Introduction
Air fine and ultrafine particle are implicated according to recent studies to acute and chronic respiratory diseases, and among the physiological pathway mechanisms triggering to these pulmonary dysfunctions, stress oxidative with inflammatory mechanisms have been revealed as one of main mechanism, that can be a risk of cardiovascular and neurological disease. Still there this evidence still there unclear, although further studies are carrying out with aims to confirm it. However, the chronic exposure to urban air fine and ultrafine PM leads to enhance reactive oxidative species in cells and tissues, with the triggering of many oxidative and inflammatory biomarkers [
1]. Epidemiological studies generally show that the fine and ultrafine particle (PM
2.5, PM
0.1) have a greater impact on health than coarse PM
10 fraction [
2,
3]. The urban ambiant fine and ultrafine particle derive mostly from combustion processes of urban trafic, some Industrial activities, and consists of carbonaceous particles with associated absorbed organic compounds and polycyclic aromatic hydrocarbons as well as reactive metals as iron, copper, nickel, zinc, and vanadium. Near to traditional classification of air pollution based on particle size and masse concentration, will chemical composition and nanostructures of air pollutants as recent complementation of this classification [
4,
5]. These air particles once in contact with the lung’s epithelium initiate inflammatory mechanisms, macrophages activation, genetically modulation of gene expression and activation of transcriptions factors [
6], that can lead with the time development of chronic respiratory diseases. The understanding of oxidative molecules from air fine or ultrafine Particles, their transmission of the target organ, and the molecular pathways generating “reactive oxygen species” (ROS) in physiological and pathological processes, is the result of experimental studies and methodological developments [
7]. Many inhalation studies on experiment Animals have revealed adverse effects associated to triggering of ROS which depend on emission source and composition of particles [
5]. Recent studies showed ROS as most important mediators of particles toxicity, with notably particular association to respiratory disease. Ultrafine and fine particles themselves contain some ROS as well as redox-active components which can lead to ROS generation on interaction with the biological and cellular components [
2,
5]. Therefore, ROS are produced in the cell´s metabolic process, and come usually from photolysis, radiolysis, and hemolytic fission, where chemical bonds break and each created newly fragment preserves one of the bounded initial electrons [
5]. ROS contribute normally in Organism to different cellular processes, such as protein phosphorylation, secondary messengers, activation of transcriptions factors, immune responses, and apoptosis [
5,
6]. Moreover mitochondria are implicated to the endogen generation of ROS through the electron transport chain, which produce the primary ROS, the superoxide anion radical (O
2-), which can interact with others molecules to generate secondary ROS such as hydrogen peroxide (H
2O
2), and the hydroxyl radical (HO*) that lead to cellular and molecular damage through lipid peroxidation, notably when the formation of ROS submerges the antioxidant defence [
5].
Indeed, Organism cells possess an antioxidant defence system that consists of the enzymes of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) as well as some non-enzymatic antioxidants such as reduced gluthatione (GSH) and vitamins A, C and E [
8,
9]. These antioxidants help the organism to tackle deleterious effect of ROS and decrease the cellular damage. SOD is one the first line of protection against ROS [
9]. It catalyzes the breakdown of superoxide anion (O
-2), into the less potent oxidant hydrogen peroxide (H
2O
2) and allows with combined action of CAT to abate the production of superoxide radicals and hydrogen peroxide and consequently protects cellular components against ROS [
10]. As lipid peroxidation has been commonly used as an indicator of ROS-mediated damage to cell membranes. Malondialdehyde (MDA) is one of the best studied end-products of peroxidation of polyunsaturated fatty acids in clinical samples and is frequently used to estimate oxidative stress conditions [
11]. It is a lipid peroxidation marker widely utilized to estimate cell damage induced by ROS [
12].
The oxidative toxic stress in organism cells is also evaluated by measuring total antioxidant capacity (TAC) and total oxidant status (TOS), which both reflect the oxidative and anti-oxidative status of the body [
13]. When Recent studies investigated the association between human exposure to air pollution and oxidative stress, they considered the antioxidative defense status or ROS levels among people in polluted areas, through using of some biomarkers (MDA, SOD, CAT, GSH), [
14,
15]. Although significant poor air quality due the accelerated urban development and rapid population growth, ist most pronounced in many developing countries, there are still today very few studies, that revealed with regard to the adverse effects of urban air pollution the changes of oxidative stress parameters among exposed people [
5]. In sub-Saharan Africa, Data, which explore or indicate triggering of oxidative stress biomarkers from chronic exposure to ambient air fine or ultrafine particles in urban surrounding of this part of World are almost nonexistent [
16].
