2.1. LF-NMR Structural Analysis for De-Novo Synthetized Organic Compiounds
Since 2020, when the benchtop NMR operating at 60 MHz (Pulsar Oxford) began to be employed in our instructional organic laboratories, the synthesis of almost all the prescribed experiments has been accompanied by
1H NMR structure characterization. Depending on the educational degree, students either receive knowledge on proton nuclear magnetic resonance under the teacher´s supervision or gain experience through generating the NMR data on their own and proceeding through the entire process towards the
1H NMR solution. Although the instalation of the benchtop-type instrument in the instructional laboratory have enabled the in-time structural analysis subsequently upon reaction completion, undergraduate students as well as gratuates and some young researchers before gaining complete practical independence have to pass out the basic expertise under the supervision of expert in both - data acqusition and data analysis. For successful data analysis the assignment of protons in the structure of elucidated organic compound with the signal (peak) in
1H NMR spectra is neccessary. Such key-step represents a challenge quite often also for skilled chemists. Non-experts are able to solve the problem only by use of available databases [
19] and for that reason, students are preparing and analysis compounds with already known structures. Also, prediction of proton spectra using on-line nmr-predictor allows students to quickly identify the correct structure [
20,
21,
22,
23].
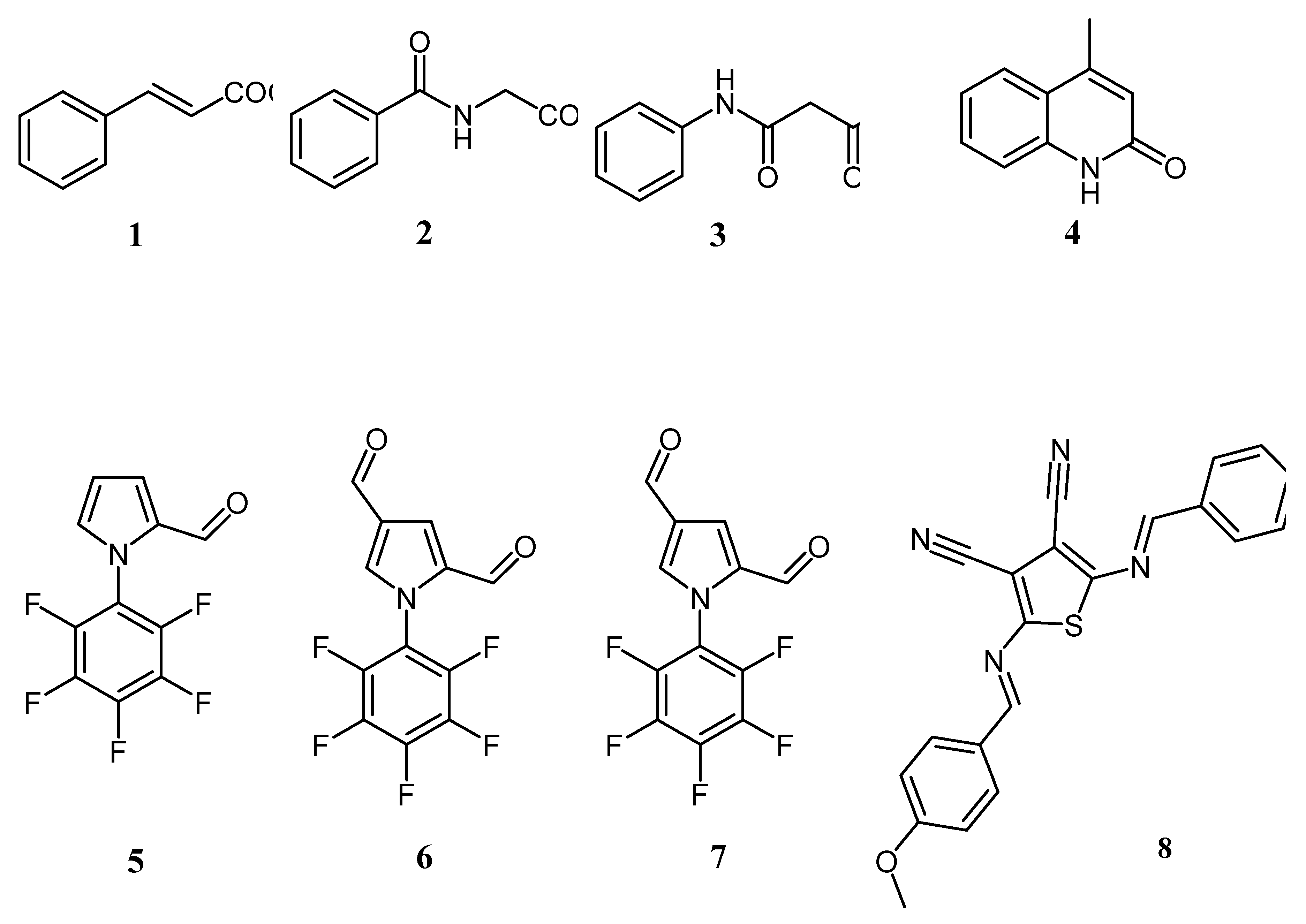
The above detailed algorithm have been applied for structure elucidation of a set of four compounds, namely cinnamic acid (
1) (
Figure 2), hippuric acid (
2) (
Figure 3), acetanilide (
3) and 4-methylquinoline-2-ol (
4) (
Figure 4), that have been prepared during the undegraduate laboratory course. At the end, beyond gaining the skill in synthesis, students were able to prepare the sample and performe the NMR acquisition finalizing their experiments with chemical structure identification as a result of correct interpretation the spectra.
