1. Introduction
Plastics are lightweight, durable, versatile, low-corrosive, and inexpensive, making them widely used in many applications and the worldwide plastic manufacturing volume is increasing [
1,
2]. The annual production of plastics has increased 200-fold from 1950 to 381 Mt in 2015, resulting in a significant amount of landfilled and dumped plastics [
3], and the accumulation of plastics is expected to continue to increase due to their slow degradation rate compared to the rate at which we consume them [
4].
In general, plastics such as polyethylene (PE) or polystyrene (PS) do not completely decompose when exposed to the natural environment but can be broken into small pieces by a variety of factors, resulting in numerous microplastics [
5,
6]. Microplastics generally refer to plastic particles smaller than 5 mm [
7], and the occurrence of microplastics in surface waters was first reported in the early 1970s by Carpenter and Smit (1972) [
8]. UNEP(United Nations Environment Programme) defined solid plastic particles of 5 mm or less that cannot be decomposed in water as microplastics [
9], and the International Organization for Standardization Environmental Aspects (ISO/TC 61/SC14) defines any solid plastic particle insoluble in water with any dimension between 1 µm and 1000 µm. Plastics are widely used in everyday life, and their high production, rapid consumption, and resistance to complete decomposition have led to large-scale release into and long-term persistence in aquatic and terrestrial environments [
4,
10,
11]. Rivers carry 70–80% of plastics, resulting in extensive sedimentation in aquatic environments such as rivers and oceans [
12].
Microplastics from consumer products such as plastic bags and food wrappers, personal care products, polyester, nylon and other fibers, materials and textile industry raw materials, etc. [
13] as well as from bath products, cosmetics, facial cleansers, etc. microplastics [
14,
15] are attracting global attention because they are very difficult to prevent because they flow into rivers and oceans through sewage pipes [
16] and can cause adverse physiological and biochemical reactions in various organisms [
17,
18]. Particularly in the aquatic environment, they are present in high concentrations [
19], and when persistent organic pollutants are adsorbed on the surface of microplastics and ingested, they are likely to have adverse effects on organisms [
20]. Increasing societal interest in the effects of these microplastics on human health and ecosystems has raised concerns about their formation, chemical interactions, and potential impacts on the environment [
20,
21], and research on their investigation and analysis has been actively conducted [
22].
Methods for removing microplastics in the aquatic environment include physical methods involving filtration and sedimentation, chemical methods to decompose and convert them, and biological methods to decompose them using microorganisms or plants [
23,
24,
25,
26,
27,
28,
29,
30]. Among these, the physical method is economical and has a high removal rate, but it is difficult to reuse the adsorbent, resulting in waste; in cases such as coagulation [
31,
32] and decomposition methods, operating costs may increase owing to the continuous injection of chemicals [
31,
32,
33].
In various aquatic environments other than sewage treatment plants, microplastics remain suspended in water bodies, while some float to the surface and denser microplastics sink to the bottom [
22,
34]. Therefore, as a sustainable maintenance plan that is suitable for application to a variety of aquatic environments, has low operating costs, is adaptable for large-scale treatment, and can treat various pollutants together, biological treatment can be considered effective. Previous research on biological methods has focused on the removal of microplastics by microorganisms or sea creatures [
25,
35,
36,
37] and considered only the environment or the interrelationship between plants and microplastics [
25,
35,
38,
39,
40]. Research on the removal of microplastics by plants is lacking. Plants alleviate pollutants by absorbing and adsorbing pollutants through their roots [
41,
42,
43,
44,
45,
46] or by slowing down wetland runoff to prevent MPs from settling in sediments [
47]. Plants are used to restore polluted environments because they can be stabilized and decomposed [
48]. In addition, aquatic plants have both direct and indirect effects on the aquatic environment. They can absorb many organic substances and increase the water purification ability of aquatic microorganisms [
49], which has a positive effect on the removal of MP from the aquatic environment.
Accordingly, this study investigated whether the amount of microplastics in the aquatic environment can be reduced using yellow irises and Buddha’s flowers, which are aquatic water-purifying plants with high ornamental value, and investigated various parameters (time, microplastic size, and plant type), focusing on their effect on the amount of microplastics in the aquatic environment.
2. Materials and Methods
2.1. Microplastics
The microplastics used in this study were white polyethylene (PE) powder, which is used in industrial and research applications, purchased from ThyssenKrupp. White PE is widely used in paints and coatings, cosmetics, and personal care applications and has high electrical conductivity and oil resistance that have ensured its use in a variety of fields, such as transparent agricultural films, disposable products, toys, and plant containers. It is a mass-produced plastic found not only in the soil but also in the aquatic environment.
There were two types of PE particle sizes in this study: 46 µm and 140 µm. As plastic waste accumulates in the environment and decomposes into smaller particles over time [
50,
51], we aimed to determine the removal range of various particle sizes.
2.2. Plant Materials and Planting
Two aquatic plants were selected to investigate their adaptability and growth in microplastics pollution sources and their ability to remove microplastics (
Table 1).
Iris pseudacorus and
Lythrum anceps (
Figure 1), which have a long flowering period and are used near watersides and in gardens, are perennial water plants that grow naturally throughout Korea’s watersides.
These two plants were selected because they have high ornamental value and are excellent at adapting to environmental stress conditions [
52,
53,
54]. These two plants could be used in future experiments to investigate their ability to remove microplastics from waterfronts and general soils.
This study was conducted for four months in a greenhouse-type growing room of a plant factory called Samyuk Eco-Farm Center at Samyuk University, located at Hwarang University in Nowon-gu, Seoul. The experimental environment was a combined solar and convection plant factory with natural light, and three 24W LED plant lamps (Designer Sunbell) were used as supplemental light in case of light shortage. To simulate the circadian cycle, lights were turned on for 10 h per day (8 a.m. to 6 p.m.). The air temperature and humidity were 25.8℃ and 97.7% on average, and the water temperature was 26.3℃ on average (
Table 2). The water temperature in the cultivation zone where
Iris pseudacorus and
Lythrum anceps were planted is shown in
Table 1 and had an average value of 26.3°C. The temperature and humidity meter was a greenhouse complex ventilation system ‘MAGMA-1000’ (MAGMA-1000’) installed in the growing room, and the water temperature measurement system was a Caple temperature recorder (CP22-WA15).
