Submitted:
16 May 2024
Posted:
17 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Computational Model
| Average strain | Radial | Circumferential | Longitudinal |
|---|---|---|---|
| Baseline Tmax 0.2 | 0.63±0.11 | -0.20±0.05 | -0.16±0.01 |
| Stiff AA Tmax 0.2 | 0.50±0.11 | -0.18±0.03 | -0.08±0.05 |
| Stiff AA and free apex Tmax 0.2 | 0.72±0.16 | -0.18±0.05 | -0.21±0.02 |
| Baseline Tmax 0.2 vs Stiff AA Tmax 0.2 | -0.13±0.02 | 0.01±0.02 | 0.08±0.06 |
| Baseline Tmax 0.2 vs Stiff AA Tmax 0.2 (%) | -20.21±2.39% | -6.78±10.86% | -48.44±36.88% |
| Baseline Tmax 0.2 vs Stiff AA and free apex Tmax 0.2 | 0.09±0.06 | 0.02±0.04 | -0.05±0.03 |
| Baseline Tmax 0.2 vs Stiff AA and free apex Tmax 0.2 (%) | 14.36±9.73% | -10.17±18.31% | 31.25±16.88% |
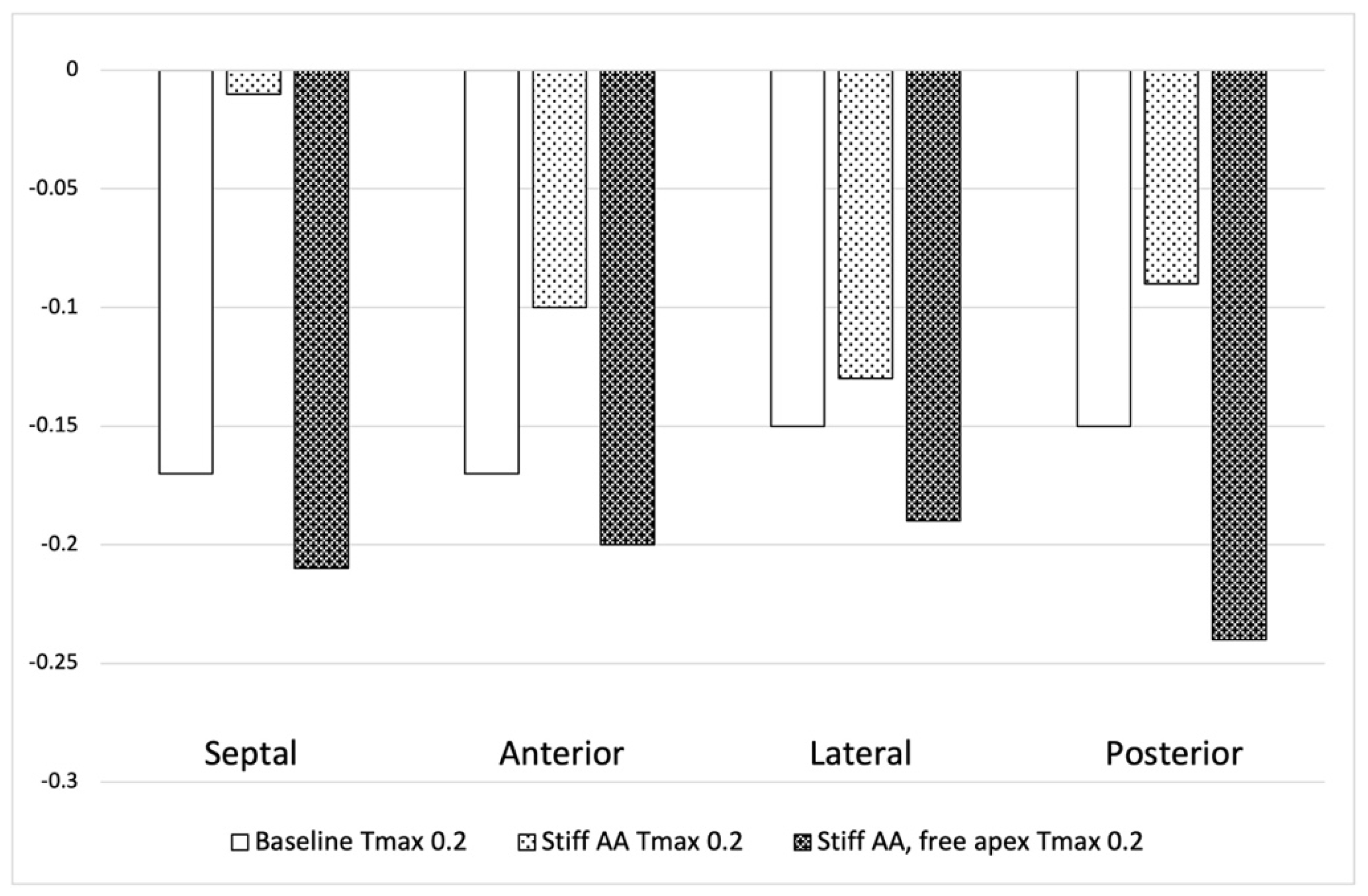
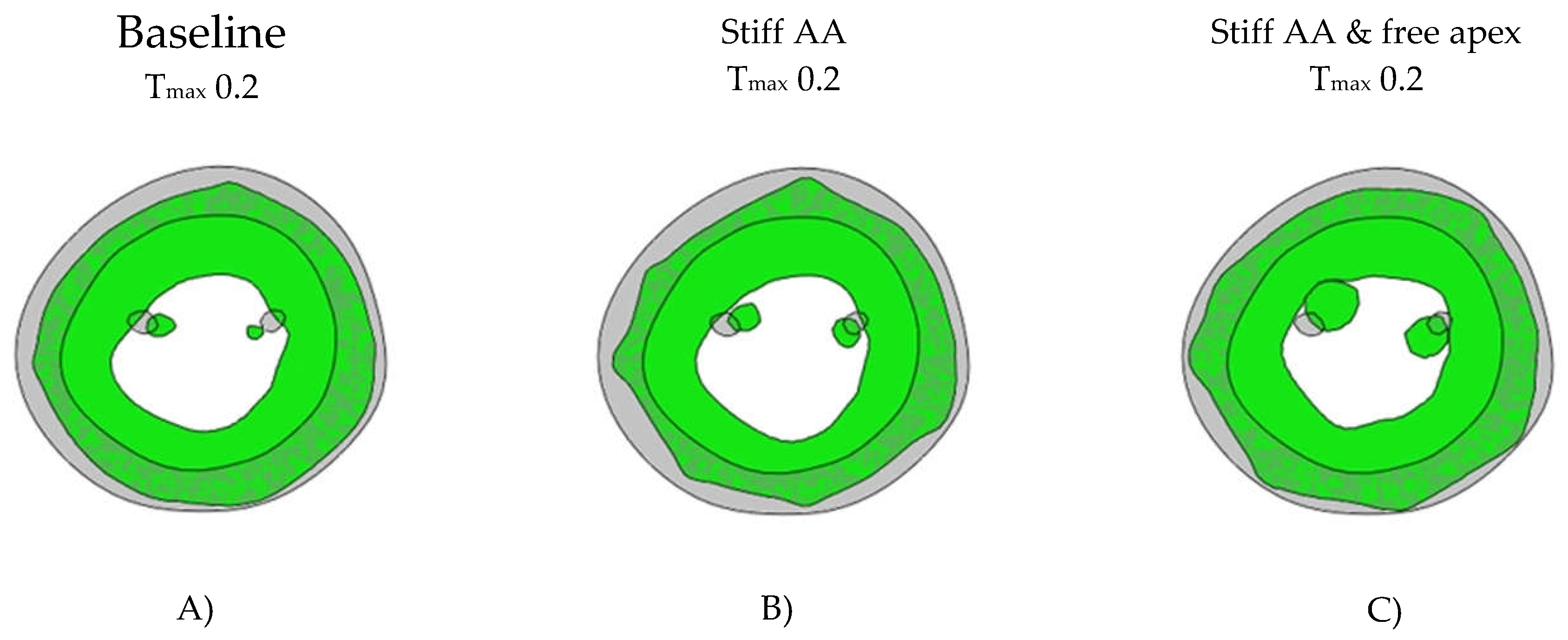
| Longitudinal strain | Septal | Anterior | Lateral | Posterior |
|---|---|---|---|---|
| Baseline Tmax 0.2 | -0.17 | -0.17 | -0.15 | -0.15 |
| Stiff AA Tmax 0.2 | -0.01 | -0.10 | -0.13 | -0.09 |
| Stiff AA and free apex Tmax 0.2 | -0.21 | -0.2 | -0.19 | -0.24 |
| Baseline Tmax 0.2 vs Stiff AA Tmax 0.2 | 0.16 | 0.07 | 0.02 | 0.06 |
| Baseline Tmax 0.2 vs Stiff AA Tmax 0.2 (%) | -94.12% | -41.18% | -13.33% | -40.00% |
| Baseline Tmax 0.2 vs Stiff AA and free apex Tmax 0.2 | -0.04 | -0.03 | -0.04 | -0.09 |
| Baseline Tmax 0.2 vs Stiff AA and free apex Tmax 0.2 (%) | 23.53% | 17.65% | 26.67% | 60.00% |
| Stress | Baseline | Stiff AA | Stiff AA and free apex |
|---|---|---|---|
| Tmax 0.2 | Tmax 0.2 | Tmax 0.2 | |
| (MPa) | 0.056±0.036 | 0.076±0.042 | 0.062±0.038 |
| vs. Baseline | 36.98±42.91% | 12.03±42.19% |
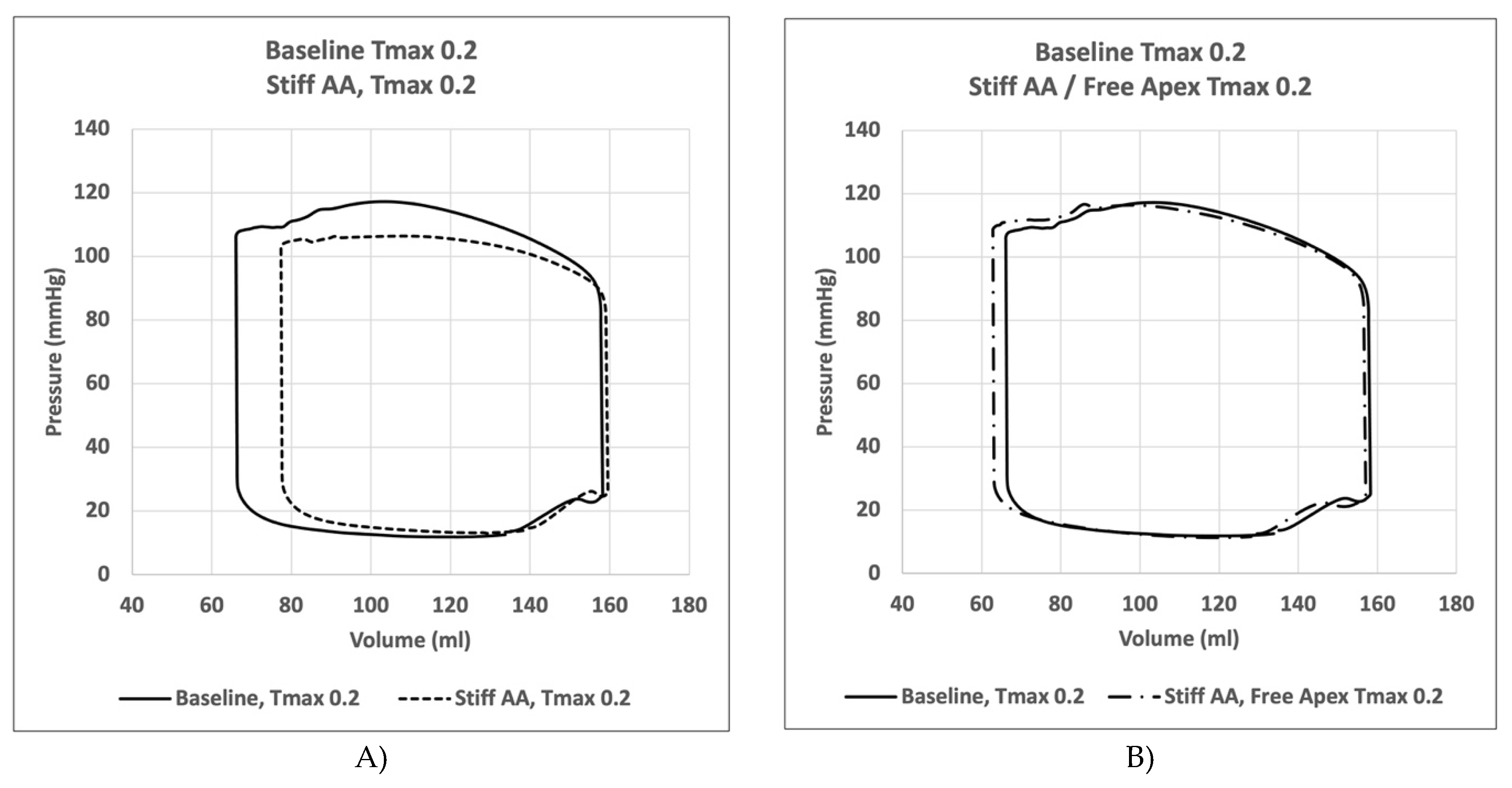
| EDP | EDV | ESP | ESV | SVed-es | SW | |
|---|---|---|---|---|---|---|
| (mmHg) | (ml) | (mmHg) | (ml) | (ml) | (Joule) | |
| Baseline Tmax 0.2 |
11.85 | 158.30 | 117.10 | 66.10 | 92.20 | 8747.50 |
| Stiff AA Tmax 0.2 |
12.86 | 159.60 | 106.40 | 77.40 | 82.20 | 7084.50 |
| Stiff AA with free apex Tmax 0.2 |
11.25 | 157.00 | 116.60 | 62.86 | 94.14 | 8923.00 |
