1. Introduction
Acanthamoeba genus amoebae are opportunistic and, despite living freely in virtually all natural environments[
1,
2,
3,
4], they can be highly pathogenic, affecting humans with diseases such as Acanthamoeba keratitis (AK), a severe keratitis[
5,
6] and Granulomatous Amebic Encephalitis (GAE), usually fatal neural disease[
7,
8,
9].
Although it presents an increase in the number of diagnosed cases[
10] and has a poor prognosis[
11], GAE is neglected, requiring more research and attention, since relevant aspects of the disease, such as pathogenesis and immune response, still need to be elucidated[
12].
Much of the research that seeks to elucidate these aspects of GAE is carried out in vitro[
13,
14,
15,
16] whose conclusions cannot always be extrapolated to clinical situations. Work using animal models for experimental infections, which bring the results obtained closer to the natural process of contagion, dissemination and infection[
17], has essentially been carried out with mice[
18,
19,
20,
21,
22], while rats and other laboratory animals are rarely used.
As with other pathologies, the use of animal models to analyze the course of GAE is essential to advance knowledge of key aspects, such as pathogenesis and immune response, necessary for improving the diagnosis and treatment of the disease. The main objective of our experiments was to test Wistar rats (Rattus norvegicus albinus) infected by intranasal instillation with a pathogenic strain of Acanthamoeba castellanii, as an animal model for GAE.
2. Material and Methods
2.1. Ethical Issues
This research was approved by the Animal Use Ethics Committee of the Universidade Federal do Pará (protocol number 3980160818), following all the norms and guidelines of the National Council for the Control of Animal Experimentation.
2.2. Amoebae and Reactivation of Strain Virulence
The amoebae used are of the
A. castellanii species (ATCC 50492) obtained from a clinical case of human keratitis and were previously identified as the T4 genotype, which is the most involved in cases of GAE[
23]. These initial cells, which were kept for a long time in PYG (Peptone-Yeast-Glucose) medium, had their virulence reactivated after passage in healthy Wistar rats, in which they were able to cause pulmonary infection. Once rescued from the lungs after maceration of these organs, these amoebae with reactivated virulence (now called T4r) were used to infect experimental animals.
2.3. Cultivation and Maintenance of Amoebas for Infection
T4r amoebae were maintained and cultured in Falcon tubes containing PYG, with replications being performed every 7 days. On the days on which the experimental infections were carried out, the number of cells in culture was adjusted after counting in the Neubauer chamber, in order to obtain 1 x 10[
6] trophozoites/mL of medium, used for inoculation in each animal[
24].
2.4. Animal Model
The animal model of GAE involved 64 healthy Wistar male rats, aged two to three months and body mass in the range of 250-300g, from the Central Animal Facility of the Institute of Biological Sciences of the Federal University of Pará (ICB-UFPA). The animals were analyzed to assess any deformity and treated against ectoparasites, and then placed in standard polypropylene cages, where they were kept in a laboratory environment with a 12-hour light/dark cycle, receiving water and food
ad libitum[
25].
2.5. Experimental Groups
Four experimental animal groups (G1 – G4) containing 16 animals each group were used. The G1 group was used to evaluate the efficiency of the infection route through the recovery of amoebae in tissues after culture in non-nutrient agar, the G2 group was used to evaluate the integrity of the blood-brain barrier (BBB) and vascular extravasation, the G3 group was used for behavioral analysis and the G4 group for histological analysis. In each group, 8 animals were immunosuppressed and infected by intranasal inoculation with 1 × 106/mL T4r trophozoites, and 8 animals were used as a control group, 4 of which were immunosuppressed and 4 were not.
2.6. Immunosuppression and Induction of Experimental Infection
The animals in each of the experimental groups G1, G2, G3 and G4 underwent immunosuppression by intraperitoneal injection of 1 daily dose of dexamethasone (5 mg/kg) for 3 consecutive days. One day after the last dose of the immunosuppressant, these mice were inoculated intranasally with 1 × 106/ml T4r trophozoites. The animals used as controls were instilled intranasally with only the sterile PYG medium, half of which (4) were also immunosuppressed and the other half were not.
2.7. Recovery of Amoebas from Non-Nutrient Agar
After 30 days of infection, the animals in group G1 were anesthetized with intraperitoneal injection of a combination of Ketamine Hydrochloride (100 mg/kg) and Xylazine Hydrochloride (5.0 mg/kg) and verified by the “tail test” for certification from an adequate state of analgesia and sedation to a surgical plan26. Then the animals were euthanized and the brain, lungs and liver were extracted from each animal. After macroscopic analysis, these organs were macerated separately and part of the resulting material was dripped onto petri dishes with non-nutrient agar covered with heat-inactivated Escherichia coli cells. The plates were incubated at room temperature for later direct analysis under a magnifying glass and mounting of slides for microscopic analysis.
2.8. Vascular Permeability Test
The integrity of the BBB after T4r infection was evaluated by the vascular permeability test using the intracardiac injection of a sterile 2.0% solution of Evans blue dye in distilled water[
27].
The animals in the G2 group used for this procedure were anesthetized and opened for exposure of the heart and injection of 500µL of a 2% solution of Evans Blue dye[
28]. Dye injection was done manually using a 1mL syringe. The removal of blood and excess dye from the animal's blood vessels was performed as previously described by Nag[
29]. The brains were then removed and placed in a Petri dish to assess the presence of vascular extravasation in the animal's encephalic microvasculature, performed with the naked eye and under a magnifying glass to capture images. Quantification of extravasated dye was performed as described by Ataíde et al.[
30].
2.9. Behavioral Test
The Rapid murine coma and behavior scale (RMCBS) was used to assess locomotor and exploratory activities, in addition to health status and the course of neural infection by T4r in rats from the G3 group. The tests were performed as previously described by Carrol et al.[
31], involving all 10 parameters recommended by the RMCBS.
2.10. Histopathology
After 30 days of infection, the animals in the G4 group were anesthetized and opened for exposure of the heart and injection of PBS, as described above. Then, 300 ml of 4% Paraformaldehyde (PFA) diluted in 0.9% PBS were perfused for tissue fixation, verified by the inoculated volume and the rigidity of the head and upper limbs of the animal[
32]. Then the brains were removed and placed in Falcon tubes with 4% PFA for 24 hours, being included in paraffin, processed and taken to microtomy to obtain 4µm sections and mount the slides stained with hematoxylin and eosin (H/E).
2.11. Statistical Analysis
The vascular extravasation quantification data were submitted to linear regression analysis to obtain the correlation coefficient (r) between optical densities and Evans blue concentrations[
33].
One-way ANOVA and Tukey's tests were also applied to verify the distribution and statistical differences between the means of the control and experimental groups. The tests were performed using the GraphPad Prism® 8.0 program and the significance level was p<0.05.
3. Results
3.1. Recovery of Amoebas from Non-Nutrient Agar
After 30 days of intranasal infection with 1x10
6 T4r cells, the animals in the G1 group were euthanized and the brain, lungs and liver were extracted from each animal. The anatomopathological analysis revealed liver and lung lesions (
Figure 1) compatible with infection by A. castellanii T4r.
One of the liver lesions was sectioned and underwent a histopathological procedure with H/E staining, showing particularities of this lesion (
Figure 2).
After analysis and photographic recording, these organs were macerated and the resulting material was dripped onto Petri dishes containing non-nutrient agar covered with heat-inactivated
E. coli cells, establishing an amoeba culture. These plates were photographed and aliquots were taken to mount slides for microscopy. The images in
Figure 3 were captured from samples of the negative control (NC - only PYG on the agar plate), the positive control (PC- Amebas in PYG on the agar plate), liver (Li - Liver macerate on the plate with agar), lung (Lu – lung macerate on agar plate) and brain (Br – brain macerate on agar plate), on days 3, 5, 20 and 48 after establishing the culture. Image analysis revealed rapid growth of amoebae in plates with lung maceration, and slower growth of amoebae in plates with liver and neural maceration.
Plates containing agar with macerated animal organs remained viable at room temperature with cysts, up to 110 days after plating, when the drying of the material caused many cracks in the middle.
T4r cells were maintained in PYG medium for carrying out the experimental infections.
Figure 4 shows the record of trophozoites and cysts characteristic of
A. castellanii, genotype T4 together in a freshly prepared slide from the maintenance medium of these cells.
3.2. Vascular Permeability Test
The integrity of the BBB after infection by
A. castellanii was assessed by direct analysis of the brain microvasculature of animals in group G2 (
Figure 5) and by quantification of Evans blue dye extracted from brains kept in formamide, performed in an ELISA reader. Extravasated Evans blue was expressed in mg dye/g tissue (
Figure 6).
3.3. Behavioral Tests
The RMCBS test was used to investigate locomotor and exploratory activity and anxiety levels of animals in response to neural infection by T4r. The animals were evaluated at intervals of 0, 1, 3, 5 and 8 weeks post-infection. The
Figure 7 summarizes the results obtained from the RMCBS.
3.4. Histopathology
After 30 days of infection, the animals in the G4 group were perfused and the brain, lungs and liver were fixed in 4% PFA and embedded in paraffin for mounting and staining on glass slides, for viewing under an optical microscope. Ten slides stained with H/E were mounted and analyzed for each perfused organ.
Histopathological analysis of brains from infected animals did not detect T4r cysts or trophozoites. Control animals showed tissue preservation in the brain regions analyzed (
Figure 8).
4. Discussion
Since the first experiments with animal models of neural infection by
Acanthamoeba were carried out by Culbertson et al.[
34], using mice and monkeys, many discoveries have been made, at the same time that new questions have arisen. Some aspects related mainly to pathogenesis, immune response and treatment remain unclear.
