Submitted:
16 May 2024
Posted:
16 May 2024
You are already at the latest version
Abstract
Keywords:
1. Physiological Modifications in Athlete’s Heart
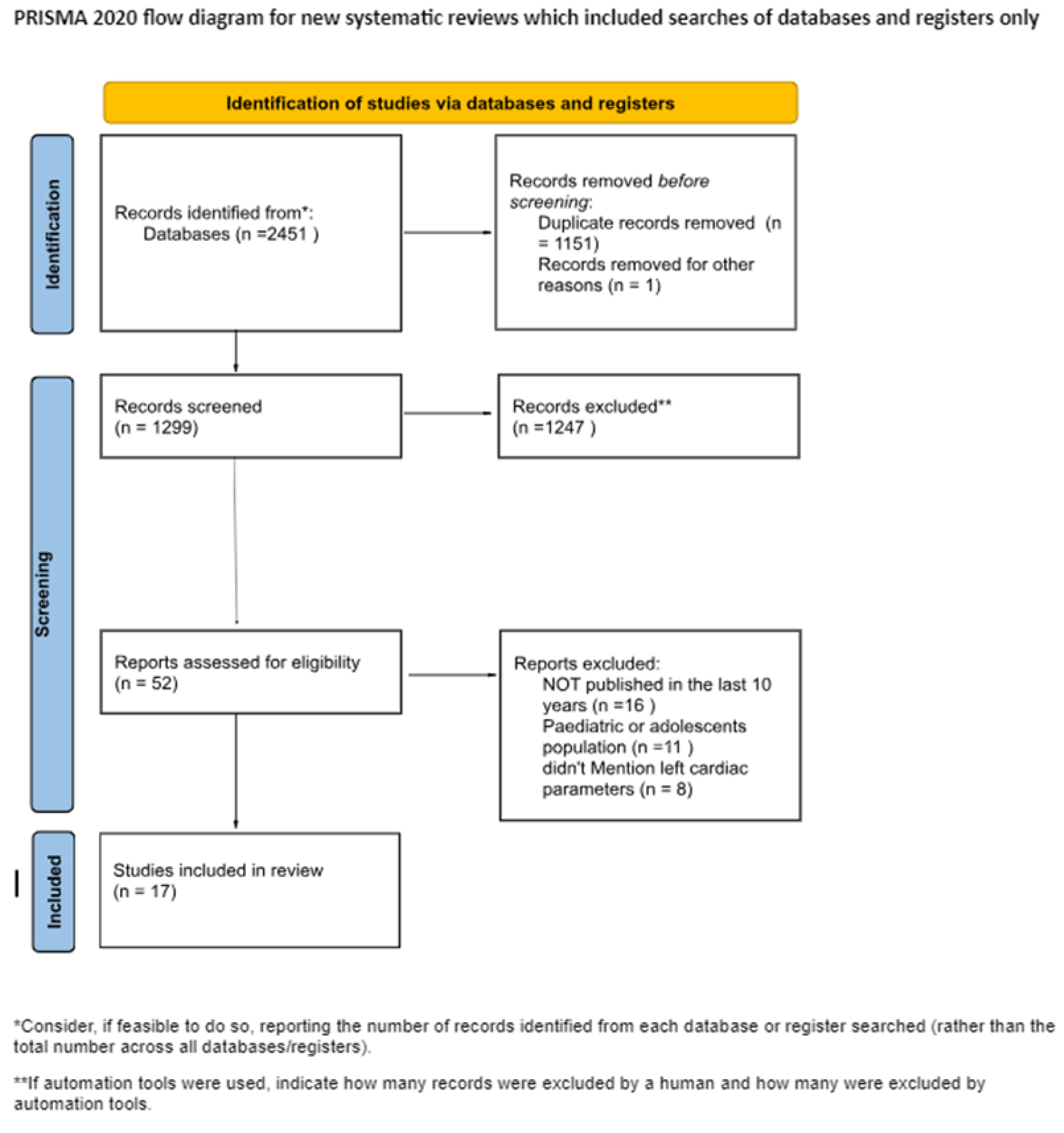
2. Materials and Methods
2.1. Inclusion/Exclusion Criteria
- 1)
- Studies that have been published in the last ten years in peer-reviewed journals;
- 2)
- We only took into consideration the adult cohorts in studies
- 3)
- Mandatory mention of left ventricular parameters
- 4)
- Mandatory mention of ultrasonography parameters
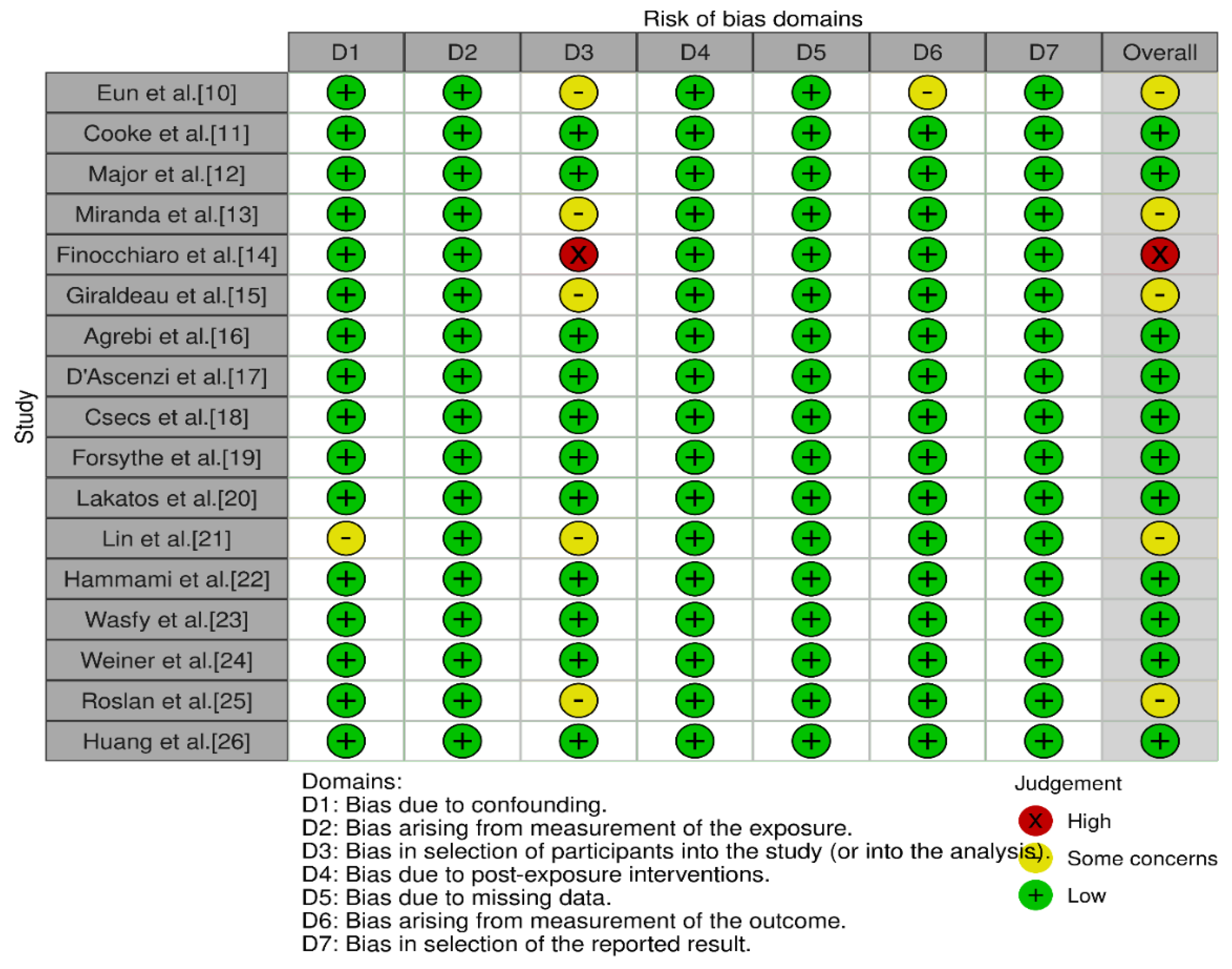
2.2. Risk of Bias
3. Results
3.1. Electrocardiographic Modifications
3.2. Imagistic Findings
3.3. Differences Between the Type of Sport and Echocardiographic Findings
3.4. Racial Differences
4. Where Adaptation Ends and Pathology Begins
5. Brief Discussion on Doping and Effects of Anabolic Drugs and How They May Alter the Athlete’s Heart
- 1)
- anabolic agents (anabolic androgenic steroids and other anabolic agents),
- 2)
- peptide factors, growth factors, and related substances (erythropoietin and agents affecting erythropoiesis, testosterone stimulating peptides, etc.),
- 3)
- beta-2 agonists (salbutamol, salmeterol, vilanterol, etc. but with some exceptions),
- 4)
- diuretics and masking agents, 5. hormone and metabolic modulators (anti-estrogenic substances, aromatase inhibitors, etc.),
- 5)
- gene and cell doping
- 6)
- manipulation of blood and blood components.
