Submitted:
16 May 2024
Posted:
17 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
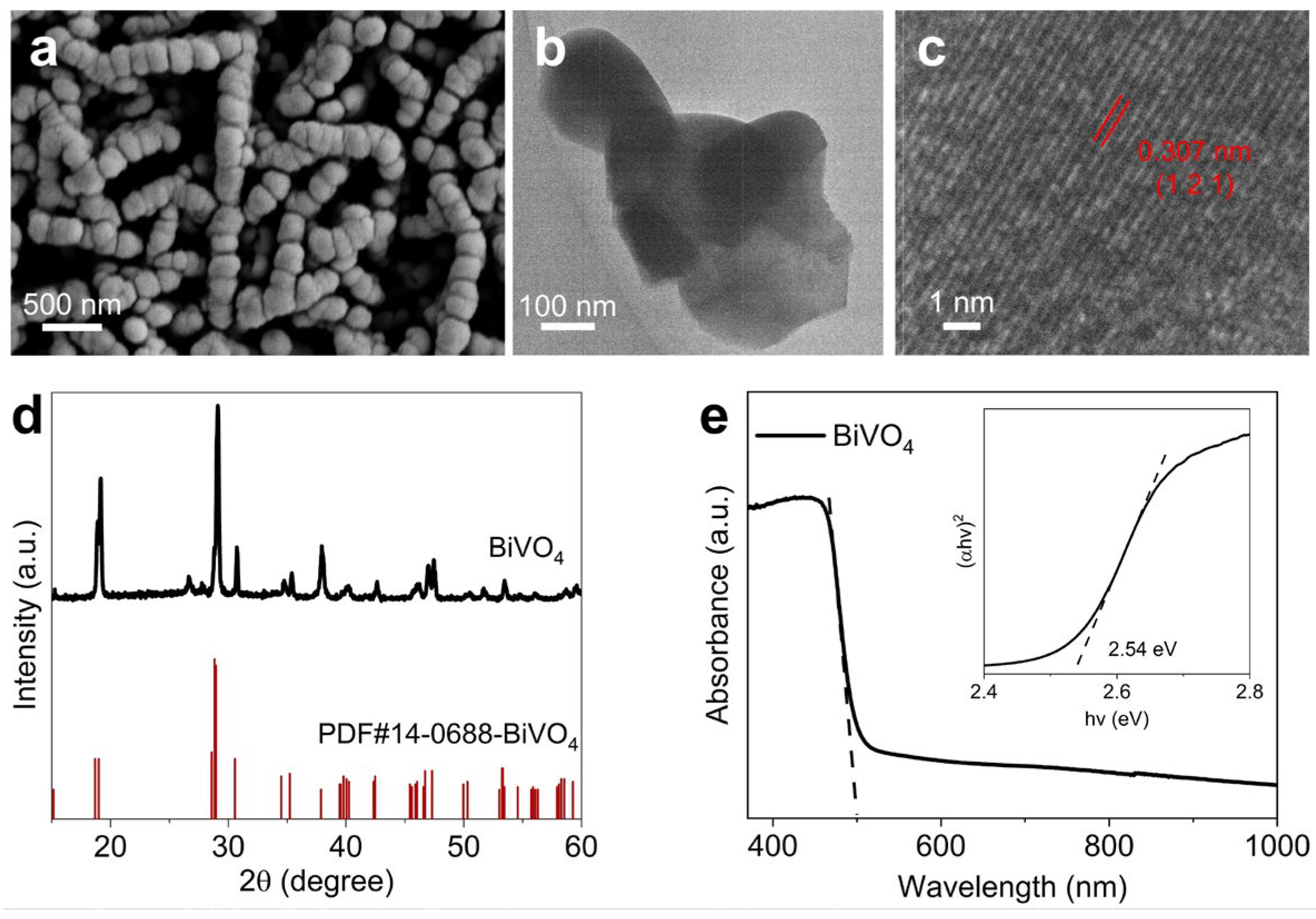
2.1. Synthesis and structural characterizations of the BiVO4 photoanode
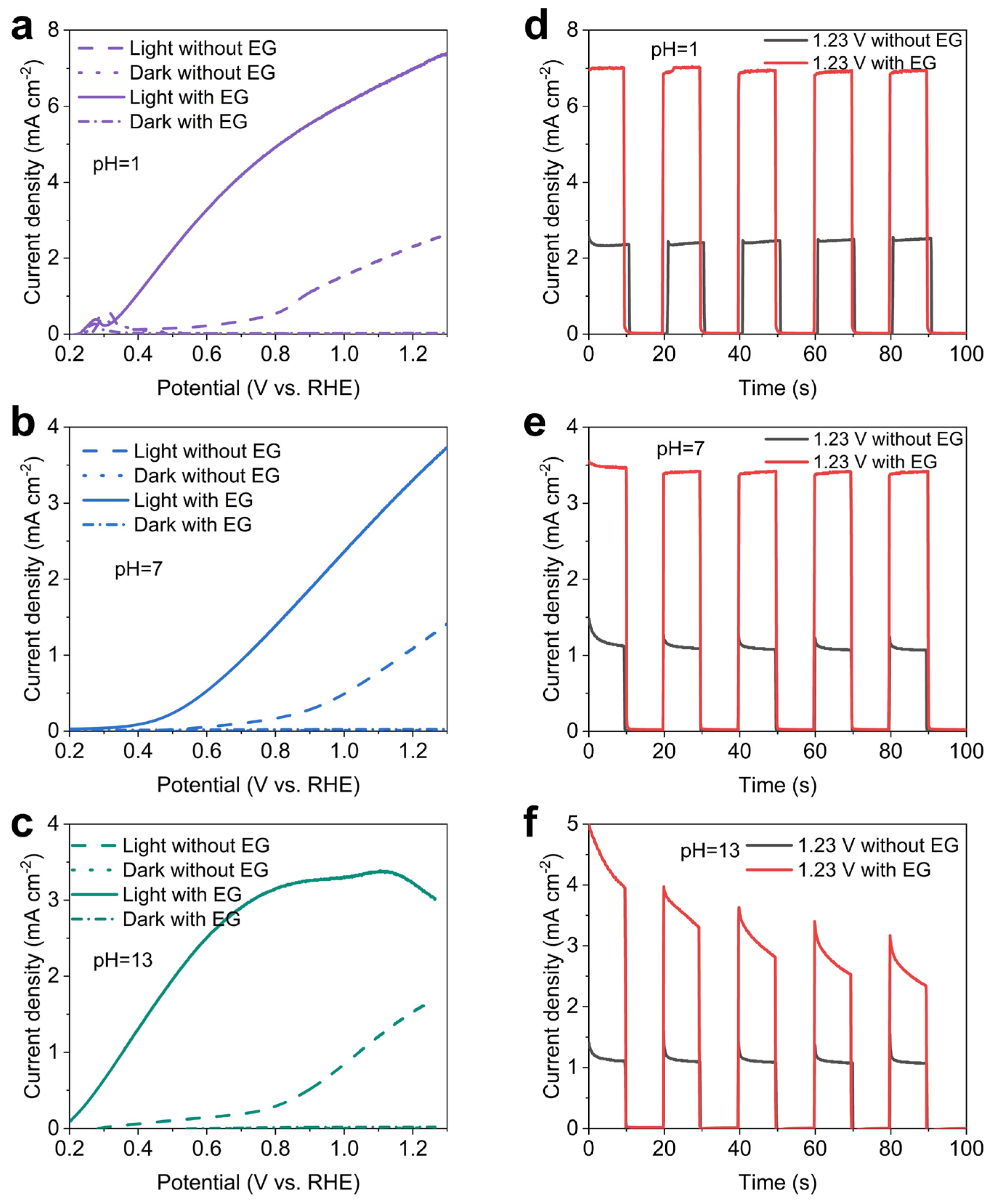
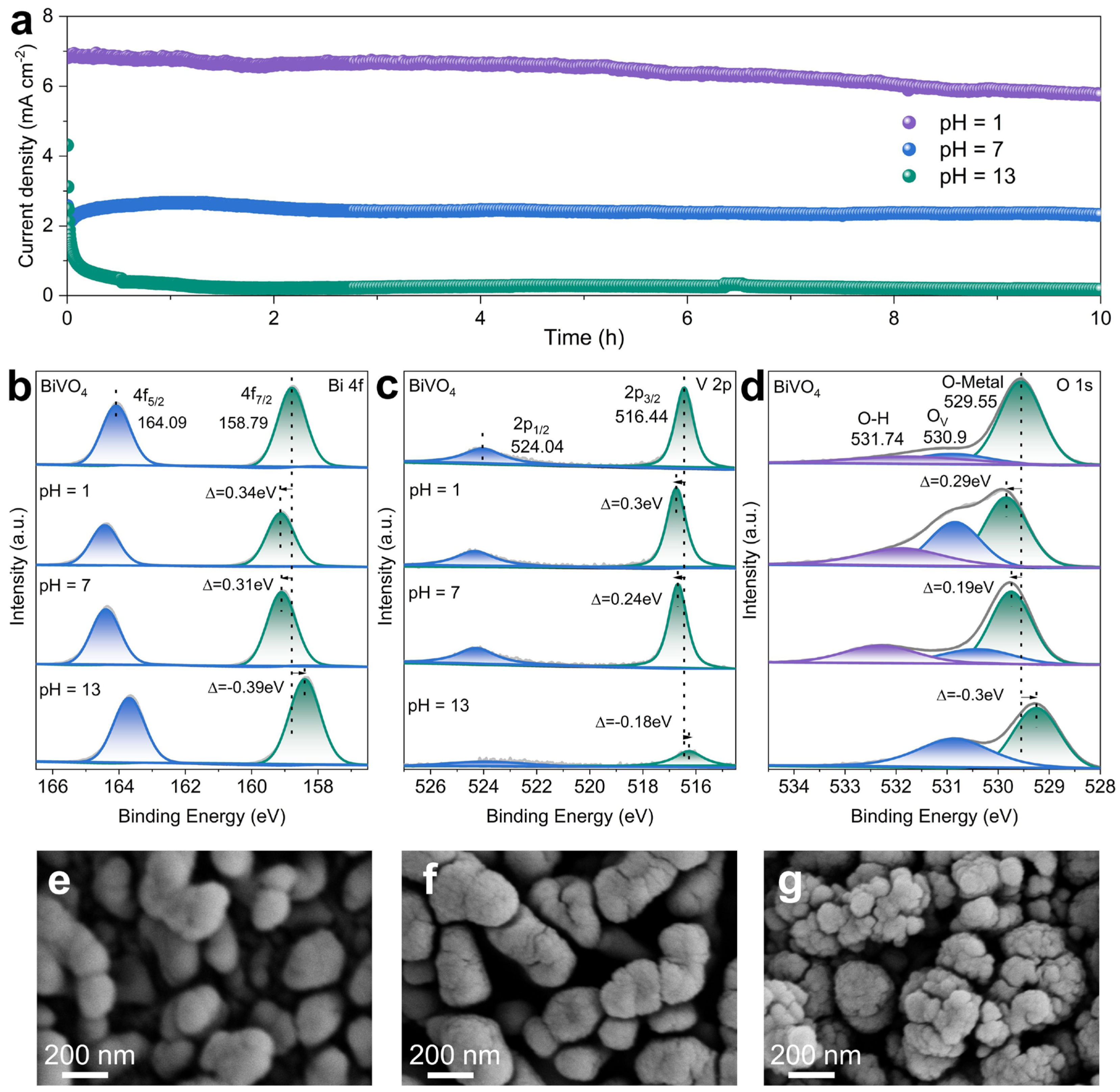
2.2. Photoelectrochemical performance of the BiVO4 photoanode
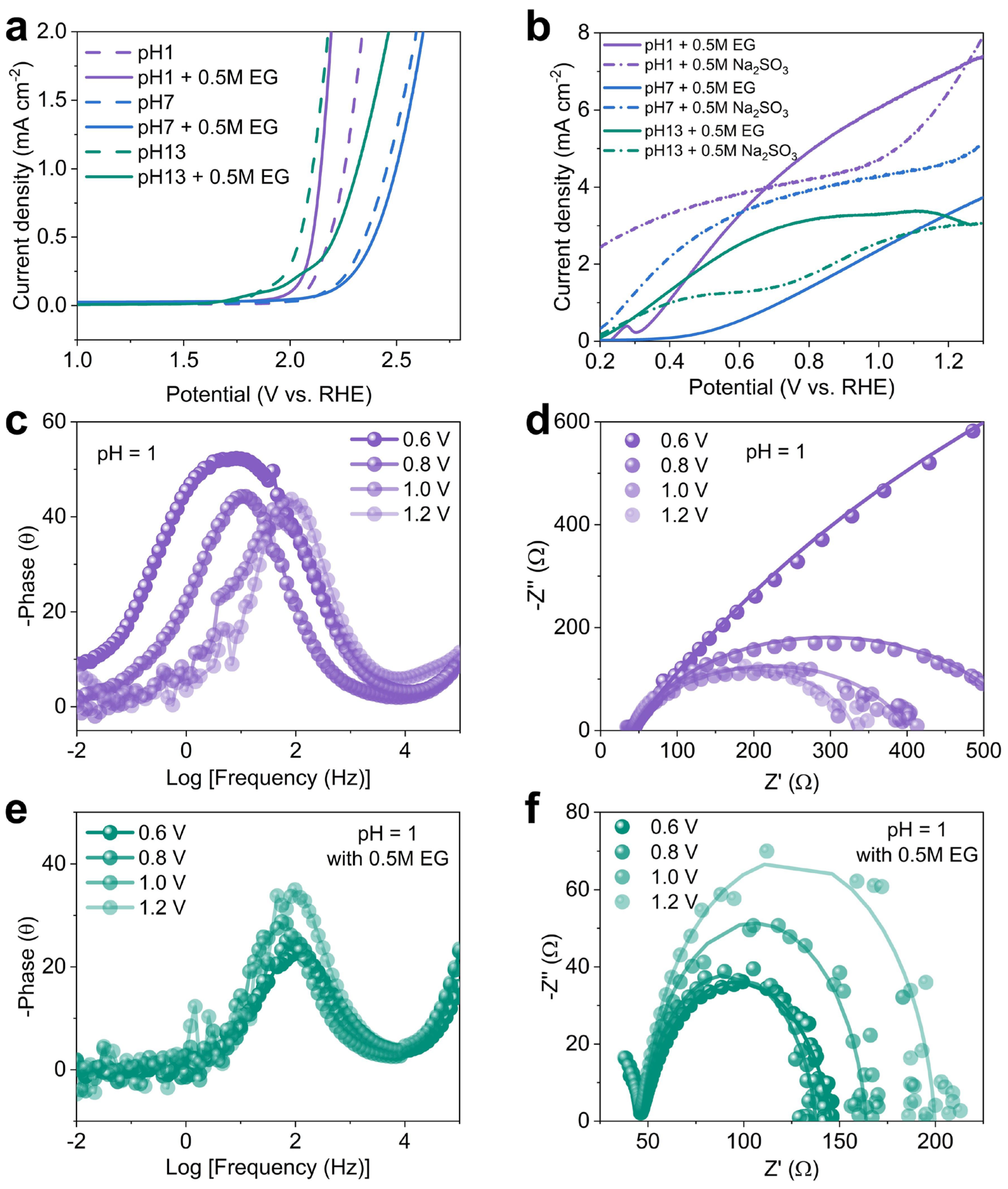
2.3. Charge transport and dynamics in PEC ethylene glycol oxidation
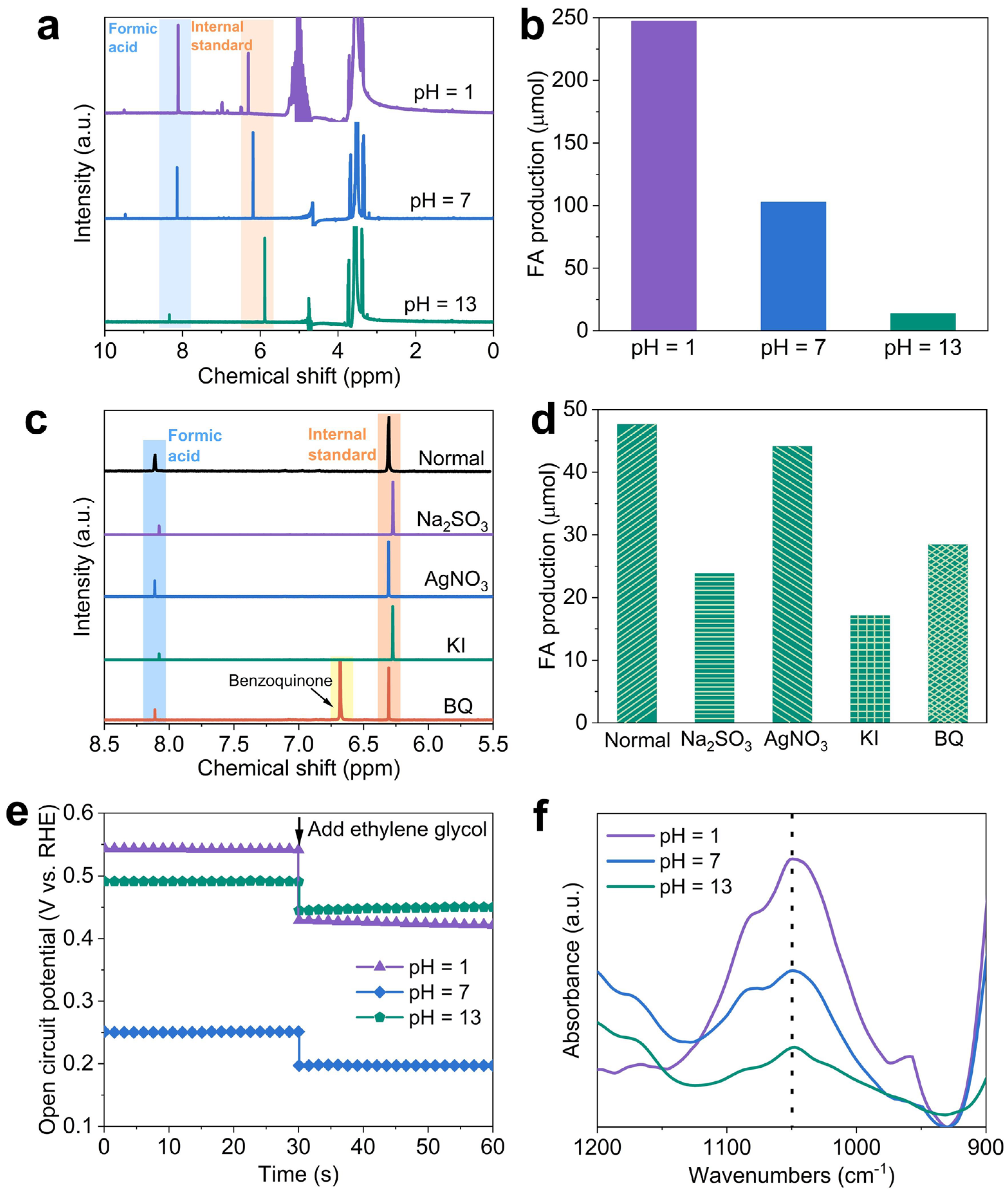
2.4. Product analysis and reaction mechanism of PEC ethylene glycol oxidation
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of the BiVO4 Photoanode
3.3. Characterization
3.4. Photoelectrochemical Measurements
3.5. The product analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, H.; Diaz, D.J.; Czarnecki, N.J.; Zhu, C.; Kim, W.; Shroff, R.; Acosta, D.J.; Alexander, B.R.; Cole, H.O.; Zhang, Y.; Lynd, N.A.; Ellington, A.D.; Alper, H.S. , Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 2022, 604, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Liu, X.; Luo, X.; Xue, Z.; Pan, H.-R.; Fu, J.; Yao, Z.-C.; Jiang, Z.; Lyu, Z.-H.; Zheng, L.; Su, D.; Zhang, J.-N.; Zhang, L.; Hu, J.-S. , Unconventional Bilateral Compressive Strained Ni–Ir Interface Synergistically Accelerates Alkaline Hydrogen Oxidation. J. Am. Chem. Soc. 2023, 145, 13805–13815. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.-H.; Huang, Y.; Song, K.; Li, T.-T.; Cui, J.-Y.; Meng, C.; Zhang, H.; Wang, J.-J. , Ir Single Atoms Boost Metal–Oxygen Covalency on Selenide-Derived NiOOH for Direct Intramolecular Oxygen Coupling. J. Am. Chem. Soc. 2024, 146, 6846–6855. [Google Scholar] [CrossRef] [PubMed]
- Roger, I.; Shipman, M.A.; Symes, M.D. , Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 0003. [Google Scholar] [CrossRef]
