Submitted:
15 May 2024
Posted:
16 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction.
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and CMV DNA Detection
2.3. Management of cCMV-Infected Newborns
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
| Maternal characteristics (n=904) | |
| Chronic diseases | 169 (18.7) |
| Pregnancy-related pathological conditions | 482 (53.4) |
| Multiple pregnancy | 78 (8.6) |
| Maternal CMV infection in pregnancy | 42 (4.6) |
| Vaginal birth | 660 (70) |
|
Neonatal characteristics (n=940) | |
| Male gender | 468 (49.8) |
| Term gestation (>37 wks) | 699 (74.4) |
| Birthweight (grams) | 2495.2±600.7 |
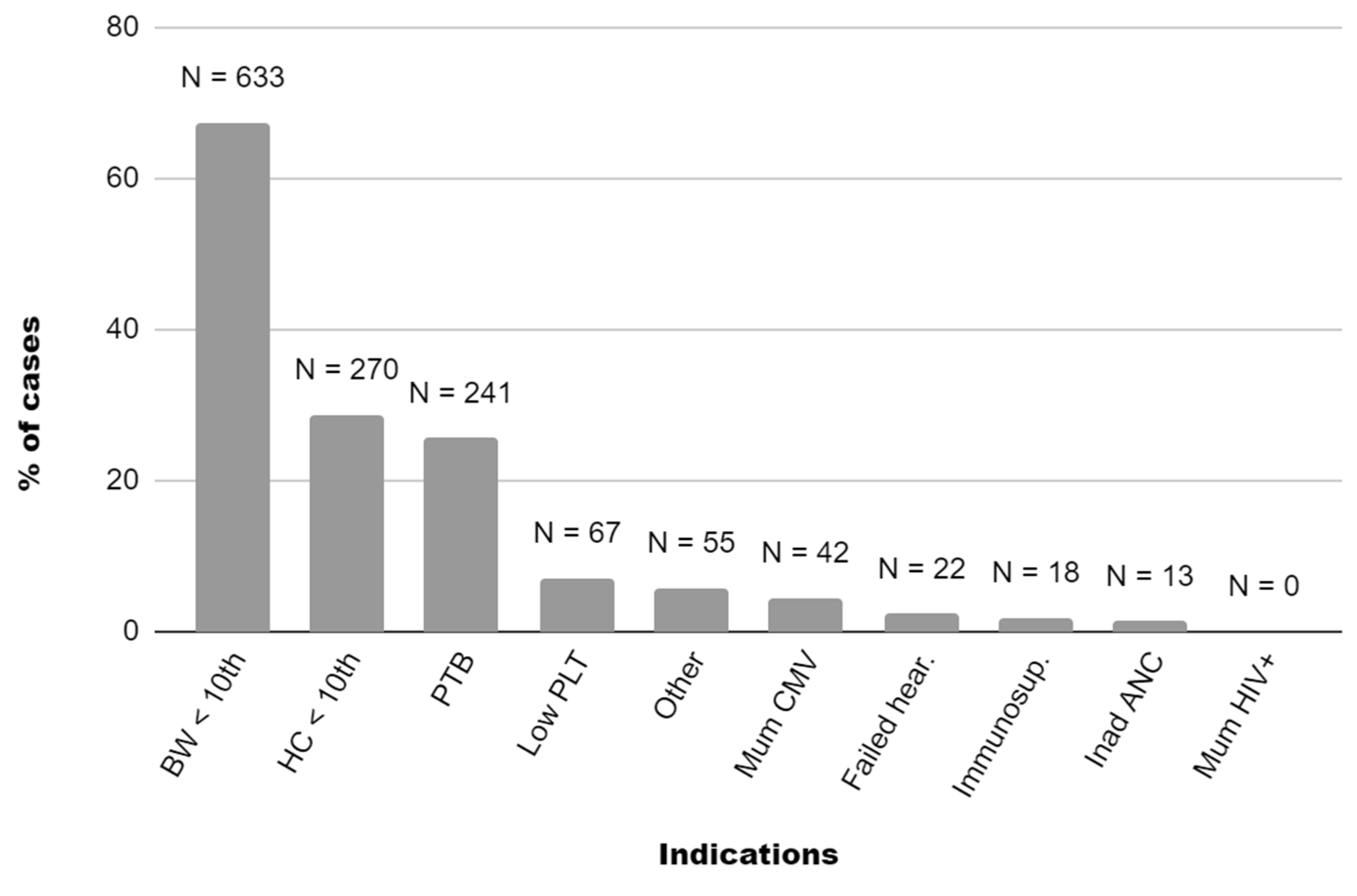
Birthweight centile
|
17.2±22.8 633 (67.3) 12 (1.3) |
Head circumference centile
|
31.2±26.6 270 (28.7) 32 (3.4) |
4. Discussion
5. Conclusions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cannon MJ, Schmid DS, Hyde TB: Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010;20(4): 202-213. [CrossRef]
- Kenneson A, Cannon MJ: Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007;17(4): 253-276.
