Submitted:
14 May 2024
Posted:
14 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
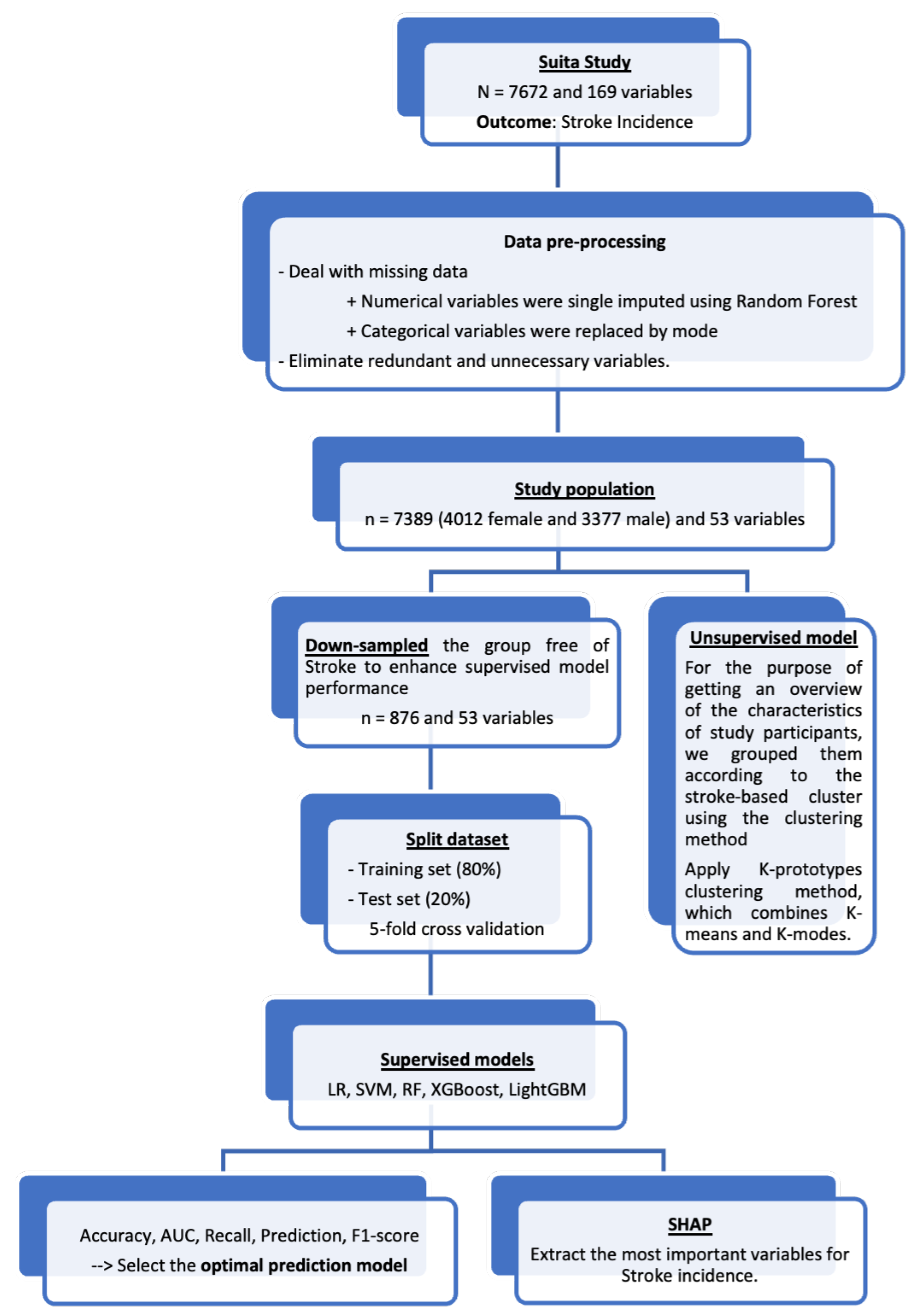
2. Materials and Methods
2.1. Study Participants
2.2. Outcomes
2.3. Risk Factors and Additional Measurements
2.4. Statistical Methods
2.5. Data Pre-Processing
2.6. Unsupervised Learning
2.7. Supervised Learning
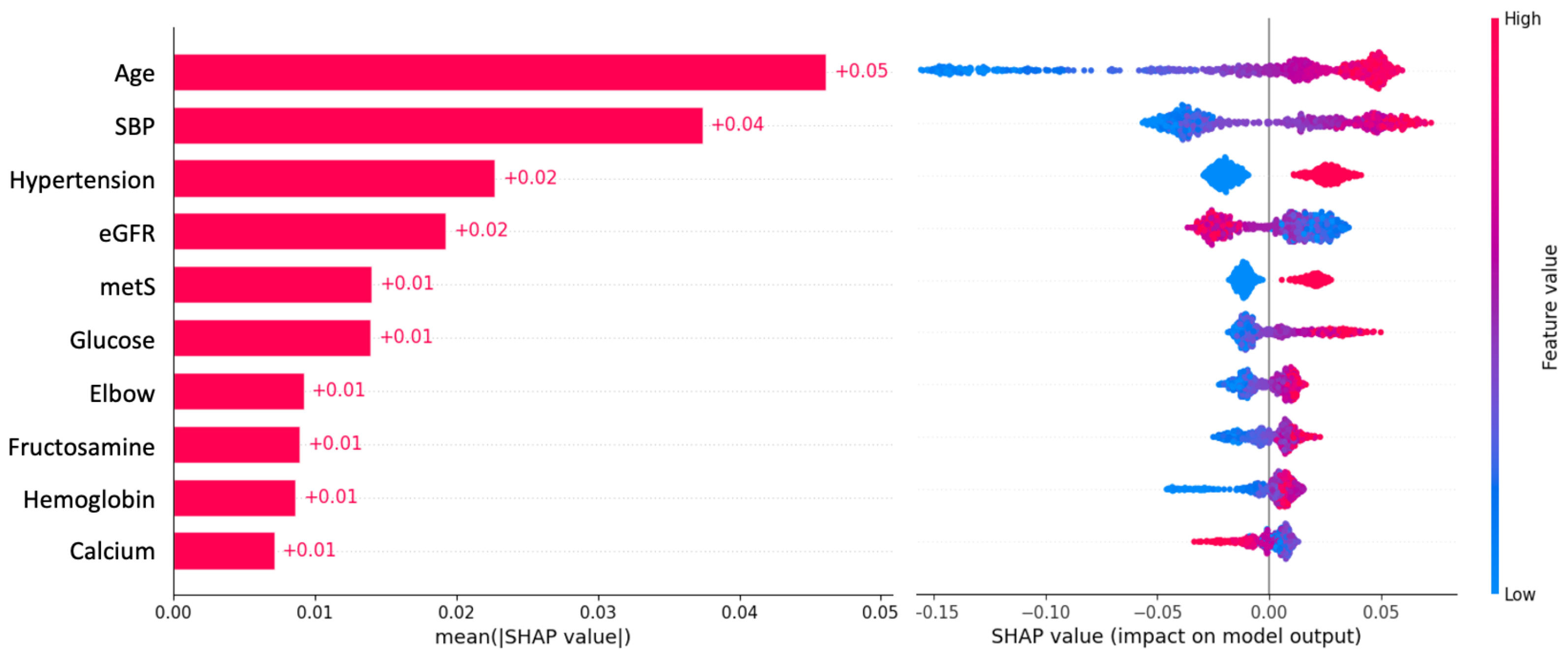
2.8. Extraction of Important Variables for Stroke Risk
3. Results
4. Discussion
4.1. Top Most Important Variables and Comparisons with Other Studies
4.2. Comparing our Important Variables and the Variables Used in Framingham and Suita Scores
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SHAP | SHapley Additive exPlanations |
| AUC | Area Under the Curve |
| VIF | Variance inflation factor |
| LR | Logistic Regression |
| SVM | Support Vector Machine |
| RF | Random Forest |
| XGBoost | eXtreme Gradient Boosting |
| Light-GBM | Light Gradient-Boosting Machine |
| BMI | Body mass index |
| SBP | Systolic blood pressure |
| DBP | Diasolic blood pressure |
| HDL-c | High-density lipoprotein cholesterol |
| eGFR | Estimated glomerular filtration rate |
| MetS | Metabolic syndrome |
Appendix A
| Performance metrics | Definition | Formula | Interpretation |
|---|---|---|---|
| Accuracy | Accuracy is a measure of how many of the total predictions made by the model are correct |
Accuracy tells us the overall correctness of predictions. However, a highly imbalanced dataset can lead to misleadingly high accuracy if the model predicts the majority class most of the time. |
|
| AUC | Area under a receiver operating characteristic (AUC-ROC) measures the ability of a model to distinguish between the positive and negative classes by varying the classification threshold |
The ROC curve plots the True Positive Rate (Recall) against the False Positive Rate at various threshold values, and AUC-ROC calculates the area under this curve. |
|
| Recall | Recall (or Sensitivity) measures the ability of the model to correctly identify positive instances out of all actual positive instances |
Recall quantifies the model’s ability to avoid missing positive cases. It’s crucial in scenarios where false negatives (missing actual positive cases) are costly or problematic. |
|
| Precision | Precision (or Positive Predictive Value) measures the accuracy of positive predictions made by the model |
Precision focuses on the accuracy of positive predictions. |
|
| F1-score | The F1-score is the harmonic mean of precision and recall. It provides a single metric that balances both precision and recall. |
