1. Introduction
Bone grafting is defined as a surgical procedure to replace missing bone with an artificial, synthetic, or natural substitute [
1]. Because tooth extraction is reported to be the most widely performed dental procedure, dental extractions cause substantial changes in alveolar ridge dimensions among the patient population [
2]. In modern implant dentistry, such alveolar defects and hard tissue deficiencies present major obstacles in providing ideal reconstructive procedures. To overcome such drawbacks, horizontal or vertical bone augmentation is performed to achieve multidimensional regeneration using various graft materials and surgical techniques [
3].
The osteogenic capacity of demineralized teeth was verified as early as 1967 [
4,
5]. Based on the potentials that fulfill the regeneration triad of osteogenesis, osteoinduction, and osteoconduction, and to overcome the limitations associated with autogenous bone grafting techniques, such as complications at the donor site and unpredictable bone resorption, tooth-derived bone graft material has been under the spotlight as a novel regeneration bone graft material for many years [
6]. Host immune response and antigenicity to this novel regenerative bone graft material, for both autogenous and allogenic sources, were negligible, and both autogenous and allogenic demineralized tooth-derived bone grafting materials are now accepted as osteoinductive and osteoconductive graft materials [
4,
5,
6,
7,
8]. Furthermore, demineralization in preparation of tooth-derived regenerative bone grafting material upregulates bone regeneration by acting as carriers of bone morphogenetic protein (BMP)-2 and providing cell adhesion domain sequences for biological recognition [
9].
Outcomes of different degrees of demineralization of dentin-derived matrix as regenerative grafting material usage have been studied. Non-demineralized dentin-derived matrix resulted in delayed osteoinduction due to hydroxyapatite blocking the release of growth factors [
10]. Conversely, in addition to providing benefits, such as reduced antigenicity and immunogenicity, demineralization of dentin-derived matrix resulted in release of various growth factors to induce bone regeneration [
11]. Demineralization of dentin-derived matrix bone graft material and perforated graft materials in block forms showed faster new bone formation compared with other types of graft material [
12].
This study aims to find out how much demineralization of dentin-derived matrix is necessary to achieve optimal bone regeneration. Evaluation of the amount and dimensional changes in the new bone of the rabbit calvaria with differentiated degree of demineralized dentin-derive matrix graft was conducted using the histomorphometric analysis.
2. Materials and Methods
Surgical Procedures
The extracted human teeth were cleaned and prepared chairside. Old restorations, caries, calculus, and soft tissue debris attached to the extracted teeth were removed using sharp manual instruments and rotary instruments with copious irrigation. A surgical mallet was then used to crush the teeth into smaller particles sized 0.8–1.0 mm. Using 0.6 N hydrochloric acid, demineralization was performed for different durations for 15 and 30 minutes, under vacuum compression with ultrasonic vibration in a vacuum-ultrasonic device (VacuaSonic System, CosmoBioMedicare Co., Seoul, Korea). The demineralized dentin-derived matrix particles were washed with phosphate-buffered saline (PBS), sterilized with a sterilization reagent, and rinsed with PBS and distilled water.
