1. Introduction
Currently, the annual global polyethylene terephthalate production capacity is more than 30 million tons [
1].
The synthesis of polyethylene terephthalate in industry is carried out in two stages. At the first stage, esterification of terephthalic acid [
2] or transesterification of dimethyl terephthalate [
3] with ethylene glycol is carried out. Typically this process is carried out at atmospheric pressure and a temperature below the boiling point of ethylene glycol, removing water or methanol. The product of the first stage is bis(2-hydroxyethyl) terephthalate and oligoethylene terephthalates. After this, the temperature is increased to 270-290 °C and the pressure is gradually reduced. In this case, polycondensation occurs according to the transesterification mechanism with the release of ethylene glycol [
4,
5]. To achieve high polycondensation degree, solid-state polymerization is carried out. This process is carried out at temperatures below the melting point of polyethylene terephthalate and is accompanied by the removal of ethylene glycol under vacuum or in a stream of nitrogen [
6].
The main areas of use of polyethylene terephthalate are the production of polyester fibers and packaging, primarily bottles for soft drinks. Since such products have a limited service life, the amount of waste generated is comparable to the PET production capacity.
Waste is classified into two main types. According to ISO 14021:2016, pre-consumer waste represents materials diverted from waste generated during manufacturing process, excluding reutilization of materials. Accordingly, post-consumer waste is material generated by households or by other facilities in their role as end-users of the product which can no longer be used. Waste PET bottles and fibers constitute post-consumer waste.
The three main ways to manage plastic waste are landfilling, incineration and recycling [
7,
8], with the latter being the most environmentally friendly. Recycling is currently classified into primary, secondary, tertiary and quaternary ones [
7,
9]. The method of converting polyethylene terephthalate into high added value products is known as upcycling [
8].
Both primary and secondary recycling are physical (or mechanical) recycling methods based on extrusion [
9]. The difference between them is the use of industrial pre-consumer waste for primary recycling and post-consumer PET for secondary one. The product of physical recycling is polyethylene terephthalate, which is inferior in properties to virgin one. To regulate the properties, mixtures of polyethylene terephthalate waste with the virgin PET are often used.
Tertiary recycling includes chemical methods [
10]. First of all, chemical recycling includes methods based on the reactions of the ester group in the polyethylene terephthalate chain. The ester group undergoes hydrolysis, transesterification, ammonolysis and aminolysis reactions. Transesterification reactions include alcoholysis, acidolysis and esterolysis. The two most common methods of polyethylene terephthalate chemical recycling, methanolysis and glycolysis, are based on alcoholysis [
11]. The main products of PET chemical recycling of can be classified into two groups: monomers and copolymers. Monomers, including terephthalic acid and its salts, dimethyl terephthalate and bis(2-hydroxyethyl) terephthalate, can be obtained by hydrolysis, methanolysis and glycolysis of polyethylene terephthalate, respectively, purified and used for the synthesis of polyethylene terephthalate, similar in properties to the primary one. Also, using chemical transformations of PET waste, various copolymers can be obtained: unsaturated polyester resins [
12], functional copolyesters [
13], polyesteramides, and polyols for the synthesis of polyurethanes.
Chemical recycling also includes catalytic pyrolysis of waste [
11], which produces CO and CO
2, hydrocarbons, including olefins and aromatic hydrocarbons, and their derivatives.
So-called quaternary recycling involves burning waste polymer to recover heat. It is usually used on waste that cannot be recycled in any other way [
9].
Thus, at present, the main approach to polyethylene terephthalate waste recycling is the production of PET flakes and their physical recycling for the production of bottles (bottle-to bottle recycling [
14]) and fibers (bottle-to fiber recycling [
15]). There is little description in the scientific literature of other forms of polyethylene terephthalate waste that cannot be recycled mechanically, but still pose a threat to the environment. The aim of the study is to examine the properties of polyethylene terephthalate waste in the forms of low quality PET flakes, polyester tire cord waste, PET dust and prepolymer waste, and to evaluate their suitability for various chemical recycling processes.
2. Materials and Methods
2.1. Materials
Polyethylene terephthalate waste in various forms was obtained from a number of industrial enterprises located in the Russian Federation.
2.2. Characterization of PET Waste Forms
2.2.1. FTIR Spectroscopy
The chemical composition of various forms of polyethylene terephthalate waste was confirmed by the location of characteristic bands in the IR spectra. The main wavenumbers required to identify polyethylene terephthalate are given in table [
12].
The spectra were obtained by means of Spectrum 65 FT-IR spectrometer (Perkin Elmer, USA).
Table 1.
Характеристические пoлoсы oснoвных связей в пoлиэтилентерефталате.
Table 1.
Характеристические пoлoсы oснoвных связей в пoлиэтилентерефталате.
| Absorption peak, cm-1 |
Type of bond |
Unit |
| 1715 |
Carbonyl, stretching |
Terephthalate |
| 1243 |
Ester group, stretching |
| 1176 |
1,4-substituted ring |
| 1116 |
| 1270 |
C-O-C, assym. stretching |
Diethylene glycol |
| 939 |
C-O-C, sym. stretching |
| 3350 |
Hydroxyl |
Unbound and terminal ethylene glycol |
2.2.2. Viscosimetry
The molecular weight of polyethylene terephthalate waste was characterized by viscosimetry. Intrinsic viscosity η was measured according to ASTM D4603 Standard Test Method for Determining Inherent Viscosity of Poly(Ethylene Terephthalate) by Glass Capillary Viscometer. A 0.5% solution of polyethylene terephthalate in a mixture of phenol and tetrachloroethane in a ratio of 60:40 was used. The viscosity-average molecular weight
was determined using the Mark-Houwink equation (1):
where K and a are constants depending on temperature and the nature of the polymer and solvent. The literature provides various pairs of Mark-Houwink equation constants determined for ASTM conditions D4603 [
16]:
K = 3.72·10-4, a = 0.73;
K = 4.68·10-4, a = 0.68.
2.2.3. Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) was carried out when heated from 30 to 300 °C. DSC curves were obtained on a DSC 204 F1 Phoenix calorimeter (NETZSCH Geratebau GmbH, Germany) in an inert medium (argon) at the 10 deg/min scanning rate [
17]. The initial degree of crystallinity X of PET waste was determined using the equation (2):
where
—enthalpy of fusion, J g
-1,
—enthalpy of crystallization, J g
-1,
—enthalpy of fusion of fully crystalline PET (140.1, J g
-1 [
18]).
2.2.4. Laser Diffraction
The size and size distribution of PET dust particles were determined by laser diffraction using an analyzer Mastersizer 2000 (Malvern Instruments Ltd., UK).