In this view, we carried out a survey in two towns of Cameroon, Douala and Dschang between February and July 2019 to investigate the possible oxidative, and pro-inflammatory responses of human cell pulmonary chronically exposed to urban air fine or ultrafine particle, trough examination of the activity of superoxide dismutase (SOD) and level of malondialdehyde (MDA) among Motorbike Drivers chronically exposed at different level of urban pollution.
Materials and Methods
Study Area
This cross-sectional study was conducted in urban area of Douala and Dschang. Douala, the economic capital of Cameroon with more than 3.5 million of inhabitants is the highest dynamic density city and one of the largest business and economic hub of Central Africa [
17]. Several intersections in Douala are places of huge daily urban traffic with thousands of vehicles and motorcycles, which are sometimes second-hand and dilapidated. In addition, Douala has two large industrial zones, some of which are often not far from inhabited areas.
On the other side, Dschang site within the Menoua Division in the West region of Cameroon, is situated about 216.3 kilometers from Douala at 1500 m above sea level. It is an agricultural and rural town in which the industrial activity is almost inexistent [
18].
Target Population and Sampling
Subjects recruited from both cities were MD with an average age of thirty years. MD from Dschang were took as control groups according to data of air quality index [
19], this city appears to be least polluted than Douala. They were mostly recruited of their stationement points at the level of great crossroads and called to respond an interviewer-administered questionnaire, on their daily level of general sensitivity of discomforts related to air pollution and invited in standard hospitals. In this view a most attention on their duration of activity, and their place of residence were taken in count. Their blood samples were taken and analysed according to human health in clinical research and GLP guidelines. In addition to the analysis of haematological data, (white blood cells and platelets), activity of superoxide dismutase (SOD) and level of malondialdehyde (MDA) were performed. Some confounding factors as consumption of Tobacco and habit to alcohol were to be considered.
Eligibity Criteria
In this Survey were included the healthy MD permanently living or working for at least five years near sites releaved to have bad air quality. On the other hand, were excluded from the study MD under treatment against several chronic respiratory and cardiovascular diseases, hepatitis, kidney ailments, also MD with a current or prior history of diabetes and others chronic metabolic disorders.
Blood Collection and Measurement
The blood of the Motorbike Drivers of both cities were taken from five milliliter of coagulated and EDTA tubes in the health examination centres at Douala and Dschang. These blood specimens were centrifuged at 3,000 rpm for 10 min within 45 min of collection. Then, serums were aliquoted, coded and stored at -80°C for the assessment of studied biological parameters.
Assement of Heamatological and Stress Oxidative Biomarkers
The stress oxidative effects of ambient air pollution in this study were evaluated through assessment of Superoxide dismutase (SOD) and Malondialdehyde (MDA). Then the mobilization of blood cells through short- or long- term inhalation of urban air fine and ultrafine particle were evaluated by measurement of haematological parameters.
Total red blood cell (RBC) count (x106/μl), Hemoglobin (Hb) content (g/dl), Total White Blood Cell (WBC) count (x103/μl) and platelet count (x103/μl) were assessed using urit 2900 plus hematology analyzer (Urit Medical Electronics, China).
MDA is a biproduct of lipid peroxidation, which was measured by colorimetric method using thiobarbituric acid reactive substances (TBARS). Indeed, serum specimens were mixed with a solution containing 1% thiobarituric acid, 1% acetic acid solution, distilled water, and heated for 60 min in a boiling water bath. The mixture was then placed on cold water to reach room temperature. Subsequently, TBARS adducts were extracted by n-butanol, followed by centrifugation at 3,500g for 10 mins. The layer, including n-butanol, was removed and the absorbance of the solution measured at 532 nm.
Measurement of SOD Activity
SOD is one of the most important antioxidant enzymes. It catalyzes the dismutation of superoxide anions (O2-) to give oxygenated water (H2O2) and molecular oxygen (O2). It was determined by the Misra and Fridovich method (1972), based on SOD's ability to inhibit or delay the self-oxidation of adrenaline in adrenochrome in a basic environment (pH 10.2). The increase in absorption read to 480 nm is proportional to the activity of superoxide dismutase. 150 µL of each test solution (serum) and solution prepared controls was introduced into the test tubes with 500 μl of carbonate-bicarbonate buffer (pH 10.2; 0.3 M; pKa 10.3), 250 μl of an EDTA solution (0.6 mM), and 350 μl of distilled water. The resulting mixture was homogenized and 250 μl of an epinephrine solution (4.5 mM) was added to initiate the reaction. Optical density was read after 30 seconds and 180 seconds at 480 nm.