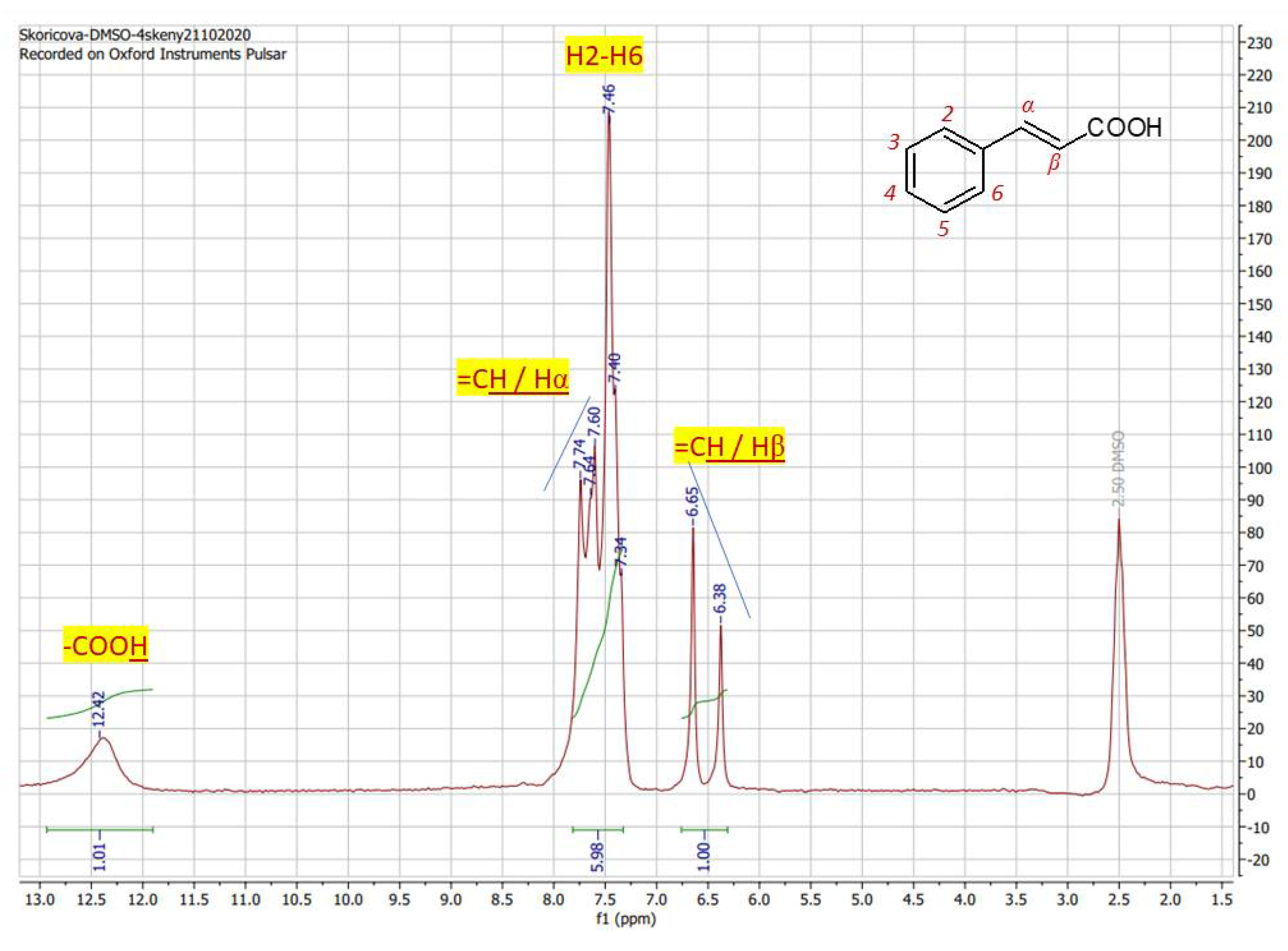
In the proton spectrum of cinnamic acid (
1)
(Figure 2) obtained from a DMSO-
d6 solution at 4 scans (8 mins) the characteristic carboxyl proton is displayed as sharp signal at 12.42 ppm, that is obvious for carboxylic acids. Aromatic peaks of five protons H2-H6 are splitted into a multiplet type of signal in the range of 7.74 – 7.34 ppm. Generally, signal splitting of aromatic hydrogen atoms is obvious also in the spectra taken at high-magnetic fields.The splitting pattern of H2/H6 in the form of doublet is expected at 7.62 ppm but is overlayed with a signal for a double bond proton Hα at 7.67 ppm. Double bond proton Hβ is observed as a clear doublet at 6.52 ppm with interaction constant
3J = 16.2 Hz confirming the
E-izomer of cinnamic acid. For such situation, when the double bond proton is overlayed by other signals the „roof“ effect is helpfull to find the appropriate pair, as is marked (blue line) within the spectrum. In spite of overlayed signals as the consequence of supressed sensitivity, spectrum of cinnamic acid obtained at low field (60 MHz) is thoroughly interpretable and represents a valid spectral result comparable to those, obtained on HF-NMR instrument. The interactions 1/6/1 (sum. = 8 protons) corresponds with formula of cinnamic acid C
9H8O
2.
Figure 2.
1H NMR spectrum of cinnamic acid in DMSO-d6 recorded at 60 MHz with proton assigment according to the structure (4 scans / 8 mins).
Figure 2.
1H NMR spectrum of cinnamic acid in DMSO-d6 recorded at 60 MHz with proton assigment according to the structure (4 scans / 8 mins).
