1. Introduction
Maintaining a safe and stable blood sugar level during the night is crucial for people with type 1 diabetes (T1D). Determining the proper predinner rapid insulin and basal insulin to adequately control both postprandial and nocturnal glucose can be a daily challenge with a high level of uncertainty. Many factors, which are difficult to measure and predict, can affect the nocturnal glycemic profile, one of them being exercise during the day [
1]. The safety and quality of life of individuals with T1D are clearly impacted by these factors [
2].
Insulin regimens requiring multiple daily injections (MDI) impose a considerable burden on people with T1D [
3]. Previous studies have shown that the timing of prandial insulin injection can affect postprandial glucose levels [
4,
5]. However, these studies were conducted in laboratory settings or relied on self-reported insulin injection times and doses. Notably, a large percentage of people with T1D do not follow the recommended prandial insulin injection timing, which can result in different glucose dynamics and increase both postprandial excursions and nocturnal hypoglycemia risk [
6].
Manufacturers recommend injecting human regular insulin 30-45 minutes before starting a meal, 15 minutes in the case of rapid insulin analogs - RI-, and at the start of a meal or within 20 minutes for second-generation ("ultrarapid") insulins - URI - [
7,
8,
9]. There is limited scientific evidence on the nocturnal glucose profile depending on the timing and type of pre-dinner rapid insulin injections. However, connected insulin pens and caps can now automatically track continuous glucose monitoring (CGM) data, as well as the dose and exact time of insulin injections [
10]. This is a promising development, as it can help people with T1D to manage their blood sugar levels more effectively and ultimately reduce their risk of complications [
11].
Insulin therapy adherence can be influenced by socioeconomic factors, treatment complexity, and fear of hypoglycemia [
12,
13]. Errors in insulin administration, such as bolus omissions and delays, can prevent optimal glycemic control [
14]. This can negatively impact the quality of life of people with diabetes and increase the risk of morbidity, mortality, and hospitalization [
15].
Although it is recommended to administer the prandial bolus injection at least 15 minutes before mealtime [
16], it may not always be feasible in real life. Fortunately, URI insulins are available which can improve postprandial dynamics even when injected after the meal has started. Our previous study, focused on postprandial glucose dynamics, confirmed a reduction in both immediate hyper- and late hypoglycemia by using URI in a real-life setting [
6].
Insulclock® is a small cap that can be attached to disposable insulin pens and is used to record the date, time, duration, and dose of insulin injections [
17]. This information is then integrated with other relevant data, such as glucose levels from CGM devices or glucometers, food intake, or physical activity, using the
Insulclock® app. Patients can review the data collected and share it with their healthcare providers to monitor trends and patterns. A multicenter randomized controlled trial has demonstrated the favourable effects of the Insulclock® system on glycemic control and variability, adherence to insulin treatment, and quality of life in people with T1D and insufficient control [
11].
The current study aimed to analyze the nocturnal glucose profile in people with T1D using multiple daily insulin injections (MDI) according to the timing of dinner rapid insulin and the type of rapid and basal insulin.
2. Materials and Methods
2.1. Design
A retrospective study analyzing anonymous real-world data derived from the electronic Insulclock® database for the six participating centers throughout Spain (Hospital General de Segovia, Segovia; Cruces University Hospital, Barakaldo; Hospital Arquitecto Marcide, Ferrol [A Coruña]; Hospital Universitario Central de Asturias, Oviedo; Hospital Universitario 12 de Octubre, Madrid; and Hospital Universitario Infanta Sofía, San Sebastián de los Reyes). At the initiation of Insulclock® use, written informed consent was obtained from each participant allowing Insulcloud S.L. to collect his/her data and use the anonymized and tabulated data for scientific purposes. The study was conducted following the ethical principles of the Declaration of Helsinki. Before any study-related activities, the Research Ethics Committee of Hospital General de Segovia, Segovia, Spain, approved the study.
2.2. Population and Database
Starting at dinner, ten hours of paired CGM and insulin injections data collected from consecutive T1D participants starting to use the connected insulin pen cap Insulclock® [
11,
17] from January to June 2022 were analyzed. Only glycemic excursions starting with a glucose level between 70 mg/dL (3.9 mmol/L) and 250 mg/dL (13.9 mmol/L) and with 10-hour data after a meal initiation were included. The Glucose Rate Increase Detector (GRID) algorithm was used to automatically detect meal glucose excursions through the rate of change (ROC) of glucose from CGM data [
18]. All patients were previously using CGM (
Freestyle Libre2®) as part of their usual diabetes care.
2.3. Outcomes
The nocturnal glucose profiles were evaluated according to time periods around postprandial excursion (PE) start. Three time periods were defined: injections from 45 to 15 minutes before (-45/-15), 15 minutes before up to PE start (-15/0), and from 0 to 45 minutes after the PE start (0/+45). Nocturnal high glucose excursions were described by the glucose area under curve (AUC) over 180 mg/dL (10 mmol/L). Hypoglycemia was evaluated with two variables: the rate of overnight periods (%) with hypoglycemic events defined as periods of glucose levels under 70 mg/dL (3.9 mmol/L) lasting more than 15 minutes, and the time below the range of glucose 70 mg/dL (3.9 mmol/L) (TBR70). The time in range 70–180 mg/dL (3.9–10.0 mmol/L) (TIR) in the nighttime period was also evaluated.
The type of insulin used was not an inclusion criterion. Nocturnal glycemic profiles were evaluated according to the insulin type used.
The administration of a second injection (correction doses) 1-5 hours after the first rapid insulin dose at dinner was also evaluated.
2.4. Statistical Analyses
Statistical analyses were performed using the SPSS software, version 25.0 (Chicago, IL). The level of statistical significance was set at a bilateral p < 0.05. Continuous variables were described by the mean and standard deviation (SD), when normally distributed, or by the median, interquartile range (IQR), when not normally distributed. Categorical variables were described by the number of valid cases and percentages. Comparisons of proportions and/or frequency distributions were performed with the Chi-square test, Mann-Whitney, Kruskal Wallis or the ANOVA test, as appropriate, with the post-hoc Bonferroni correction.