| Baseline vs. Stiff AA Tmax 0.2 vs. Tmax 0.2 |
1.01 | 1.30 | -10.70 | 11.30 | -10.00 | -1663.00 |
| Baseline vs. Stiff AA Tmax 0.2 vs. Tmax 0.2 (%) |
8.52% | 0.82% | -9.14% | 17.10% | -10.85% | -19.01% |
| Baseline vs. Stiff AA with free apex Tmax 0.2 vs. Tmax 0.2 |
-0.60 | -11.3 | -0.50 | -3.24 | 1.94 | 175.50 |
| Baseline vs. Stiff AA with free apex Tmax 0.2 vs. Tmax 0.2 (%) |
-5.06% | -0.82% | 0.43% | -4.90% | 2.10% | 2.01% |


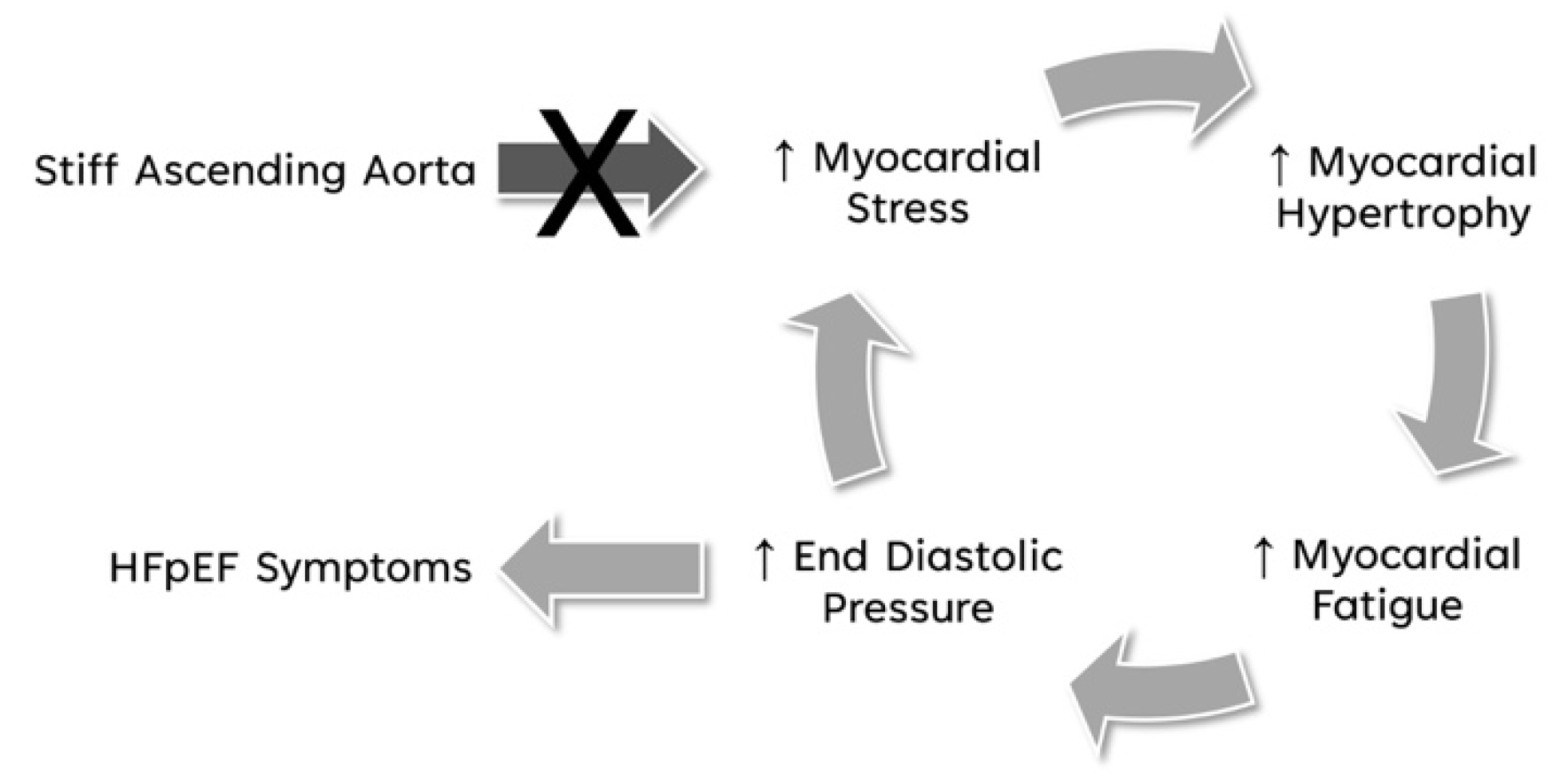
3. Results
3.1. Baseline Simulation
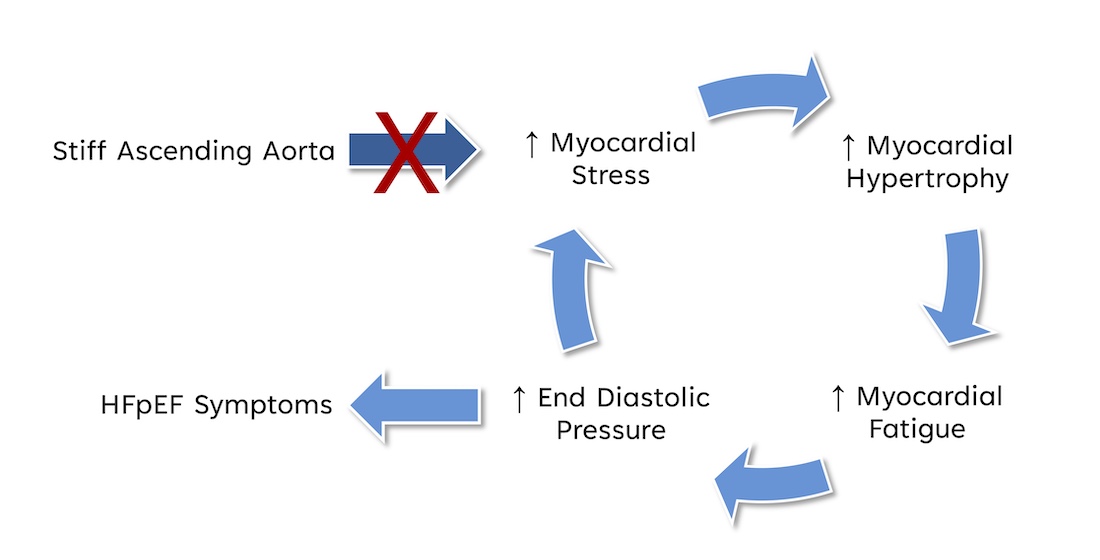
3.2. Effect of Stiff Ascending Aorta
3.3. Effect of Removing Pericardial Boundary Conditions at the Distal Half of the Pericardial Sack with Stiff Ascending Aorta
4. Discussion
5. Limitation of Study
6. Conclusion
Acknowledgments
Conflicts of Interest
References
- Carlsson, M.; Ugander, M.; Heiberg, E.; Arheden, H. The quantitative relationship between longitudinal and radial function in left, right, and total heart pumping in humans. Am J Physiol Heart Circ Physiol 2007, 293, H636–H644. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.; Ugander, M.; Mosen, H.; Buhre, T.; Arheden, H. Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 2007, 292, H1452–H1459. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.; Mahajan, A.; Saleh, S.; Hoffman, J.I.; Coghlan, C. Structure and function relationships of the helical ventricular myocardial band. J Thorac Cardiovasc Surg 2008, 136, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Plonek, T.; Rylski, B.; Nawrocki, P.; Beyersdorf, F.; Jasinski, M.; Kuliczkowski, W. Systolic stretching of the ascending aorta. Arch Med Sci 2021, 17, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Plonek, T.; Berezowski, M.; Kurcz, J.; Podgorski, P.; Sasiadek, M.; Rylski, B.; Mysiak, A.; Jasinski, M. The evaluation of the aortic annulus displacement during cardiac cycle using magnetic resonance imaging. BMC Cardiovasc Disord 2018, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Bell, V.; Mitchell, W.A.; Sigurethsson, S.; Westenberg, J.J.; Gotal, J.D.; Torjesen, A.A.; Aspelund, T.; Launer, L.J.; de Roos, A.; Gudnason, V.; et al. Longitudinal and circumferential strain of the proximal aorta. J Am Heart Assoc 2014, 3, e001536. [Google Scholar] [CrossRef] [PubMed]