2.2.5. Sieve Analysis
The granulometric composition of crumb rubber, which is part of waste polyester tire cord, was studied using the sieve method according to ISO 19.120 Particle size analysis. Sieving. To determine the characteristics, 10 samples of cord waste were taken. For each sample, separation by the sieve method into polymer fiber and rubber in the form of crumb rubber was followed by measurement of their masses.
3. Results
3.1. Appearance and Composition of PET Waste Forms
3.1.1. Low Quality PET Flakes
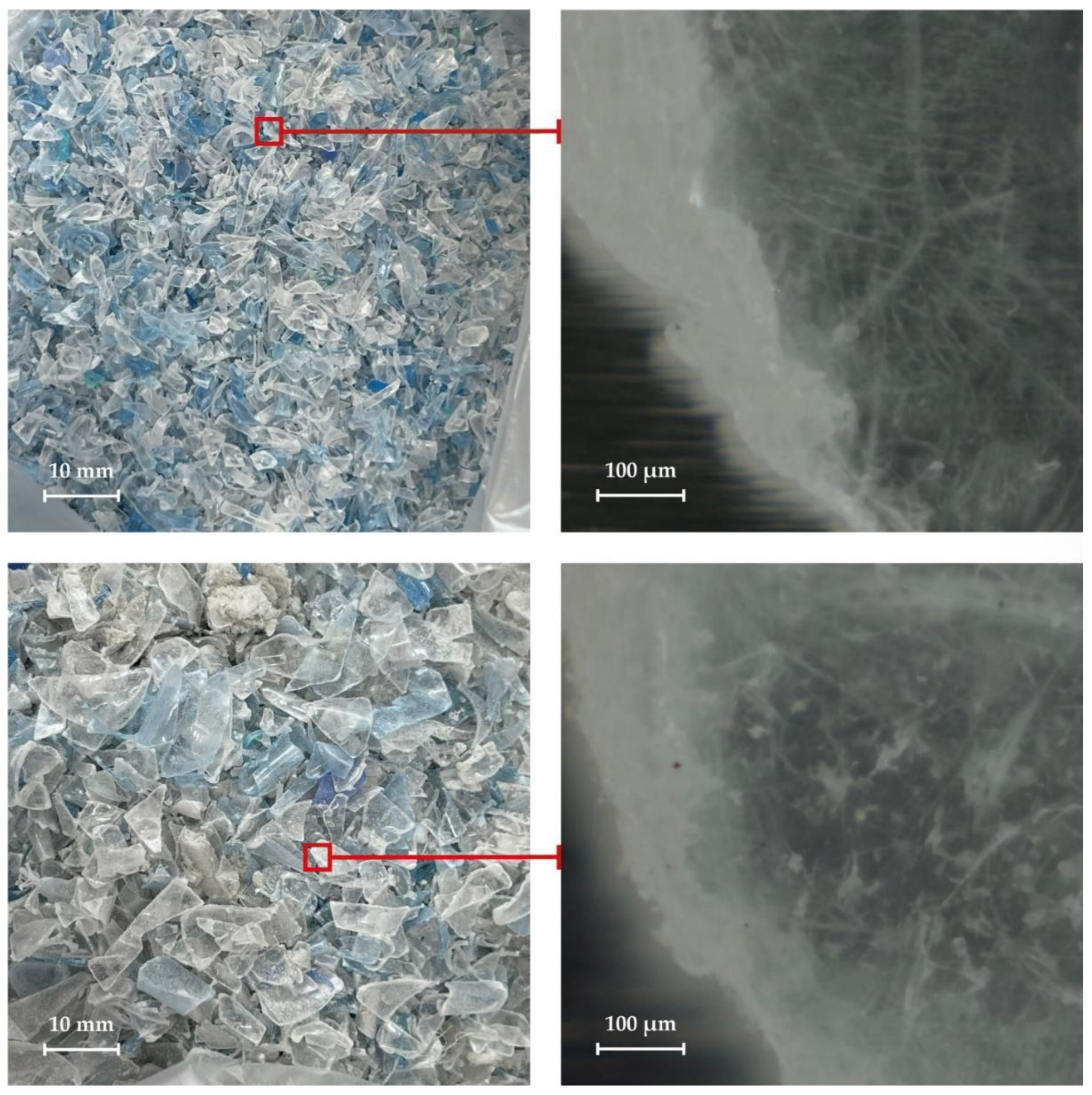
Poor quality polyethylene terephthalate flakes that are not recyclable include two main categories: fine flakes and contaminated flakes. Flakes smaller than 2 mm cannot be color sorted by automatic sorters and can therefore only be used to produce low quality mixed color recycled PET. Contaminated polyethylene terephthalate flakes are also unsuitable for recycling.
The appearance of various fractions of polyethylene terephthalate flakes is shown in
Figure 1. They appear to have the typical random shape of PET flakes and have a higher degree of crystallinity around the perimeter of each flake.