Logistic and linear regression models respectively were used to assess predictors of time below the range of glucose 70 mg/dL (3.9 mmol/L) (TBR70) and time in range 70–180 mg/dL (3.9–10.0 mmol/L) (TIR), depending on timing of insulin injection, and URI or BI type use. Simple regression models were first performed, and those variables reaching statistical significance were included in the multivariable regression models (forward selection). In the multivariable model, a p-value <0.05 was considered significant. Those variables with a variance inflation factor >5 were removed from the models.
3. Results
3.1. Population
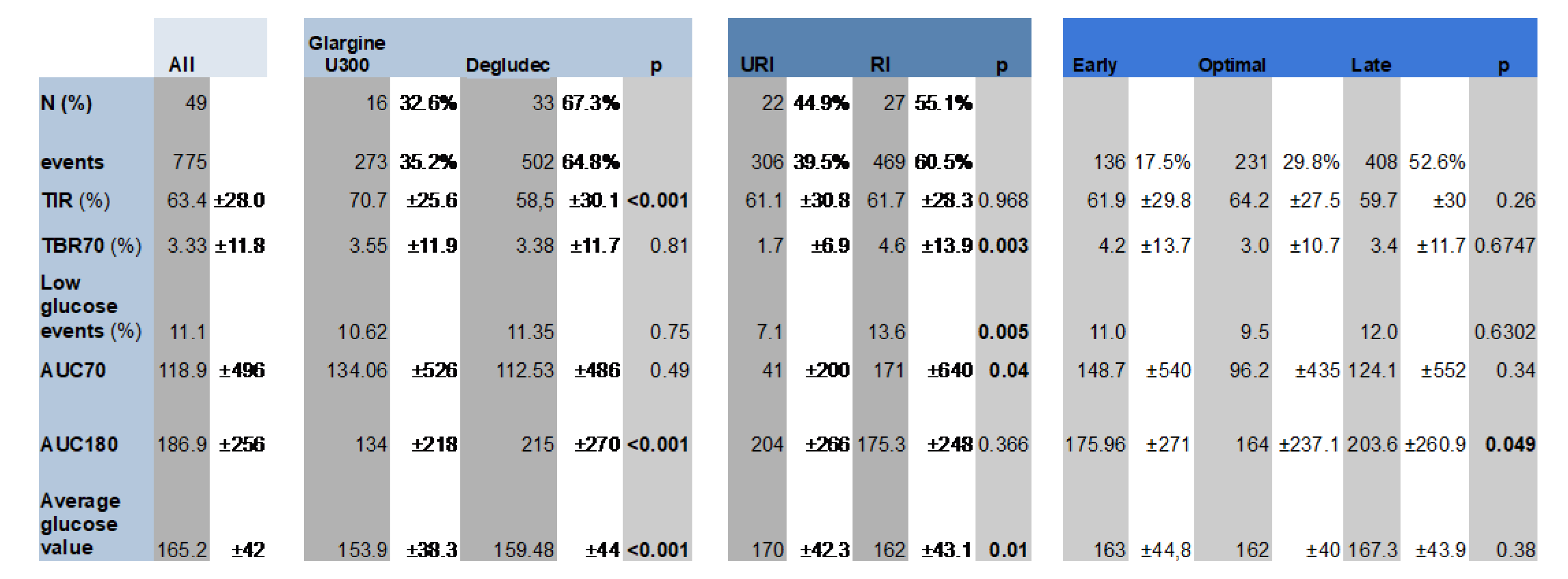
A total of 775-night periods were included, from 49 participants, 45.51 ±13.2 years old, 28 women (57.1%).
Table 1 summarizes the clinical characteristics and baseline glucometrics of the included population, both overall and according to the type of rapid and basal insulin used.
3.2. Nocturnal Glucose Dynamics Depending on the Rapid Insulin Injection Timing
The distribution of injection times rate was: -45/-15 injections in 17.5% (n=136), -15/0 injections in 29.8% (n= 231), and 0/+45 in 52.6% (n= 408).
