Submitted:
11 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area/Design
2.2. Inclusion Criteria
2.3. Samples and Data Collection
2.4. Ethical Approval
2.5. Enzyme Linked Immune Sorbent Assay (ELISA)
2.6. Nucleic acid (NA) Extraction
2.6.1. Nucleic Acid Amplification
2.6.2. Gel Electrophoresis
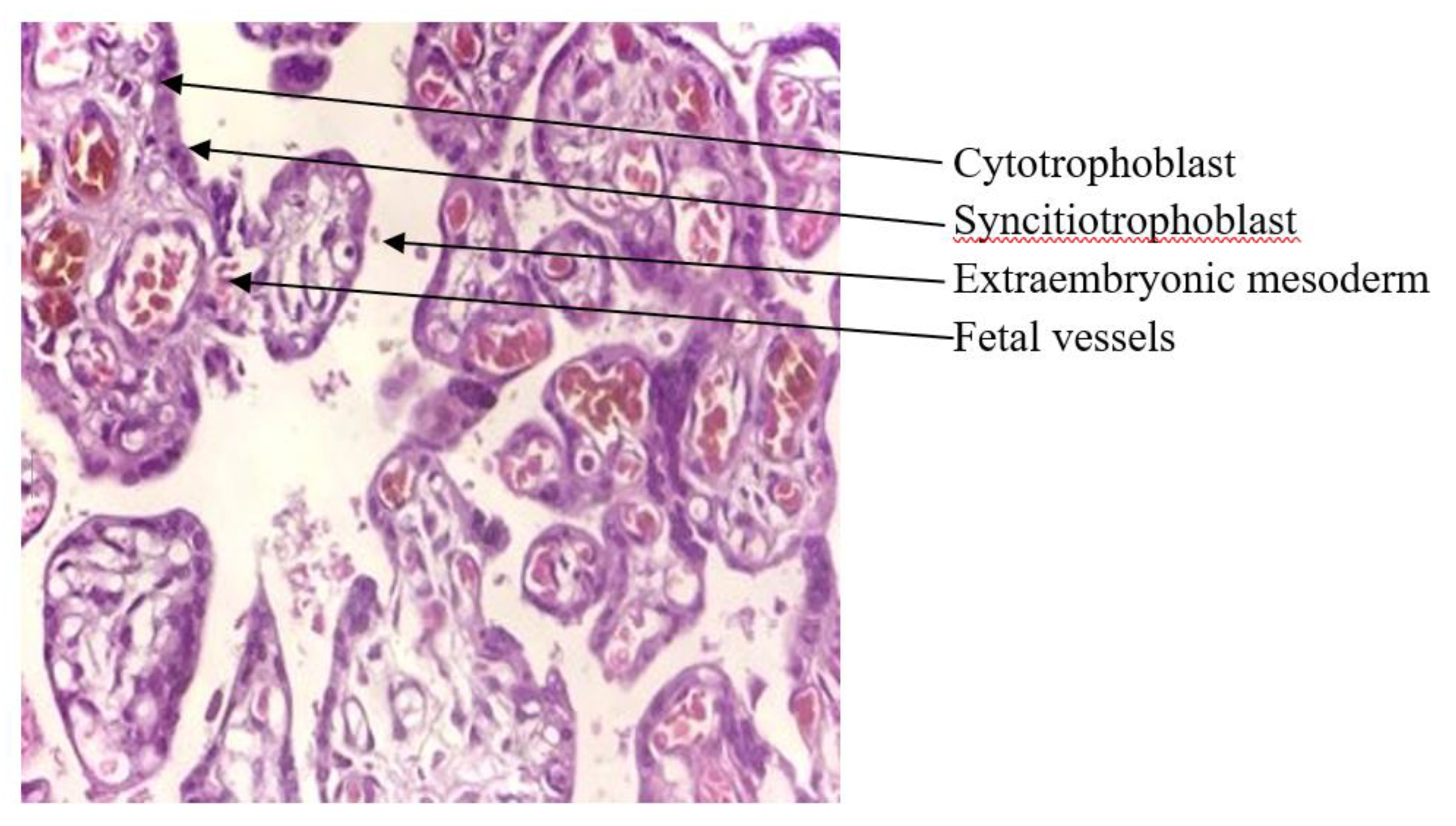
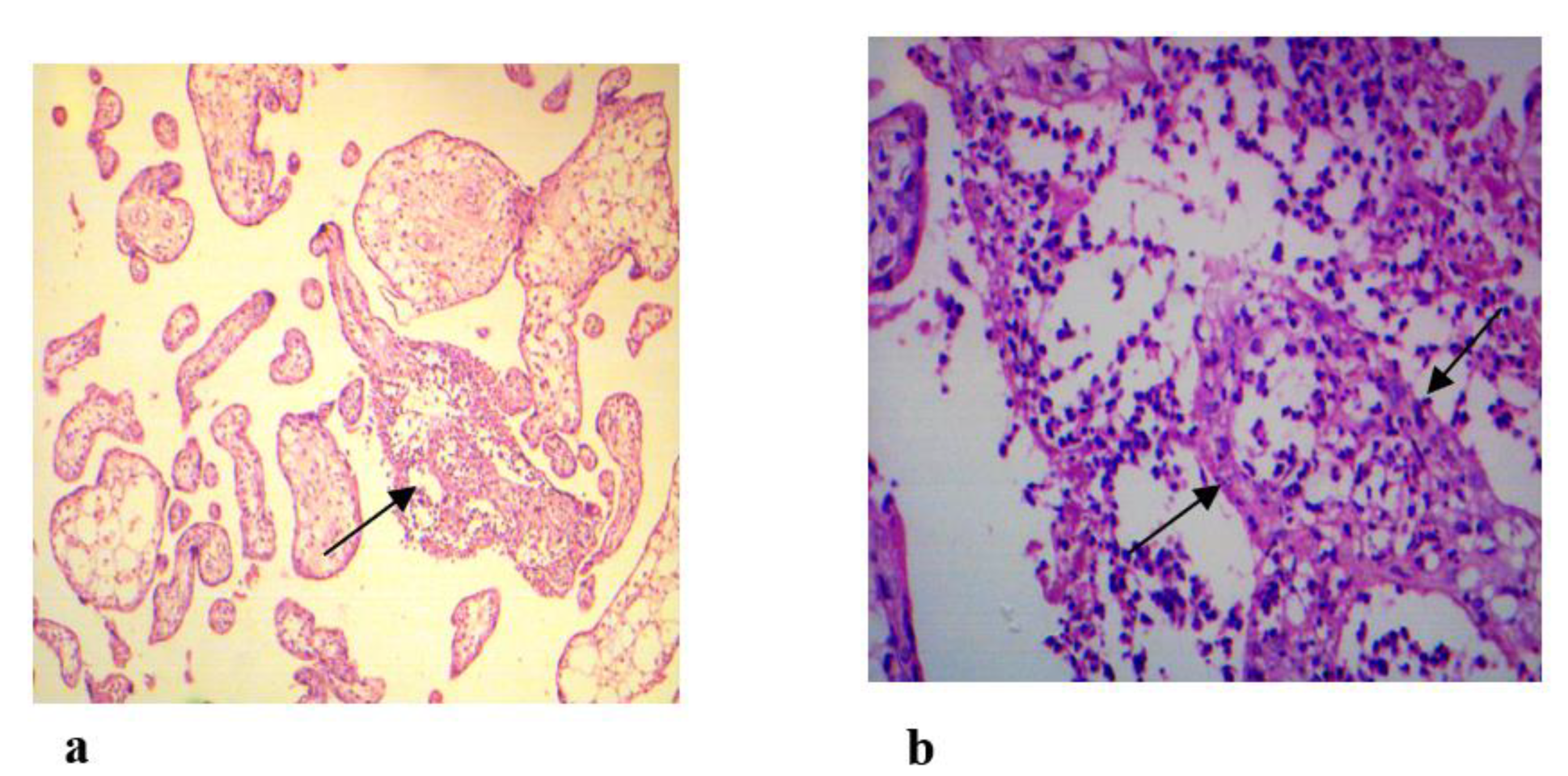
2.7. Histopathological Examination of Placenta Tissues
2.8. Data Analysis
3. Results
3.1. Demographics of Study Population
3.2. Discrepancies of ToRCH Antibodies and NA Positivity
3.3. ToRCH Pathogens and Histopathological Changes
3.4. Association CV Positivity with BOH Complications
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO, 2023; https://www.who.int/news-room/fact-sheets/detail/maternal-mortality. Accessed on date November 2, 2023.