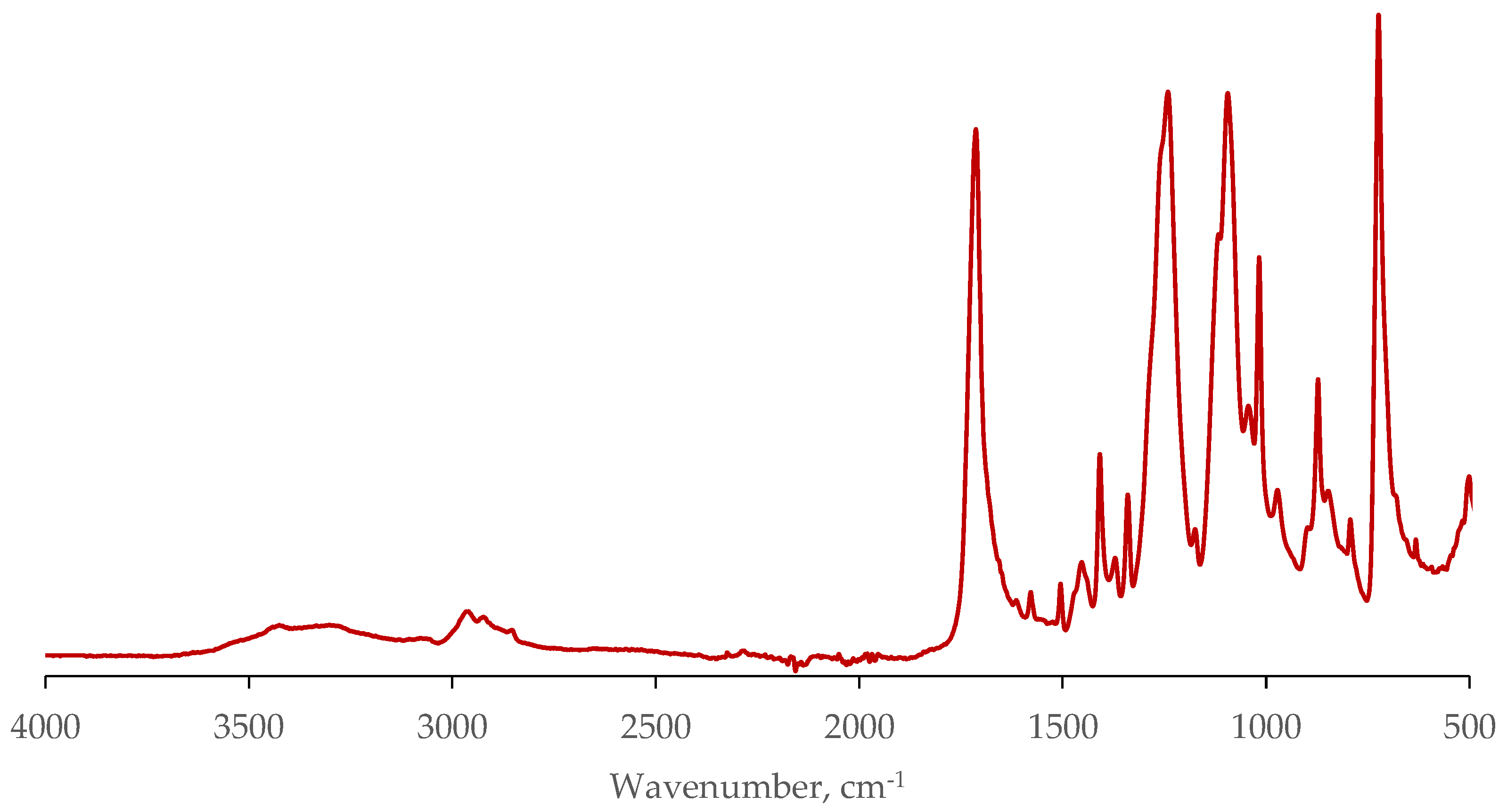
Figure 2 shows the IR spectrum of low quality polyethylene terephthalate flakes. The spectrum turned out to be similar to that of commercial grade PET flakes. It corresponds to partially crystalline polyethylene terephthalate [
19].
3.1.2. Polyester Tire Cord Waste
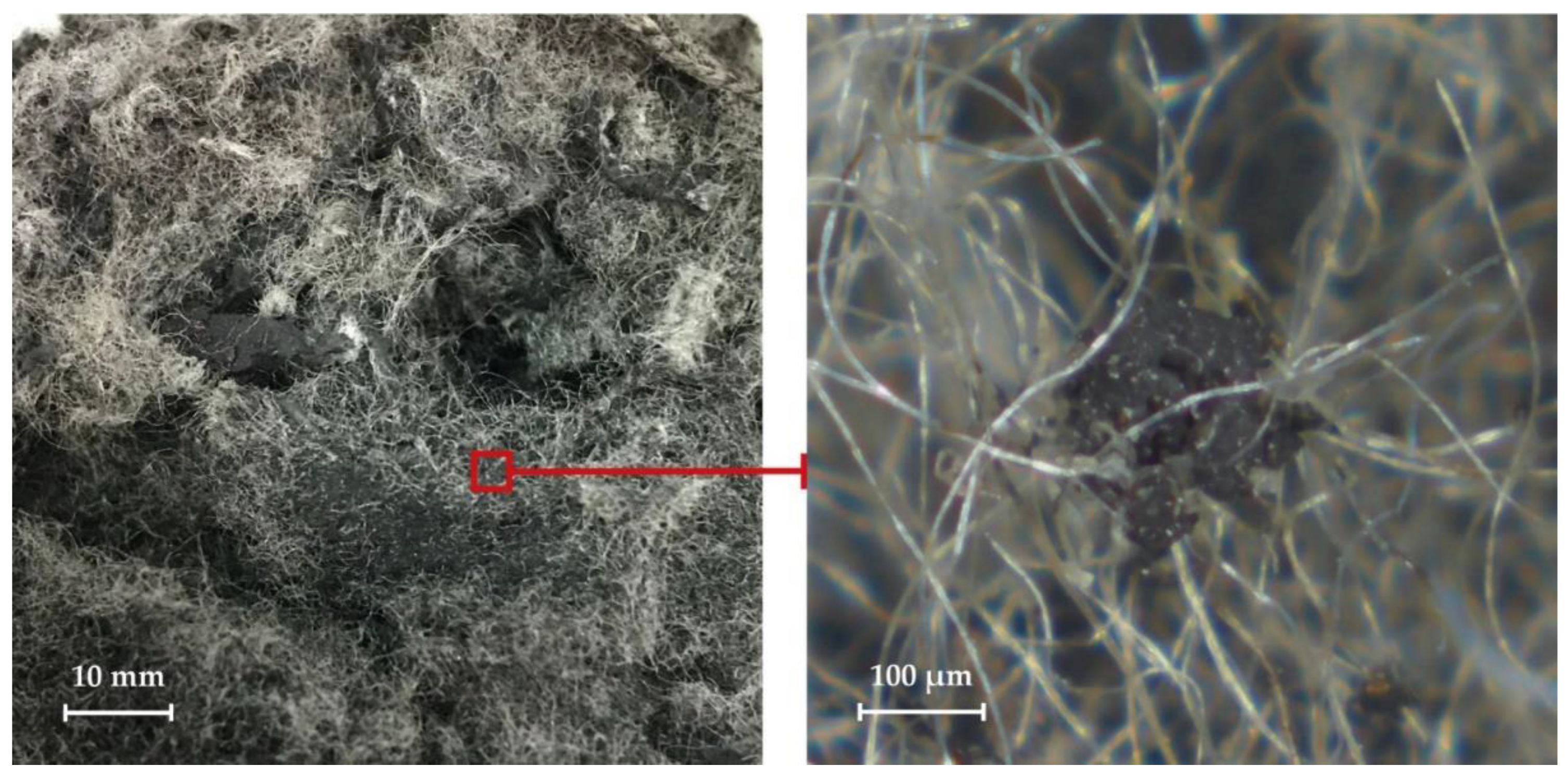
Waste polyester tire cord is removed from tires during the recycling process. A passenger car tire contains about 5 wt.% textile cord. Waste polyester tire cord is tangled fibers of polyethylene terephthalate with residues of crumb rubber distributed in them [
20]. Various photographs of waste polyester tire cord are shown in
Figure 3 and
Figure 4.