In April 2023, 60 unblooming Iris pseudacorus and Lythrum anceps in 2-inch plastic pots were purchased from a cultivation farm. The first acclimatization process was conducted in a greenhouse cultivation room for 14 days to reduce growth disorders due to environmental changes. After the first purification process, the soil attached to the roots was lightly shaken off and completely removed by rinsing with tap water, and the plants were then immersed in tap water for 14 days to perform the second purification in a hydroponic form.
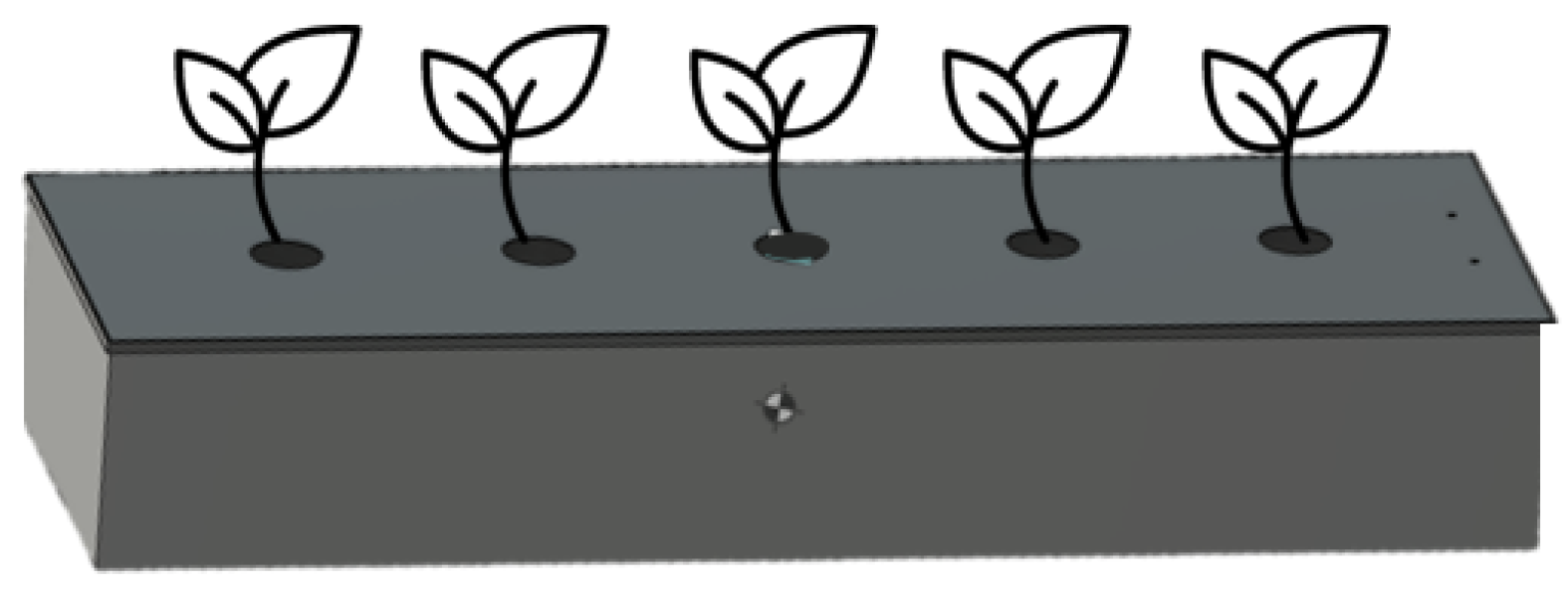
The containers for the experiments were made of stainless steel to minimize the impact of plastic, and the dimensions were 1.2m×0.2m×0.3m for width, length, and height, respectively). To float the plants in the container, a lid was made of 0.28×1.15 m. Five holes with a diameter of 5 cm were drilled at 15 cm intervals, taking into account the plant spacing (
Figure 2). To confirm the effectiveness of removing floating microplastics using plant roots, each plant was floated in a cultivation box and fixed with a sponge inserted into the cover hole of the cultivation box (
Figure 3).
The planting beds were 1.5 m x 0.9 m x 3.0 m (W*D*H) and were set up in two tiers, with
Iris pseudacorus on the right and
Lythrum anceps on the left (
Figure 4). Three boxes of plants were used as a treatment (15 weeks) and one box as a control (5 weeks) for each plant.
2.3. Exposure Experimental Set-Up
The grow boxes were filled with 70 L of tap water and treated with two types of PE powder (46 µm and 140 µm) at a concentration of 1 mg/L. The bubble generator (Resun Air-4000) was set to allow the water to flow through the planting bed, considering that the microplastics would not float or sink and be adsorbed by the plant roots, and the effect of flow rate in the natural environment.