- Bell, V.; McCabe, E.L.; Larson, M.G.; Rong, J.; Merz, A.A.; Osypiuk, E.; Lehman, B.T.; Stantchev, P.; Aragam, J.; Benjamin, E.J.; et al. Relations between aortic stiffness and left ventricular mechanical function in the community. J Am Heart Assoc 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.T.; Liu, L.; Meng, P.P.; Wang, Y.H.; Li, M.; Yang, J.; Gu, T.X.; Ma, C.Y. Effect of pericardial incision on left ventricular morphology and systolic function in patients during coronary artery bypass grafting. Cardiovasc Ultrasound 2020, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Baillargeon, B.; Rebelo, N.; Fox, D.D.; Taylor, R.L.; Kuhl, E. The living heart project: A robust and integrative simulator for human heart function. Eur J Mech A Solids 2014, 48, 38–47. [Google Scholar] [CrossRef]
- Wisneski, A.D.; Wang, Y.; Cutugno, S.; Pasta, S.; Stroh, A.; Yao, J.; Nguyen, T.C.; Mahadevan, V.S.; Guccione, J.M. Left ventricle biomechanics of low-flow, low-gradient aortic stenosis: A patient-specific computational model. Front Physiol 2022, 13, 848011. [Google Scholar] [CrossRef]
- Holzapfel, G.A.; Ogden, R.W. Constitutive modelling of passive myocardium: A structurally based framework for material characterization. Philos Trans A Math Phys Eng Sci 2009, 367, 3445–3475. [Google Scholar] [CrossRef] [PubMed]
- Sommer, G.; Schriefl, A.J.; Andra, M.; Sacherer, M.; Viertler, C.; Wolinski, H.; Holzapfel, G.A. Biomechanical properties and microstructure of human ventricular myocardium. Acta Biomater 2015, 24, 172–192. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.; Hay, I.; Dickstein, M.L.; Yi, G.H.; Wang, J.; Maurer, M.S.; Kass, D.A.; Burkhoff, D. Single-beat estimation of end-diastolic pressure-volume relationship: A novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol 2006, 291, H403–H412. [Google Scholar] [CrossRef] [PubMed]