- Gandhi MK, Khanna R: Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis 2004;4(12): 725-738. [CrossRef]
- Dollard SC, Grosse SD, Ross DS: New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007;17(5): 355-363.
- Grosse SD, Leung J, Lanzieri TM: Identification of congenital CMV cases in administrative databases and implications for monitoring prevalence, healthcare utilization, and costs. Curr Med Res Opin 2021;37(5): 769-779.
- Sorichetti B, Goshen O, Pauwels J, Kozak FK, Tilley P, Krajden M, et al.: Symptomatic Congenital Cytomegalovirus Infection Is Underdiagnosed in British Columbia. J Pediatr 2016;169: 316-317.
- Grosse SD, Ross DS, Dollard SC: Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J Clin Virol 2008;41(2): 57-62.
- Lanzieri TM, Chung W, Flores M, Blum P, Caviness AC, Bialek SR, et al.: Hearing Loss in Children With Asymptomatic Congenital Cytomegalovirus Infection. Pediatrics 2017;139(3).
- Fowler KB, Dahle AJ, Boppana SB, Pass RF: Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr 1999;135(1): 60-64.
- Fowler KB, Boppana SB: Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol 2006;35(2): 226-231.
- Ross S, Long SS, Kimberlin DW: Closer to Universal Newborn Screening for Congenital Cytomegalovirus Infection but Far Away from Antiviral Therapy in All Infected Infants. J Pediatr 2018;199: 7-9.
- Gantt S, Dionne F, Kozak FK, Goshen O, Goldfarb DM, Park AH, et al.: Cost-effectiveness of Universal and Targeted Newborn Screening for Congenital Cytomegalovirus Infection. JAMA Pediatr 2016;170(12): 1173-1180.
- Letamendia-Richard E, Périllaud-Dubois C, de La Guillonnière L, Thouard I, Cordier AG, Roque-Afonso AM, et al.: Universal newborn screening for congenital cytomegalovirus infection: feasibility and relevance in a French type-III maternity cohort. Bjog 2022;129(2): 291-299.
- Suarez D, Nielson C, McVicar SB, Sidesinger M, Ostrander B, O’Brien E, et al.: Analysis of an Expanded Targeted Early Cytomegalovirus Testing Program. Otolaryngol Head Neck Surg 2023;169(3): 679-686. [CrossRef]
- Chiereghin A, Pavia C, Turello G, Borgatti EC, Baiesi Pillastrini F, Gabrielli L, et al.: Universal Newborn Screening for Congenital Cytomegalovirus Infection - From Infant to Maternal Infection: A Prospective Multicenter Study. Front Pediatr 2022;10: 909646.
- Gantt S: Newborn cytomegalovirus screening: is this the new standard? Curr Opin Otolaryngol Head Neck Surg 2023;31(6): 382-387. Curr Opin Otolaryngol Head Neck Surg.
- Morando C, Conti G, Bubbico L, Aversa S, Araimo G, Frezza S, et al.: ORGANIZZAZIONE, ESECUZIONE E GESTIONE DELLO SCREENING NEONATALE DELLA SORDITÀ CONGENITA: GUIDA PRATICA. 2020.
- Nance WE, Lim BG, Dodson KM: Importance of congenital cytomegalovirus infections as a cause for pre-lingual hearing loss. J Clin Virol 2006;35(2): 221-225. [CrossRef]
- Berrettini S, Ghirri P, Lazzerini F, Lenzi G, Forli F: Newborn hearing screening protocol in tuscany region. Ital J Pediatr 2017;43(1): 82.
- Ciccia M, Monari C, Vitagliano G, Zarro N, Sandri F: Usefulness of a flow chart for targeted screening of congenital cytomegalovirus-related hearing loss. J Neonatal Perinatal Med 2018;11(3): 339-343.
- Fowler KB, McCollister FP, Sabo DL, Shoup AG, Owen KE, Woodruff JL, et al.: A Targeted Approach for Congenital Cytomegalovirus Screening Within Newborn Hearing Screening. Pediatrics 2017;139(2).
- Minami SB, Yamanobe Y, Nakano A, Sakamoto H, Masuda S, Takiguchi T, et al.: A High Risk of Missing Congenital Cytomegalovirus-Associated Hearing Loss through Newborn Hearing Screening in Japan. J Clin Med 2021;10(21).
- Vancor E, Shapiro ED, Loyal J: Results of a Targeted Screening Program for Congenital Cytomegalovirus Infection in Infants Who Fail Newborn Hearing Screening. J Pediatric Infect Dis Soc 2019;8(1): 55-59.