The proton spectrum of hippuric acid (
2) (
Figure 3) that posses a little bit more complex structure than the cinnamic acid exhibit significantly higher sensitivity and signal resolutiom even in an area of aromatic signals (6.5 – 8.0 ppm). Acid proton from carboxylic group is shifted to 12.53 ppm (broad singlet) as is expected for proton of the functional group of carboxylic acids. The nitrogen proton from NH group is represented by signal at 8.78 ppm. On this signal the non-standardly high resolution occurs, since the splitting with either neighboring protons of CH
2 or aromtic protons is transformed to a triplet form of signal instead of broad singlet. Aromatic protons H2/H6 are represented by doublets at 7.84 ppm with
3J = 3.6 Hz. Aromatic proton H4 is viewed at 7.43 ppm as doubked and aromatic part is complemented with doublet at 7.50 ppm with
3J = 3.6 Hz for H3/H5. Integration 1/1/2/3/2 (sum. = 9 protons) corresponds with compound formula C
9H9NO
3. ince the acetanilide (
3) acts as a substrate for subsequent synthesis of 4-methylquinoline-2-ol (
4) that is achieved upon treatment with P
2O
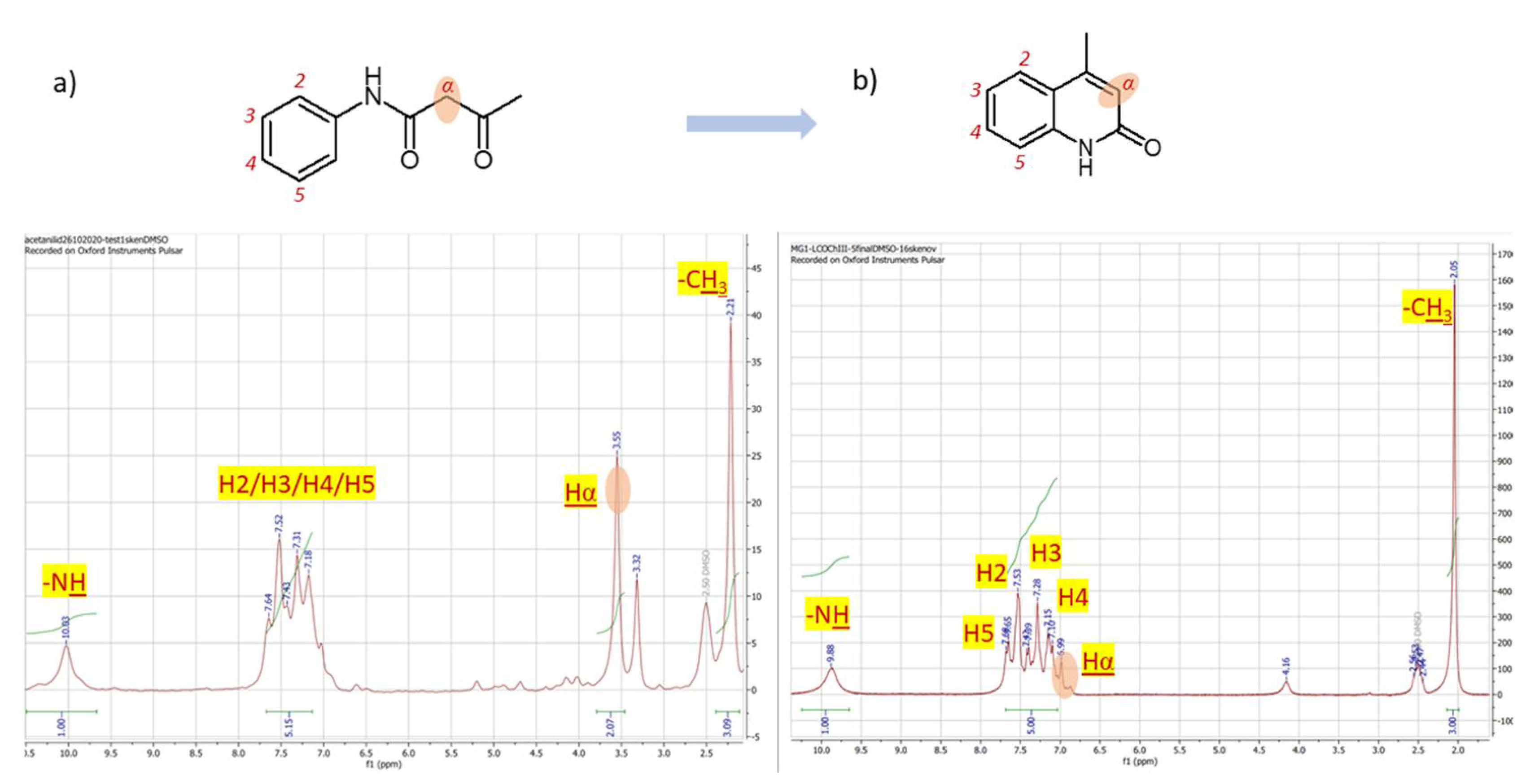
5, by observing the changes of proton spectra profiles of both compounds allows to examine the completion of reaction process. Although the resolution of proton spectra of acetanilide (
Figure 4a) is comparably lower, since only 4 scan (8 mins) were used to achieve spectrum the main difference the aliphatic protons of substrate (Fig. 6a, Hα) that are through the reaction converted to an aromatic one (
Figure 4b, H Hα) is visible immediately upon comparison of the two spectra. While the group of aliphatic protons in the structure of acetanilide is assigned with signal at 3.55 ppm (
Figure 4a) as singlet with integration equal to 2, the aromatic proton in the structure of product 4-methylquinoline-2-ol is observed in the range of aromatic protons at 6.99 ppm (
Figure 4b) with integration of one proton. Since the spectrum of 4-methylquinoline-2-ol was taken at 16 scans (32 minutes totall time) the spectrum 6b is with better resolution showing the possibility to distinguish aromatic protons as following: H5 at 7.67 ppm, H2 at 7.53 ppm, H3 at 7.28 ppm and H4 at 7.13 ppm. The structure complemented with the signal of amine group proton at 9.88 observable as broad singlet and three protons of methyl group 2.05 ppm. Methyl protons of substrate on Fig. 6a correspond with chemical shift = 2.21 ppm and NH proton at 10.03ppm. Aromatic protons H2-H5 of acetanilide are splitted in a multiplet-type signal 7.64 – 7.18 ppm. The integration 1/5/2/3 (sum. = 11) correspond with formula of acetanilide C
10H11NO
2 and 1/5/3 (sum. = 9) is in accordance with formula C
10H9NO of 4-methylquinoline-2-ol.
Figure 3.
1H NMR spectrum of hippuric acid in DMSO-d6 recorded at 60 MHz with proton assigment according to the structure (8 scans / 16mins).
Figure 3.
1H NMR spectrum of hippuric acid in DMSO-d6 recorded at 60 MHz with proton assigment according to the structure (8 scans / 16mins).
Figure 4.
1H NMR spectrum of a) acetanilide (60 MHz, DMSO-d6, 2 scans, 4 mins) and b) 4-methylquinoline-2-ol (60 MHz, DMSO-d6, 16 scans, 32 mins).
Figure 4.
1H NMR spectrum of a) acetanilide (60 MHz, DMSO-d6, 2 scans, 4 mins) and b) 4-methylquinoline-2-ol (60 MHz, DMSO-d6, 16 scans, 32 mins).