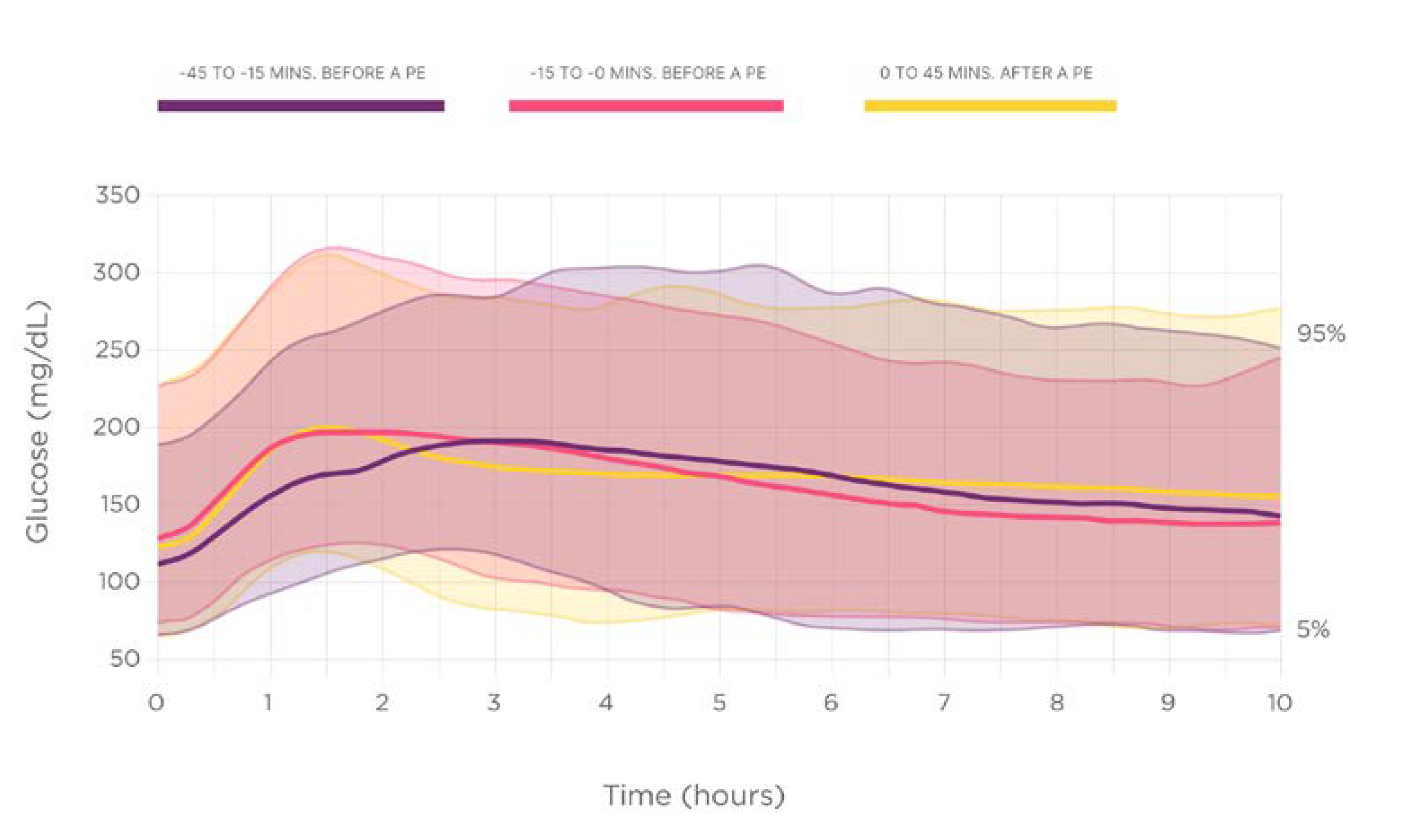
The
Figure 1 displays the nocturnal glucose dynamics depending on the prandial insulin injection time.
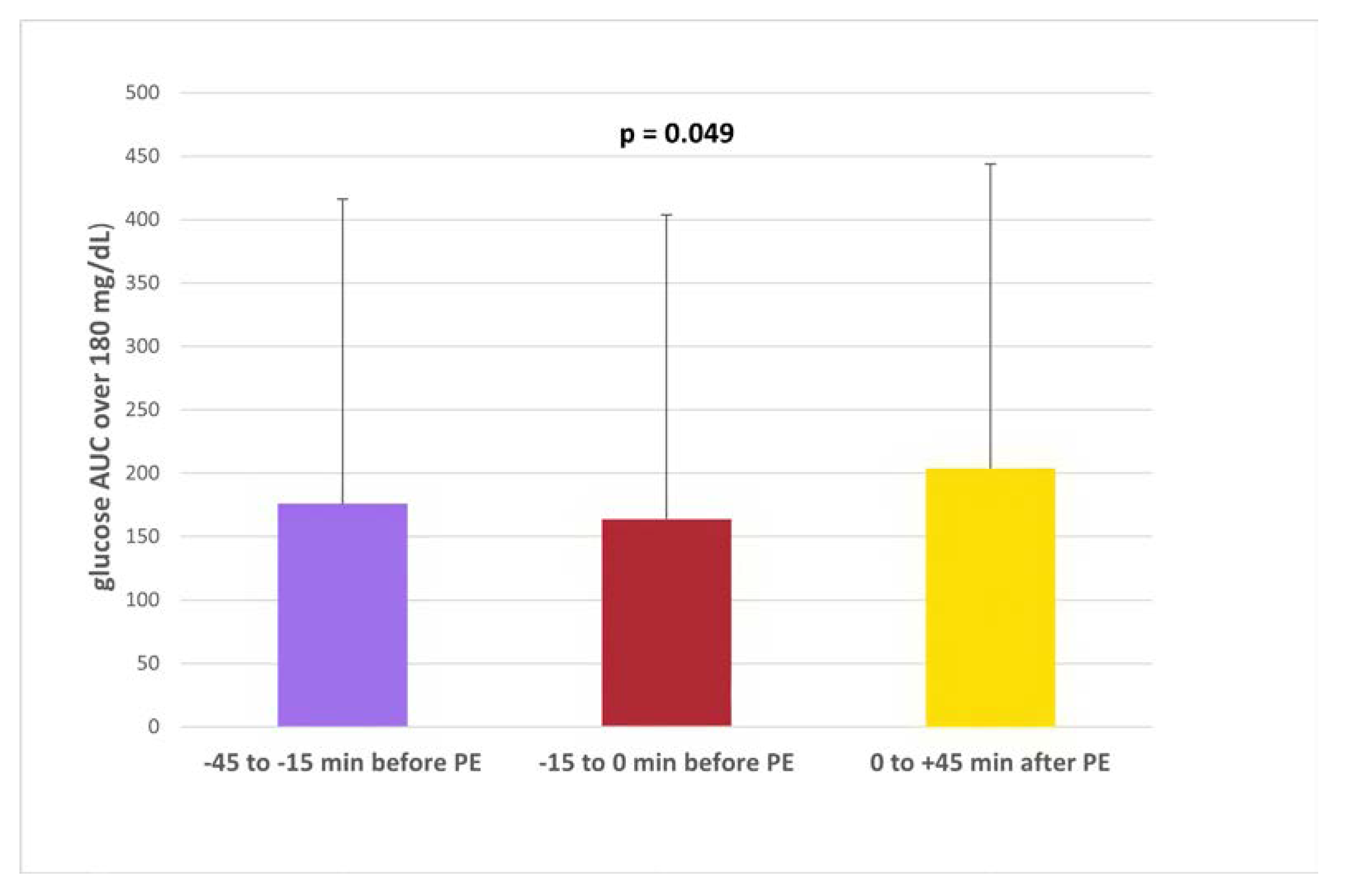
The nocturnal high glucose excursions (glucose AUC over 180 mg/dL [10 mmol/L]) results showed statistically significant differences between groups, being higher in delayed rapid insulin injections 0/+45 min, (number, %, mg/dL x h): -45-15 min (n=136, 17.5%, 175.96 ±271.0); -15-0 min (n= 231, 29.8%;,164.0 ±237.1); 0+45 min (n= 408, 52.6%, 203.6 ±260.9), overall p = 0.049 (
Figure 2).
