Submitted:
11 May 2024
Posted:
14 May 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Theoretical Equations
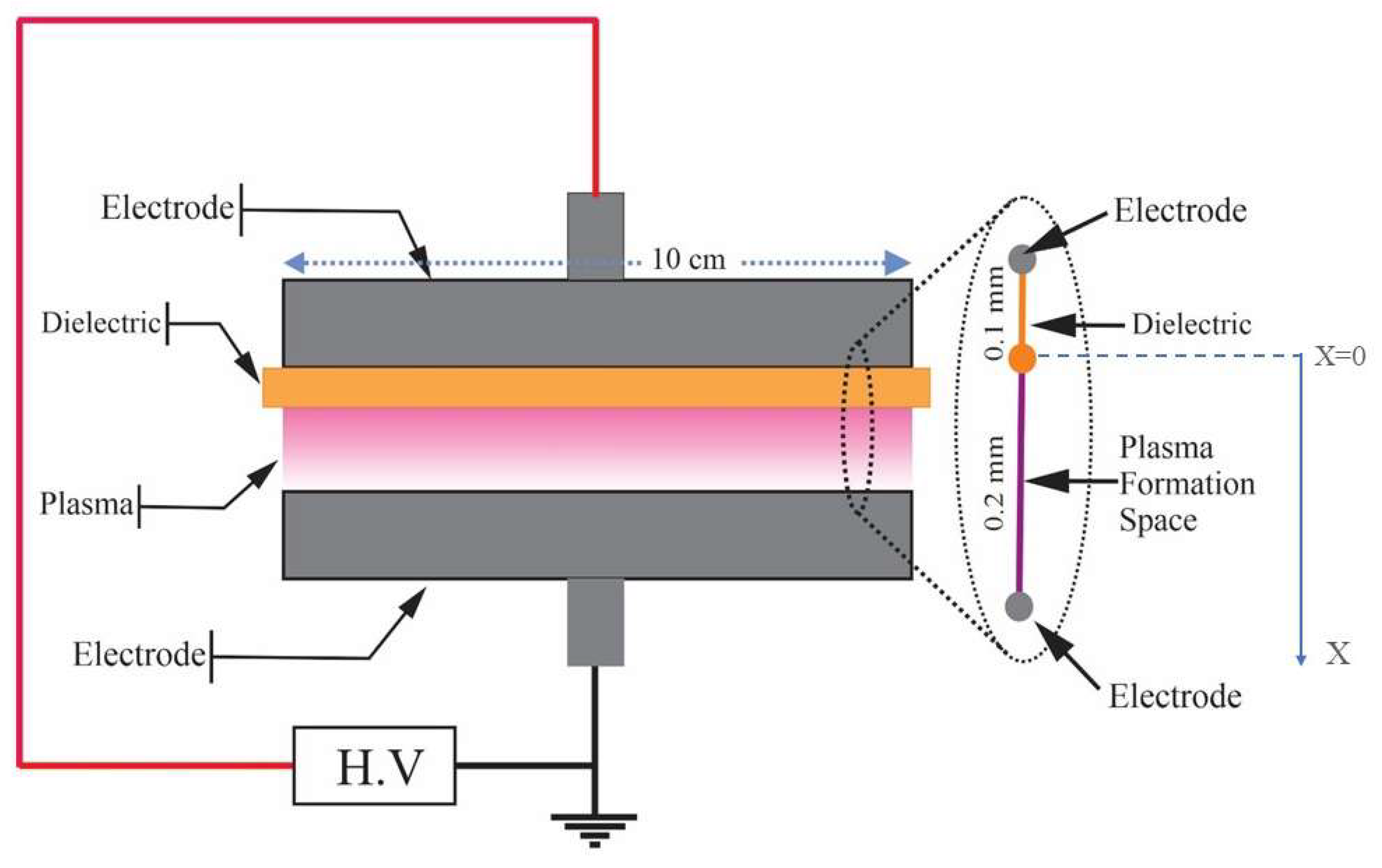
DBD Simulations in Sterilization Process
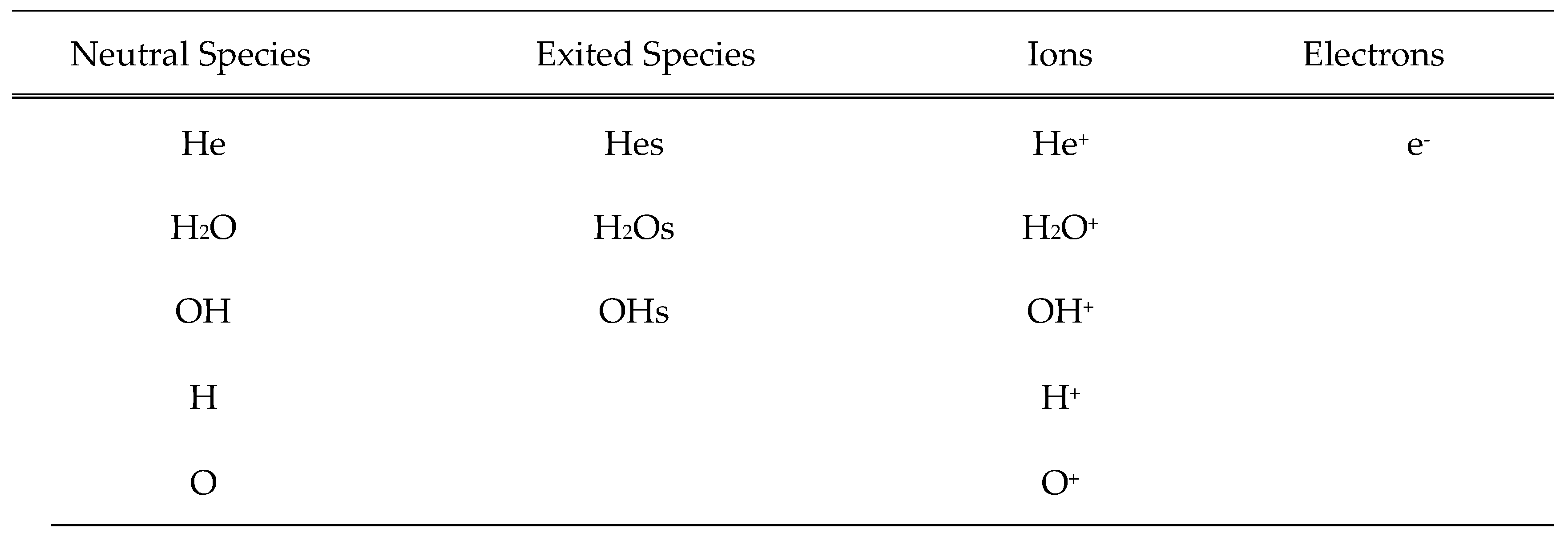
Chemical Model
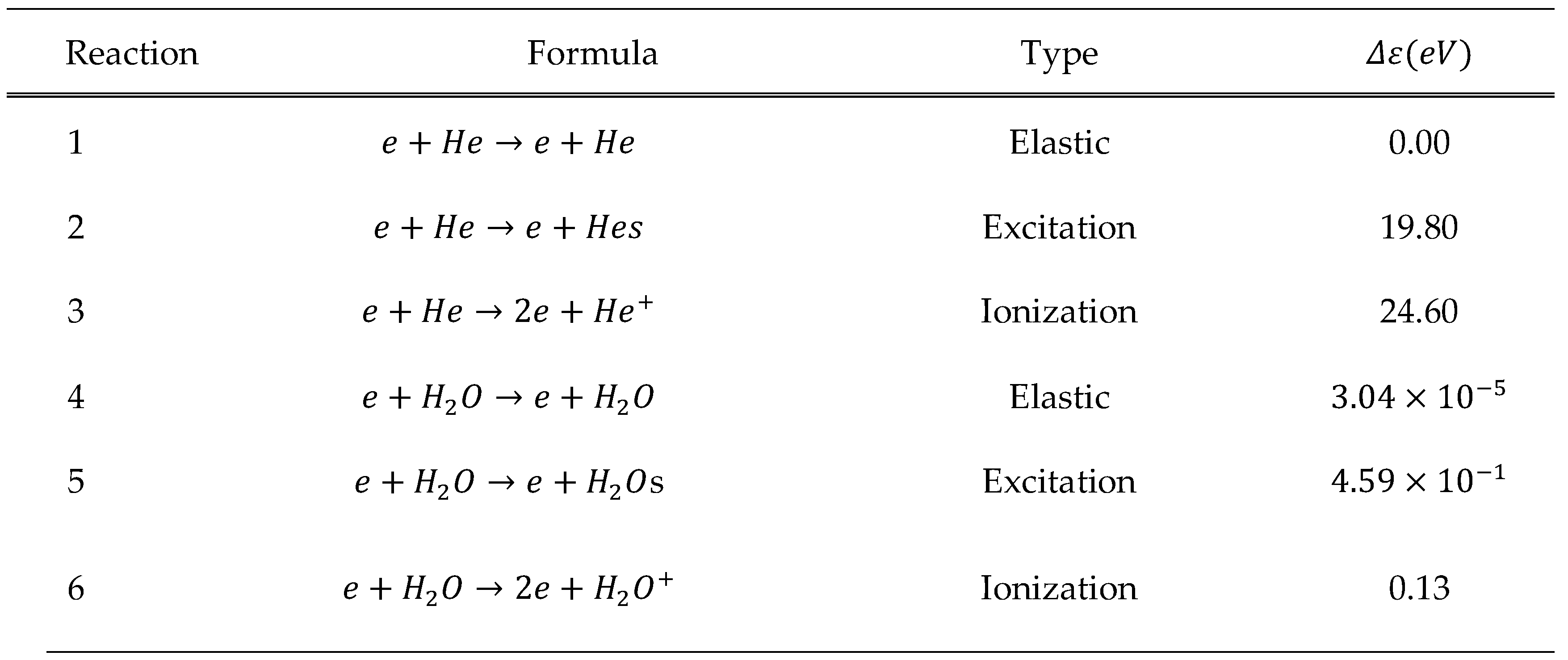
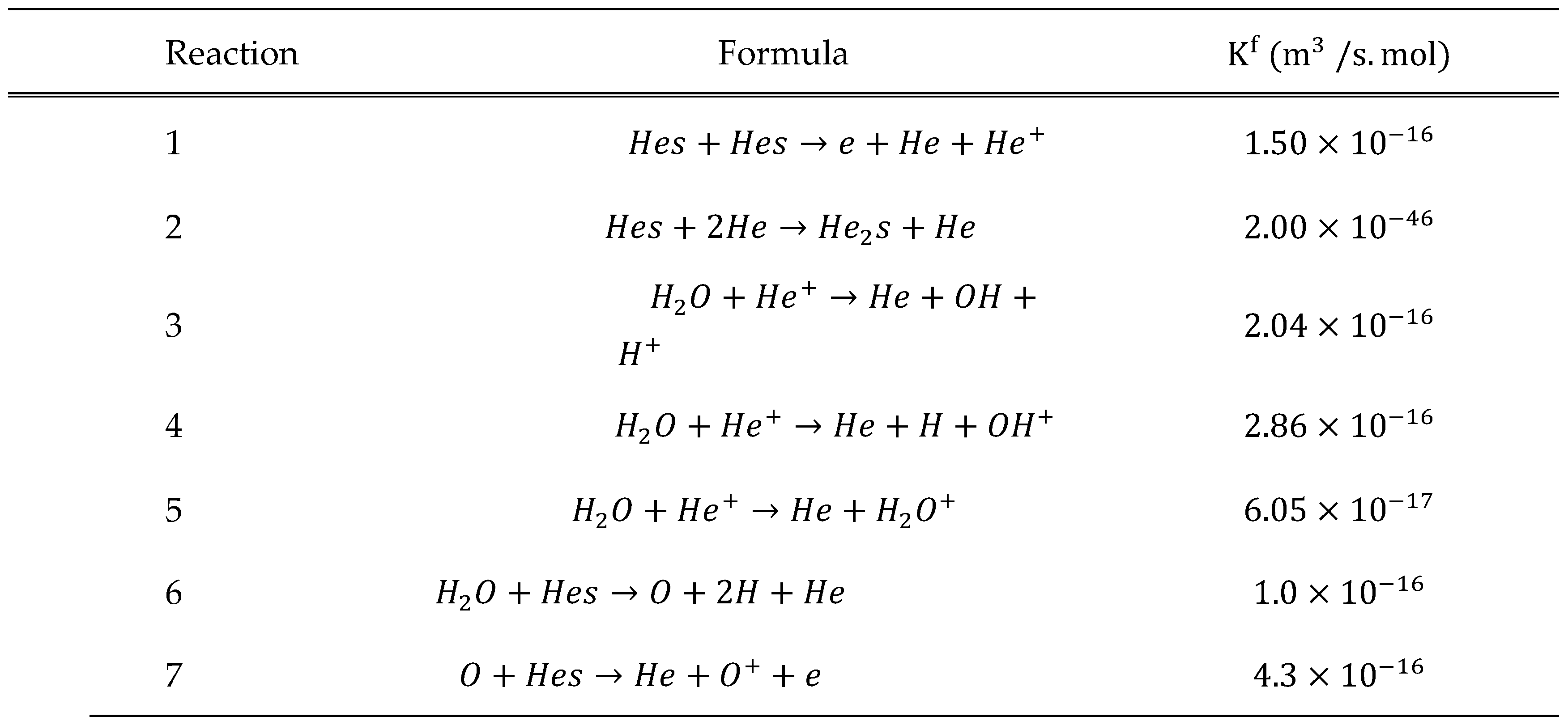
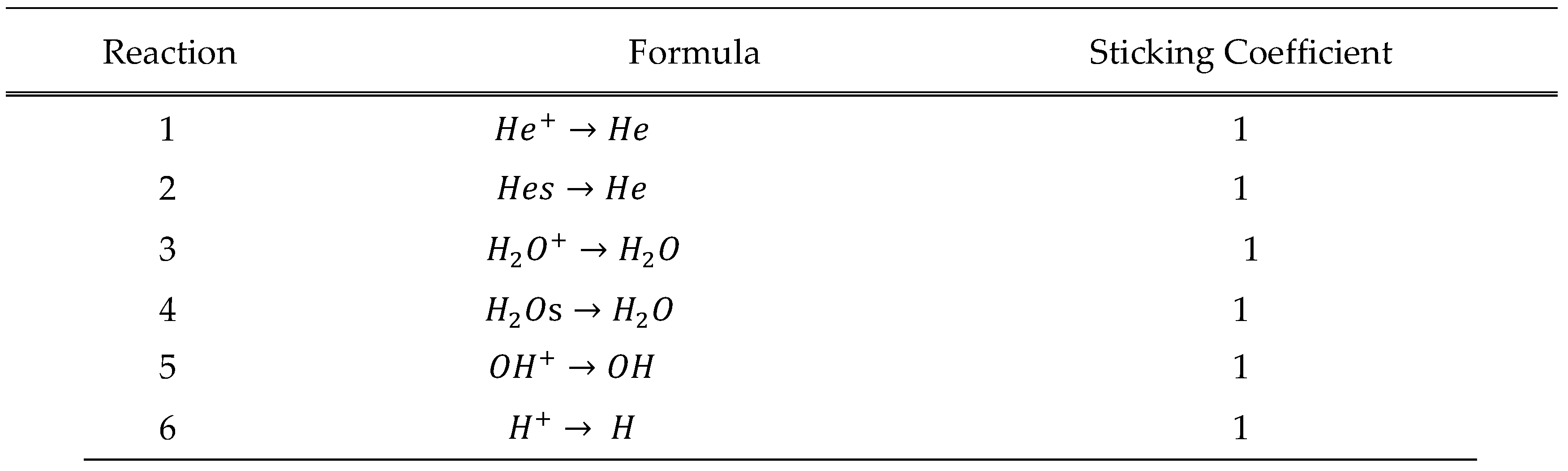
Boundary Conditions and Reactions
