Submitted:
15 May 2024
Posted:
16 May 2024
You are already at the latest version
Abstract
Keywords:
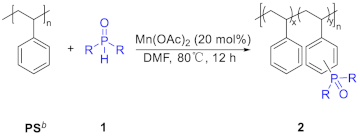
1. Introduction
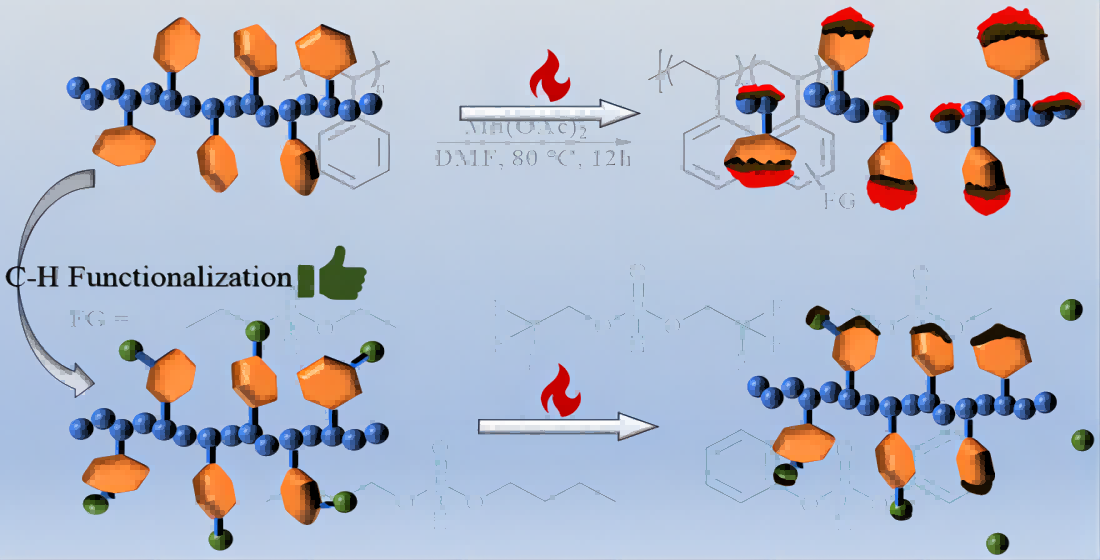
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. General Procedure for the Phosphorylation
3.3. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- (a) Maul, J.; Frushour, B. G.; Kontoff, J. R.; Eichenauer, H.; Ott, K-H.; Schade, C. Polystyrene and Styrene Copolymers. Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH 2007, 29, 475-522. (b) Geyer, R.; Jambeck, J. R.; Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. (c) Tullo, A. Polystyrene marks a milestone; parting with old annual reports and product brochures. C&EN Global Enterprise. 2021, 99(23), 32. (d) Ghoshal, T.; Parmar, P. R.; Bhuyan, T.; Bandyopadhyay, D. Polystyrene Foams: Materials, Technology, and Applications. ACS Symp. Ser. 2023, 1439, 121-141.
- (a) Levchik, S. V.; Weil, E. D. New developments in flame retardancy of styrene thermoplastics and foams. Polym. Int. 2008, 57, 431-448. (b) Wang, Y.; Zhang, J. Thermal stabilities of drops of burning thermoplastics under the UL 94 vertical test conditions. J. Hazard. Mater. 2013, 246-247, 103-109. (c) Sun, P.; Huang, X.; Xu, C. Flashpoint and burning of thin molten plastic pool above hot boundary. Appl. Therm. Eng. 2022, 215, 118931.
- (a) Rani, M.; Shim, W. J.; Han, G. M.; Jang, M.; Song, Y. K.; Hong, S. H. Hexabromocyclododecane in polystyrene based consumer products: An evidence of unregulated use. Chemosphere 2014, 110, 111-119. (b) Beach, M. W.; Kearns, K. L.; Davis, J. W.; Stutzman, J. R.; Lee, D.; Lai, Y.; Monaenkova, D.; Kram, S.; Hu, J.; Lukas, C. Stability Assessment of a Polymeric Brominated Flame Retardant in Polystyrene Foams under Application-Relevant Conditions. Environ. Sci. Technol. 2021, 55, 3050-3058.
- (a) Ezechiáš, M.; Covino, S.; Cajthaml, T. Ecotoxicity and biodegradability of new brominated flame retardants: A review. Ecotoxicol. Environ. Saf. 2014, 110, 153-167. (b) Guo, L-C.; Lv, Z.; Zhu, T.; He, G.; Hu, J.; Xiao, J.; Liu, T.; Yu, S.; Zhang, J.; Zhang, H.; Ma, W. Associations between serum polychlorinated biphenyls, halogen flame retardants, and renal function indexes in residents of an e-waste recycling area. Sci. Total Environ. 2023, 858, 159746. (c) Wang, N.; Lai, C.; Xu, F.; Huang, D.; Zhang, M.; Zhou, X.; Xu, M.; Li, Y.; Liu, S.; Huang, X.; Nie, J.; Li, H. A review of polybrominated diphenyl ethers and novel brominated flame retardants in Chinese aquatic environment: Source, occurrence, distribution, and ecological risk assessment. Sci. Total Environ. 2023, 904, 166180.