The rate of nocturnal hypoglycemic events did not differ according to the timing of rapid insulin injection, mean +SD: -45-15 min: 11.0 +0.3; -15-0 min: 9.5 +0.3; 0+45 min: 12.0 +0.3, (p = 0.630).
The time below the range of glucose 70 mg/dL (3.9 mmol/L) (TBR70) showed similar results (mean +SD): -45-15 min: 4.2 +13.7%; -15-0 min: 3.0 +10.7%; 0+45 min: 3.4 +11.7%, (p = 0.674).
3.3. Analysis According to Ultrarapid Insulin Use
Nocturnal glucose AUC over 180 mg/dL and TIR 70-180 did not reach statistical differences between URI vs RI (mean +SD): 175.3 +248 vs 204 +266 mg/dL x h (p = 0.366) and 61.7 +28.3 vs 61.1 +30.8 mg/dL x h (p = 0.968).
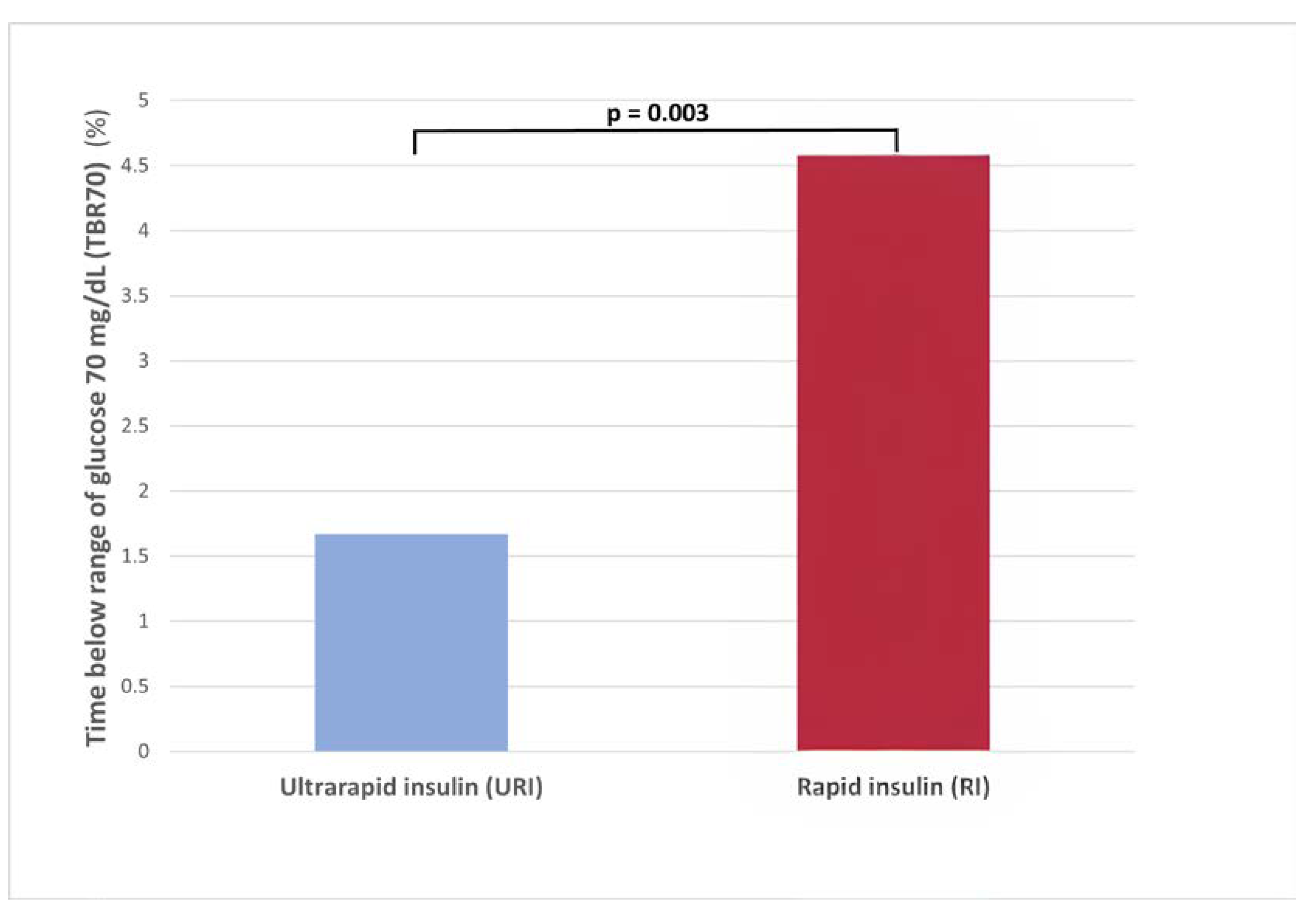
The use of URI vs RI was associated with a lower TBR70 (1.7
+6.9 vs 4.6
+13.9%; p = 0.003) (
Figure 3) and less events of hypoglycemia (7.1 vs 13.6; p = 0.005) (
Supplementary Figure S1).