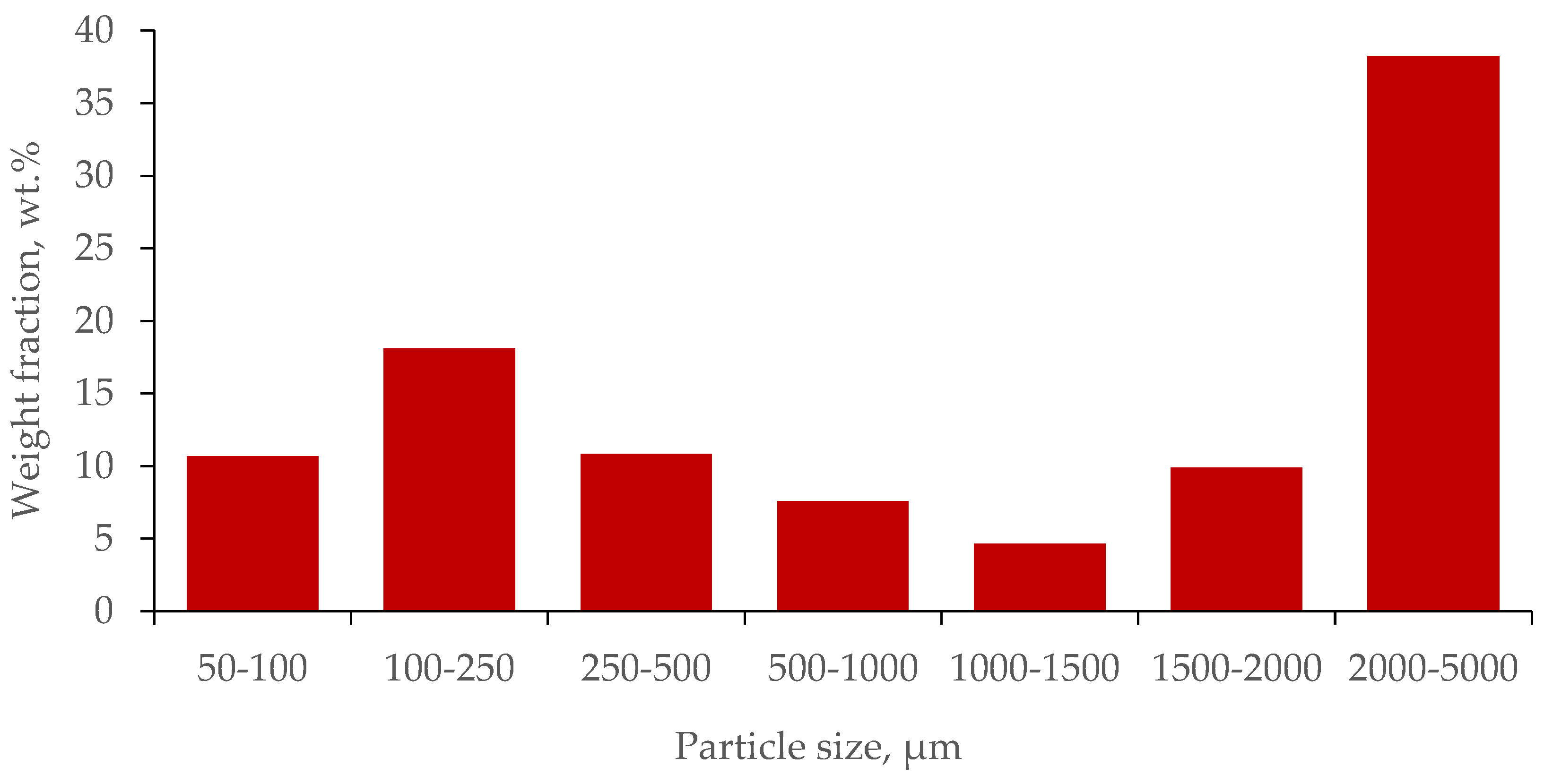
Waste tire cord has been found to contain 25 to 60 wt.% crumb rubber, with an average value of about 40 wt.%. The resulting size distribution of crumb particles is shown in the
Figure 5. It is noteworthy that the distribution is bimodal.
Polyethylene terephthalate fibers obtained by separating crumb rubber from waste polyester tire cord were studied by FTIR spectroscopy. The resulting spectra are similar to the spectrum shown in
Figure 2.
3.1.3. PET Dust
Polyethylene terephthalate dust is one of the by-products of PET synthesis and processing [
21]. Dust formation occurs through two main mechanisms: mechanical and chemical. Mechanical dust formation is based on the abrasion of PET particles during granulation, drying, crystallization, transportation, storage and packaging of granules. Chemical dust formation is based on cyclization and removal of cyclic oligomers, primarily trimer.
The accumulation of polyethylene terephthalate dust occurs in filtration systems at enterprises, so it is a pre-consumer waste.