Statistical Analyses
After having been evaluated were data classified, checked and analysed through statistical package for social science (SPSS) version 20.0. Frequencies and Mean ± standard deviation (sd) were considered as most important parameters to assess comparison of health effects of urban fine and ultrafine particle between subjects from Douala and Dschang. These both parameters were computed where appropriate. The statistical tests as Pearson’s independent Chi-square and Fisher’s exact were used. The mean of obtained parameters from Motorbike Drivers of both cities were statistically differentiated through Student´s t-test and the correlation between haematological parameters and oxidative stress markers were assessed using Pearson’s test. The influence of sites and time of exposure, the age of Subjects, level of education, the habits of consumption and the type of domestic fuel consumption for the daily kitchen, BMI, and distribution of observed symptoms on changes of oxidative stress markers. The models were adjusted for these different factors. The stress oxidative biomarkers levels were normally distributed. The significance was set at p-value < 0.05 and <0.001
Results
Demographic characteristics of subjects.
The
Table 1 presents the basis socio-demographic characteristics of MD recruited in both cities of study. The MD were majority young with age varying between 21 and 39. The mean age of participants of this study was 29.93 (± 0.82), approximatively the same in both cities. The MD in Douala were significantly more overweight than those recruited in Dschang (3.612 (P < 0.001)), according to the body mass index (BMI).
However, MD recruited in both cities had majority reached in secondary school (65.96%), with a most increased percentage in Douala. Most of them lived in Douala 3e, one of Douala's most populous neighborhoods. About 97% recruited in this town, work in the entire city.
