1. Introduction
Gastric cancer (GC) is among the most common cancers worldwide, with over one million new cases reported annually [
1] and the 3rd most deadly cancer, with an estimated 783,000 deaths in 2018 [
2]. During the last few decades, the efficacy of chemotherapy for GC has only advanced modestly, and targeted therapies have shown few successes. Many human epidermal growth factor receptor-2 (HER2)-targeting agents, such as lapatinib, pertuzumab, and T-DM1, have been tested; however, to date, only trastuzumab (Tmab) has been approved for HER2-positive GC treatment [
3]. To dissect the genomic pathophysiology of GC deeper, The Cancer Genome Atlas (TCGA) reported GC subgroups and has shown that the disease can be categorized into four genomic subtypes and suggested several candidates for targeted therapy [
4]. For example, a new agent targeting claudin-18 isoform 2 (CLDN18.2) showed promise for HER2-negative, CLDN18.2 + GC patients in the recent SPOTLIGHT trial [
5]. The monoclonal antibody zolbetuximab significantly prolonged progression-free survival (PFS) and overall survival (OS) when combined with mFOLFOX6 compared to placebo plus mFOLFOX6.
Other potential targets in GC include the hepatocyte growth factor (HGF)–hepatocyte growth factor receptor (c-MET) pathway. The MET gene encodes the hepatocyte growth factor receptor (HGFR), also known as the MET receptor, which is a receptor tyrosine kinase involved in cell growth, survival, and migration [
6]. Analysis of 444 patients with GC showed that the positivity determined by immunohistochemistry (IHC) for c-MET was 24.8% and the MET amplification rate was 2.3% [
7]. Patients with GC and MET amplification may have a worse prognosis than those without these genetic alterations [
8]. However, the criteria used to define the overexpression of MET protein have differed among many studies. In contrast to the initial phase II studies, phase III trials failed to show any clinical benefit from anti-MET monoclonal antibodies (mAbs) in GCs, even in patients with MET-positive disease in the RILOMET-1 [
9] and METGastric [
10] trials. The primary limitation of these trials was the inclusion of patients in whom the MET did not clearly drive the disease. As a result, the trials with the highest expression of MET gene amplification had fewer participants, potentially explaining the observed negative outcomes [
11].
Since the failure of these mAbs against MET, many tyrosine kinase inhibitors (TKIs) against MET have been developed for various types of cancers, including GC. Several studies have used consistent criteria to define MET amplification using FISH, and recent MET inhibitor trials for MET-amplified cancers have been successful. For non-small cell lung cancers, capmatinib [
12], tepotinib [
13] and crizotinib [