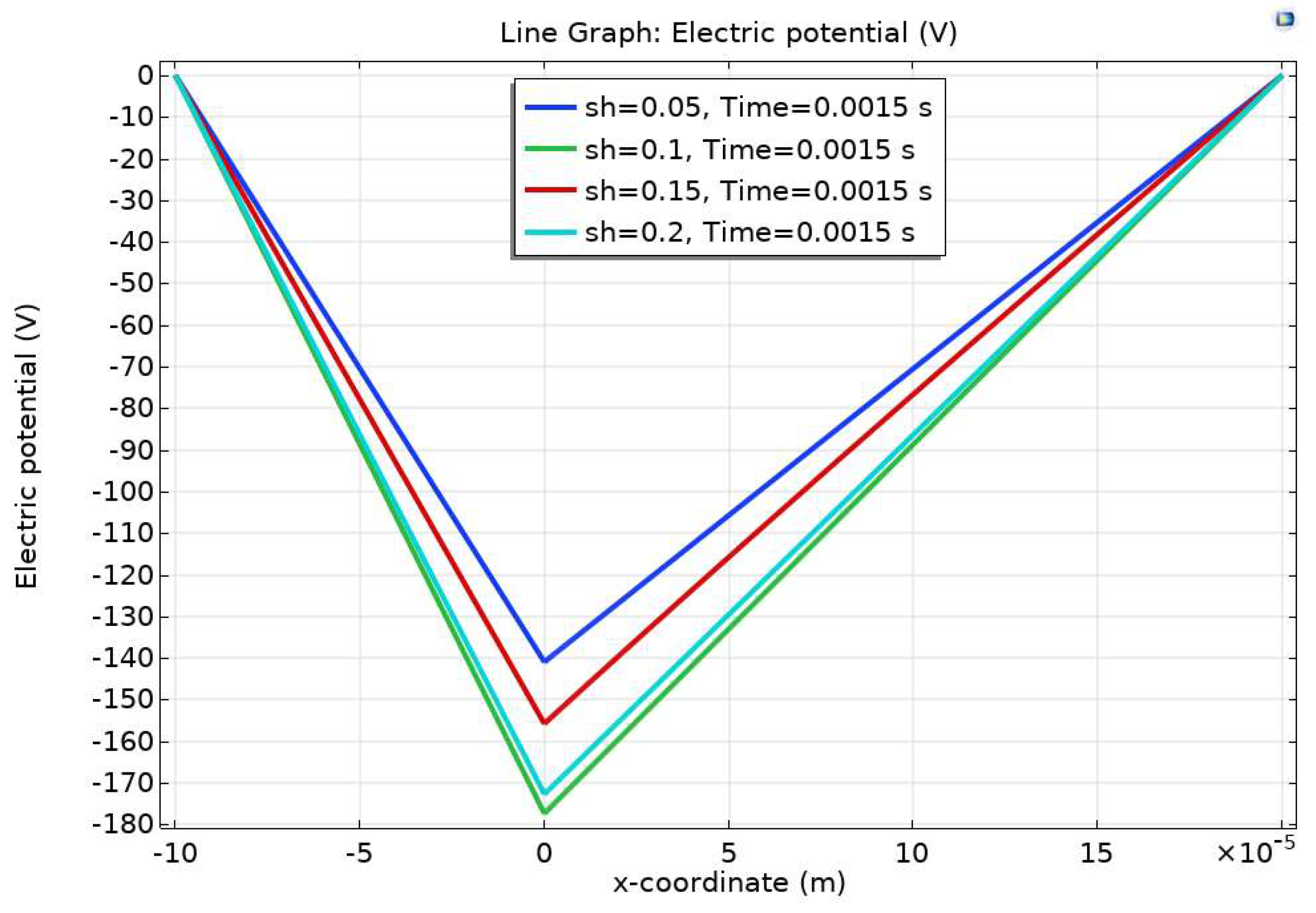
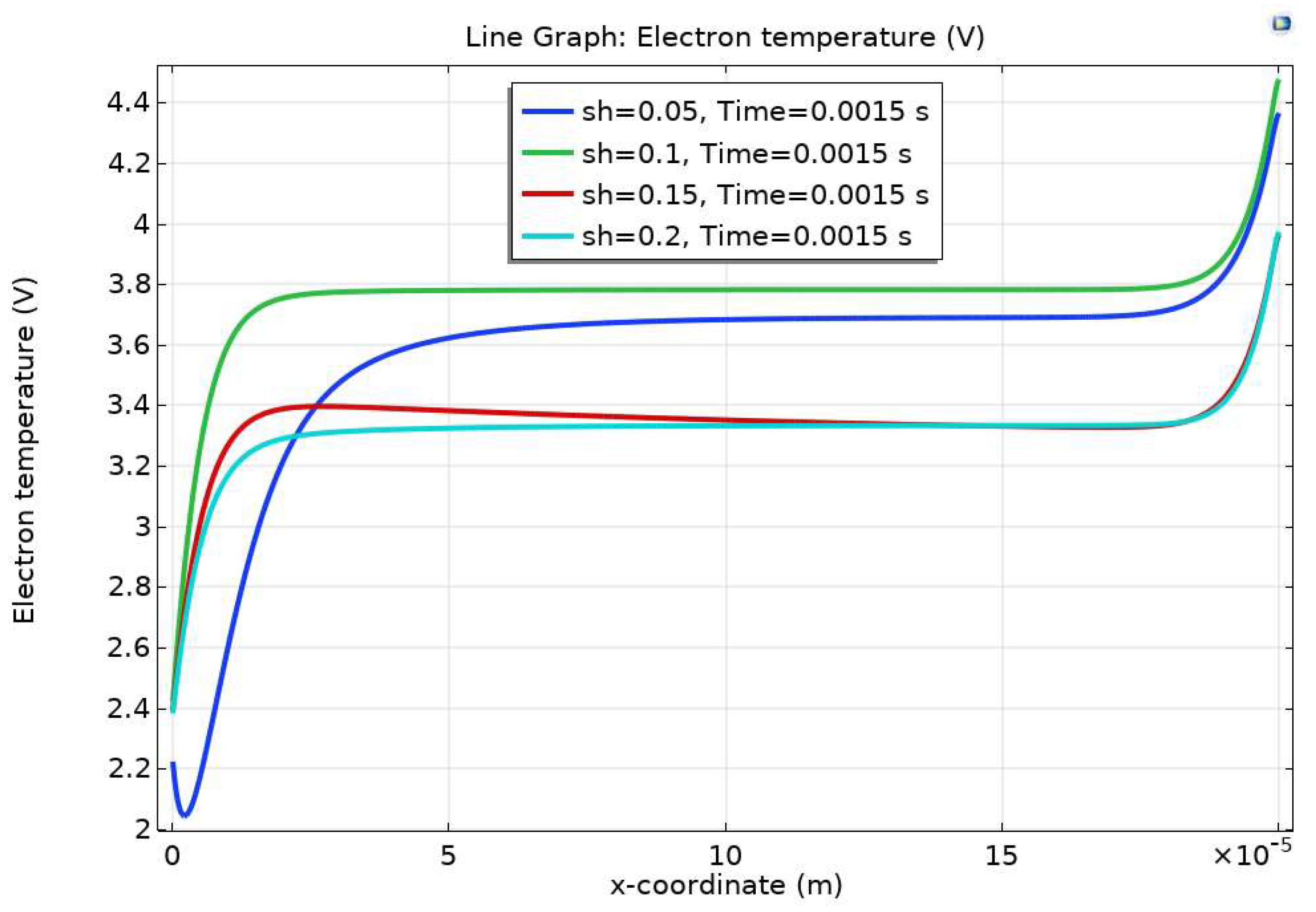
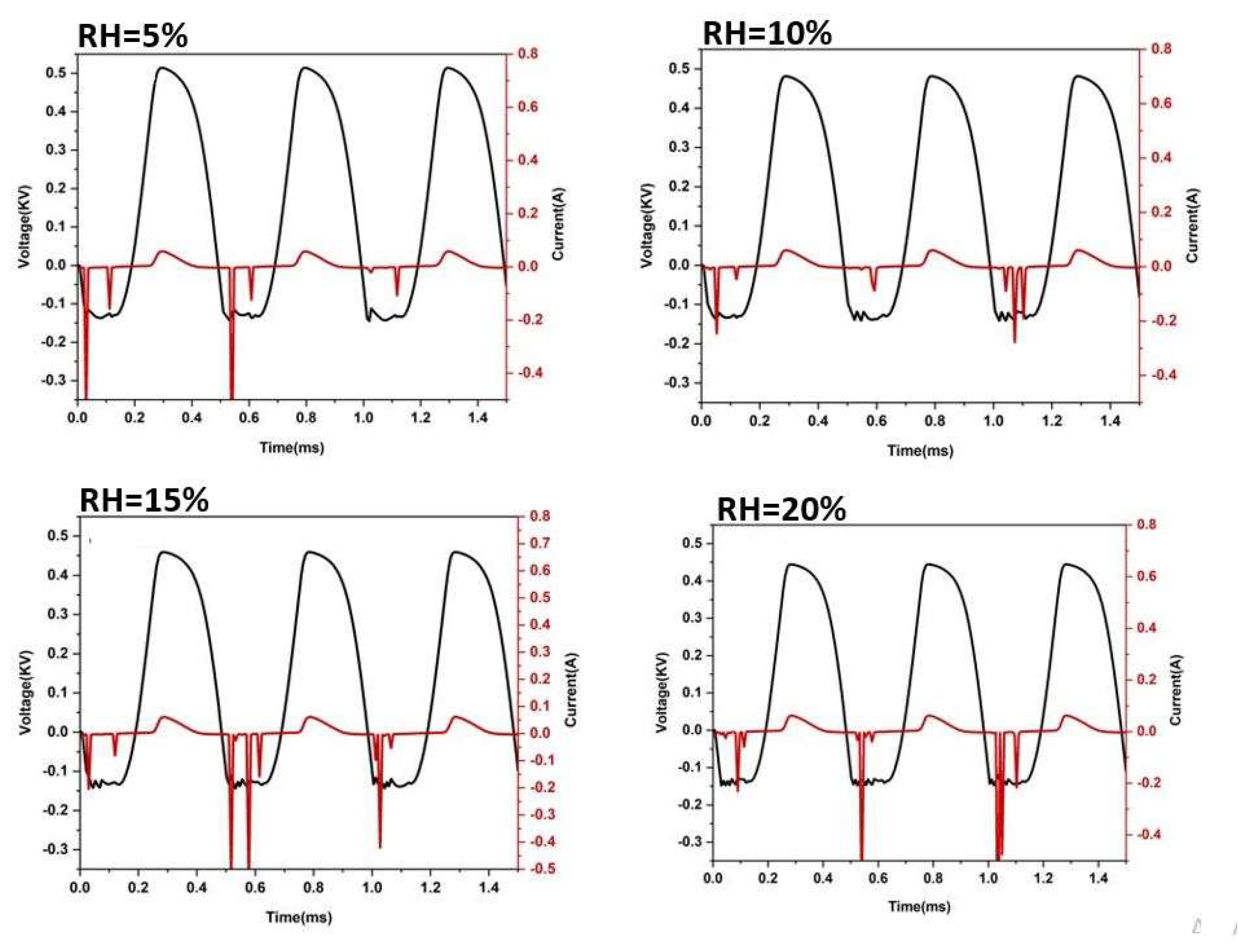
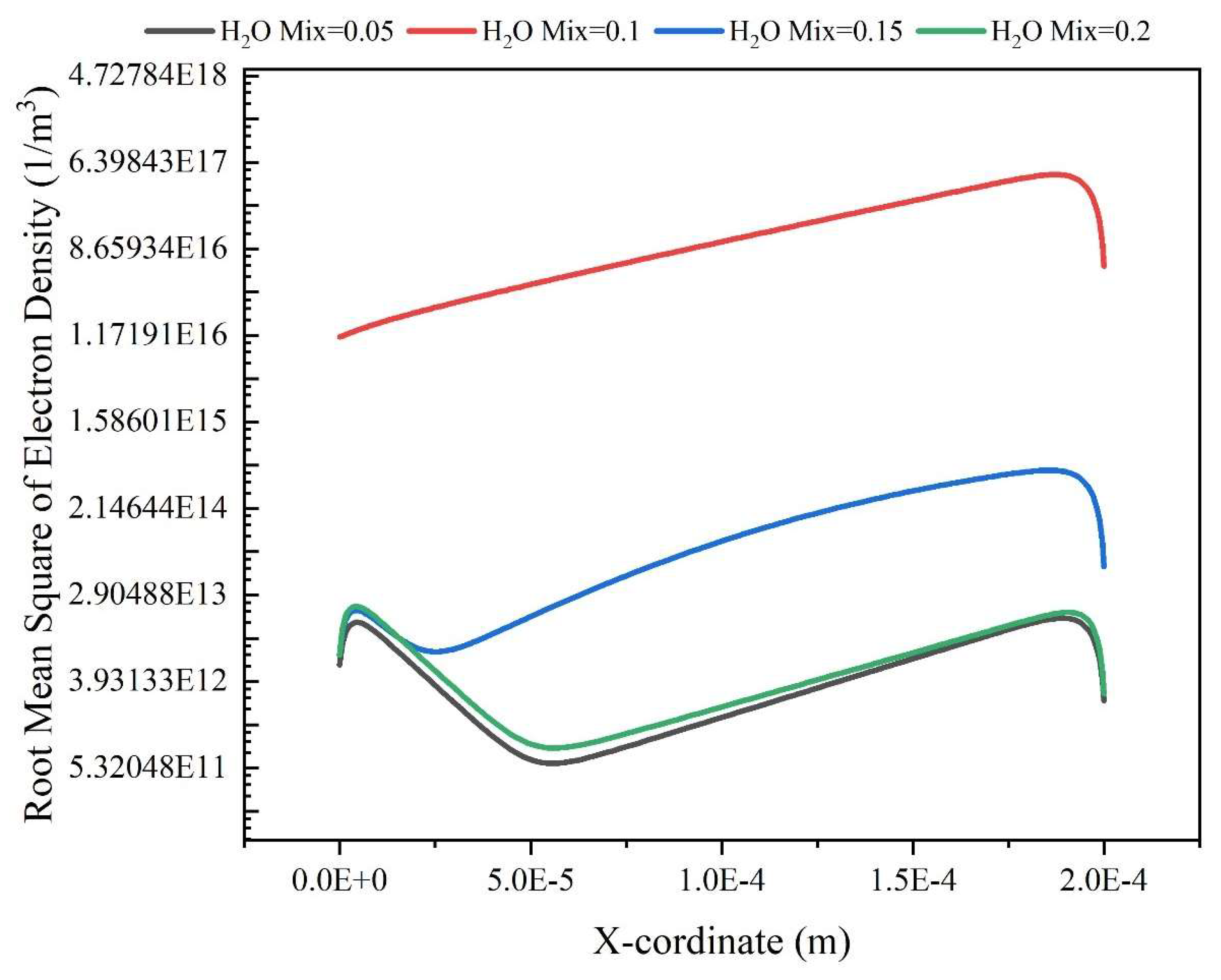
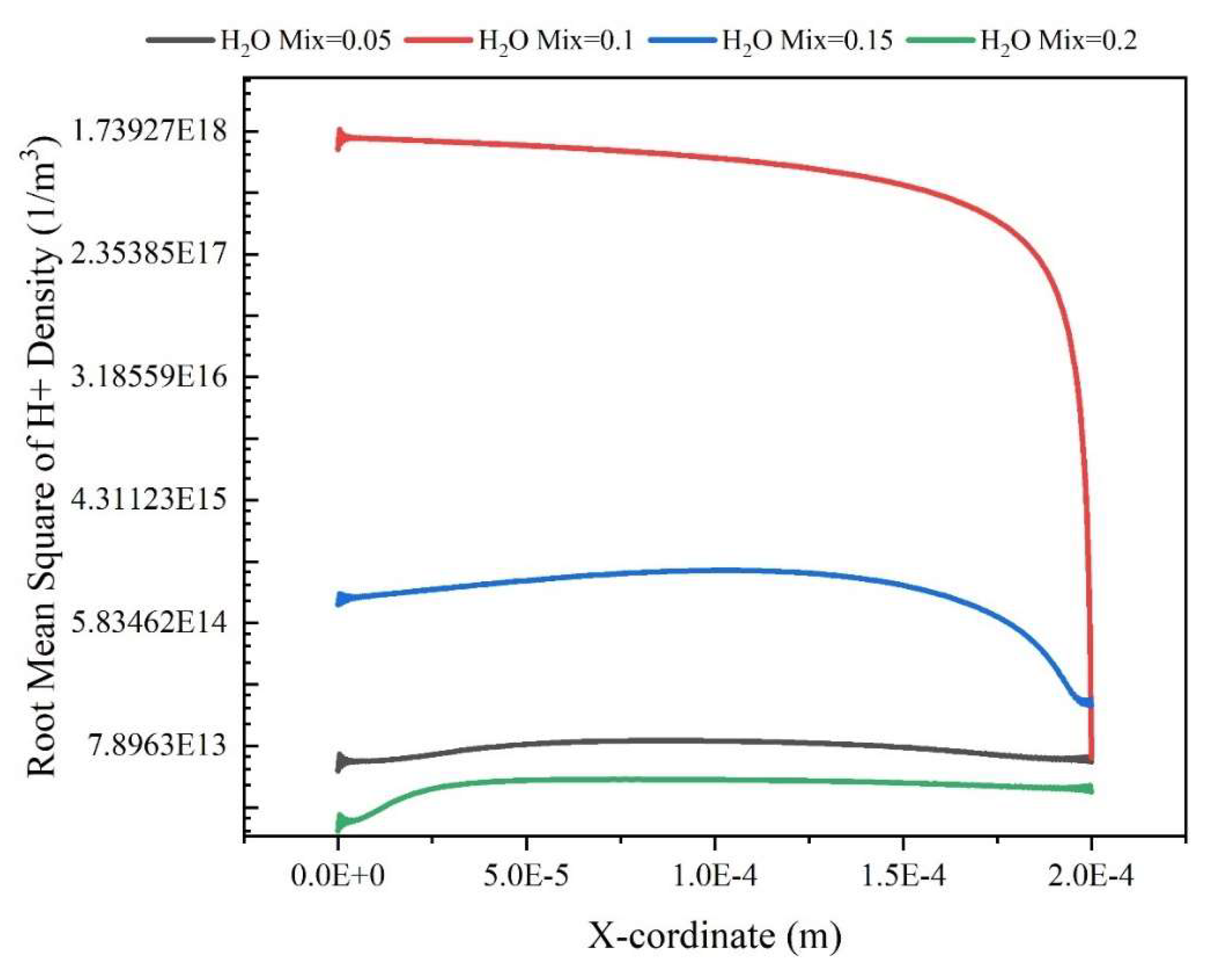
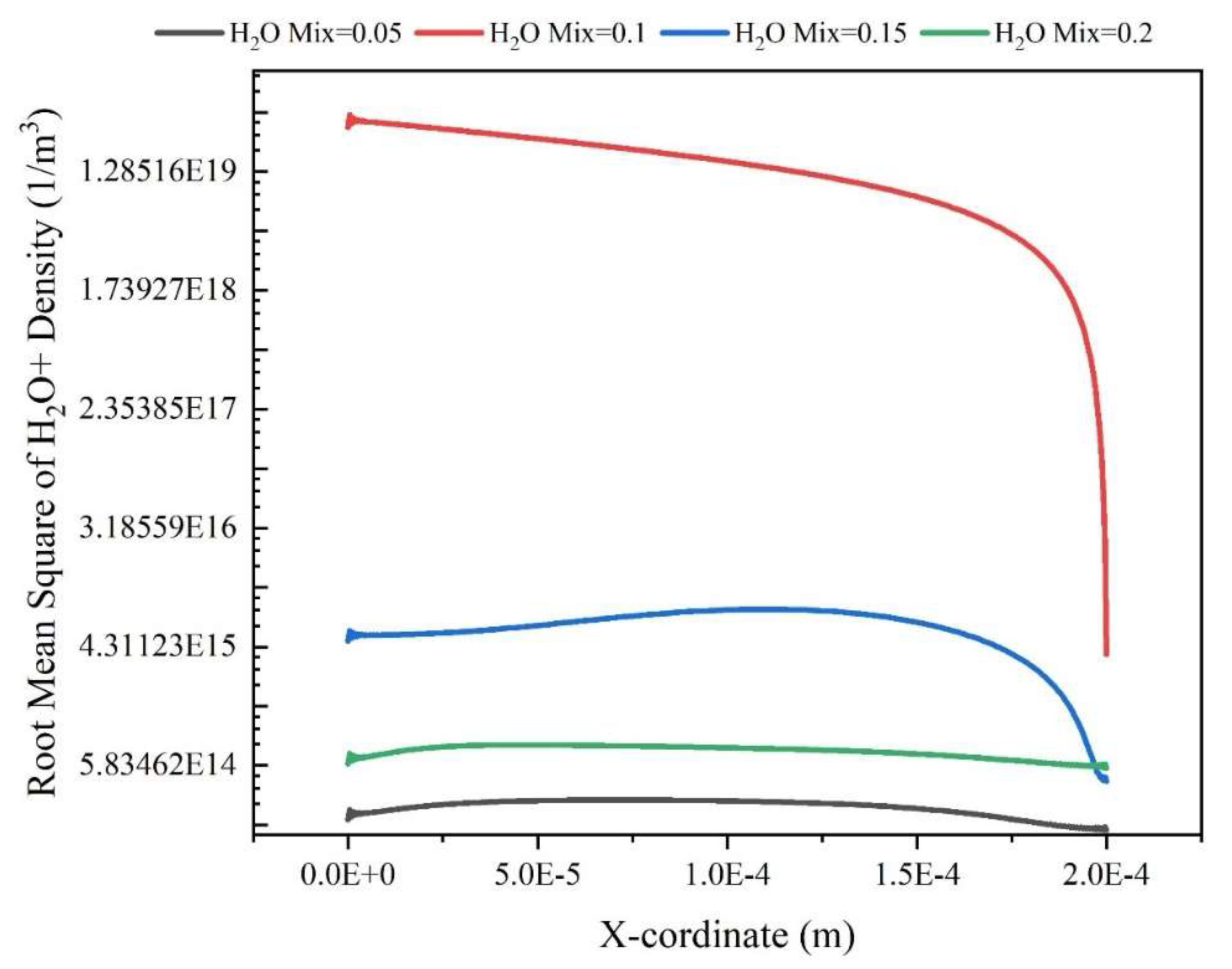
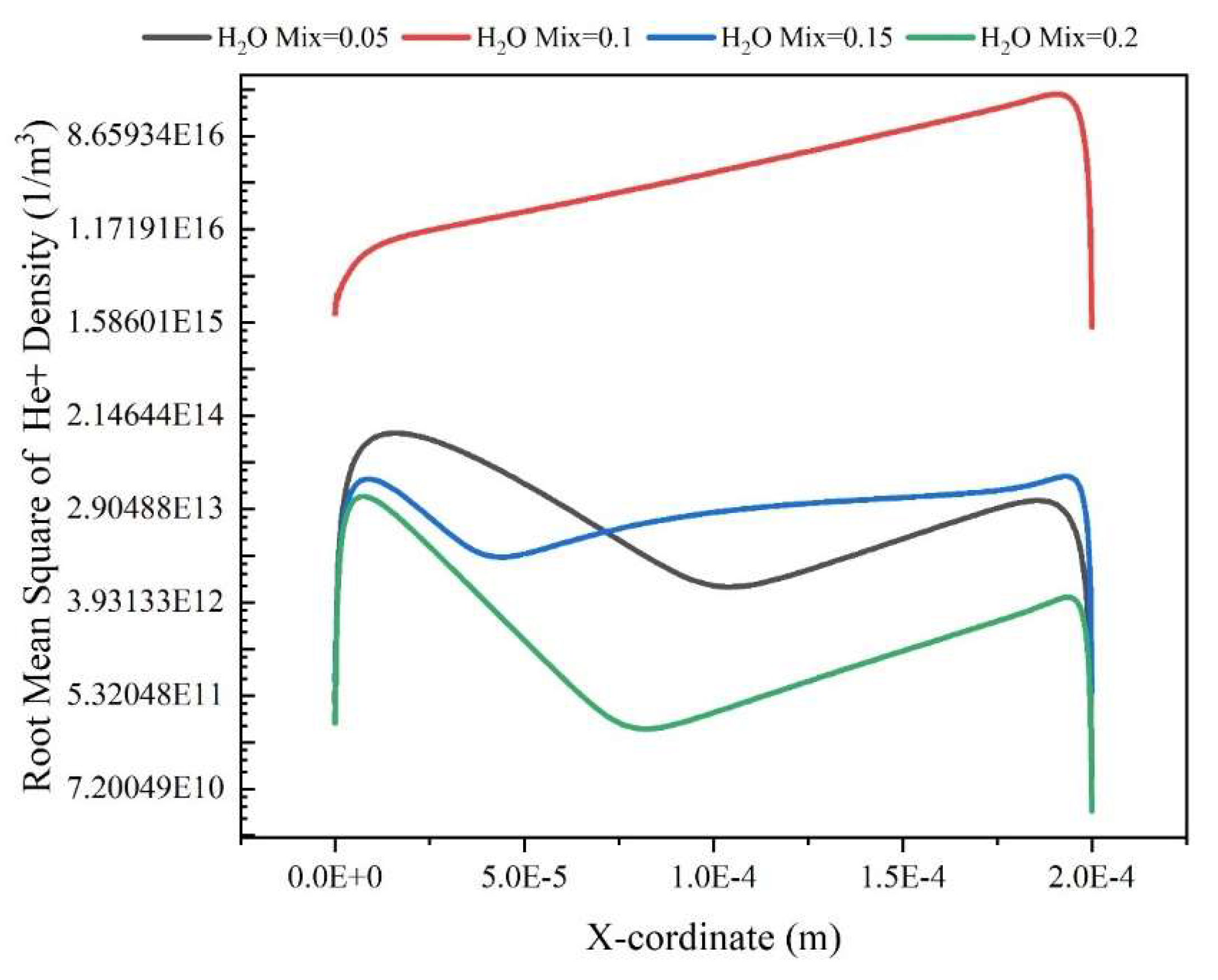
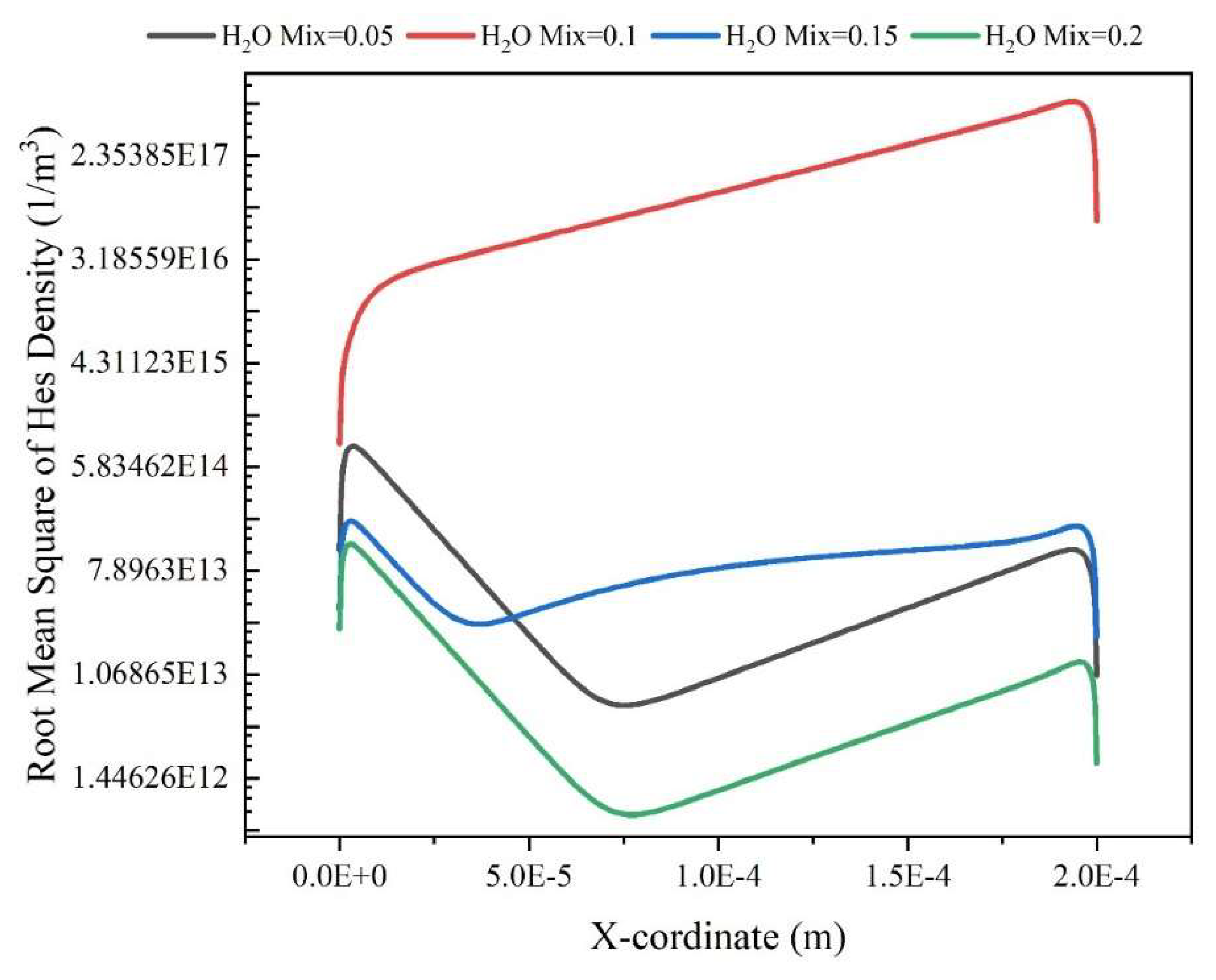
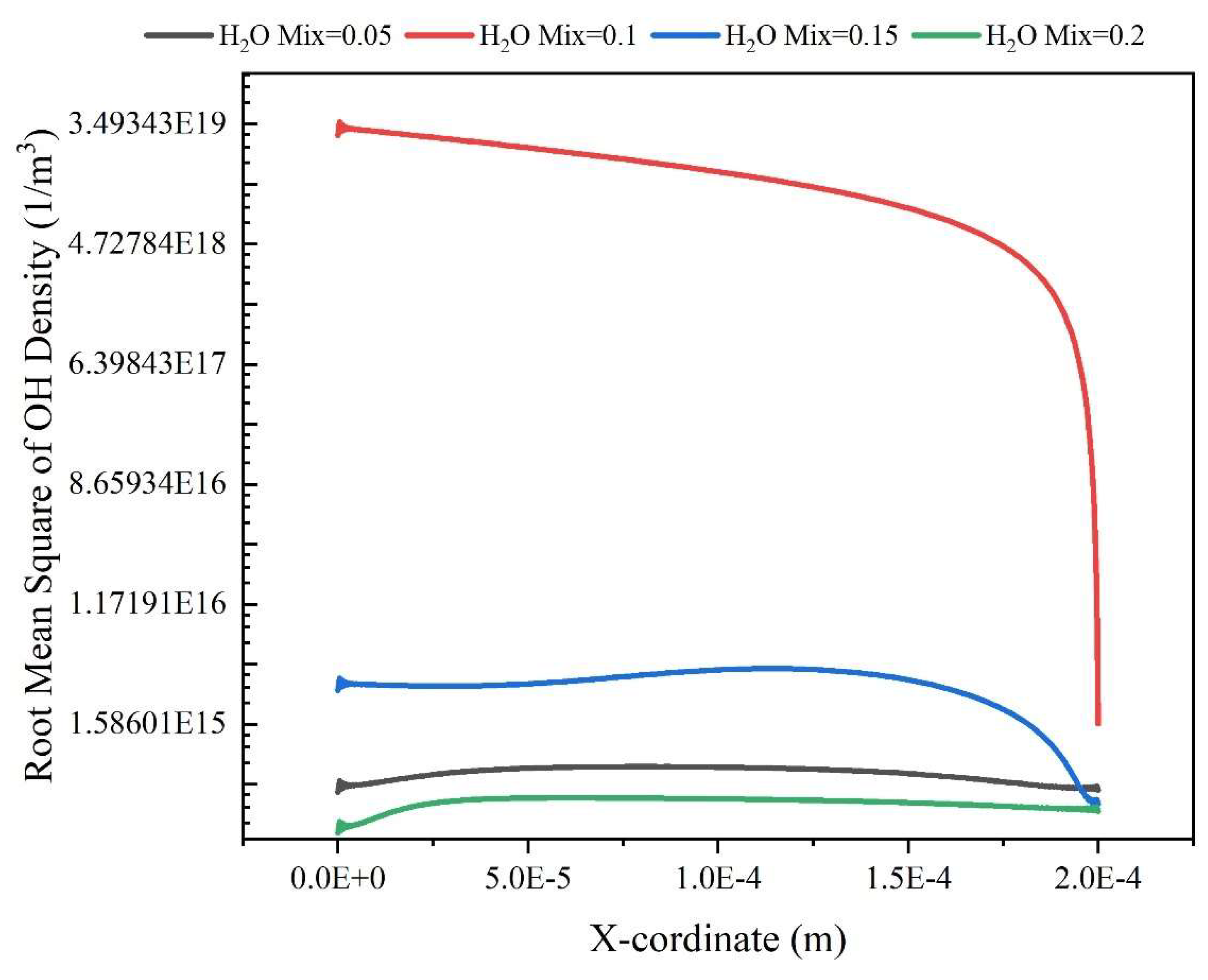
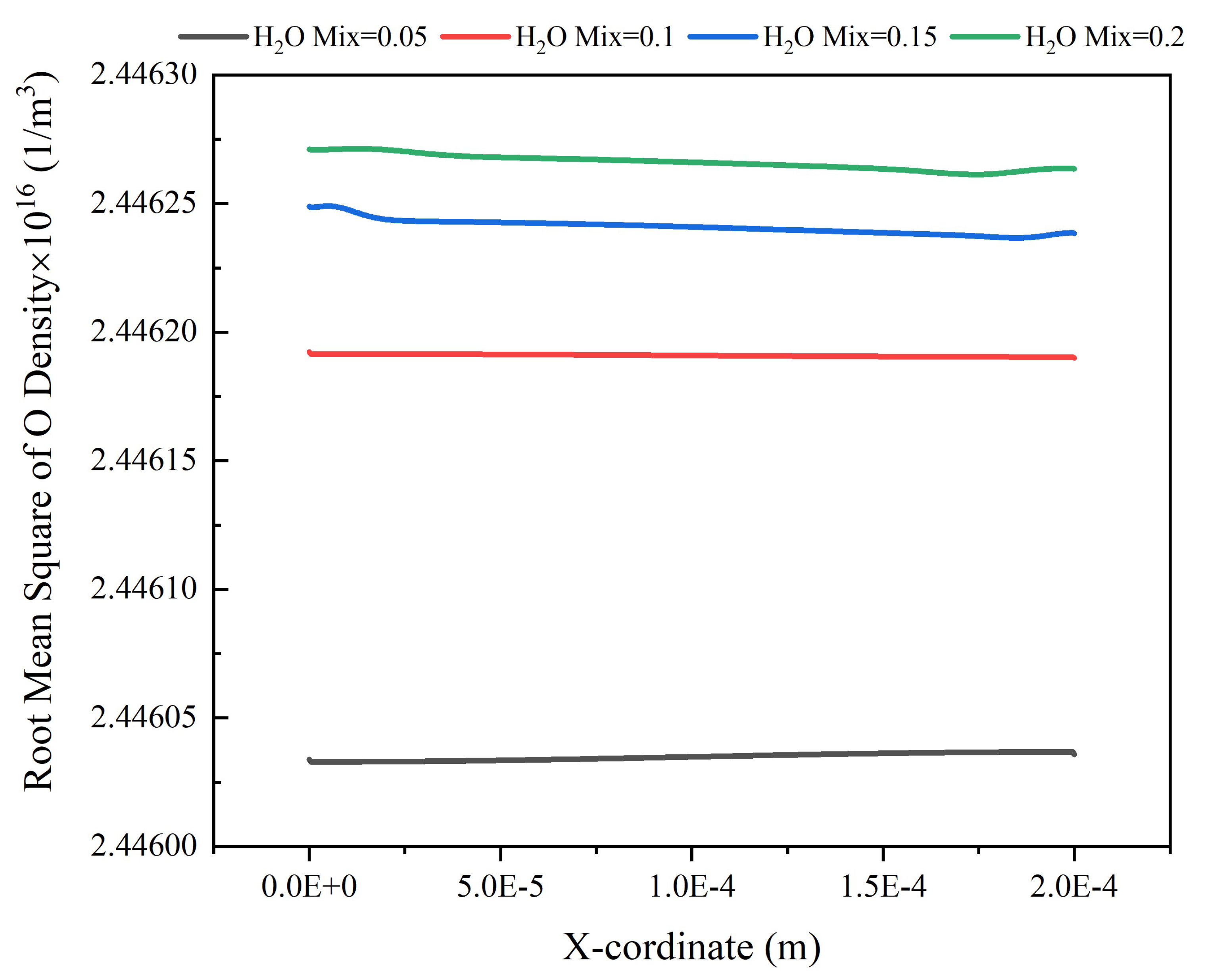
Results and Discussion
Conclusions