- Qadir M, Amir S, Jadoon S, Marwat M. Frequency and causes of perinatal mortality in a tertiary care hospital in Peshawar, Pakistan. GJMS. 2018, 16, 15–19. [Google Scholar] [CrossRef]
- Nath J, Malhotra S, Rani N. A study on bad obstetrics history-with special emphasis on etiological high-risk factors. Neonat Pediatr Med. 2021, 7, 1–3. [Google Scholar]
- Ghazaey S, Keify F, Mirzaei F, Maleki M, Tootian S, Ahadian M, Abbaszadegan MR. Chromosomal analysis of couples with repeated spontaneous abortions in northeastern iran. Int. J. fertile. Steril. 2015, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Ahmed J, Alam A, Khokhar S, Khowaja S, Kumar R, Greenow C-R. Exploring women’s experience of health care use during pregnancy and child birth to understand factors contributing to perinatal deaths in Pakistan: A qualitative study. PloS One. 2020, 15, e0232823. [Google Scholar]
- Qi Y, Zhu S, Li C, Wu H, Yue H, Zhang Y, Zhu B, Ma J, Feng Z, Kong H, Cai X. Sero epidemiology of ToRCH antibodies in the reproductive-aged women in China. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2020, 254, 114–118. [Google Scholar]
- Manjunathachar HV, Singh KN, Chouksey V, Kumar R, Sharma RK, Barde PV. Prevalence of ToRCH infections and its associated poor outcome in high-risk pregnant women of central India: time to think for prevention strategies. Indian journal of medical microbiology. 2020, 38, 379–384. [Google Scholar] [CrossRef]
- Boyer SG, Boyer KM. Update on ToRCH infections in the newborn infant. Newborn Infant Nurs. Rev. 2004, 4, 70–80. [Google Scholar] [CrossRef]
- Kumar S, Sudarshan V. Placental histopathology in high-risk pregnancy. Journal of Obstetrics and Gynecology. 2018, 39-45.
- Ghidini A, Salafia CM. Histologic placental lesions in women with recurrent preterm delivery. Acta obstetricia et gynecologica Scandinavica. 2005, 84, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Boyd TK, Redline RW. Chronic histiocytic intervillositis: a placental lesion associated with recurrent reproductive loss. Hum Pathol. 2000, 31, 1389–1396. [Google Scholar] [CrossRef]
- Shaeen SK, Tharwani ZH, Bilal W, Islam Z, Essar MY. Maternal mortality in Pakistan: challenges, efforts, and recommendations. Annals of Medicine and Surgery. 2022, 81, 104380. [Google Scholar]
- Liu Y, Wu Y, Wang F, Wang S, Zhao W, Chen L, Tu S, Qian Y, Liao Y, Huang Y, Zhang R. The Association between Previous ToRCH Infections and Pregnancy and Neonatal Outcomes in IVF/ICSI-ET: A Retrospective Cohort Study. Front. Endocrinol. 2020, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Karamat S, Khan FA, Saeed S, Jafri A, Langhani SK, Saleem A. Occurrence of Seropositivity of Toxoplasma gondii, Cytomegalovirus, and Rubella Infections in Pregnant Women. Pak. J. Med. Health Sci. 2022, 16, 25–25. [Google Scholar]
- Mahmood F, Zafar M, Noreen S, Gul S, Ullah I. Khattak S. Evaluation of Herpes, Cytomegalovirus, Rubella, and Toxoplasma Gondii Infections in Swabi, KPK, Pakistan. Pak. J. Med. Health Sci. 2022, 16, 967–967. [Google Scholar]
- Saddam GA, Rehman A, Shahzad A. Prevalence of Cytomegalovirus, Rubella and Toxoplasma gondii among Internally Displaced Women of District North Waziristan, Pakistan with Home Obstetric History. J Biomed Res Environ Sci. 2021, 2, 1231–1237. [Google Scholar] [CrossRef]
- Zeb MA, Jamal SF, Mir A, Khan AA, Ullah A. Frequency of Torch Infections during Pregnancy in Peshawar, Pakistan. Adv. Appl. Sci. Res. 2018, 9, 22–26. [Google Scholar]