After the first confirmed reports in the 1970s[
35,
36], cases of GAE were reported on all continents[
37] and the disease continues to have a very poor prognosis. According to Visvesvara et al.[
38], the high mortality that accompanies this poor prognosis in GAE is largely due to the lack of knowledge about amoebic diseases. The situation does not seem to have changed in recent years. According to Kot et al.[
39], no definitive treatment protocol against GAE has yet been established. The lack of prepared professionals and protocols for etiological recognition is at the heart of this problem, which can lead to great underreporting and errors in the diagnosis of this disease[
40,
41]. Therefore, the search for diagnostic tools and protocols for GAE is essential to improve its poor prognosis[
42,
43]. In this context, the use of animal models is essential to support the construction of these tools and protocols.
In the present work, different tests were used to evaluate Wistar rats as an animal model of GAE.
4.1. Recovery of Amoebas from Non-Nutrient Agar
Acanthamoeba castellanii T4r cells used for intranasal infection of experimental rats caused systemic infection, being recovered from the brain, lungs and liver of these animals. These results agree with Veríssimo et al.[
44] and Omaña-molina et al.[
21] who had similar results with A. castellanii seeded on non-nutrient agar plates.
It is worth mentioning that the growth of
A. castellanii occurred both on non-nutrient agar plates whose nutritional source for the amoebae were bacteria inactivated by heat, as several researchers have done previously[
45,
46,
47,
48,
49,
50,
51], and on plates containing sterile PYG as the only nutritional source. This is interesting because the methodology using PYG as a nutritional source is simpler and less expensive when bacterial strains are not available. Furthermore, growth in medium with bacterial strains may be slower[
52].
It is also important to point out that the animals used as controls in all experiments survived the entire period of tests and post-mortem examinations did not reveal any damage to the internal organs, nor were amoebae rescued from the organism of these animals.
4.2. Vascular Permeability Test
The images of the brain microvasculature of the animals and the quantification of the Evans blue dye extracted from the brains, confirmed both the efficiency of the model of intranasal infection of wistar rats by Acanthamoeba castellanii, as well as the hematogenous dissemination and ability of the amoeba to break the blood-brain barrier of the host, as did Khan and Siddiqui[
53]. Neural infections by these amoebae in humans generally occur in the lower respiratory tract and the invasion of the CNS is by the olfactory neuroepithelium, as was also observed by Kuhlencord et al.[
54] and Omaña-molina et al.[
21] or in sites of the BBB, according to findings by Sissons et al.[
55] and Khan[
56].
The passage through the BBB, despite depending on enzymatic processes and the contact of amoebae with cerebral microvascular endothelial cells (BMEC), is facilitated, in part, by the exacerbated immune response of the host which, according to BAIG[
57], in addition to violating the BBB, this response The immune system is the main cause of brain damage in the course of GAE, in addition to the action of toxins and enzymes secreted by the amoeba[
58].
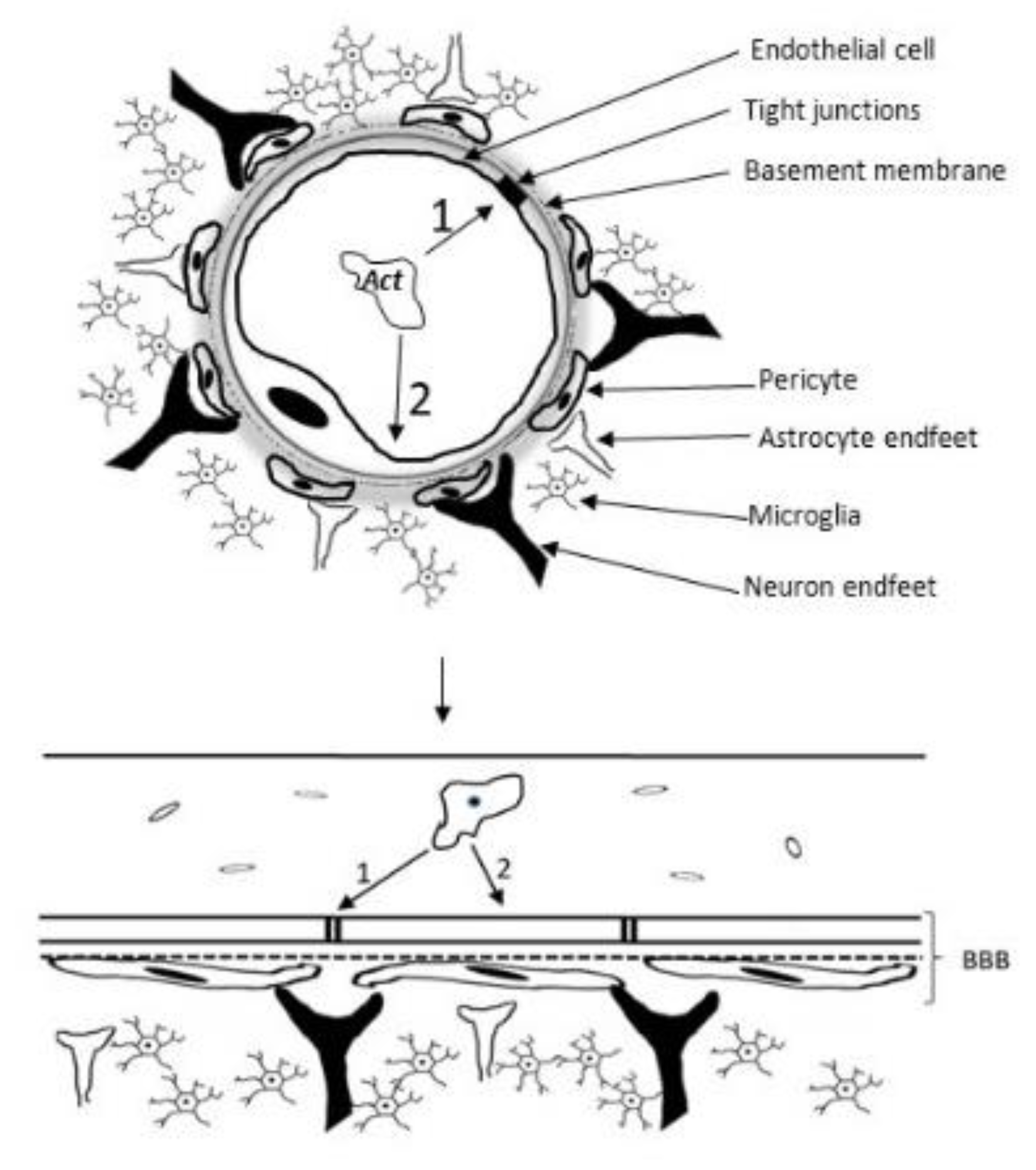
Acanthamoeba spp. degrade occludin and ZO-1, fundamental components of tight junctions and endothelial cell selectivity, indicating the use of the paracellular route as an important strategy to access the CNS (
Figure 9). This route involves the crossing of the BBB between endothelial cells, and is used by other protozoans such as
Plasmodium falciprum,
Trypanosoma spp. and
Toxoplasma gondii[
59,
60]. Acanthamoeba spp. also use the Transcellular route, where they paralyze the BMEC cell cycle resulting in necrosis or apoptosis and loss of integrity of the BBB[
61,
62].
Control animals did not show significant vascular extravasation or brains with microvasculature altered by the dye, as shown by the images.
It should be noted that an infected animal showed inflammation and necrosis of the nostrils and olfactory bulb, indicating invasion of the brain via the nasal mucosa and olfactory nerves, as has been demonstrated in other studies with experimental GAE[
63,
64].
4.3. Behavioral Tests
During the intimate relationship established by the parasites and their host animals, detectable behavioral changes may occur in the host. These alterations are assessed by the animals' performance in behavioral tests that measure the final manifestations of neural functions, such as motor coordination, memory or state of anxiety[
65]. In our research, we used the RMCBS test, an objective and quantitative scale created for use with mice, seeking to compare the results obtained with the findings of the vascular permeability test in response to neural infection of the animals by
A. castellanii T4r.
In agreement with the findings of vascular permeability assays, which demonstrated disruption of the blood-brain barrier and cerebral vascular extravasation, the RMCBS showed a significant difference (p˂ 0.01) in the mean score of control rats compared to infected animals in all tests post-infection.
The mechanisms that induce a behavioral change in parasitized animals are not completely known[
66]. Side effects of damage to the nervous system may be involved, such as altered cytokine expression of IFN-γ, TNF, IL-2 and IL-12[
67,
68] or the action of substances released by parasites that act as neurotransmitters influencing host behavior[
20,
69].
Anyway, the behavioral parameter has been fundamental to, together with other assays, determine the presence of parasites in the CNS, as well as the presence and extent of lesions resulting from parasitic invasion.
4.4. Histolopathology
Not all infected animals showed positive histopathology for the analyzed organs. Nervous tissue was the least affected, demonstrating a clear role for the BBB in defense of the CNS, as described by Daneman and Prat[
70]. In the works by Markowitz et al.[
71], histological changes in infected mice were minimal in several organs, including 9/10 animals with unchanged brains even 35 days after infection by
A. castellanii.
To increase the chances of detection of T4r by histopathology of the analyzed organs, especially the brain, the infection period was long, as recommended by Ramirez et al.[
72] and Massilamany et al.[
20]. However, this did not guarantee 100% detection of lesions, cysts or trophozoites compatible with the histopathology of
A. castellanii infection. This fact highlights the importance of molecular studies (not carried out in this work) using PCR to detect the presence of
Acanthamoeba in tissue samples from infected animals, as was done by Gianinazzi et al.[
64].
5. Conclusion
The presented results show the possibility of using wistar rats as an experimental model of GAE. However, this still represents a challenge, because, despite expressive results in the tests carried out, some infected animals did not show behavior or histopathology compatible with T4r infection, indicating the need for further research aimed at expanding model tests and including the analysis of the host's immune response, in the search for answers to the questions that still exist, helping to advance knowledge and combat this pathology.
