1. Introduction
A virus refers to an ultramicroscopic nucleoprotein entity that becomes active only when inside a living cell. Thus, it strictly defines a unique class of acellular ultra-microbial parasitic agent owing to the fact that it can never be grown on an artificial culture media. When outside the host cell the virus behaves completely as an inert particle which is referred to as the virion. This virion or the extracellular virus particle has a biology defined only if after entering the cell, utilising the machinery of its host, it is capable to produce new look alike viral progenies. A virus can be named based on its host; A group of viruses that infects plants has been termed as phytoviruses and the branch that deals with their study is referred to as plant virology.
The problem with plant viruses: The rapid growth of the human population coupled with an era of accelerating climate change, affect the virus, vector and plant interactions that creates increasing instability with in virus plant systems. Emergent viral diseases have been particularly important in causing deterioration in the food quality & yield thereby posing a humongous threat & a negative economic impact on agriculture. Understanding the diversity of viruses that remain associated with ornamental and horticultural plants is still an ongoing challenge for plant virologists.
Viral diseases of plants cause concern as they render serious losses to agricultural crops being cultivated all over the world. Net yield returns from cultivated cereals, pulses, vegetables, and fruits are lost on a large scale only because of viral infection. People no longer can adorn their homes with ornamental plants, all thanks to viral infections which causes their immediate wilting. Plant viruses have also become a nuisance for gardeners as they don’t spare the garden plants as well. But is it really that big of a problem? Well it is. The international expansion of global trade together with the increasing plant cultivar diversity have resulted in the global spread of diverse plant viruses across the planet. Every stage of plant production, right away from sowing of seeds, harvesting the produce, to marketing of the product has chances of viral introduction provided adequate care is not taken. With the current scenario of rising population and declining food resources (viruses accounting for a major share), it is likely that humanity meets its doom very soon. To prevent the same, it is highly important to gain knowledge on the existing plant virus diseases, (those that have already spread across the globe), as well as emerging plant viral diseases, review existing detection methods & control strategies & make sure that they are implemented. So, its always advisable to gain the proper knowledge on the field of plant virology and its progress/evolution from then to now. Together with that the research on prospective plant viral diseases must also be keenly emphasized and it must also be recognized and accepted as one of the significant priority areas in agricultural science.
Plant Virology—Establishment of a New Field of Science & the Timeline / Chronicle of Its Development
The field of Plant virology encompasses the study of plant viruses and all their attributes. The birth of plant virology as a scientific discipline was established through the initial foundational contributions from 3 scientists namely Mayer, Ivanowski & Beijernick those of which led to discovery as well as first description of viruses. Virus host interactions in the case of plant viruses are mainly detected in the form of symptoms. Thus in the following years, the key focus was to identify many other plant viral diseases. Subsequently, these viral diseases was found out to be transmitted from an infected plant to the next by a variety of abiotic and biotic agents, the most common being biological vectors like those of whiteflies, leaf hoppers, aphids, thrips. Plant viruses such as that of Tobacco Mosaic Virus (first virus to be crystallized, by Stanley), subsequently served as the general model in understanding the biochemical and molecular aspects of viruses as a whole. Thus, in the historical timeline of plant virology what we refer to as the biochemical age and the molecular age undoubtedly became superior in defining the lesser-known aspects of a new pathogen which had failed to obey the regular Koch’s postulates. Shortly after that the entire virus life cycle together with the nature of its spread within the host plant was understood. Gaining satisfactory knowledge on plant viruses, the next step would be to diagnose and prevent plant viral diseases. Thus the development of good viral detection methods like those of serological & molecular assays like those of EM, ELISA, PCR was then an immediate necessity to differentiate virus infected plants from healthy ones. In recent years, many agricultural areas have already witnessed the evolution of numerous plant viruses causing significant loss of cultivars those of which could have been prevented just by an early and efficient diagnosis. Recent advances with LAMP, RPA, biosensor and microarray-based techniques enabled the quicker and more accurate detection of plant viruses. Additionally, NGS and HTS have emerged to become the most remarkable of the current diagnostic systems which can provide information on all known plant viruses as well as their variants. The effective control of viruses is the only way to compensate for the loss of quality & quantity of food production. The accurate diagnosis and timely detection of the viral pathogens is critical for the management of plant viral diseases.
Through this book, we intend to discuss the important landmarks in the field of plant virology. Moving in a sequential manner, the reader shall first progress through the following sections which mark the historical timeline/chronicle of plant virology ; a) Earliest traces of plant diseases which now are a reminiscent of known viral diseases which were obscure at that time, b) Discovery of the causative agent for the suspected symptoms, c) Discerning the Biology of this new agent (Biological age), d) Purification & characterization of the agent (biophysical/ biochemical age), e) Unravelment of the molecular nature & mechanisms of this agent (Molecular age), f) Age of Viral molecular genetics, g) Age of viral diagnosis and. Following this timeline, a basic summary on plant viruses ; its morphology, nature & organisation of genomes, Viral life cycle – replication, modes of plant virus transmission will be presented.
2. Tracing Out the Earliest Historical References of Viral Disease Symptoms amongst Plants—The Protoscientific Era
It is now estimated that the viral diseases of plants have existed and continued over many centuries. It should be however noted that it was not until the late 19th century when virus as a causative agent of plant diseases was first proposed; the exact nature of this pathogen being established later only in the 20th century. The earliest reference of a typical yellowing of leaves of Eupatorium lindleyanum dating back to 752 A.D was given in a poem written by a Japanese Empress named Koken [
1] that later got translated into english by T. Inoye. Another well-known example of the western Europe was that of the tulip colour breaking / breaking of tulips which was considered as an ornamental beauty in the 16th century. In 1576 Carlos Clausius a Flemish botanist became the first scientific person to describe variegations/striping in the colour of tulip petals. This tulip petal break disease of Holland evolved to be the oldest documented known example of a disease caused by a virus in plants, all thanks to the tulipomania that existed in the 17th century which led to an exorbitant high price for striped bulbs and even paintings of the same. The craze of such bulbs led to an economic depression for even mediocre class people began to barter tons of grain and livestock in procurement of a single striped bulb often arguing that it represents class and image of their family in the society. Thus, it was this art and documentation reserve that paved way for history. It must be however noted that even when people failed to recognize the cause of the symptom planters knew that through grafting with the regular tulips the phenotype of striped ones could be reproduced. Similar other relevant examples in history includes that of a yellow stripe disease of Jasmine (now known to be caused by jasminium mottle virus) in 1692 which became highly significant as it is now recognised to be one of the earliest written account of an experimental transmission of a virus. Lawrence in, 1714 had described that these stripping can be inherited via grafting of jasmine plants on account of sap exchange. A severe potato leaf roll outbreak was seen in Great Britain in 1770. In 1791 USA recorded peach yellows. In France and Belgium Ablution leaf variegation was seen in 1869. All the above-mentioned diseases including that of a Sereh disease of sugarcane noticed in Java in the 19th century is now known to be caused by viruses.
3. Chiseling the foundations of Virology—On finding a new infectious plant entity (1882–1900)
The actual era of plant virology marked its beginning with experimentation on tobacco plants with the disease being studied named as the tobacco Mosaic disease.
3.1. A Leafspot Disease of Tobacco Plants & Its Characteristics
A Mosaic disease of tobacco was widely observed in Holland in the late 1870s that rendered them extremely bitter to taste. Considering the request of Dutch farmers, Adolf Eduard Mayer (1843-1942), a German chemist & director of the Agricultural Experiment Station at Wageningen, Netherlands (since 1879) started investigating this disease whose etiological agent was obscure at that time. Noting down the characteristics of this disease he described growth retardation & decreased yield which often followed the initial symptoms of leaf curling thus rendering them unsuitable for cigarette manufacturing. On account of appearance of a pattern of light & dark green areas on the leaf lamina, Mayer had named this as Mosaikkrankheit / tobacco Mosaic disease [
2].
3.2. Symptoms of the Disease are Transmissible Experimentally
Mayer had found out that the mosaic symptoms could be transmitted to healthy tobacco plants via the juice of infected tobacco plants by rubbing the extract of juice filtered through paper onto healthy plants (Mayer, 1886). Further as a refinement of his initial experiment, he had used a sterile thin glass capillary tube for sucking the filtered juice from infected leaves & then inserted this tube directly into the leaf vein of a healthy tobacco plant. After about 10 days he had noted the first appearance of the characteristic mosaic pattern not on the inoculated leaf but rather on the youngest leaves of the plant. Further in the subsequent days the disease had soon spread to the entire leaves of the plant. Now, he had found that the juice obtained from other leaves (those of which remained uninoculated by the tube) of this plant can be used to continue the Mosaic transmission cycle to other healthy tobacco plants [
3].
3.3. Hypothesising Bacteria as the Disease Causing Agent
On the light of experiments he had done, Mayer doubted the presence of a plant pathogen fulfilling Koch’s postulates to be responsible for tobacco mosaics. Thus, he examined the sap for bacteria or Fungi (common plant pathogens) through an optical microscope but failed to identify the agent for the mosaics. Additionally, he had also recorded that the sap infectivity was lost when it was heated to 80° C. This result made him believe a that a small bacterium was responsible for the disease (since he always failed to identify fungal hyphal structures within the sap under the light microscope) and it died at this temperature thus rendering the extract non-infectious [
3]. He finally published a detailed paper describing the disease, its symptoms and transmissibility.
3.4. The Invention of Porcelain Filters & the Classic Filtration Experiments with Sap
Charles Chamberlandt, an associate of the famous microbiologist Louis Pasteur developed an unglaced porcelin filter of size ranging between 0.1 to 1 micron that could be used to retain bacteria and clear liquid suspensions [
4].
3.5. The Possibility of Mosaic Agent Being a Toxin
In 1892, Dmitri Iwanowski, (a Russian botanist & doctoral student at St. Petersburg University, department of Natural history) was the first one to use the porcelain filters in order to characterize the agent responsible for the Mosaic disease upon being commissioned by agricultural department of Russia to investigate the newly found tobacco mosaics in regions of Crimea & Ukraine. He was successful in showing that the extract remained infectious even after passing it through the unglazed porcelain candle filters/ Chamberland filters (known to exclude bacteria on account of smaller pore size) and concluded that agent was probably a toxin [
5], for it appeared to be soluble to him as he was unable to isolate any bacteria from the infectious extract & finally published these results as a paper in the Petersburg academy of Science.
3.6. Concept of Contagium Fluidum Vivum
In 1898 Martinus Beijernick a Dutch teacher & soil microbiologist working then at Delft technical University, Netherlands (and an ally of Mayer at Wageningen till 1885) repeated the filtration experiments and agreed with Ivanowskis idea of infectious filterable agent. Along with the earlier porcelain experiments, he had also checked the filterability of the agent through agar. In the experiments he devised, a diffusion time of 10 days was given after layering the infected tobacco juice on top of a layer of thick agar. After the diffusion time the top surface was cleaned with water soon after which he carefully checked the deeper agar layers for pathogenicity (when injected into healthy tobacco plants gave the characteristic Mosaic symptoms). He thus concluded that the agent responsible was not (= fixed) or of particulate state but rather was fluidum (= dissolved state) since it was able to diffuse through agar that retained bacteria thus predicting it to be smaller than bacteria (Beijernick, 1898). He also found out that the infectious extract stayed stable for 3 months with its virulence remaining constant. In addition to that Beijernick also noted that the potency of the sap decreased on dilution, but its initial vigour got restored when it was retrieved again from newly infected plants suggesting the capability of the agent to reproduce in living tissues. Further this extract remained viable even after the injected plant parts dried but the same was found to be heat inactivated at 90°C. Thus, taking into account the properties of infectivity, filterable nature and ability to multiply, he described this as contagium
vivum fluidum or infectious living fluid and also gave the modern term virus [
6]. Thus the name Tobacco mosaic virus was first popularised as the virus that causes the tobacco mosaic disease.