This study included 15 adult male New Zealand white rabbits weighing between 2.8 and 3.2 kg (average weight 3.0 kg) as experimental subjects. The study was approved by the Animal Care and Use Committee at Catholic University Medical Center of Daegu (Approval No. DGIACUC-150911-14).
The rabbits were randomly assigned to three groups, with 5 rabbits in each group. The surgical procedures were identical for all animals. General anesthesia was administered intramuscularly using a combination of 30 mg/kg ketamine (Ketalar; Yuhan Co., Seoul, Korea) and 10 mg/kg xylazine (Rompun; Bayer Korea, Seoul, Korea), along with a 0.5 mL subcutaneous injection of lidocaine with 1:100,000 epinephrine along the calvaria midline.
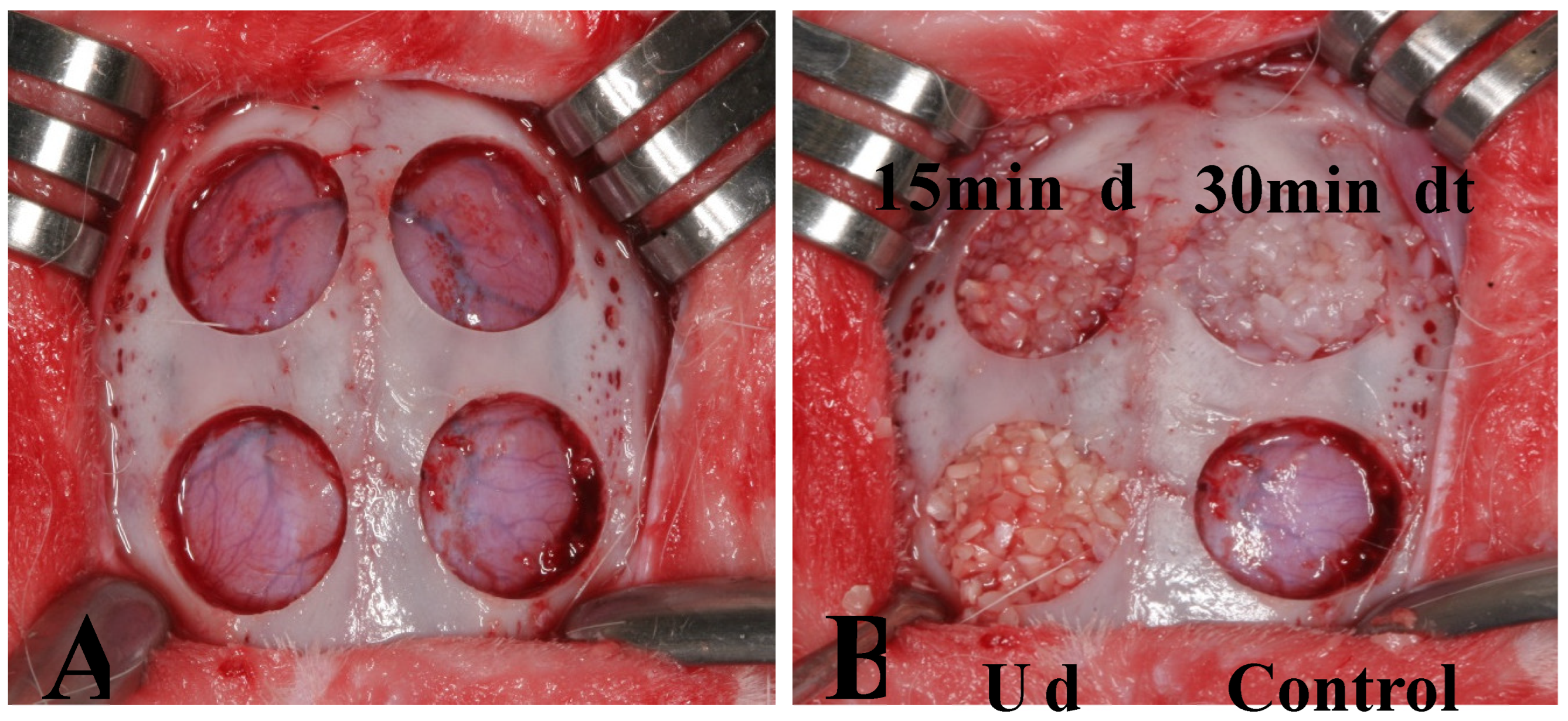
The animals were stabilized on a surgical table, and periosteal incisions were made along the sagittal midline of the calvaria to expose the frontal bone. Experimental group assignments are shown in
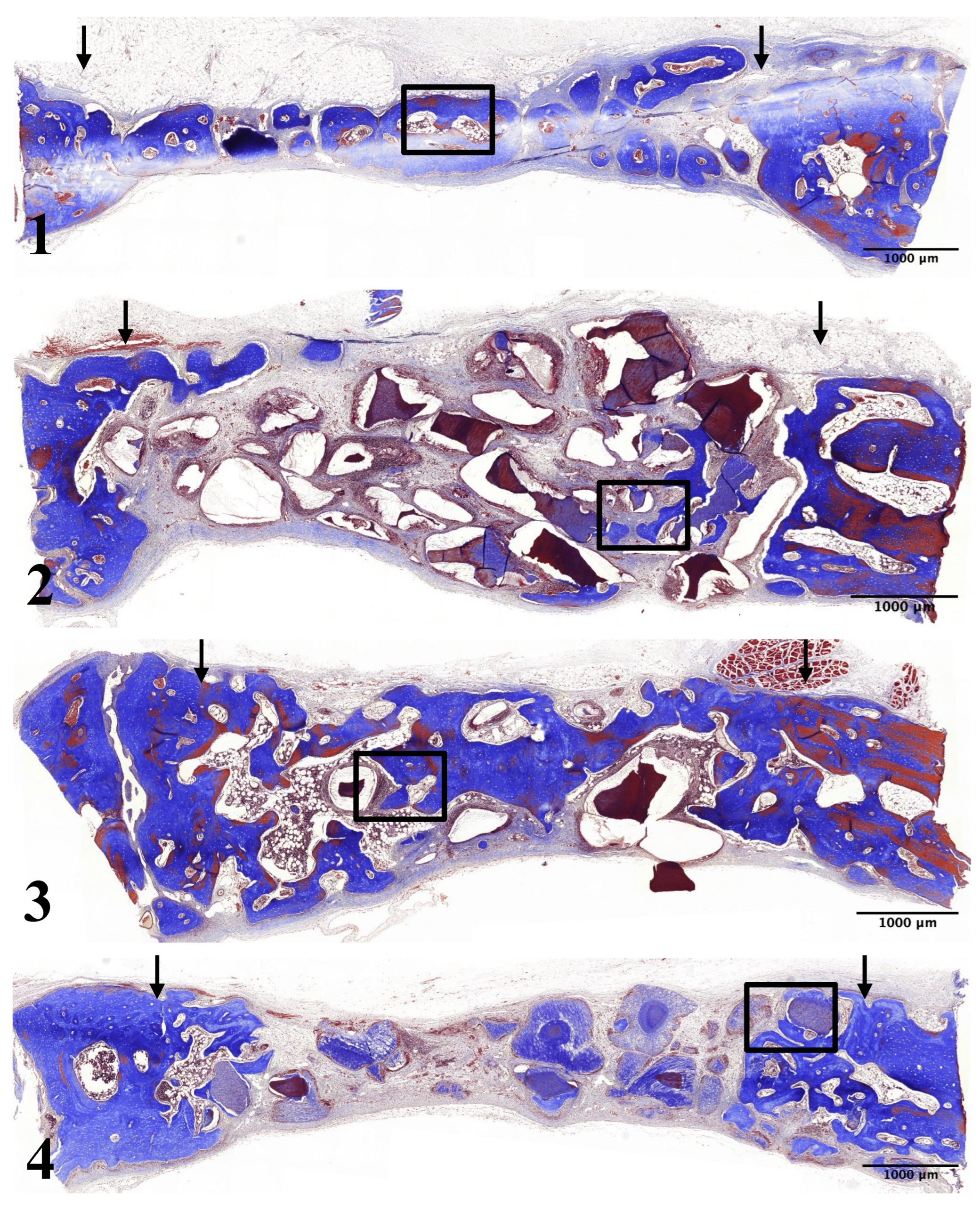
Figure 1. Four circular bone defects were prepared in each subject using 8-mm trephine burs (
Figure 1-A). Resected bones were carefully removed to avoid injury to underlying brain tissue. Then, defects were assigned to four experimental groups. In Group 1 (control group), the defect was filled with blood coagulum. In Group 2 (UdTB group), the defect was filled with non-demineralized dentin-derived matrix (Dentin grinder). In Group 3 (15 min dTB group), the defect was filled with 15-minute demineralized dentin-derived matrix (Vacuasonic). In Group 4 (30 min dTB group), the defect was filled with 30-minute demineralized dentin-derived matrix (Vacuasonic) (
Figure 1-B).
For wound closure, 4-0 nylon suture (Blue nylon, Ailee Co., Busan, Korea) was used. Intramuscular antibiotic (20 mg/kg gentamycin [Donghwa Co., Seoul, Korea]) was administered for 3 days to all subjects following the surgical procedure. The subjects in each group were euthanized at 2 weeks (n = 5), 4 weeks (n = 5), or 8 weeks (n = 5) post-surgery.
Tissue Preparation
The subjects were euthanized at 2, 4, and 8 weeks under general anesthesia. The calvarias were segmented from the cranium with a microsaw and fixed with neutral-buffered formalin for 24 hours. The specimens were washed with 0.1 M phosphate buffer, decalcified with 10% formic acid for 10 days, embedded in paraffin (Paraplast; Oxford, USA), sliced through the center of the circular preparation into 5-μm thick serial sections, and stained with hematoxylin–eosin and Masson’s trichrome staining. Newly formed bone and soft tissue changes in calvaria preparations were examined under a light microscope.
Histomorphometric Analysis
Ten randomly selected specimens from each group were photographed using an AxioCam MRc5 and Axiophot Photomicroscope (Carl Zeiss, Germany). The images were analyzed using AxioVision SE64 software (Carl Zeiss, Germany).
The following histomorphometric measurements were evaluated: the total area of augmentation, the area of new bone formation, the turnover rate of regenerative graft materials, and the bone marrow space dimension. The total area of augmentation included the area of new bone formation, graft material, fibrous tissue, and vascular tissue within the preparation. The percentage of new bone formation and the types of regenerative graft materials used were analyzed to study the efficacy of new bone formation, based on degrees of decalcification.
Statistical Analysis
A validation software was used for data processing and statistical evaluation (SPSS, version 25.0, SPSS Inc., Chicago, IL, USA). The statistical significance of differences within and between groups was assessed by one-way analysis of variance (ANOVA) using Tukey’s method. The quantitative results are presented as means ± standard deviation; a P-value of less than 0.05 was considered statistically significant.