As presented in the
Table 2, the MD recruited in both cities, never smoke majority (97.90%), but drinks alcohol regularly (91.09%), with a most significantly increased percentage among those of Douala. The majority of these have work experience ranging from 7 to 14 years (73.82%), and an average working time of 8 to 20 hours per day (85.86%).
Distributionof symptoms related to air pollution among participants.
According to data in the
Table 3, the symptoms associated to upper airways disorders, were more distributed among MD in Douala than those of Dschang. Running nose, cold, and sore throat were significantly more represented. Even more, dry cough, chest discomfort, and breathlessness representing the symptoms associated to lower airways disorders, were significantly more distributed among MD recruited in Douala than those of Dschang.
As showed, in
Table 4, symptoms associated to general discomforts as headache, eyes irritation, conjunctivitis, general tiredness, watering were significantly more distributed among MD in Douala. Nausea appears significantly more distributed among those of Dschang.
Biologicalassays.
Biological data obtained from MD including hematological parameters and oxidative stress markers as malondialdehyde, SOD, are presented in
Table 5. As shown, neutrophils in MD in Douala, appears significantly lower than those of Dschang. Monocytes were significantly more increased among MD in Douala. Differences between both categories of participants for the change of lymphocytes were not significant. On the other hand, hematocrit was significantly more increased among MD in Douala than those of Dschang.
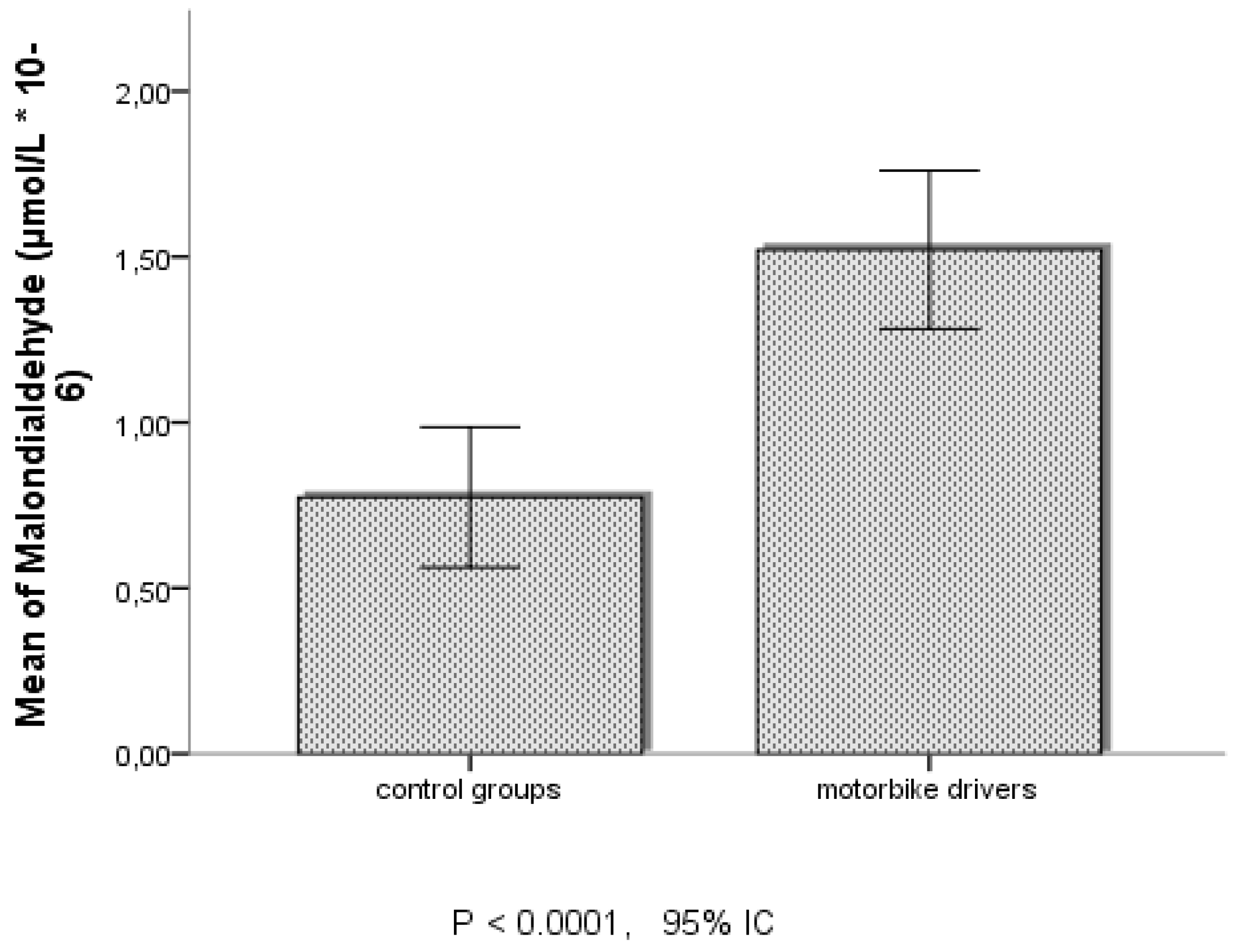
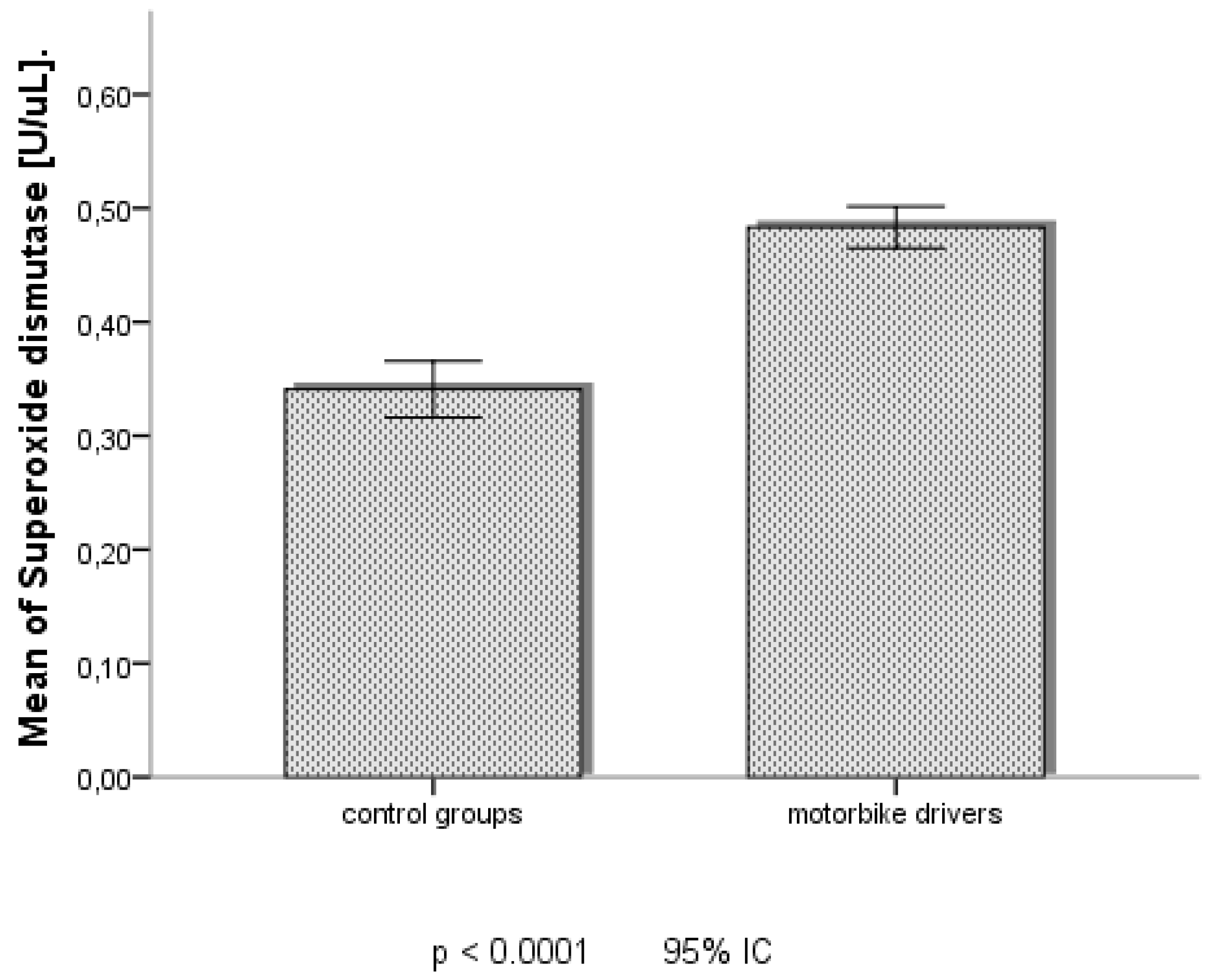
According to datas of oxidative stress markers in table and figures, MDA and SOD seemed significantly more increased among MD in Douala, than those of Dschang.