3.7. The First Description of the Virus
The experiments undertaken by these 3 scientists drew certain conclusions on conceptualizing virus as a new agent/soluble entity not visible under the common light microscope, smaller than typical bacteria retained by smallest diameter of the Chamberland filter, incapable of multiplication in artificial culture media. Thus, what was called as the virus became the first pathogen to disprove the Koch’s postulates violating the fact that it failed to grow in pure cultures outside the host plant thus making it an obligate parasite.
4. Biological Age of Plant Virology (1900–2000)—Age of Establishing Plant, Virus & Vector Relationships
In this age the classical symptoms of a virus infected plant, natural routes of virus transmission to healthy plants, methods to quantify plant viruses, methods to study virus host interactions etc were investigated.
4.1. Classical Landmarks of the Biological Age (1900–1935)
4.1.1. Quest for Diseases Similar to that of Tobacco Mosaic
During this age which is also referred to as the biological age, a variety of revelations on the nature of the plant diseases (those with reference to classical symptoms induced by a virus) were made but there were no studies to find out the exact nature or structure of the virus as such. A wide variety of plants underwent scrutinal examination for altered phenotypic changes, observable macroscopic symptoms & physiological changes. Many cultivated crops were widely screened for those symptoms that resemble thatt of tobacco mosaics.
4.1.2. Earliest Techniques Employed to Confirm Virus as Etiological Agent
During this time, it was mainly important to understand & discern whether a viral pathogen was actually responsible for a particular disease which had been found out. The techniques employed for confirmation of the viral pathogen during this age included those which enabled recreation of the disease symptoms through either sap inoculation or grafting along with the use of the early characterised ultrafiltration studies to exclude the possibility of a bacterial or fungal pathogen. Initially typical known virus disease symptoms like mosaics and leaf curl etc. were investigated amongst common cultivated plants. When examined, these External symptoms were identified in plants like tobacco, potato, cucurbits, sugar beet & legumes. Additional confirmation of virus involvement was done via analysis of typical internal symptoms through the aid of the common light microscope– identifying inclusion bodies of crystalline or amorphous type within the Mosaic regions.
4.1.3. Searching for Agents that Transmit Plant Viruses
After identifying plant viral diseases ; the next step undertaken was to discern how they are maintained & cycled from one plant to another. It was revealed as early as in 1901 that insects play a crucial role in plant virus epidemiology. The relationship between an insect & a plant virus demonstrated earlier in Japan by Hashimoto in the case of Rice dwarf disease & leaf hopper
Nephotettix apicalis was retrieved by the west. [
7] pointed out that just 5 minutes after placing a single insect from an infected plant to a healthy plant the disease shall ensue (with reference to Curly top of sugar beet & leaf hopper,
Eutettix tenella). Transmission of plant viral diseases by leaf hoppers [
8], aphids [
9,
10], by beetles [
11], by thrips [
12], by whiteflies [
13], by mites [
14] from seed [
15], from pollen [
16] was reported & confirmed.
Table 1.
A list of earliest plant virus - vector relationships .
Table 1.
A list of earliest plant virus - vector relationships .
| VECTORS |
Viruses transmitted |
References |
|
|
|
- ➢
Leaf hoppers |
|
Takami (1901)Ball (1909) |
- ➢
Aphids |
|
Doolittle (1906)Jagger (1906) |
- ➢
Beetles |
|
Smith (1924) |
- ➢
Thrips |
|
Pittman (1927) |
- ➢
Whiteflies |
|
Kirkpatrick (1931) |
- ➢
Eriophyid gall mite |
|
Amos et al. (1927) |
|
|
Doolittle & Gilbert (1919)Reddick & Stewart (1919) |
- 3.
Pollen |
|
Reddick (1931) |
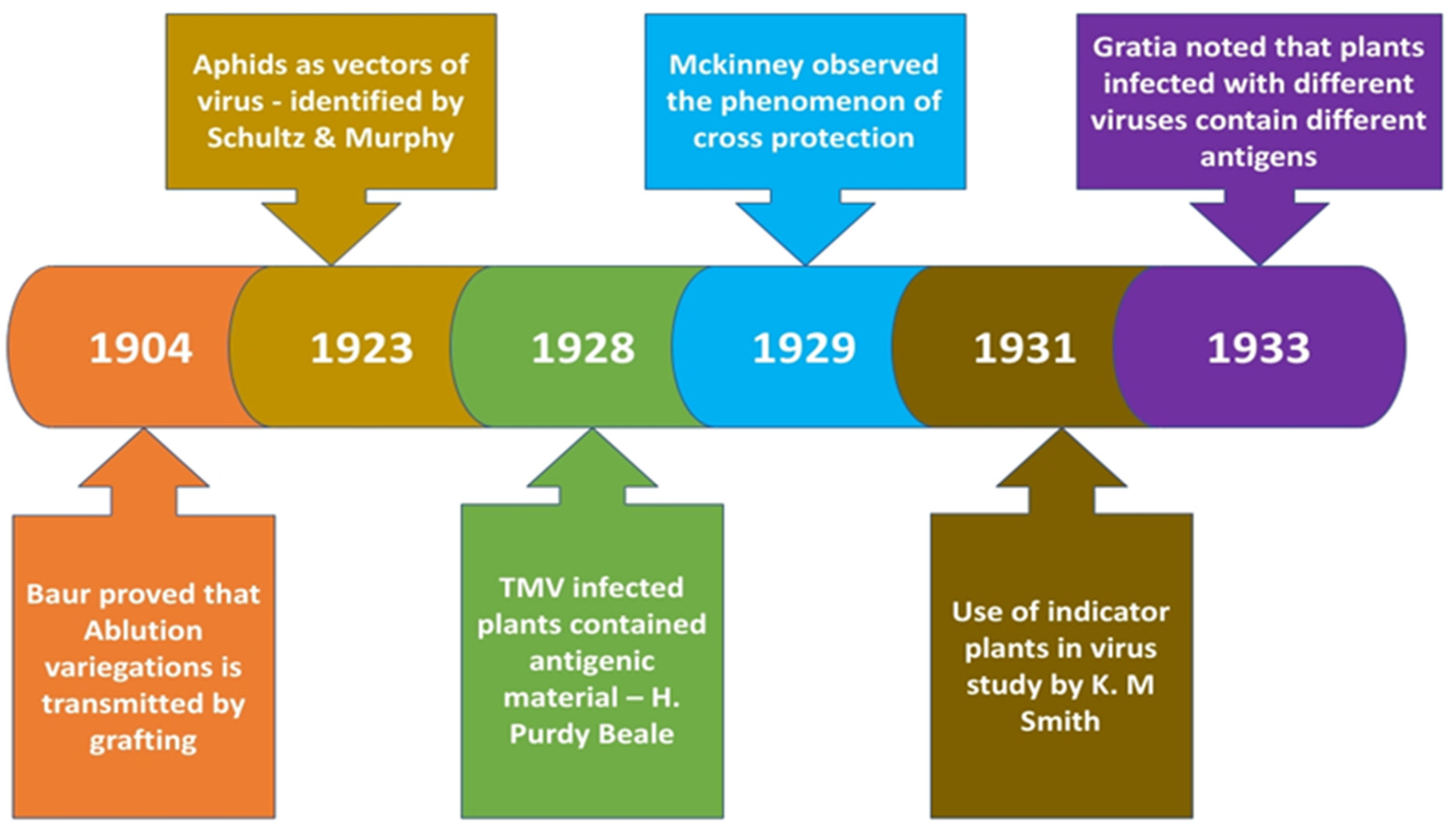
4.1.4. Hunt for an Antigen; Birth of Plant Virus Serology
This period also marked the beginning of plant serology. Initially in 1927, Dvorak took the sap from Mosaic infected plants & injected the same into experimental animals soon after which he concluded that sap from infected plants contained a component which was serologically active which was missing in healthy potato controls. Similar results were also recorded by Purdy Beale who experimented on diseased tobacco plants thus concluding that the sap exhibited properties of an antigen [
17].
4.1.5. Discovery of Cross protection principle
The principle of what is known as cross protection was introduced to the world by Mckinney in 1929, wherein he observed that tobacco plants when infected by viral sap (retrieved from green mosaics) failed to reproduce the characteristic yellow mosaic symptoms upon challenging the same with the sap obtained from tobacco yellow mosaics [
18]. Tobaccos inoculated with a mild strain of potato virus X was also immune from subsequent inoculations with severe strains of the virus.
Quantifying the Virus—The Development of the First Biological Assay for a Plant Virus
The most significant experiment done in this period was by Holmes who discovered that local lesions developed in tobacco leaves when these were injected with infected sap. The no of local lesions was in proportion with the concentration of virus in the inoculum injected [
19]. This old technique still forms the basis of quantitative assay of many plant viruses.
4.1.6. Use of Differential hosts, indicator hosts & Filter plants
It was shown during this period that same host can be infected by more than a single type of virus and the same disease symptoms can be caused by different viruses. Initially it was shown earlier in 1925 by James Johnson that young tobacco leaves were infected by Inoculating the sap from healthy potato plants (the agent being initially termed as healthy potato virus but later called as virus X by Smith). Thus, James had concluded that the virus may be carried within the potato plants without any symptoms but when this was injected into tobacco it resulted in overt symptoms. Thus, tobacco became the ideal alternative host which reacted accurately to sap inoculation: henceforth such plants were termed as differential hosts. Another use of this so-called hosts became more evident in 1931 through the works of Erwin F Smith who used plants other than potato (named later as indicator hosts) for his study on potato Mosaic disease [
20]. Smith had showed that 2 different viruses with distinct properties (which he named X & Y) when in combination resulted in potato virus disease. Smith had used the indicator plants for filtering 1 virus from the other in a sap containing crude viral complex thus he obtained virus X free from virus Y when he needle inoculated the mixture into Datura stramonium (functioning as unfavorable host for virus Y). Hence plants like Datura came to be known as filter plants. Similarly, it was also reported that when a tobacco plant gets infected with a mixture of virus X & virus Y, pure culture of the latter can be obtained first from the youngest leaves because the virus Y travels faster to the growing points. Thus, indicator plants helped in gaining much information about many unknown viruses. Smith had also used the property of selective / restricted vector multiplication for separating potato virus Y from potato virus X, on account of virus Y being transmissible through the aphid Myzus persicae whereas virus X wasn’t – thus enabling virus Y to be purified free of virus X from crude viral complex.
4.1.7. Cross Protection Test
Elaborating on the cross-protection idea introduced by Mckinney, in 1934 Kunkel developed a cross protection test to gather useful information about interaction of different viruses that infect a host [
21]. Initially the leaf lamina is marked into two portions & the individual viruses under study are needle inoculated into two portions. The development of virus induced local lesions are then noted. The test was often referred to as half leaf test when one out of the 2 inoculated viruses induce localized lesions. If the second virus fails to induce lesions it means both the viruses are somewhat related. Further if both the viruses induced lesions and if they tend to increase towards the mid rib then they are unrelated.
Figure 1.
A series of findings made in the biological age before the advancement of biochemical age of Virology.
Figure 1.
A series of findings made in the biological age before the advancement of biochemical age of Virology.