14] are currently recommended for patients with high level MET amplification or MET exon 14 skipping mutations in the US. The VIKTORY trial was a basket study of patients with GC based on clinical sequencing [
15]. In this study, savolitinib, a class I c-MET inhibitor and small-molecule receptor TKI, showed an overall response rate of 50% (10 of 20) in the MET-amplified arm. This indicates that patients with MET-amplified GC can experience additional advantages from targeted therapy, potentially leading to improved OS compared to those who receive conventional second-line chemotherapy as part of their standard care. Although these MET TKIs show potential as targeted therapies for MET-amplified GC, none have been approved for GC treatment due to the lack of confirmatory clinical trials.
This study aimed to evaluate the efficacy of capmatinib and savolitinib against MET-amplified GC cell lines in vitro and in vivo.
2. Results
2.1. Identification of MET-Amplified GC Cells and Assessment of Their Sensitivity to Capmatinib and Savolitinib
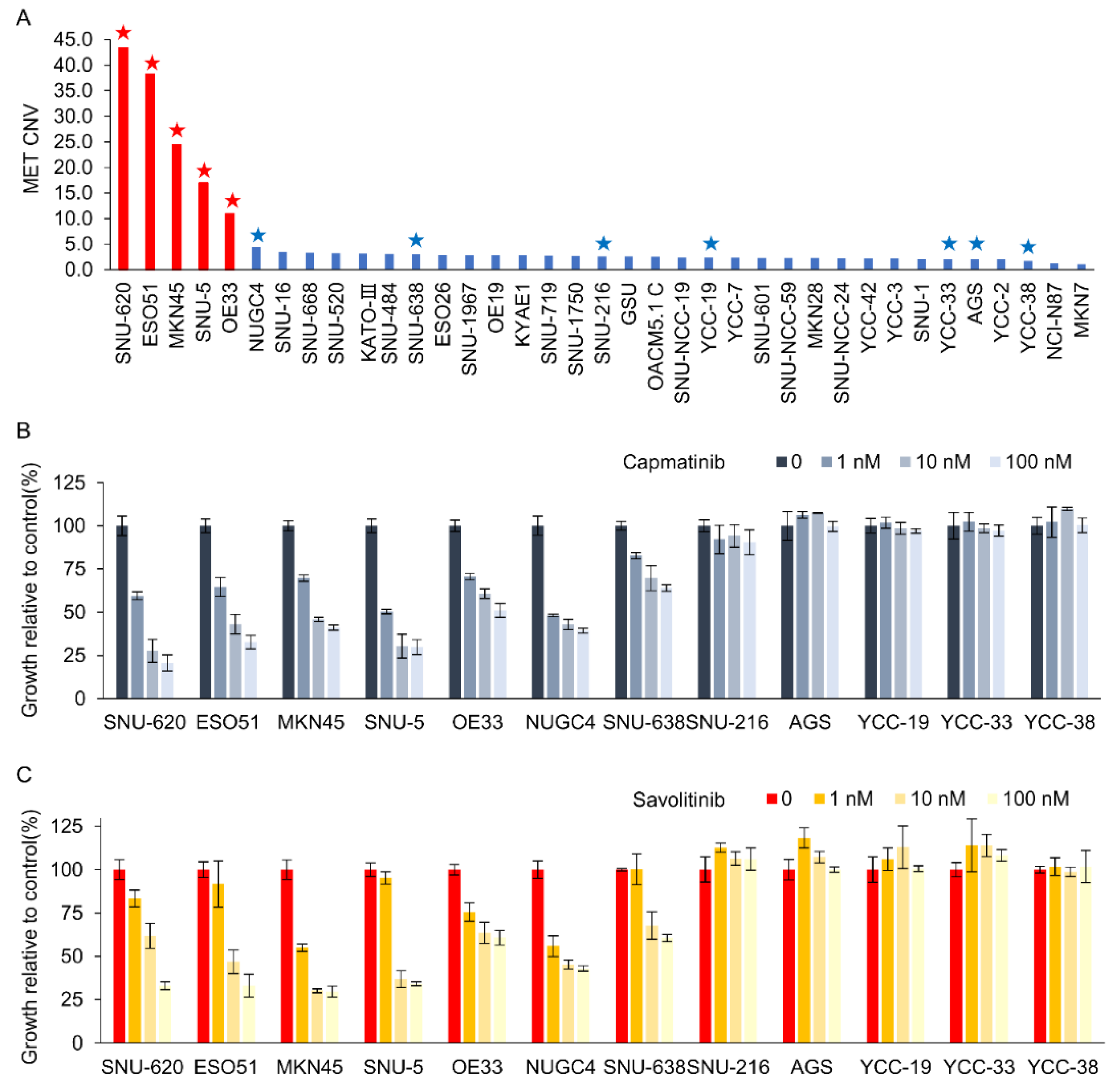
We evaluated the 37 GC cell lines for MET amplification using droplet digital polymerase chain reaction (ddPCR). MET protein overexpression was not significantly correlated with MET amplification in these cells (
Supplementary Figure S1). MET overexpression is not the right biomarker to identify MET-positive GC cells for MET TKIs in clinical trials [
11]. Here, ddPCR revelaed that five GC cell lines exhibited MET copy numbers > 10 (
Figure 1A). Subsequently, CCK-8 assays were performed on cells treated with capmatinib (
Figure 1B) and savolitinib (
Figure 1C). MET-amplified GC cells exhibited dose-dependent responses to these agents. Notably, NUGC-4 and SNU-638 cells, which exhibited MET overexpression but not amplification, only showed modest responses to MET TKIs.
2.2. Clonogenic Cell Survival Assay Revealed that Capmatinib and Savolitinib Inhibited MET-Positive GC Cell Growth
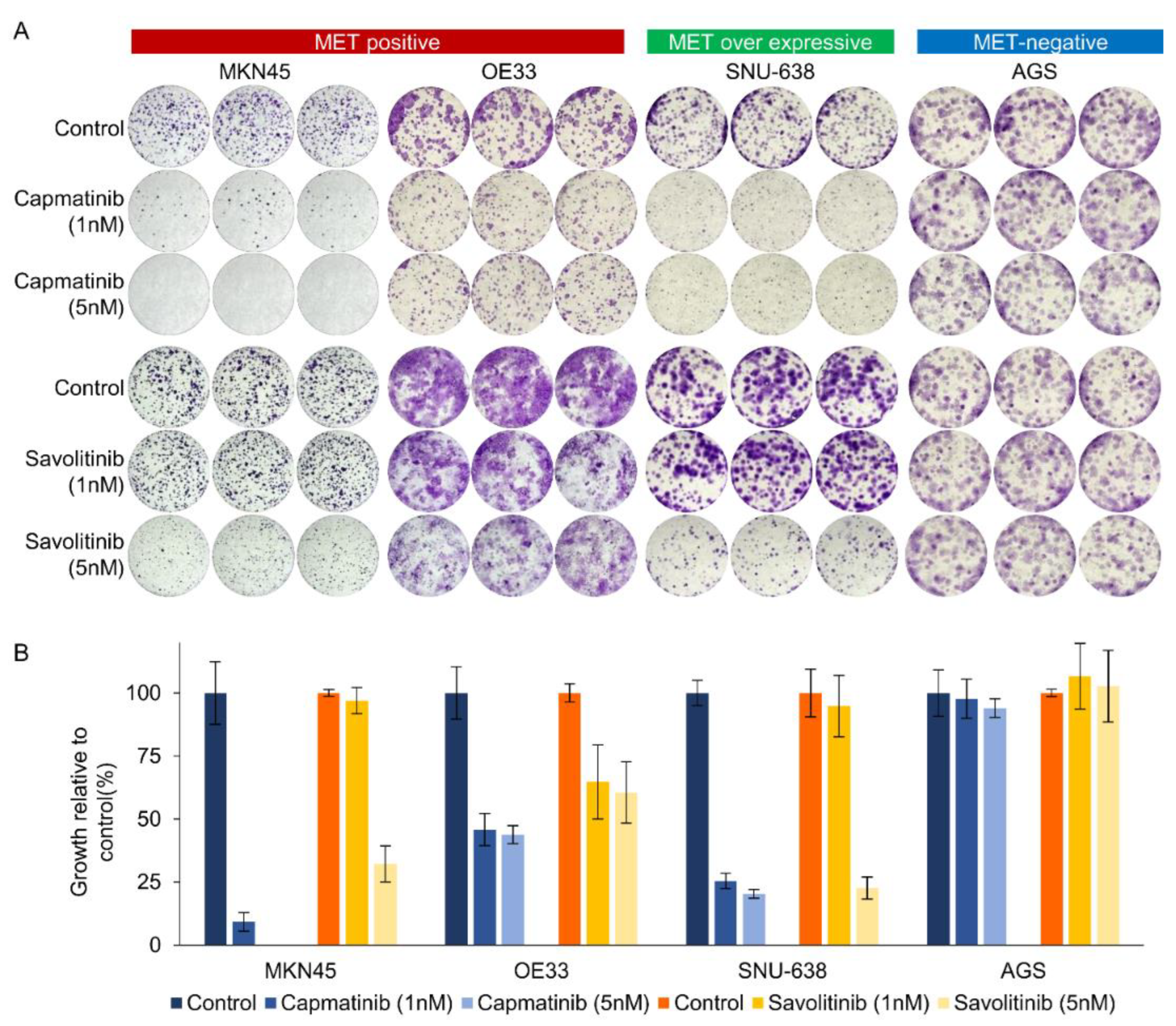
Next, we performed clonogenic cell survival assays on MKN45, OE33, SNU638, and AGS cells. After 2 weeks of culture, MET-positive GC (MKN45 and OE33) cells showed significant decrease in colony formation in a dose-dependent manner after both capmatinib and savolitinib treatments (
Figure 2A). AGS cells (human gastric adenocarcinoma-derived cells) were selected as the MET-negative models. Notably, MET-overexpressing GC (SNU-638) cells, but not AGS cells, were responsive to MET TKIs. Although other MET-positive GC (SNU-5, SNU-620, and ESO51) cells showed good responses to MET TKIs, MET-overexpressing GC (NUGC-4) cells were only modestly sensitive to MET TKIs (
Supplementary Figure S2). Overall, MET-overexpressing GC cells showed variable sensitivity, whereas MET-positive GC cells showed high sensitivity to MET TKIs.