3. Results
Histological Analysis
Host or lamellar bone was stained red, while woven or newly formed bone was stained blue with Masson’s trichrome staining. Regenerative graft material particles stained with various colors and differentiated well from the surrounding tissue in Masson’s trichrome staining. No signs of inflammation were found in any experimental group under light microscopy.
2-Week Results
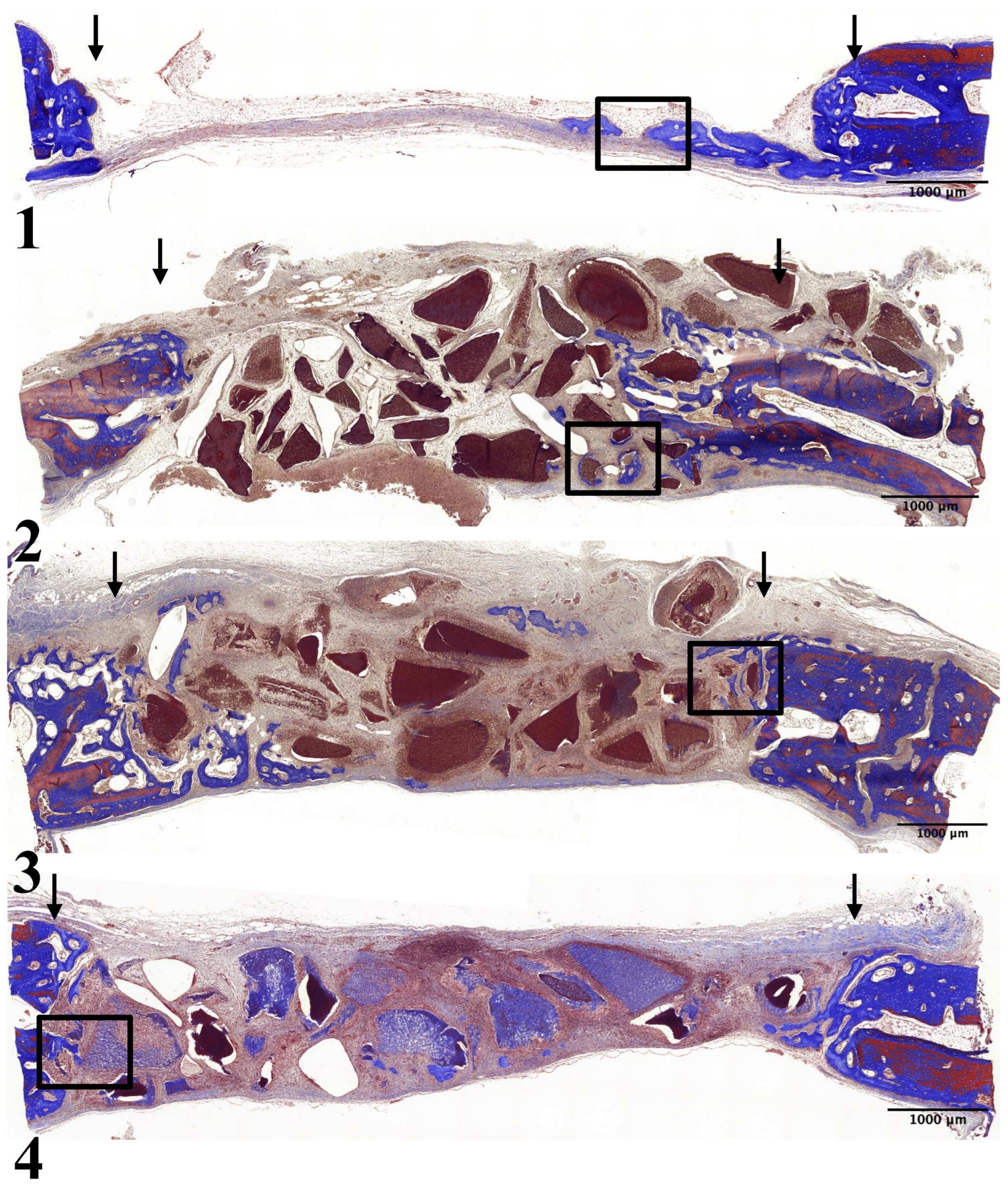
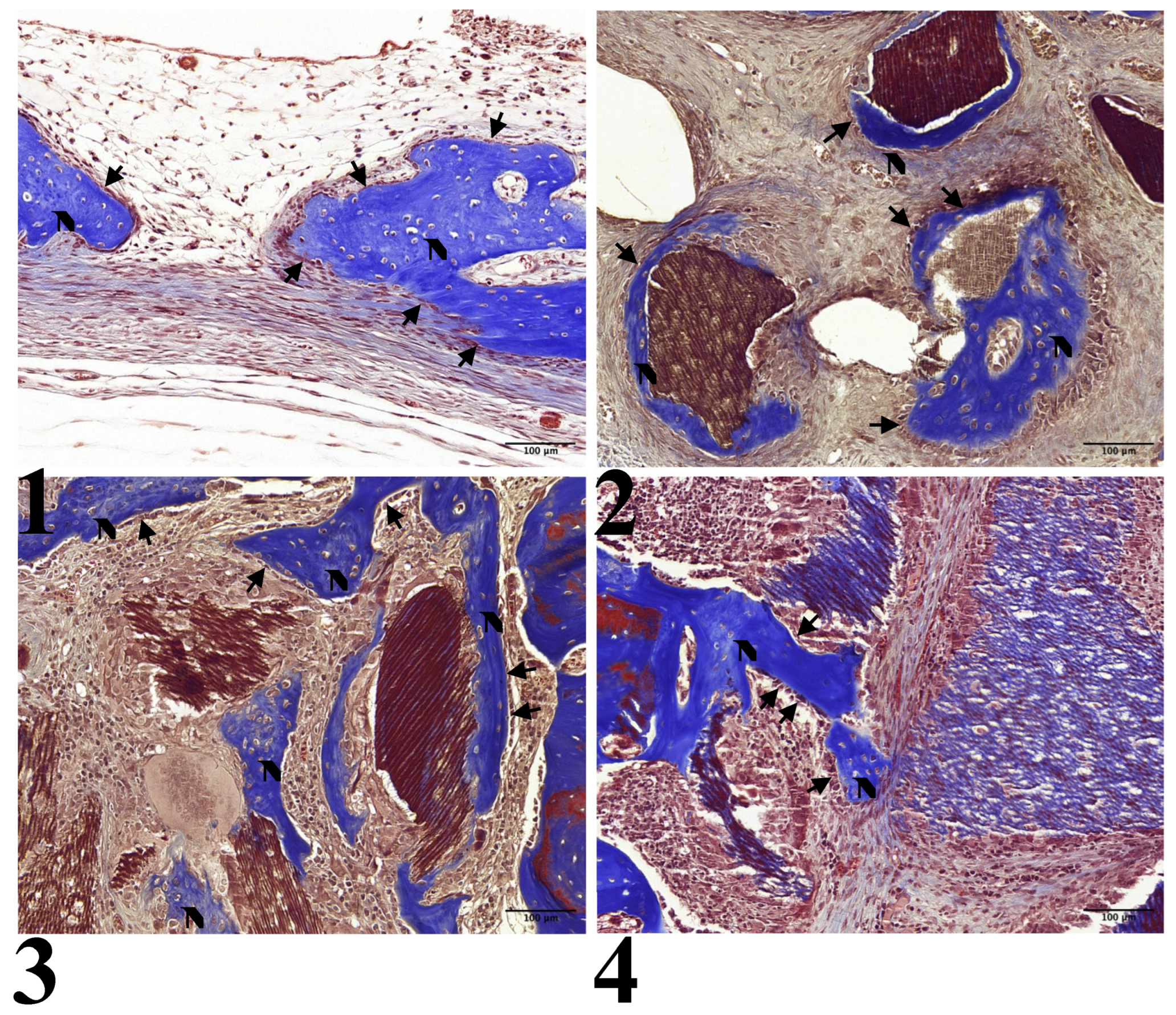
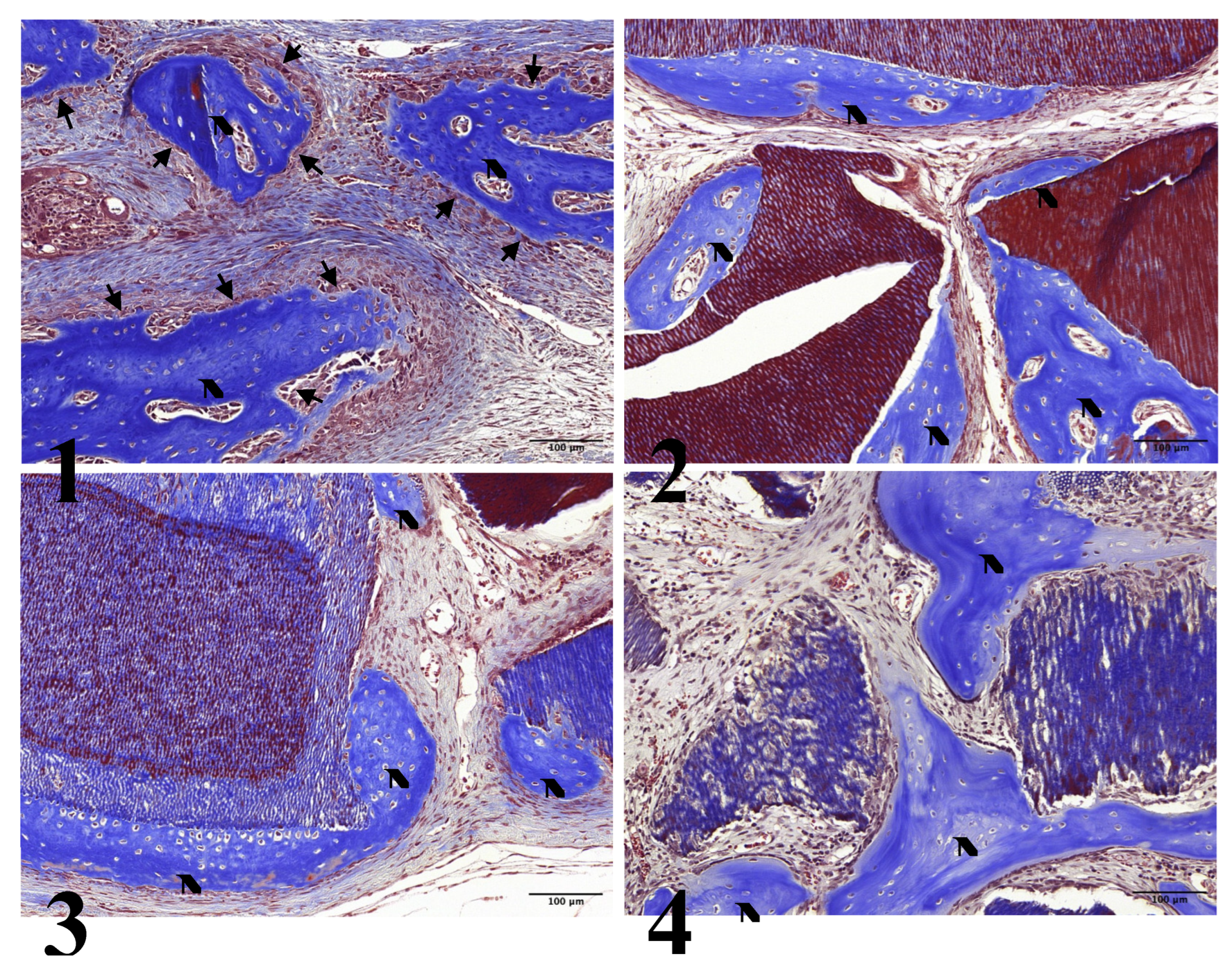
In all experimental groups, limited new bone formation was observed, concentrated around the preparation margin. The amount of newly formed bone was similar across experimental groups. In Group 1, the center of the preparation was somewhat depressed and flattened by surrounding connective tissue and dura mater. Newly formed bone was partially identified on the surface of the preparation margin, accompanied by osteoblasts on the surface of the newly formed bone (
Figure 2-1, 3-1).
All other groups showed comparable results with partial new bone formation at the preparation margin and on the surfaces of the graft particles: Group 2 (
Figure 2-2, 3-2), Group 3 (
Figure 2-3, 3-3), and Group 4 (
Figure 2-4, 3-4). All groups showed osteoblasts on the surface of the newly formed bone.