3.4. Analysis According to the Type of Second-Generation Basal Insulin
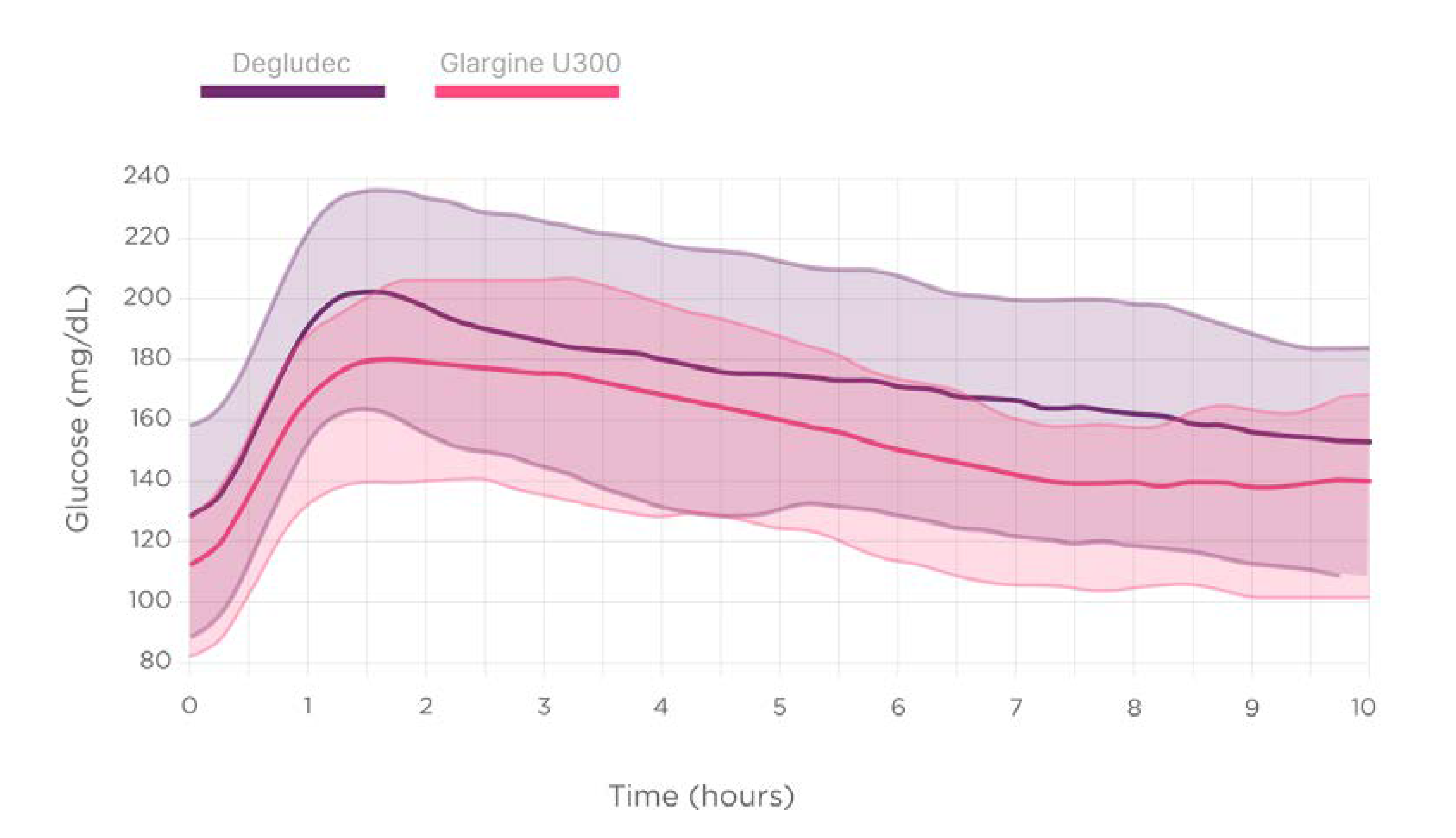
Figure 4 describes the post-dinner and nocturnal glucose dynamics depending on the second-generation BI type.
Statistically significant differences were not detected regarding the second-generation BI use on un-adjusted glucometrics describing nocturnal high glucose excursions (glucose AUC) over 180 mg/dL (10 mmol/L), nocturnal hypoglycemic events, the time below the range of glucose 70 mg/dL (3.9 mmol/L) (TBR70), nor time in range 70–180 mg/dL (3.9–10.0 mmol/L) (TIR).
However, the multivariable logistic regression model also including timing of injection, BI type, and pre-dinner glucose, identified the use of Gla-300 as independently associated with a higher TIR 70-180 during the night (Adjusted R-squared: 0.22, p <0.001).
3.5. Analysis According to Second Insulin (Correction Dose) Use
A second (correction) injection was detected in 18.6% of the overnight periods analized (n= 144). It was associated with higher hypoglycemia events (18.1 vs 9.5%; p 0.003), TBR70 (5.5 +14.2 vs 3.0 +11.1%; p 0.003), glucose AUC over 180 mg/dL (226.1 +257.8 vs 178.0 +255.3 mg/dL x h; p 0.001), and lower TIR (56.0 +27.4 vs 62.7 +29.6 mg/dL x h; p 0.004).
3.6. Multivariable Analysis
The multivariable logistic regression models (Supplementary
Table 1), including timing of injection, use of a second injection, rapid and basal insulin type, confirmed:
The use of URI was independently associated with a reduced risk (-52%) of events of glucose < 70 mg/dL (3.9 mmol/L) (Adjusted R-squared: 0.017; p 0.003), and with a lower (-64%) TBR70 (Adjusted R-squared: 0.016; p 0.003).
-
Not adding a second (correction) injection after the dinner was independently associated with:
- ∘
a reduction in overnight hypoglycemia: -47% events of glucose < 70 mg/dL); Adjusted R-squared: 0.017; p 0.003, and -61% TBR70, (Adjusted R-squared: 0.016; p 0.003).