Author Contributions
Acknowledgments
Ethics Approval
Consent to Participate
Consent for Publication
Competing Interests
Data Availability
References
- Z. Machala and M. J. Pavlovich, “A New Phase in Applied Biology,” Trends Biotechnol., vol. 36, no. 6, pp. 577–578, 2018. [CrossRef]
- J. M. Jung et al., “Cold Plasma Treatment Promotes Full-thickness Healing of Skin Wounds in Murine Models,” Int. J. Low. Extrem. Wounds, vol. 22, no. 1, pp. 77–84, 2023. [CrossRef]
- S. K. Dubey et al., “Cold atmospheric plasma therapy in wound healing,” Process Biochem., vol. 112, pp. 112–123, 2022. [CrossRef]
- S. Cha and Y. S. Park, “Plasma in dentistry,” Clin. Plasma Med., vol. 2, no. 1, pp. 4–10, 2014. [CrossRef]
- R. Mehrabifard, H. Mehdian, and mahdi bakhshzadmahmodi, “Effect of non-thermal atmospheric pressure plasma on MDA-MB-231 breast cancer cells,” Pharm. Biomed. Res., vol. 3, no. 3, pp. 12–16, 2017. [CrossRef]
- R. Mehrabifard, H. Mehdian, K. Hajisharifi, and E. Amini, “Improving Cold Atmospheric Pressure Plasma Efficacy on Breast Cancer Cells Control-Ability and Mortality Using Vitamin C and Static Magnetic Field,” Plasma Chem. Plasma Process., vol. 40, no. 2, pp. 511–526, Nov. 2020. [CrossRef]
- D. Sersenová, Z. Machala, V. Repiská, and H. Gbelcová, “Selective apoptotic effect of plasma activated liquids on human cancer cell lines,” Molecules, vol. 26, no. 14, 2021. [CrossRef]
- D. Dobrynin et al., “Physical and biological mechanisms of direct plasma interaction with living tissue,” New J. Phys., vol. 11, no. 11, p. 115020, 2009. [CrossRef]
- M. Amini, M. Momeni, and A. Jahandideh, “Floating Discharge Plasma for Healing of Tendon Injury,” High Temp., vol. 58, no. 6, pp. 795–799, 2020. [CrossRef]
- M. Schröder, A. Ochoa, and C. Breitkopf, “ Numerical simulation of an atmospheric pressure RF-driven plasma needle and heat transfer to adjacent human skin using COMSOL ,” Biointerphases, vol. 10, no. 2, p. 029508, Jun. 2015. [CrossRef]
- X. Lu et al., “On the chronological understanding of the homogeneous dielectric barrier discharge,” High Volt., vol. 8, no. 6, pp. 1132–1150, 2023. [CrossRef]
- .