- Jamil S, Ahmed B, Ali S, Bashir S, Mahmood N, Idress M. Assessment of ELISA and real time PCR in diagnosis of Cytomegalovirus and Herpes Simplex Virus in Pregnant Women of Peshawar, Pakistan. Int. J. Biosci. 2017, 11, 35–42. [Google Scholar] [CrossRef]
- Mujtaba G, Khurshid A, Sharif S, Alam MM, Aamir U-B, Shaukat S, Angez M, Rana MS, Umair M, Shah AA, Zaidi SSZ. Distribution of cytomegalovirus genotypes among neonates born to infected mothers in Islamabad, Pakistan. PLoS One. 2016, 11, e0156049. [Google Scholar]
- Shah AA, Muhammad H, Farooqi N, Gul N, Khan AA, Nabi G, Khan SN. Association of ToRCH Agents with Spontaneous Abortion in Patients with Bad Obstetric History. WJZ. 2015, 10, 291–294. [Google Scholar]
- Ali S, Khan FA, Mian AA, Afzal MS. Seroprevalence of cytomegalovirus, herpes simplex virus and rubella virus among pregnant women in KPK province of Pakistan. JIDC. 2014, 8, 389–390. [Google Scholar] [CrossRef]
- Sadek OA, Abdel-Hameed ZM, Kuraa HM. Molecular detection of Toxoplasma gondii DNA in raw goat and sheep milk with discussion of its public health importance in Assiut Governorate. Assiut Vet. Med. J. 2015, 61, 166–177. [Google Scholar] [CrossRef]
- Munro SC, Hall B, Whybin LR, Leader L, Robertson P, Maine GT, Rawlinson WD. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J. Clin. Microbial. 2005, 43, 4713–4718. [Google Scholar] [CrossRef] [PubMed]
- Coyle PV, Desai A, Wyatt D, McCaughey C, O’Neill HJ. A comparison of virus isolation, indirect immunofluorescence and nested multiplex polymerase chain reaction for the diagnosis of primary and recurrent herpes simplex type 1 and type 2 infections. J. Virol. Methods. 1999, 83, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Bosma TJ, Corbett KM, O'Shea S, Banatvala JE, Best JM. PCR for detection of rubella virus RNA in clinical samples. J. Clin. Microbial. 1995, 33, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Abdoli A, Pirestani M. Are pregnant women with chronic helminth infections more susceptible to congenital infections? Front Immunol. 2014, 5, 53. [Google Scholar]
- Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014, 370, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Krishnan L, Nguyen T, McComb S. From mice to women: the conundrum of immunity to infection during pregnancy. J Reprod Immunol. 2013, 97, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Sappenfield E, Jamieson DJ, Kourtis AP. Pregnancy and susceptibility to infectious diseases. Infect Dis Obstet Gynecol doi. 2013, 2013, 752852. [Google Scholar]
- Obaid HM, Juma S-A. ToRCH screening test in pregnant women of Kirkuk city. MJS. 2016, 27, 17–25. [Google Scholar]
- Sadik MS, Fatima H, Jamil K, Patil C. Study of ToRCH profile in patients with bad obstetric history. Biol Med. 2012, 4, 95. [Google Scholar]
- Kishore J, Misra R, Paisal A, Pradeep Y. Adverse reproductive outcome induced by Parvovirus B19 and ToRCH infections in women with high-risk pregnancy. JIDC. 2011, 5, 868–873. [Google Scholar] [CrossRef]
- Vora KS, Gupta P, Saiyed S, Prajapati B, Natesan S. Prevalence of ToRCH Infections during Pregnancy: A Prospective Cohort Study in Tribal Region of Gujarat, India. ASWH. 2020, 2, 16–22. [Google Scholar]
- Kakayi ST, Haji HO, Al-Daoody AAK. Incidence of Miscarriage in Pregnant Women due to ToRCH Co-Infection in Erbil City/Iraq. JUBPAS. 2021, 29, 1–15. [Google Scholar]
- AL-Saeed AT, Abdulmalek IY, Ismail HG. ToRCH Infections in Mothers with BOH as a Cause of Neonatal Miscarriages and Fetal Malformation in Duhok Province, Kurdistan Region/Iraq. Sci J Univ Zakho. 2015, 3, 183–193.