2.2. Synthesis and Combined 1H / 19F NMR for Structure Analysis of Novel Pentafluorophenylpyrroles 5-7
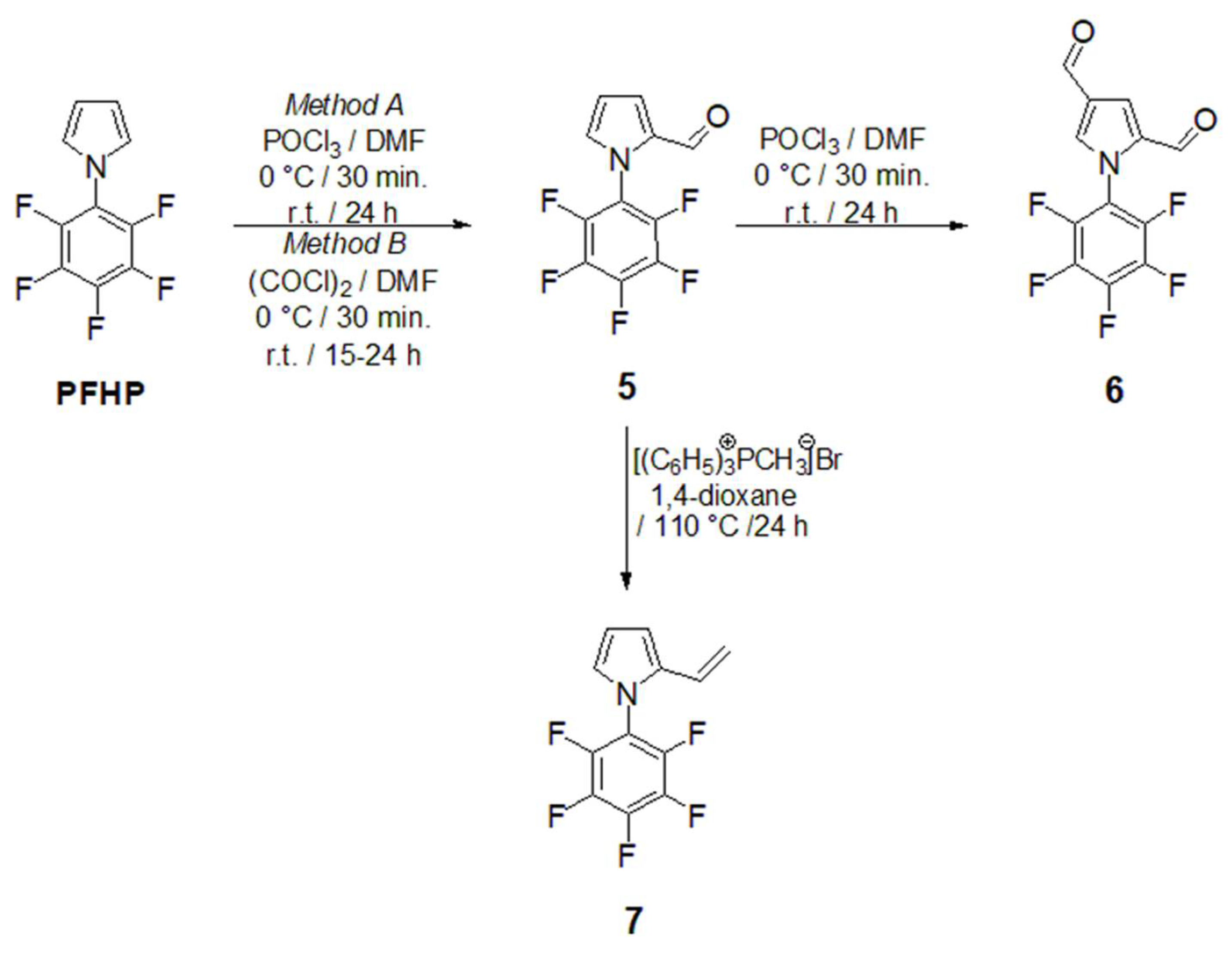
Compounds that possess intermolecular interactions such as hydrogen bonds, charge–transfer (CT) interactions, and Lewis acid–base interactions are most favoured in electronic materials design. Particularly, oligo- and polymers have been identified as promising opto-electronic materials due to their potential mechanical flexibility, structural tunability, solution processability and cost-effectiveness. Polyfluorobenzenes are attractive among the known and utilized organic materials as they exhibit stacking interactions on surfaces leading to long-range order in covalent organic frameworks [
24,
25]. The synthesis of such compounds is therefore still urgent and the presence of a formyl group is important in subsequent structural modifications since it undergoes a number of reactions. Therefore, 1-pentafluorophenyl-1
H-pyrrole-2-carbaldehyde (
5) was chosen as the substrate for the synthesis of novel formyl and vinyl substituted derivatives - 1-pentafluorophenyl-1
H-pyrrole-2,4-dicarbaldehyde (
6) and 1-pentafluorophenyl-2-vinyl-1
H-pyrrole (
7) (
Scheme 1). The synthesis of aldehyde
5 followed the Vilsmeier-Haack-Arnold (VHA) protocol and the reaction proceeded at 80 °C for 8 hours [
26]. Herein, the slight modification of approach towards the monoformylated compound
5 is presented (
Scheme 1) and the reaction proceeded at a lower temperature (25 °C) and extended reaction time (24 hours,
Method A,
Scheme 1). This manner is more convenient when compared to that originally described [
26] in terms of more convenient isolation and with a yield increase of up to 90 %. Trials towards the synthesis of pyrrole-2-carbaldehyde
5 were extended with the approach described for the formylation of pyrrole-type derivatives. The reaction relies on the use of oxalyl chloride (COCl)
2 instead of phosporus oxychloride (POCl
3) (
Method B) according to [
27]. Nevertheless, the same product
5 has been isolated in a lower yield with the modified process under the standard VHA conditions (82 % vs. 92 %). As the formyl group possesses a significant deactivation effect on a pyrrole core through subsequent electrophilic formylation under the standard VHA reaction condition (Scheme 1), the Cα/Cβ biscarbonyl
6 was isolable in a significant minority in a mixture with substrate
5.
Under the conditions of the Wittig reaction the formyl group (-CH=O) is transformed into the vinylene-type substituent (-HC=CH-). Nowadays it is known that derivatives with vinylene-linkers are important for opto-electronic materials since the linkage ensures effective π-conjugation. Our target vinylene-type derivative
7 is rather important as a substrate for subsequent polymerization reaction towards polyfluorofenylpyrroles. Its synthesis was performed from a monoformylated substrate
5 under the standard Wittig-type reaction behaviour (
Scheme 1). The reaction produced a mixture of desired product
7 and starting material
5 in the ratio of
5 :
7 = 6 : 1. Although the reactions towards
6 and
7 proceeded only with partial conversion, such type of derivatives combines the polarized aromatic backbone (C
6F
5) with a modifiable pyrrole core.