Changingof heamatological parameters and oxidative stress markers in motorbikes drivers in Douala
As shown in
Table 6, it was observed significant decrease of neutrophils with changing of SOD and MDA. Monocytes increase significantly with variations of both oxidative stress makers. Correlations between changing of oxidative stress and others hematological parameters as red blood cells, hemoglobin, hematocrit, lymphocytes and platelets were not observed.
Exposurefactors and changing of oxidative stress markers in motorbikes drivers in Douala
Data of
Table 7 revealed significant correlation between place of habitation and changing of SOD and MDA. These both oxidative stress markers seemed to be not influenced by work experience in years and average working time in hour per day
Respiratory disorders and changing of oxidative stress markers among motorbike drivers in Douala
As shown results of data in
Table 9, significant correlation between distribution of cold and changing of oxidative stress markers SOD, MDA was observed among MD. Distribution of others symptoms as sinusitis, running nose, current fever and sore throat were no significantly correlated with changing of both oxidative stress markers.
However, distribution of dry cough seemed significantly correlated with changing of MDA. It was no found significant correlation between distribution of wheezing breath, chest discomfort and variations of oxidative stress markers among MD. Distribution of breathlessness was almost significantly correlated with changing of SOD.
General discomforts and changing of oxidative stress markers among motorbike drivers in Douala
It is observed on data of
Table 9, an absence of significant correlation between distribution of headache, eyes irritation, watering, dizziness, conjunctivitis, general tiredness, nausea and the changing of both oxidative stress markers, among MD.
Discussion
The results of this study show how a short– or long-term exposure under polluted sites in urban areas could lead with the time the development of acute or chronical respiratory and cardiovascular diseases among Motorbike drivers living and working in urban areas. The assessment of some biological markers revealed the role of stress oxidative and proinflammatory mechanism as main pathways in the initiation and evolution of theses pathologies.
Of this view it was important to settle the evidence that chronically inhalation of fine and ultrafine pollutants in urban areas could biologically lead to cell production of reactive oxygen species, that through Fenton reaction activates cumulative compounded of peroxidation mechanisms, with the initiation and propagation of oxidated or ionised free radical molecules oft dangerous for the biological compounds as proteins, lipid, DNA or body cells. MDA evaluated in this study is one of the specific and sensitive biomarkers which allowed to show this molecular aspect among exposed subjects. However, these free radical chain reactions evolve restrict or control processes either partly by compartmentation, or by antioxidant defence and the production of enzyme systems that could reduce intracellular concentration of these reactive oxygen species. Among the enzyme systems is SOD implicated in the reduction of superoxide anion free radical into molecular oxygen and hydrogen peroxide (H
2O
2), that lead to decrease of O
2- level which is implicated to huge damage of. Cells. as a group of metalloenzymes SOD is found in all biological compartments and form the front line of defence against reactive oxygen species [
20,
21]. The biological changes of MDA and SOD evaluated among Motorbike Drivers confirm the evidence that the daily exposed under urban polluted air by fine and ultrafine particles lead to advanced free radical cell damages, particularly at level of pulmonary cells. The facts appear to be more pronounced among Motorbike Drivers of Douala than those of Dschang. The results of these clinical observations of stress oxidative with exposure against fine or ultrafine particles corroborate with results of Rezaei
et al., 2020, who revealed more increased change of blood MDA among healthy occupationally bus drivers, taxi drivers, policeman, and urban service workers exposed to outdoor air pollution in Iran [
8]. The same evidence was prior shown by Sorensen et al., 2003 in female students after environmental exposure to air pollutants [
22]. Furthermore, our study showed a significant correlation between change of MDA with place of habitation and frequences of symptoms of cold and dry cough among Motorbike Drivers. The others exposure factors and symptoms were not significantly correlated. This data revealed that Motorbike Drivers is not only daily exposed at level of the sites of activity but also where they live. It would be so important in the next studies to establish the profile of repartition of fine or ultrafine particles in urban air of Douala according to different areas. The results on the increase of biological SOD among Motorbikes Drivers of this Study corroborate with those of Davel et al., 2012 on the Rats which showed that exposure to PM
2,5 causes enhancing of protein expression of cu/Zn- and Mn-SOD in the pulmonary artery [
23]. This evidence differs however those of Rezaei