The F1-score combines the strengths of precision and recall into a single metric. |
References
- WHO. The top 10 causes of death, 2020. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. International Journal of Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, M.O.; Thrift, A.G.; Mahal, A.; Ishida, M.; Martins, S.; Johnson, W.D.; Pandian, J.; Abd-Allah, F.; Yaria, J.; Phan, H.T.; et al. Primary stroke prevention worldwide: translating evidence into action. The Lancet Public Health 2022, 7, e74–e85. [Google Scholar] [CrossRef] [PubMed]
- Ambale-Venkatesh, B.; Yang, X.; Wu, C.O.; Liu, K.; Hundley, W.G.; McClelland, R.; Gomes, A.S.; Folsom, A.R.; Shea, S.; Guallar, E.; Bluemke, D.A.; Lima, J.A. Cardiovascular Event Prediction by Machine Learning. Circulation Research 2017, 121, 1092–1101. [Google Scholar] [CrossRef]
- Kim, J.T.; Kim, N.R.; Choi, S.H.; Oh, S.; Park, M.S.; Lee, S.H.; Kim, B.C.; Choi, J.; Kim, M.S. Neural network-based clustering model of ischemic stroke patients with a maximally distinct distribution of 1-year vascular outcomes. Scientific Reports 2022, 12, 9420. [Google Scholar] [CrossRef] [PubMed]
- Dritsas, E.; Trigka, M. Stroke Risk Prediction with Machine Learning Techniques. Sensors 2022, 22, 4670. [Google Scholar] [CrossRef]
- Tazin, T.; Alam, M.N.; Dola, N.N.; Bari, M.S.; Bourouis, S.; Khan, M.M. Stroke Disease Detection and Prediction Using Robust Learning Approaches. Journal of Healthcare Engineering 2021, 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, Y.; Kamide, K.; Okamura, T.; Watanabe, M.; Higashiyama, A.; Kawanishi, K.; Okayama, A.; Kawano, Y. Impact of High-Normal Blood Pressure on the Risk of Cardiovascular Disease in a Japanese Urban Cohort. Hypertension 2008, 52, 652–659. [Google Scholar] [CrossRef]
- Kokubo, Y.; Watanabe, M.; Higashiyama, A.; Nakao, Y.M.; Kobayashi, T.; Watanabe, T.; Okamura, T.; Okayama, A.; Miyamoto, Y. Interaction of Blood Pressure and Body Mass Index With Risk of Incident Atrial Fibrillation in a Japanese Urban Cohort: The Suita Study. American Journal of Hypertension 2015, 28, 1355–1361. [Google Scholar] [CrossRef]
- Nakao, Y.M.; Miyamoto, Y.; Ueshima, K.; Nakao, K.; Nakai, M.; Nishimura, K.; Yasuno, S.; Hosoda, K.; Ogawa, Y.; Itoh, H.; Ogawa, H.; Kangawa, K.; Nakao, K. Effectiveness of nationwide screening and lifestyle intervention for abdominal obesity and cardiometabolic risks in Japan: The metabolic syndrome and comprehensive lifestyle intervention study on nationwide database in Japan (MetS ACTION-J study). PLOS ONE 2018, 13, e0190862. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Cui, R.; Takamoto, I.; Kiyama, M.; Saito, I.; Okamura, T.; Miyamoto, Y.; Higashiyama, A.; Kiyohara, Y.; Ninomiya, T.; Yamada, M.; Nakagawa, H.; Sakurai, M.; Shimabukuro, M.; Higa, M.; Shimamoto, K.; Saito, S.; Daimon, M.; Kayama, T.; Noda, M.; Ito, S.; Yokote, K.; Ito, C.; Nakao, K.; Yamauchi, T.; Kadowaki, T. Risk Classification for Metabolic Syndrome and the Incidence of Cardiovascular Disease in Japan With Low Prevalence of Obesity: A Pooled Analysis of 10 Prospective Cohort Studies. Journal of the American Heart Association 2021, 10. [Google Scholar] [CrossRef]
- Imai, E.; Horio, M.; Nitta, K.; Yamagata, K.; Iseki, K.; Hara, S.; Ura, N.; Kiyohara, Y.; Hirakata, H.; Watanabe, T.; Moriyama, T.; Ando, Y.; Inaguma, D.; Narita, I.; Iso, H.; Wakai, K.; Yasuda, Y.; Tsukamoto, Y.; Ito, S.; Makino, H.; Hishida, A.; Matsuo, S. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clinical and Experimental Nephrology 2007, 11, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Martin-Morales, A.; Yamamoto, M.; Inoue, M.; Vu, T.; Dawadi, R.; Araki, M. Predicting Cardiovascular Disease Mortality: Leveraging Machine Learning for Comprehensive Assessment of Health and Nutrition Variables. Nutrients 2023, 15, 3937. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. Extensions to the k-Means Algorithm for Clustering Large Data Sets with Categorical Values. Data Mining and Knowledge Discovery 1998, 2, 283–304. [Google Scholar] [CrossRef]
- Landwehr, N.; Hall, M.; Frank, E. Logistic Model Trees. Machine Learning 2005, 59, 161–205. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Saito, T.; Suzuki, M.; Ishioka, T.; Tomisawa, Y.; Nakaya, N.; Abo, M. Support Vector Machine-Based Classifier for the Assessment of Finger Movement of Stroke Patients Undergoing Rehabilitation. Journal of Medical and Biological Engineering 2020, 40, 91–100. [Google Scholar] [CrossRef]
- Su, P.Y.; Wei, Y.C.; Luo, H.; Liu, C.H.; Huang, W.Y.; Chen, K.F.; Lin, C.P.; Wei, H.Y.; Lee, T.H. Machine Learning Models for Predicting Influential Factors of Early Outcomes in Acute Ischemic Stroke: Registry-Based Study. JMIR Medical Informatics 2022, 10, e32508. [Google Scholar] [CrossRef] [PubMed]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.Y. LightGBM: A Highly Efficient Gradient Boosting Decision Tree. Advances in Neural Information Processing Systems 2017, 30. [Google Scholar]
- Nouraei, H.; Nouraei, H.; Rabkin, S.W. Comparison of Unsupervised Machine Learning Approaches for Cluster Analysis to Define Subgroups of Heart Failure with Preserved Ejection Fraction with Different Outcomes. Bioengineering 2022, 9, 175. [Google Scholar] [CrossRef]