6. Conclusions
7. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- La Gerche, A.; Wasfy, M.M.; Brosnan, M.J.; Claessen, G.; Fatkin, D.; Heidbuchel, H.; Baggish, A.L.; Kovacic, J.C. The athlete’s heart—challenges and controversies: JACC focus seminar 4/4. Journal of the American College of Cardiology 2022, 80, 1346–1362. [Google Scholar] [CrossRef]
- Bernheim, A.; Zuber, M.; Knechtle, B.; Linka, A.; Faeh-Gunz, A.; De Pasquale, G.; Seifert, B.; Pfyffer, M.; Naegeli, B.; Jost, C.A. Structural and functional cardiac alterations in Ironman athletes: New insights into athlete's heart remodeling. International journal of cardiology 2013, 164, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, A.; Darvizeh, F.; Cundari, G.; Rovere, G.; Ferrandino, G.; Nicoletti, V.; Cilia, F.; De Vizio, S.; Palumbo, R.; Esposito, A. Advanced cardiac imaging in athlete’s heart: Unravelling the grey zone between physiologic adaptation and pathology. La radiologia medica 2021, 1–14. [Google Scholar] [CrossRef]
- Ribeiro, I.; Botanico, J. Physiologic left ventricular cavity dilatation in elite athletes. Annals of Internal Medicine 1999, 131, 546. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Maron, B.J.; Spataro, A.; Proschan, M.A.; Spirito, P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. New England Journal of Medicine 1991, 324, 295–301. [Google Scholar] [CrossRef]
- Mont, L.; Sambola, A.; Brugada, J.; Vacca, M.; Marrugat, J.; Elosua, R.; Pare, C.; Azqueta, M.; Sanz, G. Long-lasting sport practice and lone atrial fibrillation. European heart journal 2002, 23, 477–482. [Google Scholar] [CrossRef]
- Levine, B.D.; Lane, L.D.; Buckey, J.C.; Friedman, D.B.; Blomqvist, C.G. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation 1991, 84, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Pelliccia, A.; Heidbuchel, H.; Sharma, S.; Link, M.; Basso, C.; Biffi, A.; Buja, G.; Delise, P.; Gussac, I. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. European heart journal 2010, 31, 243–259. [Google Scholar] [CrossRef]
- ROBINS-E Development Group (Higgins J, M.R., Rooney A, Taylor K, Thayer K, Silva R, Lemeris C, Akl A, Arroyave W, Bateson T, Berkman N, Demers P, Forastiere F, Glenn B, Hróbjartsson A, Kirrane E, LaKind J, Luben T, Lunn R, McAleenan A, McGuinness L, Meerpohl J, Mehta S, Nachman R, Obbagy J, O'Connor A, Radke E, Savović J, Schubauer-Berigan M, Schwingl P, Schunemann H, Shea B, Steenland K, Stewart T, Straif K, Tilling K, Verbeek V, Vermeulen R, Viswanathan M, Zahm S, Sterne J). Risk Of Bias In Non-randomized Studies - of Exposure (ROBINS-E). Launch version. Available online: https://www.riskofbias.info/welcome/robins-e-tool.
- Eun, L.Y.; Chae, H.W. Assessment of myocardial function in elite athlete’s heart at rest-2D speckle tracking echocardiography in Korean elite soccer players. Scientific Reports 2016, 6, 39772. [Google Scholar] [CrossRef]
- Cooke, S.; Samuel, T.J.; Cooper, S.M.; Stöhr, E.J. Adaptation of myocardial twist in the remodelled athlete's heart is not related to cardiac output. Experimental Physiology 2018, 103, 1456–1468. [Google Scholar] [CrossRef]
- Major, Z.; Csajági, E.; Kneffel, Z.; Kováts, T.; Szauder, I.; Sidó, Z.; Pavlik, G. Comparison of left and right ventricular adaptation in endurance-trained male athletes. Acta Physiologica Hungarica 2015, 102, 23–33. [Google Scholar] [CrossRef]
- Miranda, D.P.; Dos Santos, M.J.; Salemi, V.M.C.; de Oliveira, E.P.C.; Verberne, H.J.; da Rocha, E.T. Differential effects of variation in athletes training on myocardial morphophysiological adaptation in men: Focus on 123I-MIBG assessed myocardial sympathetic activity. Journal of Nuclear Cardiology 2014, 21, 570–577. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Dhutia, H.; D’Silva, A.; Malhotra, A.; Steriotis, A.; Millar, L.; Prakash, K.; Narain, R.; Papadakis, M.; Sharma, R. Effect of sex and sporting discipline on LV adaptation to exercise. JACC: Cardiovascular Imaging 2017, 10, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Giraldeau, G.; Kobayashi, Y.; Finocchiaro, G.; Wheeler, M.; Perez, M.; Kuznetsova, T.; Lord, R.; George, K.P.; Oxborough, D.; Schnittger, I. Gender differences in ventricular remodeling and function in college athletes, insights from lean body mass scaling and deformation imaging. The American journal of cardiology 2015, 116, 1610–1616. [Google Scholar] [CrossRef]
- Agrebi, B.; Tkatchuk, V.; Hlila, N.; Mouelhi, E.; Belhani, A. Impact of specific training and competition on myocardial structure and function in different age ranges of male handball players. PLoS One 2015, 10, e0143609. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Biella, F.; Lemme, E.; Maestrini, V.; Di Giacinto, B.; Pelliccia, A. Female athlete’s heart: sex effects on electrical and structural remodeling. Circulation: Cardiovascular Imaging 2020, 13, e011587. [Google Scholar] [CrossRef]
- Csecs, I.; Czimbalmos, C.; Toth, A.; Dohy, Z.; Suhai, I.F.; Szabo, L.; Kovacs, A.; Lakatos, B.; Sydo, N.; Kheirkhahan, M. The impact of sex, age and training on biventricular cardiac adaptation in healthy adult and adolescent athletes: cardiac magnetic resonance imaging study. European Journal of Preventive Cardiology 2020, 27, 540–549. [Google Scholar] [CrossRef]
- Forsythe, L.; MacIver, D.H.; Johnson, C.; George, K.; Somauroo, J.; Papadakis, M.; Brown, B.; Qasem, M.; Oxborough, D. The relationship between left ventricular structure and function in the elite rugby football league athlete as determined by conventional echocardiography and myocardial strain imaging. International journal of cardiology 2018, 261, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, B.K.; Molnár, A.Á.; Kiss, O.; Sydó, N.; Tokodi, M.; Solymossi, B.; Fábián, A.; Dohy, Z.; Vágó, H.; Babity, M. Relationship between cardiac remodeling and exercise capacity in elite athletes: incremental value of left atrial morphology and function assessed by three-dimensional echocardiography. Journal of the American Society of Echocardiography 2020, 33, 101–109.e101. [Google Scholar] [CrossRef]
- Lin, J.; Wang, F.; Weiner, R.B.; DeLuca, J.R.; Wasfy, M.M.; Berkstresser, B.; Lewis, G.D.; Hutter, A.M.; Picard, M.H.; Baggish, A.L. Blood pressure and LV remodeling among American-style football players. JACC: Cardiovascular Imaging 2016, 9, 1367–1376. [Google Scholar] [CrossRef]
- Hammami, N.; Frih, B.; Rahali, H.; Mkacher, W.; Rezgui, T.; Čular, D.; Bouassida, A. Effects of taekwondo style practice on cardiac remodeling and isokinetic thigh strength in elite women players. Science & sports 2021, 36, 479. e471–479. e479. [Google Scholar]
- Wasfy, M.M.; Weiner, R.B.; Wang, F.; Berkstresser, B.; Lewis, G.D.; DeLuca, J.R.; Hutter, A.M.; Picard, M.H.; Baggish, A.L. Endurance exercise-induced cardiac remodeling: not all sports are created equal. Journal of the American Society of Echocardiography 2015, 28, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.B.; DeLuca, J.R.; Wang, F.; Lin, J.; Wasfy, M.M.; Berkstresser, B.; Stoehr, E.; Shave, R.; Lewis, G.D.; Hutter Jr, A.M. Exercise-induced left ventricular remodeling among competitive athletes: a phasic phenomenon. Circulation: Cardiovascular Imaging 2015, 8, e003651. [Google Scholar] [CrossRef] [PubMed]
- Roslan, A.; Stanislaus, R.; Sin, T.Y.; Aris, F.A.; Ashari, A.; Shaparudin, A.A.; Shah, W.F.W.R.; Beng, K.H.; Jhung, L.T.; Aktifanus, A.T.J. Echocardiography and strain analysis in Malaysian elite athletes versus young healthy adults. IJC Heart & Vasculature 2023, 47, 101242. [Google Scholar]