Author Contributions
Conceptualization, S.LB. and E.J.O.B; methodology, S.LB., K.R.H.M.O., C.P.B and E.J.O.B; investigation, S.L.B, E.S.M., F.A.V.S., N.S.F.M. and B.J.A.A.; resources, S.L.B, E.S.M., F.A.V.S. and E.J.O.B.; data curation, A.M.H., C.P.B., S.A.S.M. and S.S.D.; writing—original draft preparation, , S.L.B. and E.J.O.B..; writing—review and editing, S.L.B., A.C.F.P. and E.S.M.; visualization, , S.L.B, E.S.M., F.A.V.S., K.R.H.M.O and E.J.O.B.; supervision, E.J.O.B; project administration, E.J.O.B..; funding acquisition, S.LB. and E.J.O.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Federal University of Pará, the Federal institute of Education of Pará, and the authors.
Institutional Review Board Statement
This research was approved by the Animal Use Ethics Committee (Protocol number 3980160818) of the Federal University of Pará, following all the norms and guidelines of the National Council for the Control of Animal Experimentation.Informed Consent Statement: Not applicable.
Data Availability Statement
The data presented in this study are available in this article and on request from the corresponding author.
Acknowledgments
The authors would like to thank the Laboratory of Entomology and Tropical Parasitology of the Federal University of Sergipe for providing the strain used and the vivariums of the Institute of Biological Sciences and the Laboratory of Neuroplasticity of the Institute of Health Sciences of the Federal University of Pará for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- BROWN, Tim J; CURSONS, Ray T M; KEYS, Elizabeth A. Amoebae from antarctic soil and water. Applied And Environmental Microbiology, Washington, v. 44, n. 2, p.491-493, 1982.
- VISVESVARA, Govinda S; STEHR-GREEN, J K. Epidemiology of free-living ameba infection. The Journal Of Protozoology, New York, v. 37, n. 4, p.25-33, 1990. [CrossRef]
- RODRíGUEZ-ZARAGOZA, S; MAYZLISH, E.; STEINBERGER, Y.. Seasonal Changes in Free-Living Amoeba Species in the Root Canopy of Zygophyllum dumosum in the Negev Desert, Israel. Microbial Ecology, On Line, v. 49, n. 1, p.134-141, 2005.
- MULEC, Janez et al. Acanthamoeba and other free-living amoebae in bat guano, an extreme habitat. Parasitology Research, [s.l.], v. 115, n. 4, p.1375-1383, 17 dez. 2015. Springer Nature. [CrossRef]
- GALARZA, Carlos et al. Cutaneous acanthamebiasis infection in immunocompetent and immunocompromised patients. International Journal Of Dermatology, [s.l.], v. 48, n. 12, p.1324-1329, dez. 2009. Wiley. [CrossRef]
- LORENZO-MORALES, Jacob; KHAN, Naveed A.; WALOCHNIK, Julia. An update onAcanthamoebakeratitis: diagnosis, pathogenesis and treatment. Parasite, [s.l.], v. 22, n. 10, p.1-8, 2015. EDP Sciences. [CrossRef]
- SHIRWADKAR, Charudatt G. et al. AcanthamoebaEncephalitis in Patient with Systemic Lupus, India. Emerging Infectious Diseases, [s.l.], v. 12, n. 6, p.984-986, jun. 2006. Centers for Disease Control and Prevention (CDC). [CrossRef]
- CATEAU, E. et al. Free-living amoebae: what part do they play in healthcare-associated infections?. Journal Of Hospital Infection, [s.l.], v. 87, n. 3, p.131-140, jul. 2014. Elsevier BV. [CrossRef]
- KOT K, ŁANOCHA-ARENDARCZYK NA, KOSIK-BOGACKA DI. Amoebas from the genus Acanthamoeba and their pathogenic properties. Ann Parasitol. 2018;64(4):299-308.
- KALRA, S.K., SHARMA, P., SHYAM, K., TEJAN, N., GHOSHAL, U., Acanthamoeba and its pathogenic role in granulomatous amebic encephalitis, Experimental Parasitology (2019). [CrossRef]
- CARLESSO, Ana Maris et al. Potentially pathogenic acanthamoeba isolated from a hospital in Brazil. Current Microbiology, New York, v. 60, n. 3, p.185-190, 2010. [CrossRef]
- DUGGAL, Shalini Dewan et al. Role of Acanthamoeba in Granulomatous Encephalitis. Journal Of Infectious Diseases & Immune Therapies, Austin, v. 1, n. 1, p.1-7, 2017.
- TEIXEIRA, Lais Helena et al. Prevalence of potentially pathogenic free-living amoebae from Acanthamoeba and Naegleria genera in non-hospital, public, internal environments from the city of Santos, Brazil. Braz J Infect Dis, Salvador, v. 13, n. 6, p.395-347, 2009.
- CAUMO KS, MONTEIRO KM, OTT TR, MASCHIO VJ, WAGNER G, FERREIRA HB, ROTT MB. Proteomic profiling of the infective trophozoite stage of Acanthamoeba polyphaga. Acta Trop. 2014 Dec;140:166-72. [CrossRef]
- RAMÍREZ-RICO G, MARTÍNEZ-CASTILLO M, DE LA GARZA M, SHIBAYAMA M, SERRANO-LUNA J. Acanthamoeba castellanii Proteases are Capable of Degrading Iron-Binding Proteins as a Possible Mechanism of Pathogenicity. J Eukaryot Microbiol. 2015 Sep-Oct;62(5):614-22. [CrossRef]
- CASTRO-ARTAVIA E, RETANA-MOREIRA L, LORENZO-MORALES J, ABRAHAMS-SANDÍ E. Potentially pathogenic Acanthamoeba genotype T4 isolated from dental units and emergency combination showers. Mem Inst Oswaldo Cruz. 2017;112(12):817-821. [CrossRef]
- ARMSTRONG, M. The pathogenesis of human Acanthamoeba infection. Infect. Dis. Rev. v.2, p. 65 – 73, 2000.
- DE JONCKHEERE, Johan F. Growth characteristics, cytopathic effect in cell culture, and virulence in mice of 36 type strains belonging to 19 different Acanthamoeba spp. Appl. Environ. Microbiol., v.39, p.681–685,1980.
- JANITSCHKE K, MARTÍNEZ AJ, VISVESVARA GS, SCHUSTER F. Animal model Balamuthia mandrillaris CNS infection: contrast and comparison in immunodeficient and immunocompetent mice: a murine model of "granulomatous" amebic encephalitis. J Neuropathol Exp Neurol. 1996 Jul;55(7):815-21.
- MASSILAMANY, Chandirasegaran et al. SJL Mice Infected with Acanthamoeba castellanii Develop Central Nervous System Autoimmunity through the Generation of Cross-Reactive T Cells for Myelin Antigens. Plos One, [s.l.], v. 9, n. 5, p.1-11, 30 maio 2014. Public Library of Science (PLoS). [CrossRef]
- OMAÑA-MOLINA, Maritza et al. In vivo CNS infection model of Acanthamoeba genotype T4: the early stages of infection lack presence of host inflammatory response and are a slow and contact-dependent process. Parasitology Research, [s.l.], v. 116, n. 2, p.725-733, 3 dez. 2017. Springer Nature. [CrossRef]
- ŁANOCHA-ARENDARCZYK, N., KOLASA-WOŁOSIUK, A., WOJCIECHOWSKA-KOSZKO, I. et al. Changes in the immune system in experimental acanthamoebiasis in immunocompetent and immunosuppressed hosts. Parasites Vectors 11, 517 (2018). [CrossRef]
- ALVES, Daniella de Sousa Mendes Moreira et al. Experimental infection of T4 Acanthamoeba genotype determines the pathogenic potential. Parasitology Research, [s.l.], v. 115, n. 9, p.3435-3440, 11 maio 2016. Springer Nature. [CrossRef]
- BECKER-FINCO, Alessandra et al. Physiological, morphological, and immunochemical parameters used for the characterization of clinical and environmental isolates of Acanthamoeba. Parasitology. 2013 Mar;140(3):396-405. [CrossRef]
- LINO-DE-OLIVEIRA, Cilene et al. Effects of acute and chronic fluoxetine treatments on restraint stress-induced Fos expression. Brain research bulletin,v.55, n.6, p. 747-54, 2001.
- GAGE, Gregory J.; KIPKE, Daryl R.; SHAIN, William. Whole Animal Perfusion Fixation for Rodents. Journal Of Visualized Experiments, [s.l.], n. 65, p.1-9, 30 jul. 2012. MyJove Corporation. [CrossRef]
- RADU, Maria; CHERNOFF, Jonathan. An in vivo Assay to Test Blood Vessel Permeability. Journal Of Visualized Experiments, [s.l.], n. 73, p.1-4, 16 mar. 2013. MyJove Corporation. [CrossRef]
- FLIERL, Michael A; STAHEL, Philip F; BEAUCHAMP, Kathryn M; MORGAN, Steven J; SMITH, Wade R; SHOHAMI, Esther. Mouse closed head injury model induced by a weight-drop device. Nature Protocols, [S.L.], v. 4, n. 9, p. 1328-1337, 27 ago. 2009. Springer Science and Business Media LLC. [CrossRef]
- NAG, Sukriti. Blood–Brain Barrier Permeability Using Tracers and Immunohistochemistry. Blood-Brain Barrier,, [S.L.], p. 133-144, 2003. Humana Press. [CrossRef]
- ATAÍDE BJA, KAUFFMANN N, MENDES NSF, et al. Melatonin Prevents Brain Damage and Neurocognitive Impairment Induced by Plasmodium Berghei ANKA Infection in Murine Model of Cerebral Malaria. Front Cell Infect Microbiol. 2020 Sep 30;10:541624. [CrossRef]
- CARROLL RW, WAINWRIGHT MS, KIM KY, KIDAMBI T, GÓMEZ ND, TAYLOR T, et al. (2010) A Rapid Murine Coma and Behavior Scale for Quantitative Assessment of Murine Cerebral Malaria. PLoS ONE 5 (10): e13124. [CrossRef]
- SILVA, Bruno Pires Ferreira e et al. Ethanol exposition in the perinatal to aguardente into rats cerebral cortex. Revista Paraense de Medicina, Belém, v. 20, n. 1, p.7-14, 2006.