2.4. Analyzing the Adsorption of Microplastics by Plants and the Concentration of Microplastics in Aquatic Environments
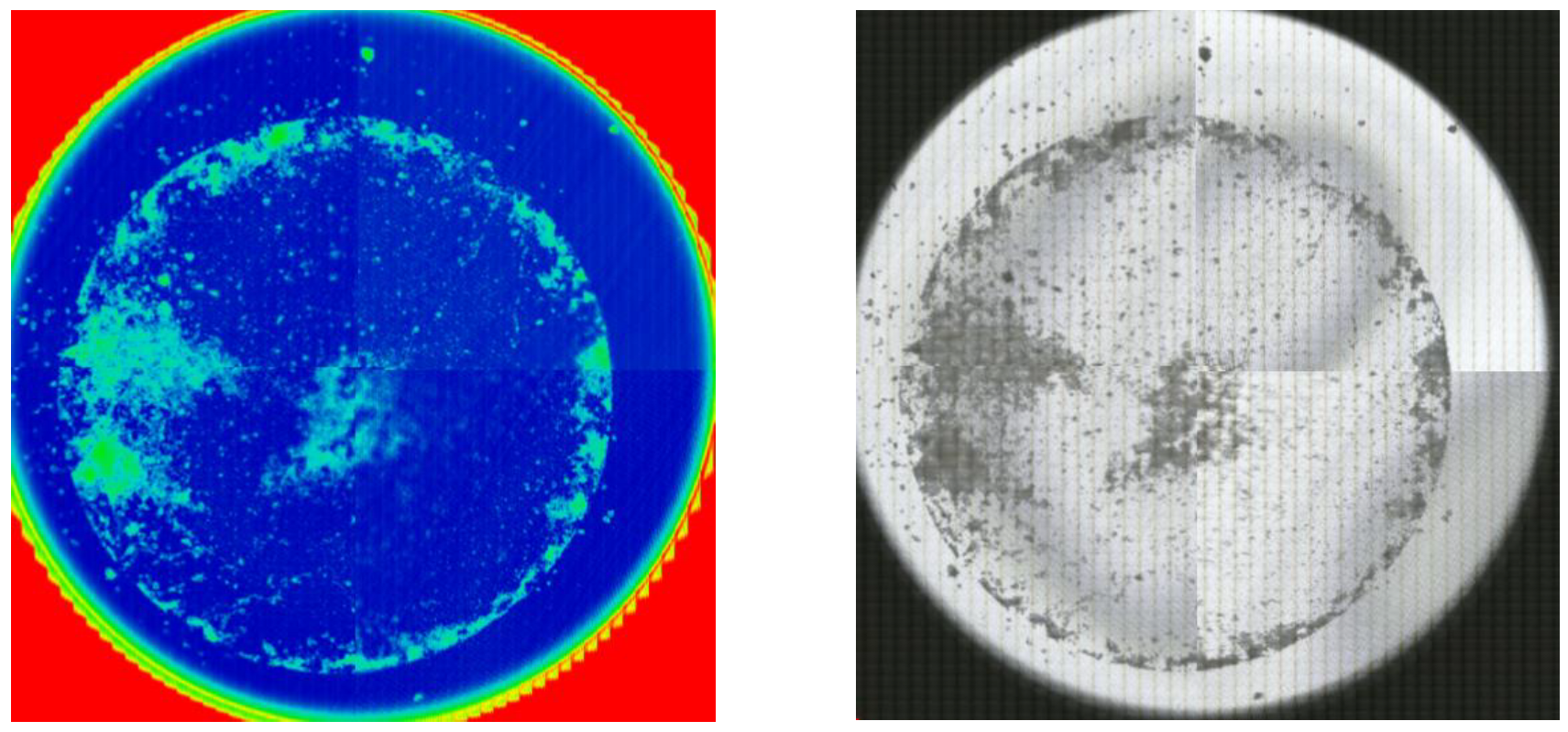
To analyze the microplastic removal content in the aquatic environment, pre- and post-treatment water quality was analyzed using FT-IR with a microscope (NicoletTM iN10MX FT-IR microscope, Thermo Fisher Scientific, Waltham, MA, USA).
Samples were placed in 500 mL beakers and filtered under reduced pressure through a metal mesh filter (20 µm, 25diameter). The filter was divided into four compartments across the front of the filter using the Omnic Picta Program (Thermo Fisher Scientific Inc., Waltham, MA, USA) to obtain IR spectra of all areas by ultra-fast mapping. The IR spectra obtained from each compartment were library-matched with the plastic IR spectra and considered as plastic when the matching rate was more than 70%, and quantitative data were collected by counting (
Figure 5).
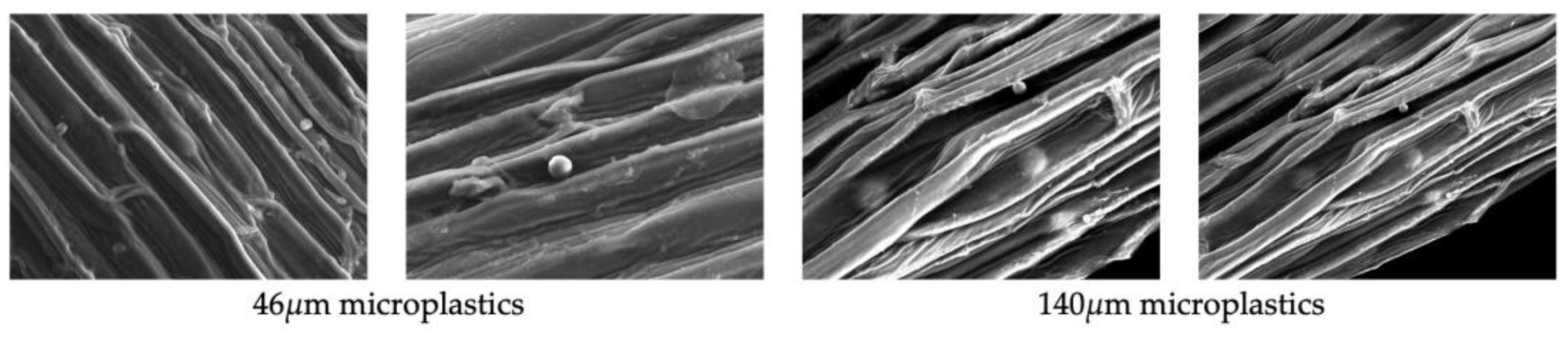
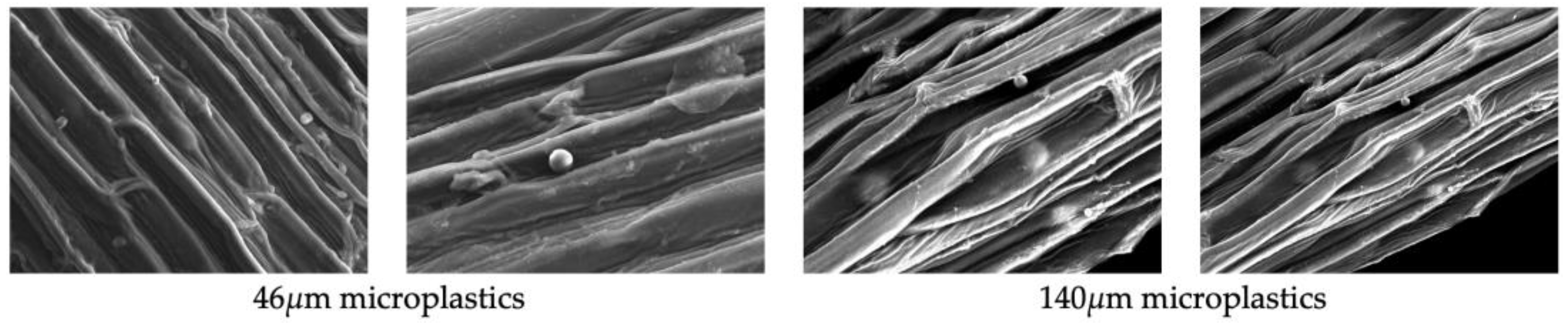
To identify the adsorbed microplastic particles from the plant roots, some roots of Iris pseudacorus and Lythrum anceps were removed and analyzed using a JEOL Scanning Electron Microscope JSM-6510. The adsorption analysis was performed once post-mortem (90 days).
2.5. Grow Survey
Pre- and post-planting growth surveys were conducted during May through August to examine water quality adaptation of aquatic plants. Height was measured from the base to the tip of the longest leaf, and Width was measured at a point 15 cm above the base in the planted state. Leaf number was measured for Iris pseudacorus and branch number for Lythrum anceps.