4-Week Results
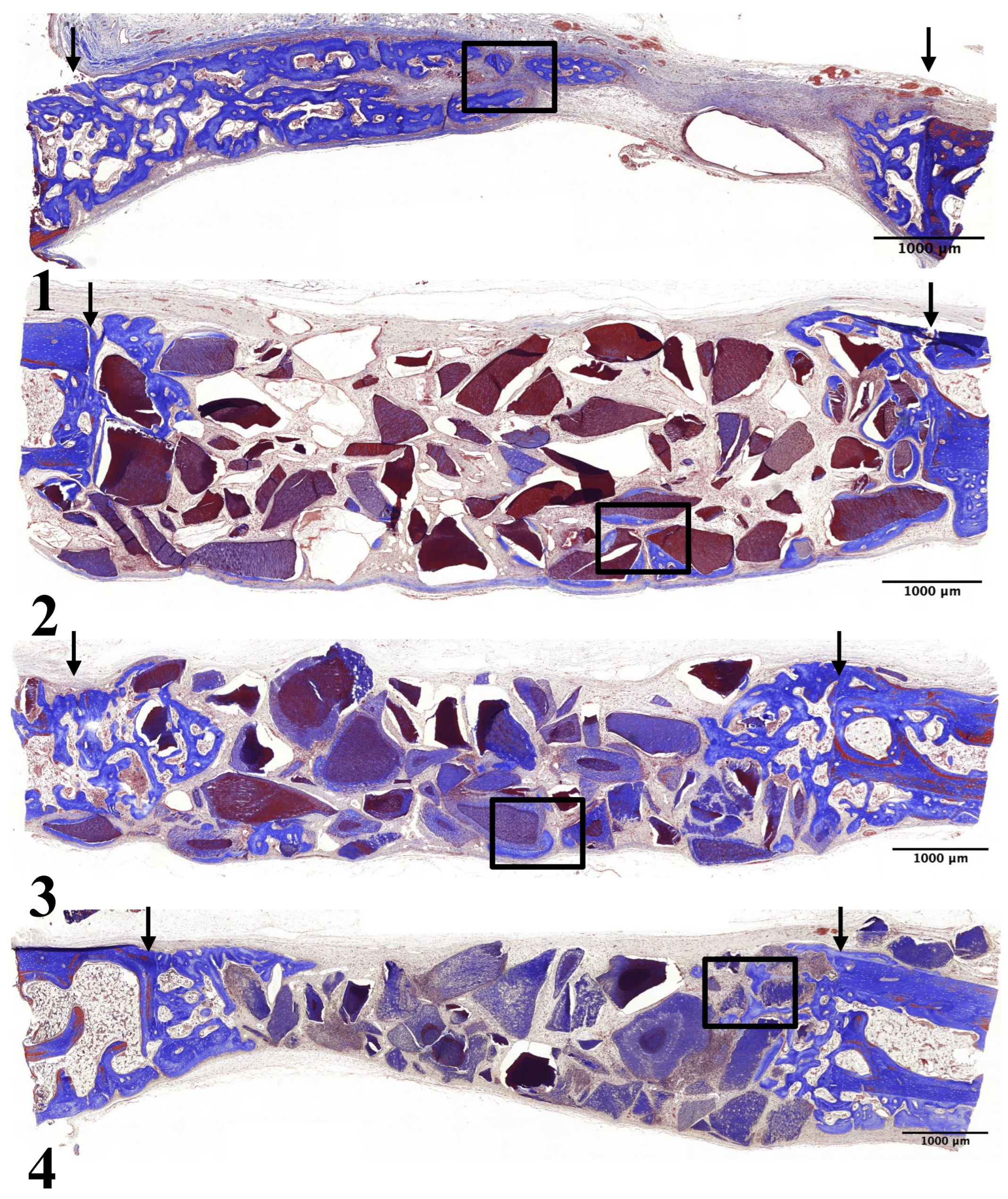
In Group 1, newly formed bone was thicker, including at the center of the preparation. Many osteoblasts were observed on the surface of the newly formed bone (
Figure 4-1, 5-1).
In Group 2, newly formed bone was thicker and observed on the surfaces of UdTB particles. The density of newly formed bone increased compared to the 2-week result. However, most new bone formation was observed only near the preparation margins (
Figure 4-2, 5-2).
In Group 3, the thickness and density of newly formed bone increased compared to the 2-week period. Newly formed bone was observed at the center of the defect. The size and density of 15-min demineralized dTB particles were comparable to the 2-week result (
Figure 4-3, 5-3).
In Group 4, newly formed bone was thicker and observed on the surfaces of 30-min demineralized dTB particles. The density of newly formed bone increased compared to the 2-week result. However, the majority of the newly formed bone was found only near the preparation margins. The density of 30-min demineralized dTB particles was comparable to the 2-week result (
Figure 4-4, 5-4).
8-Week Results
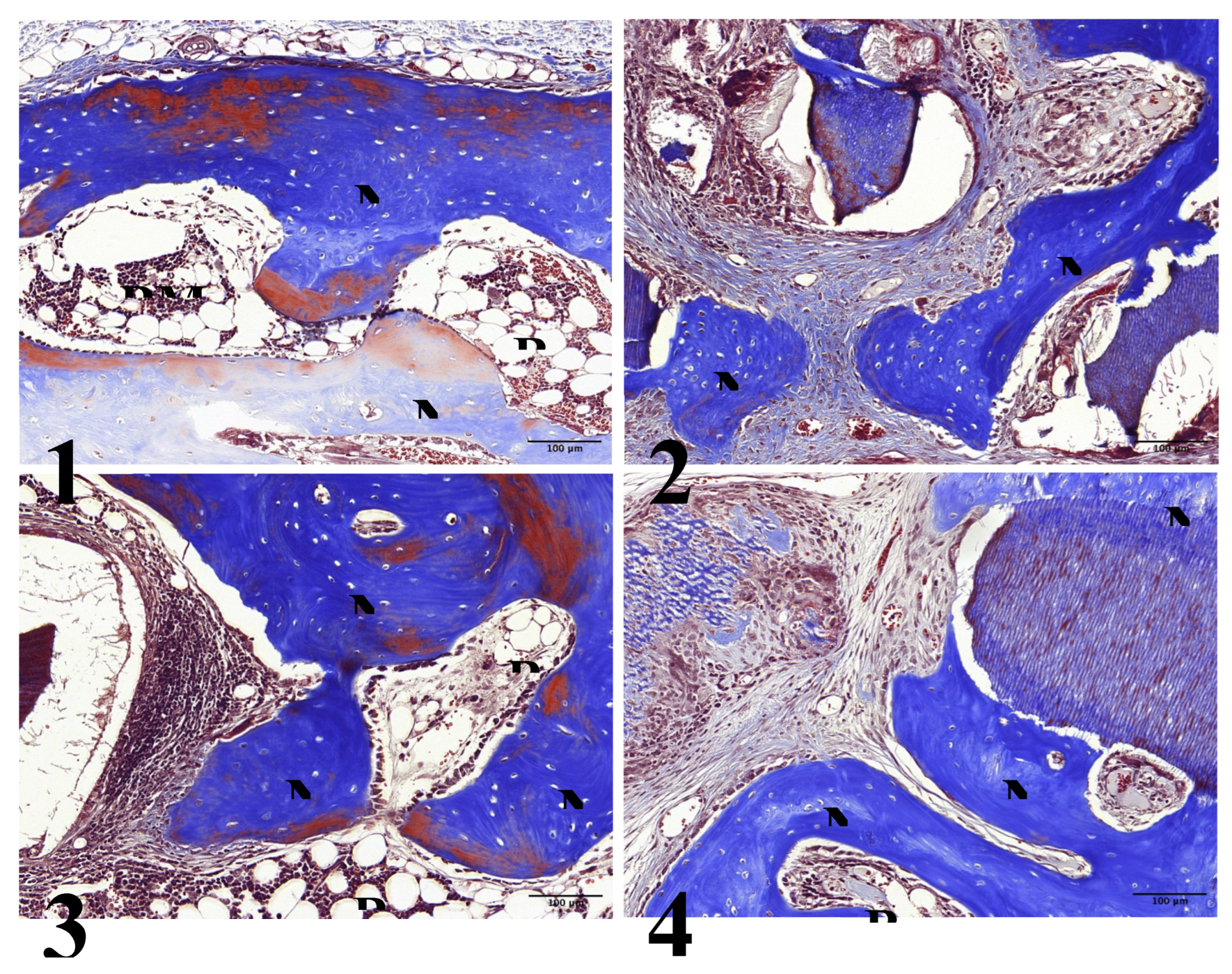
In Group 1, new bone formation was observed from the preparation margin to the center. Thicker bone formation was noted, and the bone marrow space containing adipose tissue was observed between newly formed bone cells (
Figure 6-1, 7-1).