et al., 2020, that showed a decreased change of SOD, among exposed people in polluted surroundings in Iran [
8]. It is to notice that the magnitude of antioxidative response depends upon the several factors as duration of exposure, ambient fine or ultrafine particle concentration or toxicity, and the susceptibility of the subjects under polluted air [
24]. During low or acute exposure level of air pollutants may increase SOD-system in the early phase to reduce the oxidative damage while chronic exposure, or highly toxic air fine or ultrafine particle may lead exhaustion of endogenous antioxidant responses [
23]. For this reason, it could be observed with chronical exposures the progressively decrease of implication of enzyme SOD-system, that leave place to some endogenous antioxidant responses as NF-E2-related factor-2 (Nrf2), Nuclear factor-κB (NF-κB), NAD(P)H quinone oxidoreductase 1 (Nqo1), glutamate-cysteine ligase modifier subunit (Gclm), activation protein-1 (AP-1) and CREB-binding proteins (CBPs), that are regulated and influenced by redox status and implicated in the transcriptional regulation of a wide range of genes that are involved in oxidative stress and cellular response mechanisms [
24,
25,
26,
27].
Furthermore, the reduced activity of SOD as observed in this study may also result from a decrease in their resistance or the imbalance due to the deleterious impacts of the accumulation of superoxide anion and hydrogen peroxide initiated to air fine or ultrafine particles and their oxidative metabolites [
21]. The progressively loss of SOD activity reflects particularly increased oxidative and nitrative stress and could be observed in asthmatic patients. This evidence suggests that SOD serves as a surrogate marker of oxidative stress, notably in the case of asthma severity [
27]
. Although in this Study any motorbike drivers with Asthma were observed.
However, near to evidence of oxidative stress related to exposure to fine or ultrafine particles observed among the motorbike drivers in this study, it was also observed change of neutrophils, and monocytes. Clinically according to previous studies, the inflammatory effects of PM
10 have been revealed in experimental animal whom direct instillation into lung was done PM
10 provoke airway inflammation through the release of mediators that exacerbate lung disease in susceptible individuals [
27,
28]. Moreover, fine and ultrafine particles have been showed to be implicated to direct stimulation of Macrophage and epithelial cells to produce some inflammatory cytokines as TNF-α, TGF-β1, GM-CSF, PDGF, IL-6, and IL-8. An extensive literature confirms the generation of reactive oxygen and nitrogen species in airway epithelial cells and macrophages of the lung and have been reviewed extensively elsewhere [
27,
28], particularly in subjects with previous chronic diseases [
29]. The pathways of oxidative stress with reactive oxygen species are responsible for acute and chronic lung inflammation [
26]. The results of some studies suggest that oxidative stress, inflammation, and tissue damage are direct correlated with exposures to air fine or ultrafine particles [
30,
31]. This pathway mobilizes white blood cells and biomarkers implicated in the mechanism of phagocytosis. Then, an innate and adaptive immune response is induced by alveolar macrophages [
32]. The effects are more pronounced in patients with chronic pulmonary diseases [
33].
It was also revealed implication of PM
2.5 in the systemic inflammatory response through the release of inflammatory cytokines and chemokines from lung immune cells into the circulation [
28,
34]. The underlying mechanisms by which pulmonary oxidative stress leads to systemic inflammation in response to air pollution is still here not fully understood and should be more studied. Furthermore, the effects of air fine or ultrafine particulate on epithelial barrier function and tight junction expression in nasal mucosa in human has not been still here studied. But what is known is the loss of barrier function in the human nasal epithelium through decreased expression of tight junction proteins and increased of proinflammatory cytokines [
27], which rise mobilization of inflammatory cells and mediators. PM
10 and diesel exhausted particle, that consists of polyaromatic hydrocarbons (hydrophobic molecules) increase also lung inflammation by inhaled allergens or respiratory viral infection by acting as adjuvants. Several cytokines and chemokines were increased in animal experiments and human studies when lymphocytes and macrophages or monocytes were co-stimulated with particulates in the presence of specific allergens [
27]. The results of our study corroborate this evidence, especially in complex urban areas as Douala and Dschang, in which air could contain a mixture of pollutants or aerosols, and the proximity of different source could have more influence on the change of blood stress oxidative or inflammatory markers as indicated by the factor place of habitation of the study. This evidence should be further explored through additional studies on assessment of air pollution in these areas and the influence of each compound of aerosols on the evolution of pollution-related respiratory or cardiovascular and neurotoxicity disease.