2.6. Statistical Analysis
Statistical analysis was performed using the SPSS 26.0 (IBM Co., Armonk, NY, USA) program. Normality and homogeneity of variance were tested by Shapiro-Wilk and Levene tests, respectively. As a result, parametric statistical methods were used. Paired sample t-tests were performed to determine the significant difference between pre- and post-microplastic removal for each plant species and microplastic particle size. One-way ANOVA was performed to test the significant effect of microplastic concentration level according to microplastic particle size, and Duncan’s test was applied. Descriptive statistical analysis was conducted to determine the changes in plant growth following microplastic removal.
3. Results
3.1. Plants Remove Microplastics from Aquatic Environment
We measured the removal rate of microplastics in water and found that different plant species had a positive effect on microplastic removal depending on the size of the microplastic particles. The removal rate
of Iris pseudacorus decreased by 72.1% from 252.9 to 70.4, and the removal rate of
Lythrum anceps decreased by 70.2% from 203.5 to 60.5. When measuring removal by microplastic size, 46 µm decreased from 222.6 to 78.0, a 64.9% reduction, and 140 µm decreased from 233.7 to 52.9, a 77.3% reduction. The correlation between microplastic removal rate by plant type and microplastic removal rate by microplastic particle size showed a significant difference between
Iris pseudacorus (
t=11.068,
p<.001),
Lythrum anceps (t=13.113,
p<.001), and 46 µm (
t=10.773,
p<.001) and 140 µm (
t=12.344,
p<.001) by microplastic particle size (
Table 3).
Pre- and post-measurements of the effectiveness of Iris pseudacorus and Lythrum anceps in removing microplastics from the water showed that Iris pseudacorus and Lythrum anceps had a positive impact on reducing microplastics in the water.
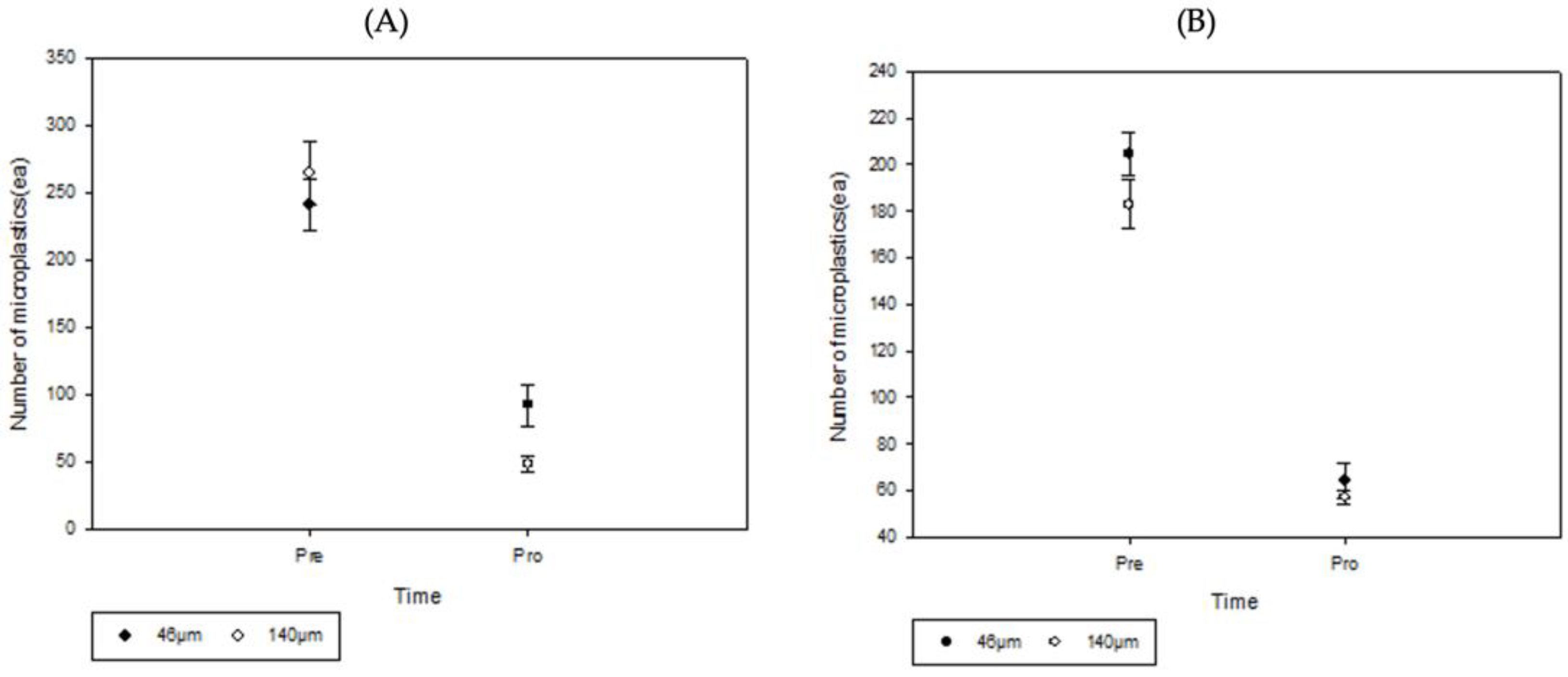
The microplastic removal rate for the plots planted with
Iris pseudacorus decreased by 79.7% from 240.99 to 92.20 for the 46 µm microplastic size, and by 81.5% from 264.82 to 48.76 for the 140 µm microplastic size (
Figure 6). The microplastic removal rate of the
Lythrum anceps planted plots decreased by 68.7% from 204.3 to 63.92 for the microplastic size of 46 µm, and by 68.7% from 182.76 to 57.16 for the microplastic size of 140 µm (
Figure 6).
The correlation of microplastic removal rate with microplastic particle size by plant showed a significant difference between
Iris pseudacorus (
F=39.014,
p<.001) and
Lythrum anceps (
F=33.801,
p<.001) depending on the microplastic particle size (
Table 4).