4.2. Additional Inputs to the Biological Age; with Reference to Plant Virus Vectors (1939–2000)
These inputs refer to the non classical advancements made in the field of plant virology specially in relation to plant virus and their vectors. Although not widely recognised, the landmarks listed below holds key importance for they progressed contemporary to the biochemical/Biophysical age of plant virology
4.2.1. Investigating Vector—Virus Interactions
The first concepts of virus vector interactions were introduced by Roberts & Watson, wherein they had also coined the 2 terms “persistent “ and non persistent to describe the time span the vector remains infective following acquisition [
22]. During the early years 4 hypothetical mechanisms for aphid based transmission of nonpersistent viruses was suggested those of which included a) biological transmission, b) transmission through salivary apparatus [
23], c) transmission by regurgitation & d) mechanical transmission [
24]. Majority of reports [
25,
26] using Myzus persicae & Brevicoryne brassicae vectors had shown that the total time in between initiation of a feed & completion of successful inoculation will not be longer than 1-2 minutes. Discovery of nematodes as soil borne plant virus vectors [
27].
4.2.1.1. Testing Whether Viruses Are Carried by Mouth Parts (= Mechanical Transmission)
The Retention test: The stylets of an aphid are shed during moulting and are replaced by new ones in advancing instars. If a virus is carried within the stylets then it cannot be transmitted after a molt – testing this employing M. persicae & beet yellow net virus showed the absence of virus in turnip indicator plants on which the aphid feeded. Artificial stylet wetting test: This test involved dipping the stylets of aphids into a concentrated solution of TMV abd permitting them to feed on test plants. The absence of a plant viral infection was taken as an evidence of virus not being transmitted via the stylet.
4.2.1.2. Insights on Biological Transmission of Viruses
Electron micrographs of rice stunt virus in a section of its insect vector tissues gave the first logical evidence for the multiplication of such viruses within the cells of their arthropod vectors [
28]. Another contribution was made by Herold, when he published elecron micrographs of virus like particles concentrated in the cytoplasm & the vicinity of the nucleus within the corn leaves. The particles which were arranged in a regular array gave rise to the microcrystal that were 242 microns in length and 48 microns in width (placing them in virus category). The unequivocal demonstration that plant viruses establish & even can cause diseases in their vectors came via the study undertaken by Jensen in 1958 wherein he noted that colonies of Colladonus molanus when made to feed on plants infected with the Western X-disease peach yellow leaf roll strain resulted in the vector exhibiting reduced longevity (life span) along with continuing virus transmission until each of them died. It was also shown that a single leafhopper species can potentially transmit 2 unrelated plant viruses when both Wound tumour virus & potato dwarf viruses were shown to be transmitted by Agallia constricta [
29]. The leaf hopper Dalbulus maidis, the vector for Corn-stunt virus, exhibit a property of surviving only for 4 days on healthy asters, but longer periods on those asters infected with aster yellows [
30]. Sylvester had lateron recognised a semipersistent form of virus transmission. Vector region analysis showed that persistent viruses passed through the insect gut wall and hemocoel to accumulate in the salivary glands and thus become circulative whereas the non persistent viruses doesn’t pass through insects interior but gets associated with anterior portion of feeding canal & are thus termed as non circulative or stylet borne. It was later also recognised that there are 2 types of types of circulative viruses, those of which can replicate in the vector termed propogative & those of which which doesn’t replicate in the vector nonpropogative.
4.2.1.3. Examples of Viruses & Their Key Proteins Involved in Biological & Mechanical Transmission
Potyviruses (a non persistent virus) are shown to be transmitted via a virus encoded nonstructural protein termed as the helper component protein; often abbreviated as HC-Pro that binds the virus particles to aphid mouth parts [
31,
32]. Caulimoviruses can persist in the aphid vectors for several hours in addition to which they also use an attachment mechanism using association of virus particles with aphid mouth parts. An HC-Pro called P2 [
33] binds to aphid stylets and to a 2nd nonstructural viral protein termed P3. Other stylet borne viruses such as that of the Cucumoviruses require only the viral Coat protein & don’t require an HC-Pro for aphid mediated non persistent transmission [
34]. The 2b protein was another HC-Pro that was found to be essential for the persistent non circulative transmission of Tobraviruses by trichodorid nematodes. [
35] & [
36] has shown that tospoviruses of the Bunyaviridae family replicate in their vector ie, thrips and involve in Propogative transmission. The long persistence of luteovirus without replication in aphid vectors was reported as a case of circulative nonpropogative transmission [
37,
38].
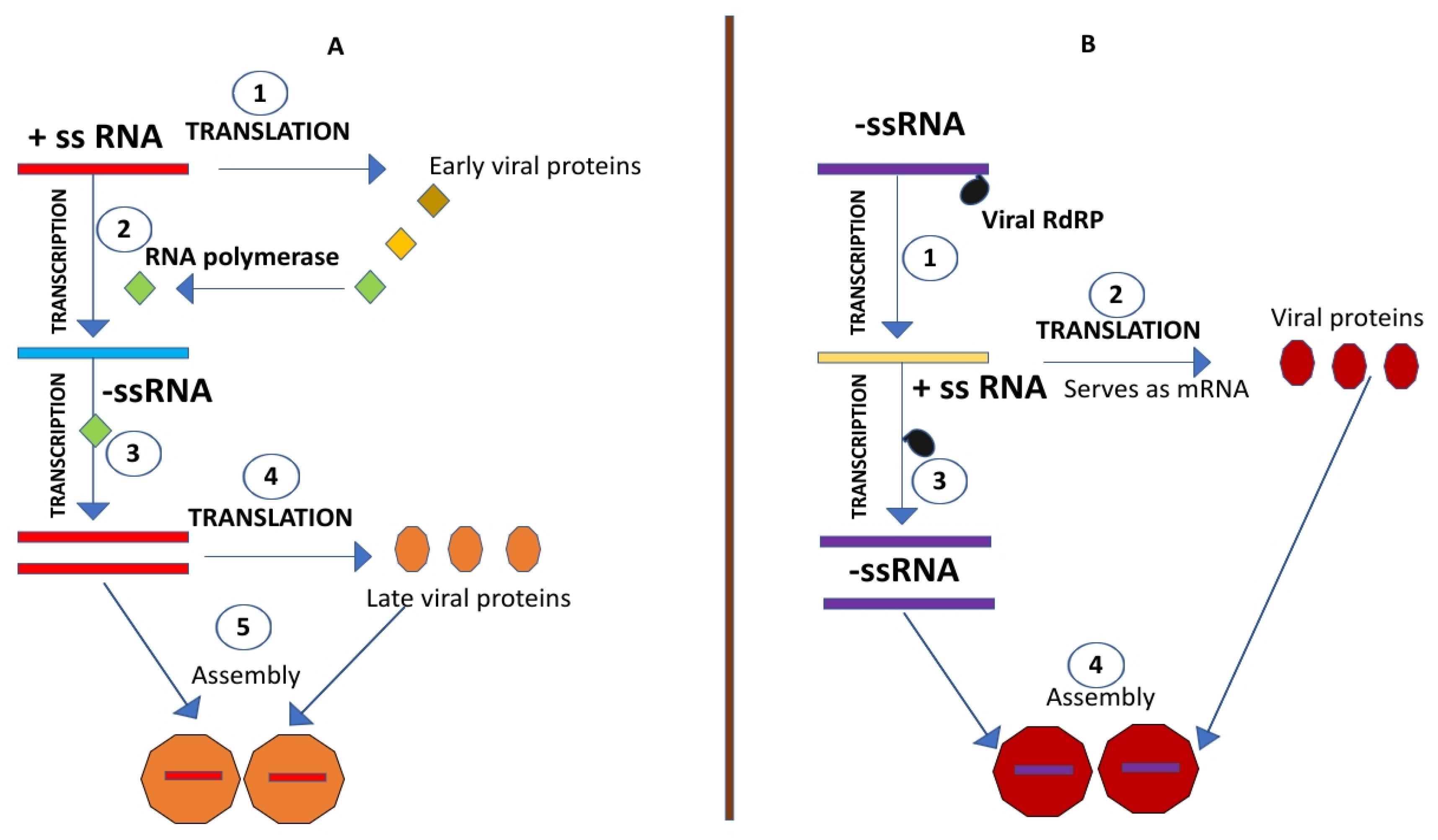
5. Biochemical / Biophysical Age of Plant Virology (1930-1968)—Defining Physics and Chemistry of the Virus Using TMV as a Model
This age started since the 1930s and marked the basis of virus purification & chemical characterization. Details on the exact viral structure, size, the molecular basis of its infectivity and methods for viral clarification & concentration became available.
5.1. Attempts to Purify the Virus from the Extract via Precipitation
Based on the Holmes infectivity assay described earlier, it was Vinson & Peter from the Philadelphia Boyce Thompson institute who had first precipitated TMV from crude sap of infected tobacco (highly concentrated) with particular salts like lead acetate & Safranin together with Acetone. This technique was an elaboration of the earlier technique developed by Vinson in 1927 wherein he noted that addition of Acetone (in non-inactivating concentrations / higher dilution) to the infective sap would concentrate it further. In a refinement of the same, the purer form of the virus precipitate was obtained by Vinson & Peter in 1929 by addition of safranine, magnesium sulphate, ammonium sulphate and iron acetate was finally eluted out in amyl alcohol. This precipitate showed properties of a protein for when tested the former also moved under an electric field. In 1931, adding acetic acid together with acetone to purified salted out sap, they obtained some infective crystals which failed to retain the infectivity when crystallized for the second time. Thus in 1932 when they reported their results in Journal of Medical Association, they predicted these crystals to be of protein nature not retaining their infectivity when recrystallized. Adding onto this Purdy Beale at the same institute raised neutralizing Abs against TMV in rabbits reaffirming on the proteinaceous nature.
5.2. Principles of Refraction to Understand TMV
The earliest biophysical experiment that gave an idea of the shape of TMV was undertaken in 1932 at California University, Berkeley by Takahashi & Rawlins wherein they made a TMV rich crude plant juice to flow in between crossed nicol prisms. They had noted that the flowing TMV extract bestowed double refraction, a pattern that was shown to happen only with Rod like particles as reported earlier by Freundlich [
39].