- ∘
a reduction in overnight hyperglycemia: -21% glucose AUC over 180 mg/dL (Adjusted R-squared: 0.017; p <0.001).
- ∘
more time in the recommended glucose range 70-180 mg/dL (TIR): +6,7% (62.7 +29.6 mg/dL vs 56.0 +27.4); (Adjusted R-squared: 0.048; p <0.001).
Additionally, using glargine U300 instead of degludec was associated with higher TIR (70.7 vs 58.5%, +12,2%) after adjusting by baseline glucose before dinner (Adjusted R-squared: 0.22, p < 0.001).
4. Discussion
People with T1D need to maintain a safe and flat nocturnal blood glucose level avoiding both hyperglycemia and hypoglycemia. Prandial pre-dinner rapid and basal insulins can have a direct impact on the blood glucose levels during the nocturnal period. In this study, we have found that taking rapid insulin at the right time before dinner and using an `ultrarapid´ insulin analog is associated to improved glucose control during the night. Additionally, regarding second-generation basal insulin, using Gla-300 instead of degludec was associated with achieving more time in the recommended glucose levels target during the night.
Several studies have been conducted to determine the effect of prandial insulin timing on postprandial glucose dynamics [
4,
16]. However, less scientific evidence is available evaluating the relationship between recommended timing for pre-dinner prandial insulin injection and the nocturnal glucose profile beyond the postprandial period. A study comparing preprandial vs. postprandial insulin glulisine in patients initiating basal-bolus regimen for type 2 diabetes showed that nocturnal hypoglycaemia rates were higher in the postprandial administration group [
19]. Another randomized, open-labeled, cross-over trial found no statistically significant differences on nighttime hypoglycemic episodes between insulin aspart administered before or after meals in children and adolescents with T1D [
20]. The PRONTO-T1D study in patients with type 1 diabetes showed that the rate of hypoglycaemia was significantly lower for mealtime ultrarapid lispro (URLi) compared to post-meal URLi in the late postprandial period (>4 hours after the meal) [
21]. Data presented in this report showed higher nocturnal glucose (AUC over 180 mg/dL) in delayed pre-dinner rapid insulin injections.
Second-generation rapid (`ultrarapid´) insulins can improve postprandial glucose dynamics [
22,
23]. Two different `ultrarapid´ rapid insulin analogs, Fast-acting insulin aspart (Fiasp©) and insulin Ultra-rapid lispro (URLi) have been marketed. There is limited scientific evidence on the impact of pre-dinner rapid insulin injection type on the whole nocturnal glucose profile, particularly in the T1D population under MDI regime. In the PRONTO-T1D CGM substudy mealtime URLi decreased night-time TBR70 mg/dl compared with mealtime Lispro [
24]. However, the same study pointed out to increasing glucose levels from evening to early morning in the group with mealtime URLi [
24]. A meta-analysis of randomized controlled trials comparing faster-acting insulin aspart (Fiasp) to insulin aspart in people with diabetes mellitus showed that nocturnal hypoglycaemic episodes were not different [
25].
Similarly, second-generation BI analogs, with longer, flatter and less variable profiles, are currently available for use in T1D population [
26]. The studies comparing insulin degludec and insulin glargine 300 U/mL have shown conflicting results on their stability, variability and clinical endpoints in the T1D population [
27,
28]. We recently published a retrospective study showing improved nocturnal CGM glucometrics with glargine 300 in comparison with IDeg-100 in patients with T1D in a real-world setting [
29]. The present analysis supports these results.
According to a research study, many individuals with T1D add corrective insulin at least once a week, with 57% of adults and 65% of children reporting the need for it [
3]. In this current study, 18.6% of the participants took a corrective insulin dose after dinner. However, avoiding this action could significantly reduce both overnight hypoglycemia and hyperglycemia, leading to better safety, glycemic control, and overall quality of life.
Results regarding insulin injection timing and type impact on glycemic control should be confirmed and quantified in real-life settings combining CGM data and automatically tracked insulin dose and time information. Connected insulin pens and caps provide an opportunity to assemble this information and provide a more accurate picture of nocturnal glucose levels [
10]. The real-world nature and the methodology (CGM and connected insulin pen data) used in this study are its main strengths. The limitations include that meal content is not analyzed and could influence the results. Only excursions starting with a glucose level between 70 and 250 mg/dL were included. Additionally, we must understand that we are detecting glucose excursions by CGM sensors in interstitial fluid not the meal start itself. There is a known lag time between the glucose detected in interstitial fluid and glucose in blood, particularly in rapid changing periods (33). Moreover, a first physiological delay exists from food ingestion to blood glucose appearance. Some previous studies described both lags to be about ten minutes each, on average (34). Thus, while real insulin bolus timing is correctly appraised, 10 to 20 minutes should be added to estimated meal times . The absence of an analysis by insulin dose could be taken as a limitation. However, the study research work hypothesis starts by assuming that the dose selection is made depending on the carbohydrate counting and carbohydrate/insulin ratio and insulin sensitivity factor previously set for every subject, as per standard of care.
5. Conclusions
Timing of rapid insulin injection in relation with dinner initiation is related to nocturnal high glucose excursions. Avoiding second (correction) rapid insulin doses after dinner could significantly reduce both overnight hypoglycemia and hyperglycemia. The use of ultrarapid insulin can reduce the risk of nocturnal low glucose values. Second-generation 'ultrarapid' insulins should be recommended for people with T1D to facilitate improved glucose control. However, further research is needed to determine the optimal timing of insulin injection and the effect of different types of insulin on nocturnal glucose levels.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1: Supplementary Figure S1, Rate of events of hypoglycemia < 70 mg/dL; Supplementary Table S1. Multivariable regression models results.
Author Contributions
FGP designed and supervised the study, researched data, analyzed data and wrote the manuscript. XV, CA supervised the study, researched data, analyzed data and reviewed the manuscript. EFR, LC, PP and SA researched data and reviewed the manuscript. AV, JJPG, LRV, and RC analyzed data and reviewed the manuscript.