Considering the close absolute values of the gyromagnetic ratio of isotopes
1H and
19F with γ (
1H) = 26.75105 and γ (
19F) = 25.18034 rad·s
-1T
-1, in combination with the fact that γ in combination with the field strength has the main impact on NMR sensitivity,
19FNMR spectroscopy utilizes the substantial resolution and sensitivity. Therefore, the presence of the fluorine atom in any organic molecule opens a convenient route for structural identification by
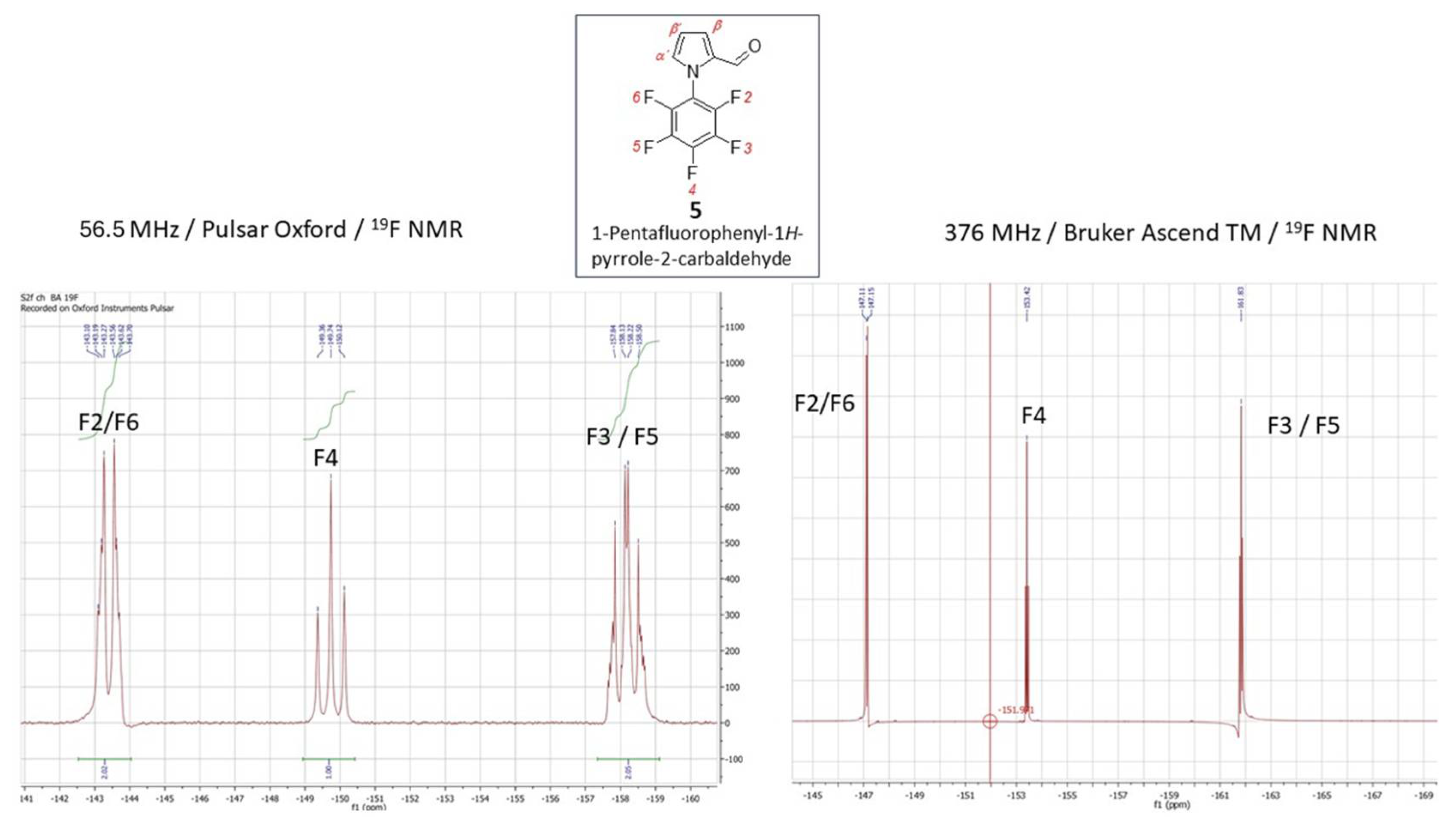
19F NMR. Given the synthetic importance of pentafluorophenylpyrroles as building blocks for functional oligo- and polymers, the effectiveness of their structural characterization force the process of materials design. Using the LF
19F NMR we were able to identify the structure of previously reported pentafluorophenyl-1
H-pyrrole-2-carbaldehyde (
5) (
Figure 5, left). The
19F signals at the chemical shifts δ = -143.40 ppm (dq,
JF-F = 22.9,
JH-F = 4.8 Hz) for F2/F6, -149.74 ppm and -158.17 ppm (q,
JF-F = 22.9, 4.8 Hz) for F3/F5, respectively, coupling to the proton of carbonyl group on the pyrrole (CH=O) and the Cα-proton of the pyrrole core, that are accompanied with the triplet at -149.74 ppm, were explicitly acquired from LF-NMR data (
Figure 5, left). In combination with
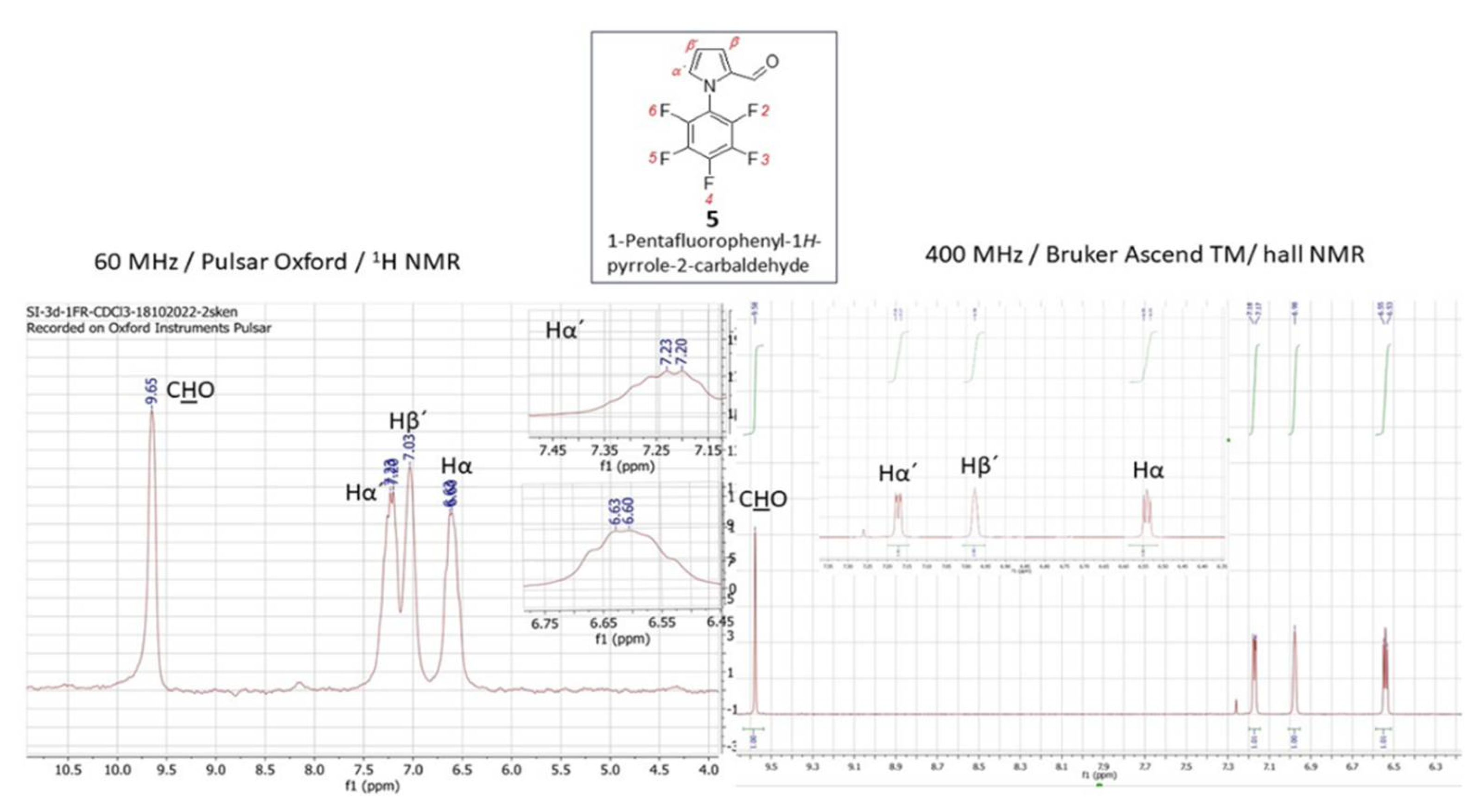
1H LF-NMR (
Figure 6, left) from which the proton signals are readily assigned and identified the data achieved from the benchtop NMR in this particular case were of comparably higher-quality than those which have been achieved by HF-NMR (
Figure 5 and 6 – right). Additionally, the challenging aspect of combined the
1H/
19F LF-NMR experiment increases the pedagogical value in terms of accessing the slightly underrated fluorine NMR spectroscopy in the interest of undergraduate students when dealing with research into fluorine-containing derivatives. In the case of novel 1-pentafluorophenyl-1