- van der Weiden S, de Jong EP, Te Pas AB, Middeldorp JM, Vossen AC, Rijken M, et al.: Is routine TORCH screening and urine CMV culture warranted in small for gestational age neonates? Early Hum Dev 2011;87(2): 103-107.
- Zhang Y, Egashira T, Egashira M, Ogiwara S, Tomino H, Shichijo A, et al.: Expanded targeted screening for congenital cytomegalovirus infection. Congenit Anom (Kyoto) 2023;63(3): 79-82.
- Akiva MH, Hyde De Souza H, Lamarre V, Boucoiran I, Gantt S, Renaud C, et al.: Identifying Clinical Criteria for an Expanded Targeted Approach to Screening for Congenital Cytomegalovirus Infection-A Retrospective Study. Int J Neonatal Screen 2023;9(3).
- Masarweh K, Felszer-Fisch C, Shinwell E, Hasanein J, Peniakov M, Weiner SA, et al.: The Yield of Targeted Examination for the Detection of Symptomatic Congenital Cytomegalovirus Infection. Isr Med Assoc J 2021;23(5): 318-322.
- Levit Y, Dym L, Yochpaz S, Manor Y, Adler A, Halutz O, et al.: Assessment of Risk Indicators for Targeted Cytomegalovirus Screening in Neonates. Neonatology 2020;117(6): 750-755.
- McCrary H, Shi K, Newberry I: Outcomes from an expanded targeted early cytomegalovirus testing program. . J Pediatr Infect Dis 2020;15(4): 189-194.
- Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, et al.: Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 2016;48(3): 333-339.
- Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al.: International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014;384(9946): 857-868.
- Istituto Superiore di Sanita’: La gravidanza fisiologica. Antenatal care for uncomplicated pregnancy. Sistema Nazionale Linee Guida (SNLG). 2011.
- Agenzia Italiana del Farmaco (AIFA). Inserimento del medicinale «Valaciclovir» (originatore o biosimilare) nell’elenco dei medicinali erogabili a totale carico del Servizio Sanitario Nazionale, per la prevenzione dell’infezione fetale e il trattamento della malattia fetale da citomegalovirus. In: (AIFA) AIdF, ed. Determina n 142618/2020, 20A07138) Gazzetta Ufficiale - Serie Generale n.322 del 30 dicembre 2020. 2020.
- AMCLI, SIGO, SIMaST, SIMIT, SIN, SIP: Percorsi diagnostico-assistenziali in Ostetricia-Ginecologia e Neonatologia. Citomegalovirus. 2012.
- Luck SE, Wieringa JW, Blázquez-Gamero D, Henneke P, Schuster K, Butler K, et al.: Congenital Cytomegalovirus: A European Expert Consensus Statement on Diagnosis and Management. Pediatr Infect Dis J 2017;36(12): 1205-1213.
- Fernicola F, Carli A, Arienti F, Viola Vasarri M, Lanteri L, Scandella G, et al.: Fatal severe persistent pulmonary hypertension with lung microvasculature parietal hyperplasia in a neonate with congenital cytomegalovirus infection treated in-utero with valacyclovir: A case report. Eur J Obstet Gynecol Reprod Biol 2024;294: 245-246.
- Diener ML, Zick CD, McVicar SB, Boettger J, Park AH: Outcomes From a Hearing-Targeted Cytomegalovirus Screening Program. Pediatrics 2017;139(2).
- Adler SP, Marshall B: Cytomegalovirus infections. Pediatr Rev 2007;28(3): 92-100.
- Istituto Superiore di Sanità: La gravidanza fisiologica, parte I. Antenatal care for uncomplicated pregnancy, part I. Sistema Nazionale Linee Guida (SNLG). 2023.
- Lazzarotto T, Gabrielli L, Lanari M, Guerra B, Bellucci T, Sassi M, et al.: Congenital cytomegalovirus infection: recent advances in the diagnosis of maternal infection. Hum Immunol 2004;65(5): 410-415.
- Dalmartello M, Parazzini F, Pedron M, Pertile R, Collini L, La Vecchia C, et al.: Coverage and outcomes of antenatal tests for infections: a population based survey in the Province of Trento, Italy. J Matern Fetal Neonatal Med 2019;32(12): 2049-2055.
- Maltezou PG, Kourlaba G, Kourkouni Ε, Luck S, Blázquez-Gamero D, Ville Y, et al.: Maternal type of CMV infection and sequelae in infants with congenital CMV: Systematic review and meta-analysis. J Clin Virol 2020;129: 104518.
- Boucoiran I, Yudin M, Poliquin V, Caddy S, Gantt S, Castillo E: Guideline No. 420: Cytomegalovirus Infection in Pregnancy. J Obstet Gynaecol Can 2021;43(7): 893-908.