5.3. Quest for the Biochemical & Biophysical Nature of TMV.
5.3.1. Crystallisation of TMV
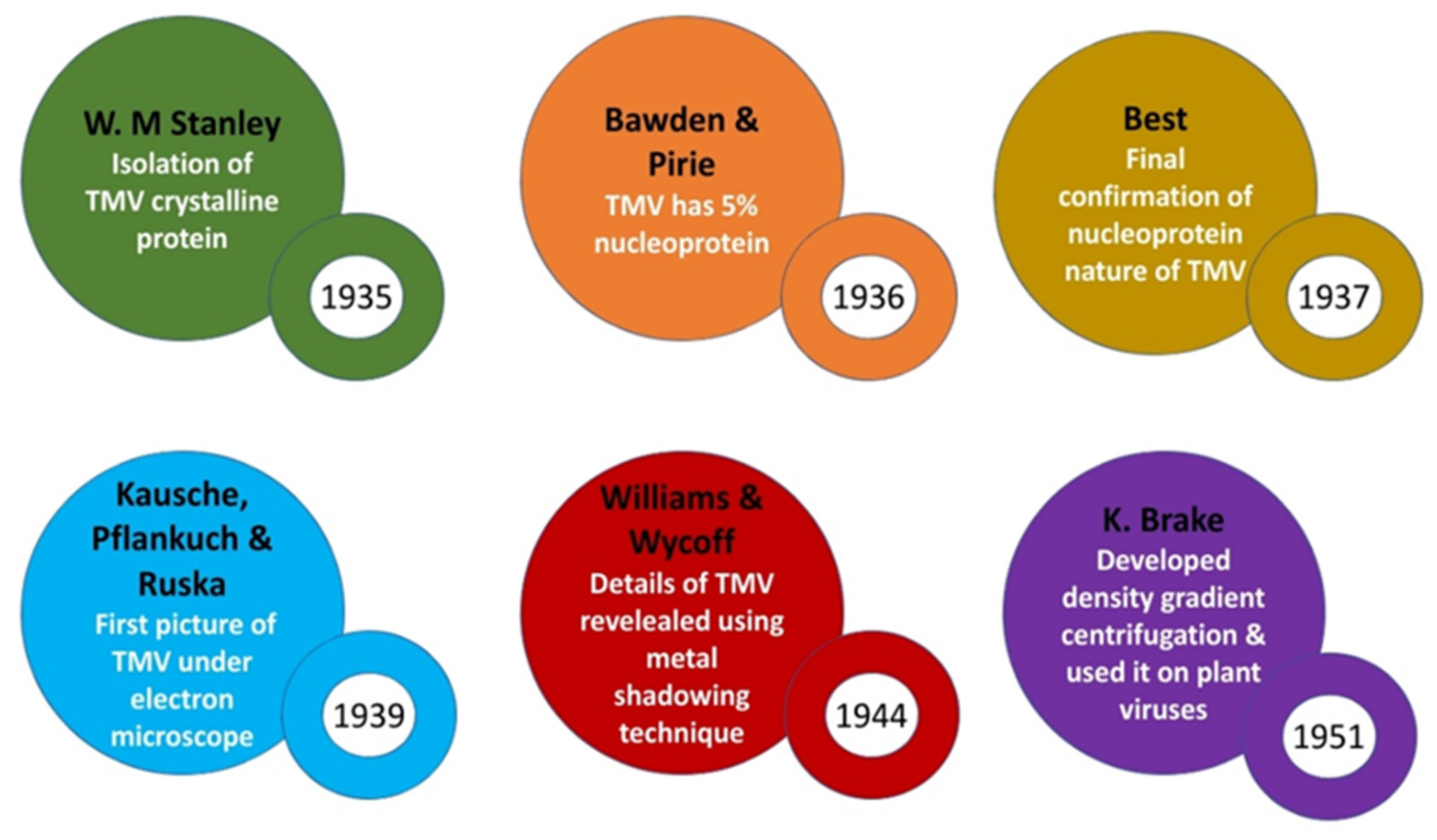
It was Wendel Meridith Stanley working with the so-called Princeton group at the Rockefeller institute for Medical Research in New Jersey who became a key figure of the Biochemical age. Stanley had started his experiments with TMV in 1933 and soon in 1934 he had showed that TMV infectivity was lost in presence of pepsin (freshly crystallized by Northop) thus arguing on the proteinaceous nature of the virus. Together with this he had also studied the infected tobacco juice at a wide range of pH and recorded its inactivation rates closely followed the rate of inactivation of proteins. In 1935, Stanley first precipitated TMV in the form of crystals resembling needles by adding ammonium sulphate to highly concentrated infected juice (made by processing approximately 4000 Kg of diseased tobacco leaves). These crystals were about 0.03mm in length when observed under a magnification of 400X of the light microscope [
40].
5.3.2. Voting for an Enzymatic / Proteinaceous Nature of TMV
Upon initial analysis of the crystalline material with classical tests for biomolecules, Stanley had recorded a positive biuret test (for protein) & negative Fehling & Molisch test results (for carbohydrate). Finding approximately 20% nitrogen in his crystals he immediately declared to the world that the precipitate he had obtained was indeed that of a protein. He also showed that these crystals (when redissolved - in the suspension form) produced the disease when rubbed onto healthy plants (up to a billionth dilution) & thus disproving the earlier notion of virus being a living soluble liquid & also postulated that what was then called as virus was an autocatalytic protein/enzyme capable of multiplying in living cells [
40].
5.3.3. Studying Physical Properties of TMV
Discerning the nature of the pathogen the next immediate step that was undertaken by Stanley was to understand the size and shape of the protein. The samples of crystalline TMV obtained by Stanley was soon taken to the University of Uppsala, Sweden by Svedberg who predicted the molecular weight of the protein to be 17 million by studying its rate of sedimentation at a force of 4000000g under an analytical ultracentrifuge. Further a significantly large asymmetry constant also hypothesized the virus to be of rod shape.
5.3.4. Blow to the Protein Alone Nature of TMV
However the proteinaceous chemical nature of crystals deduced by Stanley didn’t sustain for long for it was soon proved to be vogue when studies undertaken by F. C Bawden & N. W Pirie from UK Rothamsted Institute (working with common, enation & aucuba strains of TMV) also showed the invariable presence of 2.5% carbohydrate & 0.5% phosphorous besides protein in the TMV crystals they had purified [
41]. They also noted that the latter 2 components got released separately upon heat denaturation of TMV. Despite several efforts they were unable to obtain a fully active virus preparation that was phosphorous free. The presence of phosphorous in purified virus precipitate pointed towards the presence of a nucleic acid as an integral component of the virus. UV spectral analysis also showed maximum absorption at the nucleic acid region for the virus. Working independent of Bawden & Pirie, best from Australia, made a significant contribution whereby he first speculated TMV as an enzyme complex that is made up of a large protein-based part (=apoenzyme) and a small acid prosthetic group (which became inactivated in weakly alkaline solutions on account of its hydroxyl ion concentration).
5.3.5. Leads from X-ray Diffraction Studies
Based on the 1st X ray diffraction analysis of TMV undertaken by Wycoff & Corey by samples given by Bawden & Pirie, it was concluded that TMV was made of repeated structures resembling a rod like morphology [
41]. Thus Bawden & Pirie voted for TMV suspension to be a crystalline nucleoprotein with rod or cigar shaped constituent particles (also agreed by crystallographers Bernal & Fankuchen in 1937 by their X ray analysis of TMV). It was also shown by Bernal & Fankuchen that the so-called Stanley crystals were regular only in the two dimensions meaning they weren’t actual 3D crystals – thus they more accurately described them as paracrystals. First true 3D image of crystallized Tomato Bushy Stunt virus (a spherical virus) was also obtained shortly by Bawden & Pirie with the help of Bernal & Fankuchen in 1938. Meanwhile the molecular weight & shape of the TMV protein was first proposed.
5.3.6. Understanding TMV Constituents
In 1939 Max Lauffer along with Stanley actually separated TMV into a protein & nucleic acid part soon after which the nucleic acid was identified to be RNA by H. S Loring, post-doctoral student of Stanley by his experiments involving marked decrease in infectivity of TMV when treated with ribonuclease.
5.3.7. The First Image of TMV via the Aid of the Electron Microscope
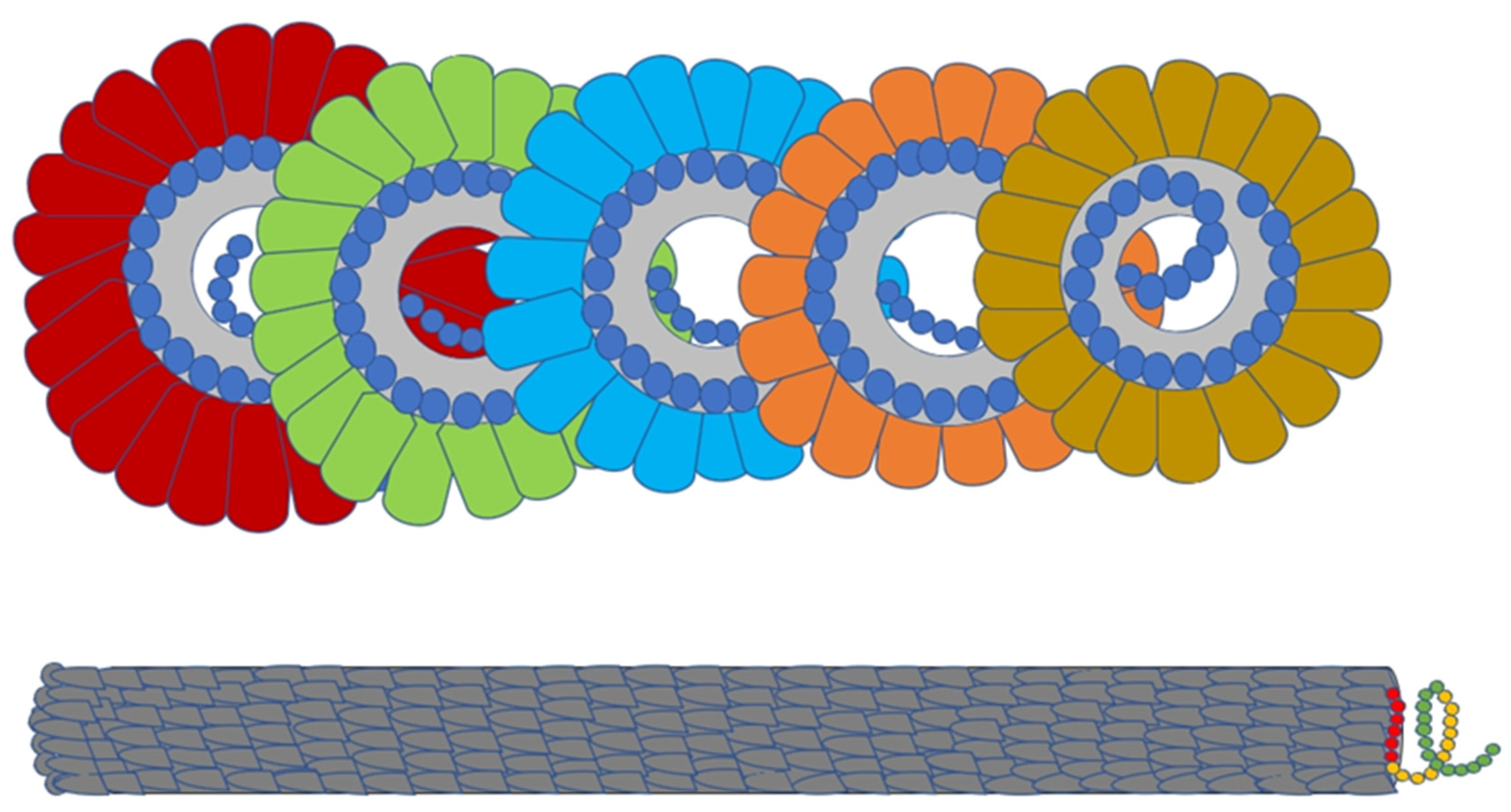
The direct visualization of TMV became possible when a new electron microscope (designed by E. Ruska & Von Borris) with a resolution of 10nm & magnification of 1 lakh was introduced to Berlin via the Siemens company. Finally the first vague electron microscopic image / electron micrograph of TMV came in 1939 which supported the rod like morphology of TMV (predicted earlier by diffraction analysis) in addition to giving a rough estimate of the size of TMV Rods [
42]. In 1941, Bernal & Fankuchen moved one step further when through their improvisation in X Ray diffraction analysis, they discerned the diameter of TMV Rods to be approximately 15nm and a length of roughly ten times the width. Further a higher resolution image of TMV (under electron microscope) with minute details was obtained later in 1944 by Williams & Wycoff by a specimen preparation technique called shadow casting where heavy metals like lead, gold or palladium were vaporized in vacuum & was allowed to deposit on the particle thus resulting in a shadow of the particle. These electron microscopic images also confirmed TMV to be Rods having a diameter of 15nm & a length of 300nm [
43].