- (a) Veen, I.; Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119-1153. (b) Velencoso, M. M.; Battig, A.; Markwart, J. C.; Schartel, B.; Wurm, F. R. Molecular Firefighting-How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450-10467. (c) Zhang, C.; Jiang,Y.; Li, S.; Huang, Z.; Zhan, X-Q.; Ma, N.; Tsai, F-C. Recent trends of phosphorus-containing flame retardants modified polypropylene composites processing. Heliyon 2022, 8, e11225.
- (a) Yan, Y-W.; Huang, J-Q.; Guan, Y-H.; Shang, K.; Jian, R-K.; Wang, Y-Z. Flame retardance and thermal degradation mechanism of polystyrene modified with aluminum hypophosphite. Polym. Degrad. Stab. 2014, 99, 35-42. (b) Qian, X.; Zheng, K.; Lu, L.; Wang, X.; Wang, H. A novel flame retardant containing calixarene and DOPO structures: Preparation and its application on the fire safety of polystyrene. Polym. Adv. Technol. 2018, 29, 2715-2723. (c) Wang, X.; Tu, H.; Xiao, H.; Lu, J.; Xu, J.; Gu, G. A novel halogen-free flame-retardant fabrication for the study of smoke suppression and flame retardancy of polystyrene. Polymer 2023, 283, 126240.
- (a) Dumitrascu, A.; Howell, B. A.; Flame-retarding vinyl polymers using phosphorus-functionalized styrene monomers. Polym. Degrad. Stab. 2011, 96, 342-349. (b) Tai,Q.; Song, L.; Hu, Y.; Yuen, R. K. K.; Feng, H.; Tao, Y. Novel styrene polymers functionalized with phosphorus–nitrogen containing molecules: Synthesis and properties. Mater. Chem. Phys. 2012, 134, 163-169. (c) Sun, J.; Wang, C.; Hong, Y-L.; Tan, Z-W.; Liu, C-M. Phosphine Oxide-Containing Multifunctional Polymer via RAFT Polymerization and Its High-Density Post-Polymerization Modification in Water. ACS Appl. Polym. Mater. 2021, 3, 3214-3226. (d) Baby, A.; Tretsiakova-McNally, S.; Joseph, P.; Arun, M.; Zhang, J.; Pospiech, D. The influence of phosphorus- and nitrogen- containing groups on the thermal stability and combustion characteristics of styrenic polymers. J. Therm. Anal. Calorim. 2023, 148, 229-241.
- Thomas, G. L.; Böhner C.; Ladlow, M.; Spring, D. R. Synthesis and utilization of functionalized polystyrene resins. Tetrahedron 2005, 61, 12153-12159. [CrossRef]
- (a) Gauthier, M. A.; Gibson, M. I.; Klok, H-A. Synthesis of Functional Polymers by Post-Polymerization Modification. Angew. Chem. Int. Ed. 2009, 48, 48-58. (b) Günay, K. A.; Theato, P.; Klok, H-A. Standing on the Shoulders of Hermann Staudinger: Post-polymerization Modification from Past to Present. J. Polym. Sci. A Polym. Chem. 2013, 51, 1-28. (c) Blasco, E.; Sims, M. B.; Goldmann, A. S.; Sumerlin, B. S.; Barner-Kowollik, C. 50th Anniversary Perspective: Polymer Functionalization. Macromolecules 2017, 50, 5215-5252. (d) Ohn, N.; Kim, J. G.; Mechanochemical Post-Polymerization Modification: Solvent-Free Solid-State Synthesis of Functional Polymers. ACS Macro Lett. 2018, 7, 561-565.
- (a) Williamson, J. B.; Lewis, S. E.; Johnson Ⅲ, R. R.; Manning, I. M.; Leibfarth, F. A. C-H Functionalization of Commodity Polymers. Angew. Chem. Int. Ed. 2019, 58, 8654-8668. (b) Zhao, Y.; Li, D.; Jiang X. Chemical Upcycling of Polyolefins through C-H Functionalization. Eur. J. Org. Chem. 2023, 26, e202300664 (1 of 15).