- S. Hajikhani, R. Mehrabifard, and H. Soltani Ahmadi, “A numerical analysis of the impact of gas pressure and dielectric material on the generation of body force in an air gas plasma actuator,” Radiat. Phys. Eng., vol. 5, no. 2, pp. 51–60, Apr. 2024. [CrossRef]
- F. Sohbatzadeh, A. Hosseinzadeh Colagar, S. Mirzanejhad, and S. Mahmodi, “E. coli, P. aeruginosa, and B. cereus Bacteria Sterilization Using Afterglow of Non-Thermal Plasma at Atmospheric Pressure,” Appl. Biochem. Biotechnol., vol. 160, no. 7, pp. 1978–1984, Apr. 2010. [CrossRef]
- J. R. Roth, “Industrial plasma engineering: Volume 2: Applications to nonthermal plasma processing,” Ind. Plasma Eng. Vol. 2 Appl. to Nonthermal Plasma Process., vol. 2, pp. 1–628, Jan. 2001.
- S. Gadkari, ; Xin, ; T., S. Gu, and X. Tu, “Fluid model for a partially packed dielectric barrier discharge plasma reactor,” Phys. Plasmas, vol. 24, p. 93510, 2017. [CrossRef]
- J. Pan, L. Li, B. Chen, Y. Song, Y. Zhao, and X. Xiu, “Numerical simulation of evolution features of the atmospheric-pressure CF4 plasma generated by the pulsed dielectric barrier discharge,” Eur. Phys. J. D 2016 706, vol. 70, no. 6, pp. 1–8, Jun. 2016. [CrossRef]
- R. Abidat, S. Rebiai, and L. Benterrouche, “Numerical simulation of atmospheric Dielectric Barrier Discharge in helium gas using COMSOL Multiphysics,” 2013 3rd Int. Conf. Syst. Control. ICSC 2013, pp. 134–139, 2013. [CrossRef]
- Y. B. Golubovskii, V. A. Maiorov, J. Behnke, and J. F. Behnke, “Modelling of the homogeneous barrier discharge in helium at atmospheric pressure,” J. Phys. D. Appl. Phys., vol. 36, no. 1, p. 39, Dec. 2002. [CrossRef]
- S. Tappi et al., “Effect of cold plasma generated with different gas mixtures on safety, quality and nutritional aspects of fresh sea bream fillets,” Innov. Food Sci. Emerg. Technol., vol. 89, 2023. [CrossRef]
- Y. Ucar, Z. Ceylan, M. Durmus, O. Tomar, and T. Cetinkaya, “Application of cold plasma technology in the food industry and its combination with other emerging technologies,” Trends Food Sci. Technol., vol. 114, pp. 355–371, 2021. [CrossRef]
- Schmidt, S. Bekeschus, H. Jablonowski, A. Barton, K. D. Weltmann, and K. Wende, “Role of Ambient Gas Composition on Cold Physical Plasma-Elicited Cell Signaling in Keratinocytes,” Biophys. J., vol. 112, no. 11, pp. 2397–2407, 2017. [CrossRef]
- R. Mehrabifard, “Two-dimensional simulation of Argon dielectric barrier discharge (DBD) in plasma actuator structure with COMSOL Multiphysics,” Radiat. Phys. Eng., vol. 4, no. 4, pp. 43–50, 2023. [CrossRef]
- G. V. Naidis, “Modelling of OH production in cold atmospheric-pressure He-H2O plasma jets,” Plasma Sources Sci. Technol., vol. 22, no. 3, 2013. [CrossRef]
- K. Shuaibov, A. I. Dashchenko, and I. V. Shevera, “Emission from a DC glow discharge in a He/H2O mixture,” Tech. Phys., vol. 46, no. 11, pp. 1484–1487, 2001. [CrossRef]
- X. Pei, Y. Lu, S. Wu, Q. Xiong, and X. Lu, “A study on the temporally and spatially resolved OH radical distribution of a room-temperature atmospheric-pressure plasma jet by laser-induced fluorescence imaging,” Plasma Sources Sci. Technol., vol. 22, no. 2, 2013. [CrossRef]
- E. Ilik, C. Durmus, and T. Akan, “Adding Water Droplets into Atmospheric Pressure Plasma Jet of Helium,” IEEE Trans. Plasma Sci., vol. 47, no. 11, pp. 5000–5005, 2019. [CrossRef]
- K. Ding, M. A. Lieberman, and A. J. Lichtenberg, “Hybrid model of neutral diffusion, sheaths, and the α to γ transition in an atmospheric pressure He/H2O bounded rf discharge,” J. Phys. D. Appl. Phys., vol. 47, no. 30, 2014. [CrossRef]
- L. C. Pitchford et al., “LXCat: an Open-Access, Web-Based Platform for Data Needed for Modeling Low Temperature Plasmas,” Plasma Process. Polym., vol. 14, no. 1–2, 2017. [CrossRef]
- M. Hayashi, “Electron Collision Cross-Sections for Molecules Determined from Beam and Swarm Data,” Swarm Stud. Inelast. Electron-Molecule Collisions, pp. 167–187, 1987. [CrossRef]
- R. Mehrabifard, Z. Kabarkouhi, F. Rezaei, K. Hajisharifi, H. Mehdian, and E. Robert, “Physical understanding of the static magnetic field’s synergistic enhancement of cold atmospheric pressure plasma treatment,” Apr. 2023. [CrossRef]
- H. Halfmann, N. Bibinov, J. Wunderlich, and P. Awakowicz, “A double inductively coupled plasma for sterilization of medical devices,” J. Phys. D. Appl. Phys., vol. 40, no. 14, p. 4145, Jun. 2007. [CrossRef]
- F. Sohbatzadeh and H. Soltani, “Time-dependent one-dimensional simulation of atmospheric dielectric barrier discharge in N2/O2/H2O using COMSOL Multiphysics,” J. Theor. Appl. Phys., vol. 12, no. 1, pp. 53–63, 2018. [CrossRef]
- M. M. Iqbal and M. M. Turner, “Influence of Gap Spacing between Dielectric Barriers in Atmospheric Pressure Discharges,” Contrib. to Plasma Phys., vol. 55, no. 6, pp. 444–458, 2015. [CrossRef]
- S. Kanazawa et al., “LIF imaging of OH radicals in DC positive streamer coronas,” Thin Solid Films, vol. 515, no. 9, pp. 4266–4271, 2007. [CrossRef]
- Y. J. Hong et al., “Measurement of hydroxyl radical density generated from the atmospheric pressure bioplasma jet,” J. Instrum., vol. 7, no. 03, p. C03046, Mar. 2012. [CrossRef]
- S. Schröter et al., “Chemical kinetics in an atmospheric pressure helium plasma containing humidity,” Phys. Chem. Chem. Phys., vol. 20, no. 37, pp. 24263–24286, 2018. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