- Fernandez-Lozano, C.; Hervella, P.; Mato-Abad, V.; Rodríguez-Yáñez, M.; Suárez-Garaboa, S.; López-Dequidt, I.; Estany-Gestal, A.; Sobrino, T.; Campos, F.; Castillo, J.; Rodríguez-Yáñez, S.; Iglesias-Rey, R. Random forest-based prediction of stroke outcome. Scientific Reports 2021, 11, 10071. [Google Scholar] [CrossRef]
- Sirsat, M.S.; Fermé, E.; Câmara, J. Machine Learning for Brain Stroke: A Review. Journal of Stroke and Cerebrovascular Diseases 2020, 29, 105162. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, Z.; Zhang, Y.; Shang, J.; Yu, L.; Fu, P.; Liu, Y.; Li, X.; Wang, H.; Ren, L.; Zhang, W.; Hou, H.; Tan, X.; Wang, W. Rapid triage for ischemic stroke: a machine learning-driven approach in the context of predictive, preventive and personalised medicine. EPMA Journal 2022, 13, 285–298. [Google Scholar] [CrossRef]
- Nugroho, A.W.; Arima, H.; Miyazawa, I.; Fujii, T.; Miyamatsu, N.; Sugimoto, Y.; Nagata, S.; Komori, M.; Takashima, N.; Kita, Y.; Miura, K.; Nozaki, K. The Association between Glomerular Filtration Rate Estimated on Admission and Acute Stroke Outcome: The Shiga Stroke Registry. Journal of Atherosclerosis and Thrombosis 2018, 25, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Penn, A.M.; Croteau, N.S.; Votova, K.; Sedgwick, C.; Balshaw, R.F.; Coutts, S.B.; Penn, M.; Blackwood, K.; Bibok, M.B.; Saly, V.; Hegedus, J.; Yu, A.Y.X.; Zerna, C.; Klourfeld, E.; Lesperance, M.L. Systolic blood pressure as a predictor of transient ischemic attack/minor stroke in emergency department patients under age 80: a prospective cohort study. BMC Neurology 2019, 19, 251. [Google Scholar] [CrossRef] [PubMed]
- Arafa, A.; Kokubo, Y.; Sheerah, H.A.; Sakai, Y.; Watanabe, E.; Li, J.; Honda-Kohmo, K.; Teramoto, M.; Kashima, R.; Nakao, Y.M.; Koga, M. Developing a Stroke Risk Prediction Model Using Cardiovascular Risk Factors: The Suita Study. Cerebrovascular Diseases 2022, 51, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Guzik, A.; Bushnell, C. Stroke Epidemiology and Risk Factor Management. CONTINUUM: Lifelong Learning in Neurology 2017, 23, 15–39. [Google Scholar] [CrossRef]
- Turana, Y.; Tengkawan, J.; Chia, Y.C.; Nathaniel, M.; Wang, J.; Sukonthasarn, A.; Chen, C.; Minh, H.V.; Buranakitjaroen, P.; Shin, J.; et al. Hypertension and stroke in Asia: A comprehensive review from HOPE Asia. The Journal of Clinical Hypertension 2021, 23, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Saver, J.L.; Chang, K.H.; Liao, H.W.; Chang, S.C.; Ovbiagele, B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ 2010, 341, c4249–c4249. [Google Scholar] [CrossRef]