- Huang, K.-C.; Lin, C.-E.; Lin, L.-Y.; Hwang, J.-J.; Lin, L.-C. Data-driven clustering supports adaptive remodeling of athlete's hearts: An echocardiographic study from the Taipei Summer Universiade. Journal of the Formosan Medical Association 2022, 121, 1495–1505. [Google Scholar] [CrossRef]
- Lisman, K.A. Electrocardiographic evaluation in athletes and use of the Seattle criteria to improve specificity. Methodist DeBakey cardiovascular journal 2016, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Basu, J.; Malhotra, A. Interpreting the athlete’s ECG: current state and future perspectives. Current treatment options in cardiovascular medicine 2018, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Drezner, J.A.; Sharma, S.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C. International criteria for electrocardiographic interpretation in athletes: consensus statement. British journal of sports medicine 2017, 51, 704–731. [Google Scholar] [CrossRef]
- Tseng, W.-Y.I.; Su, M.-Y.M.; Tseng, Y.-H.E. Introduction to cardiovascular magnetic resonance: technical principles and clinical applications. Acta Cardiologica Sinica 2016, 32, 129. [Google Scholar]
- Lang, C.; Atalay, M.K. Cardiac magnetic resonance imaging and computed tomography: state of the art in clinical practice. Rhode Island Medical Journal 2014, 97, 28. [Google Scholar]
- Fogante, M.; Agliata, G.; Basile, M.C.; Compagnucci, P.; Volpato, G.; Falanga, U.; Stronati, G.; Guerra, F.; Vignale, D.; Esposito, A. Cardiac imaging in athlete’s heart: the role of the radiologist. Medicina 2021, 57, 455. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Haskell, W.; Snell, P.; Van Camp, S.P. Task Force 8: classification of sports. Journal of the American College of Cardiology 2005, 45, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, M.; Carre, F.; Kervio, G.; Rawlins, J.; Panoulas, V.F.; Chandra, N.; Basavarajaiah, S.; Carby, L.; Fonseca, T.; Sharma, S. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. European heart journal 2011, 32, 2304–2313. [Google Scholar] [CrossRef] [PubMed]
- Basavarajaiah, S.; Boraita, A.; Whyte, G.; Wilson, M.; Carby, L.; Shah, A.; Sharma, S. Ethnic differences in left ventricular remodeling in highly-trained athletes: relevance to differentiating physiologic left ventricular hypertrophy from hypertrophic cardiomyopathy. Journal of the American College of Cardiology 2008, 51, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Kervio, G.; Pelliccia, A.; Nagashima, J.; Wilson, M.G.; Gauthier, J.; Murayama, M.; Uzan, L.; Ville, N.; Carré, F. Alterations in echocardiographic and electrocardiographic features in Japanese professional soccer players: comparison to African-Caucasian ethnicities. European journal of preventive cardiology 2013, 20, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Magalski, A.; Maron, B.J.; Main, M.L.; McCoy, M.; Florez, A.; Reid, K.J.; Epps, H.W.; Bates, J.; Browne, J.E. Relation of race to electrocardiographic patterns in elite American football players. Journal of the American College of Cardiology 2008, 51, 2250–2255. [Google Scholar] [CrossRef] [PubMed]
- Magalski, A.; McCoy, M.; Zabel, M.; Magee, L.M.; Goeke, J.; Main, M.L.; Bunten, L.; Reid, K.J.; Ramza, B.M. Cardiovascular screening with electrocardiography and echocardiography in collegiate athletes. The American journal of medicine 2011, 124, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Dhutia, H.; Yeo, T.-J.; Finocchiaro, G.; Gati, S.; Bulleros, P.; Fanton, Z.; Papatheodorou, E.; Miles, C.; Keteepe-Arachi, T. Accuracy of the 2017 international recommendations for clinicians who interpret adolescent athletes’ ECGs: a cohort study of 11 168 British white and black soccer players. British journal of sports medicine 2020, 54, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.J.; Semsarian, C.; Orchard, J.W.; La Gerche, A.; Orchard, J.J. The impact of ethnicity on athlete ECG interpretation: a systematic review. Journal of Cardiovascular Development and Disease 2022, 9, 183. [Google Scholar] [CrossRef]
- Leo, T.; Uberoi, A.; Jain, N.A.; Garza, D.; Chowdhury, S.; Freeman, J.V.; Perez, M.; Ashley, E.; Froelicher, V. The impact of ST elevation on athletic screening. Clinical Journal of Sport Medicine 2011, 21, 433–440. [Google Scholar] [CrossRef]
- Malhotra, A.; Oxborough, D.; Rao, P.; Finocchiaro, G.; Dhutia, H.; Prasad, V.; Miller, C.; Keavney, B.; Papadakis, M.; Sharma, S. Defining the normal spectrum of electrocardiographic and left ventricular adaptations in mixed-race male adolescent soccer players. Circulation 2021, 143, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, N.; Papadakis, M.; Carre, F.; Kervio, G.; Panoulas, V.F.; Ghani, S.; Zaidi, A.; Gati, S.; Rawlins, J.; Wilson, M.G. Cardiac adaptation to exercise in adolescent athletes of African ethnicity: an emergent elite athletic population. British journal of sports medicine 2013, 47, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Miragoli, M.; Goldoni, M.; Demola, P.; Paterlini, A.; Li Calzi, M.; Gioia, M.I.; Visioli, F.; Rossi, S.; Pela, G. Left ventricular geometry correlates with early repolarization pattern in adolescent athletes. Scandinavian journal of medicine & science in sports 2019, 29, 1727–1735. [Google Scholar]
- Noseworthy, P.A.; Weiner, R.; Kim, J.; Keelara, V.; Wang, F.; Berkstresser, B.; Wood, M.J.; Wang, T.J.; Picard, M.H.; Hutter Jr, A.M. Early repolarization pattern in competitive athletes: clinical correlates and the effects of exercise training. Circulation: Arrhythmia and Electrophysiology 2011, 4, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Waase, M.P.; Mutharasan, R.K.; Whang, W.; DiTullio, M.R.; DiFiori, J.P.; Callahan, L.; Mancell, J.; Phelan, D.; Schwartz, A.; Homma, S. Electrocardiographic findings in national basketball association athletes. JAMA cardiology 2018, 3, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Drezner, J.A.; Ackerman, M.J.; Anderson, J.; Ashley, E.; Asplund, C.A.; Baggish, A.L.; Börjesson, M.; Cannon, B.C.; Corrado, D.; DiFiori, J.P. Electrocardiographic interpretation in athletes: the ‘Seattle criteria’. British journal of sports medicine 2013, 47, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.C.; Weeks, K.L.; Pretorius, L.; McMullen, J.R. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacology & therapeutics 2010, 128, 191–227. [Google Scholar]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. Journal of molecular and cellular cardiology 2016, 97, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Marijon, E.; Uy-Evanado, A.; Reinier, K.; Teodorescu, C.; Narayanan, K.; Jouven, X.; Gunson, K.; Jui, J.; Chugh, S.S. Sudden cardiac arrest during sports activity in middle age. Circulation 2015, 131, 1384–1391. [Google Scholar] [CrossRef]
- Wasfy, M.M.; Hutter, A.M.; Weiner, R.B. Sudden cardiac death in athletes. Methodist DeBakey cardiovascular journal 2016, 12, 76. [Google Scholar] [CrossRef]
- Cadwallader, A.B.; Murray, B. Performance-enhancing drugs I: understanding the basics of testing for banned substances. International journal of sport nutrition and exercise metabolism 2015, 25, 396–404. [Google Scholar] [CrossRef] [PubMed]
- The List of Prohibited Substances and Methods (List) indicates what substances and methods are prohibited in sport and when. Available online: https://www.wada-ama.org/en/prohibited-list#search-anchor.