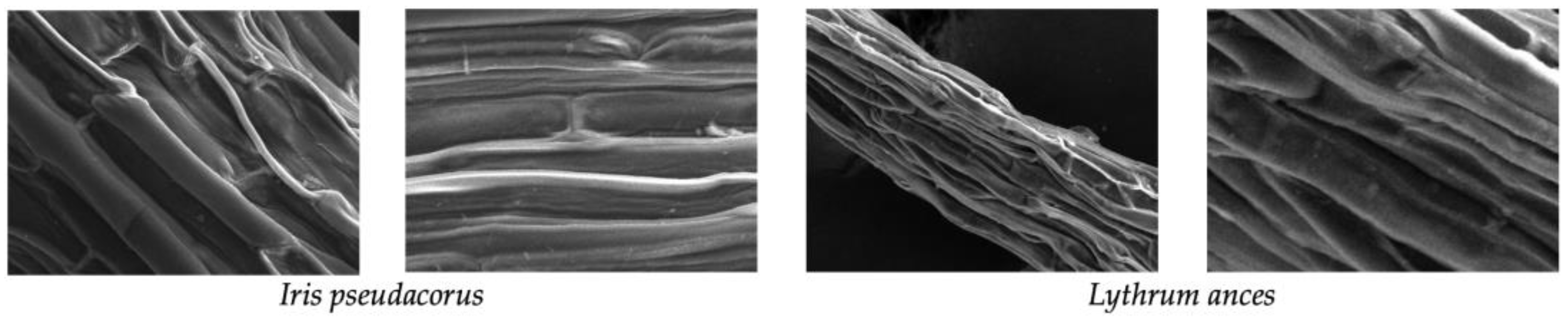
Scanning electron microscopy (SEM) was used to identify adsorbed particles on the roots of
Iris pseudacorus and
Lythrum anceps (
Figure 7,
Figure 8 and
Figure 9). This confirmed that the microplastic particles were removed by adsorption on the plant roots.
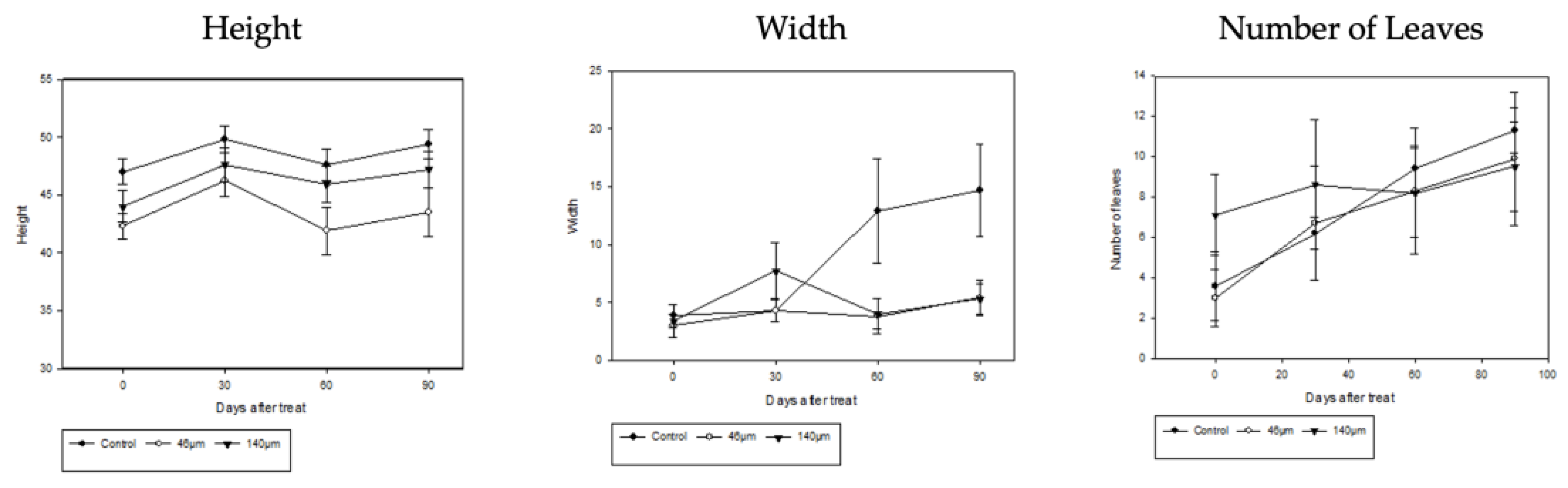
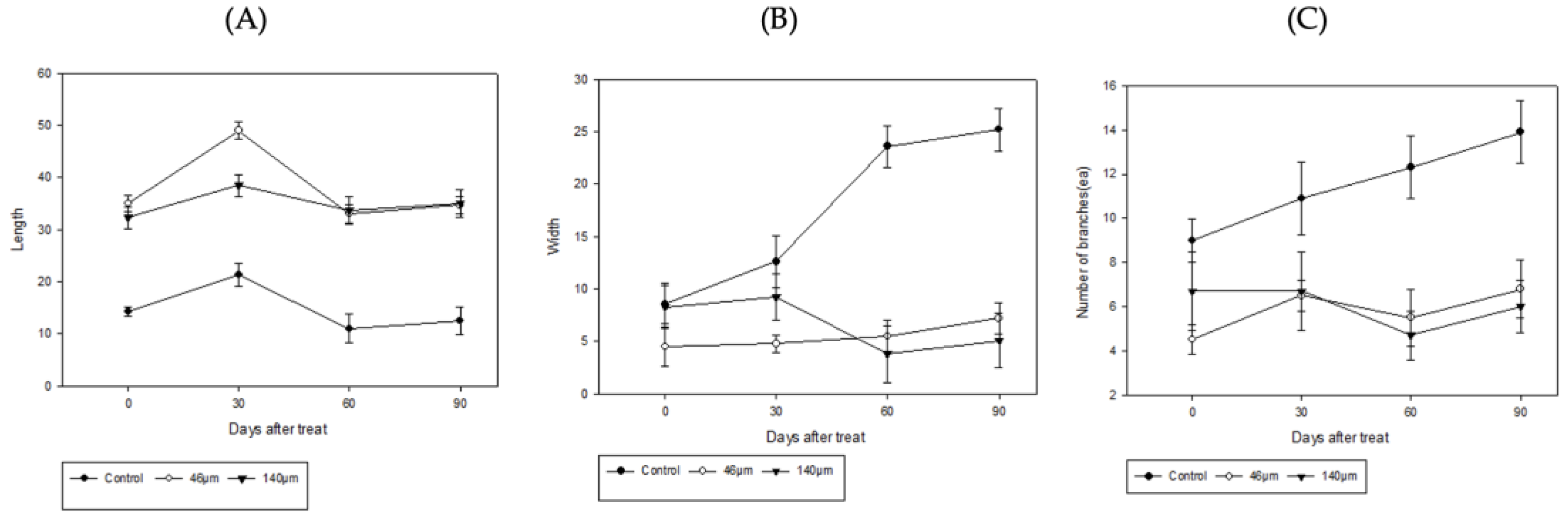
3.2. Plant Growth as a Function of Microplastic Concentration
When
Iris pseudacorus and
Lythrum anceps were grown in microplasticized tap water for 4 months from May to August, both the Height, width, and number of leaves of
Iris pseudacorus increased over time (
Figure 10). At a microplastic particle size of 140 µm, the initial width of the
Lythrum anceps decreased from 8.3 cm to 5.1 cm. In addition, both the height and leaf number of the
Lythrum anceps increased over time (
Figure 11). The correlation of plant growth changes (height, width, branch number, and leaf number) with microplastic concentration was not statistically significant.