2.3. Savolitinib Inhibits the Phosphorylation of MET and Its Downstream Molecules, Protein Kinase B (AKT), Extracellular Signal-Regulated Kinase (ERK), and S6
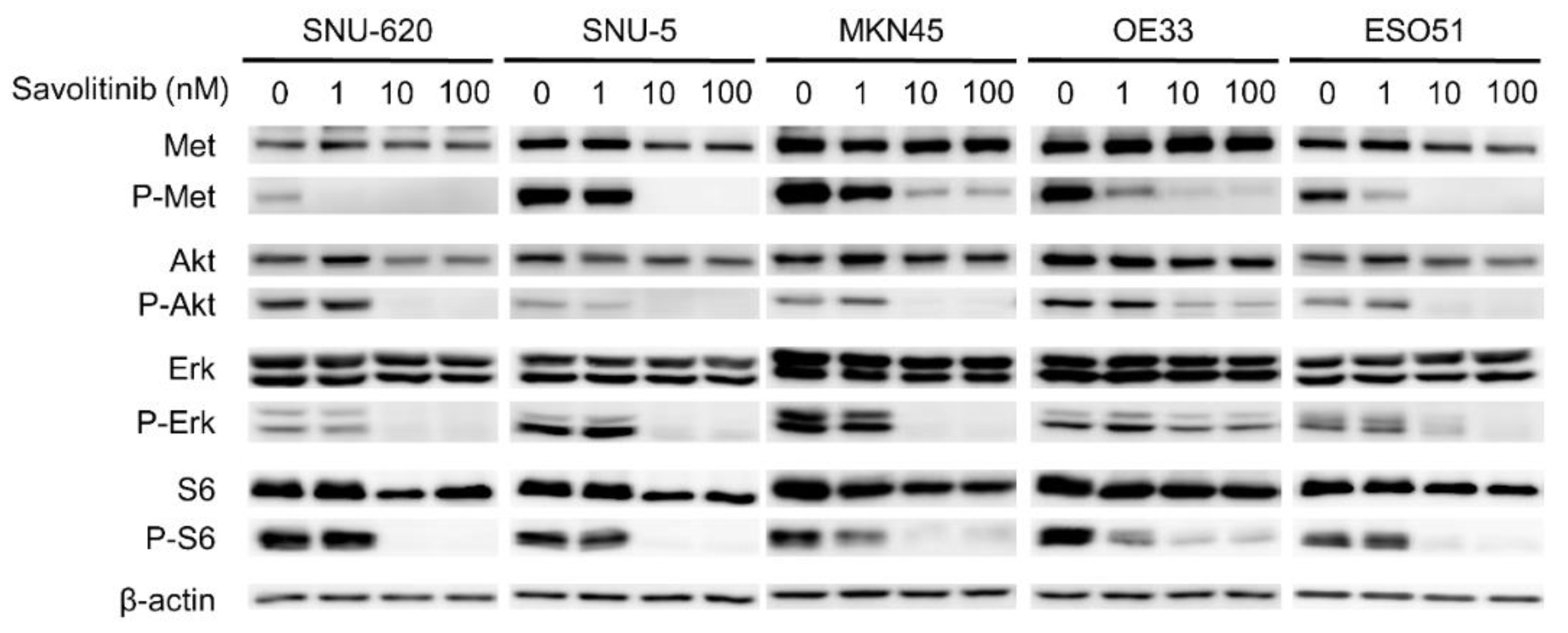
Savolitinib exhibited promising activity in Chinese patients with advanced non-small cell lung cancer (NSCLC) with MET ex14-skipping alterations, including pulmonary sarcomatoid carcinoma, in a phase II trial [
16]. Based on the results of this trial, on June 22, 2021, savolitinib was conditionally approved for the treatment of NSCLC with METex14-skipping alterations in patients who have progressed or are intolerant to platinum-based chemotherapy in China. The anti-proliferative effects of the drug are mediated by the inhibition of phosphorylated c-Met and downstream signaling via the ERK and AKT pathways [
17]. To evaluate the effect of savolitinib on MET-positive GC cells, the signals related to MET expression were assessed via western blotting. We treated these GC cells with 1, 10 and 100 nM of savolitinib for 24 h. Savolitinib effectively inhibited MET and its downstream signaling molecules, AKT, ERK, and S6 (
Figure 3). Similarly, capmatinib showed clear inhibition of MET and its downstream signaling molecules in MET-positive MKN45 and OE33 cells (
Supplementary Figure S3).