Funding
The study concept and design were developed, and the data collection, analysis and interpretation performed thanks to the unconditional efforts of all the investigators. No funding from the companies producing the mentioned products was received. The APC was funded by Insulcloud S.L.
Institutional Review Board Statement
The Research Ethics Committee of Hospital General de Segovia, Segovia, Spain, approved the study (REC code 22-0252, 15th September 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors acknowledge the editorial assistance of Nicole Quinn, Always English S.L.
Conflicts of Interest
Fernando Gómez-Peralta has taken part in advisory panels for Insulcloud S.L., Sanofi and Novo Nordisk; has received research support from Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals and Lilly; and has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Co. and Lilly. Cristina Abreu has received research support from Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals and Lilly and has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals, AstraZeneca Pharmaceuticals LP, and Bristol-Myers Squibb Co. Elsa Fernández-Rubio has acted as a speaker for Novo Nordisk, Lilly, and AstraZeneca Pharmaceuticals. Laura Cotovad has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals, AstraZeneca Pharmaceuticals LP, and Lilly. Pedro Pujante has received research support from Novo Nordisk, and has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals, AstraZeneca Pharmaceuticals LP, and Lilly. Sharona Azriel has acted as a speaker for Amgen, Abbott, Dexcom, Sanofi, Novo Nordisk, Novartis, Roche, Boehringer Ingelheim Pharmaceuticals, AstraZeneca Pharmaceuticals LP. and Lilly. Rosa Corcoy declares that she has received consultancy fees from Novo Nordisk and speakers’ fees or support for attending meetings or research from Novo Nordisk, Lilly, Sanofi, Roche, Medtronic and Novalab-Air liquide. The remaining authors have no conflict of interest to disclose.
References
- Campbell, M.D.; Walker, M.; Bracken, R.M.; Turner, D.; Stevenson, E.J.; Gonzalez, J.T.; Shaw, J.A.; West, D.J. Insulin Therapy and Dietary Adjustments to Normalize Glycemia and Prevent Nocturnal Hypoglycemia after Evening Exercise in Type 1 Diabetes: A Randomized Controlled Trial. BMJ Open Diabetes Research and Care 2015, 3, e000085. [Google Scholar] [CrossRef]
- Jennum, P.; Stender-Petersen, K.; Rabøl, R.; Jørgensen, N.R.; Chu, P.-L.; Madsbad, S. The Impact of Nocturnal Hypoglycemia on Sleep in Subjects With Type 2 Diabetes. Diabetes Care 2015, 38, 2151–2157. [Google Scholar] [CrossRef]
- Lane, W.; Lambert, E.; George, J.; Rathor, N.; Thalange, N. Exploring the Burden of Mealtime Insulin Dosing in Adults and Children With Type 1 Diabetes. Clin Diabetes 2021, 39, 347–357. [Google Scholar] [CrossRef]
- Mozzillo, E.; Franceschi, R.; Di Candia, F.; Ricci, A.; Leonardi, L.; Girardi, M.; Rosanio, F.M.; Marcovecchio, M.L. Optimal Prandial Timing of Insulin Bolus in Youths with Type 1 Diabetes: A Systematic Review. Journal of Personalized Medicine 2022, 12, 2058. [Google Scholar] [CrossRef]
- Slattery, D.; Amiel, S.A.; Choudhary, P. Optimal Prandial Timing of Bolus Insulin in Diabetes Management: A Review. Diabetic Medicine 2018, 35, 306–316. [Google Scholar] [CrossRef]
- Gómez-Peralta, F.; Valledor, X.; López-Picado, A.; Abreu, C.; Fernández-Rubio, E.; Cotovad, L.; Pujante, P.; García-Fernández, E.; Azriel, S.; Corcoy, R.; et al. Ultrarapid Insulin Use Can Reduce Postprandial Hyperglycemia and Late Hypoglycemia, Even in Delayed Insulin Injections: A Connected Insulin Cap-Based Real-World Study. Diabetes Technol Ther 2024, 26, 1–10. [Google Scholar] [CrossRef]
- Actrapid-Epar-Summary-Public_en.Pdf.
- EMA Humalog Available online:. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/humalog (accessed on 16 January 2023).
- EMA Fiasp Available online:. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/fiasp (accessed on 1 April 2023).