- Lu, T.; Li, T.; Shi, D.; Sun, J.; Pang, H.; Xu, L.; Yang, J.; Tang, Y. , In situ establishment of Co/MoS2 heterostructures onto inverse opal-structured N,S-doped carbon hollow nanospheres: Interfacial and architectural dual engineering for efficient hydrogen evolution reaction. SmartMat 2021, 2, 591–602. [Google Scholar] [CrossRef]
- Lewis, N.S. , Research opportunities to advance solar energy utilization. Science 2016, 351, aad1920. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Shi, W.; Liu, Y.; Li, D.; Yin, H.; Chi, H.; Luo, Y.; Ta, N.; Fan, F.; Wang, X.; Li, C. , Unassisted Photoelectrochemical Cell with Multimediator Modulation for Solar Water Splitting Exceeding 4% Solar-to-Hydrogen Efficiency. J. Am. Chem. Soc. 2021, 143, 12499–12508. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-T.; Cui, J.-Y.; Xu, M.; Song, K.; Yin, Z.-H.; Meng, C.; Liu, H.; Wang, J.-J. , Efficient Acidic Photoelectrochemical Water Splitting Enabled by Ru Single Atoms Anchored on Hematite Photoanodes. Nano Lett. 2024, 24, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, Y.; Yang, G.; Zhou, H.; Li, R.; Chen, S.; Li, H.; Li, L.; Fontaine, O.; Deng, J. , Take full advantage of hazardous electrochemical chlorine erosion to ultrafast produce superior NiFe oxygen evolution reaction electrode. Chem. Eng. J. 2022, 446, 136833. [Google Scholar] [CrossRef]
- Wang, X.; Xing, C.; Liang, Z.; Guardia, P.; Han, X.; Zuo, Y.; Llorca, J.; Arbiol, J.; Li, J.; Cabot, A. , Activating the lattice oxygen oxidation mechanism in amorphous molybdenum cobalt oxide nanosheets for water oxidation. J. Mater. Chem. A 2022, 10, 3659–3666. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Du, R.; Xing, C.; Qi, X.; Liang, Z.; Guardia, P.; Arbiol, J.; Cabot, A.; Li, J. , Cobalt Molybdenum Nitride-Based Nanosheets for Seawater Splitting. ACS Appl. Mater. Interfaces 2022, 14, 41924–41933. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Jiao, Y.; Yan, H.; Xie, Y.; Wu, A.; Dong, X.; Guo, D.; Tian, C.; Fu, H. , Interfacial Engineering of MoO2-FeP Heterojunction for Highly Efficient Hydrogen Evolution Coupled with Biomass Electrooxidation. Adv. Mater. 2020, 32, 2000455. [Google Scholar] [CrossRef]
- Korley, L.T.J.; III, T.H.E.; Helms, B.A.; Ryan, A.J. , Toward polymer upcycling—adding value and tackling circularity. Science 2021, 373, 66–69. [Google Scholar] [CrossRef]
- Ellis, L.D.; Rorrer, N.A.; Sullivan, K.P.; Otto, M.; McGeehan, J.E.; Román-Leshkov, Y.; Wierckx, N.; Beckham, G.T. , Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 2021, 4, 539–556. [Google Scholar] [CrossRef]
- Kalathil, S.; Miller, M.; reisner, E. , Microbial Fermentationof Polyethylene Terephthalate (PET) Plastic Waste for the Productionof Chemicals or Electricity**. Angew. Chem. Int. Ed. 2022, 61, e202211057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, R.; Yan, M.; Wang, X.; Wei, X.; Wang, Y.; Wang, L.; Zhang, J.; Yin, S. , One-Pot Synthesis Inorganic – Organic Hybrid PdNi Bimetallenes for PET Electrocatalytic Value-Added Transformation. Advanced Functional Materials 2024, 2401796. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; Sun, R.; Wang, A.; Wang, X.; Zhang, T. , Synthesis of ethylene glycol and terephthalic acid from biomass for producing PET. Green Chem. 2016, 18, 342–359. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. , Palladium-Based Electrocatalysts for Alcohol Oxidation in Half Cells and in Direct Alcohol Fuel Cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Ma, X.; Han, X.; Xing, C.; Qi, X.; He, R.; Arbiol, J.; Pan, H.; Zhao, J.; Deng, J.; Zhang, Y.; Yang, Y.; Cabot, A. , Selective Ethylene Glycol Oxidation to Formate on Nickel Selenide with Simultaneous Evolution of Hydrogen. Adv. Sci. 2023, 10, 2300841. [Google Scholar] [CrossRef]
- Liu, W.-J.; Xu, Z.; Zhao, D.; Pan, X.-Q.; Li, H.-C.; Hu, X.; Fan, Z.-Y.; Wang, W.-K.; Zhao, G.-H.; Jin, S.; Huber, G.W.; Yu, H.-Q. , Efficient electrochemical production of glucaric acid and H2 via glucose electrolysis. Nat. Commun. 2020, 11, 265. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zhang, W.; Yao, X.; Liang, Q. , Hydrogel Enabled Dual-Shielding Improves Efficiency and Stability of BiVO4 Based Photoanode for Solar Water Splitting. Advanced Functional Materials 2024, 2314973. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Zhang, Y.; Xu, L.; Wang, T.; Xiao, X.; Wang, S.; Wang, L.; Huang, W. , A BiVO4 Photoanode with a VOx Layer Bearing Oxygen Vacancies Offers Improved Charge Transfer and Oxygen Evolution Kinetics in Photoelectrochemical Water Splitting. Angew. Chem. Int. Ed. 2023, 62, e202217346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yu, S.; Dai, Y.; Huang, X.; Chou, L.; Lu, G.; Dong, G.; Bi, Y. , Nitrogen-incorporation activates NiFeOx catalysts for efficiently boosting oxygen evolution activity and stability of BiVO4 photoanodes. Nat. Commun. 2021, 12, 6969–6976. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chang, M.; Zhao, Z.; Niu, F.; Zhang, Z.; Sun, Z.; Zhang, L.; Hu, K. , Selective Valorization of Glycerol to Formic Acid on a BiVO4 Photoanode through NiFe Phenolic Networks. ACS Appl. Mater. Interfaces 2023, 15, 11678–11690. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Dong, C.; Kim, S.; Lu, Y.; Wang, Y.; Yu, Z.; Gu, Y.; Gu, Z.; Lee, D.K.; Zhang, K.; Park, J.H. , Photo-Electrochemical Glycerol Conversion over a Mie Scattering Effect Enhanced Porous BiVO4 Photoanode. Adv. Mater. 2023, 35, 2209955. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-G.; Kao, C.-C.; Kuo, J.-L.; Chiu, C.-c.; Chiang, C.-Y. , Unveiling the crystallographic facet dependence of the photoelectrochemical glycerol oxidation on bismuth vanadate. Applied Catalysis B: Environmental 2020, 278, 119303. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.-C.; Cai, W.; Ma, J.; Yang, H.B.; Xiao, H.; Li, J.; Xiong, Y.; Huang, Y.; Liu, B. , Selective photoelectrochemical oxidation of glycerol to high value-added dihydroxyacetone. Nat. Commun. 2019, 10, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.L.; Marinho, J.Z.; dos Santos, A.L.R.; de Faria, A.M.; Souza, R.A.C.; Wang, C.; Patrocinio, A.O.T. , Photoelectrochemical reforming of glycerol by Bi2WO6 photoanodes: Role of the electrolyte pH on the H2 evolution efficiency and product selectivity. Appl. Catal. A., Gen. 2022, 646, 118867. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.-S. , Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Guo, Y.H.; Liu, M.; Liu, X.L.; Zhang, H.P.; Jiang, W.Y.; Wang, P.; Zheng, Z.K.; Liu, Y.N.; Cheng, H.F.; Dai, Y.; Wang, Z.Y.; Huang, B.B. , Boosting H2 Production from a BiVO4 Photoelectrochemical Biomass Fuel Cell by the Construction of a Bridge for Charge and Energy Transfer. Adv. Mater. 2022, 34, 2201594. [Google Scholar] [CrossRef]
- Dotan, H.; Sivula, K.; Grätzel, M.; Rothschild, A.; Warren, S.C. , Probing the photoelectrochemical properties of hematite (α-Fe2O3) electrodes using hydrogen peroxide as a hole scavenger. Energy Environ. Sci. 2011, 4, 958–964. [Google Scholar] [CrossRef]