- Hassan, N.; Salem, M.; Sayed, M. Doping and effects of anabolic androgenic steroids on the heart: histological, ultrastructural, and echocardiographic assessment in strength athletes. Human & experimental toxicology 2009, 28, 273–283. [Google Scholar]
- La Gerche, A.; Brosnan, M.J. Cardiovascular effects of performance-enhancing drugs. Circulation 2017, 135, 89–99. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.D.R.; de Bairros, A.V.; Yonamine, M. Blood doping: risks to athletes’ health and strategies for detection. Substance Use & Misuse 2014, 49, 1168–1181. [Google Scholar]
- Malhotra, A.; Dhutia, H.; Gati, S.; Yeo, T.-J.; Dores, H.; Bastiaenen, R.; Narain, R.; Merghani, A.; Finocchiaro, G.; Sheikh, N. Anterior T-wave inversion in young white athletes and nonathletes: prevalence and significance. Journal of the American College of Cardiology 2017, 69, 1–9. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; Culasso, F.; Spataro, A.; Caselli, G. Athlete's heart in women: echocardiographic characterization of highly trained elite female athletes. Jama 1996, 276, 211–215. [Google Scholar] [CrossRef]
| Article | Patients | Type of sport / Intensity | LEFT VENTRICULAR MASS+ INDEX | VOLUMES | STRAIN | LEFT VENTRICULAR ECHO FINDINGS | RIGHT VENTRICULAR ECHO FINDINGS |
|---|---|---|---|---|---|---|---|
| Hammami et al., 2021 | 20 | STRENGTH | LVEF(%) (WD) 59,33 ± 7,23 (ITF) 63,06 ± 7,40 LVEDD (ml) (WD) 124,63 ± 32,70 (ITF) 117,76 ± 9,65 LVESD (ml) (WD) 51,55 ± 12,29 (ITF) 50,57 ± 10,26 IST (mm) (WD) 7,07 ± 0,62 (ITF) 8,19 ± 1,37 (p<0,05) PWIVS (mm) (WD) 6,85 ± 0,62 (ITF) 7,43 ± 0,74 |
RVEDD (mm) (WD) 48,94 ± 4,35 (ITF) 47,59 ± 2,86 RVESD (mm) (WD) 28,38 ± 3,39 (ITF) 30,00 ± 2,07 RVL (mm) (WD) 65,35 ± 8,51 (ITF) 64,37 ± 3,44 |
|||
| Finocchiaro et al., 2017 | 1,083 | ENDURANCE+ MIXED TYPE EXERCISE REGIMEN | LVM/BSA (g/m2) 83 ± 17 (p=0.001) | LVEDD (mm) 49 ± 4 (p<0.001) LVEDD/BSA (mm/m^2) 28.6 ± 2.7(p<0.001) IVs (mm) 8.7 ± 1.2 (p<0.001) PW (mm) 8.4 ± 1.2 (p<0.001) RWT: 0.35 ± 0.04 (p<0.001) RWT >0.42: 34 (8) (p=0.04) |
|||
| Giraldeau et al., 2015 | 315 | STRENGTH + MIXED TYPE EXERCISE REGIMEN | LVM (g)111 +/- 20 (0.001) LVM/BSA (g/m^2) 65 +/- 9(0.001) |
Stroke volume (ml) 75 +/- 12(0.001) LV end-diastolic volume absolute (ml) 142 +/- 23(0.001) LV end-diastolic volume indexed to BSA (ml/m2) 83 +/- 12(0.001) LV Mass-to-Volume ratio 0.79 +/- 0.09 (0.015) LV Mass-to-Volume ratio (Allometric correction) 0.24 +/- 0.03 (0.035) Left atrial volume absolute (ml) 47.5 +/- 11.7 (0.001) RA volume (ml) 41,2 +/- 10,9 (p<0,0001) RA volume indexed BSA (ml/m^2) 24,1 +/- 5,7 (p=0,02) |
2D GLS manual strain (%) (F) -21.6 +/- 1.9(0.02) ; LV GLS (%) (F) -22.0 +/- 1.9(0.02); Diastolic early SR (%/s) (F)1.81 +/- 0.40(<0.01); RV GLS (%) -24,9 +/- 2,6 (p=0,76) LA LS contraction (%) -11,2 +/- 4,4 (p=0,65) RA LS contraction (%) -13,2 +/- 5,3 (p=0,056) |
LVEF (%) (F) 66 +/- 4 (0.004); Interventricular sept thickness (mm) (F) 6,1 +/- 1,0 (0.001); Posterior wall thickness (mm) (F) 7,0 +/- 1,1(0.001) Left ventricular internal diameter (mm) (F) 47,1 +/- 4,2(0.001) |
RV area (cm^2) 22,8 +/- 3,5 (p<0,0001) RV dimensions basal (cm) 3,7 +/- 0,5 (p<0,0001) RV dimensions mid (cm) 2,9 +/- 0,5 (p<0,0001) RV longitudinal (cm) 8,2 +/- 0,7 (p<0,0001) |
| D'Ascenzi et al., 2020 | 720 | ENDURANCE+ STRENGTH+ MIXED TYPE | LVM(g) 143.9±31.4 (96–202) (0.0001) LVM index, g/m2 81.8±15.1 (60–111) (0.0001) |
LV ejection fraction, % 67±6 (57–77) (0.0001) LV end-diastolic diameter, mm (F) 48.8±3.3 (44–54) (0.0001) LV end-diastolic diameter index, mm/m2 28.6±2.4 (25–33) (0.0001) LV end-systolic diameter, mm 30.2±3.3 (25–36) (0.0001) LV end-systolic diameter index, mm/m2 17.7±1.9 (15–21) (0.0001) IVS, mm 8.7±0.8 (7–10) (0.0001) Posterior wall, mm 8.4±0.8 (7–10) (0.0001) RWT 0.35±0.03 (0.31–0.39) (0.0001) Left atrial diameter, mm 32.3±3.8 (26–38) (0.0001) |
RV basal diameter (mm) 35.1±4.8 (27–44) (p<0.0001) RV midcavity diameter (mm) 23.9±4.2 (18–31) (p<0.0001) RV longitudinal diameter, (mm) 79.4±9.3 (65–95) (p<0.0001) RV diastolic area (cm^2) 19.5±5.2 (13–27) (p<0.0001) RV systolic area (cm^2) 9.1±2.7 (5–14) (p<0.0001) |
||
| Roslan et al., 2023 | 100 | ENDURANCE+ MIXED TYPE EXERCISE REGIMEN | LVM index (g/m2) 71,57 +/- 28,21 (p<0.001) | LVEDV index (mls/m^2) 57,25 +/- 11,55 (p<0,001) LVESV index (mls/m^2) 24,03 +/- 7,55 (p<0,001) |
GLS (%) 21,91 +/- 2,54 (p=0,53) LArS (%) 48,07 +/- 10,06 (p=0,004) LAcS (%) 36,3 +/- 8,30 (p=0,232) RVGLS (%) 22,85 +/- 3,08 (p=0,34) |