2.4. Effects of Savolitinib on MET-Positive GC MKN45 Xenografts
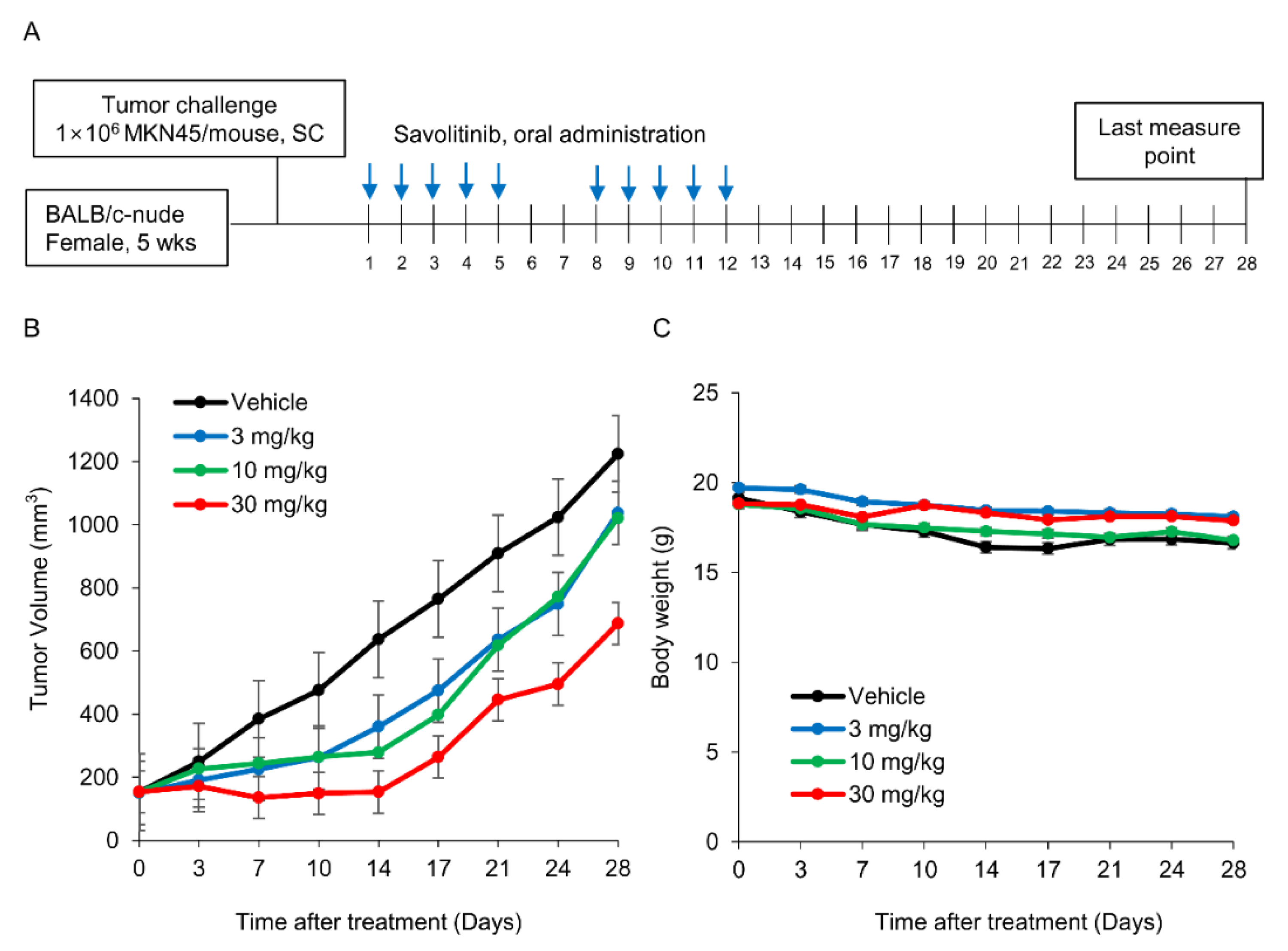
Next, we tested the efficacy of savolitinib in vivo. MKN45 xenografts were treated with savolitinib at the specified doses for 14 d (
Figure 4A). Savolitinib monotherapy significantly inhibited the growth of xenografts (
Figure 4B), but the body weights of mice were similar among the tested groups (
Figure 4C). These findings suggest that savolitinib inhibits the growth of MET-positive GC cells in vivo.
2.5. Combined Effects of Savolitinib and Trastuzumab on HER2/MET-Positive OE33 Cells
OE33 cells exhibit reduced sensitivity to the HER2 inhibitor, lapatinib, when used as a single agent [
18]. We previously confirmed their relative resistance to Tmab [
19]. Tmab monotherapy does not significantly inhibit ERK and S6 activity on OE33 cells [
19]. Consistently, MET co-amplifications are significantly associated with worse outcomes, indicating resistance to anti-HER2 targeted therapy for HER2+ GC in a post hoc analysis of 327 samples from JACOB trial [
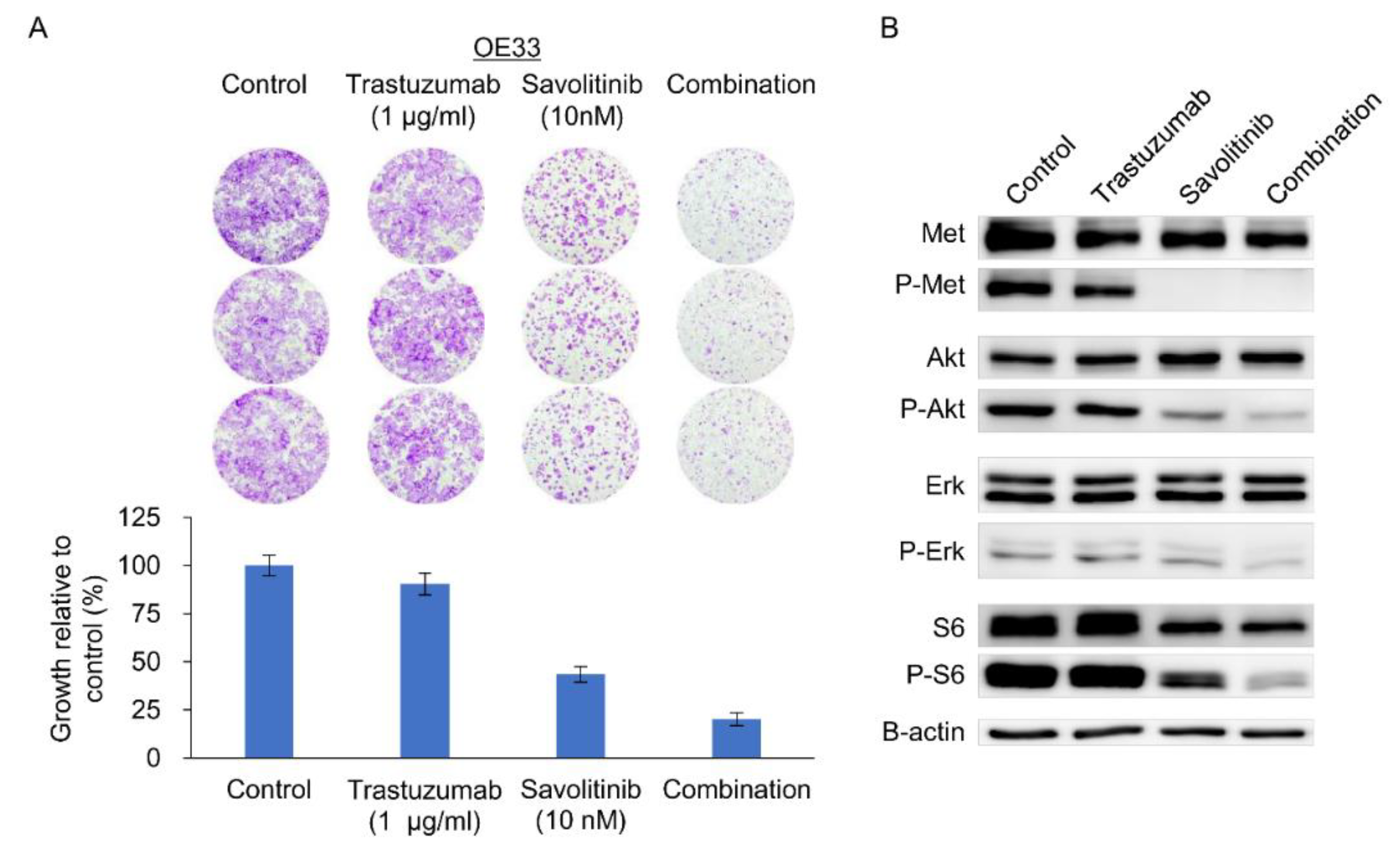
20]. Here, treatment of OE33 cells with Tmab, savolitinib, or their combination effectively suppressed the growth of colonies (
Figure 5A) and the downstream molecules, AKT, ERK and S6 (
Figure 5B). Therefore, Tmab and savolitinib combination can effectively target the HER2/MET-positive OE33 cells.