In Group 2, thicker bone formation was observed even on the surfaces of UdTB particles. The newly formed bone had a higher density than the 4-week result. However, in this group, new bone formation was absent at the center of the preparation (
Figure 6-2, 7-2).
In Group 3, a significant increase in the thickness and density of newly formed bone was observed compared to the 4-week result. Increased bone formation extended from the preparation margin to the center. Some mature lamellar bone cells were found inside the newly formed bone, and an increased amount of bone marrow space containing adipose tissue was observed between the newly formed bone cells. However, the size and density of 15-min demineralized dTB particles decreased compared to the 4-week result (
Figure 6-3, 7-3).
In Group 4, new bone formation on the surface of 30-min demineralized dTB particles increased compared to the 4-week result. However, a significant increase in new bone formation was not found at the center of the preparation. The density of 30-min demineralized dTB particles decreased compared to the 4-week result, and bone marrow space containing adipose tissue was observed between newly formed bone cells and 30-min demineralized dTB particles (
Figure 6-4, 7-4).
Histomorphometric Analysis
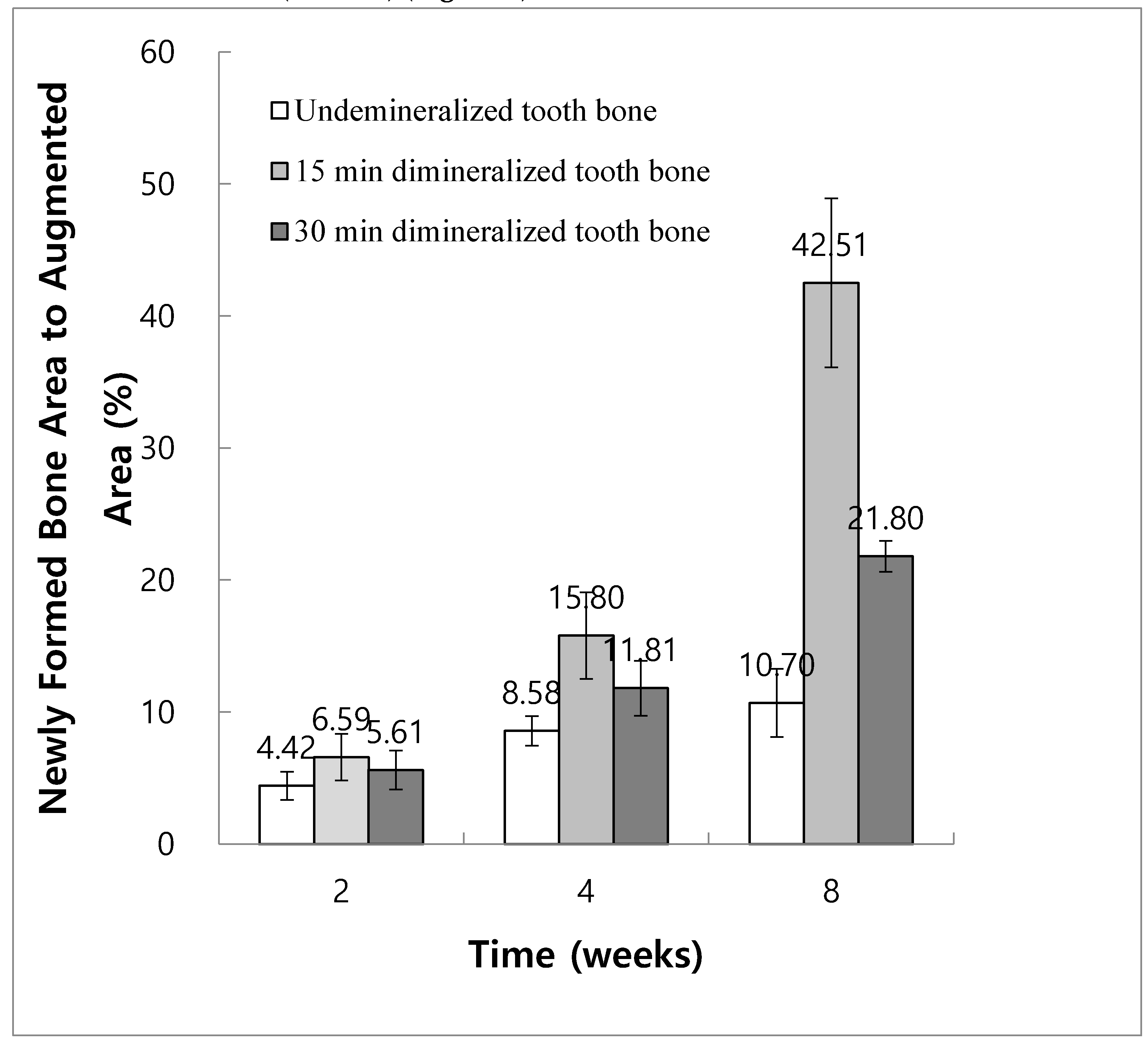
In Group 2, the ratios of newly formed bone area to augmented area at 2, 4, and 8 weeks were 4.42% ± 1.06%, 8.58% ± 1.11%, and 10.70% ± 2.58%, respectively. In Group 3, the ratios of newly formed bone area to augmented area at 2, 4, and 8 weeks were 6.59% ± 1.76%, 15.80% ± 3.28%, and 42.51% ± 6.40%, respectively. In Group 4, the ratios of newly formed bone area to augmented area at 2, 4, and 8 weeks were 5.61% ± 1.48%, 11.81% ± 2.09%, and 21.80% ± 1.18%, respectively.
The results of one-way ANOVA and post hoc comparisons showed a statistically significant increase in new bone area at 8 weeks compared to 2 and 4 weeks for both Groups 3 and 4 (P < 0.05). However, in Group 2, the difference in the new bone area at 8 weeks was not significant compared with that at 2 and 4 weeks (P < 0.05) (
Figure 8).
At 4 weeks, new bone formation in Group 3 was significantly higher than that in Group 2. At 8 weeks, Group 3 showed significantly higher new bone formation than other experimental groups, followed by Groups 4 and 2 (
Figure 8).