5.3.8. Aid of Centrifugation to Isolate Plant Viruses
In 1951 a very crucial technique named density gradient centrifugation pioneered by K. Brakke led to direct isolation of plant viruses after sap clarification. This technique involved layering of viral extract on top of a gradient created by using different concentrations of high-density salts like lithium chloride or Caesium chloride in centrifuge tubes & centrifuging at a speed of nearly 30,000 to 45,000 rpm [
44]. Thus, the virus got separated (at a position where its density matched with that of the salt) & thus was withdrawn using a needle syringe.
Figure 2.
A picture showing highlights of biochemical & biophysical breakthroughs on TMV (1935-51).
Figure 2.
A picture showing highlights of biochemical & biophysical breakthroughs on TMV (1935-51).
5.3.9. Attempts to Discern the Structral Organisation of TMV.
The biochemical age continued till the late 1950s as further refinements of the virus structure & arrangement was made through electron microscopic & X Ray crystallographic studies. The X-ray studies undertaken in the 1950s by James Watson made him believe that TMV was probably helical. Soon in 1954 he had published a paper in this regard, but he failed to accurately discern the number of subunits per each turn of such a spiral structure. In the following year obtaining samples from repolymerized nucleic acid free TMV particles Rosalind Franklin obtained a detailed high-quality X Ray Crystallography from which the first true picture of TMV quaternary structure could be deduced 1956 [
45]. In the same year along with confirming the earlier hypothesis by Watson that TMV had a helical structure, Franklin & Caspar moved one step further when they provided evidence that the helix was a hollow one rather than a solid [
46]. It was also found that the TMV RNA was single stranded & was actually wound (spiralled) along the inner surface of the hollow cylinder made by protein capsid:- with analogy to a ‘thread spiraling inside a donut hole’ [
47].
Table 2.
Early technical advances & findings that aided in building the foundation for biochemical age of plant virology.
Table 2.
Early technical advances & findings that aided in building the foundation for biochemical age of plant virology.
| Techniques / Findings |
Developer/Researcher |
Year |
| Biochemical methods |
1. Salt precipitation of TMV
2. [Pb (C2H3O2)2 + safranin]
[Safranin+MgSO4+(NH4)2 SO4+Fe(C2H3O2)2]
|
Vinson & Peter |
1929 – 1931 |
Crystallisation of TMV,
[Saturated (NH4)2SO4]
Tests for chemical nature
❖ + Biuret test for protein
❖ - Fehling & Molisch test for
Carbohydrates
|
W. M Stanley |
1935
|
| TMV was shown to contain about 2.5 % carbohydrate and 0.5% phosphorous. |
Norman & Pierie |
1936 |
| TMV can be seperated into protein & nucleic acid parts. |
Stanley & Max Lauffer |
1939 |
| The nucleic acid part in TMV is that of ribonucleic acid |
H. S Loring |
1940 |
| Biophysical methods |
Nicol Bifringence studies
TMV has Rod like morphology
|
Takahashi & Rawlins |
1932 |
X-Ray diffraction analysis
Stanley crystals of TMV are cigar shaped but were regular only in 2 dimensions ; hence werent true crystals
|
Bernal & Fankuchen |
1937 |
X-Ray diffraction analysis
First true 3D image of tomato bushy stunt virus (spherical) obtained.
|
Bernal & Fankuchen |
1938 |
Electron Microscopy
1st image of TMV
|
Kaushe et al. |
1939 |
X-Ray diffraction analysis
Diameter of TMV rods is 15nm & length is 150nm - 250nm
|
Bernal & Fankuchen |
1941 |
Electron microscopy
Shadow casting technique for better resolution predicts TMV dianeter to be around 300nm
|
Corey & Wycoff |
1944 |
Ultracentrifugation
Density gradient (CsCl or LiCl) ultracentrifugation can be used for direct isolation of viruses.
|
Brakke |
1951 |
Electron Microscopy
Negative staining technique
|
Brenner & Horne |
1959 |
Electron Microscopy
Freeze–etch & Freeze–fracture techniques were developed to discern 3D ultra structure information on viruses
|
Steere |
1957-60 |
| Serological methods |
Histochemical methods
Detection using fluorescent antibodies
|
Coons et al. |
1942 |
Gel diffusion tests
Ouchterlony method of plant virus detection
|
Outchterlony |
1949 |
| Immunoelectrophoresis technique |
Grabar & Williams |
1953 |
| Radioimmunology technique |
Berson & Yallow |
1960 |
Serologically specific EM
Leaf Dip method of electron microscopy
|
Ball & Brake |
1968 |
6. Molecular age of Plant Virology (1943–1995)
The molecular age became one of the most crucial ages in the history of plant virology. Having the chemistry and physics of the virus already established; scientists of that time wished to understand, how the virus carries out its essential processes within the host such as key viral factors for disease initiation, establishment, progression & transmission. The scientists with curiosity wanted to know how the virus create progeny. The viral macromolecule which harbors the genetic information had to be found out. Experiments were undertaken with the classical TMV model.
6.1. Decoding the Basis of Viral Infectivity; Understanding Whether the Nucleic Acid Dictates the Protein or Vice-Versa?
The doubt on which one of the TMV constituents i.e., protein or RNA is responsible for the transfer of genetic information was indeed present amongst the scientists of that time. The biological differences between the different strains of TMV were attributed to the differences in their nucleic acid.
6.1.1. Splitting TMV to Pieces
Schramm in 1943 had done a simple experiment to test which TMV component was capable of reproduction. Employing a slight alkaline solution, he had spitted TMV into pieces. He also recorded that when the pH was shifted back to acidic the pieces reassembled to their original form. Schramm had then recorded the incapability of both the pieces & reaggregated forms to produce new TMV rods. In a further refinement of the same, Schramm obtained a nucleic acid free TMV protein by using a nucleotidase (obtained from Calf intestine). He recorded that even though the virus crystallized as before it had lost its infectivity. Thus, he asserted on nucleic acid being the genetic molecule within the TMV.
6.1.2. Presence of 2 Components within the 2 Bands/Fractions after Ultracentrifugation of Turnip Yellow Mosaic Virus
The year 1949 marked the isolation and crystallization of Turnip yellow Mosaic Virus by Roy Markham & K. Smith. Even though the virus exhibited homogenecity in migration under regular electrophoresis, sedimentation studies with the same using an analytical ultracentrifuge indicated the presence of 2 components within two layered fractions. The heavier fraction was thicker (comprised of 70 to 80% of total material) and was shown to be nucleoproteins consisting of 37% of RNA while the lighter fraction was solely that of proteins [
48]. Experimenting further they found that only the RNA containing part was infectious. Although these experiments were a breakthrough, they weren’t much decisive for it only gave the understanding that RNA component was essential for infection. Hence there lingered a doubt amongst the scientific world whether RNA alone was the infective entity or was it the association of RNA and protein that is responsible for the infective nature of the TMV pathogen?
6.1.3. Experiments with TMV Coat Protein Variants Give a Breakthrough
In order to understand the infective component in detail Harris & Knight in 1952 devised a new experiment wherein they first developed a dethronized TMV by treating normal TMV with carboxy peptidase (thus removing the C-terminal threonine of TMV coat protein). Initial experiments with these coat protein variant TMV showed they possessed identical infectivity with that of regular TMV (thus there was no loss of infectivity). Further analyzing the progeny obtained after Inoculating the plants with dethronized TMV showed that these were of the normal type (those with C terminal threonine). This experiment gave an idea that viral RNA controlled the specify of the viral protein [
49].
6.1.4. Artificially Reconstituting TMV—Creating a Virus in the Lab
In 1955, Fraenkel Conrat & Williams had obtained a pure RNA fraction of TMV by disrupting the TMV particles with detergent. A pure protein fraction was also obtained by undertaking the alkali treatment of TMV particles. Independently testing these fractions for infectivity, they had noted both to be of non-infectious nature. However, on mixing both the fractions an infectious nucleoprotein with the properties of TMV was made. Thus, they claimed to artificially create the virus by reconstitution by associating 2 kinds of molecules [
50].
6.1.5. Unequivocal Proof of TMV RNA as Its Genetic Material
Having their doubts still unresolved Alfred Gierer and Gerhard Schram in 1956 had efficiently separated TMV RNA from protein by shaking the TMV Preparation with a water saturated phenol solution (RNA remains while protein gets extracted in phenol). Also treating TMV with acid or alkali yielded the viral protein in native form. Further separately Inoculating the two constituents into the tobacco leaf they had found that only RNA of TMV had produced the characteristic lesions (those resembling that of intact TMV) on the inoculated leaf. However, the strength/ index of infectivity of the RNA fraction was significantly lower when compared to intact TMV particles indicating that RNA was labile. They had also recognized that the TMV lesions fail to develop when the purified RNA had been treated with RNAse [
51]. RNAse was known to have no effect on intact TMV particles. Further there was no loss of infectivity in TMV RNA portion even after treatment with antiserum or undertaking ultracentrifugation (to exclude TMV particles completely). In the light of the above revelations Gierer & Schram stated that infectivity was not created denovo by the mixing of 2 different components (as stated by Fraenkel & Conrat) but rather it was solely the inherent function of the labile TMV RNA which following the association of protein was protected from inactivation. Thus, they concluded that the viral genome of TMV must be comprised of RNA. Fraenkel too when analysed his TMV RNA fraction closely recorded its infectious nature.
6.1.6. Reconstitution Experiment with 2 Strains of TMV
In 1957, motivated by the inferences Gierer & Schramm gave, F. Conrat & B. Singer performed reconstitution experiments with 2 strains of TMV (differing in amino acid composition) namely the common strain (causing green mosaic) & Holmes rib grass virus / plantago strain (producing ring spot lesions). They first broke these viruses respectively into RNA & protein component parts by employing their purification procedures of detergent (SDS) disruption & alkali/acetic acid treatment. Following this they reconstituted hybrid viruses, obtaining reciprocal chimeras first with an RNA from common strain enclosed in HR protein and the other with HR RNA enclosed in common TMV protein. These reassembled chimeric viruses were then used to infect tobacco leaves separately. Upon analysis of progeny it was found that daughter viruses were always phenotypically & genotypically identical to the parent strain from which their RNAs had been obtained i.e., Common TMV RNA even if enclosed inside the HR protein gave only daughter viruses containing common RNA & Common protein and produced green mosaic on leaves [
52]. Thus, it was unequivocally proved that specificity of viral proteins was determined by RNA alone & proteins carry no genetic information at all.
6.2. Establishing the Heritability, Mutability & Integrity of TMV Genome
6.2.1. How Warty is TMV RNA?
Having discovered the genetic basis of viral infectivity, it then became essential to understand its integrity. It was immediately shown that a single break rendered in the TMV RNA is enough to cause loss of its infectivity [
53].
6.2.2. Chemical Mutagens Induce the Formation of a New TMV Strain
Induced mutation studies were undertaken further with TMV (vulgare strain) & TMV mutants were first reported through deamination of RNA (mostly C to U & A to I transitions) with the help of nitrous acid [
54]. For the selection of mutants, they had used the primary method of enumeration of local necrotic lesions induced within the differential host Java tobacco. The vulgare strain of TMV generally causes systemic response in its natural host but with Java tobacco it causes primary chlorotic lesions which gets immediately followed by systemic symptoms which are secondary, whereas the other strains of TMV cause only local necrotic lesions. Thus, on analysis of chemically treated vulgare strain, a proportion of the same caused only local necrotic lesions within java tobacco pointing out towards the fact that they were variants. Experiments with dilution of vulgare preparation was used to further confirm that this result is not because of reduction in virulence of the strain but rather is due to the formation of a new variant. In a further refinement of the chemistry of nitrous acid in inducing mutations within TMV, Schuster & Schramm proved that it was oxidative deamination within the nitrogen bases of the TMV RNA that was responsible for the creation of mutant type (wherein adenine, Guanine & cytokine was converted into hypoxanthine, Xanthine & uracil respectively). In 1960, Siegel used size of lesion as a characteristic to identify TMV variants wherein mutants were selected based on smaller lesion diameter than those induced by the regular/parent strain. During this time, it was also a question of doubt whether the variants were mutants at both the genetic as well as the physical level. But if this doubt had to be clarified it was essential to know those amino acids that make up the TMV capsid.