3. Discussion
Our study demonstrated that the growth of MET-amplified GC cells was effectively suppressed by savolitinib and capmatinib. Here, we suggest suitable cell line-based models for future studies of MET-positive GC. MET inhibitors in combination with trastuzumab exhibited synergistic therapeutic effects on HER2- and MET-amplified GC cells. Although the incidence of MET amplification is relatively low in patients with GC, it could be a promising target for next-generation sequencing.
Determining which patients with GC will benefit from c-MET inhibitors remains difficult. As discussed briefly, MET mAbs have failed in large phase III clinical trials, possibly because of the definition of MET-positive cases. Rilotumumab with epirubicin, cisplatin and capecitabine (ECX) improved PFS to a median of 5.7 months compared to 4.2 months compared to ECX and Hazard Ratio for PFS was 0.60 (80% confidence interval 0.45 – 0.79, P = 0.016) [
21]. Subsequent phase III trial defined MET-positive tumors when the tumor showed ≥25% of tumor cells with membrane staining of ≥1+ staining intensity[
9]. MET gene amplification was assessed in exploratory analyses by fluorescence in-situ hybridization (FISH) and amplification was defined as a MET:CEN7 ratio of 2.0 or greater. The trial was terminated early because of the higher number of deaths in the rilotumumab group than in the placebo group. Moreover, no biomarkers (MET IHC, amplification, or MET copies) showed a distinct effect of rilotumumab. In the METGastric phase 3 trial, onartuzumab was evaluated as a first-line treatment in combination with mFOLFOX6 in patients with GC [
10]. MET-positive tumors were defined by IHC as at least 50% of the tumor cells showing weak, moderate, and/or strong staining intensity (MET 1 +/2 +/3 +). Unfortunately, this trial was terminated prematurely because of the lack of efficacy in a phase 2 trial [
22]. The addition of onartuzumab to mFOLFOX6 did not improve OS, PFS, or ORR compared to mFOLFOX6 alone, irrespective of MET expression status. Based on these observations, we hypothesized that a positive IHC screening for cMET is not a suitable biomarker for patients with GC.
MET amplification has been the subject of extensive research on both primary and acquired resistance to many types of targeted therapies. 49 GC cell lines were screened for MET amplification, and c-MET and p-MET [
23]. We identified six GC cell lines with MET amplification and two c-MET-overexpressing cell lines. The authors also tested MET-targeting antibodies (Sym015 and SAIT301) and two small molecules (foretinib and tivantinib) in GC cells. However, none of these agents are currently approved for the treatment of GC. MET amplification is well known induces acquired resistance to gefitinib [24, 25]. Therefore, combination therapy comprising a MET inhibitor and an EGFR-TKI could be a logical treatment strategy to overcome acquired MET-mediated resistance to EGFR-TKIs. In a recent TATTON trial, the combination therapy of savolitinib and osimertinib showed encouraging anti-tumor activity in patients with MET-amplified/overexpressed EGFR-mutated advanced NSCLC who previously experienced disease progression on EGFR TKIs [
26]. MET plays a role in trastuzumab resistance in HER2-positive breast cancer (BC) cells [
27]. Inhibition of MET enhances the sensitivity of cells to trastuzumab-mediated growth inhibition, whereas MET activation protects cells against the effects of trastuzumab. These findings highlight the importance of MET in influencing the response to trastuzumab treatment in patients with HER2-positive BC. Similarly, HGF/MET-mediated acquired resistance to lapatinib has been reported as a mechanism of resistance to HER2-targeted agents in GC cells [28, 29]. MET amplification has also been suggested as a candidate genomic alteration for predicting primary trastuzumab resistance in patients with HER2-positive GC [
30]. In the present study, we thoroughly analyzed the protein expression and gene amplification of MET and HER2 in 37 GC cell lines. Based on this analysis, we identified only HER2/MET dual-positive OE33 cells, which are primarily resistant to trastuzumab [
31], and suggested a potential combination of MET and HER2 inhibition to overcome trastuzumab resistance. A previous study of MET-amplified GC cells did not include a dual positive cell line to test this combination [
23].