- Jasim M, Majeed HA, Ali AI. Performance of Serological Diagnosis of ToRCH Agents in Aborted versus non aborted Women of West province in Iraq Tikrit Med J. 2011, 17. [Google Scholar]
- Jahromi AS, Kazemi A, Manshoori G, Madani A, Moosavy S, Seddigh B. Seroprevalence of rubella virus in women with spontaneous abortion. Am. J. Infec. Dis. 2011, 7, 16–19. [Google Scholar] [CrossRef]
- 38. Nirmal K, Saha R, Ramachandran VG. Khan AM. ToRCH infection in antenatal women: A 5-year hospital-based study. EJMS.. 2017; 54-57.
- Ali MS, Qowaider SR, Almal NYB. and Kahald FA. Seroprevalence of Antibodies to Cytomegalovirus, Rubella Virus and T. gondii among aborted women in El-Beida City. Saudi J Biomed Res. 2020, 5, 357–362. [Google Scholar] [CrossRef]
- Manandhar T, Hò G-T, Pump WC, Blasczyk R and Bade-Doeding C. Battle between Host Immune Cellular Responses and HCMV Immune Evasion. Int. J. Mol. Sci. 2019; 20: 3626.
- Goodrum, F. Human Cytomegalovirus Latency: Approaching the Gordian Knot. Annu Rev Virol. 2016, 3, 333–357. [Google Scholar] [CrossRef]
- Deftereou TE, Trypidi A, Alexiadi CA, Theotokis P, Manthou ME, Meditskou S, Simopoulou M, Lambropoulou M, Maria L. Congenital herpes simplex virus: A histopathological view of the placenta. Cureus. 2022, 14, e29101. [Google Scholar]
- Jackson SE, Mason GM, Wills MR. Human cytomegalovirus immunity and immune evasion. Virus Research. 2011, 157, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Al-Adnani M, Marnerides A. Chronic inflammatory conditions of the placenta. Diagnostic Histopathology. 2023, 29, 554–562. [Google Scholar] [CrossRef]
- Chudnovets A, Liu J, Narasimhan H, Liu Y, Burd I. Role of inflammation in virus pathogenesis during pregnancy. J Virol. 2020, 95, 10–128. [Google Scholar]
- Pretorius V, Wright CA, Hall DR, Schubert PT. Chronic villitis of unknown aetiology: Association with adverse pregnancy outcomes in a high-risk population in South Africa. In Obstetrics and Gynaecology Forum. 2019, 29, 7–12. [Google Scholar]
- Haseeb A, Khattak AA, Hayat A, ur Rehman M, Bano SA, Ahmed B, Khan I, Rehman A, Ullah R. and Barki S. Seroprevalence of ToRCH in Aborted versus non-aborted Women. J Infect. 2011, 63, 200–206.