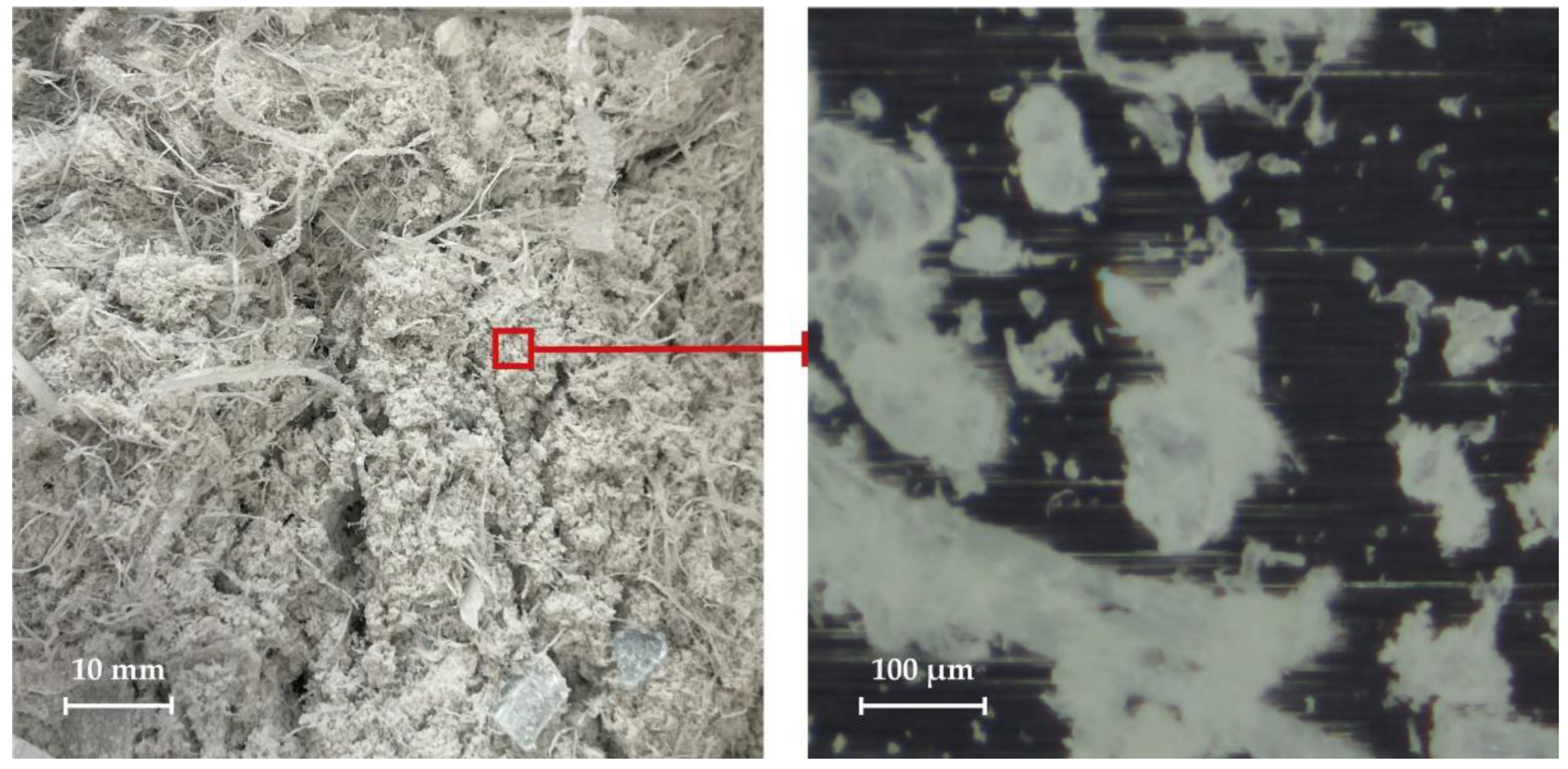
The appearance and shape of various polyethylene terephthalate dust particles are shown in
Figure 6 and
Figure 7.
According to the results of laser diffraction, the size of polyethylene terephthalate dust particles ranged from 1.9 to 1096.5 μm. The share of the main fraction with sizes from 239.9 to 316.2 μm was 11.6 vol.%. The particle size distribution is unimodal.
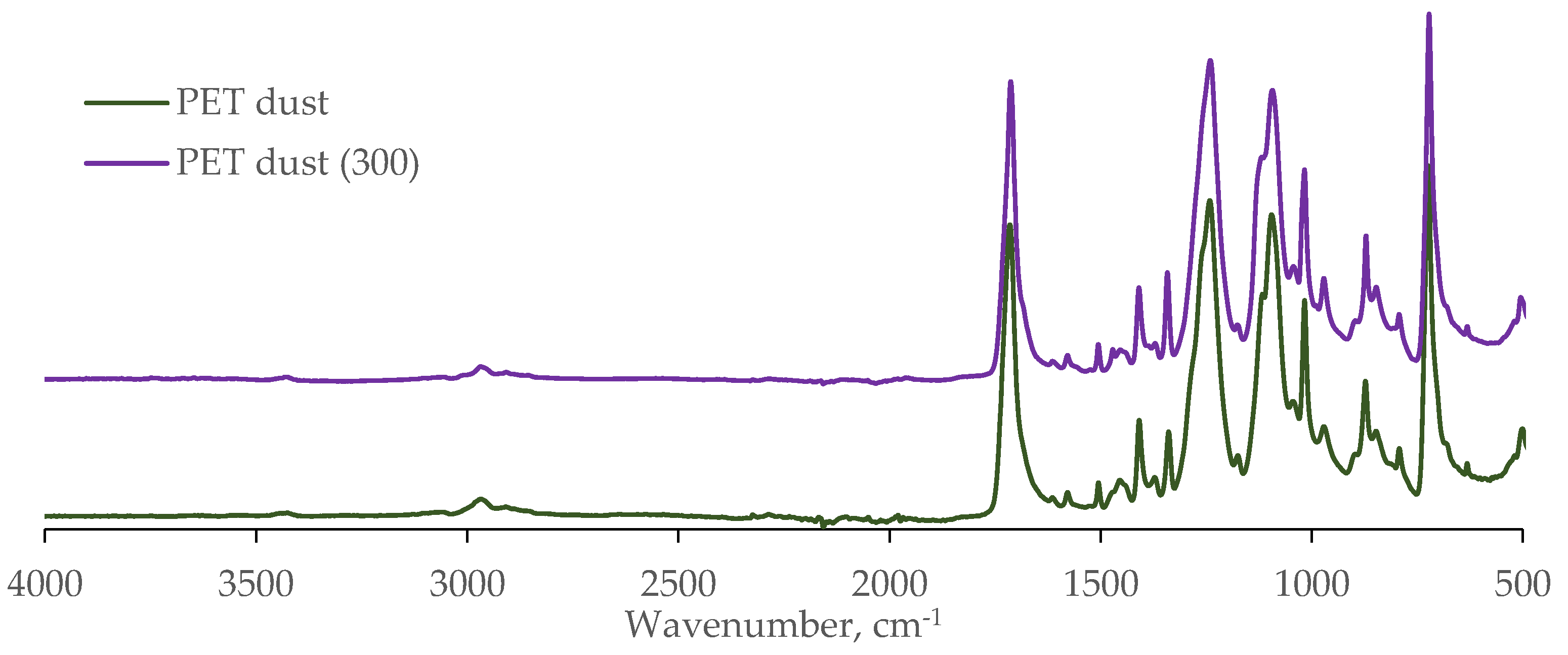
The FTIR spectrum also corresponds to literature data for partially crystalline polyethylene terephthalate (PET dust,
Figure 8).
3.1.4. Prepolymer Waste
Another form of pre-consumer waste is prepolymer waste. The formation of this waste occurs at the polycondensation stage during unloading of the polymer, sampling, distillation and condensation of ethylene glycol. Often this type of waste contains a large amount of free ethylene glycol, which wets the PET surface.