MET inhibitors are effective against MET-driven cancers; however, they also pose toxicity concerns. Capmatinib, crizotinib, and tepotinib monotherpaies are approved for the treatment of metastatic NSCLC with high-level MET exon 14 skipping mutations. In the GEOMETRY mono-1 trial with capmatinib [
12], 66% of patients experienced emergent adverse events (TEAE) of grade ≥ 3. Serious adverse reactions occurred in 51% of patients, with dyspnea (7%), pneumonia (4.8%), and pleural effusion (3.6%) being the most prevalent. Tragically, one patient (0.3%) experienced a fatal adverse reaction attributed to pneumonitis. In the VISION trial with tepotinib [
32], 34.8% of patients encountered TEAE of grade ≥ 3. Peripheral edema was the most prevalent TEAE (67.1%), with 11.2% of the patients experiencing grade ≥ 3 peripheral edema. Fatal adverse reactions, including pneumonitis, hepatic failure, and dyspnea from fluid overload, were observed in one patient (1.0%). Amivantamab-vmjw (amivantamab) is a bispecific EGFR/MET antibody approved for patients with advanced NSCLC with EGFR exon 20 insertion mutations after therapy [
33]. Serious adverse reactions were observed in 30% of the patients. Permanent discontinuation due to an adverse reaction were observed in 11% of patients, with the most common (≥1%) adverse reactions leading to permanent discontinuation being pneumonia, infusion-related reaction, pneumonitis/interstitial lung disease, dyspnea, pleural effusion, and rash. Fatal adverse reactions were reported in three patients (2.3%), with two fatal events attributed to pneumonia and one recorded as sudden death. The safety profiles revealed by these findings are unsatisfactory for targeted agents. However, these findings provide important insights for the careful selection of patients for MET-targeted therapy and facilitate the development of better inhibitors with low toxicity.
4. Materials and Methods
4.1. Cell Lines and Reagents
Among the 37 human gastric and esophageal adenocarcinoma cell lines obtained for this study [
19], three GC (SNU-620, MKN-45, and SNU-5) and two esophageal adenocarcinoma (ESO51 and OE33) cell lines were selected for analysis. AGS, MKN-45, SNU-620, SNU638, and SNU-5 cells were obtained from the Korean Cell Line Bank (Seoul, South Korea). ESO51 and OE33 cells were purchased from Sigma Aldrich (St. Louis, MO, USA). YCC-19, YCC-33, and YCC-38 cell lines were provided by Professor Sun-Young Rha (Yonsei Cancer Center, Republic of Korea). NUGC-4 cells were purchased from the RIKEN BRC Cell Bank (Tsukuba, Ibaraki, Japan). All cells were cultured in the Roswell Park Memorial Institute-1640 (RPMI-1640) medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin. Cultured cells were maintained at 37 °C in an atmosphere of 5% CO
2. Trastuzumab was purchased from Orient Bio (Gyeonggi-do, South Korea). Capmatinib (#S2788) and savolitinib (#S7674) were purchased from Selleck Chemicals (Houston, TX, USA).
4.2. ddPCR
Next, ddPCR assay was performed by the LOGONE Bio-Convergence Research Foundation (Seoul, South Korea) using the QX200 system (Bio-Rad, Hercules, CA, USA), according to the manufacturers’ recommendations. The reaction mixture in a volume of 20 µL consisted of 4× ddPCR multiplex supermix (5 μL) for Probes (Bio-Rad, Hercules, CA, USA), primers, probes, and DNA template. For MET copy number analysis, the concentrations of primers and probes were kept in the range of 900–250 nM. All primer sequences are listed in
Supplementary Table S1. All procedures were as previously described [
19]. CNV values were determined from the copy number of each reference gene using the following equation:
4.3. Cell proliferation Assay
Cells (5×103 cells/well) were seeded in 96-well plates and treated with capmatinib or savolitinib for a specific period. Cell survival rate was measured using the cell counting kit-8 (CCK-8; Dojindo, Japan) and calculated using the following formula: ([A450 of experimental group − A450 of background group]/[A450 of control group − A450 of background group]) × 100.
4.4. Clonogenic Cell Survival Assay and Culture in Soft Agar
Clonogenic assays were performed as previously described [
34]. Cells at a density of 2500–5000 cells per well were seeded overnight in 24-well plates. The next day, the cells were treated with capmatinib or savolitinib. The medium was replaced with a fresh medium every three days. Control cells were treated with an equivalent amount of dimethyl sulfoxide. After 10–14 d, the cells were stained with a crystal violet solution (Cat. No. V5265; Sigma-Aldrich) diluted with distilled water to a concentration of 0.1%. The cells were destained with distilled water and quantified using the ImageJ Software 1.53i (National Institutes of Health, Bethesda, MA, USA). For the anchorage-independent colony formation assay, 2.5 × 10
3 to 7.5 × 10
3 cells were suspended in 0.5 mL of 0.4% top agar that was layered on top of 1 mL of 1% base agar in each well in 12-well multi-well plates, as previously described [
19].
4.5. Western Blotting Analysis
Cells (2–8 × 105) were seeded in 100-mm dishes. Proteins were harvested as previously described. Western blotting was performed to determine the expression of relevant signaling pathways, such as the HER2 signaling pathway. Primary antibodies against the following molecules were purchased from Cell Signaling Technology (Beverley, MA, USA): p-MET-Tyr 1234/1235 (#3126), MET (#4560), p-HER2-Tyr 1221/1222 (#2249), HER2 (#2165), p-AKT-Ser 473 (#4060), AKT (#2217), p-ERK-Thr 202/Tyr 204 (#9106), p-S6-Ser 235/236 (#2211) and S6 (#2217). Antibodies against AMPK (#sc-19128), ERK (#sc-271269), and actin (#sc-47778) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Secondary antibodies were purchased from Thermo Fisher Scientific. Membranes were blocked with the blocking buffer (5% non-fat dry milk in Tris-buffered saline containing 0.01% Tween-20 (TBST]) for 1 h at room temperature and incubated with primary antibodies diluted in 5% bovine serum albumin in TBST (1:1000) overnight at 4 °C. The membranes were washed multiple times with TBST and incubated again with the corresponding horseradish peroxidase-conjugated secondary antibodies diluted in 3% non-fat dry milk in TBST (1:5000) for 1 h at room temperature. The membranes were washed multiple times with PBST and visualized using an enhanced chemiluminescence detection kit (Amersham Biosciences, Arlington Heights, IL, USA).