- Sack, K.L.; Baillargeon, B.; Acevedo-Bolton, G.; Genet, M.; Rebelo, N.; Kuhl, E.; Klein, L.; Weiselthaler, G.M.; Burkhoff, D.; Franz, T.; et al. Partial lvad restores ventricular outputs and normalizes lv but not rv stress distributions in the acutely failing heart in silico. Int J Artif Organs 2016, 39, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.C.; Ratcliffe, M.B.; Zhang, P.; Wallace, A.W.; Fata, B.; Hsu, E.W.; Saloner, D.; Guccione, J.M. Mri-based finite-element analysis of left ventricular aneurysm. Am J Physiol Heart Circ Physiol 2005, 289, H692–H700. [Google Scholar] [CrossRef] [PubMed]
- Strocchi, M.; Gsell, M.A.F.; Augustin, C.M.; Razeghi, O.; Roney, C.H.; Prassl, A.J.; Vigmond, E.J.; Behar, J.M.; Gould, J.S.; Rinaldi, C.A.; et al. Simulating ventricular systolic motion in a four-chamber heart model with spatially varying robin boundary conditions to model the effect of the pericardium. J Biomech 2020, 101, 109645. [Google Scholar] [CrossRef] [PubMed]
- Pagoulatou, S.Z.; Ferraro, M.; Trachet, B.; Bikia, V.; Rovas, G.; Crowe, L.A.; Vallee, J.P.; Adamopoulos, D.; Stergiopulos, N. The effect of the elongation of the proximal aorta on the estimation of the aortic wall distensibility. Biomech Model Mechanobiol 2021, 20, 107–119. [Google Scholar] [CrossRef]
- Moore, C.C.; Lugo-Olivieri, C.H.; McVeigh, E.R.; Zerhouni, E.A. Three-dimensional systolic strain patterns in the normal human left ventricle: Characterization with tagged mr imaging. Radiology 2000, 214, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Kohn, J.C.; Lampi, M.C.; Reinhart-King, C.A. Age-related vascular stiffening: Causes and consequences. Front Genet 2015, 6, 112. [Google Scholar] [CrossRef]
- Oishi, Y.; Miyoshi, H.; Mizuguchi, Y.; Iuchi, A.; Nagase, N.; Oki, T. Aortic stiffness is strikingly increased with age >/= 50 years in clinically normal individuals and preclinical patients with cardiovascular risk factors: Assessment by the new technique of 2d strain echocardiography. J Cardiol 2011, 57, 354–359. [Google Scholar] [CrossRef]