H-pyrrole-2,4-dicarbaldehyde (
6) and pentafluorophenyl-2-vinyl-1
H-pyrrole (
7) the capability of LF-NMR regarding both
19F and
1H is rather limited. These compounds were synthesized for the first time, and, to the best of our knowledge, their spectral data were previously unknown by either LF or by HF-NMR. Moreover, the reactions towards both derivatives
6 and
7 have proceeded as an incomplete conversion with desired products in minor abundance in a mixture with the substrate
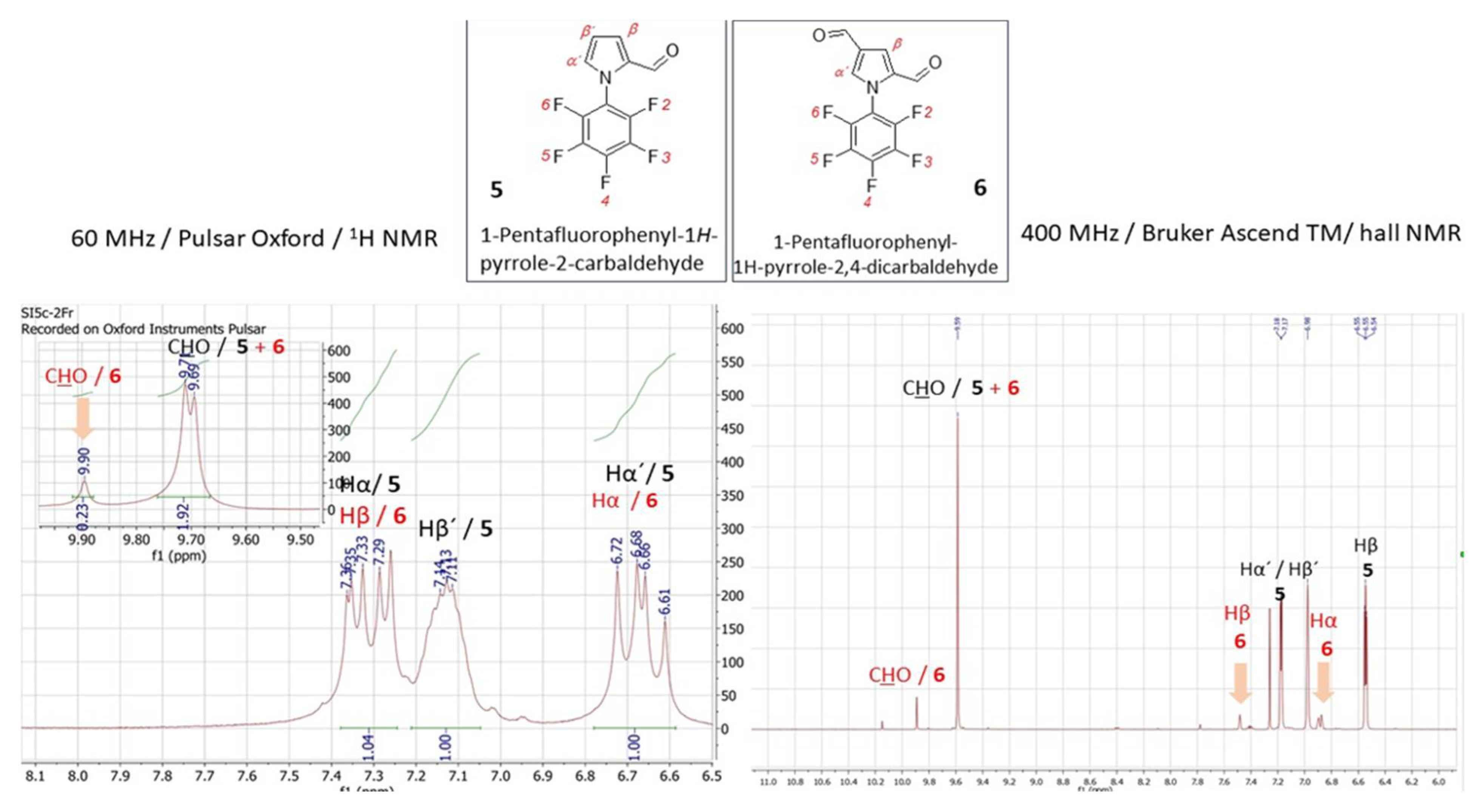
5. Therefore, the spectral data characterizes an appropriate mixture of
5/
6 (
Figure 7) or
5/
7 and the structure from both LF/HF-NMR can only be deduced. However, with the spectra of pure
5 at hand, the signal estimation for novel compounds relies on the exclusion principle. According to the signal splitting to a doublet with δ = 9.69 and 9.71 ppm with an additional chemical shift at 9.90 ppm in the LF-proton NMR (
Figure 7, left) and a signal with δ= 9.59 (2xH) ppm with an additional chemical shift at 9.85 ppm in the HF-proton NMR (
Figure 8, right) illustrated for compound
6 (Figure8), it can be assumed that the mixture contains more formylated products. From the most shielded proton at 9.90 ppm (LF-NMR) or 9.85 ppm (HF-NMR) that is a part of bisformylated derivative
6, the ratio of substrate
5 / product
6 was determined to be 8 : 1 from both LF- and HF-proton NMR. More convenient manipulation of the spectral width enables a better signal assignment for pyrrole-core protons at 7.33 ppm and 6.67 ppm for Hβ and Hα, respectively. In the case of novel compounds
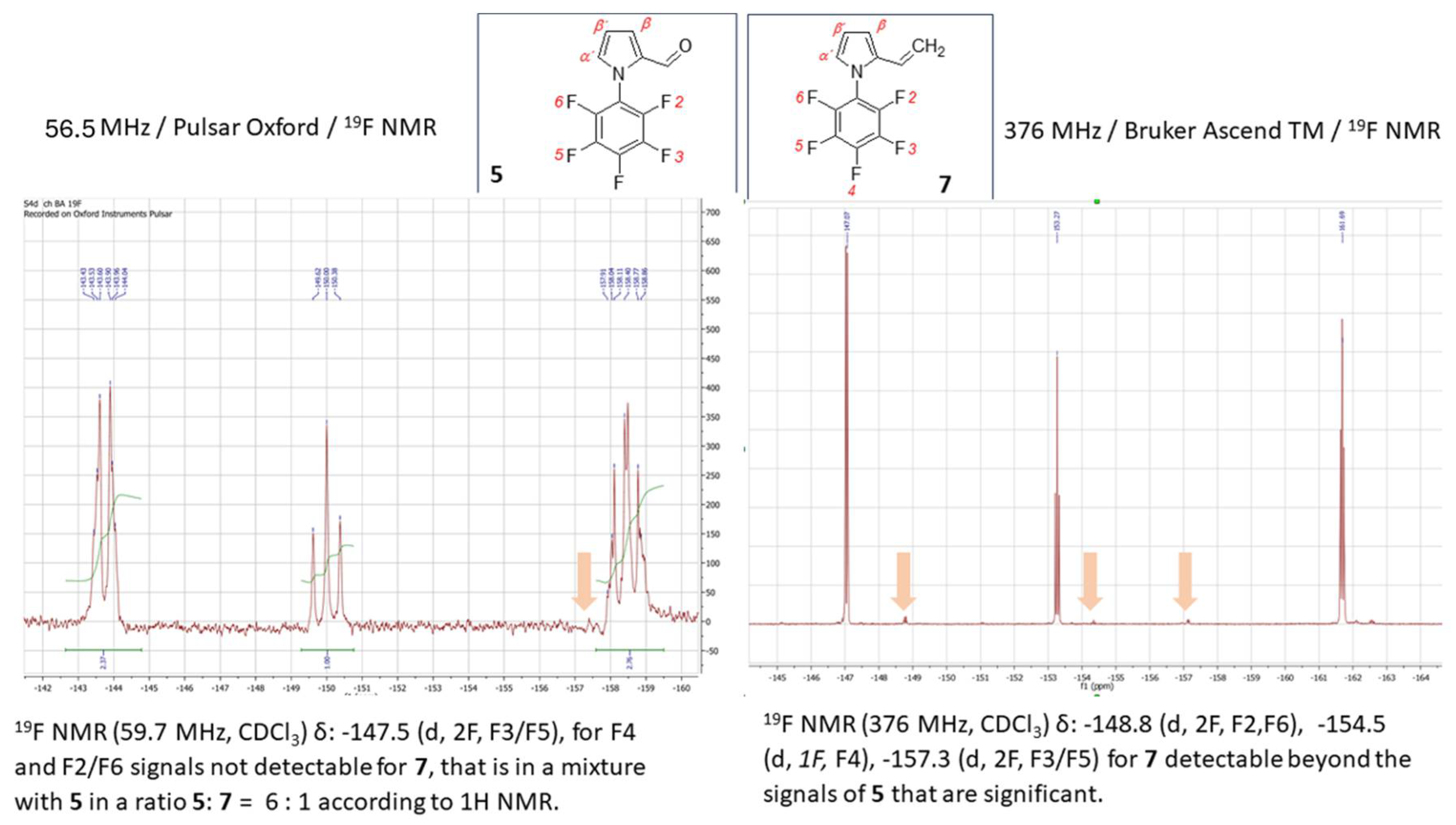
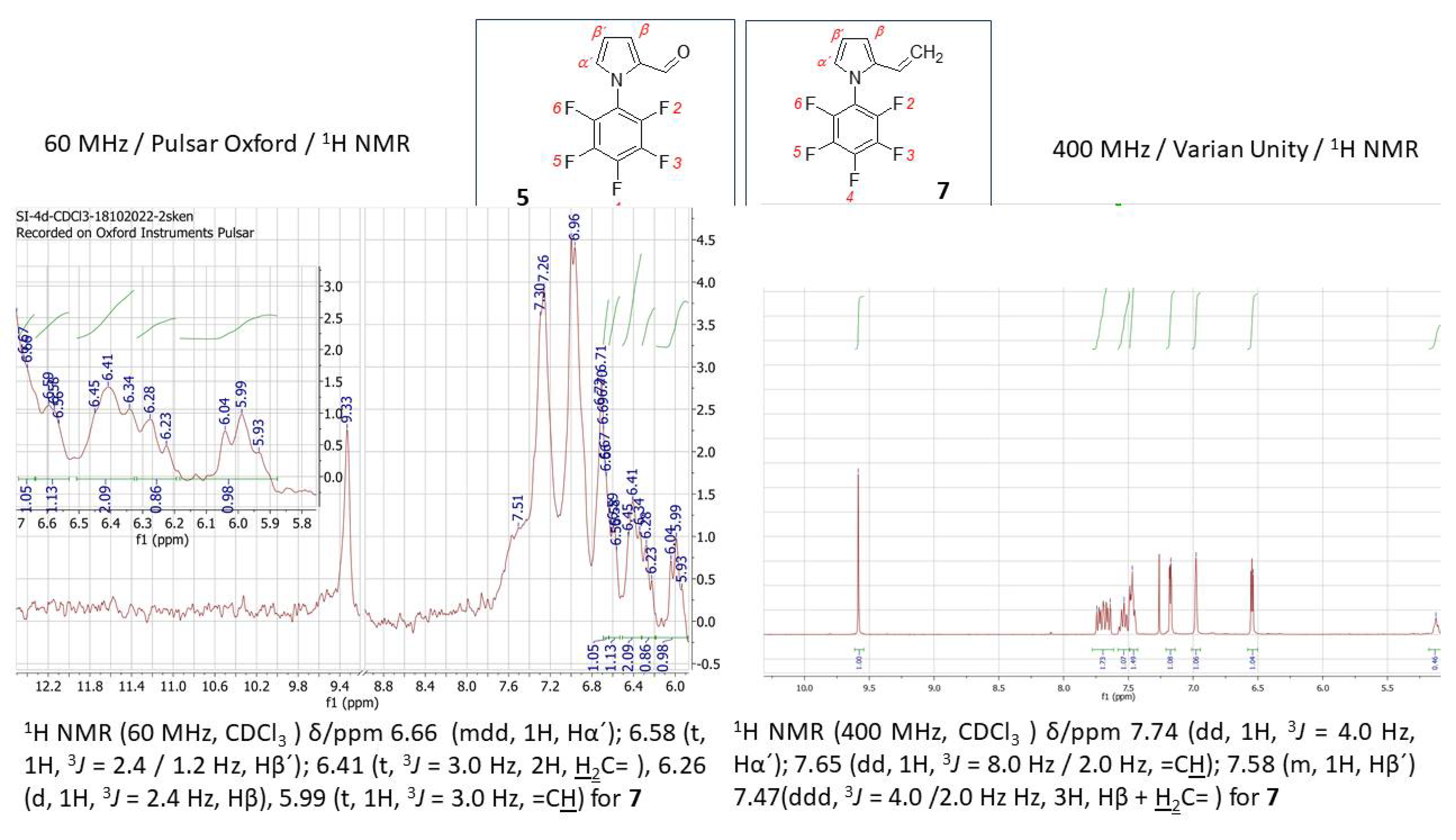
6 and