- GEHLEN, Marcelo Luiz et al.. Avaliação espectrofotométrica do azul de Evans na reação inflamatória da córnea: estudo experimental em coelhos. Arquivos Brasileiros de Oftalmologia, [S.L.], v. 67, n. 2, p. 219-225, abr. 2004. FapUNIFESP (SciELO). [CrossRef]
- CULBERTSON, C G et al. Experimental Infection of Mice and Monkeys by Acanthamoeba. Am. J. Pathol., Philadelphia, v. 35, n. 1, p.185-197, 1959.
- MARTÍNEZ, Augusto Julio et al. Meningoencephalitis due to Acanthamoeba sp. Pathogenesis and clinico-pathological study. Acta Neuropathologica, Berlim, v. 37, n. 3, p.183-191, 1977.
- DUMA, R J; HELWIG, W B; MARTINEZ, A J. Meningoencephalitis and brain abscess due to a free-living amoeba. Annals Of Internal Medicine, Philadelphia, v. 88, n. 4, p.468-473, 1978.
- MARCIANO-CABRAL, Francine; CABRAL, Guy A. Acanthamoeba spp. as Agents of Disease in Humans. Clinical Microbiology Reviews, Washington, v. 16, n. 2, p.273-307, 2003.
- VISVESVARA, Govinda S.; MOURA, Hercules; SCHUSTER, Frederick L.. Pathogenic and opportunistic free-living amoebae: Acanthamoebaspp.,Balamuthia mandrillaris,Naegleria fowleri, and Sappinia diploidea. Fems Immunology & Medical Microbiology, [s.l.], v. 50, n. 1, p.1-26, jun. 2007. Oxford University Press (OUP). [CrossRef]
- KOT K, ŁANOCHA-ARENDARCZYK N, KOSIK-BOGACKA D. Immunopathogenicity of Acanthamoeba spp. in the Brain and Lungs. Int J Mol Sci. 2021 Jan 27;22(3):1261. [CrossRef]
- MAYCOCK, Nicholas J. R.; JAYASWAL, Rakesh. Update on Acanthamoeba Keratitis. Cornea, [s.l.], v. 35, n. 5, p.713-720, maio 2016. Ovid Technologies (Wolters Kluwer Health). [CrossRef]
- KRÓL-TURMIŃSKA K, OLENDER A. Human infections caused by free-living amoebae. Ann Agric Environ Med. 2017 May 11;24(2):254-260. [CrossRef]
- BAIG AM, IQBAL J, KHAN NA. In vitro efficacies of clinically available drugs against growth and viability of an Acanthamoeba castellanii keratitis isolate belonging to the T4 genotype. Antimicrob Agents Chemother. 2013 Aug;57(8):3561-7. [CrossRef]
- MATSUI, Takahiro et al. A case report of granulomatous amoebic encephalitis by Group 1 Acanthamoeba genotype T18 diagnosed by the combination of morphological examination and genetic analysis. Diagnostic Pathology, [s.l.], v. 13, n. 1, p.1-6, 10 maio 2018. Springer Nature. [CrossRef]
- VERÍSSIMO C de M, MASCHIO VJ, CORREA AP, BRANDELLI A, ROTT MB. Infection in a rat model reactivates attenuated virulence after long-term axenic culture of Acanthamoeba spp. Mem Inst Oswaldo Cruz. 2013 Nov;108(7):832-5. [CrossRef]
- CURSONS RT, BROWN TJ, KEYS EA, MORIARTY KM, TILL D. Immunity to pathogenic free-living amoebae: role of humoral antibody. Infect Immun. 1980;29(2):401-407. [CrossRef]
- OMAÑA-MOLINA Maritza et al. Induction Of Morphological And Electrophysiological Changes In Hamster Cornea After In Vitro Interaction With Trophozoites Of Acanthamoeba Spp. Infect Immun. 2004 Jun;72(6):3245-51. [CrossRef]
- CORSARO D, VENDITTI D. Phylogenetic evidence for a new genotype of Acanthamoeba (Amoebozoa, Acanthamoebida). Parasitol Res. 2010 Jun;107(1):233-8. [CrossRef]
- NUPRASERT, Warisa et al. Identification of a Novel T17 Genotype of Acanthamoeba from Environmental Isolates and T10 Genotype Causing Keratitis in Thailand. Journal Of Clinical Microbiology, Washington, v. 48, n. 12, p.4636-4640, 2010.
- RISLER, Arnaud; COUPAT-GOUTALAND, Bénédicte; PÉLANDAKIS, Michel. Genotyping and phylogenetic analysis of Acanthamoeba isolates associated with keratitis. Parasitology Research, [s.l.], v. 112, n. 11, p.3807-3816, 20 ago. 2013. Springer Nature. [CrossRef]
- MASCHIO, Vinicius José et al. Acanthamoeba T4, T5 and T11 Isolated From Mineral Water Bottles in Southern Brazil. Current Microbiology, [s.l.], v. 70, n. 1, p.6-9, 15 ago. 2015. Springer Nature. [CrossRef]
- BEHERA, Himanshu Sekhar; SATPATHY, Gita; TRIPATHI, Manjari. Isolation and genotyping of Acanthamoeba spp. from Acanthamoeba meningitis/ meningoencephalitis (AME) patients in India. Parasites & Vectors, [s.l.], v. 9, n. 442, p.1-6, 9 ago. 2016. Springer Nature. [CrossRef]
- MORRISON, Annie O. et al. Disseminated Acanthamoeba Infection Presenting With Cutaneous Lesions in an Immunocompromised Patient. American Journal Of Clinical Pathology, [s.l.], v. 145, n. 2, p.266-270, 22 jan. 2016. Oxford University Press (OUP). [CrossRef]
- KHAN, Naveed Ahmed; SIDDIQUI, Ruqaiyyah. Acanthamoeba affects the integrity of human brain microvascular endothelial cells and degrades the tight junction proteins. International Journal For Parasitology, [s.l.], v. 39, n. 14, p.1611-1616, dez. 2009. Elsevier BV. [CrossRef]
- KUHLENCORD A, MERGERIAN H, BOMMER W. Studies on the pathogenesis of Acanthamoeba-associated meningoencephalitis. Zentralbl Bakteriol. 1989 Jul;271(2):256-60. [CrossRef]
- SISSONS, James et al. Identification and properties of proteases from an Acanthamoeba isolate capable of producing granulomatous encephalitis. Bmc Microbiology, [s.l.], v. 6, n. 1, p.1-8, 2006. Springer Nature. [CrossRef]
- KHAN, Naveed Ahmed. Acanthamoeba and the blood-brain barrier: the breakthrough. Journal Of Medical Microbiology, [s.l.], v. 57, n. 9, p.1051-1057, 1 set. 2008. Microbiology Society. [CrossRef]
- BAIG, Abdul Mannan. Pathogenesis of amoebic encephalitis: Are the amoebae being credited to an ‘inside job’ done by the host immune response?. Acta Tropica, [s.l.], v. 148, p.72-76, ago. 2015. Elsevier BV. [CrossRef]
- KRISTENSSON K, MASOCHA W, BENTIVOGLIO M. Mechanisms of CNS invasion and damage by parasites. Handb Clin Neurol. 2013;114:11-22. [CrossRef]
- ELSHEIKHA, H.M. E KHAN, N.A., Protozoa traversal of the blood–brain barrier to invade the central nervous system, FEMS Microbiology Reviews, v.34, n.4, 2010. [CrossRef]
- MASOCHA W, KRISTENSSON K. Passage of parasites across the blood-brain barrier. Virulence. 2012;3(2):202-212. [CrossRef]
- SISSONS J, ALSAM S, JAYASEKERA S, KIM KS, STINS M, KHAN NA. Acanthamoeba induces cell-cycle arrest in host cells. J Med Microbiol. 2004 Aug;53(Pt 8):711-717. [CrossRef]
- SISSONS J, KIM KS, STINS M, JAYASEKERA S, ALSAM S, KHAN NA. Acanthamoeba castellanii Induces Host Cell Death via a Phosphatidylinositol 3-Kinase-Dependent Mechanism. Infect Immun . 2005;73(5):2704-2708. [CrossRef]
- KIDERLEN AF, LAUBE U. Balamuthia mandrillaris, an opportunistic agent of granulomatous amebic encephalitis, infects the brain via the olfactory nerve pathway. Parasitol Res. 2004 Sep;94(1):49-52. [CrossRef]
- GIANINAZZI, Christian et al. Potentially human pathogenic Acanthamoeba isolated from a heated indoor swimming pool in Switzerland. Experimental Parasitology, [s.l.], v. 121, n. 2, p.180-186, fev. 2009. Elsevier BV. [CrossRef]
- LALONDE R, KIM HD, FUKUCHI K. Exploratory activity, anxiety, and motor coordination in bigenic APPswe + PS1/DeltaE9 mice. Neurosci Lett. 2004 Oct 14;369(2):156-61. [CrossRef]
- KAUSHIK M, LAMBERTON PH, WEBSTER JP. The role of parasites and pathogens in influencing generalised anxiety and predation-related fear in the mammalian central nervous system. Horm Behav. 2012 Aug;62(3):191-201. [CrossRef]
- HAMILTON CM, BRANDES S, HOLLAND CV, PINELLI E. Cytokine expression in the brains of Toxocara canis-infected mice. Parasite Immunol. 2008 Mar;30(3):181-5. [CrossRef]
- BAIG, Abdul Mannan. Granulomatous amoebic encephalitis: ghost response of an immunocompromised host?. Journal Of Medical Microbiology, [s.l.], v. 63, n. 12, p.1763-1766, 19 set. 2014. Microbiology Society. [CrossRef]
- WEBSTER JP. The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizophr Bull. 2007;33(3):752-756. [CrossRef]
- DANEMAN R, PRAT A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. Published 2015 Jan 5. [CrossRef]
- MARKOWITZ, Sheldon M. et al. Experimental Acanthamoeba infections in mice pretreated with methylprednisolone or tetracycline. The American Jounal of Pathology, v.92, n.3, p.733-44, 1978.