- Wuyts, F.L.; Vanhuyse, V.J.; Langewouters, G.J.; Decraemer, W.F.; Raman, E.R.; Buyle, S. Elastic properties of human aortas in relation to age and atherosclerosis: A structural model. Phys Med Biol 1995, 40, 1577–1597. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E.; Levy, B.I.; Struijker-Boudier, H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 2003, 107, 2864–2869. [Google Scholar] [CrossRef] [PubMed]
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005, 25, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.T.; Wenzelburger, F.; Lee, E.; Heatlie, G.; Leyva, F.; Patel, K.; Frenneaux, M.; Sanderson, J.E. The pathophysiology of heart failure with normal ejection fraction: Exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol 2009, 54, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Thierry, C. Gillebert, M., PHD, Marc L. De Buyzere. Hfpef, diastolic suction, and exercise. JACC: CARDIOVASCULAR IMAGING 2012, 5, 871–873. [Google Scholar]
- Yotti, R.; Bermejo, J.; Antoranz, J.C.; Desco, M.M.; Cortina, C.; Rojo-Alvarez, J.L.; Allue, C.; Martin, L.; Moreno, M.; Serrano, J.A.; et al. A noninvasive method for assessing impaired diastolic suction in patients with dilated cardiomyopathy. Circulation 2005, 112, 2921–2929. [Google Scholar] [CrossRef]
- Kelly, R.P.; Tunin, R.; Kass, D.A. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ Res 1992, 71, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Hay, I.; Fetics, B.; Kass, D.A. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: Implications for systolic and diastolic reserve limitations. Circulation 2003, 107, 714–720. [Google Scholar] [CrossRef]
- Chow, B.; Rabkin, S.W. The relationship between arterial stiffness and heart failure with preserved ejection fraction: A systemic meta-analysis. Heart Fail Rev 2015, 20, 291–303. [Google Scholar] [CrossRef]