4.6. Establishment of a Xenograft Mouse Model
All animal procedures were approved by and performed according to the protocols of the Seoul National University Institutional Animal Care and Use Committee (SNU-200622-1). Five-week-old athymic nude mice were obtained from Orient Bio (Seongnam-si, Republic of Korea) and acclimated for 14 d. Human MKN-45 GC cells were cultured in the RPMI-1640 medium containing 10% fetal bovine serum and resuspended in 50% Cultrex Basement Membrane Extract in phosphate-buffered saline. The mice were subcutaneously injected with viable MKN-45 cells (2×106 cells/mouse) into the right flank. When the average tumor size reached approximately 150 mm3, the mice were randomly divided into vehicle control and treatment groups, with six mice per group. The day of randomization was defined as day 0. The size of the tumor was measured twice weekly with digital calipers, and the volume of the tumor was calculated using the following formula: volume (mm3) = length (mm) × width (mm)2 × 0.5. All mice were observed daily during the treatment period.
4.7. Statistical Analyses
Statistical analyses were conducted using SPSS version 29.0 (IBM SPSS Statistics for Windows; IBM Corp. Armonk, NY, USA). Data are presented as means ± standard error (SE). The statistical significance of differences was determined using the Kruskal–Wallis test, One-way analysis of variance, and Student’s t-test, as appropriate. All statistical analyses were two-tailed. Statistical significance was set P < 0.05.
5. Conclusions
In conclusion, our findings demonstrated that the MET TKIs, savolitinib and capmatinib, effectively suppressed the proliferation of MET-amplified human GC cells and inhibited tumor growth. However, further investigation is necessary to explore the potential benefits of combining anti-HER2 agents with MET-TKIs for HER2- and MET-positive GC treatment. Although currently available MET-targeted agents show therapeutic potential for patients with specific types of MET-driven cancers, further research is necessary to develop strategies to overcome the significant toxicity of MET inhibitors.
6. Patents
Not applicable.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1. Expression levels of MET in 37 gastric cancer (GC) cells determined via western blotting analysis. MET-positive GC (OE33: MKN-45, SNU-5, SNU-620, and ESO51) cell lines overexpressed MET. Several GC cells without MET amplification exhibited variable MET expression. Figure S2. Long-term effects of capmatinib and savolitinib on MET-positive and -overexpressing GC cells determined via soft agar assay. Cells were treated with capmatinib and savolitinib for 2–3 weeks, and relative colony numbers were calculated. Figure S3. Effects of capmatinib on MET-positive GC cells assessed via immunoblotting analysis.
Author Contributions
J.-S.K.: Conceptualization, methodology, resources, investigation, data curation, formal analysis, validation, software, writing – original draft, writing – review and editing, visualization, supervision, project administration, funding acquisition. M.Y.K.: Conceptualization, methodology, investigation, data curation, formal analysis, validation, writing – review and editing, visualization. S.H.: Conceptualization, methodology, resources, investigation, data curation, validation, writing – review and editing, visualization, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by the Boramae Medical Center (Grant No. 03-2020-3).
Institutional Review Board Statement
No human participants were included in this study. All animal experiments were approved by an Institutional Review Board.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
Some results of this study were presented at the 2022 Annual Meeting of the American Association of Cancer Research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J. Clin. 2021, 71, 209–249. [CrossRef]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: A population-based modelling study. EClinicalMedicine 2022, 47, 101404. [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [CrossRef]
- Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209.
- Shitara K, Lordick F, Bang YJ, Enzinger P, Ilson D, Shah MA, Van Cutsem E, Xu RH, Aprile G, Xu J, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2023;401:1655-1668.
- Bradley, C.A.; Salto-Tellez, M.; Laurent-Puig, P.; Bardelli, A.; Rolfo, C.; Tabernero, J.; Khawaja, H.A.; Lawler, M.; Johnston, P.G.; Van Schaeybroeck, S.; et al. Targeting c-MET in gastrointestinal tumours: Rationale, opportunities and challenges. Nat. Rev. Clin. Oncol. 2017, 14, 562–576. [CrossRef]
- Yang, Y.; Wang, C.; Dai, C.; Liu, X.; Li, W.; Huang, M.; Zhao, X.; Ji, D.; Li, J.; Guo, W. Amplification and expression of c-MET correlate with poor prognosis of patients with gastric cancer and upregulate the expression of PDL1. Acta Biochim. et Biophys. Sin. 2021, 53, 547–557. [CrossRef]
- Peng, Z.; Zhu, Y.; Wang, Q.; Gao, J.; Li, Y.; Li, Y.; Ge, S.; Shen, L. Prognostic Significance of MET Amplification and Expression in Gastric Cancer: A Systematic Review with Meta-Analysis. PLOS ONE 2014, 9, e84502. [CrossRef]
- Catenacci, D.V.T.; Tebbutt, N.C.; Davidenko, I.; Murad, A.M.; Al-Batran, S.-E.; Ilson, D.H.; Tjulandin, S.; Gotovkin, E.; Karaszewska, B.; Bondarenko, I.; et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1467–1482. [CrossRef]
- Shah MA, Bang YJ, Lordick F, Alsina M, Chen M, Hack SP, Bruey JM, Smith D, McCaffery I, Shames DS, et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The METGastric Randomized Clinical Trial. JAMA Oncol. 2017;3:620-627.
- Van Herpe F, Van Cutsem E. The Role of cMET in Gastric Cancer-A Review of the Literature. Cancers (Basel). 2023;15.
- Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, Tan DSW, Hida T, de Jonge M, Orlov SV, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383:944-957.
- Blaszkowska M, Specht-Szwoch Z, Dziadziuszko R. Tepotinib for advanced non-small-cell lung cancer with MET exon 14 skipping mutations. ESMO Open. 2022;7:100449.
- Camidge, D.R.; Otterson, G.A.; Clark, J.W.; Ou, S.-H.I.; Weiss, J.; Ades, S.; Shapiro, G.I.; Socinski, M.A.; Murphy, D.A.; Conte, U.; et al. Crizotinib in Patients With MET-Amplified NSCLC. J. Thorac. Oncol. 2021, 16, 1017–1029. [CrossRef]
- Lee, J.; Kim, S.T.; Kim, K.; Lee, H.; Kozarewa, I.; Mortimer, P.G.; Odegaard, J.I.; Harrington, E.A.; Lee, J.; Lee, T.; et al. Tumor Genomic Profiling Guides Patients with Metastatic Gastric Cancer to Targeted Treatment: The VIKTORY Umbrella Trial. Cancer Discov. 2019, 9, 1388–1405. [CrossRef]
- Lu, S.; Fang, J.; Li, X.; Cao, L.; Zhou, J.; Guo, Q.; Liang, Z.; Cheng, Y.; Jiang, L.; Yang, N.; et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, phase 2 study. Lancet Respir. Med. 2021, 9, 1154–1164. [CrossRef]
- Gavine PR, Ren Y, Han L, Lv J, Fan S, Zhang W, Xu W, Liu YJ, Zhang T, Fu H, et al. Volitinib, a potent and highly selective c-Met inhibitor, effectively blocks c-Met signaling and growth in c-MET amplified gastric cancer patient-derived tumor xenograft models. Mol Oncol. 2015;9:323-333.