- RAMIREZ E, CAMPOY E, MATUZ D, ROBLES E. Acanthamoeba isolated from contaminated groundwater. J Eukaryot Microbiol. 2006;53 Suppl 1:S10-1. [CrossRef]
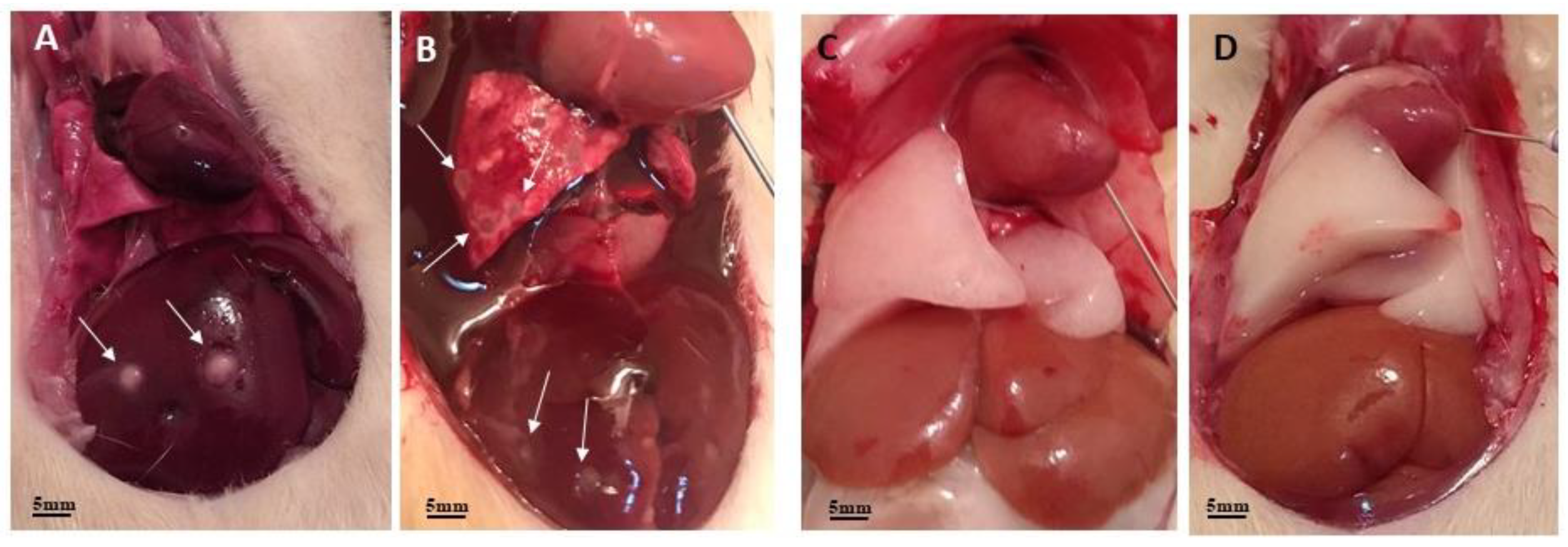
Figure 1.
Anatomopathological analysis of the liver and lungs of experimental animals. Images A and B show several liver and lung lesions (arrows) compatible with T4r infection. Images C and D show these organs preserved in control animals.
Figure 1.
Anatomopathological analysis of the liver and lungs of experimental animals. Images A and B show several liver and lung lesions (arrows) compatible with T4r infection. Images C and D show these organs preserved in control animals.
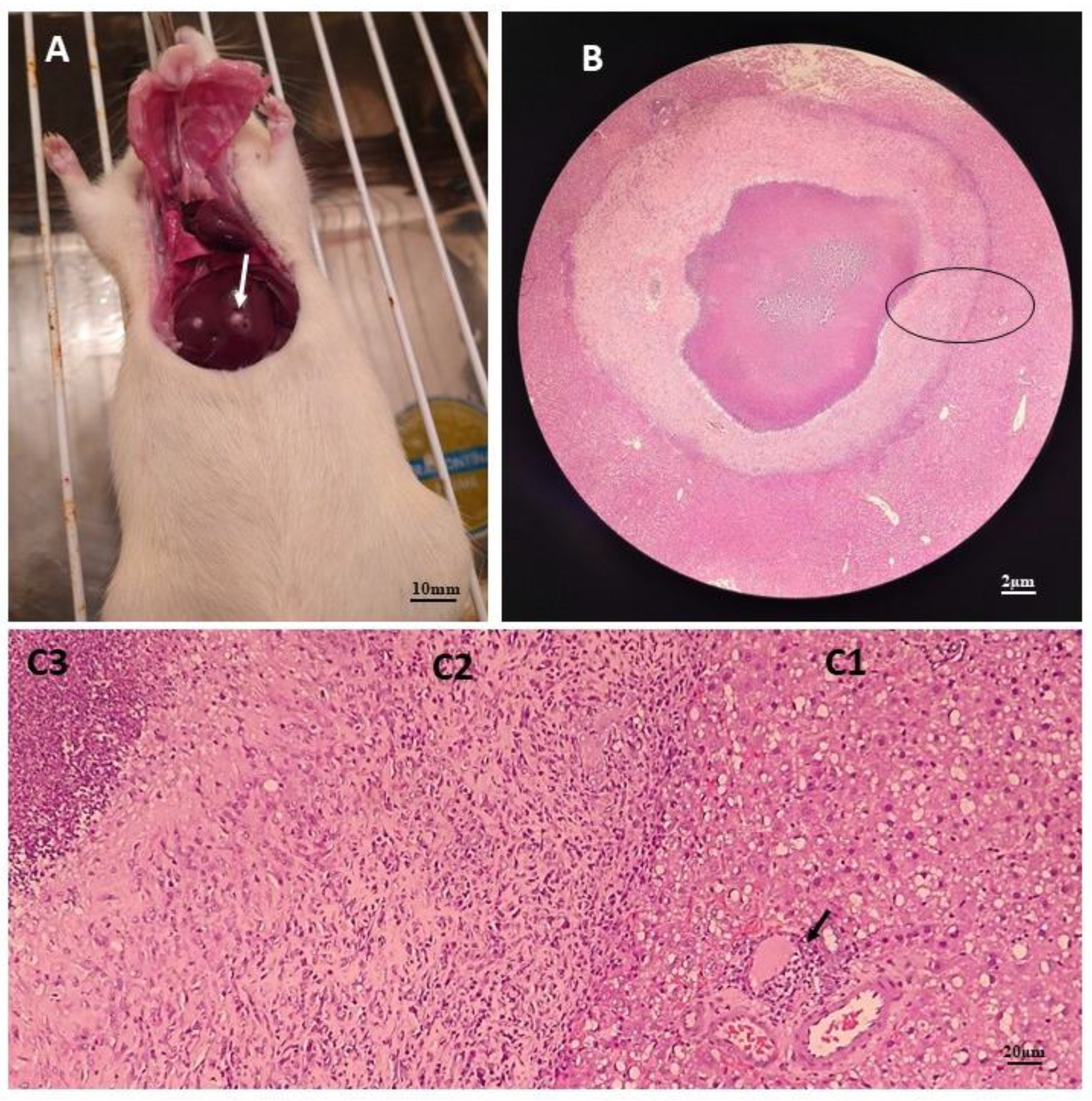
Figure 2.
Anatomopathological and histopathological images of the liver of an infected rat. Image A shows an animal undergoing surgery with liver lesions compatible with A. castellanii infection; Image B shows a histological section with an overview of one of the liver lesions, showing three well-defined areas, at a final magnification of 32x. Image C shows these three areas divided into; (C1) which represents the peripheral area and shows acidophilic hepatocytes with hyperchromatic nuclei, in addition to mild steatosis. The arrow points to an inflammatory focus; (C2) is the intermediate area characterized by a halo of fibrosis and (C3) which represents the central area of the lesion, characterized by the presence of necrosis with a large number of degenerated neutrophils, in a histological section after H/E staining with a final increase of 200x.
Figure 2.
Anatomopathological and histopathological images of the liver of an infected rat. Image A shows an animal undergoing surgery with liver lesions compatible with A. castellanii infection; Image B shows a histological section with an overview of one of the liver lesions, showing three well-defined areas, at a final magnification of 32x. Image C shows these three areas divided into; (C1) which represents the peripheral area and shows acidophilic hepatocytes with hyperchromatic nuclei, in addition to mild steatosis. The arrow points to an inflammatory focus; (C2) is the intermediate area characterized by a halo of fibrosis and (C3) which represents the central area of the lesion, characterized by the presence of necrosis with a large number of degenerated neutrophils, in a histological section after H/E staining with a final increase of 200x.
Figure 3.