- Hundley, W.G.; Kitzman, D.W.; Morgan, T.M.; Hamilton, C.A.; Darty, S.N.; Stewart, K.P.; Herrington, D.M.; Link, K.M.; Little, W.C. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol 2001, 38, 796–802. [Google Scholar] [CrossRef]
- Desai, A.S.; Mitchell, G.F.; Fang, J.C.; Creager, M.A. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail 2009, 15, 658–664. [Google Scholar] [CrossRef]
- Karagodin, I.; Aba-Omer, O.; Sparapani, R.; Strande, J.L. Aortic stiffening precedes onset of heart failure with preserved ejection fraction in patients with asymptomatic diastolic dysfunction. BMC Cardiovasc Disord 2017, 17, 62. [Google Scholar] [CrossRef]
- Yip, G.; Wang, M.; Zhang, Y.; Fung, J.W.; Ho, P.Y.; Sanderson, J.E. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: Time for a redefinition? Heart 2002, 87, 121–125. [Google Scholar] [CrossRef]
- DeVore, A.D.; McNulty, S.; Alenezi, F.; Ersboll, M.; Vader, J.M.; Oh, J.K.; Lin, G.; Redfield, M.M.; Lewis, G.; Semigran, M.J.; et al. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: Insights from the relax trial. Eur J Heart Fail 2017, 19, 893–900. [Google Scholar] [CrossRef]
- Shah, A.M.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; Liu, L.; Pitt, B.; Pfeffer, M.A.; Solomon, S.D. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 2015, 132, 402–414. [Google Scholar] [CrossRef]
- Morris, D.A.; Boldt, L.H.; Eichstadt, H.; Ozcelik, C.; Haverkamp, W. Myocardial systolic and diastolic performance derived by 2-dimensional speckle tracking echocardiography in heart failure with normal left ventricular ejection fraction. Circ Heart Fail 2012, 5, 610–620. [Google Scholar] [CrossRef]
- Wang, J.; Fang, F.; Wai-Kwok Yip, G.; Sanderson, J.E.; Feng, W.; Xie, J.M.; Luo, X.X.; Lee, A.P.; Lam, Y.Y. Left ventricular long-axis performance during exercise is an important prognosticator in patients with heart failure and preserved ejection fraction. Int J Cardiol 2015, 178, 131–135. [Google Scholar] [CrossRef] [PubMed]
- DuPont, J.J.; Kenney, R.M.; Patel, A.R.; Jaffe, I.Z. Sex differences in mechanisms of arterial stiffness. Br J Pharmacol 2019, 176, 4208–4225. [Google Scholar] [CrossRef]
