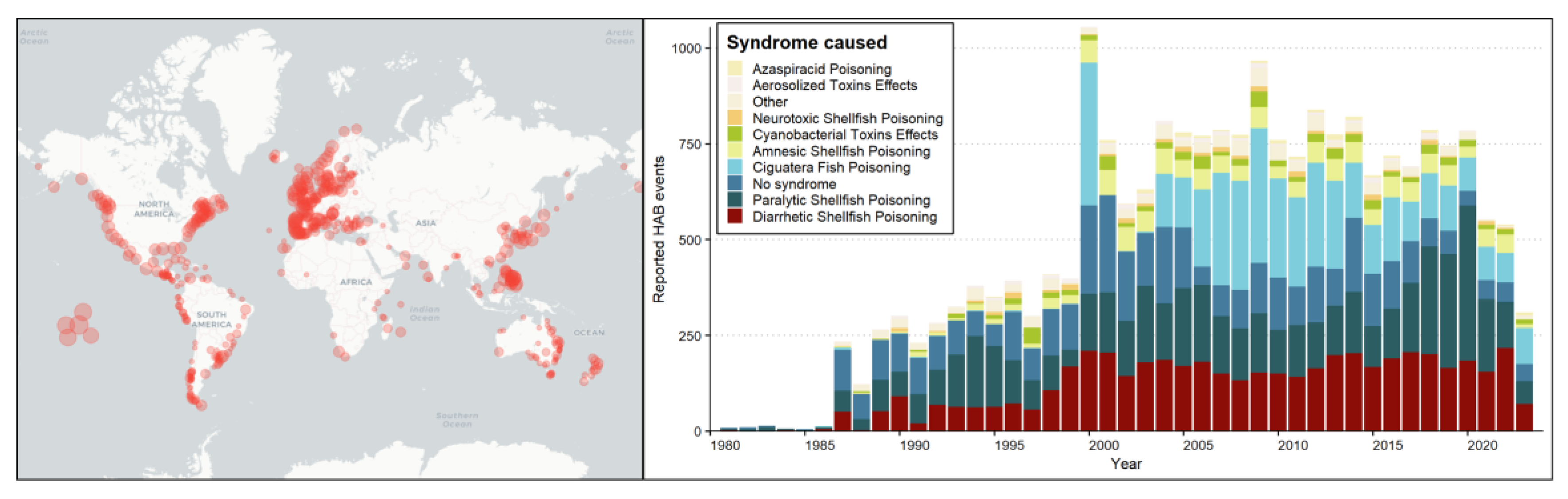
| Metal | Metalloenzyme | Metal | Metalloenzyme | |
|---|---|---|---|---|
|
Fe |
Fe-S proteins (e.g., aconitase) |
Mn |
Oxygen evolving enzymes | |
| Ferredoxin | Superoxide dismutase | |||
| Superoxide dismutase | Arginase | |||
| Nitrate reductase | Phosphotransferases | |||
| Nitrite reductase |
Cu |
Plastocyanin | ||
| Chelatase | Ferrous oxidase | |||
| Peroxidase | Amine oxidase | |||
|
Zn |
Carbonic anhydrase | Cytochrome oxidase | ||
| Alkaline phosphatase | Ascorbate oxidase | |||
| RNA polymerase | Superoxide dismutase | |||
| tRNA synthase |
Co |
Carbonic anhydrase | ||
| Reverse transcriptase | Cobalamin | |||
| Carboxypeptidase | Alkaline phosphatase | |||
| Superoxide dismutase | Se | Gluthathione peroxidase | ||
| Alcohol dehydrogenase | V | Bromoperoxidase | ||
| Aminopeptidase | Nitrogenase | |||
| Ni | Urease | Cd | Carbonic anhydrase | |
| Superoxide dismutase | Alkaline phosphatase |
| Species | Metals | Reference |
|---|---|---|
| Akashiwo sanguinea | Co (Vitamin B12) | [204] |
| Alexandrium | Zn | [205] |
| Alexandrium catenella | Cu, Sn (as butyltin) | [206] |
| Cu, Cd | [207] | |
| Zn, Pb | [208] | |
| Fe | [209] | |
| Co (Vitamin B12) | [204] | |
| Alexandrium minutum | Fe, Cu | [197] |
| Se | [210] | |
| Cu | [211] | |
| Co (Vitamin B12) | [204] | |
| Alexandrium tamarense | Fe | [212] |
| Amphidinium carterae | Cu | [213] |
| Aurecoccus anophagefferens | Zn, Cu, Ni, Cd | [214] |
| Se | [215] | |
| Co (Vitamin B12) | [204] | |
| Fe | [216] | |
| Aureoumbra lagunensis | Co (Vitamin B12) | [204] |
| Chaetoceros compressus | Cu | [217] |
| Chatonella antiqua | Fe | [218] |
| Chatonella marina | Zn, Cu, Cd, Pb, Hg | [219] |
| Co (Vitamin B12) | [204] | |
| Chatonella ovata | Zn, Cu, Cd, Pb, Hg | [219] |
| Choomonas | Cu, As | [220] |
| Cochlodinium polykrikodes | Cu | [221,222] |
| Cu and Pb | [223] | |
| Co (Vitamin B12) | [204] | |
| Dinophysis acuminata | Cu | [217] |
| Fibrocasa japonica | Co (Vitamin B12) | [204] |
| Gymnodinium aureolum | Co (Vitamin B12) | [204] |
| Gymnodinium breve | Cu, Ti, Zr | [224] |
| Gymnodium catenatum | Se | [210,225] |
| Fe, Cu, Se | [226] | |
| Zn, Cu, Cd, Pb, Hg | [219] | |
| Gymnodinium instriatum | Co (Vitamin B12) | [204] |
| Gymnodinium nagasakiense | Se | [227,228] |
| Gymnodinium sanguineum | Fe | [229,230] |
| Zn, Cu, Cd, Pb, Hg | [219] | |
| Heterosigma akashiwo | Fe | [218] |
| Heterocapsa artica | Co (Vitamin B12) | [204] |
| Heterocapsa circularisquama | Fe | [218] |