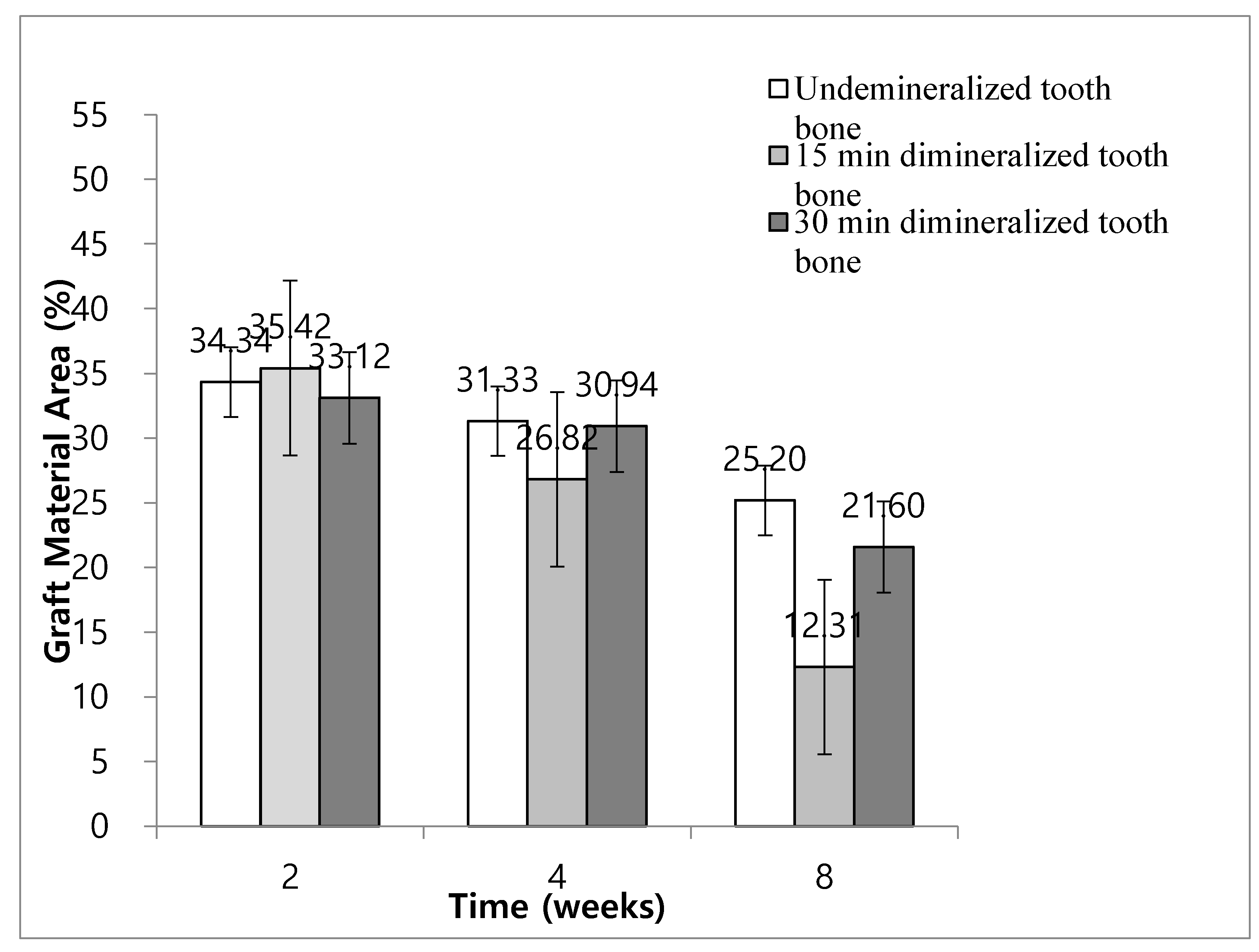
In Group 2, the ratios of graft material area to total augmented area at weeks 2, 4, and 8 were 34.34% ± 4.96%, 31.33% ± 4.02%, and 25.20% ± 4.35%, respectively. In Group 3, the ratios of graft material area to total augmented area at weeks 2, 4, and 8 were 35.42% ± 4.46%, 26.82% ± 4.05%, and 12.31% ± 4.53%, respectively. In Group 4, the ratios of graft material area to total augmented area at weeks 2, 4, and 8 were 33.12% ± 4.29%, 30.94% ± 6.16%, and 21.60% ± 4.58%, respectively.
In Groups 3 and 4, the ratio of graft material to total augmented area at 8 weeks was significantly lower than that at weeks 2 and 4 (P < 0.05). In Group 2, the ratio of graft material to total augmented area at 8 weeks was not significantly different from the results of weeks 2 and 4 (P < 0.05) (
Figure 9). The ratio of graft material to total augmented area decreased significantly in Group 3 at 8 weeks compared with Groups 2 and 4. At 8 weeks, the ratio of graft material to total augmented area decreased significantly in Group 4 compared to that in Group 2 (
Figure 9).
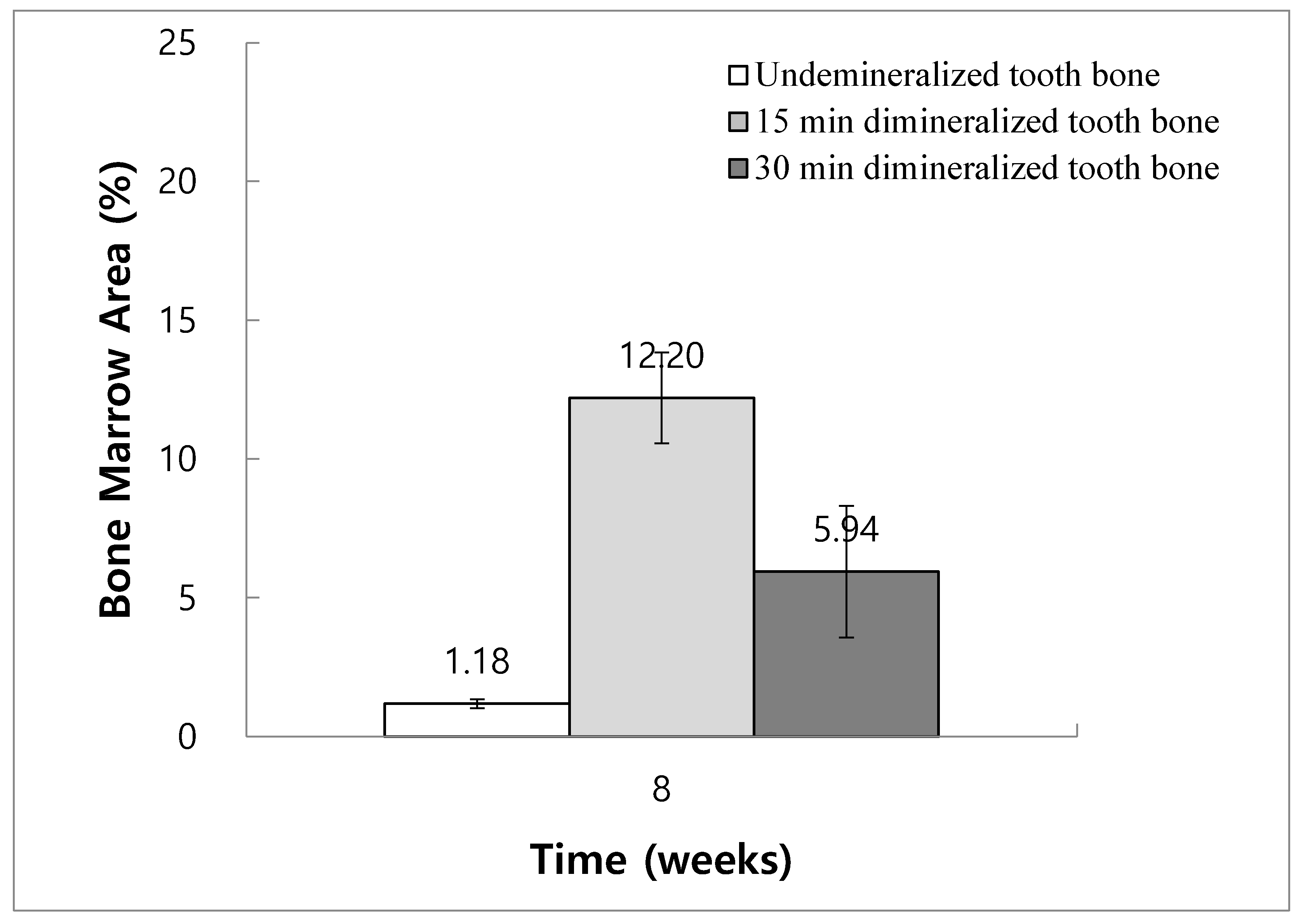
The ratios of bone marrow area to total augmentation area at 8 weeks in Groups 2, 3, and 4 were 1.18% ± 0.16%, 12.20% ± 1.64%, and 5.94% ± 2.37%, respectively. The bone marrow area increased significantly in Group 3 at 8 weeks compared with Groups 2 and 4 (
Figure 10).