4. Discussion
This study was conducted to verify the effectiveness of microplastic remediation in the aquatic environment using aquatic plants. Currently, microplastics are classified as contaminants of emerging concern, and most microplastics generated in our daily lives flow into various aquatic environments, such as sewage treatment plants and rivers, through sewage. Microplastics in sewage treatment plants are treated using sedimentation or filtration, microbial treatment, and reverse osmosis; however, these are generally not effective or highly efficient [
55,
56,
57]. In addition, research is needed on economical and efficient methods that can be applied not only to sewage treatment plants but also to various aquatic environments such as rivers, soil, and coastal waters.
Phytoremediation is the most environmentally efficient method for removal of pollutants from water bodies such as rivers and streams, with the expectation of aesthetic effects [
25,
58]. Therefore, research is needed on methods to reduce microplastic pollution in the aquatic environment using plants that can adsorb microplastics through their roots or leaves [
54,
59,
60].
In this study, we found that the concentration of microplastics in the water was reduced in the planter boxes planted with
Iris pseudacorus and
Lythrum anceps. The removal of microplastics was confirmed by their adsorption by
Iris pseudacorus and
Lythrum anceps roots. Plants absorb nutrients through their roots and adsorb suspended solids [
61,
62], which can promote the purification process of pollutants in the aquatic environment. This is consistent with the results of previous studies [
63,
64,
65,
66]. Waterside plants, such as yellow irises and Buddha’s flowers, are rooted at the bottom of the aquatic environment, and parts of their leaves and stems are within the aquatic environment, making them effective at removing microplastics floating in the aquatic environment or settling on the bottom.
We measured the change in microplastic concentration as a function of microplastic size, and found that larger microplastic particle size had a significant effect on reducing microplastic concentration in both Iris pseudacorus and Lythrum anceps. This suggests that larger particles of floating microplastics are better adsorbed by dense plant roots exposed to water. However, in the actual environment, yellow irises and Buddha’s flowers are often rooted in the soil rather than underwater; therefore, additional research is needed on the adsorption of microplastics in the aquatic environment that has settled in the soil.
As a result of measuring plant growth changes to check the growth status of plants in an aquatic environment polluted with microplastics, it was confirmed that the height, width, and number of branches (leaf number for
Iris pseudacorus) of
Iris pseudacorus and
Lythrum anceps all increased. The size of the microplastic particles did not negatively impact plant growth, which is consistent with previous studies [
46,
49,
67,
68]. As a result, it appears that microplastics in the aquatic environment did not have a significant negative impact on the growth of the roots of yellow irises and Buddha’s flowers in the water; therefore, aboveground growth was also smooth. However, microplastics can affect plant biomass and seed germination [
11], and it is necessary to consider their impact on plants continuously exposed to the toxicity of microplastics. In particular,
Iris pseudacorus and
Lythrum anceps are highly ornamental plants with prolonged flowering, so it is worth investigating the effects of long-term exposure to microplastics on flowering, coloration, and fragrance.
The microplastics used in this study were larger than the pore size of plant root cell walls, 4 nm [
69], and were, therefore, unlikely to be absorbed into the root cell walls during the study period. Therefore, removal through absorption was not confirmed. However, there have been cases where microplastics have been broken down into nanoparticles and absorbed into cell walls [
70]; therefore, it is necessary to confirm their absorption through plant roots. In addition, when applied to the actual environment, microplastics remain attached to the plant surface in water. Therefore, there is a need to further evaluate the stability of microplastics in the aquatic environment when plants are removed from the aquatic environment or when they age.
5. Conclusions
While research on aquatic microplastic removal by plants is still in its infancy, the results of this study confirm that removing microplastics from the water through aquatic plants is effective and does not significantly affect plant growth. However, the potential loss of microplastics through adsorption by plants should also be considered during plant removal in extreme weather conditions or after plant restoration. Future experiments will need to test the method in real-world conditions and consider possible limitations, such as the adsorption of microplastics by plant roots over time, removal of microplastics through plant uptake, and sensitivity to other contaminants.
In conclusion, we believe that the method proposed in this study will be useful for removing microplastics using aquatic plants near waterfronts.
Author Contributions
Conceptualization, Y.K. and S.C.; methodology, Y.K. and K.P; software, S.C..; validation, K.P. and J.B..; formal analysis, J.B.; investigation, S.C. and J.B..; resources, Y.K. and K.P.; data curation, S.C.; writing—original draft preparation, Y.K.; writing—review and editing, S.C and K.P..; visualization, S.C..; supervision, Y.K. and K.P..; project administration, Y.K..; funding acquisition, Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Sahmyook University Project (RI12023016) in 2023.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Sueran Choi, upon reasonable request.
Acknowledgments
We would like to express our gratitude to Sahmyook University for enabling us to conduct this research, and to SU-AgRI, the school enterprise of Sahmyook University, for providing the research facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Laist, D.W. Impacts of Marine Debris: Entanglement of Marine Life in Marine Debris Including a Comprehensive List of Species with Entanglement and Ingestion Records. In Marine Debris: Sources, Impacts, and Solutions; Coe, J.M., Rogers, D.B., Eds.; Springer New York: New York, NY, 1997; pp. 99–139. [Google Scholar]
- Hammer, J.; Kraak, M.H.S.; Parsons, J.R. Plastics in the Marine Environment: The Dark Side of a Modern Gift. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer New York: New York, NY, 2012; pp. 1–44. [Google Scholar]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.; Yang, A.; Jiang, Z.; Ning, D.; Fahad, S. New insights in to the environmental behavior and ecological toxicity of microplastics. J Hazard Mater Adv 2023, 10, 100298. [Google Scholar] [CrossRef]
- Leslie, H.A. Plastic in Cosmetics. Are we polluting the environment through our personal care?; United Nations Environment Programme (UNEP): 2015.