Photomicrographs of plates and slides mounted with material from amoeba culture in agar. (NC): Negative control plates show no amoebae; (PC): Positive control plates containing T4r cells maintained in PYG show, in PC3, multiple trophozoites at the culture addition site; in PC5, trophozoites in the process of encysting; in PC20, cysts formed in the trail of E. coli and in PC48, cysts characteristic of A. castellanii in optical microscopy with final magnification of 400x; (Li): Here the plate was seeded with liver material from T4r-infected animal. Li3 and Li5 show the site of addition of liver material; Li20 and Li48 show T4r cysts in a slide visualized in MO with a final magnification of 400x. (Lu): Here the agar plate was seeded with lung material from an infected animal; Lu3 shows lung addition site; Lu5 shows cysts in E. coli tracks; Lu20 shows numerous mature cysts and Lu48 shows cysts characteristic of A. castellanii in a slide stained with Lugol and visualized in MO with a final magnification of 400x; (Br): Here the plate was seeded with encephalic material from the brain of an infected animal. Br3 shows site of material addition; Br5 shows E. coli trail; Br20 shows several trophozoites and cysts and Br48 shows a cyst characteristic of A. castellanii in a slide stained with Lugol and visualized in MO with a final magnification of 400x.
Figure 3.
Photomicrographs of plates and slides mounted with material from amoeba culture in agar. (NC): Negative control plates show no amoebae; (PC): Positive control plates containing T4r cells maintained in PYG show, in PC3, multiple trophozoites at the culture addition site; in PC5, trophozoites in the process of encysting; in PC20, cysts formed in the trail of E. coli and in PC48, cysts characteristic of A. castellanii in optical microscopy with final magnification of 400x; (Li): Here the plate was seeded with liver material from T4r-infected animal. Li3 and Li5 show the site of addition of liver material; Li20 and Li48 show T4r cysts in a slide visualized in MO with a final magnification of 400x. (Lu): Here the agar plate was seeded with lung material from an infected animal; Lu3 shows lung addition site; Lu5 shows cysts in E. coli tracks; Lu20 shows numerous mature cysts and Lu48 shows cysts characteristic of A. castellanii in a slide stained with Lugol and visualized in MO with a final magnification of 400x; (Br): Here the plate was seeded with encephalic material from the brain of an infected animal. Br3 shows site of material addition; Br5 shows E. coli trail; Br20 shows several trophozoites and cysts and Br48 shows a cyst characteristic of A. castellanii in a slide stained with Lugol and visualized in MO with a final magnification of 400x.
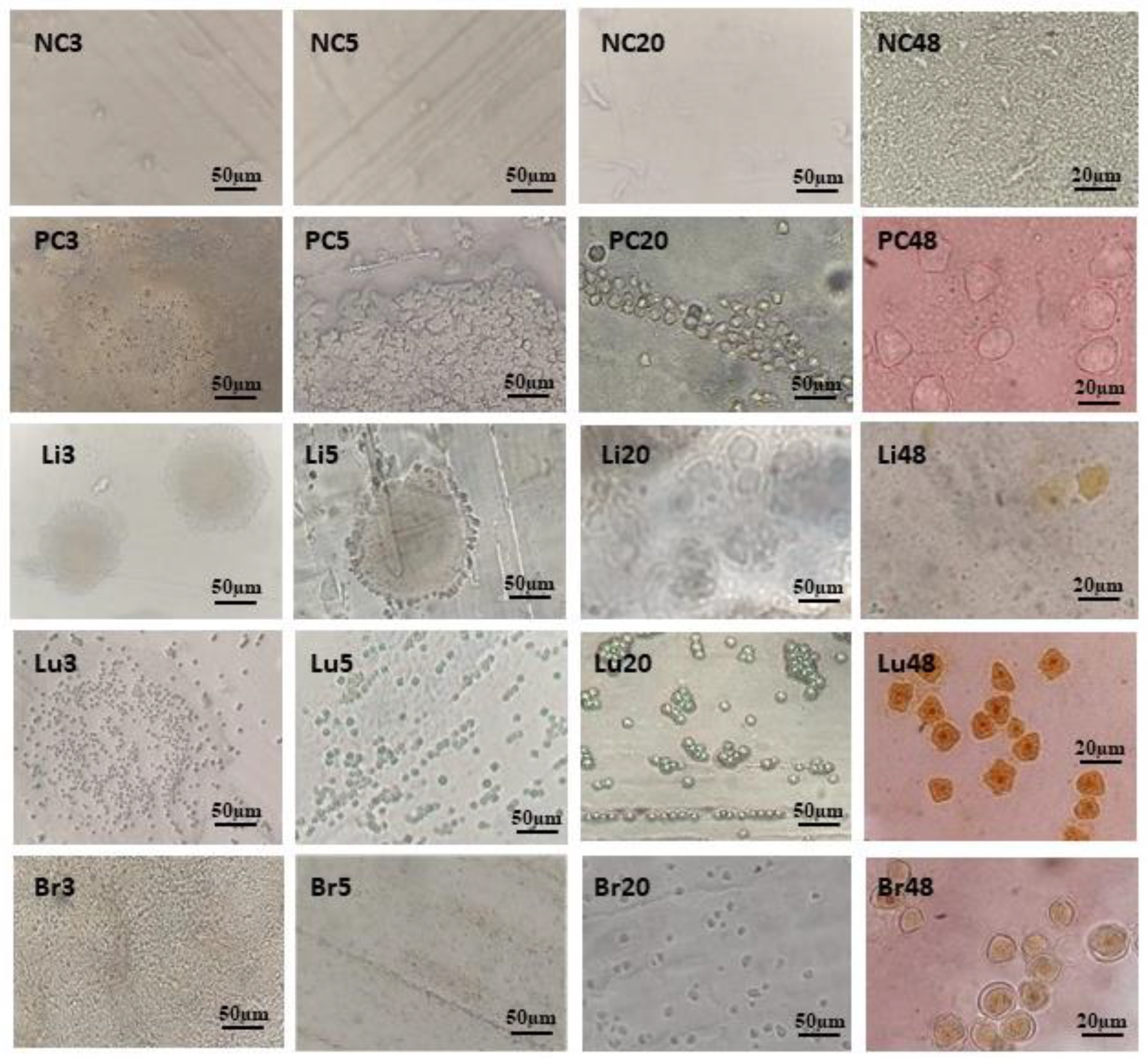
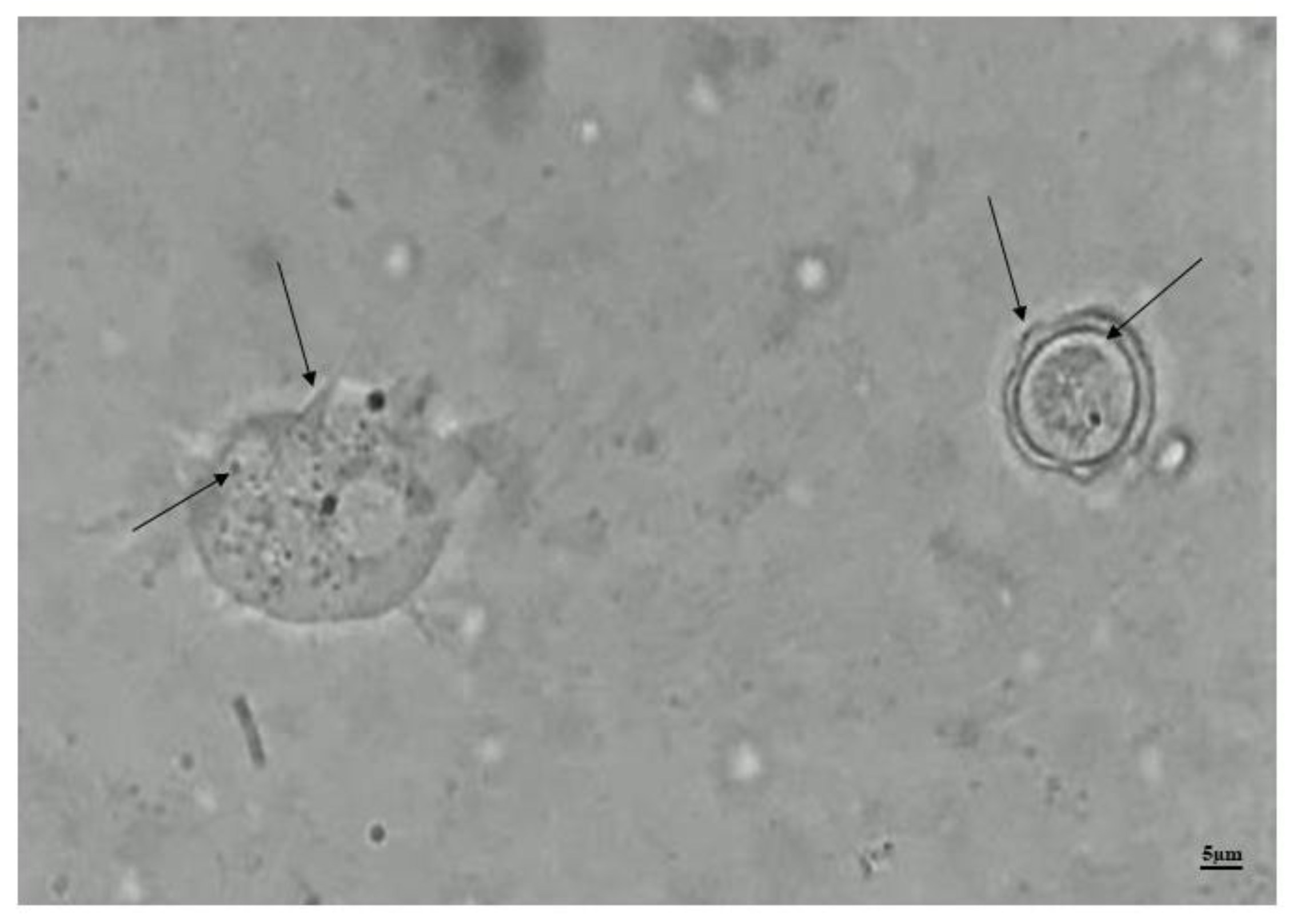
Figure 4.