IVSd (cm) 0,77 +/- 0,11 (p=0,11) PWTd (cm) 0,8 +/- 0,31 (p=0,035) LVIDd (cm) 4,56 +/- 0,32 (p=0,009) IVSs (cm) 1,07 +/-0,14 (p=0,943) PWTs (cm)1,25 +/- 0,17 (p=0,095) LVIDs (cm) 2,94 +/- 0,29 (p<0,001) RWT 0,35 +/- 0,13 (p=0,131) |
|
| Lakatos et al., 2020 | 138 | MIXED TYPE EXERCISE REGIMEN | LVM index (g/m^2) 88.9 ± 9.5 (p<0.001) | LVSVi (mL/m^2) 46.1 ± 5.9 (p<0.05) LVEDVi (mL/m^2) 79.7 ± 8.3 (p<0.001) LVESVi (mL/m^2) 33.6 ± 5.3 (p<0.001) |
LA GLS (%) 34.3 ± 7.8 (p<0.01) LV GLS (%) −19.4 ± 1.9 (p<0,001) LV GCS (%) −28.1 ± 3.4 (p<0.05) |
LVEF (%) 57.9 ± 4.7 (p<0.01) LVMi (g/m^2) 88.9 ± 9.5 (p<0.001) |
|
| Huang et al., 2022 | 598 | ENDURANCE+ STRENGTH+ MIXED TYPE | LVM index (g/m^2) 79.7±18.8 (p<0.001) | LV stroke volume index (mL/m^2) 39.3 ± 7.8 (p<0.001) LVEDV index (mL/m^2) 67.3 ± 12.1 (p<0.001) LVESV index (mL/m^2) 28.0 ± 6.3 (p<0.001) |
LVGLS (%) −23.7 ± 2.3 (p<0.001) LVGCS (%) −28.5 ± 4.1 (p=0.778) RVLS (%) -27,9 +/- 4,4 (p=0,358) |
LVEF (%) 58.5 ± 5.4 (p=0.06) IVSd (mm) 8.6 ± 1.3 (p<0.001) LVPWd (mm) 8.7 ± 1.3 (p<0.001) LVIDd index (mm/m^2) 27.9 ± 2.9 (p<0.001) LVIDs index (mm/m^2) 18.4 ± 2.5 (p<0.017) |
RVEDA index (cm^2/m^2) 11,3 +/- 1,9 (p<0,001) RVESA index (cm^2/m^2) 6,7 +/- 1,3 (p<0,001) |
| Article | Patients | Type of sport / Intensity | LEFT VENTRICULAR MASS+ INDEX | VOLUMES | STRAIN | LEFT VENTRICULAR ECHO FINDINGS | RIGHT VENTRICULAR ECHO FINDINGS |
|---|---|---|---|---|---|---|---|
| Eun et al., 2016 | 29 | MIXED TYPE EXERCISE REGIMEN | LVM (g) 216.7±21.9 (p=0,001) LVM index (g/height2) 110.2±9.4 (p=0,001) |
Stroke volume (mL) 89.3±15.4 (p=0,01) End-diastolic volume (mL/m2) 152.0±19.4 (p=0,001) End-systolic volume (mL/m2) 62.7±12.7 (p=0,001) |
Longitudinal strain: −17.6+/- 1.8 (p=ns) | Ejection Fraction (%) 62.3±4.2 (p=ns) Interventricular septal thickness (mm) 10.4±0.8 (p=0,01) Posterior wall thickness (mm) 10.6±0.7 (p=0,001) End-diastolic diameter (mm) 53.1±0.7 (p=0,001) End-systolic diameter (mm) 36.5±1.8 (p=0,001) Relative wall thickness 4.0±0.3 (p=ns) Cardiac index (L/m2) 2.5±0.6 (p=ns) End-systolic WS (mmHg) 240.9±32.6 (p=0,001) End-systolic MWS (g/cm2) 21.5±1.5 (p=ns) |
RV Systolic Area (m2) 14.9±3.7 (p=0,005) RV Diastolic Area (m2) 26.1±7.1 (p=0,002) |
| Cooke et al., 2018 | 11 | ENDURANCE | LVM(g) 181 +/- 27 (p= 0,001) LVM index 95 +/- 12 (p=0,001) |
Stroke volume(ml) 87±14 (p< 0,01) Stroke volume index(ml m−2) 46±9 (p<0,01) End-diastolic volume(ml) 148±20 (p<= 0,01) End-diastolic volume index(ml m−2) 78±12 (p<= 0,05) End-systolic volume(ml)61±16 (p=0,0001) End-systolic volume index(ml m−2) 32±8 (p=0,01) |
Peak twist(degrees) 13.4±6.6 (p=0,0001) Peak torsion(degrees cm−1) 1.5±0.8 (p=0,0001) LV shortening(Δcm) 1.9±0.5 (p=0,0001) Sphericity index 1.7±0.1 (p=0,0001) End-diastolic LV length(cm) 9.3±0.7 (p=0,0001) Cardiac output(L min−1) 4.3±0.8 (p=0,0001) Cardiac index(L min−1 m−2) 2.3±0.5 (p=0,0001) |
||
| Major et al., 2014 | 52 | ENDURANCE | LVM(g/m^3) 99,4 +/- 17,3 (p,0,001) | LV rel. Stroke Volume (ml/m^3) 36,6 +/- 8,9 (p<0,01) |
rel.LVLADd (mm/m) 63.8 ± 5.6 (p<0,05) |
rel.RVLADd (mm/m) 63.4 ± 6.3 (p<0,001) RVLA.Fr.Sh. (%) 25.9 ± 6.3 (p= NS) rel.RVSADd (mm/m) 27.3 ± 3.6 (p<0,001) RVSA.Fr.Sh. (%) 20.3 ± 8.4 (p= NS) RVAd (cm2 ) 28.0 ± 5.0 (p<0,001) RVFAC (%) 42.7 ± 6.8 (p= NS) |
|
| Miranda et al., 2014 | 40 | ENDURANCE+ STRENGTH | LVM index (g m^-2) (endurance) 79,25 +/- 20,75 (p=0,01) LVM index (g m^-2) (strength) 88,74 +/- 28,71 (p<0,01) |
Left ventricular ejection fraction (%) (endurance) 62,70 +/- 6,34 (p=0,628) Left ventricular ejection fraction (%) (strength) 61,35 +/- 4,69 (p=0,163) Septum thickness (mm) (endurance) 8,40 +/- 1,60 (p=0,041) Septum thickness (mm) (strength) 9,75 +/- 1,86 (p=0,01) Posterior wall thickness (mm) (endurance) 8,50 +/- 1,43 (p=0,01) Posterior wall thickness (mm) (strength) 10 +/- 2 (p=0,01) Left ventricular end diastolic dimension (mm) (endurance) 48,65 +/- 4,70 (p=0,055) Left ventricular end diastolic dimension (mm) (strength) 49,85 +/- 4,20 (p=0,01) Left ventricular end systolic dimension (mm) (endurance) 30,20 +/- 3,25 (p=0,042) Left ventricular end systolic dimension (mm) (strength) 31,25 +/- 3,66 (p=0,004) |
|||
| Forsythe et al., 2018 | 139 | MIXED TYPE EXERCISE REGIMEN | LVM (g) 191 ± 31 (p<0,001) LVM index (g/(m^2)^2.7) 38 ± 7 (p<0,001) LVM index (g/m2) 87 ± 13 (p<0,001) |
Stroke Volume (ml) 92 ± 16 (p<0,001) LVEDV (ml) 157 ± 25 (p<0,001) LVEDV (ml/(m^2)^1.5) 48 ± 7 (p<0,001) LVESV (ml) 65 ± 13 (p<0,001) LVESV (ml/(m^2)^1.5) 20 ± 4 (p<0,001) |