- Turbadkar D, Mathur M. and Pele M. Seroprevelance of ToRCH infection in bad obstetric history. Indian J Med Microbiol. 2003, 21, 108–110. [Google Scholar]
- Irina T, Valeriu D. The role of ToRCH infection in early miscarriage. morphological study. German international journal of science. 2023, 1, 58–62. [Google Scholar]
- Mahmoud AM, Hagag HM, Ismail KA, Alharthi AM, Altalhi AA, Jaafer NF, Alharthi HH, Elwethenani AA, Khan KH, Al-ajmani SH, et al. Prevalence of Infectious Agents Causing Abortion in Pregnant Women Using Serological Tests and Histopathological Analysis. Appl Microbiol. 2023, 3, 698–708. [Google Scholar] [CrossRef]
- Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, Brosens JJ. Recurrent pregnancy loss. Nat. Rev. Dis. Prim. 2020, 6, 98. [Google Scholar] [CrossRef]
| Variables | Group A n (%) |
Group B n (%) |
|
|---|---|---|---|
|
Age Groups (Years) |
G-I = 18-27 | 13 (19.11) | 9 (13.23) |
| G-II = 28-37 | 24 (35.29) | 10 (14.70) | |
| G-III = 38-47 | 6 (8.82) | 6 (8.82) | |
| Trimesters | 1st | 18 (41.86) | - |
| 2nd | 19 (44.18) | - | |
| 3rd | 6 (13.95) | - | |
|
Antibodies (Positivity) |
IgM | 11 (16.17) | 1 (1.47) |
| IgG | 10 (14.70) | 21 (30.88) | |
| IgM and IgG | 1 (1.47) | - | |
| NA | Positive | 26 (38.23) | 19 (27.94) |
| BOH Complications | RSAs | 23 (33.82) | 9 (13.23) |
| Stillbirth | 8 (11.76) | 7 (10.29) | |
| IUGR | 2 (2.94) | - | |
| Neonatal death | 4 (5.88) | 5 (7.35) | |
| CA | 6 (8.82) | 4 (5.88) | |
| Groups (n) |
T.gondii | RV | CMV | HSV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM n (%) |
IgG n (%) |
NA n (%) |
IgM n (%) |
IgG n (%) |
NA n (%) |
IgM n (%) |
IgG n (%) |
NA n (%) |
IgG n (%) |
NA n (%) |
|
|
Group A (43) |
2 (4.65) | 4 (9.30) | 6 (13.95) | 6 (13.95) | 3 (6.97) | 8 (18.6) | 3 (6.97) | 1 (2.32) | 6 (13.95) | 2 (4.65) | 6 (13.95) |
|
Group B (25) |
- | 10 (40) | 3 (12) | 1 (4) | 6 (24) | 4 (16) | - | 3 (12) | 6 (24) | 2 (8) | 6 (24) |
| Total (68) | 2 (2.94) | 14 (20.6) | 9 (13.23) | 7 (10.29) | 9 (13.23) | 12 (17.64) | 3 (4.41) | 4 (5.88) | 12 (17.64) | 4 (5.88) | 12 (17.64) |
|
p-value |
0.062 | 0.088 | 0.113 | 1.000 | |||||||
| Category |
T. gondii n (%) |
RV n (%) |
CMV n (%) |
HSV n (%) |
Total n (%) |
|---|---|---|---|---|---|
| Cat-I | 1 (1.47) | - | - | - | 1 (1.47) |
| Cat-II | 2 (2.94) | 4 (5.88) | 3 (4.41) | - | 9 (13.23) |
| Cat-III | - | 3 (4.41) | - | - | 3 (4.41) |
| Cat-IV | 7 (10.29) | 3 (4.41) | - | - | 10 (14.70) |
| Cat-V | 6 (8.82) | 6 (8.82) | 4 (5.88) | 4 (5.88) | 20 (29.41) |
| Cat-VI | 1 (1.47) | 2 (2.94) | 5 (7.35) | 8 (11.76) | 16 (23.52) |
| Total | 17 (25) | 18 (26.47) | 12 (17.64) | 12 (17.64) | 59 86.76) |
| Groups (n) |
T.gondii | RV | CMV | HSV | ||||
|---|---|---|---|---|---|---|---|---|
| Ab/CV | NA/CV | Ab/CV | NA/CV | Ab/CV | NA/CV | Ab/CV | NA/CV | |
|
Group A (43) |
6/3 | 6/2 | 9/3 | 8/4 | 4/2 | 6/2 | 2/1 | 6/1 |
|
Group B (25) |
10/1 | 3/1 | 7/2 | 4/2 | 3/0 | 6/1 | 2/2 | 6/1 |
| Total = 68 | 16/4 | 9/3 | 16/5 | 12/6 | 7 /2 | 12/3 | 4/3 | 12/2 |
| BOH complications (n) |
Group A n (%) |
Group B n (%) |
Total n (%) |
|---|---|---|---|
| RSAs (32) | 4 (12.5) | 3 (9.37) | 7 (21.87) |
| Stillbirths (15) | 1 (6.66) | 1 (6.66) | 2 (13.33) |
| IUGR (2) | 1 (50) | - | 1 (50) |
| Neonatal deaths (9) | 2 (22.2) | 1 (11.1) | 3 (33.3) |
| CA (10) | 1 (10) | - | 1 (10) |
| p-value | 0.826 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