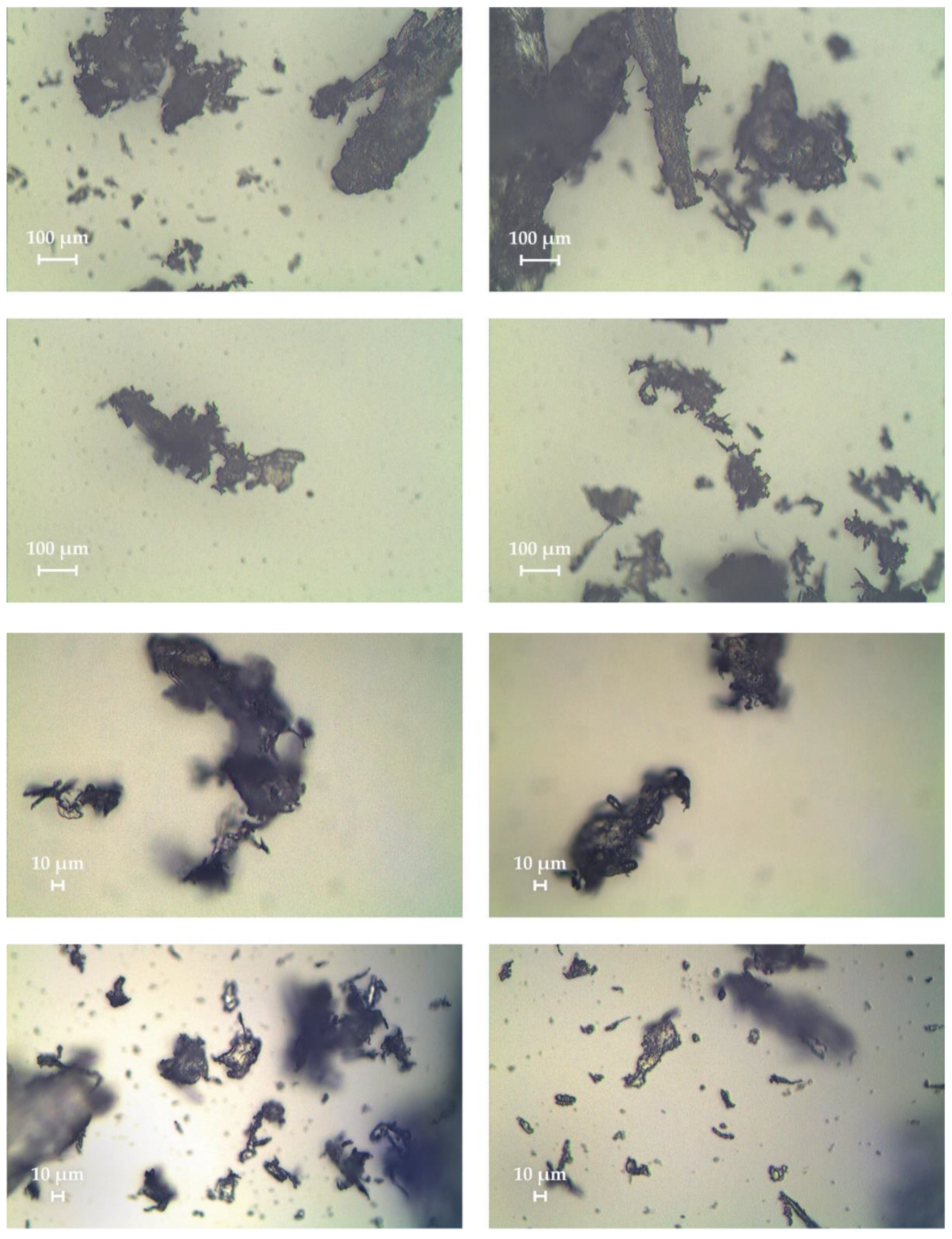
The appearance of various prepolymer fragments is shown in
Figure 9 and
Figure 10. It can be noted that this waste is the most diverse. There are both opaque fragments of low molecular weight polymer and transparent drops, fibers and pieces. Also, different fragments have different degrees of degradation, since their color varies from transparent white to dark brown.
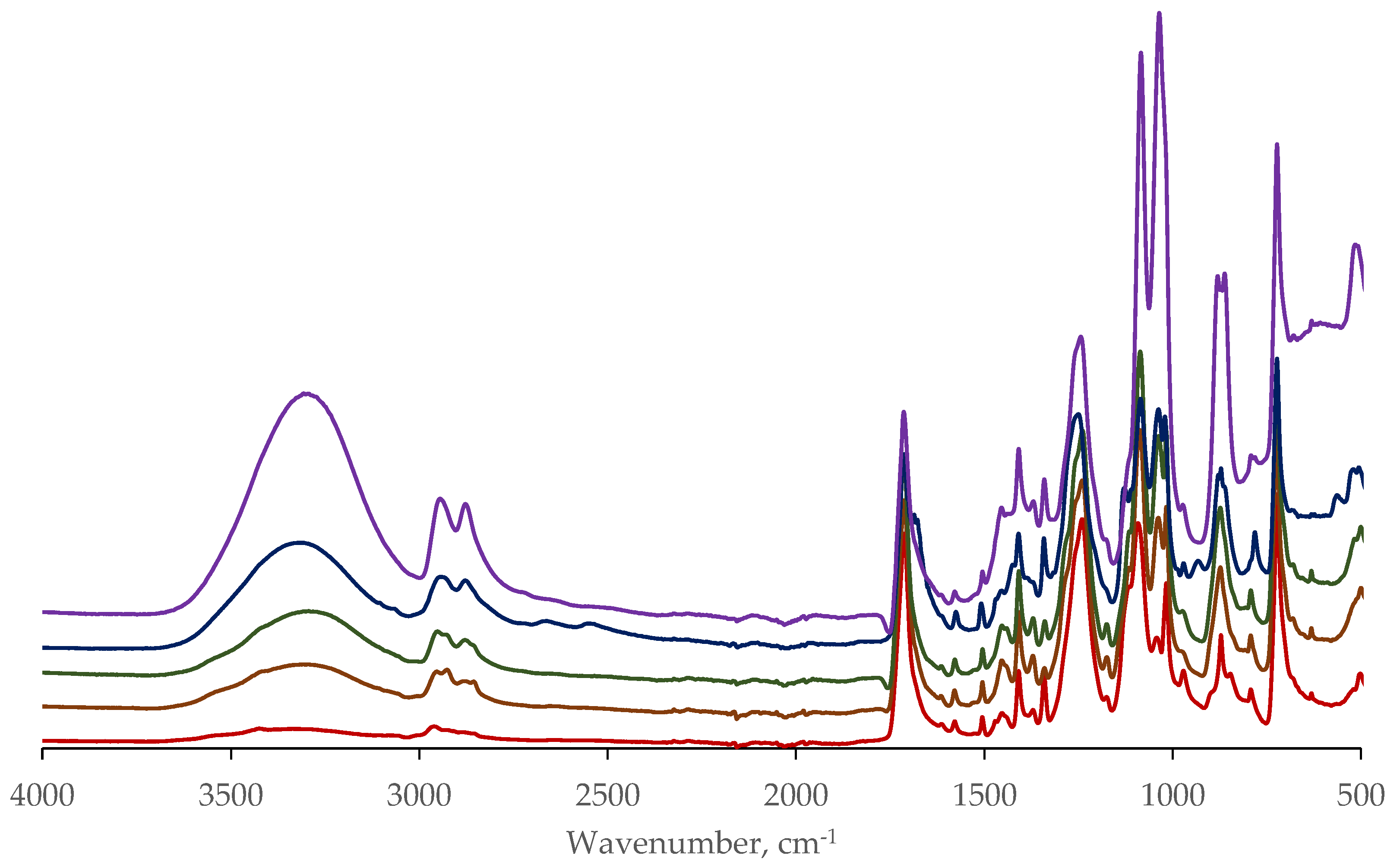
Figure 11 shows the FTIR spectra of various waste prepolymer fragments. The main difference lies in the region of the 3350 cm
-1 band, corresponding to the terminal hydroxyl groups. The difference may be due to both the different molecular weight of the prepolymer fragments and the presence of residual ethylene glycol.