Mean Wall Thickness (mm) 9 ± 1 (p<0,001) Maximum wall thickness (mm) 10 ± 1 (p<0,001) LVIDd (mm) 56 ± 4 (p<0,001) LVIDd index (mm/(m^2)^0.5) 37±2 (p<0,001) LVIDs (mm) 38 ± 3 (p<0,001) LVIDs index (mm/(m2) 26 ± 2 (p=0,017) |
||
| Lin et al., 2016 | 87 | MIXED TYPE EXERCISE REGIMEN | LVM (g) (lineman) 265 ± 43 (p<0.001) LVM (g) (non-lineman) 217 ± 35 (p<0.001) LVM index (gm/m^2) (lineman) 109 ± 15 (p<0.001) LVM index (gm/m^2) (non-lineman) 101 ± 15 (p<0.001) |
PWT (mm) (lineman) 11.0 ± 1.2 (p<0.001) PWT (mm) (non lineman) 10.4 ± 1.1 (p=0,01) IVS thickness (mm) (lineman) 10.6 ± 1.2 (p<0.001) IVS thickness (mm) (non lineman) 9.8 ± 1.4 (p=0,004) Relative wall thickness (lineman) 0.40 ± 0.06 (p<0.001) |
|||
| Wasfy et al., 2014 | 71 | ENDURANCE | LVM (g) (runners) 159 +/- 20 LVM (g) (rowers) 209 +/- 26 (p<0,001) LVM index (g/m^2) (runners) 88 +/- 11 LVM index (g/m^2) (runners) 108 +/- 13 (p<0,001) |
Stroke volume (mL) (runners) 102 +/- 13 Stroke volume (mL) (rowers) 100 +/- 15 Stroke volume index (mL/m^2) (runners) 57 +/- 6 Stroke volume index (mL/m^2) (rowers) 52 +/- 8 (p<0,001) LVEDV (mL) (runners) 182 +- 21 LVEDV (mL) (rowers) 172 +- 25 (p<0,05) LVEDV index (mL/m2) (runners) 101 +- 10 LVEDV index (mL/m2) (rowers) 89 +/- 13 (p<0,001) |
Interventricular septum (mm) (runners) 7.7 +- 1 Interventricular septum (mm) (rowers) 9.4 +_ 1 (p<0,001) Posterior wall (mm) (runners) 8.4 +-1 Posterior wall (mm) (rowers) 9.7 +_1 (p<0,001) Wall thickness (average) (mm) (runners) 8.1 +- 1 Wall thickness (average) (mm) (rowers) 9.5 +-1 (p<0,001) Wall thickness/BSA (mm/m2) (runners) 4.5 +_ 1 Wall thickness/BSA (mm/m2) (rowers) 4.9 +- (p<0,001) Relative wall thickness (runners) 0.32 +_0.04 Relative wall thickness (rowers) 0.37 +_0.04 LVIDd (mm) (runners) 50.3 +/- 3 LVIDd (mm) (rowers) 52.4 +/- 3 (p<0,001) LVIDd/BSA (mm/m2) (runners) 27.9 +/- 2 LVIDd/BSA (mm/m2) (rowers) 27.1 +/- 2 (p<0,01) LVIDs (mm) (runners) 34.4 +/- 3 LVIDs (mm) (rowers) 35.5 +/- 2 (p<0,05) LVIDs/BSA (mm/m2) (runners)19.1 +/- 6,2 LVIDs/BSA (mm/m2) (rowers) 18.3 +/- 6,1 (p<0,001) |
RV basal diameter (mm) (runners) 45.1+- 6 RV basal diameter (mm) (rowers) 45.1 +- 4 RV diastolic area (cm2) (runners) 27.4+- 4 RV diastolic area (cm2) (rowers) 26.2 +-3 Diastolic area/BSA (cm2/m2) (runners) 14.7 +-3 Diastolic area/BSA (cm2/m2) (rowers) 13.5 +-2 RV systolic area (cm2) (runners) 15.7 +_3 RV systolic area (cm2) (rowers) 15.8 +_3 Systolic area/BSA (cm2/m2) (runners) 8.7 +_1 Systolic area/BSA (cm2/m2) (rowers) 8.1 +_2 p=NS |
|
| Weiner et al., 2015 | 12 | ENDURANCE | Stroke volume (mL) 138.1±20.4 (p<0.001) | LV longitudinal strain (%) −19.9±2.5 (p=0.04) |
Cardiac output (L/min) 7.0±1.1 (p=0.5) LV peak Sm (cm/s) 8.9±1.3 (p<0.001) |
||
| Finocchiaro et al., 2017 | 1,083 | ENDURANCE+ MIXED TYPE EXERCISE REGIMEN | LVM index (g/m2) 101+/-21 (p<0.001) |
LVEDD (mm) 54+/-5 (p<0.001) LVEDD/BSA (mm/m) 27.2+/-2.7 (p<0.001) LVEDD/BSA (mm/m) 27.2+/-2.7 (p<0.001) IVS (mm) 10.0+/-1.3 (p<0.001) Maximal LVWT >12 mm: 26 (40) (p<0.001) LVEDD (mm) 54+/-5 (p<0.001) IVS (mm) 10.0+/-1.3 (p<0.001) |
|||
| Giraldeau et al., 2015 | 315 | STRENGTH+ MIXED TYPE EXERCISE REGIMEN | LVM (g) 159 +/- 26 (p<0,001) LVM index (g/m^2) 77 +/- 10 (p<0,001) LVM index to LBM: 2,2 +/- 0,3 (p=0,63) |
Stroke volume (mL) 98 +/- 20 (p<0,001) LVEDV (ml) 193 +/- 33 (p<0,001) LVEDV index to BSA (ml/m^2) 94 +/- 1(p<0,001) LA volume (ml) 59,1 +/- 16,8 (p<0,001) LA volume index BSA: 28,6 +/- 7,5 (p= 0,54) LV mass to volume ration 0,83 +/- 0,11 (p=0,015) RA volume (mL) 56,1 +/- 15,3 (p<0,0001) RA volume index BSA 27,2 +/- 6,7 (p<0,02) |
LV global longitudinal strain (GLS) (%) -20,6 +/- 2,0 (p=0,02) LV systolic strain rate (%/s) -1,06 +/- 0,18 (p=0,67) LV diastolic early strain rate (%/s) 1,56 +/- 0,43 (p<0,01) RV global longitudinal strain (%) -24,7 +/- 2,6 (p=0,76) RV systolic strain rate (%/s) -1,5 +/- 0,3 (p=0,58) RV diastolic early strain rate (%/s) 1,78 +/- 0,60 (p=0,043) LA LG contraction(%) 10,8 +/- 3,8 (p=0,65) RA LG contraction (%) -15,2 +/- 4,2 (p=0,056) |
LVEF (%) 63 +/- 6 (p=0,0004) IVS (mm) 7.1 +/- 0.1 (p< 0.001) PW (mm) 8.2 +/- 1.5 (p< 0.001) LVID (mm) 52.2 +/- 4.1 (p< 0.001) |
RV area (cm^2) 29,7 +/- 3,9 (p<0,0001) RV dimensions basal (cm) 4,3 +/- 0,5 (p<0,0001) RV dimensions mid (cm) 3,6 +/- 0,5 (p<0,0001) RV longitudinal (cm) 9,1 +/- 0,9 (p<0,0001) |
| Agrebi et al., 2015 | 36 | MIXED TYPE EXERCISE REGIMEN | LVM (g) 238,73 +/- 29,57 LVM index (g/m^2) 119,66 +/- 12,67 |