Photomicrograph of Trophozoite and cyst of A. castellanii in PYG. On the left in the image we have a trophozoite showing acantapodia and contractile vacuoles and on the right, a cyst showing ecto and endocyst, visualized by MO, with final magnification of 1000x.
Figure 4.
Photomicrograph of Trophozoite and cyst of A. castellanii in PYG. On the left in the image we have a trophozoite showing acantapodia and contractile vacuoles and on the right, a cyst showing ecto and endocyst, visualized by MO, with final magnification of 1000x.
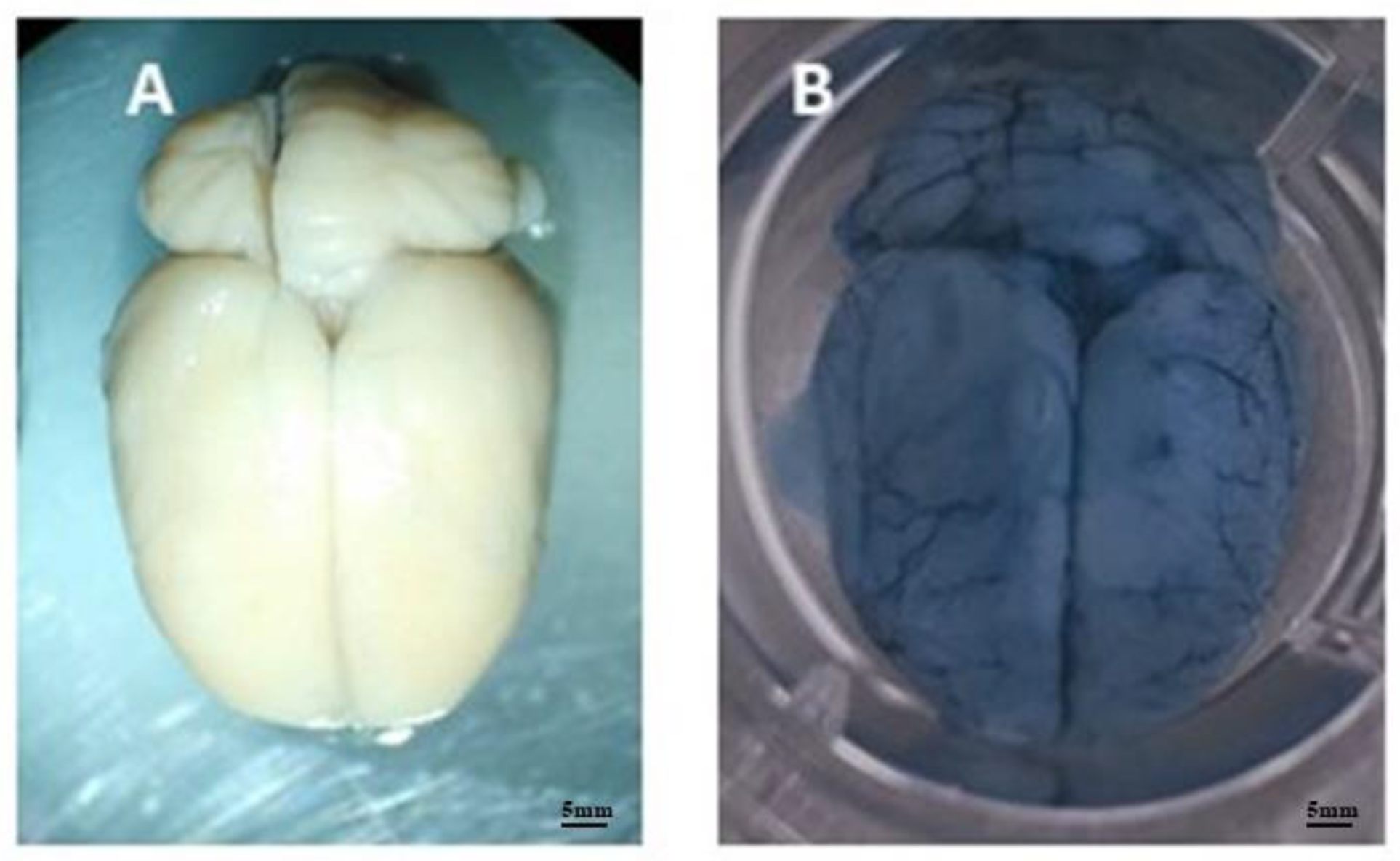
Figure 5.
Photographs of the encephalic vasculature of an infected and non-infected animal. Image A shows the brain of a control rat without signs of vascular leakage. Image B shows a rat brain 8 weeks after infection, showing significant leakage of Evans blue dye.
Figure 5.
Photographs of the encephalic vasculature of an infected and non-infected animal. Image A shows the brain of a control rat without signs of vascular leakage. Image B shows a rat brain 8 weeks after infection, showing significant leakage of Evans blue dye.
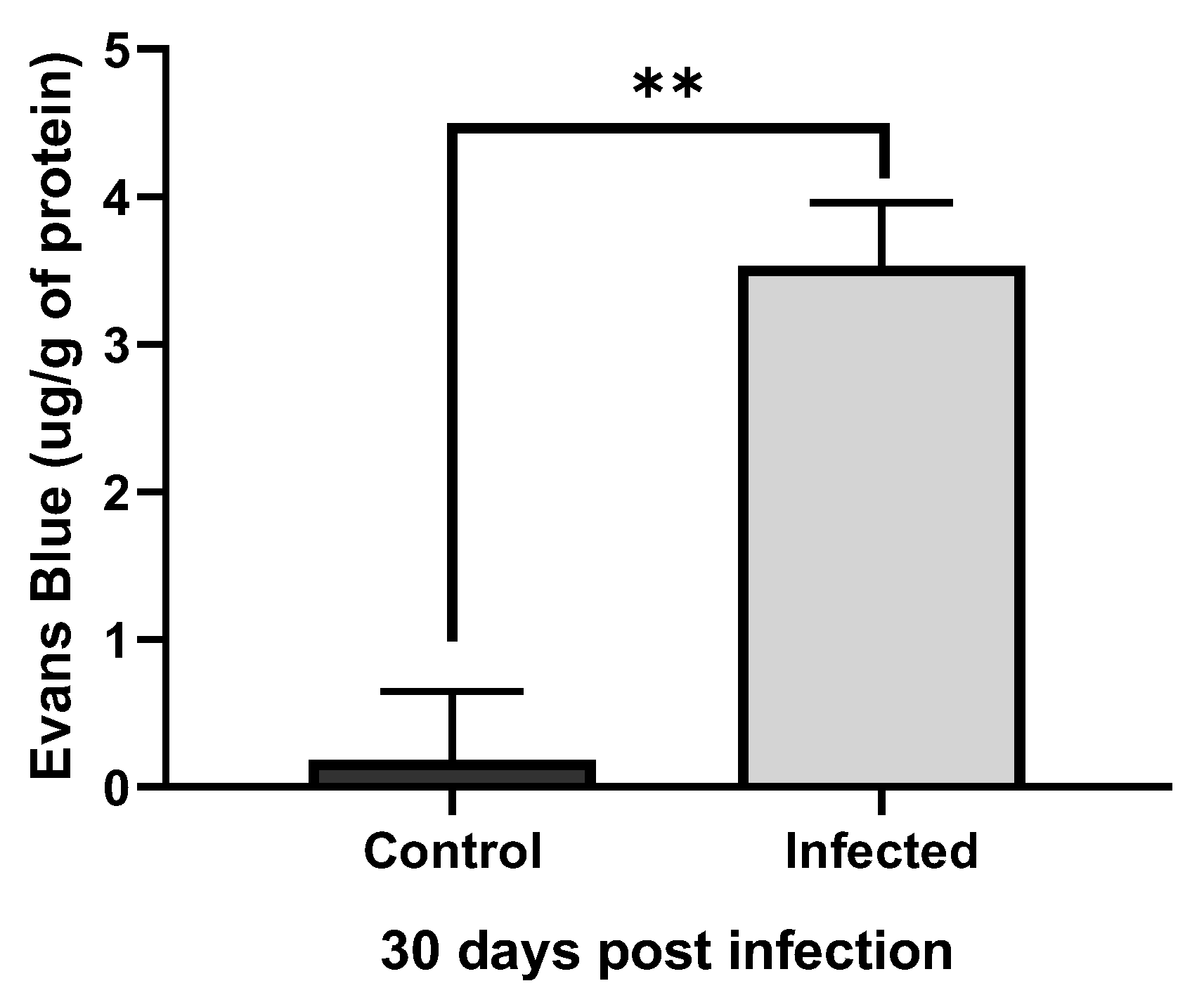
Figure 6.
Graph with vascular extravasation results from control and infected animals. Comparison of the mean quantification of extravasated dye in the brain of control rats and rats infected by T4r shows a significant difference (p˂ 0.01).
Figure 6.
Graph with vascular extravasation results from control and infected animals. Comparison of the mean quantification of extravasated dye in the brain of control rats and rats infected by T4r shows a significant difference (p˂ 0.01).