- Lee, D.K.; Choi, K.-S. , Enhancing long-term photostability of BiVO4 photoanodes for solar water splitting by tuning electrolyte composition. Nat. Energy. 2018, 3, 53–60. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, W.; Bi, Y.; Liang, Y.; Fu, J.; Wang, L.; Zhou, Q. , Accelerating Surface Reaction Kinetics and Enhancing Bulk as well as Surface Charge Carrier Dynamics in Al/ Al2O3-Coated BiVO4 Photoanode. Advanced Functional Materials 2024, 2403396. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Yang, H.; Huang, Y. , Systematic Constructing FeOCl/BiVO4 Hetero-Interfacial Hybrid Photoanodes for Efficient Photoelectrochemical Water Splitting. Small 2024, 2402406. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Dong, C.; Kou, Y.; Hu, C.; Zang, J.; Zhu, J.; Ma, B.; Li, Y.; Ding, Y. , Boosting photoelectrocatalytic oxygen evolution activity of BiVO4 photoanodes via caffeic acid bridged to NiFeOOH. Applied Catalysis B: Environment and Energy 2024, 353. [Google Scholar] [CrossRef]
- Pan, J.B.; Wang, B.H.; Wang, J.B.; Ding, H.Z.; Zhou, W.; Liu, X.; Zhang, J.R.; Shen, S.; Guo, J.K.; Chen, L.; Au, C.T.; Jiang, L.L.; Yin, S.F. , Activity and Stability Boosting of an Oxygen-Vacancy-Rich BiVO4 Photoanode by NiFe-MOFs Thin Layer for Water Oxidation. Angew. Chem. Int. Ed. 2020, 60, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, P.; Yun, J.H.; Hu, Y.; Wang, L. , An Electrochemically Treated BiVO4 Photoanode for Efficient Photoelectrochemical Water Splitting. Angew. Chem. Int. Ed. 2017, 56, 8500–8504. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, S.A.; Yang, J.W.; Sohn, W.; Kim, J.; Cheon, W.S.; Park, J.; Cho, J.H.; Lee, C.W.; Jun, S.E.; Park, S.H.; Moon, J.; Kim, S.Y.; Jang, H.W. , Boosted charge transport through Au-modified NiFe layered double hydroxide on silicon for efficient photoelectrochemical water oxidation. J. Mater. Chem. A 2023, 11, 17503–17513. [Google Scholar] [CrossRef]