4. Discussion
The significance of selecting appropriate regenerative graft material, along with the optimal preparation method for clinical application, cannot be overstated in achieving optimal regeneration outcomes. In this study, we delved into the effectiveness of new bone formation in rabbit calvaria using various graft materials: absence of graft material (Group 1), non-demineralized dentin-derived graft material (Group 2), 15-minute demineralized dentin-derived graft material (Group 3), and 30-minute demineralized dentin-derived graft material.
Regenerative bone grafting materials are typically classified into four main types: autogenous, allogenic, xenogenic, and alloplastic. Autogenous bone grafts are considered the gold standard due to their ability to fulfill the essential components of the regenerative triad: osteoconduction, osteoinduction, and osteogenesis [
13]. Additionally, autogenous bone graft material offers the advantage of reduced immunological reactions [
14]. However, the use of autogenous bone grafts has its drawbacks, including potential complications at the donor site, unpredictable bone resorption, and limitations in availability [
15,
16,
17,
18].
In contrast, allogenic or xenogenic grafts do not require additional surgery time for preparation or harvesting but may exhibit batch variabilities and carry the risk of disease transmission and immune-related challenges, such as immunologic rejection [
18,
19]. Synthetic materials provide precise control over characteristics such as porosity and hydrophilicity or hydrophobicity through controlled manufacturing processes. Nevertheless, their efficacy often depends on the addition of bioactive factors, such as platelet-rich plasma, to facilitate regeneration [
19].
To address the limitations associated with autogenous regenerative graft materials and seek a viable alternative with comparable biological properties, researchers have explored extracted human teeth as novel graft materials. These teeth possess similar physicochemical properties to bone, particularly dentin, which shares a comparable chemical composition with bone. Dentin consists of 70%–75% inorganic, 20% organic, and 10% water, while alveolar bone comprises 65% inorganic, 25% organic, and 10% water [
6,
9,
20,
21,
22,
23,
24,
25,
26,
27].
BMP, transforming growth factor-b (TGF-b), and insulin-like growth factor II (IGF-II) have been identified in both human bone and tooth dentin [
28]. BMP and TGF-b initiate bone induction through endochondral bone differentiation [
29].
The osteogenic capacity of demineralized tooth-derived regenerative bone grafting material was first recognized in 1967. Recent studies have confirmed both autogenous and allogenic demineralized teeth as highly osteoinductive and osteoconductive graft materials [
5,
6,
26,
30,
31]. Demineralized dentin-derived matrix graft material has demonstrated superior regeneration efficacy compared to other commonly used graft materials, such as Bio-Oss and B-TCP [
32,
33]. Furthermore, demineralization of dentin-derived matrix graft material has been shown to enhance its regenerative properties by reducing antigenicity and immunogenicity while accelerating bone formation [
8,
11,
12].
However, excessive demineralization during preparation can negatively impact the physicochemical properties and clinical outcomes of demineralized tooth-derived graft materials. Over-demineralization may lead to detrimental alterations in the crystallinity of hydroxyapatite and graft mineral, affecting factors such as BMP and TGF-b.34 For instance, a study comparing the outcomes of 10-minute and 30-minute demineralization protocols revealed that the latter resulted in excessive loss of mineralized components [
34].
Hence, it is imperative to identify and establish an optimal preparation protocol for tooth-derived graft materials to consistently achieve the best clinical results.
In this study, the ratio of newly formed bone to the total augmented area served as a measure of the graft material’s regenerative capacity. Higher ratios indicate faster bone formation and greater regeneration efficiency. Histomorphometric analysis revealed more new bone formation in the demineralized graft Groups (3 and 4) compared to the non-demineralized graft group (Group 2). Notably, the 15-minute demineralization graft group (Group 3) exhibited significantly more new bone formation than the 30-minute demineralization graft group (Group 4) at 8 weeks. Overall, demineralization led to more new bone formation than non-demineralization, with the 15-minute demineralization group showing superior results compared to the 30-minute demineralization group.
The ratio of graft material area to the total augmented area reflects the turnover rate of the graft material. Lower ratios indicate faster graft material turnover and more efficient remodeling. Histomorphometric analysis demonstrated a significant advantage of demineralization over non-demineralization: both the 15-minute and 30-minute demineralization groups exhibited faster graft material turnover compared to the non-demineralized graft group. Moreover, the 15-minute demineralization group outperformed the 30-minute demineralization group at 8 weeks. Overall, demineralization facilitated faster graft material turnover, with the 15-minute demineralization protocol being more effective than the 30-minute protocol.
A higher ratio of bone marrow area to the total augmentation area indicates the efficacy of the regenerative graft material and summarizes the regeneration progression. Histomorphometric analysis revealed significant regeneration advantages in the 15-minute demineralization group (Group 3) compared to the 30-minute demineralization and non-demineralized graft groups (Groups 4 and 2, respectively).
In Group 1, 100% autogenous bone regeneration occurred, albeit with less volume than in other groups. This suggests that natural wound healing can regenerate some bone volume without additional graft material placement. Additionally, in Group 4, noticeable loss of graft volume was observed due to excessive demineralization of the dentin-derived matrix graft material. However, Group 4 still yielded more bone regeneration than control Group 1, indicating that dentin-derived matrix as a graft material enhances overall regeneration outcomes, even without optimal demineralization.
This study has several limitations. Firstly, a longer-term, more extensive study is required to compare outcomes over extended periods. Secondly, a more comprehensive classification may be necessary to address various clinical scenarios requiring different grafting material preparation guidelines. Thirdly, the additional use of bioactive ingredients, such as platelet-rich fibrin or concentrated growth factors, could further enhance graft material healing processes, necessitating further studies to investigate their efficacy with differently prepared dentin-derived matrix graft materials.