LV end diastolic diameter (mm) 54,80 +/- 4,18 LV end systolic diameter (mm) 35,10 +/- 1,11 Posterior wall thickness (mm) 8,10 +/- 1,10 LV ejection time (msec) 279±38.14 |
RV diameter (mm) 18,65 +/- 4,74 | ||
| Roslan et al., 2023 | 100 | ENDURANCE+ MIXED TYPE EXERCISE REGIMEN | LVM index (g/m^2) 93,53 +/- 15,85 (p<0,001) | LVEDV index (mls/m^2) 67,36 +/- 14,75 (p<0,001) LVESV index (mls/m^2) 27,84 +/- 8,14 (p<0,001) |
GLS (%) 19,96 +/- 2,37 (p=0,16) LArS (%) 44,12 +/- 9,55 (p<0,001) LAcS (%) 32,53 +/- 6,45 (p=0,038) RVGLS (%) 21,04 +/- 2,77 (p=0,43) |
IVSd (cm) 0,95 +/- 0,12 (p=0,003) PWTd (cm) 0,91 +/- 0,11 (p<0,001) LVIDd (cm) 5,05 +/- 0,36 (p<0,001) IVSs (cm) 1,27 +/- 0,17 (p=0,473) PWTs (cm)1,42 +/- 0,16 (p=0,086) LVIDs (cm) 3,4 +/- 0,39 (p<0,001) RWT 0,36 +/-0,05 (p=0,705) |
|
| Huang et al., 2022 | 598 | ENDURANCE+ STRENGTH+ MIXED TYPE | LVM index (g/m^2) 97,4 +/- 21,0 (p<0,001) | LV stroke volume index (ml/m^2) 43,7 +/- 9,0 (p<0,001) LVEDV index (ml/m^2) 75,7 +/- 13,5 (p<0,001) LVESV index (mll/m^2) 32,0 +/- 6,6 (p<0,001) |
LVGLS (%) -22,4 +/- 2,2 (p<0,001) LVGCS (%) -28,4 +/- 4,5 (p=0,778) RVLS (%) -27,5 +/- 4,7 (p=0,358) |
IVSd (mm) 9,7 +/- 1,4 (p<0,001) LVPWd (mm) 9,9 +/- 1,5 (p<0,001) LVIDd index (mm/m^2) 26,9 +/- 2,6 (p;0,001) LVIDs index (mm/m^2) 16,7 +/- 1,7 (p<0,017) |
RVEDA index (cm^2/m^2) 12,9 +/- 2,4 (p<0,001) RVESA index (cm^2/m^2) 7,7 +/- 1,5 (p<0,001) |
| Title | Patients | Type of sport / Intensity | LEFT VENTRICULAR MASS+ INDEX | VOLUMES | STRAIN | LEFT VENTRICULAR ECHO FINDINGS | RIGHT VENTRICULAR ECHO FINDINGS |
|---|---|---|---|---|---|---|---|
| Eun et al., 2016 | 29 | MIXED TYPE EXERCISE REGIMEN | LV Mass (g) 216.7±21.9 (p=0,001) Mass index (g/height2) 110.2±9.4 (p=0,001) |
Stroke volume (mL) 89.3±15.4 (p=0,01) End-diastolic volume (mL/m2) 152.0±19.4 (p=0,001) End-systolic volume (mL/m2) 62.7±12.7 (p=0,001) |
Longitudinal strain: −17.6+1.8 (p=ns) | Ejection Fraction (%) 62.3±4.2 (p=ns) Interventricular septal thickness (mm) 10.4±0.8 (p=0,01) Posterior wall thickness (mm) 10.6±0.7 (p=0,001) End-diastolic diameter (mm) 53.1±0.7 (p=0,001) End-systolic diameter (mm) 36.5±1.8 (p=0,001) Relative wall thickness 4.0±0.3 (p=ns) Cardiac index (L/m2) 2.5±0.6 (p=ns) End-systolic WS (mmHg) 240.9±32.6 (p=0,001) End-systolic MWS (g/cm2) 21.5±1.5 (p=ns) |
RV Systolic Area (m2) 14.9±3.7 (p=0,005) RV Diastolic Area (m2) 26.1±7.1 (p=0,002) |
| Cooke et al., 2018 | 11 | ENDURANCE | LV mass (g) 181 +/- 27 (p= 0,001) LV mass index 95 +/- 12 (p=0,001) |
Stroke volume(ml) 87±14 (p< 0,01) Stroke volume index(ml m−2) 46±9 (p<0,01) End-diastolic volume(ml) 148±20 (p<= 0,01) End-diastolic volume index(ml m−2) 78±12 (p<= 0,05) End-systolic volume(ml)61±16 (p=0,0001) End-systolic volume index(ml m−2) 32±8 (p=0,01) |
Peak twist(degrees) 13.4±6.6 (p=0,0001) Peak torsion(degrees cm−1) 1.5±0.8 (p=0,0001) LV shortening(Δcm) 1.9±0.5 (p=0,0001) Sphericity index 1.7±0.1 (p=0,0001) End-diastolic LV length(cm) 9.3±0.7 (p=0,0001) Cardiac output(L min−1) 4.3±0.8 (p=0,0001) Cardiac index(L min−1 m−2) 2.3±0.5 (p=0,0001) |
||
| Major et al., 2014 | 52 | ENDURANACE | LV mass (g/m^3) 99,4 +/- 17,3 (p,0,001) | LV rel. Stroke Volume (ml/m^3) 36,6 +/- 8,9 (p<0,01) |
rel.LVLADd (mm/m) 63.8 ± 5.6 (p<0,05) |
rel.RVLADd (mm/m) 63.4 ± 6.3 (p<0,001) RVLA.Fr.Sh. (%) 25.9 ± 6.3 (p= NS) rel.RVSADd (mm/m) 27.3 ± 3.6 (p<0,001) RVSA.Fr.Sh. (%) 20.3 ± 8.4 (p= NS) RVAd (cm2 ) 28.0 ± 5.0 (p<0,001) RVFAC (%) 42.7 ± 6.8 (p= NS) |
|
| Miranda et al., 2014 | 40 | ENDURANCE+ STRENGTH | Left ventricular mass index (g m^-2) (endurance) 79,25 +/- 20,75 (p=0,01) Left ventricular mass index (g m^-2) (strength) 88,74 +/- 28,71 (p<0,01) |
Left ventricular ejection fraction (%) (endurance) 62,70 +/- 6,34 (p=0,628) Left ventricular ejection fraction (%) (strength) 61,35 +/- 4,69 (p=0,163) Septum thickness (mm) (endurance) 8,40 +/- 1,60 (p=0,041) Septum thickness (mm) (strength) 9,75 +/- 1,86 (p=0,01) Posterior wall thickness (mm) (endurance) 8,50 +/- 1,43 (p=0,01) Posterior wall thickness (mm) (strength) 10 +/- 2 (p=0,01) Left ventricular end diastolic dimension (mm) (endurance) 48,65 +/- 4,70 (p=0,055) Left ventricular end diastolic dimension (mm) (strength) 49,85 +/- 4,20 (p=0,01) Left ventricular end systolic dimension (mm) (endurance) 30,20 +/- 3,25 (p=0,042) Left ventricular end systolic dimension (mm) (strength) 31,25 +/- 3,66 (p=0,004) |
|||