- (a) Isono, T.; Baba, E.; Tanaka, S.; Miyagi, K.; Dazai, T.; Li, F.; Yamamoto, T.; Tajima, K.; Satoh, T. Installation of the adamantyl group in polystyrene-block-poly(methyl methacrylate) via Friedel-Crafts alkylation to modulate the microphase-separated morphology and dimensions. Polym. Chem. 2023, 14, 2675-2684. Zhang, Z.; Zhang, Y.; Zeng, R.; Photoinduced iron-catalyzed C-H alkylation of polyolefins. Chem. Sci. 2023, 14, 9374-9379. (b)Tizpar, S.; Abbasian, M.; Taromi, F. A.; Entezami, A. A. Grafting of Poly(methyl methacrylate) or Polyacrylonitrile onto Polystyrene Using ATRP Technique. J. Appl. Polym. Sci. 2006, 100, 2619-2627. (c) Niu, M.; Li, T.; Xu, R.; Gu, X.; Yu, D.; Wu, Y. Synthesis of PS-g-POSS Hybrid Graft Copolymer by Click Coupling via “ Graft Onto” Strategy. J. Appl. Polym. Sci. 2013, 129, 1833-1844. (d) Lewis, S. E.; Jr, W. B. E.; Leibfarth, F. A. Upcycling aromatic polymers through C-H fluoroalkylation. Chem. Sci. 2019, 10, 6270-6277. (e) Chalk, A. J. Abnormal Reactions of Polystyrene Metalated with Butyllithium-N, N, N’, N’ -tetramethylethylenediamine. J. Polym. Sci. B: Polym. Lett. 1968, 6, 649-651. (f) Lochmann, L.; Fréchet, J. M. J. Controlled Functionalization of Polystyrene: Introduction of Reactive Groups by Multisite Metalation with Superbase and Reaction with Electrophiles. Macromolecules 1996, 29, 1767-1771. (g) Fahs, G. B.; Benson, S. D.; Moore, R. B. Blocky Sulfonation of Syndiotactic Polystyrene: A Facile Route toward Tailored Ionomer Architecture via Postpolymerization Functionalization in the Gel State. Macromolecules 2017, 50, 2387-2396. Si, J.; Hao, N.; Zhang, M.; Cheng, S.; Liu, A.; Li, L.; Ye, X. Universal Synthetic Strategy for the Construction of Topological Polystyrenesulfonates: The Importance of Linkage Stability during Sulfonation. ACS Macro Lett. 2019, 8, 730-736. (h) Xue, B.; Huang, P-P.; Zhu, M-Z.; Fu, S-Q.; Ge, J-H.; Li, X.; Liu, P-N. Highly Efficient and para-Selective C-H Functionalization of Polystyrene Providing a Versatile Platform for Diverse Applications. ACS Macro Lett. 2022, 11, 1252-1257.
- Shin, J.; Jensen, S. M.; Ju, J.; Lee, S.; Xue, Z.; Noh, S. K.; Bea, C. Controlled Functionalization of Crystalline Polystyrenes via Activation of Aromatic C-H Bonds. Macromolecules 2007, 40, 8600-8608.
- King, E. R.; Hunt, S. B.; Hamernik, L. J.; Gonce, L. E.; Wiggins, J. S.; Azoulay, J. D. Gold-Catalyzed Post-Polymerization Modification of Commodity Aromatic Polymers. JACS Au 2021, 1, 1342-1347.
- Dou, B.; Xu, Y.; Wang, J. Gold-Catalyzed Precise Bromination of Polystyrene. J. Am. Chem. Soc. 2023, 145, 10422-10430.
- Xu, W.; Zou, J-P.; Zhang, W. Manganese(III)-mediated direct phosphonylation of arenes. Tetrahedron Lett. 2010, 51, 2639-2643.
- (a) Moulay, S. Functionalized Polystyrene and Polystyrene-Containing Material Platforms for Various Applications. Polym. Plast. Technol. Eng. 2018, 57, 1045-1092. (b) McCoy, M.; Recycling polystyrene. C&EN 2018, 96, 13. (c) Billiet, S.; Trenor, S. R. 100th Anniversary of Macromolecular Science Viewpoint: Needs for Plastics Packaging Circularity. ACS Macro Lett. 2020, 9, 1376-1390. (d) Jehanno, C.; Alty, J. W.; Roosen, M.; Meester, S. D.; Dove, A. P.; Chen, E. Y.-X.; Leibfarth, F. A.; Sardon, H. Critical advances and future opportunities in upcycling commodity polymers. Nature 2022, 603, 803-814. (e) Goring, P. D.; Priestley, R. D. Polymer Recycling and Upcycling: Recent Developments toward a Circular Economy. JACS Au 2023, 3, 2609-2611.
- (a) G. Keglevich, E. Jablonkai and L. B. Bal’azs, A “green” variation of the Hirao reaction: the P–C coupling of diethyl phosphite, alkyl phenyl-Hphosphinates and secondary phosphine oxides with bromoarenes using a P-ligand-free Pd(OAc)2 catalyst under microwave and solvent-free conditions RSC Adv. 2014, 4, 22808-22816. (b) Q. Tai, L. Songa, Y. Hua, R. K.K. Yuen, H. Feng and Y. Tao, Novel styrene polymers functionalized with phosphorus–nitrogen containing molecules: Synthesis and properties: Mater. Chem. Phys. 2012, 134, 163-169.
- Shapiro, A, J.; O’Dea, R. M.; Epps, T. H. III. Thermogravimetric Analysis as a High-Throughput Lignocellulosic Biomass Characterization Method. ACS Sustainable Chem. Eng. 2023, 11, 17216-17223.
- (a) Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H.; Lv, P.; Jie, G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer 2010, 51, 2435-2445. (b) Blake, N.; Turner, Z. R.; Buffet, J-C.; O’Hare, D. Flame retardant phosphonate-functionalised polyethylenes. Polym. Chem. 2023, 14, 3175-3185.