Conclusion
In conclusion this study carried out at Douala and Dschang revealed one more time that the pathway of oxidative stress is a most important indicator in the development of systemic toxicity related to acute or long-term urban air pollution exposure. More recent studies either on Human or animals showed that fine or ultrafine are implicated in the evolution of airway inflammation and increase respiratory disorders. The oxidative cellular damage and their reactive oxygen species could explain the fine or ultrafine particles-induced human health effects. The variations of markers SOD and MDA, and white blood cells as Neutrophils and Monocytes prove that Motorbike Drivers in these chosen urban cities are daily exposure to risk of chronic respiratory disease, that could chronically lead to more complicated disorders. for this reason, it is very important to carry out more campaigns or studies of sensibilisation among active people in these urban cities. Moreover, additional studies are needed to explore and clarify the different mechanisms by which fine or ultrafine particle initiate health effects.
Authors’ Contributions: All authors contributed from the project conception to proofreading the manuscript.
Acknowledgements
This study was supported by research unit of Laboratory of Biochemistry of the Medicinal Plants, Food Science and Nutrition (LABPMAN) of University of Dschang, and of Desbrest Institute of Epidemiology and Public Health, University of Montpellier, INSERM, and Division of Respiratory Medicine, Allergology, and Thoracic Oncology, University Hospital of Montpellier, Montpellier, France.
Conflict of Interest
The authors declare having no conflict of interest.
References
- Araujo, J.A. Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Qual. Atmosphere Heal. 2010, 4, 79–93. [Google Scholar] [CrossRef]
- Leni, Z.; Cassagnes, L.E.; Daellenbach, K.R.; El Haddad, I.; Vlachou, A.; Uzu, G.; Prévôt, A.S.H.; Jaffrezo, J.-L.; Baumlin, N.; Salathe, M.; et al. Oxidative stress-induced inflammation in susceptible airways by anthropogenic aerosol. PLOS ONE 2020, 15, e0233425. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Kuntic, M.; Hahad, O.; Delogu, L.G.; Rohrbach, S.; Di Lisa, F.; Schulz, R.; Münzel, T. Effects of air pollution particles (ultrafine and fine particulate matter) on mitochondrial function and oxidative stress – Implications for cardiovascular and neurodegenerative diseases. Arch. Biochem. Biophys. 2020, 696, 108662. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Ghorani-Azam, A.; Riahi-Zanjani, B. Effects of air pollution on human health and practical measures for prevention in Iran. J. Res. Med Sci. 2016, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Leni, Z.; Künzi, L.; Geiser, M. Air pollution causing oxidative stress. Curr. Opin. Toxicol. 2020, 20–21, 1–8. [Google Scholar] [CrossRef]
- Sierra-Vargas, M.P.; Montero-Vargas, J.M.; Debray-García, Y.; Vizuet-De-Rueda, J.C.; Loaeza-Román, A.; Terán, L.M. Oxidative Stress and Air Pollution: Its Impact on Chronic Respiratory Diseases. Int. J. Mol. Sci. 2023, 24, 853. [Google Scholar] [CrossRef] [PubMed]
- Rajper, S.A.; Ullah, S.; Li, Z. Exposure to air pollution and self-reported effects on Chinese students: A case study of 13 megacities. PLOS ONE 2018, 13, e0194364. [Google Scholar] [CrossRef] [PubMed]
- Vandchali, N.R.; Koolivand, A.; Ranjbar, A.; Zarei, P.; Fathi, M.; Malekafzali, S.; Mollamohammadi, N.; Jalali-Mashayekhi, F. Oxidative toxic stress and p53 level in healthy subjects occupationally exposed to outdoor air Pollution - a cross-sectional study in Iran. J. Neurol. Sci. 2020, 27, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Ito, Y.; Suzuki, K.; Sasaki, R.; Otani, M.; Aoki, K. Mortality Rates from Cancer or All Causes and SOD Activity Level and Zn/Cu Ratio in Peripheral Blood: Population-based Follow-up Study. J. Epidemiology 2002, 12, 14–21. [Google Scholar] [CrossRef]
- Katerji, M.; Filippova, M.; Duerksen-Hughe, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxidative Med. Cell. Longev. 2018, 2019, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Motor, S.; Ozturk, S.; Ozcan, O.; Gurpinar, A.B.; Can, Y.; Yuksel, R.; Yenin, J.Z.; Seraslan, G.; Ozturk, O.H. Evaluation of total antioxidant status, total oxidant status and oxidative stress index in patients with alopecia areata. . 2014, 7, 1089–93. [Google Scholar]