- An, D.; Kim, J. Proposing policy for the prevention of marine pollution from microplastics. J Environ Policy Admin 2018, 26, 77–102. [Google Scholar]
- Carpenter, E.J.; Smith, K.L. Plastics on the Sargasso Sea Surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef] [PubMed]
- (UNEP), U.N.E.P. Plastic in Cosmetics [Fact Sheet]; Nairobi, 2015.
- Rochman, C.M. Microplastics research—from sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Science Advances 2018, 4, eaap8060. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Bai, X.; Ye, Z. Removal and generation of microplastics in wastewater treatment plants: A review. J Clean Prod 2021, 291, 125982. [Google Scholar] [CrossRef]
- Gregory, M.R. Plastic ‘scrubbers’ in hand cleansers: a further (and minor) source for marine pollution identified. Mar Pollut Bull 1996, 32, 867–871. [Google Scholar] [CrossRef]
- Zitko, V.; Hanlon, M. Another source of pollution by plastics: Skin cleaners with plastic scrubbers. Mar Pollut Bull 1991, 22, 41–42. [Google Scholar] [CrossRef]
- Andrady, A.L. Persistence of Plastic Litter in the Oceans. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, 2015; pp. 57–72. [Google Scholar]
- Hong, Y.; Wu, S.; Wei, G. Adverse effects of microplastics and nanoplastics on the reproductive system: A comprehensive review of fertility and potential harmful interactions. Sci Total Environ 2023, 903, 166258. [Google Scholar] [CrossRef] [PubMed]
- Franzellitti, S.; Canesi, L.; Auguste, M.; Wathsala, R.H.G.R.; Fabbri, E. Microplastic exposure and effects in aquatic organisms: A physiological perspective. Environ Toxicol Pharmacol 2019, 68, 37–51. [Google Scholar] [CrossRef]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in water systems: A review of their impacts on the environment and their potential hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar Pollut Bull 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Zoh, K.-D. A review on occurrence and environmental risk assessment for microplastics in freshwater systems. Korean J Public Health 2019, 56, 10–24. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res 2015, 75, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Pico, Y.; Alfarhan, A.; Barcelo, D. Nano- and microplastic analysis: Focus on their occurrence in freshwater ecosystems and remediation technologies. Trends Anal Chem 2019, 113, 409–425. [Google Scholar] [CrossRef]
- Ma, P.; Wei Wang, m.; Liu, H.; Feng Chen, y.; Xia, J. Research on ecotoxicology of microplastics on freshwater aquatic organisms. Environ Pollut Bioavailability 2019, 31, 131–137. [Google Scholar] [CrossRef]
- Li, L.; Xu, G.; Yu, H.; Xing, J. Dynamic membrane for micro-particle removal in wastewater treatment: Performance and influencing factors. Sci Total Environ 2018, 627, 332–340. [Google Scholar] [CrossRef]
- Perren, W.; Wojtasik, A.; Cai, Q. Removal of Microbeads from Wastewater Using Electrocoagulation. ACS Omega 2018, 3, 3357–3364. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Monnot, M.; Sun, Y.; Asia, L.; Wong-Wah-Chung, P.; Doumenq, P.; Moulin, P. Microplastics in different water samples (seawater, freshwater, and wastewater): Removal efficiency of membrane treatment processes. Water Res 2023, 232, 119673. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram Int 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, C.; Li, H.; Offiong, N.-A.O.; Bi, Y.; Zhou, R.; Ren, H. A systematic review of electrocoagulation technology applied for microplastics removal in aquatic environment. Chem Eng J 2023, 456, 141078. [Google Scholar] [CrossRef]
- Elkhatib, D.; Oyanedel-Craver, V.; Carissimi, E. Electrocoagulation applied for the removal of microplastics from wastewater treatment facilities. Sep Purif Technol 2021, 276, 118877. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A. A Procedure for Measuring Microplastics using Pressurized Fluid Extraction. Environ Sci Technol 2016, 50, 5774–5780. [Google Scholar] [CrossRef] [PubMed]
- Mai Lei, M.L.; Bao LianJun, B.L.; Shi Lei, S.L.; Wong, C.; Zeng, E. A review of methods for measuring microplastics in aquatic environments. Environ Sci Pollut Res 25, 11319–11332.
- Chorghe, D.; Sari, M.A.; Chellam, S. Boron removal from hydraulic fracturing wastewater by aluminum and iron coagulation: Mechanisms and limitations. Water Res 2017, 126, 481–487. [Google Scholar] [CrossRef]
- Spacilova, M.; Dytrych, P.; Lexa, M.; Wimmerova, L.; Masin, P.; Kvacek, R.; Solcova, O. An Innovative Sorption Technology for Removing Microplastics from Wastewater. Water 2023, 15, 892. [Google Scholar] [CrossRef]
- Sundbæk, K.B.; Koch, I.D.W.; Villaro, C.G.; Rasmussen, N.S.; Holdt, S.L.; Hartmann, N.B. Sorption of fluorescent polystyrene microplastic particles to edible seaweed Fucus vesiculosus. J Appl Phycol 2018, 30, 2923–2927. [Google Scholar] [CrossRef]
- Boyajian, G.E.; Carreira, L.H. Phytoremediation: a clean transition from laboratory to marketplace? Nat Biotech 1997, 15, 127–128. [Google Scholar] [CrossRef]
- Singh, O.; Labana, S.; Budhiraja, R.; Jain, R. Phytoremediation: an overview of metallic ion decontamination from soil. Appl Microbiol Biotechnol 2003, 61, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Fehn, S.; Steegmüller, T.; Rauwolf, S.; Löwe, H.; Pflüger-Grau, K.; Berensmeier, S. Immobilization of PETase enzymes on magnetic iron oxide nanoparticles for the decomposition of microplastic PET. Nanoscale Adv 2021, 3, 4395–4399. [Google Scholar] [CrossRef] [PubMed]
- Chia, W.Y.; Ying Tang, D.Y.; Khoo, K.S.; Kay Lup, A.N.; Chew, K.W. Nature’s fight against plastic pollution: Algae for plastic biodegradation and bioplastics production. Eviron Sci Ecotech 2020, 4, 100065. [Google Scholar] [CrossRef] [PubMed]