7 the better signal distinguishing between the desired product and the substrate is achieved from the fluorine spectra (
Figure 7). While in the
19F HF-NMR signals are distributed significantly from each other (
Figure 7, right) with product determination at -148.2 ppm for F2/F6, -153.5 ppm for F4 and -159.8 ppm for F3/F5, the effective adjustment of the spectral width allows the same signal designation from
19F LF-NMR (
Figure 7, left) with chemical shifts at -143.5 ppm for F2/F6, -149.0 ppm for F4 and finally at -158.0 ppm for F3/F5. Surprisingly, the signal splitting in LF-NMR for compound
6 is of better quality compared to HF-NMR data, assuming that H-F splitting of F2/4 with the neighbouring protons of the pyrrole core is equal to
JH-F = 22.5 Hz. In the case of vinylated derivative, that have been isolated in a reaction mixture in minority, the best resolution is achievable via high-field NMR data for both,
1H and
19F NMR spectra (
Figure 9 and
Figure 10)
2.3 Synthesis and Throughout Structural Characterization of Thiophene-Centered Azomethine 8.
Among the various small molecules synthesized for organic electronics, azomethines with imine linkage (-C=NH-) known as Schiff bases are recognized as an alternative for typical conjugated materials containing vinylene-type bonding (-HC=CH-) [
28,
29]. Azomethines with a thiophene core are observed undergoing a reversible electrochemical oxidation process, exhibit a narrow energy band gap, high thermal stability, and generally act as p-type (hole transporting) materials [
30]. The general route towards azomethines is comparably more convenient as an approach than their vinylene-type counterparts. Based on previous results, we have found that the phosphorus oxychloride (P
2O
5) exhibits a notable efficiency in Schiff-type condensation towards thiophene-centred azomethines [