| Heterocapsa triquetra | Cu | [217] |
| Co (Vitamin B12) | [204] | |
| Karenia brevis | Fe, Cu, Co, Cd, Hg | [201] |
| Karenia mikimotoi | Co (Vitamin B) | [204] |
| Karenia selliformis | Mn, Zn, Cu, Co, Se | [231] |
| Karlodinium veneficum | Co (Vitamin B12) | [204] |
| Katodinium rotundatum | Cu, As | [220] |
| Lingulodium polyedrum | Fe | [209] |
| Cd | [232] | |
| Mo | [233] | |
| Ostreopsis cf. ovata | Cu, Sn (as butyltin) | [206] |
| Ostreopsis siamensis | Mn, Zn, Cu, Co, Se | [231] |
| Phaeocystis globosa | Co (Vitamin B12) | [204] |
| Polykrikos hartmannii | Co (Vitamin B12) | [204] |
| Protoceratium reticulatum | Mn, Zn, Cu, Co, Se | [231] |
| Fe, Co, Se | [234] | |
| Prorocentrum dentatum | Fe | [235] |
| Prorocentrum donghaiense | Co (Vitamin B12) | [204] |
| Prorocentrum lima | Mn, Zn, Cu, Co, Se | [231] |
| Prorocentrum micans | Cu | [213] |
| Prorocentrum minimum | Co (Vitamin B12) | [204] |
| Pseudo-nitzschia sp. | Fe, Cu | [175,198] |
| Pseudo-nitzschia australis | Mn, Zn, Cu, Co, Se | [231] |
| Fe, Cu | [236] | |
| Pseudo-nitzschia delicatissima | Fe, Cu | [237] |
| Cu | [238] | |
| Pseudo-nitzschia multiseries | Fe | [239] |
| Cu | [240,241] | |
| Co (Vitamin B12) | [204] | |
| Pseudo-nitzschia pungens | Co (Vitamin B12) | [204] |
| Rhizosolenia setigera | Cu | [217] |
| Scrippsiella trochoidea | Fe | [242] |
| Co (Vitamin B12) | [204] | |
| Skeletonema costatum | Fe | [235] |
| Takayama acrotrocha | Co (Vitamin B12) | [204] |
| Thalassiosira pseudonana | Cu, As | [220] |
| Trichodesmium | Ni | [190,243] |
| Species | Metals | Reference |
|---|---|---|
| Anabaena lemmermannii | Cu | [199] |
| Anabaenopsis sp. | Cu | [244] |
| Aphanizomenon schindlerii | Fe | [245] |
| Aphanizonmenon sp. | Cu | [199] |
| Chlamydomonas reinhardtii | Cu | [199,246] |
| Cylindrospermopsis raciboskii | Cu | [199] |
| Closterium ehrenbergii | Cu | [247] |
| Desmodesmus | Cu, Fe | [248] |
| Lyngbya wollei | Cu | [249] |
| Microcystis sp. | Cu, Fe | [248] |
| Zn | [250] | |
| Microcystis aeruginosa | Fe, Zn, Al, Cd, Cr, Cu, Mn, Ni, and Sn | [251] |
| Co, Cu, Fe, Mn, Mo | [252] | |
| Ca, Cu, Pb, Cd | [253] | |
| Fe, Mn, As | [254] | |
| Fe, Zn | [255] | |
| Fe, Cu | [200] | |
| Cu, Zn | [202,256] | |
| Zn, Cd | [257] | |
| Ni | [258] | |
| Fe | [259,260,261] | |
| Cu | [199,202,203,244,262,263,264,265] | |
| Zn | [266,267] | |
| As | [268] | |
| Oscillatoria sp | Cu | [199] |
| Pseudokirchneriella subcapitata | Cu | [199] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