- Lee, S.A.; Lee, T.H.; Kim, C.; Choi, M.-J.; Park, H.; Choi, S.; Lee, J.; Oh, J.; Kim, S.Y.; Jang, H.W. , Amorphous Cobalt Oxide Nanowalls as Catalyst and Protection Layers on n-Type Silicon for Efficient Photoelectrochemical Water Oxidation. ACS Catal. 2019, 10, 420–429. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Kuznetsov, D.A.; Pflug, N.C.; Fedorov, A.; Mueller, C.R. , Solar-driven valorisation of glycerol on BiVO4 photoanodes: effect of co-catalyst and reaction media on reaction selectivity. J. Mater. Chem. A 2021, 9, 6252–6260. [Google Scholar] [CrossRef]
- Klahr, B.; Gimenez, S.; Zandi, O.; Fabregat-Santiago, F.; Hamann, T. , Competitive Photoelectrochemical Methanol and Water Oxidation with Hematite Electrodes. ACS Appl. Mater. Interfaces 2015, 7, 7653–7660. [Google Scholar] [CrossRef] [PubMed]
- Heo, N.; Jun, Y.; Park, J.H. , Dye molecules in electrolytes: new approach for suppression of dye-desorption in dye-sensitized solar cells. Sci. Rep. 2013, 3, 1712–1717. [Google Scholar] [CrossRef]
- Wang, X.; Wei, X.; Zhang, R.; Yan, M.; Wei, R.; Zhang, X.; Zhu, Z.; Wang, Y.; Zhao, X.; Yin, S. , Defect-rich Pd@PdOs nanobelts for electrocatalytic oxidation of ethylene glycol. Inorg. Chem. Front. 2024, 11, 2562–2569. [Google Scholar] [CrossRef]