- Khalil A HP, Jones CE, et al., on behalf of the Royal College of Obstetricians and Gynaecologists: Scientific Impact Paper No. 56: Congenital Cytomegalovirus Infection: Update on Screening, Diagnosis and Treatment (2nd edition). Peer review draft. 2023.
- Kimberlin D, Barnett E, Lynfield R, Sawyer M: Cytomegalovirus Infection. In Red Book: 2021–2024 Report of the Committee on Infectious Diseases. American Academy of Pediatrics: Itasca, IL, USA. 2021.
- Gantt S, Bitnun A, Renaud C, Kakkar F, Vaudry W: Diagnosis and management of infants with congenital cytomegalovirus infection. Paediatr Child Health 2017;22(2): 72-74.
- Boppana SB, Ross SA, Shimamura M, Palmer AL, Ahmed A, Michaels MG, et al.: Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med 2011;364(22): 2111-2118.
- Ross SA, Michaels MG, Ahmed A, Palmer AL, Sánchez PJ, Bernstein DI, et al.: Contribution of Breastfeeding to False-Positive Saliva Polymerase Chain Reaction for Newborn Congenital Cytomegalovirus Screening. J Infect Dis 2018;217(10): 1612-1615.
| Patients | n. 1 | n. 2 | n. 3 | n. 4 | n. 5 | n. 6 | n. 7 | n. 8 | n. 9 | n. 10 | n. 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal CMV status at beginning of pregnancy |
Seropositive | Unknown | Unknown | Seronegative | Seropositive | Unknown | Unknown | Sero positive |
Sero negative |
Sero negative |
Sero negative |
| Indication for CMV testing | Mat. CMV NPI (peri-conception/1st trim.) FGR (amnio+) BW<10 PTB Low PLT Petechiae |
FGR BW&HC<10 PTB Low PLT Petechiae Hepatitis |
FGR BW<10 |
Mat. CMV PI (24-28 weeks) |
Mat. CMV NPI (peri-conception/1st trim.) |
Mat. CMV PI (unknown timing) FGR (amnio+) BW&HC<10 Low PLT |
FGR BW<10 Low PLT Petechiae |
Mat. CMV NPI (peri-conception/1st trim.) Fetal ascites (amnio+) PTB Ascites |
Mat. CMV PI (1st trim.) |
Mat. CMV PI (26-30 weeks) BW&HC <10 |
Mat. CMV PI (1st trim.) |
| VCV in pregnancy | Yes | No | No | No | No | Yes | No | Yes | Yes | No | Yes |
| Gender | Female | Female | Male | Male | Female | Female | Male | Male | Female | Male | Male |
| Delivery mode | CS | CS | CS | VB | VB | VB | CS | CS | VB | VB | VB |
| GA (weeks) |
33 5/7 | 33 6/7 | 37 1/7 | 37 5/7 | 40 3/7 | 38 1/7 | 37 5/7 | 36 1/7 | 39 3/7 | 41 4/7 | 39 6/7 |
| BW centile | 4 | 0 | 4 | 45 | 12 | 0 | 2 | 92 | 61 | 4 | 67 |
| HC centile | 35 | 1 | 25 | 70 | 33 | 0 | 70 | 97 | 79 | 0 | 74 |
| NBHS result | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| CMV-positive specimens | Saliva, urine | Urine | Urine | Saliva, urine | Saliva, urine | Saliva, urine | Saliva, urine | Saliva, urine | Saliva, urine | Urine | Saliva, urine |
| Saliva VL (copies/mL) |
19,498 | - | - | 50,000,000 | 44,296,505 | 20,575,137 | 2,699,368 | 13,619,118 | 18,000,000 | - | 13,000,000 |
| Urine VL (copies/mL) | 386,791 | 177,000,000 | 22,581,862 | 3,947,803 | 5,100,000 | 212,000,000 | 5,650,371 | 88,000,000 | 4,700,260 | 309,977 | 95,000,000 |
| Whole blood VL (copies/mL) | 477 | 155,808 | 22,581,862 | 2,289 | Neg. | 4,161 | 13,899 | 975 | 4,128 | 390 | 17,150 |
| Additional CNS findings (TCUS & MRI) |
Left periventricular cystic lesion, ventriculo megaly |
Ventriculo megaly, polymicro gyria |
None | None | None | None | None | None | None | None | None |
| GCV ValGCV |
Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | No |
| Follow-up (length in months) |
Deceased on 11th day of life | Cognitive delay and motor impairment (31 mo) |
Mild speech delay (36 mo) |
Abnormal bilateral ABR threshold at 2 months, then regular (25 mo) |
Regular (26 mo) |
Mild cognitive delay and motor impairment (15 mo) |
Regular (6 mo) |
Regular (9 mo) |
Regular (9 mo) |
Abnormal left ear ABR threshold at 3 weeks, then regular (7 mo) |
Regular (6 mo) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