6.2.3. Sequencing of TMV Coat Protein
The first sequencing of the TMV coat protein was undertaken in 1960 independently by workers in two laboratories i.e., Frankel Conrat & Tsugita at the Virus laboratory, California University, Berkeley & H. G Wittmann at the Max Planck institute of Biology in Tubingen. Wittmann & Braunitzer had first separated tryptic peptides of the virus proteins using ion exchange chromatography and separately determined the amino acid composition of each peptide whereas Conrat & Tsugita had used an automatic amino acid analyzer to directly determine the composition of the TMV coat protein. Finally, both the groups arrived at the same 158 amino acid sequence for TMV coat protein of the common strain [
55,
56].
6.3. Decoding the Mechanism of Formation of Viral Proteins from the Genome; the Attempt to Crack the Viral Genetic Code Using TMV
Even though TMV RNA was known to harbour genetic information (and with RNA mutations affecting coat protein) it had to be known how exactly this information is transferred to the protein level.
6.3.1. The Cell Free Translation Systems—Nirenberg & Matthaei Experiment
The famous Nirenberg & Matthaei experiment undertaken in 1961, at the National institute of Health by Marshall W Nirenberg & J. Heinrich Matthaei showed that RNA controlled the production of specific types of protein. They had created a cell free translation system from E. Coli extracts & had used synthetic nucleic acid polymers to build amino acid into proteins. Initially adding an artificially synthesized poly Uracil nucleotide oligomer to the in vitro cell free extract a protein composed of entirely Phenylalanine amino acid was retrieved. Similar experiments with the other 3 RNA nucleotide bases also yielded a poly chain of single amino acids. This experiment of in vitro translation showed that RNA was the messenger that conveyed the genetic information into the proteins.
6.3.2. Addition of TMV RNA to Cell Free Translation System
Subsequently in 1962, Nirenberg, Conrat & A. Tsugita had used purified TMV genomic RNA to see if any TMV proteins are produced in vitro. About 75-fold increase in protein production was observed post addition of TMV RNA part of which was shown to precipitate with TMV antiserum pointing out the synthesis of TMV coat protein within the experimental system. This experiment also concluded that the single stranded genome of TMV was actually that of messenger sense (+) in addition to revealing the universal nature of codon biology: owing to the fact that a viral RNA got effectively translated even by a bacterial system. The viral genetic code was also verified and elaborated further (Discerning relative positions/order of individual bases within the codon) with the help of TMV mutants i.e., by comparing & contrasting the amino acid changes in the mutant & regular TMV coat protein [
57].
6.4. A Closer Look on TMV Genome as a Functional mRNA; Its Potential Role in Synthesizing Viral Proteins Those of Which Are Important in TMV Life Cycle
TMV became the pioneer pure RNA molecule that was chosen for various studies. In understanding the base composition of this molecule, the first of the techniques developed by Sugiyama & Frankel Conrat in 1961 was directed to determine the terminal sequence of TMV genome. Upon analysis they determined that the 3’end of the TMV genome was made up of an adenosine residue which was unphosphorylated, a finding which was also confirmed by Stein Schneider in 1966.
6.4.1. Digestion and Gel Run of TMV RNA
Several unique oligo nucleotides were generated via the digestion of TMV RNA with ribonuclease T1 [
58]. The presence of a terminal 7-methyl guanosine cap (cap 0) at the 5’end was discovered later [
59,
60] shortly after which it was established that losing the cap (through phosphodiesterase treatment) rendered loss in infectivity of TMV RNA. Even before the appearance of an invitro translation system there were studies undertaken to know about the proteins which appeared in tobacco mosaic virus infected plants [
61]. A gel electrophoretic analysis of proteins from TMV infected tobacco & spindle tuber virus infected potato was also undertaken [
62].
6.4.2. Aid of Wheat Germ Extract Translation System to Study Translation of TMV RNA
Further in 1976 an efficient invitro translation system of wheat germ extract was used to study the protein synthesis from TMV genome. It was initially found that despite multiple efforts coat protein never got translated due to which it was concluded that TMV genomic RNA wasn’t an efficient template for CP translation. As early as in 1972, Jackson had noticed that TMV infected tobacco tissues had a small RNA which was found to be directly translated in in vivo (owing to that fact that it was attached to polyribosomes when purified). When this RNA called as the LMC RNA was tested invitro it was found to give the CP (pointing out towards a sub genomic RNA). Upon analysis it was found that this RNA was also capped at the 5’end just like that of the genomic RNA [
59,
60]. Also, Beachy & Zaitlin in 1977 had noted RNAs of discrete weights like those of 0.68 MDa (termed as I-2 RNA /intermediate length RNA – 2) & 0.9-1.6 MDa (termed as I-1 RNA/intermediate RNA 1). When purified I-2 RNA was translated in vitro it gave rise to a protein of mol weight 30K [
63]. Thus, it was confirmed that I-2 RNA functioned as subgenomic mRNA for 30K protein. Also, translation of the whole TMV RNA in cell free systems like those of wheat germ extract [
64] gave only 2 proteins of mol weights 130K & 180K suggesting their presence towards 5’end of TMV RNA. Thus, it was postulated that 130K, 180K, 30K & coat protein was involved in playing a role in TMV life cycle, but it had to be also proved via experimentation with live cells.
6.5. Creation of a Plant Cell Based In Vitro Experimental System to Understand TMV Life Cycle within the lab, A Prerequisite to the Study of Plant Viral Replication & Translation
Until the early 1970s the molecular & replication studies with the plant viruses were lagging much behind when compared to the bacterial and animal viral counterparts owing to the fact that an effective invitro plant culture system wasn’t available wherein synchronization of infection can be established.
6.5.1. Development of Plant Protoplast Systems & the Study of Growth Curve if a Plant Virus
In 1971, Takabe & associates (after a refinement of E. C Cocking’s experiments in 1966) effectively developed the plant protoplast systems of tobacco through which the study of one step growth curve studies of plant virus became feasible[
65,
66,
67]. The exact details on the replication of many plant viruses became available shortly after this. Studies with the TMV infected protoplasts first showed that proteins of size 130kDa & 180kDa [
68] to be present within them. Upon closer examination it was found out that synthesis of 130K was found to predominate over 180K & also 180K was produced because of a read through via the termination codon of 130K [
69]. After the nuclease treatment of whole TMV RNA followed by electrophoresis the shortest RNA was sequenced which gave information on the last 1000 nucleotides of the 3’end of the TMV RNA [
69]. This cistron of 1kb was that of the Coat protein. Subsequently the 30K protein was also finally detected from TMV infected protoplasts [
69].
6.6. Other Recognised Landmarks in the Molecular Age of Plant Virology
6.6.1. Discovery of Satellites
Basil Kassanis was the first one to notice very small virus particles within some of the cultures of the larger Tobacco Necrosis Virus (TNV). These agents termed as satellite viruses were almost spherical, had a size of roughly 17nm & were able to replicate only in the presence of of the larger TNV virions of 30nm size which acted as a helper virus [
70]. In 1971, another subviral agent ie, naked RNAs was found in close association with preparations of numerous other viruses by Schneider [
71]. These were termed as Satellite RNAs ; those RNAs which don’t encode a capsid of their own. Although first satellite termed as RNA 5 of CMV was seen along with Cucumber mosaic virus, later studies that followed also showed the presence of dispensable but biologically active satellite RNAs within the preparations of many other viruses.
6.6.2. Discovery of Viroids
When the cause of the famous Potato spindle tuber disease was examined a free RNA molecule (without a protein coat) of smaller genome size ; and molecular weight about 25-110 kDa was found [
72].. A year later when Citrus excortis disease was studied, its causative agent was found to exhibit properties similar to that of Potato Spindle Tuber Viroid [
73].
6.6.3. More Kinds of Plant Viruses to Encounter
6.6.3.1. Discovery of dsRNA Viruses
The ds RNA genome in a plant virus was first shown to be present in that of wound tumour virus [
74]. It was also found out that this plant tumour viral RNA can be electrophoretically resolved into 12 fragments (in 7.5% Polyacrylamide gel) totaling to a combined molecular weight of 16 mega Daltons [
74].
6.6.3.2. Discovery of Multipartite Viruses
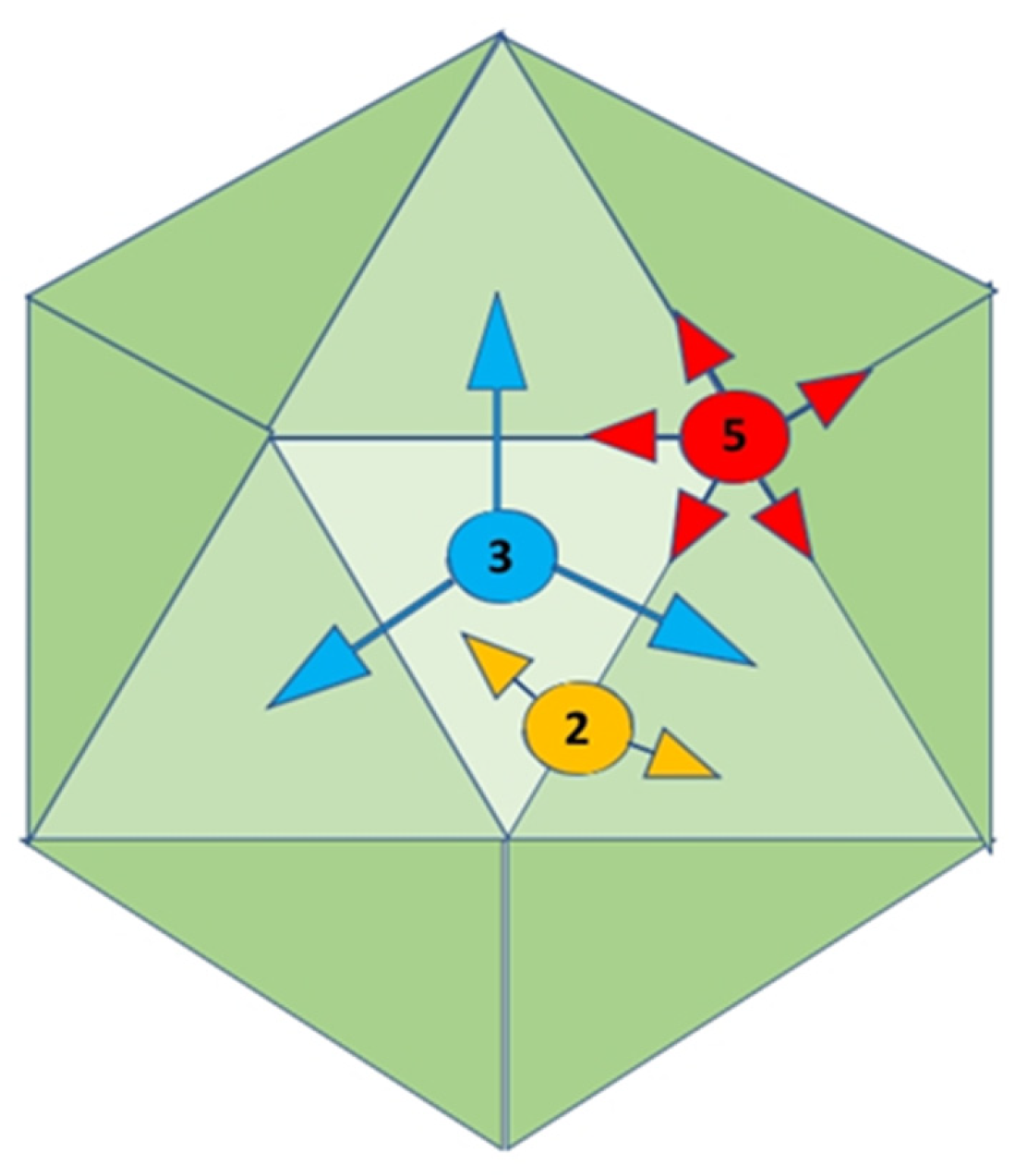
This molecular age also marked the basis for the discovery of plant viruses with segmented genomes. Discovery of multi component/multipartite viruses was shown through the aid of sucrose density fractionation that in contrast to TMV where only one size of RNA molecule (= a single particle) was required to initiate infection, 2 sizes of Tobacco rattle virus (described as long & short Rods) was a must to facilitate a normal infection & subsequent progeny generation [
75]. Thus Tobacco rattle virus was known to harbour a bipartite genome. Other examples include Cowpea mosaic virus (=bipartitite) & Alfalfa mosaic virus (tripartite).