- Chao, C.H.; Wu, C.L.; Huang, W.Y. Association between estimated glomerular filtration rate and clinical outcomes in ischemic stroke patients with high-grade carotid artery stenosis. BMC Neurology 2021, 21, 124. [Google Scholar] [CrossRef]
- Hajhosseiny, R.; Matthews, G.K.; Lip, G.Y. Metabolic syndrome, atrial fibrillation, and stroke: Tackling an emerging epidemic. Heart Rhythm 2015, 12, 2332–2343. [Google Scholar] [CrossRef]
- Carson, A.P.; Muntner, P.; Kissela, B.M.; Kleindorfer, D.O.; Howard, V.J.; Meschia, J.F.; Williams, L.S.; Prineas, R.J.; Howard, G.; Safford, M.M. Association of Prediabetes and Diabetes With Stroke Symptoms. Diabetes Care 2012, 35, 1845–1852. [Google Scholar] [CrossRef]
- Ribeiro, R.T.; Macedo, M.P.; Raposo, J.F. HbA1c, Fructosamine, and Glycated Albumin in the Detection of Dysglycaemic Conditions. Current Diabetes Reviews 2015, 12, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Rawlings, A.M.; Lutsey, P.L.; Maruthur, N.; Pankow, J.S.; Steffes, M.; Coresh, J. Fructosamine and Glycated Albumin and the Risk of Cardiovascular Outcomes and Death. Circulation 2015, 132, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, A.; Lubas, A.; Smoszna, J.; Niemczyk, S. Risk Factors Associated with All-Cause Death Among Dialysis Patients with Diabetes. Medical Science Monitor 2021, 27. [Google Scholar] [CrossRef] [PubMed]
- Panwar, B.; Judd, S.E.; Warnock, D.G.; McClellan, W.M.; Booth, J.N.; Muntner, P.; Gutiérrez, O.M. Hemoglobin Concentration and Risk of Incident Stroke in Community-Living Adults. Stroke 2016, 47, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Jee, S.H.; Yun, J.E.; Baek, S.J.; Lee, D.C. Hemoglobin Concentration and Risk of Cardiovascular Disease in Korean Men and Women - The Korean Heart Study. Journal of Korean Medical Science 2013, 28, 1316. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.V.; Tripathi, B.; Agarwal, Y.; Kabi, B.; Kumar, R. Association of serum calcium levels with clinical severity of ischemic stroke at the time of admission as defined by NIHSS score: A cross-sectional, observational study. Journal of Family Medicine and Primary Care 2022, 11, 6427. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Xun, P.; Fly, A.D.; Bidulescu, A.; Tsinovoi, C.L.; Judd, S.E.; McClure, L.A.; Cushman, M.; Unverzagt, F.W.; He, K. Calcium Intake and Serum Calcium Level in Relation to the Risk of Ischemic Stroke: Findings from the REGARDS Study. Journal of Stroke 2019, 21, 312–323. [Google Scholar] [CrossRef]