- Masiá, P.; Sol, D.; Ardura, A.; Laca, A.; Borrell, Y.J.; Dopico, E.; Laca, A.; Machado-Schiaffino, G.; Díaz, M.; Garcia-Vazquez, E. Bioremediation as a promising strategy for microplastics removal in wastewater treatment plants. Mar Pollut Bull 2020, 156, 111252. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Moore, F.; Keshavarzi, B.; Hopke, P.K.; Naidu, R.; Rahman, M.M.; Oleszczuk, P.; Karimi, J. PET-microplastics as a vector for heavy metals in a simulated plant rhizosphere zone. Sci Total Environ 2020, 744, 140984. [Google Scholar] [CrossRef] [PubMed]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci Total Environ 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.; Andreou, D.; Green, I.D.; Britton, J.R. Microplastics in freshwater fishes: Occurrence, impacts and future perspectives. Fish Fish 2021, 22, 467–488. [Google Scholar] [CrossRef]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.-J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci Total Environ 2020, 703, 134699. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D.L. Environmentally relevant concentrations of polyethylene microplastics negatively impact the survival, growth and emergence of sediment-dwelling invertebrates. Environ Pollut 2018, 236, 425–431. [Google Scholar] [CrossRef]
- Kalčíková, G. Beyond ingestion: Adhesion of microplastics to aquatic organisms. Aquat Toxicol 2023, 258, 106480. [Google Scholar] [CrossRef]
- Kalčíková, G.; Žgajnar Gotvajn, A.; Kladnik, A.; Jemec, A. Impact of polyethylene microbeads on the floating freshwater plant duckweed Lemna minor. Environ Pollut 2017, 230, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Bae, B.; Kim, Y. Feasibility Test for Phytoremediation of Heavy Metals-Contaminated Soils using Various Stabilizers. J Korean Geo-Environ Soc 2012, 13, 59–70. [Google Scholar]
- Mateos-Cárdenas, A.; Scott, D.T.; Seitmaganbetova, G.; Frank, N.A.M.v.P.; John, O.H.; Marcel, A.K.J. Polyethylene microplastics adhere to Lemna minor (L.), yet have no effects on plant growth or feeding by Gammarus duebeni (Lillj.). Sci Total Environ 2019, 689, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment. Environ Sci Technol Lett 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Datu, S.S.; Supriadi, *!!! REPLACE !!!*; Tahir, A. Microplastic in Cymodocea rotundata seagrass blades. Int J Environ Agric Biotech 2019, 4, 1758–1761. [Google Scholar] [CrossRef]
- Kim, Y. Evaluation of the water purification capacity in Iris pseudacorus and Acorus calamu. Korean Journal of Horticultural Science & Technology 2008, 26, 172–176. [Google Scholar]
- Schlüter, U.; Crawford, R.M.M. Long-term anoxia tolerance in leaves of Acorus calamus L. and Iris pseudacorus L. J Exp Bot 2001, 52, 2213–2225. [Google Scholar] [CrossRef]
- Ryu, B.Y. Garden and Plant; Flora: Republic of Korea, 2016. [Google Scholar]
- Mejía, A.C.; Velasco, A.C.; Sánchez, P.Z.; Cisneros, B.J. Photo-Oxidation Treatment of the Reject Stream of a Nanofiltration Membrane System. In Membranes: Materials, Simulations, and Applications; Maciel-Cerda, A., Ed.; Springer International Publishing: Cham, 2017; pp. 105–111. [Google Scholar]
- Landaburu-Aguirre, J.; García-Pacheco, R.; Molina, S.; Rodríguez-Sáez, L.; Rabadán, J.; García-Calvo, E. Fouling prevention, preparing for re-use and membrane recycling. Towards circular economy in RO desalination. Desalination 2016, 393, 16–30. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Hadibarata, T. Microplastics removal through water treatment plants: Its feasibility, efficiency, future prospects and enhancement by proper waste management. Environmental Challenges 2021, 5, 100264. [Google Scholar] [CrossRef]
- Ra, K.H.; Kwon, S.H.; Lee, J.H. Aquatic Plants for Wastewater Treatment. J Environ Health Sci 1996, 22, 49–55. [Google Scholar]
- Hoellein, T.J.; Shogren, A.J.; Tank, J.L.; Risteca, P.; Kelly, J.J. Microplastic deposition velocity in streams follows patterns for naturally occurring allochthonous particles. Sci Rep 2019, 9, 3740. [Google Scholar] [CrossRef] [PubMed]
- Woodall, L.C.; Gwinnett, C.; Packer, M.; Thompson, R.C.; Robinson, L.F.; Paterson, G.L.J. Using a forensic science approach to minimize environmental contamination and to identify microfibres in marine sediments. Mar Pollut Bull 2015, 95, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, C.; Wang, X.; Cheng, H.; Xing, J.; Li, Y.; Wang, L.; Ge, T.; Du, A.; Wang, Z. Water Spinach (Ipomoea aquatica F.) Effectively Absorbs and Accumulates Microplastics at the Micron Level—A Study of the Co-Exposure to Microplastics with Varying Particle Sizes. Agriculture 2024, 14, 301. [Google Scholar] [CrossRef]
- Lu, B.; Xu, Z.; Li, J.; Chai, X. Removal of water nutrients by different aquatic plant species: An alternative way to remediate polluted rural rivers. Ecol Eng 2018, 110, 18–26. [Google Scholar] [CrossRef]
- Maggioni, L.A.; Fontaneto, D.; Bocchi, S.; Gomarasca, S. Evaluation of water quality and ecological system conditions through macrophytes. Desalination 2009, 246, 190–201. [Google Scholar] [CrossRef]
- Petrucio, M.; Esteves, F. Uptake rates of nitrogen and phosphorus in the water by Eichhornia crassipes and Salvinia auriculata. Revista Brasil Biol 2000, 60, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cui, Y.; Dong, Y. Phytoremediation of Polluted Waters Potentials and Prospects of Wetland Plants. Acta Biotechnol 2002, 22, 199–208. [Google Scholar] [CrossRef]
- Mant, C.; Costa, S.; Williams, J.; Tambourgi, E. Phytoremediation of chromium by model constructed wetland. Bioresour Technol 2006, 97, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Dovidat, L.C.; Brinkmann, B.W.; Vijver, M.G.; Bosker, T. Plastic particles adsorb to the roots of freshwater vascular plant Spirodela polyrhiza but do not impair growth. Limnol Oceanogr Lett 2020, 5, 37–45. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ Sci Technol 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Carpita, N.C. Limiting Diameters of Pores and the Surface Structure of Plant Cell Walls. Science 1982, 218, 813–814. [Google Scholar] [CrossRef] [PubMed]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials 2021, 11, 2935. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).