3.2. Molecular Weight of PET Waste Forms
For each form of waste, 5 samples of polyethylene terephthalate were selected and examined by viscometry. Samples taken from polyester tire cord did not include crumb rubber. The intrinsic viscosity intervals obtained as a result of measurements are given in
Table 2. To calculate the viscosity-average molecular weight
, both sets of constants given in paragraph 2.2.2 were used. The ranges of viscosity-average molecular weights are also given in
Table 2.
3.3. Thermal Characteristics and Crystallinity of PET Waste Forms
The data obtained by processing DSC curves for samples of various polyethylene terephthalate waste forms are systematized in
Table 3.
According to the literature, when measured by DSC, depending on the size and shape of the particles, the glass transition temperature of polyethylene terephthalate lies in the range of 70-85 °C, and the melting point is 245-255 °C. The degree of crystallinity of polyethylene terephthalate can reach up to 45-50% [
22]. The temperature characteristics of waste low-grade PET flakes, polyester tire cord fibers, PET dust and prepolymer are consistent with those expected for polyethylene terephthalate.
The DSC curve for the dust fraction also has a shoulder at 267.2 °C at the peak of melting. When measuring the same sample again after cooling, the shoulder is absent. Since the FTIR spectra of PET dust before (PET dust,
Figure 8) and after (PET dust (300),
Figure 8) heating to 300 °C coincide, we can conclude that this shoulder corresponds to the removal of cyclic polyethylene terephthalate oligomers, corresponding in chemical composition to linear PET.
Thus, all forms of polyethylene terephthalate waste considered, including dust and prepolymer waste, are PET polymers and not oligomers.
4. Discussion
The obtained intrinsic viscosity values for PET flakes fall within the range for bottle-grade PET (0.7 to 0.85 dl g
-1) [
23]. Low quality flakes can be chemically recycled by any methods known for polyethylene terephthalate. In this case, it is important to take into account the color of the flakes and use them either to obtain products whose color is not important, or to carry out additional purification in the case of obtaining monomers. It is important to note that these flakes can also be recycled by physical methods to produce mixed-color products.
Unlike flakes, waste polyester tire cord cannot be physically recycled. Currently, they are reused as heat or sound insulating materials, additives and fillers, or subjected to pyrolysis [
24]. Chemical processing of tire cord waste is possible under conditions in which devulcanization of crumb rubber also occurs [
25]. The devulcanization process occurs due to the rupture of C-S and S-S bonds, which have lower energy than C-C and C=C bonds [
26]. The breaking of bonds occurs under the influence of temperature, mechanical stress, chemical agents, microwave irradiation, ultrasound, and enzymes [
27]. By chemical processing of polyester tire cord, both monomers – in particular, bis(2-hydroxyethyl) terephthalate [
20] – and oligomers – unsaturated polyester resins [
28] – were obtained. It is shown that the presence of crumb rubber does not affect the transesterification reaction, and due to the side effects of devulcanization, it is possible to achieve complete dissolution of the crumb in the reaction mixture.
Polyethylene terephthalate dust has the widest range of viscosities and molecular weights, as shown in
Table 2. It also includes cyclic oligomers, which is confirmed by literature data [
21], as well as by DSC and FTIR methods. However, due to the purity and large specific surface area of the particles, PET dust can apparently be successfully recycled by most known chemical methods. It is noteworthy that the small and branched surface of the particles can allow the process to be carried out at high speed.
The prepolymer has the lowest molecular weight among waste forms and also contains ethylene glycol. The most promising methods of chemical processing for it are those based on reducing the molecular weight and implying the presence of ethylene glycol in the reaction mixture or its removal. Thus, the most promising are hydrolysis with the production of terephthalic acid or its salts and distillation of ethylene glycol, alcoholysis with the production of dialkyl terephthalates and distillation of ethylene glycol, or glycolysis with the production of bis(2-hydroxyethyl) terephthalate.
Author Contributions
Conceptualization, K.K.; methodology, K.K. and R.T.; software, G.A. and D.I.; validation, R.T., G.A. and D.I.; investigation, K.K..; writing—original draft preparation, K.K.; writing—review and editing, A.G.; visualization, K.K. and A.M.; supervision, R.T. and A.G.; project administration, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Production Capacity of Polyethylene Terephthalate Worldwide from 2014 to 2024. Available online: https://www.statista.com (accessed on 15 March 2024).