- Rohrmann, S.; Garmo, H.; Malmström, H.; Hammar, N.; Jungner, I.; Walldius, G.; Hemelrijck, M.V. Association between serum calcium concentration and risk of incident and fatal cardiovascular disease in the prospective AMORIS study. Atherosclerosis 2016, 251, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Burgess, S.; Michaëlsson, K. Association of Genetic Variants Related to Serum Calcium Levels With Coronary Artery Disease and Myocardial Infarction. JAMA 2017, 318, 371. [Google Scholar] [CrossRef]
- Jahangiry, L.; Farhangi, M.A.; Rezaei, F. Framingham risk score for estimation of 10-years of cardiovascular diseases risk in patients with metabolic syndrome. Journal of Health, Population and Nutrition 2017, 36, 36. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Itaya, T.; Terasawa, Y.; Kohriyama, T. Association between the Suita Score and Stroke Recurrence in Patients with First-ever Ischemic Stroke: A Prospective Cohort Study. Internal Medicine 2022, 61, 7905-21. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Okamura, T.; Watanabe, M.; Nakai, M.; Takegami, M.; Higashiyama, A.; Kokubo, Y.; Okayama, A.; Miyamoto, Y. Predicting Coronary Heart Disease Using Risk Factor Categories for a Japanese Urban Population, and Comparison with the Framingham Risk Score: The Suita Study. Journal of Atherosclerosis and Thrombosis 2014, 21, 784–798. [Google Scholar] [CrossRef] [PubMed]
| Overall | Stroke incidence | |||
|---|---|---|---|---|
| No | Yes | p-value | ||
| (n = 7389) | (n = 6951, 94.1%) | (n = 438, 5.9%) | ||
| Age, Years | 56 [44, 65] | 55 [44, 65] | 66 [58, 72] | <.0001 |
| male | 3377 (45.7%) | 3143 (45.2%) | 234 (53.4%) | <.0001 |
| BMI, kg/m2 | 22.5 (3.01) | 22.5 (3.00) | 23.1 (3.21) | <.0001 |
| SBP, mmHg | 124 [110, 138] | 123 [110, 137] | 137 [122, 153] | <.0001 |
| DBP, mmHg | 77.7 (12.2) | 77.4 (12.0) | 81.2 (13.4) | <.0001 |
| Smoking, n (%) | 0.004 | |||
| Current | 2140 (29.5) | 1999 (29.2) | 141 (33.3) | |
| Past | 1162 (16.0) | 1075 (15.7) | 87 (20.5) | |
| Never | 3963 (54.5) | 3767 (55.1) | 196 (46.2) | |
| Glucose, mg/dL | 95.0 [90.0, 102.0] | 95.0 [89.0, 101.0] | 99.0 [92.0, 107.0] | <.0001 |
| Fructosamine, mol/L | 253 (22.3) | 253 (22.2) | 258 (23.7) | <0.001 |
| Elbow, mm | 6.3 (0.6) | 6.3 (0.6) | 6.4 (0.5) | 0.008 |
| Calcium, mg/dL | 9.4 (0.4) | 9.4 (0.4) | 9.3 (0.4) | 0.039 |
| Hemoglobin, g/dL | 13.9 (1.5) | 13.9 (1.5) | 14.1 (1.4) | 0.001 |
| TG, mg/dL | 99.0 [71.0, 144.0] | 98.0 [70.0, 143.0] | 112.5 [82.0, 163.8] | <.0001 |
| non-HDL-c, mg/dL | 152.6 (36.9) | 152.2 (36.9) | 158.9 (36.8) | 0.0002 |
| eGFR, mL/min/1.73 m2 | 90.0 [73.7, 104.6] | 90.3 [74.4, 104.8] | 80.0 [66.6, 95.0] | <.0001 |