- Hassan, S.; Williams, F.; Awasthi, N.; Schwarz, M.A.; Schwarz, R.E.; Li, J.; von Holzen, U. Combination effect of lapatinib with foretinib in HER2 and MET co-activated experimental esophageal adenocarcinoma. Sci. Rep. 2019, 9, 1–11. [CrossRef]
- Kim, J.-S.; Kim, M.Y.; Hong, S. Synergistic Effects of Metformin and Trastuzumab on HER2 Positive Gastroesophageal Adenocarcinoma Cells In Vitro and In Vivo. Cancers 2023, 15, 4768. [CrossRef]
- Pietrantonio, F.; Manca, P.; Bellomo, S.E.; Corso, S.; Raimondi, A.; Berrino, E.; Morano, F.; Migliore, C.; Niger, M.; Castagnoli, L.; et al. HER2 Copy Number and Resistance Mechanisms in Patients with HER2-positive Advanced Gastric Cancer Receiving Initial Trastuzumab-based Therapy in JACOB Trial. Clin. Cancer Res. 2023, 29, 571–580. [CrossRef]
- Iveson, T.; Donehower, R.C.; Davidenko, I.; Tjulandin, S.; Deptala, A.; Harrison, M.; Nirni, S.; Lakshmaiah, K.; Thomas, A.; Jiang, Y.; et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014, 15, 1007–1018. [CrossRef]
- Shah, M.A.; Cho, J.-Y.; Tan, I.B.; Tebbutt, N.C.; Yen, C.-J.; Kang, A.; Shames, D.S.; Bu, L.; Kang, Y.-K. A Randomized Phase II Study of FOLFOX With or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction. Oncol. 2016, 21, 1085–1090. [CrossRef]
- Kim, H.J.; Kang, S.K.; Kwon, W.S.; Kim, T.S.; Jeong, I.; Jeung, H.; Kragh, M.; Horak, I.D.; Chung, H.C.; Rha, S.Y. Forty-nine gastric cancer cell lines with integrative genomic profiling for development of c-MET inhibitor. Int. J. Cancer 2018, 143, 151–159. [CrossRef]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.-M.; Zhao, X.; Christensen, J.; et al. MET Amplification Leads to Gefitinib Resistance in Lung Cancer by Activating ERBB3 Signaling. Science 2007, 316, 1039–1043. [CrossRef]
- Ke, E.-E.; Zhou, Q.; Zhang, Q.-Y.; Su, J.; Chen, Z.-H.; Zhang, X.-C.; Xu, C.-R.; Yang, J.-J.; Tu, H.-Y.; Yan, H.-H.; et al. A Higher Proportion of the EGFR T790M Mutation May Contribute to the Better Survival of Patients with Exon 19 Deletions Compared with Those with L858R. J. Thorac. Oncol. 2017, 12, 1368–1375. [CrossRef]
- Hartmaier RJ, Markovets AA, Ahn MJ, Sequist LV, Han JY, Cho BC, Yu HA, Kim SW, Yang JC, Lee JS, et al. Osimertinib + Savolitinib to Overcome Acquired MET-Mediated Resistance in Epidermal Growth Factor Receptor-Mutated, MET-Amplified Non-Small Cell Lung Cancer: TATTON. Cancer Discov. 2023;13:98-113.
- Shattuck DL, Miller JK, Carraway KL, 3rd, Sweeney C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008;68:1471-1477.
- Chen, C.-T.; Kim, H.; Liska, D.; Gao, S.; Christensen, J.G.; Weiser, M.R. MET Activation Mediates Resistance to Lapatinib Inhibition of HER2-Amplified Gastric Cancer Cells. Mol. Cancer Ther. 2012, 11, 660–669. [CrossRef]
- Park, J.; Choi, Y.; Ko, Y.S.; Kim, Y.; Pyo, J.-S.; Jang, B.G.; Kim, M.A.; Lee, J.-S.; Chang, M.S.; Park, J.-W.; et al. FOXO1 Suppression Is a Determinant of Acquired Lapatinib-Resistance in HER2-Positive Gastric Cancer Cells through MET Upregulation. Cancer Res. Treat. 2018, 50, 239–254. [CrossRef]
- Pietrantonio, F.; Fuca, G.; Morano, F.; Gloghini, A.; Corso, S.; Aprile, G.; Perrone, F.; De Vita, F.; Tamborini, E.; Tomasello, G.; et al. Biomarkers of Primary Resistance to Trastuzumab in HER2-Positive Metastatic Gastric Cancer Patients: the AMNESIA Case-Control Study. Clin. Cancer Res. 2018, 24, 1082–1089. [CrossRef]
- Park, K.-S.; Hong, Y.S.; Choi, J.; Yoon, S.; Kang, J.; Kim, D.; Lee, K.-P.; Im, H.-S.; Lee, C.H.; Seo, S.; et al. HSP90 inhibitor, AUY922, debilitates intrinsic and acquired lapatinib-resistant HER2-positive gastric cancer cells. BMB Rep. 2018, 51, 660–665. [CrossRef]
- Mazieres J, Paik PK, Garassino MC, Le X, Sakai H, Veillon R, Smit EF, Cortot AB, Raskin J, Viteri S, et al. Tepotinib Treatment in Patients With MET Exon 14-Skipping Non-Small Cell Lung Cancer: Long-term Follow-up of the VISION Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2023;9:1260-1266.
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.-Y.; Kim, S.-W.; Lee, C.K.; et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J. Clin. Oncol. 2021, 39, 3391–3402. [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).