- Samargandy, S.; Matthews, K.A.; Brooks, M.M.; Barinas-Mitchell, E.; Magnani, J.W.; Janssen, I.; Hollenberg, S.M.; El Khoudary, S.R. Arterial stiffness accelerates within 1 year of the final menstrual period: The swan heart study. Arterioscler Thromb Vasc Biol 2020, 40, 1001–1008. [Google Scholar] [CrossRef]
- Coutinho, T.; Borlaug, B.A.; Pellikka, P.A.; Turner, S.T.; Kullo, I.J. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol 2013, 61, 96–103. [Google Scholar] [CrossRef]
- van Ommen, A.; Canto, E.D.; Cramer, M.J.; Rutten, F.H.; Onland-Moret, N.C.; Ruijter, H.M.D. Diastolic dysfunction and sex-specific progression to hfpef: Current gaps in knowledge and future directions. BMC Med 2022, 20, 496. [Google Scholar] [CrossRef]
- Tran, P.; Banerjee, P. Myocardial fatigue at a glance. Curr Heart Fail Rep 2023, 20, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Linekar, A.; Dandekar, U.; Barker, T.; Balasubramanian, S.; Bhaskara-Pillai, J.; Shelley, S.; Maddock, H.; Banerjee, P. Profiling the biomechanical responses to workload on the human myocyte to explore the concept of myocardial fatigue and reversibility: Rationale and design of the power heart failure study. J Cardiovasc Transl Res 2024, 17, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Burkhoff, D.; Topkara, V.K.; Sayer, G.; Uriel, N. Reverse remodeling with left ventricular assist devices. Circ Res 2021, 128, 1594–1612. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.S.; O’Toole, M.L.; Hiller, W.D.; Hackney, K.; Reichek, N. Cardiac fatigue after prolonged exercise. Circulation 1987, 76, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Kleinnibbelink, G.; van Dijk, A.P.J.; Fornasiero, A.; Speretta, G.F.; Johnson, C.; Hopman, M.T.E.; Sculthorpe, N.; George, K.P.; Somauroo, J.D.; Thijssen, D.H.J.; et al. Exercise-induced cardiac fatigue after a 45-minute bout of high-intensity running exercise is not altered under hypoxia. J Am Soc Echocardiogr 2021, 34, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Oxborough, D.; Birch, K.; Shave, R.; George, K. “Exercise-induced cardiac fatigue”--a review of the echocardiographic literature. Echocardiography 2010, 27, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Maddock, H.; Banerjee, P. Myocardial fatigue: A mechano-energetic concept in heart failure. Curr Cardiol Rep 2022, 24, 711–730. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Tran, P.; Ennis, S.; Powell, R.; McGuire, S.; McGregor, G.; Kimani, P.K.; Weickert, M.O.; Miller, M.A.; Cappuccio, F.P.; et al. Rising arterial stiffness with accumulating comorbidities associates with heart failure with preserved ejection fraction. ESC Heart Fail 2023, 10, 2487–2498. [Google Scholar] [CrossRef]
- Daou, D.; Gillette, T.G.; Hill, J.A. Inflammatory mechanisms in heart failure with preserved ejection fraction. Physiology (Bethesda) 2023, 38, 0. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Carter, R.E.; Melenovsky, V.; DeSimone, C.V.; Gaba, P.; Killu, A.; Naksuk, N.; Lerman, L.; Asirvatham, S.J. Percutaneous pericardial resection: A novel potential treatment for heart failure with preserved ejection fraction. Circ Heart Fail 2017, 10, e003612. [Google Scholar] [CrossRef] [PubMed]
- Watkins, M.W.; LeWinter, M.M. Physiologic role of the normal pericardium. Annu Rev Med 1993, 44, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Walkowiak, O.A.; Head, W.T.; Kwon, J.H.; Kavarana, M.N.; Rajab, T.K. A review of animal models for post-operative pericardial adhesions. Front Surg 2022, 9, 966410. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Suri, R.M.; Burkhart, H.M.; Greason, K.L.; Dearani, J.A.; Schaff, H.V.; Sundt, T.M., 3rd. Identifying patients at particular risk of injury during repeat sternotomy: Analysis of 2555 cardiac reoperations. J Thorac Cardiovasc Surg 2010, 140, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Shabetai, R. The pericardium; Grune & Stratton: New York, 1981. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