| Hypertension, n (%) | 2295 (31.1) | 2054 (29.5) | 241 (55.0) | <.0001 |
| Diabetes, n (%) | 898 (12.2) | 798 (11.5) | 100 (22.8) | <.0001 |
| MetS, n (%) | 1811 (24.5) | 1630 (23.4) | 181 (41.3) | <.0001 |
| Overall | Stroke Risk | ||||
|---|---|---|---|---|---|
| High | Medium | Low | p-value | ||
| (n = 7389) | (n = 1974) | (n = 2565) | (n = 2850) | ||
| Stroke incidence, n (%) | 438 (5.9) | 179 (9.1) | 169 (6.6) | 90 (3.2) | <0.001 |
| Age, Years | 56 [44, 65] | 63 [55, 71] | 55 [44, 63] | 50 [40, 62] | <0.001 |
| Gender | <0.001 | ||||
| Male, n (%) | 3377 (45.7) | 211 (10.7) | 2497 (97.3) | 669 (23.5) | |
| Female, n (%) | 4012 (54.3) | 1763 (89.3) | 68 (2.7) | 2181 (76.5) | |
| BMI, kg/m2 | 22.5 (3.0) | 24.0 (2.7) | 23.8 (2.6) | 20.3 (2.1) | <0.001 |
| Body fat, % | 23.2 (6.0) | 28.6 (5.6) | 20.6 (4.1) | 21.8 (5.3) | <0.001 |
| SBP, mmHg | 126.3 (20.8) | 138.7 (20.0) | 129.0 (19.1) | 115.4 (16.8) | <0.001 |
| DBP, mmHg | 77.6 (11.8) | 82.2 (10.8) | 81.3 (11.4) | 71.1 (9.7) | <0.001 |
| Smoking, n (%) | <0.001 | ||||
| Current | 2140 (29.0) | 194 (9.8) | 1300 (50.7) | 646 (22.7) | |
| Past | 1162 (15.7) | 157 (8.0) | 746 (29.1) | 259 (9.1) | |
| Never | 4087 (55.3) | 1623 (82.2) | 519 (20.2) | 1945 (68.2) | |
| eGFR, mL/min/1.73 m2 | 90.8 (23.7) | 86.9 (23.9) | 89.1 (22.0) | 94.9 (24.4) | <0.001 |
| Hemoglobin, g/dL | 13.9 (1.5) | 13.3 (1.1) | 15.3 (1.0) | 13.1 (1.3) | <0.001 |
| TG, mg/dL | 99 [71, 144] | 116 [87, 159.8] | 129 [91, 186] | 73 [57, 95] | <0.001 |
| non-HDL-c, mg/dL | 152.4 (36.1) | 172.2 (34.2) | 155.2 (34.2) | 136.2 (31.3) | <0.001 |
| HDL-c, mg/dL | 54.6 (14.0) | 53.3 (13.1) | 48.8 (12.5) | 60.7 (13.3) | <0.001 |
| Glucose, mg/dL | 95 [90, 101] | 97 [92, 104] | 98 [92.9, 105] | 91 [87, 96] | <0.001 |
| Fructosamine, mol/L | 253.2 (22.3) | 258.3 (23.0) | 251.9 (23.3) | 250.8 (20.4) | <0.001 |
| Elbow, mm | 6.3 (0.6) | 6.1 (0.5) | 6.8 (0.4) | 6.0 (0.5) | <0.001 |
| Calcium, mg/dL | 9.3 (0.4) | 9.5 (0.4) | 9.4 (0.4) | 9.2 (0.4) | <0.001 |
| Hypertension, n (%) | 2295 (31.1) | 1063 (53.9) | 920 (35.9) | 312 (10.9) | <0.001 |
| Diabetes, n (%) | 898 (12.2) | 334 (16.9) | 455 (17.7) | 109 (3.8) | <0.001 |
| MetS, n (%) | 1811 (24.5) | 762 (38.6) | 997 (38.9) | 52 (1.8) | <0.001 |
| Accuracy | AUC | Recall | Precision | F1-score | |
|---|---|---|---|---|---|
| LR | 0.64 | 0.68 | 0.64 | 0.64 | 0.64 |
| RF | 0.70 | 0.71 | 0.70 | 0.70 | 0.70 |
| SVM | 0.68 | 0.73 | 0.68 | 0.68 | 0.68 |
| XGBoost | 0.68 | 0.71 | 0.68 | 0.68 | 0.68 |
| LightGBM | 0.66 | 0.70 | 0.66 | 0.67 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