- Liu, T.; Gu, X.; Wang, J.; Feng, L. Modeling and Analysis of New Reactor Concepts for Poly(Ethylene Terephthalate) Esterification Process. Chemical Engineering and Processing - Process Intensification 2019, 135, 217–226. [Google Scholar] [CrossRef]
- Ha, K.; Rhee, H. Optimal Reaction Conditions for the Minimization of Energy Consumption and By-product Formation in a Poly(Ethylene Terephthalate) Process. J Appl Polym Sci 2002, 86, 993–1008. [Google Scholar] [CrossRef]
- Kim, I.S.; Woo, B.G.; Choi, K.Y.; Kiang, C. Two-phase Model for Continuous Final-stage Melt Polycondensation of Poly(Ethylene Terephthalate). III. Modeling of Multiple Reactors with Multiple Reaction Zones. J Appl Polym Sci 2003, 90, 1088–1095. [Google Scholar] [CrossRef]
- Chicheva, D.S.; Krasnykh, E.L.; Shakun, V.A. Kinetic Regularities of Neopentyl Glycol Esterification with Acetic and 2-Ethylhexanoic Acids. Fine Chemical Technologies 2024, 19, 28–38. [Google Scholar] [CrossRef]
- Ma, Y.; Agarwal, U.S.; Sikkema, D.J.; Lemstra, P.J. Solid-State Polymerization of PET: Influence of Nitrogen Sweep and High Vacuum. Polymer 2003, 44, 4085–4096. [Google Scholar] [CrossRef]
- Bhanderi, K.K.; Joshi, J.R.; Patel, J.V. Recycling of Polyethylene Terephthalate (PET Or PETE) Plastics—An Alternative to Obtain Value Added Products: A Review. Journal of the Indian Chemical Society 2023, 100, 100843. [Google Scholar] [CrossRef]
- Muringayil Joseph, T.; Azat, S.; Ahmadi, Z.; Moini Jazani, O.; Esmaeili, A.; Kianfar, E.; Haponiuk, J.; Thomas, S. Polyethylene Terephthalate (PET) Recycling: A Review. Case Studies in Chemical and Environmental Engineering 2024, 9, 100673. [Google Scholar] [CrossRef]
- Kirshanov, K.; Toms, R.; Aliev, G.; Naumova, A.; Melnikov, P.; Gervald, A. Recent Developments and Perspectives of Recycled Poly(Ethylene Terephthalate)-Based Membranes: A Review. Membranes 2022, 12, 1105. [Google Scholar] [CrossRef]
- Babaei, M.; Jalilian, M.; Shahbaz, K. Chemical Recycling of Polyethylene Terephthalate: A Mini-Review. J Environ Chem Eng 2024, 12, 112507. [Google Scholar] [CrossRef]
- Bohre, A.; Jadhao, P.R.; Tripathi, K.; Pant, K.K.; Likozar, B.; Saha, B. Chemical Recycling Processes of Waste Polyethylene Terephthalate Using Solid Catalysts. ChemSusChem 2023, 16. [Google Scholar] [CrossRef]
- Kirshanov, K.; Toms, R.; Melnikov, P.; Gervald, A. Unsaturated Polyester Resin Nanocomposites Based on Post-Consumer Polyethylene Terephthalate. Polymers 2022, 14, 1602. [Google Scholar] [CrossRef] [PubMed]
- Kirshanov, K.A.; Gervald, A.Yu.; Toms, R.V.; Lobanov, A.N. Obtaining Phthalate Substituted Post-Consumer Polyethylene Terephthalate and Its Isothermal Crystallization. Fine Chemical Technologies 2022, 17, 164–171. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Worrell, E.; Patel, M.K. Open-Loop Recycling: A LCA Case Study of PET Bottle-to-Fibre Recycling. Resour Conserv Recycl 2010, 55, 34–52. [Google Scholar] [CrossRef]
- Farah, S.; Kunduru, K.R.; Basu, A.; Domb, A.J. Molecular Weight Determination of Polyethylene Terephthalate. In Poly(Ethylene Terephthalate) Based Blends, Composites and Nanocomposites; Elsevier, 2015; pp. 143–165. [Google Scholar]
- Aliev, G.; Toms, R.; Melnikov, P.; Gervald, A.; Glushchenko, L.; Sedush, N.; Chvalun, S. Synthesis of L-Lactide from Lactic Acid and Production of PLA Pellets: Full-Cycle Laboratory-Scale Technology. Polymers 2024, 16, 624. [Google Scholar] [CrossRef] [PubMed]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The Role of Crystalline, Mobile Amorphous and Rigid Amorphous Fractions in the Performance of Recycled Poly (Ethylene Terephthalate) (PET). Polym Degrad Stab 2012, 97, 98–107. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Toms, R.V.; Balashov, M.S.; Golubkov, S.S.; Melnikov, P.V.; Gervald, A.Yu. Modeling of Poly(Ethylene Terephthalate) Homogeneous Glycolysis Kinetics. Polymers 2023, 15, 3146. [Google Scholar] [CrossRef] [PubMed]
- Kirshanov, K.; Toms, R.; Melnikov, P.; Gervald, A. Investigation of Polyester Tire Cord Glycolysis Accompanied by Rubber Crumb Devulcanization. Polymers 2022, 14, 684. [Google Scholar] [CrossRef] [PubMed]
- Geller, Y.A.; Antanenkova, I.S.; Aparushkina, M.A. Analysis of the Influence of Dust Formation on Technological Processes in the Production of PET. J Phys Conf Ser 2021, 2057, 012136. [Google Scholar] [CrossRef]
- Rieckmann, T.; Volker, S. Poly(ethylene terephthalate) polymerization—Mechanism, catalysis, kinetics, mass transfer and reactor design. In Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Scheirs, J., Long, T.E., Eds.; John Wiley & Sons: London, UK, 2003; pp. 31–116. [Google Scholar] [CrossRef]
- Bartolome, L.; Imran, M.; Cho, B.G.; Al-Masry, W.A.; Kim, D.H. Recent Developments in the Chemical Recycling of PET. In Material Recycling - Trends and Perspectives; Achilias, D., Ed.; InTech: Rijeka, Croatia, 2012; pp. 65–82. [Google Scholar]
- Kirshanov, K.; Toms, R.; Gervald, A. Prospects of Polyester Tire Cord Waste Utilization. Kauchuk i Rezina 2022, 81, 148–154. [Google Scholar]
- Kirshanov, K.; Aliev, G.; Izmalkov, D.; Borisenko, D.; Toms, R.; Gervald, A. Polyester Tire Cord Waste Chemical Recycling. Part 1. Review. Kauchuk i Rezina 2023, 82, 28–37. [Google Scholar]
- Xiao, Z.; Pramanik, A.; Basak, A.; et al. Material recovery and recycling of waste tyres—A review. Clean Mat 2022, 5, 100115. [Google Scholar] [CrossRef]
- Bockstal, L.; Berchem, T.; Schmetz, Q.; Richel, A. Devulcanisation and reclaiming of tires and rubber by physical and chemical processes: A review. J Clean Prod 2019, 236, 117574. [Google Scholar] [CrossRef]
- Kirshanov, K.; Aliev, G.; Balashov, M. Obtaining Unsaturated Resins by Chemical Recycling of Waste Polyester Tire Cord. Part 1. Kauchuk i Rezina 2023, 82, 122–127. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).