- Deng, K.; Lian, Z.; Wang, W.; Yu, J.; Yu, H.; Wang, Z.; Xu, Y.; Wang, L.; Wang, H. , Lattice Strain and Charge Redistribution of Pt Cluster/Ir Metallene Heterostructure for Ethylene Glycol to Glycolic Acid Conversion Coupled with Hydrogen Production. Small 2023, 20, 2305000. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Shan, Z.; Dong, C.; Lu, Y.; Meng, W.; Zhang, G.; Cai, B.; Su, G.; Park, J.H.; Zhang, K. , Covalent organic frameworks bearing Ni active sites for free radical-mediated photoelectrochemical organic transformations. Sci. Adv. 2023, 9, eadi9442. [Google Scholar] [CrossRef]
- Luo, L.; Chen, W.; Xu, S.-M.; Yang, J.; Li, M.; Zhou, H.; Xu, M.; Shao, M.; Kong, X.; Li, Z.; Duan, H. , Selective Photoelectrocatalytic Glycerol Oxidation to Dihydroxyacetone via Enhanced Middle Hydroxyl Adsorption over a Bi2O3-Incorporated Catalyst. J. Am. Chem. Soc. 2022, 144, 7720–7730. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, D.; Zhang, B.; Xue, Z.; Mu, T. , Substrate molecule adsorption energy: An activity descriptor for electrochemical oxidation of 5-Hydroxymethylfurfural (HMF). Chem. Eng. J. 2022, 433, 133842. [Google Scholar] [CrossRef]
- Zhou, P.; Lv, X.; Tao, S.; Wu, J.; Wang, H.; Wei, X.; Wang, T.; Zhou, B.; Lu, Y.; Frauenheim, T.; Fu, X.; Wang, S.; Zou, Y. , Heterogeneous-Interface-Enhanced Adsorption of Organic and Hydroxyl for Biomass Electrooxidation. Adv. Mater. 2022, 34, 2204089. [Google Scholar] [CrossRef]
- Shi, K.; Si, D.; Teng, X.; Chen, L.; Shi, J. , Pd/NiMoO4/NF electrocatalysts for the efficient and ultra-stable synthesis and electrolyte-assisted extraction of glycolate. Nat. Commun. 2024, 15, 2899. [Google Scholar] [CrossRef]
- Schnaidt, J.; Heinen, M.; Denot, D.; Jusys, Z.; Jürgen Behm, R. , Electrooxidation of glycerol studied by combined in situ IR spectroscopy and online mass spectrometry under continuous flow conditions. J. Electroanal. Chem 2011, 661, 250–264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