- Rabha, R.; Ghosh, S.; Padhy, P.K. Indoor air pollution in rural north-east India: Elemental compositions, changes in haematological indices, oxidative stress and health risks. Ecotoxicol. Environ. Saf. 2018, 165, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Katoto, P.D.; Byamungu, L.; Brand, A.S.; Mokaya, J.; Strijdom, H.; Goswami, N.; De Boever, P.; Nawrot, T.S.; Nemery, B. Ambient air pollution and health in Sub-Saharan Africa: Current evidence, perspectives and a call to action. Environ. Res. 2019, 173, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Arsène Delors, F. G and Gilbert B. F. (2020). Port and industrial activities in douala – cameroon: socio-economic mutations and environmental consequences, Revista Universitară de Sociologie. 2020. [Google Scholar]
- Mayi, M.P.A.; Bamou, R.; Djiappi-Tchamen, B.; Fontaine, A.; Jeffries, C.L.; Walker, T.; Antonio-Nkondjio, C.; Cornel, A.J.; Tchuinkam, T. Habitat and Seasonality Affect Mosquito Community Composition in the West Region of Cameroon. Insects 2020, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Copernicus Atmosphere Monitoring Service (2019), https://air.plumelabs. 2019.
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. (Qassim) 2018, 12, 88–93. [Google Scholar] [PubMed]
- Van Raamsdonk, J.M.; Hekimi, S. Deletion of the Mitochondrial Superoxide Dismutase sod-2 Extends Lifespan in Caenorhabditis elegans. PLOS Genet. 2009, 5, e1000361. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.; Daneshvar, B.; Hansen, M.; O Dragsted, L.; Hertel, O.; Knudsen, L.; Loft, S. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ. Heal. Perspect. 2003, 111, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Davel, A.P.; Lemos, M.; Pastro, L.M.; Pedro, S.C.; de André, P.A.; Hebeda, C.; Farsky, S.H.; Saldiva, P.H.; Rossoni, L.V. Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology 2012, 295, 39–46. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Bevan, G.H.; Palanivel, R.; Das, L.; Rajagopalan, S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020, 34, 101545. [Google Scholar] [CrossRef] [PubMed]
- X. Xu, C. X. Xu, C. Liu, Z. Xu, K. Tzan, M. Zhong, A. Wang, M. Lippmann, L.C. Chen, S. Rajagopalan, Q. Sun (2011) Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue Toxicol. Sci., 124 (2011), pp.
- Delfino, R.J.; Staimer, N.; Vaziri, N.D. Air pollution and circulating biomarkers of oxidative stress. Air Qual. Atmosphere Health 2010, 4, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Leikauf, G.D.; Kim, S.-H.; Jang, A.-S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. 2020, 52, 329–337. [Google Scholar] [CrossRef] [PubMed]
- J.C. Hogg, S. J.C. Hogg, S. van Eeden, Pulmonary and systemic response to atmospheric pollution, Respirology 14 (2009) 336–346.
- Kampfrath, T.; Maiseyeu, A.; Ying, Z.; Shah, Z.; Deiuliis, J.A.; Xu, X.; Kherada, N.; Brook, R.D.; Reddy, K.M.; Padture, N.P.; et al. Chronic Fine Particulate Matter Exposure Induces Systemic Vascular Dysfunction via NADPH Oxidase and TLR4 Pathways. Circ. Res. 2011, 108, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary Oxidative Stress, Inflammation and Cancer: Respirable Particulate Matter, Fibrous Dusts and Ozone as Major Causes of Lung Carcinogenesis through Reactive Oxygen Species Mechanisms. Int. J. Environ. Res. Public Heal. 2013, 10, 3886–3907. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, R.S.; Bevan, G.H.; Palanivel, R.; Das, L.; Rajagopalan, S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020, 34, 101545. [Google Scholar] [CrossRef] [PubMed]
- R. Miyata, S.F. R. Miyata, S.F. van Eeden, The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter, Toxicol. Appl. Pharmacol. 257 (2011) 209–226.
- Gao, N.; Xu, W.; Ji, J.; Yang, Y.; Wang, S.-T.; Wang, J.; Chen, X.; Meng, S.; Tian, X.; Xu, K.-F. Lung function and systemic inflammation associated with short-term air pollution exposure in chronic obstructive pulmonary disease patients in Beijing, China. Environ. Heal. 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Becker, S.; Mundandhara, S.; Devlin, R.B.; Madden, M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: Further mechanistic studies. Toxicol. Appl. Pharmacol. 2005, 207, 269–275. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).