- Tejera-Pérez, C.; Chico, A.; Azriel-Mira, S.; Lardiés-Sánchez, B.; Gomez-Peralta, F. ; Área de Diabetes-SEEN Connected Insulin Pens and Caps: An Expert’s Recommendation from the Area of Diabetes of the Spanish Endocrinology and Nutrition Society (SEEN). Diabetes Ther 2023. [CrossRef]
- Gomez-Peralta, F.; Abreu, C.; Fernández-Rubio, E.; Cotovad, L.; Pujante, P.; Gaztambide, S.; Bellido, D.; Menéndez Torre, E.; Ruiz-Valdepeñas, S.; Bello, H.; et al. Efficacy of a Connected Insulin Pen Cap in People With Noncontrolled Type 1 Diabetes: A Multicenter Randomized Clinical Trial. Diabetes Care 2023, 46, 206–208. [Google Scholar] [CrossRef]
- Martyn-Nemeth, P.; Schwarz Farabi, S.; Mihailescu, D.; Nemeth, J.; Quinn, L. Fear of Hypoglycemia in Adults with Type 1 Diabetes: Impact of Therapeutic Advances and Strategies for Prevention - a Review. Journal of Diabetes and its Complications 2016, 30, 167–177. [Google Scholar] [CrossRef]
- Datye, K.A.; Boyle, C.T.; Simmons, J.; Moore, D.J.; Jaser, S.S.; Sheanon, N.; Kittelsrud, J.M.; Woerner, S.E.; Miller, K.M. Timing of Meal Insulin and Its Relation to Adherence to Therapy in Type 1 Diabetes. J Diabetes Sci Technol 2018, 12, 349–355. [Google Scholar] [CrossRef]
- Peyrot, M.; Rubin, R.R.; Kruger, D.F.; Travis, L.B. Correlates of Insulin Injection Omission. Diabetes Care 2010, 33, 240–245. [Google Scholar] [CrossRef]
- Robinson, S.; Newson, R.S.; Liao, B.; Kennedy-Martin, T.; Battelino, T. Missed and Mistimed Insulin Doses in People with Diabetes: A Systematic Literature Review. Diabetes Technol Ther 2021, 23, 844–856. [Google Scholar] [CrossRef]
- Cobry, E.; McFann, K.; Messer, L.; Gage, V.; VanderWel, B.; Horton, L.; Chase, H.P. Timing of Meal Insulin Boluses to Achieve Optimal Postprandial Glycemic Control in Patients with Type 1 Diabetes. Diabetes Technol Ther 2010, 12, 173–177. [Google Scholar] [CrossRef]
- Gomez-Peralta, F.; Abreu, C.; Gomez-Rodriguez, S.; Ruiz, L. Insulclock: A Novel Insulin Delivery Optimization and Tracking System. Diabetes Technology and Therapeutics 2019, 21, 209–214. [Google Scholar] [CrossRef]
- Harvey, R.A.; Dassau, E.; Zisser, H.; Seborg, D.E.; Doyle, F.J. Design of the Glucose Rate Increase Detector: A Meal Detection Module for the Health Monitoring System. Journal of Diabetes Science and Technology 2014, 8, 307–320. [Google Scholar] [CrossRef]
- Ratner, R.; Wynne, A.; Nakhle, S.; Brusco, O.; Vlajnic, A.; Rendell, M. Influence of Preprandial vs. Postprandial Insulin Glulisine on Weight and Glycaemic Control in Patients Initiating Basal-Bolus Regimen for Type 2 Diabetes: A Multicenter, Randomized, Parallel, Open-Label Study (NCT00135096). Diabetes Obes Metab 2011, 13, 1142–1148. [Google Scholar] [CrossRef]
- Danne, T.; Axel Schweitzer, M.; Keuthage, W.; Kipper, S.; Kretzschmar, Y.; Simon, J.; Wiedenmann, T.; Ziegler, R. Impact of Fast-Acting Insulin Aspart on Glycemic Control in Patients with Type 1 Diabetes Using Intermittent-Scanning Continuous Glucose Monitoring Within a Real-World Setting: The GoBolus Study. Diabetes Technol Ther 2021, 23, 203–212. [Google Scholar] [CrossRef]
- Klaff, L.; Cao, D.; Dellva, M.A.; Tobian, J.; Miura, J.; Dahl, D.; Lucas, J.; Bue-Valleskey, J. Ultra Rapid Lispro Improves Postprandial Glucose Control Compared with Lispro in Patients with Type 1 Diabetes: Results from the 26-Week PRONTO-T1D Study. Diabetes, Obesity and Metabolism 2020, 22, 1799–1807. [Google Scholar] [CrossRef]
- Chow, E.; Chan, J.C.N. Targeting Postprandial Glucose Control Using Ultra-Rapid Insulins: Is Faster Better? Science Bulletin 2022, 67, 2392–2394. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Piras de Oliveira, C.; Juneja, R.; Ribeiro, A.; Chigutsa, F.; Blevins, T. What Is the Value of Faster Acting Prandial Insulin? Focus on Ultra Rapid Lispro. Diabetes Obes Metab 2022, 24, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Malecki, M.T.; Cao, D.; Liu, R.; Hardy, T.; Bode, B.; Bergenstal, R.M.; Bue-Valleskey, J. Ultra-Rapid Lispro Improves Postprandial Glucose Control and Time in Range in Type 1 Diabetes Compared to Lispro: PRONTO-T1D Continuous Glucose Monitoring Substudy. Diabetes Technol Ther 2020, 22, 853–860. [Google Scholar] [CrossRef]
- Pal, R.; Banerjee, M.; Bhadada, S.K. Glycaemic Efficacy and Safety of Mealtime Faster-Acting Insulin Aspart Administered by Injection as Compared to Insulin Aspart in People with Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Diabet Med 2021, 38, e14515. [Google Scholar] [CrossRef]
- Bolli, G.B.; DeVries, J.H. New Long-Acting Insulin Analogs: From Clamp Studies to Clinical Practice. Diabetes Care 2015, 38, 541–543. [Google Scholar] [CrossRef]
- Owens, D.R.; S Bailey, T.; Fanelli, C.G.; Yale, J.-F.; Bolli, G.B. Clinical Relevance of Pharmacokinetic and Pharmacodynamic Profiles of Insulin Degludec (100, 200 U/mL) and Insulin Glargine (100, 300 U/mL) - a Review of Evidence and Clinical Interpretation. Diabetes Metab 2019, 45, 330–340. [Google Scholar] [CrossRef]
- Battelino, T.; Edelman, S.V.; Nishimura, R.; Bergenstal, R.M. Comparison of Second-Generation Basal Insulin Analogs: A Review of the Evidence from Continuous Glucose Monitoring. Diabetes Technol Ther 2021, 23, 20–30. [Google Scholar] [CrossRef]
- Gomez-Peralta, F.; Chico Ballesteros, A.; Marco Martínez, A.; Pérez Corral, B.; Conget Donlo, I.; Fuentealba Melo, P.; Zaragozá Arnáez, F.; Matabuena Rodríguez, M. Insulin Glargine 300 U/Ml versus Insulin Degludec 100 U/Ml Improves Nocturnal Glycaemic Control and Variability in Type 1 Diabetes under Routine Clinical Practice: A Glucodensities-Based Post Hoc Analysis of the OneCare Study. Diabetes Obes Metab 2024, 26, 1993–1997. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).