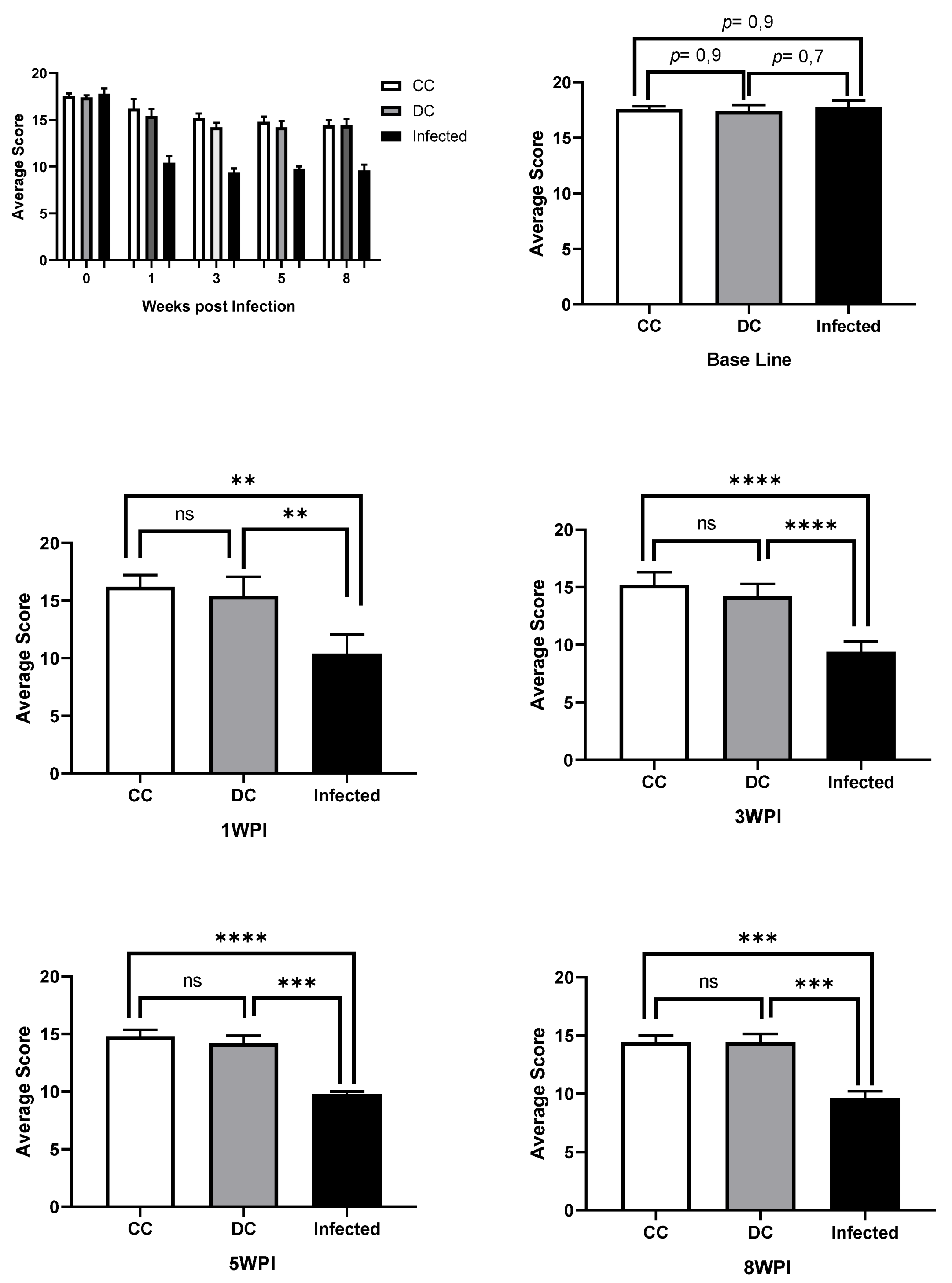
Figure 7.
Image with comparative graphs of the results of the RMCBS behavioral test in control and infected rats. (CC): Clean control rats; (DC): Control rats with application of dexamethasone and (Infected) rats infected with T4r. There was no statistical difference between any of the 3 groups at baseline (week 0). There was no statistical difference between CC and DC in any of the tests. There was a statistical difference between control animals (CC and DC) and infected animals in all post-infection tests, with the smallest difference in the 1WPI (p˂ 0.01) and the largest in the 3WPI (p˂ 0.0001). WPI (week post infection).
Figure 7.
Image with comparative graphs of the results of the RMCBS behavioral test in control and infected rats. (CC): Clean control rats; (DC): Control rats with application of dexamethasone and (Infected) rats infected with T4r. There was no statistical difference between any of the 3 groups at baseline (week 0). There was no statistical difference between CC and DC in any of the tests. There was a statistical difference between control animals (CC and DC) and infected animals in all post-infection tests, with the smallest difference in the 1WPI (p˂ 0.01) and the largest in the 3WPI (p˂ 0.0001). WPI (week post infection).
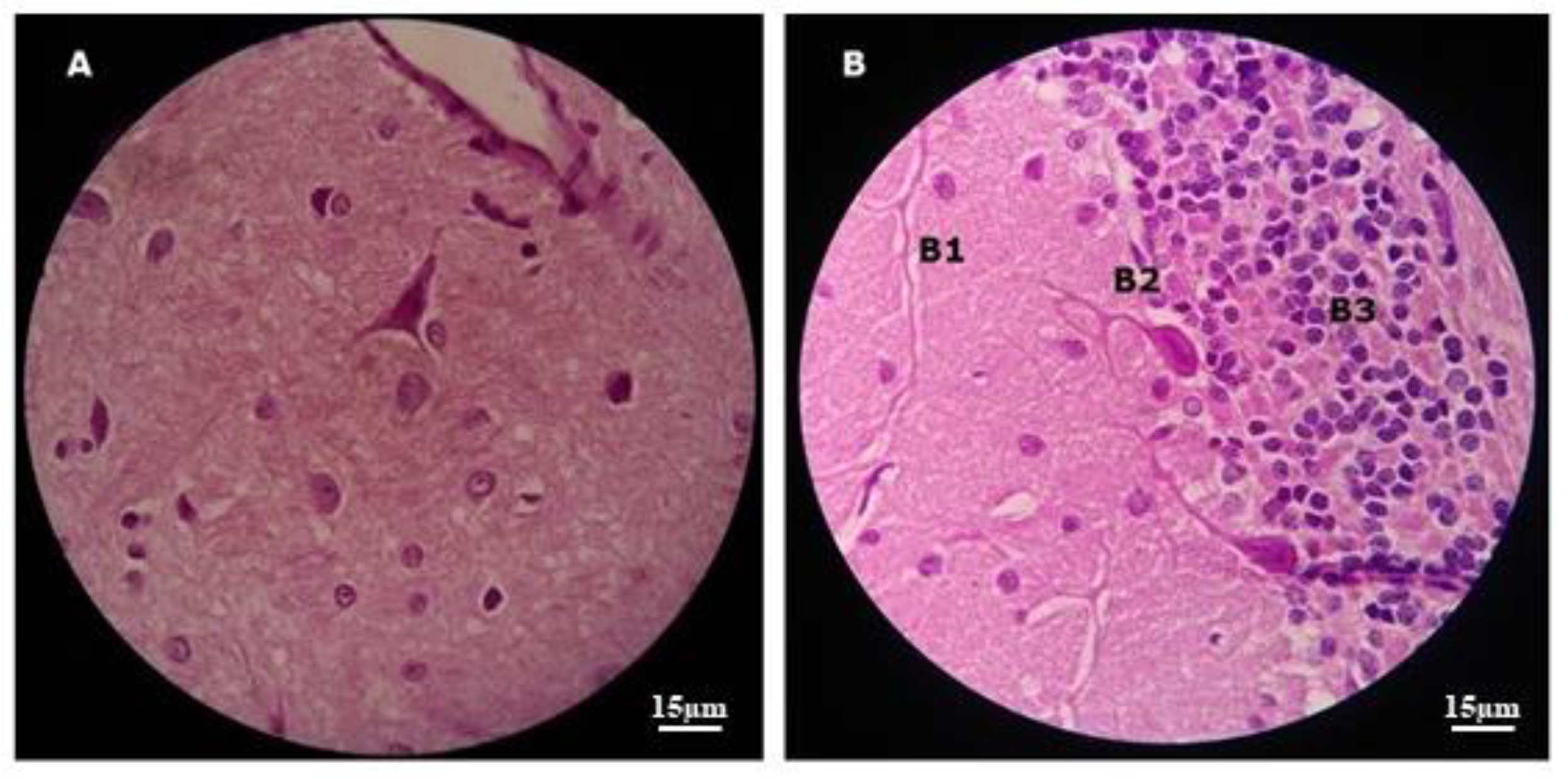
Figure 8.
Images with histopathological sections of the brain of a control animal after H/E staining: In (A) we can see the cerebral cortex with preserved tissue architecture; In (B) there is the cerebellum with tissue preservation in the molecular (B1), Purkinje (B2) and granulosa (B3) layers. Original 1000x increase in MO.
Figure 8.
Images with histopathological sections of the brain of a control animal after H/E staining: In (A) we can see the cerebral cortex with preserved tissue architecture; In (B) there is the cerebellum with tissue preservation in the molecular (B1), Purkinje (B2) and granulosa (B3) layers. Original 1000x increase in MO.
Figure 9.
Scheme of a Neurovascular unit showing the elements of the blood-brain barrier and the passageways of Acanthamoeba (Act) to the brain parenchyma: 1- Paracellular Way: Tight junction elements such as Occludins and ZO-1 are degraded through Acanthamoeba proteases , in a contact-independent mechanism. (Note Serine proteases, metalloproteases and Ecto-ATPases facilitate transmigration and passage to deeper regions of the brain. 2- Transcellular pathway: MBP binds to BMEC receptors, altering its cycle and causing its death, in a contact-dependent mechanism.
Figure 9.
Scheme of a Neurovascular unit showing the elements of the blood-brain barrier and the passageways of Acanthamoeba (Act) to the brain parenchyma: 1- Paracellular Way: Tight junction elements such as Occludins and ZO-1 are degraded through Acanthamoeba proteases , in a contact-independent mechanism. (Note Serine proteases, metalloproteases and Ecto-ATPases facilitate transmigration and passage to deeper regions of the brain. 2- Transcellular pathway: MBP binds to BMEC receptors, altering its cycle and causing its death, in a contact-dependent mechanism.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).