31]. From here, we followed a procedure utilizing P
2O
5 in ethanol (
Scheme 2). Hence, the yield of previously described compound 2,5-
bis-[(4-methoxy-benzylidene)-amino]-thiophene-3,4-di-carbonitrile (
8) increased up to 60% from 56% [
30].
Chemical structure of the 2,5-bis-[(4-methoxy-benzylidene)-amino]-thiophene-3,4-dicarbo-nitrile (
8) have been for the first time fully assigned by the combination of routine
1H NMR and 2D – COSY (1H-1H) spectra acquired at LF, as is shown on
Figure 11.
Interestingly, one of the structure determining protons assigned to the proton of azometine-linkage (-HC=N-) appear in 1D spectrum as doublet at δ = 8.62 ppm, and a corresponding cross-peak signal was observed in the 2D COSY spectrum. The aromatic protons in the structure of 8a are readily detectable in both 1H and 2D spectrum with the appropriate signal splitting at δ(H2) = 8.52 ppm, δ(H6) = 8.26 ppm and δ(H5) = 8.12 ppm. Interestinlgy, the signal of the proton H3 is splitted into an unexpected signal splitting pattern at δ(H3-phenyl-core 1) = 7.70 ppm as doublet while the same proton on the other phenyl core is separated unconventionally to doublet with δ = 7.83 ppm with integration of 2/3 and δ = 7.58 integrated to 1/3. Such type of equivocal dyadic cleavage in aromatic fragment occurs probably as the consequence of the sample overheating due to the long duration of NMR measurement. It is obvious, also during the acquisition at the high magnetic fields, that the long irradiation affects the sample overheating causing the spin dephasing because of the internal magnetic field (B1) inhomogeneity. As result of such behaviour the signal degradation with a broader bandwidth occurs within the spectra as have occurred by both, 1H and 2D NMR experiments for 8.