6.6.3.3. Discovery of Plant dsDNA Viruses
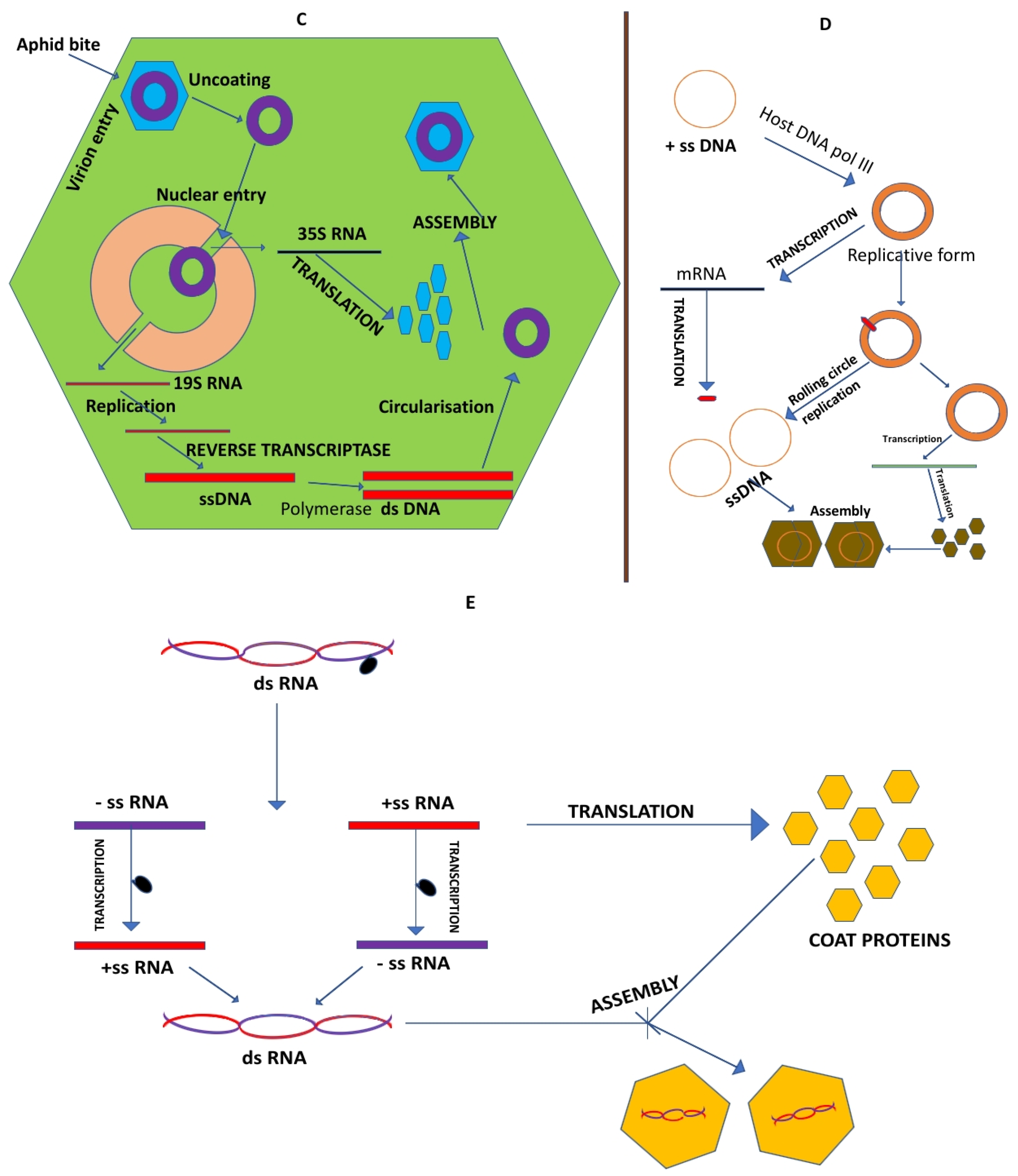
The first DNA genome in a plant virus was reported in that of Cauliflower Mosaic Virus [
76]. Shepherd while working with the purification of CaMV had first discovered that the nucleic acid of this virus wasn’t readily hydrolyzed by the weak acid /alkali (marking an indicative of the absence of an RNA genome), but instead gave a positive diphenylamine test (evidence for the presence of DNA). Further even on treating the purified virus with pancreatic ribonuclease the virus retained infectivity and gave diphenylamine test whereas on the other hand experimenting with DNAse completely abolished infectivity. Finally, hydrolyzed virus readily gave 4 distinguishable spots when subjected to descending chromatography, one of which corresponded to the thymine reference (A DNA base).
6.6.3.4. Discovery of Plant ssDNA Viruses
The occurrence of plant virus with single stranded DNA genome was first reported by Goodman in that of Geminiviruses. Through experiments with exonucleases the insensitivity of geminiviral DNA had been clearly established, thus proving that the geminiviral genetic material was composed of a single stranded DNA with circular (= nonlinear) topology [
77,
78,
79].
6.7. Seminal Discoveries on Molecular Requirements/Faculties for Replication of Different Plant Viruses
6.7.1. Replication of RNA Viruses—The Requirements of Specific RDRP Varies (from lp awasti)
In the year 1963, Brome Mosaic Virus became the first eukaryotic virus from which an RdRP was first identified and characterised, from the barley seedlings infected by the virus [
80]. SDS PAGE analysis showed a predominant band on a 34.5 KDa molecular component [
81]. To understand the faculties for the recruitment of RdRP to the RNA, DNA templates were generated wherein in it was showed that the presence of one additional nucleotide at the 3’end which had to be an initiation nucleotide [
82]. On the other hand, as reported by Collomer, ds RNAs of CMV and its satellite, have an unpaired terminal guanosine which together with other proximal downstream elements are mandatory for its RdRP recognition.
6.7.2. Replication of DNA Viruses
Plant DNA viruses are those that fall in either dsDNA viruses containing RT or ssDNA genome families. Replication of CaMV requires a reverse transcriptase wherein a full length transcript is an intermediate. The ORF V of CaMV encodes the RT enzyme [
83].
SsDNA viruses (Geminiviruses) was reported to undergo a rolling circle mechanism for replication [
84] with the aid of just a single viral encoded replication initiator protein (Rep) [
85].
7. Age of Viral Molecular Genetics (1980–2000)
The age of viral molecular genetics is basically an offshoot of the Molecular age. The key details of viral nucleic acids, the relative location of genes, introduction of viral gene cloning, characterizing the functions of all viral proteins, other viral genome associated properties all came into limelight.
7.1. Locating the Genes/Cistrons of Various Plant Viral Proteins within the TMV Genome; An Application of Recently Developed DNA Sequencing & Cloning Methods
7.1.1. Cloning and Sequencing of TMV RNA
In the 1970s the discovery of reverse transcriptase by Baltimore, Temin & Mizutani showed that RNA could act as template to synthesize DNA thus paved way for cloning RNA as cDNA. Also, this period led to the isolation & characterization of Restriction endonuclease thus enabling DNA modification. The complete TMV RNA (vulgare strain) was cloned into M13 bacteriophage and then sequenced by Sanger sequencing and overlapped each short stretches of nucleotides through the aid of the bioinformatics tools and determined to be of 6395 nucleotides. The computer finally caked the pieces together to reveal the entire sequence. Lastly the cistrons for these proteins were accurately located on the TMV genome [
69].
7.1.2. Building a Complete Genetic Map of TMV Genome
The genome organization of TMV was first given through cloning & sequencing experiments wherein the terminal 5’ end of TMV consisted of the cap structure after which an untranslated leader sequence of 68 nucleotides followed till the first ORF of 130K protein. The termination codon of 130K protein was leaky and the read through protein was 180K protein. The terminal codons of 180K protein was found to overlap with that of the 30K protein. Finally, a 3rd ORF which starts after 2 nucleotides from the 30K protein encodes the 17K coat protein that located in the 3’end of the TMV genome. At the 3’ end an extremity a non-coding region of 200 nucleotides with an ability to fold into a tRNA like structure was present [
69]. Hence finally it was established that the RNA genome of TMV common strain encodes 4 gene products. Shortly after the sequencing of TMV, the complete genome sequence of tomato mosaic virus strain of TMV was made available [
86]. Wilson in 1984 had initially proposed a Co translational disassembly hypothesis following fresh infection of plant cells which predicts the Coat proteins to get released first from the 5’ end of the TMV rod soon after which the ribosomes bind to the freshly exposed ORF1 to start translation. This hypothesis was proved to be true when [
87] also reported the similar finding that occur within TMV.
7.2. Cracking the Functions of TMV Proteins; A Potential Use of Reverse Genetics as a Gene Manipulation System
7.2.1. Reverse Genetics Enters the Arena
Even though the complete amino acid sequences of the entire TMV coded proteins could be discerned employing the sequenced nucleotide information the functions of all other proteins other than the coat protein remained obscure because it wasn’t possible to obtain pure proteins in high concentrations from TMV infected cells. The only solution to this problem at that time was to create a system in which gene could be manipulated via artificial induction of changes at the genotype level & then analyzing the phenotype. An RNA manipulation system was first time established with Brome Mosaic Virus RNAs wherein infectious RNAs of BMV were transcribed in vitro from the full-length cDNA clones [
88]. Following the success of gene manipulation with BMV [
88] the method was soon employed to TMV. In 1986, full-length genome of infectious TMV RNA was synthesized. Initially infectious RNA was obtained using the E. coli RNA polymerase which later got substituted with T7 RNA polymerase [
89]. Manipulation of genomic RNA can thus be achieved by modifying cDNA clones through deletion/insertion (indels) or substitution of nucleotides. Thus, this method enabled to study the function of a multitude of virus coded proteins.
7.2.2. Finding Out the Proteins Involved in TMV Replication
As early as in 1984, the 130K & 180K proteins were hypothesized to take part in TMV replication because these proteins shared a significant homology with already known RNA dependent RNA polymerases/RdRPs [
90]. However, the decisive evidence of these were a part of the RNA replicase complex was established through a mutant wherein both 30K & coat protein genes were deleted [
91]. Even though these proteins were deleted progeny viral RNAs were synthesized showing that replication had still happened. In 1986, Ishikawa et al. had been successful in creating several mutations at the Amber (UAG) termination codon of the 130K gene. They had noted that mutants which failed to produce 180K (only 130K) protein wasn’t infectious whereas mutants with only 180K & no 130K retained only very low levels of infectivity. Thus, a balanced expression of both the genes was necessary for efficient replication of TMV RNA. Finally, in 1997 Osman & Buck had isolated the TMV RNA polymerase Complex and showed that 130K & 180K were its constituents.