| Agrebi et al., 2015 | 36 | MIXED TYPE EXERCISE REGIMEN | LV mass (g) 238,73 +/- 29,57 LV mass index (g/m^2) 119,66 +/- 12,67 |
LV end diastolic diameter (mm) 54,80 +/- 4,18 LV end systolic diameter (mm) 35,10 +/- 1,11 Posterior wall thickness (mm) 8,10 +/- 1,10 LV ejection time (msec) 279±38.14 |
RV diameter (mm) 18,65 +/- 4,74 | ||
| Forsythe et al., 2018 | 139 | MIXED TYPE EXERCISE REGIMEN | LV Mass (g) 191 ± 31 (p<0,001) LV Mass index (g/(m^2)^2.7) 38 ± 7 (p<0,001) LV mass index (g/m2) 87 ± 13 (p<0,001) |
Stroke Volume (ml) 92 ± 16 (p<0,001) LVEDV (ml) 157 ± 25 (p<0,001) LVEDV (ml/(m^2)^1.5) 48 ± 7 (p<0,001) LVESV (ml) 65 ± 13 (p<0,001) LVESV (ml/(m^2)^1.5) 20 ± 4 (p<0,001) |
Mean Wall Thickness (mm) 9 ± 1 (p<0,001) Maximum wall thickness (mm) 10 ± 1 (p<0,001) LVIDd (mm) 56 ± 4 (p<0,001) LVIDd index (mm/(m^2)^0.5) 37±2 (p<0,001) LVIDs (mm) 38 ± 3 (p<0,001) LVIDs index (mm/(m2) 26 ± 2 (p=0,017) |
||
| Lakatos et al., 2020 | 138 | MIXED TYPE EXERCISE REGIMEN | LV mass index (g/m2) 98.7 ± 15.6 (p<0.001) | Stroke volume index (mL/m2) 49.3 ± 7.8 (p<0.05) LVEDV index(mL/m2) 89.0 ± 13.1 (p<0.001) LVESV index (mL/m2) 39.7 ± 6.8 (p<0.001) RV Stroke volume index (ml/m2) 49.1 ± 6.6 (p<0.01) RVEDV index (ml/m2) 92.9 ± 13.7 (p<0.001) RVESVi index (ml/m2) 43.8 ± 9.0 (p<0.001) |
LV global longitudinal strain (%) −18.3 ± 1.8 (p<0.001) LV global contraction strain (%) −26.9 ± 2.4 (p<0.05) |
LVEF (%) 55.4 ± 3.5 (p<0.01) | RVEF (%) 53.1 ± 4.2 (p<0.001) |
| Lin et al., 2016 | 87 | MIXED TYPE EXERCISE REGIMEN | LV mass (g) (lineman) 265 ± 43 (p<0.001) LV mass (g) (non-lineman) 217 ± 35 (p<0.001) LV mass index (gm/m^2) (lineman) 109 ± 15 (p<0.001) LV mass index (gm/m^2) (non-lineman) 101 ± 15 (p<0.001) |
PWT (mm) (lineman) 11.0 ± 1.2 (p<0.001) PWT (mm) (non lineman) 10.4 ± 1.1 (p=0,01) IVS thickness (mm) (lineman) 10.6 ± 1.2 (p<0.001) IVS thickness (mm) (non lineman) 9.8 ± 1.4 (p=0,004) Relative wall thickness (lineman) 0.40 ± 0.06 (p<0.001) |
|||
| Hammami et al., 2021 | 20 | STRENGTH | LVEF(%) (WD) 59,33 ± 7,23 (ITF) 63,06 ± 7,40 LVEDD (ml) (WD) 124,63 ± 32,70 (ITF) 117,76 ± 9,65 LVESD (ml) (WD) 51,55 ± 12,29 (ITF) 50,57 ± 10,26 IST (mm) (WD) 7,07 ± 0,62 (ITF) 8,19 ± 1,37 (p<0,05) PWIVS (mm) (WD) 6,85 ± 0,62 (ITF) 7,43 ± 0,74 |
RVEDD (mm) (WD) 48,94 ± 4,35 (ITF) 47,59 ± 2,86 RVESD (mm) (WD) 28,38 ± 3,39 (ITF) 30,00 ± 2,07 RVL (mm) (WD) 65,35 ± 8,51 (ITF) 64,37 ± 3,44 |
|||
| Wasfy et al., 2014 | 71 | ENDURANCE | LV mass (g) (rowers) 209 +/- 26 (p<0,001) LV mass index (g/m^2) (runners) 88 +/- 11 LV mass index (g/m^2) (runners) 108 +/- 13 (p<0,001) |
Stroke volume (mL) (runners) 102 +/- 13 Stroke volume (mL) (rowers) 100 +/- 15 Stroke volume index (mL/m^2) (runners) 57 +/- 6 Stroke volume index (mL/m^2) (rowers) 52 +/- 8 (p<0,001) LV mass (g) (runners) 159 +/- 20 LVEDV (mL) (runners) 182 +- 21 LVEDV (mL) (rowers) 172 +- 25 (p<0,05) LVEDV index (mL/m2) (runners) 101 +- 10 LVEDV index (mL/m2) (rowers) 89 +/- 13 (p<0,001) |
Interventricular septum (mm) (runners) 7.7 +- 1 Interventricular septum (mm) (rowers) 9.4 +_ 1 (p<0,001) Posterior wall (mm) (runners) 8.4 +-1 Posterior wall (mm) (rowers) 9.7 +_1 (p<0,001) Wall thickness (average) (mm) (runners) 8.1 +- 1 Wall thickness (average) (mm) (rowers) 9.5 +-1 (p<0,001) Wall thickness/BSA (mm/m2) (runners) 4.5 +_ 1 Wall thickness/BSA (mm/m2) (rowers) 4.9 +- (p<0,001) Relative wall thickness (runners) 0.32 +_0.04 Relative wall thickness (rowers) 0.37 +_0.04 LVIDd (mm) (runners) 50.3 +/- 3 LVIDd (mm) (rowers) 52.4 +/- 3 (p<0,001) LVIDd/BSA (mm/m2) (runners) 27.9 +/- 2 LVIDd/BSA (mm/m2) (rowers) 27.1 +/- 2 (p<0,01) LVIDs (mm) (runners) 34.4 +/- 3 LVIDs (mm) (rowers) 35.5 +/- 2 (p<0,05) LVIDs/BSA (mm/m2) (runners)19.1 +/- 6,2 LVIDs/BSA (mm/m2) (rowers) 18.3 +/- 6,1 (p<0,001) |
RV basal diameter (mm) (runners) 45.1+- 6 RV basal diameter (mm) (rowers) 45.1 +- 4 RV diastolic area (cm2) (runners) 27.4+- 4 RV diastolic area (cm2) (rowers) 26.2 +-3 Diastolic area/BSA (cm2/m2) (runners) 14.7 +-3 Diastolic area/BSA (cm2/m2) (rowers) 13.5 +-2 RV systolic area (cm2) (runners) 15.7 +_3 RV systolic area (cm2) (rowers) 15.8 +_3 Systolic area/BSA (cm2/m2) (runners) 8.7 +_1 Systolic area/BSA (cm2/m2) (rowers) 8.1 +_2 p=NS |
|
| Weiner et al., 2015 | 12 | ENDURANCE | Stroke volume (mL) 138.1±20.4 (p<0.001) | LV longitudinal strain (%) −19.9±2.5 (p=0.04) | LV ejection fraction (%) 63.3±2.9 (p=0.23) Cardiac output (L/min) 7.0±1.1 (p=0.5) LV peak Sm (cm/s) 8.9±1.3 (p<0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