- Yang, B.; Wang, L.; Guo, Y.; Zhang, Y.; Wang, N.; Cui, J.; Tian, L. Synthesis of a novel phosphate-containing highly transparent PMMA copolymer with enhanced thermal and flame retardant properties. Polym. Adv. Technol. 2020, 31, 472-481. [CrossRef]
- (a) Wilkie, C. A.; Chigwada, G.; Gilman, Sr. J. W.; Lyon, R. E. High-throughput techniques for the evaluation of fire retardancy. J. Mater. Chem. 2006, 16, 2023-2030. (b) Lyon, R. E.; Walters, R. N.; Stoliarov, S. I. Screening Flame Retardants for Plastics Using Microscale Combustion Calorimetry. Polym. Eng. Sci. 2007, 47, 1501-1510. (c) Price, D.; Cunliffe, L. K.; Bullett, K. J.; Hull, T. R.; Milnes, G. J.; Ebdon, J. R.; Hunt, B. J.; Joseph, P. Thermal behaviour of covalently bonded phosphate and phosphonate flame retardant polystyrene systems. Polym. Degrad. Stab. 2007, 92, 1101-1114.
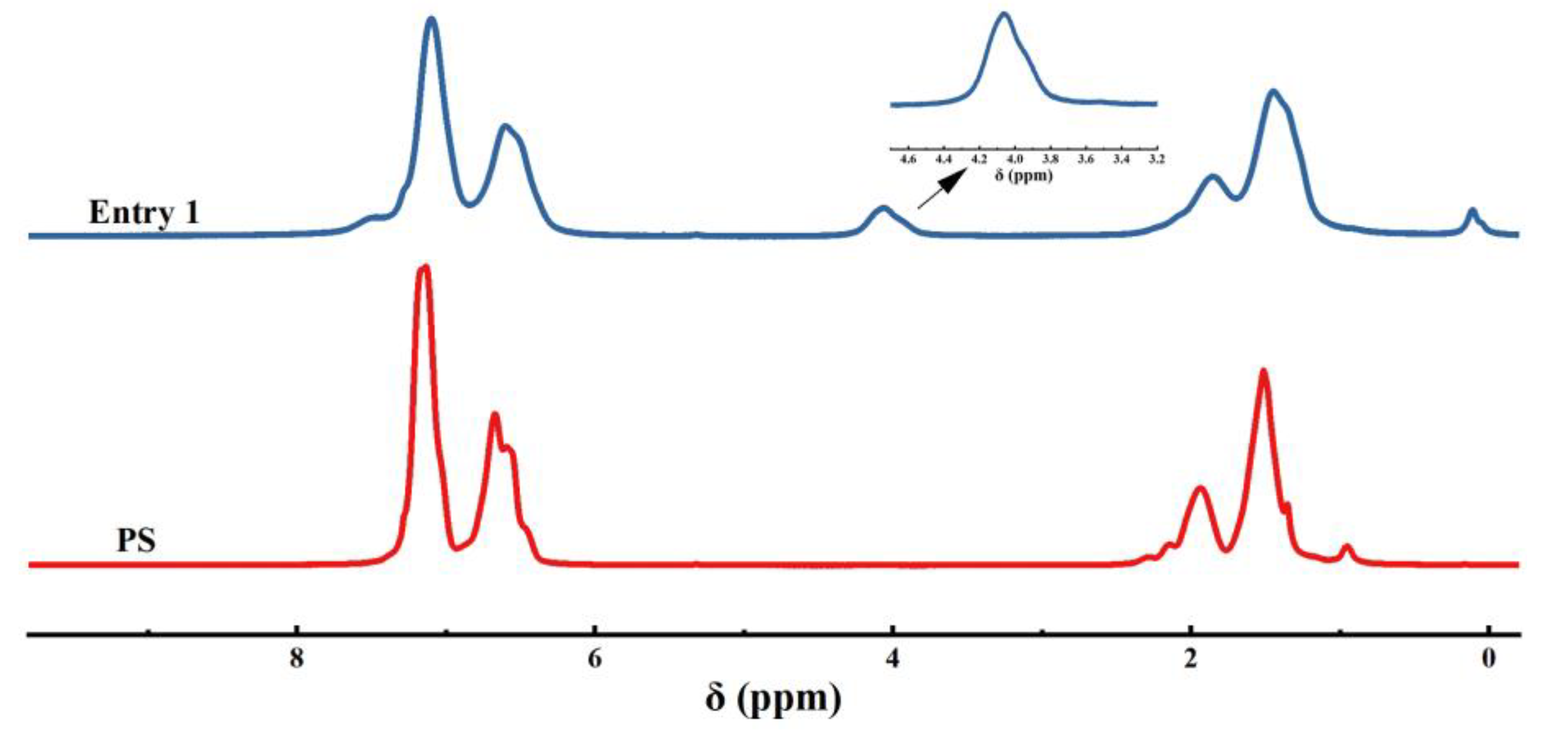
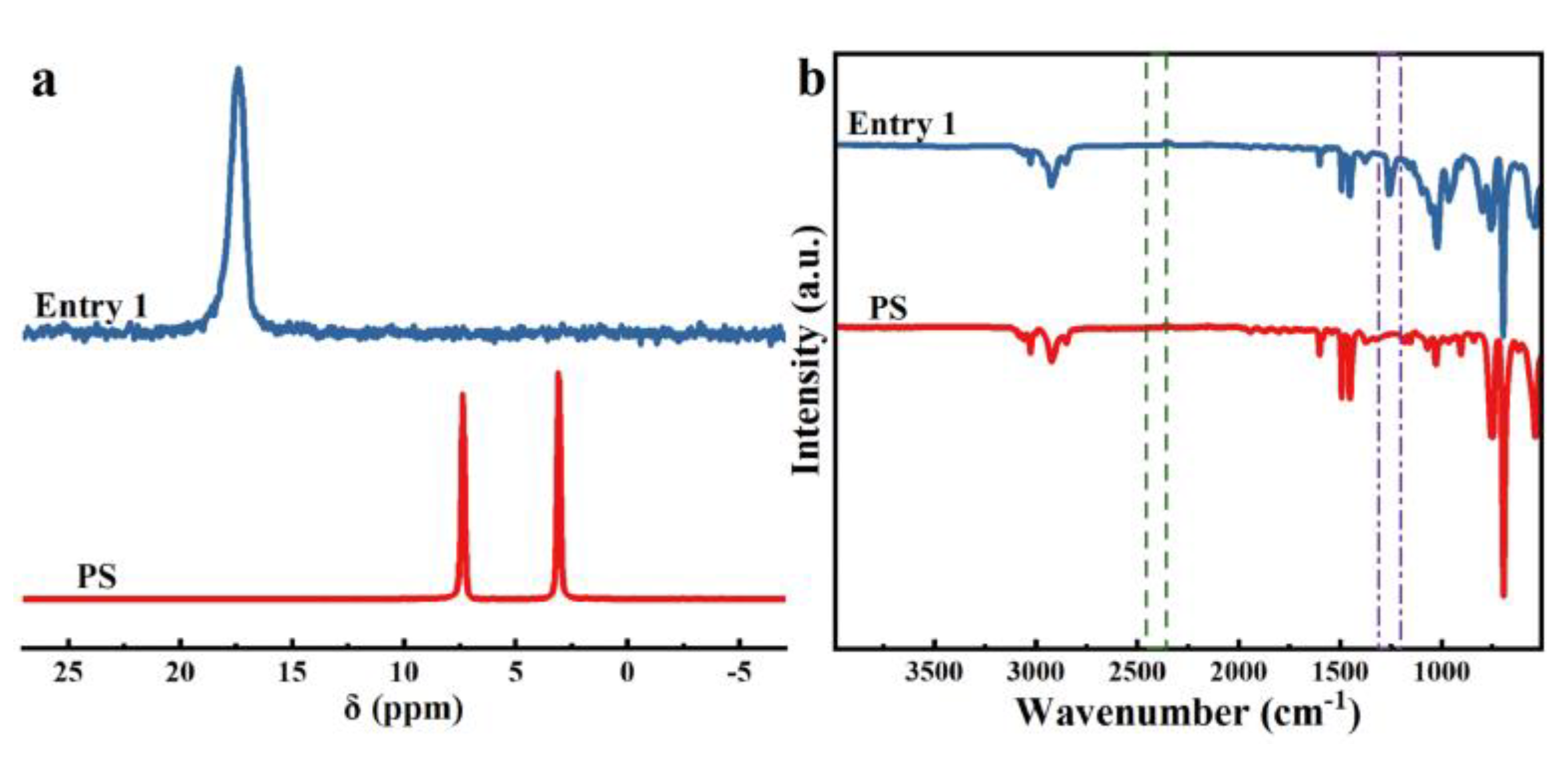
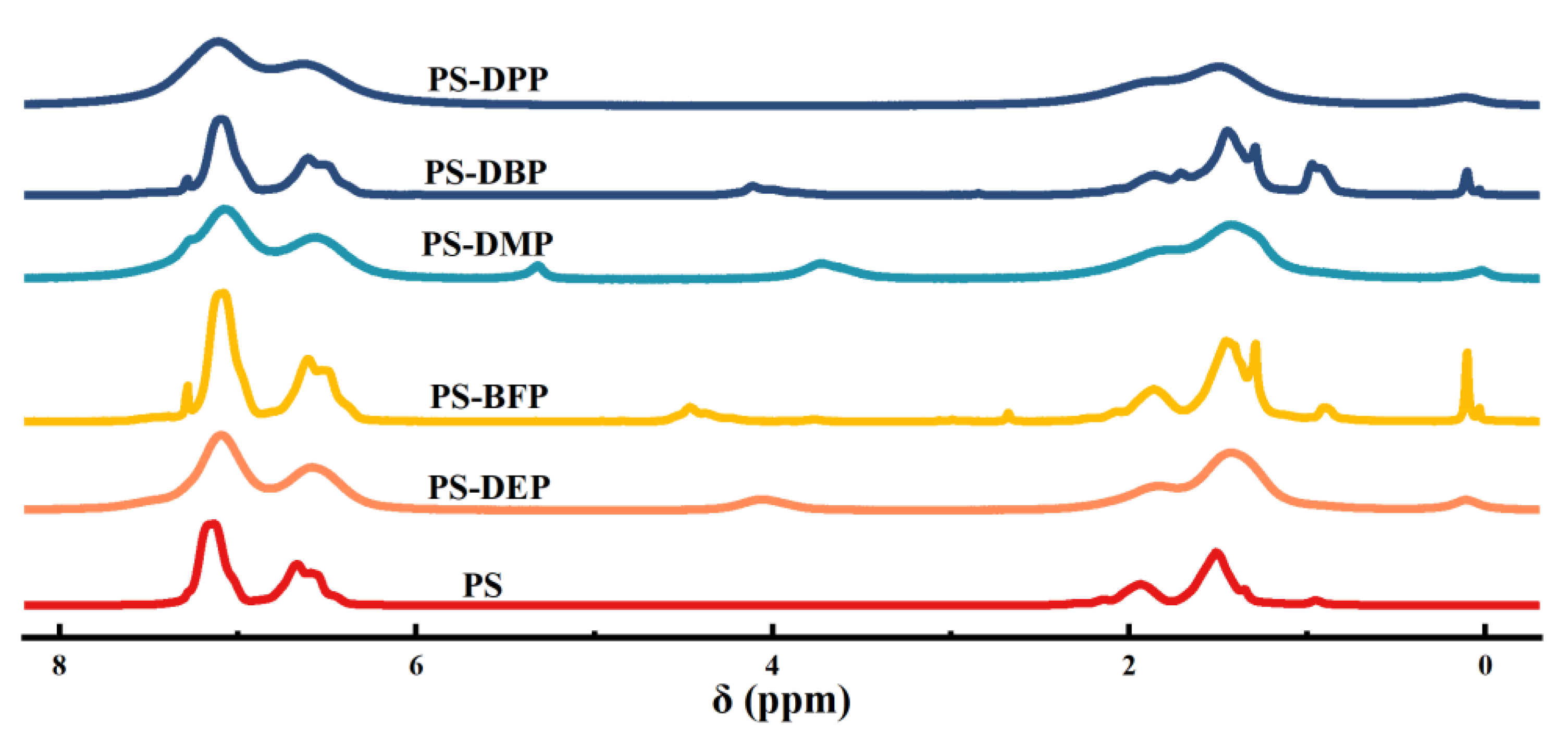
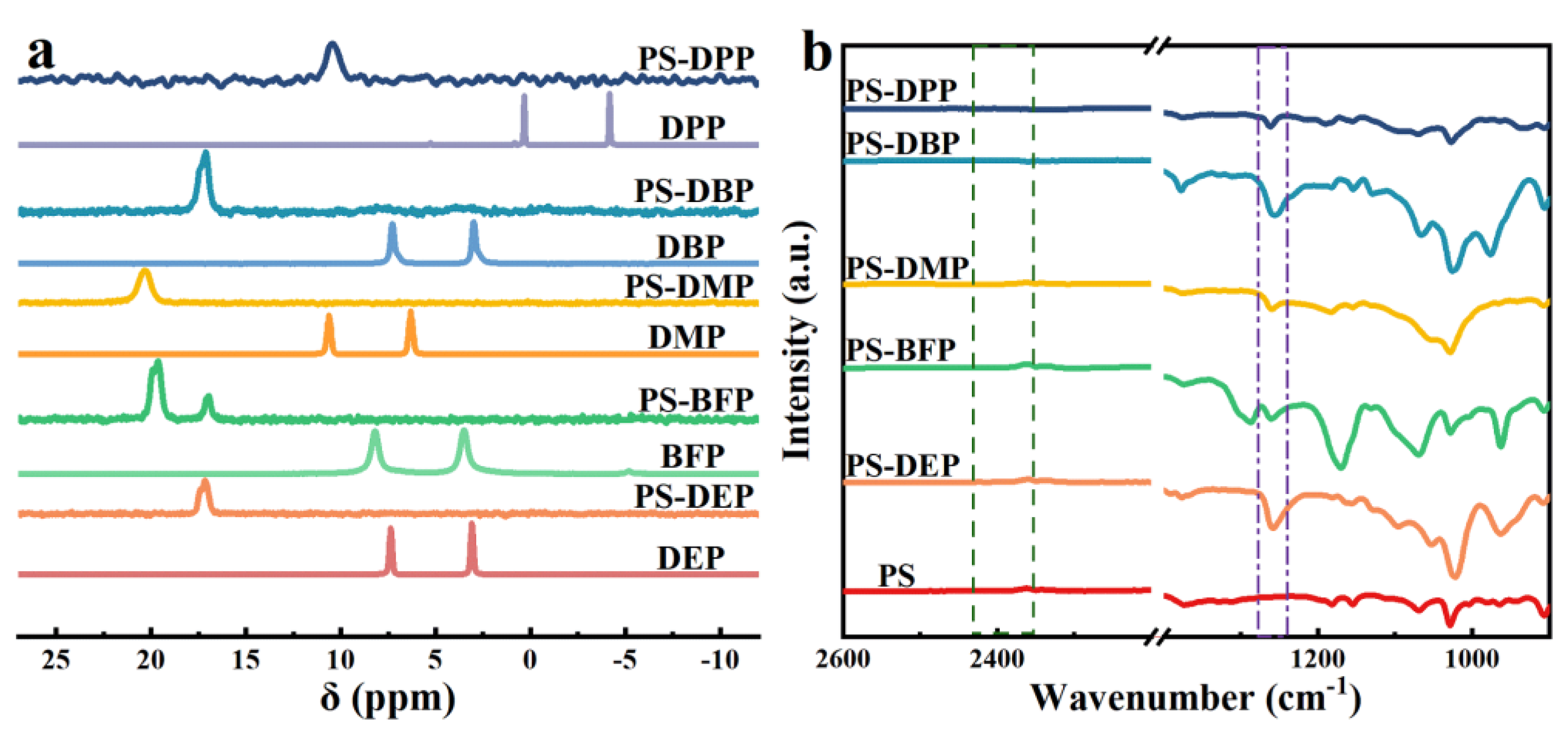
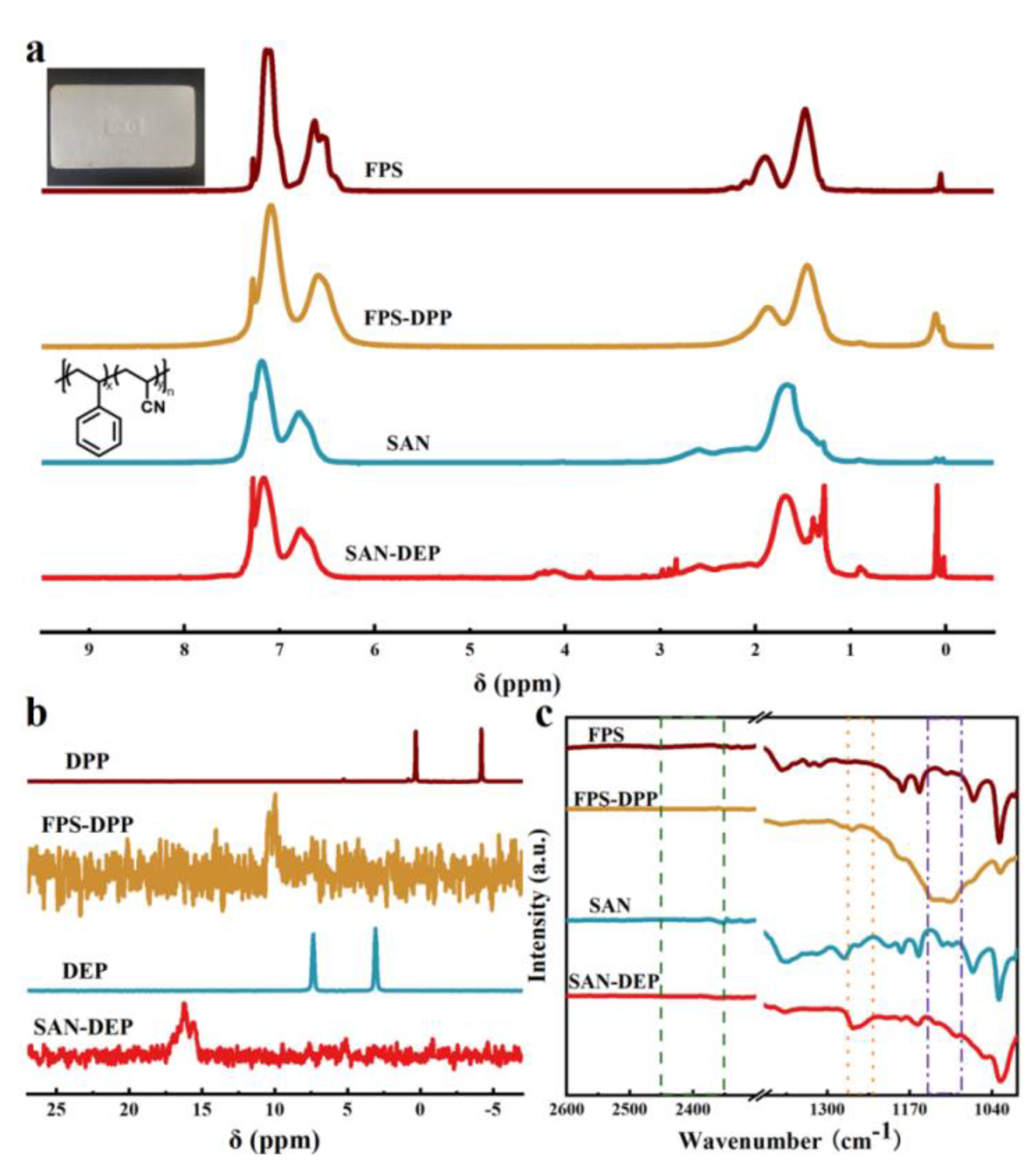
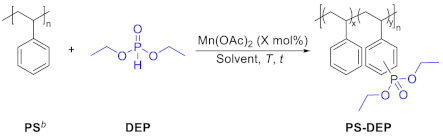
| PS-DEP c | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Solvent | [DEP]/[PS] | [Mn(OAc)2] |
T (℃) |
t (h) |
Mn (kg·mol-1) |
Ð | Functionalization d (%) |
| 1 | DMF | 1 | 0.2 | 80 | 12 | 135.0 | 1.88 | 10 |
| 2 | DMF | 0.75 | 0.2 | 80 | 12 | 156.2 | 1.91 | 5 |
| 3 | DMF | 2 | 0.2 | 80 | 12 | 147.7 | 1.99 | 6.75 |
| 4 | DMF | 3 | 0.2 | 80 | 12 | - | - | 0 |
| 5 | DMF | 1 | 0.4 | 80 | 12 | 142.8 | 1.86 | 7.75 |
| 6 | DMF | 1 | 1 | 80 | 12 | - | - | 1 |
| 7 | DMF | 2 | 1 | 80 | 12 | 146.0 | 1.66 | 6 |
| 8 | DMF | 2 | 2 | 80 | 12 | - | - | 0 |
| 9 | DMF | 2 | 3 | 80 | 12 | - | - | 0 |
| 10 | DMF | 1 | 0.2 | 60 | 12 | - | - | 0 |
| 11 | DMF | 1 | 0.2 | 90 | 12 | 152.0 | 1.88 | 4.25 |
| 12 | THF | 1 | 0.2 | 80 | 12 | 192.2 | 1.74 | 1 |
| 13 | toluene | 1 | 0.2 | 80 | 12 | 23.8 | 3.73 | 2.25 |
| 14 | DCE | 1 | 0.2 | 80 | 12 | - | - | 0 |
| 15 | HOAc | 1 | 0.2 | 80 | 12 | - | - | 0 |
| 16 | DMF: HOAc = 10: 1 | 1 | 0.2 | 80 | 12 | 92.9 | 2.09 | 13 |
| 17e | DMF | 1 | 0.2 | 80 | 12 | - | - | 0 |
| 18 f | DMF | 1 | 0.2 | 80 | 12 | - | - | 0 |
| 2 c | Nitrogen atmosphere e | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | 1 | 2 |
Mn (kg·mol-1) |
Ð | Functionalization d (%) |
Td, 5% (℃) |
Td, 50% (℃) |
Td ,max (℃) |
Residue (%) |
| 1 | - | PS | - | - | - | 377.5 | 418.5 | 424 | 2.48 |
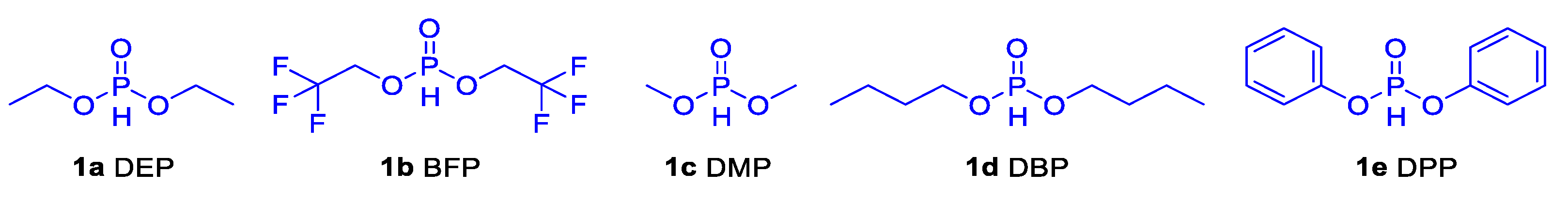
| 2 | DEP | PS-DEP | 134915 | 1.88 | 10 | 282.8 | 444.7 | 354, 463 | 10.36 |
| 3 | BFP | PS-BFP | 133.4 | 2.00 | 8.5 | n.d | n.d | n.d | n.d |
| 4 | DMP | PS-DMP | 140.8 | 1.97 | 8.3 | 131 | 424.9 | 426 | 12.15 |
| 5 | DBP | PS-DBP | 139.9 | 1.74 | 11 | 245.9 | 430.3 | 440 | 2.57 |
| 6 | DPP | PS-DPP | 176.2 | 1.74 | 13.6 | 395 | 424.5 | 424 | 14.44 |
| Sample | HRC (J/g·K) |
pHRR (W/g) |
THR (kJ/g) |
TpHRR (℃) |
|---|---|---|---|---|
| PS | 1043.4 | 1051.1 | 46.5 | 433.5 |
| PS-BFP | 536.7 | 539.2 | 35.9 | 458.1 |
| PS-DMP | 596.5 | 600.4 | 40.5 | 437.8 |
| PS-DPP | 642.3 | 647.1 | 40.6 | 434.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