7.2.3. 30K Protein of TMV Genome Dictates Virus Movement
Study on the function performed by the 30K protein was based on a temperature sensitive mutant of TMV called Ls1 wherein it was noted that at 32 °C, the virus replicated & assembled as usual in the protoplast but failed to spread from one cell to another in the leaves (a function possible only at 20°C). On analysis it was found that the mutation leading to the state was due to a Serine to proline missense mutation at the 154th position of the 30K protein. This suggested that the function encoded by a regular 30K protein was cell to cell movement. In order to confirm the same first a transgenic tobacco expressing the wild type of 30K protein was used to see if it’s presence would help/complement the Ls1 mutant to spread from cell to cell systemically & at non permissive temperature [
92]. Secondly numerous frameshift mutations within the 30K gene was also undertaken and the phenotype of the protein was noticed. Both the experiments invariantly proved the 30K protein to have a movement function thus later came to be known as the movement protein (MP). MP was found to accumulate in large numbers in the infected leaf plasmodesmata. In 1989, Wolf et al. had reported that the molecular size exclusion limit for MP positive transgenic tobacco for inter cell transport was about 10 times higher when compared to those of the tobacco controls. In 1992, Citovsky et al. also deciphered another property of MP when he demonstrated the invitro property of MP to bind to single stranded nucleic acid and form an elongated structure. Later in 1995, Heinlein et al. together with McLean et al. reported the direct association of MP with the microtubules of TMV infected protoplasts. Later on, movement protein was discovered in almost all plant virus examined.
7.2.4. Multifaceted Roles of Coat Protein
The function of the coat protein or CP was known earlier for it was known to protect the RNA genome by forming a virus particle. However, in due course of time the multifunctional role of the CP was established by reverse genetics. A TMV mutant lacking the CP gene was created that found to be defective in systemic movement [
91]. Further long-distance movement was found to be hindered in almost all CP mutants [
93]. Together with these studies during the same period massive amount of work was done on understanding the replication & translation of ds RNA viruses and also viruses with DNA as genome along with discovering roles of other virus gene coded products (mostly that of virulence genes).
7.3. Applications of the Genetic Age of Plant Virology
7.3.1. Coat Protein Mediated Resistance—Discovery of Viral Gene Induced Resistance of Plants to Subsequent Viral Infections: A Transgene Mediated Response
Working with plant viral genes Powel Abel and co-workers in 1986 showed that viral coat protein genes (key mediator) when transferred into plant nuclear DNA could confer virus resistance [
94] ; Transgenic Tobacco plants expressing the coat protein (CP) gene sense RNA showed remarkable time lag for symptom development after inoculation. Later on the mechanisms of such a transgene resistance (viral mediated) was explored by [
95] following which some crop plants like papaya resistant to papaya ringspot virus in Hawaii with transgenic virus resistance could be commercialized [
96]. It is now recognised that other virus encoded proteins (like those of RDRP), antiviral ribozymes & even antisense RNAs can lead to transgenic resistance.
7.3.2. Discovery of Viral Genome-Based Inactivation of Host Gene Silencing
Gene silencing as a method of regulating virus replication within plants was discovered in 1993. Subsequently on analysis of plant viral genomes some virus derived RNA molecules were found to typically encode a suppressor for gene silencing [
97].
7.3.3. Mapping of Viral Recombinants & Assessing Viral Genome Mutation Frequencies
Finally, the availability of a vast multitude of virus genome sequences made it possible to also map the frequency of mutation and recombination together with inferring the phylogenetic relationships between plant viral families and genera.
7.3.4. Application of TMV as Gene Vector
The foundation of the genetic engineering system enabled the development of TMV as a gene vector for it then became highly possible to insert foreign genes into this viral genome. To name a few experiments the CP gene of TMV had been replaced by Chloramphenicol acetyl transferase (CAT) gene [
91]. Alternatively, a TMV which fluoresce under U. V light due to the insertion of the green fluorescent protein (GFP) from the jelly fish enabled in recording the spread of virus in the infected tissues. Not only TMV but many other plant viruses have been developed by various research labs some of which have been used to even express the epitope of animal viruses or say the malarial epitope [
98].
7.3.4. Cauliflower Mosaic Virus & Its Application in Plant Genetic Studies
When Arabidopsis thaliana was a new model, plant genetic studies was still in its infancy. CaMV inserts its double stranded DNA into plant cells inorder to ensure high amount of gene products. Studies undertaken confired that selective sequences adjacent to the 35S gene in CaMV was actually responsible for greater gene expression activity in plants. Thus, CaMV 35S promoter became one of the most commonly used cloning vector to check the function of new genes when inserted in plant.
8. Diagnostic Age of Plant Virology—Advent of Modern Technologies that Finds Applications in Plant Virology (1980s to Present)
The 2 major breakthroughs in the filed of virus diagnostics came with the discoveries of modern serological assays in the form of ELISA & Nucleic acid based assays mainly in the form of PCR.
- I.
Direct & indirect ELISA has evolved to become one of the most frequent methods to diagnose plant viral diseases [
99]. Swanson had employed ELISA to differentiate closely related viruses or their strains through virus/strain specific antibodies. Double Antibody Sandwich – ELISA format has evolved as the most commonly used type of ELISA for both virus diagnosis as well as viral quantification. ELISA also has been used in the screening of infections in imported plant materials or germplasm.
- II.
Lateral flow assay is another variant of ELISA which has simpliflied the detection and characterisation of llant viruses [
100]. The first LFA described for any plant pathogens was for CMV & TMV [
101]. LFA based diagnostic kits are now used for routine field detection of plant viruses.
- III.
The discovery of PCR technique in 1984 by Karry Mullis [
102] as an invitro technique enabled sensitive detection of plant DNA viruses in the coming years. The cycler was able to amplify the given plant viral DNA sequence to about a million fold in just 2-4 hours (using 3 steps namely Denaturation, annealing & extension which gets repeated in a cyclic pattern) provided specific forward & reverse primers are employed. Multiplex PCRs wherein several viruses are diagnosed (through the aid of multiple primer pairs) in a single reaction has been reportedly used to detect large no of plant viruses. Reverse transcription – PCR wherein a preliminary step of reverse transcription is required for cDNA synthesis from the RNA was used in the same year by Ahmed Hadidi to show that viroids from pome fruits and satellite RNAs from temperature fruits got amplified thus enabling their easy detection. Real time PCR has succesfully engaged quantitative detection of Citrus tristeza virus & Citus yellow vein clearing virus [
103].
Since, PCR virus detection requires temperature cycling a rate limiting step, newer isothermal amplification methods were introduced.
- IV.
Loop mediated isothermal amplification (LAMP) first described in 2000 by Notomi et al., (a technique that uses 4-6 orimers to amplify the target at constant temperature of 60°C) has been developed & standardised for some viruses infecting apples, citrus, banana, grapes etc. Reverse transcription loop mediated isothermal amplification was highly successful at detecting plant RNA viruses [
104].
- V.
Another isothermal method for plant virus detection is that of Rolling circke Amplification (RCA) originally described in 1995 by Fire & Xu as a method of generating ss circular DNA products from from a circular or linear DNA at constant temperature. For plant viruses, multiprimed RCA had been applied first to bipartitite geminiviruses infecting plants in view of amplifying its DNA B component. RCA followed immediately by RFLP was used for the diagnosis of geminiviruses that have small ss circular DNA genomes [
105].
- VI.
Helicase dependent amplification (HDA) first developed in 2004 by Vincent et al.; now greatly employed for rapid detection of plant viruses & viroids is another technique (that employs a DNA helicase +single stranded binding proteins +endonuclease) to create ssDNA as a target template for annealing & extension with 2 primers at a constant temperature of 65°C.
- VII.
Recombinant polymerase assays: since its very first advent in 2006 [
106], it has been used for the rapid detection of plant pathogens. Dozens of plant RNA viruses including members of Bromoviridae, Luteoviridae, Potyviridae, Reoviridae, Virgoviridae, Closteroviridae etc have been directly reported by RPA. Also several plant DNA viruses that fall in the families Caulimoviridae, Geminiviridae & Nanoviridae have been detetected using RPA [
107]. The detection of Viroids by RPA was first reported in 2016.
- VIII.
RNA array microaarays were used for the first time for a plant virus detection in 2003 [
108], & for viriod detection in 2012 [
109]
- IX.
NGS in the recent years has accelarated hypothesis free viral detection and also has reduced costs when it comes to large scale surveys. The first use of NGS in the field of plant virology was in 2009. Multiple viral infections referred to as the pepper viromes from pepper plants have been reported using the Illumina ‘s HiSeq platform that aids in detection of multiple viruses (known & obscure) at a time. Smith et al. in 2014 was the first to use the Next generation sequencing technology to sequence the entire genome of an isolate of barley stripe mosaic virus from barley grains that were around 750 years old. Recent developments enable metagenomic samples like soil to be analysed directly by NGS technology wherein light can also be shed on evolutionary history of plant viruses.
- X.
Deep sequencing on siRNAs isolated from the samples that are infected has allowed the full or partial recovery of genomic sequences of viruses. These siRNA sequencing based methods have already been used to detect many plant viral infections in fruits and vegetables [
110].
9. A Bird’s Eye View on Plant Virology; Compiling Up the Knowledge We Have Gained from History
Plant viruses are submicroscopic intercellular transmissible obligate pathogens which consists of a genetic material (nucleic acid core) inside a protein coat officially termed as the capsid. Viruses are known to occur in 2 phases i.e., intracellular (within the host) wherein viruses may or may not be complete & extracellular inert phase (outside the host) which is usually represented by a complete structure referred to as the virion. The viruses occupy a twilight zone between the world of living & the non-living. The ability to reproduce, mutate and cause the disease to support their living nature whereas lack of respiratory and enzymatic activities together with an acellular organization & ability to be crystallized like chemicals support their non-living nature.
9.1. The Morphology of Various Plant Viruses
The techniques like those of the electron microscope & X Ray diffraction described earlier, enabled us to study the size, shape & architecture of the viruses. The size of plant virus ranges from 17nm (alfalfa mosaic virus) to 1250 X 40nm (Beet yellow virus). Plant viruses are known to occur in different shapes which are normally a characteristic of their species. Broadly they are divided into isometric (same distance from the centre to each & every corner of the particle) & anisometric forms. When observed under an electron microscope they can be spherical (e.g., Tomato Spotted Wilt Virus), Rod shaped (e.g., Tobacco mosaic virus), Bacilliform (e.g., Banana streak virus). Based on their architecture/symmetry of capsid covering the nucleic acid, Plant viruses are classified into helical (cylindrical or elongate) & cuboidal (rounded or polyhedral). Helical forms are anisometric whereas cuboidal forms are isometric in nature. The elongated viruses can be further differentiated into rigid Rods (e.g., Tobacco rattle virus), flexuous Rods (e.g., Potato virus X) & Filamentous Rods (e.g., Rice stripe virus). The protein based protective coat outside the nucleic acid core is referred to as the capsid. Capsid in turn is made up of protein subunits called capsomeres. Plant viral capsids are made of only one kind of protein (or capsomere type). Outside the nucleic acids the capsomeres are known to arrange themselves in either helical or geometric forms. Plant virus capsids (like that of animal viruses) have antigenic properties which provides the basis for the serological differentiation from other viruses & different strains of related viruses. Most of the plant viruses are naked in nature (or without a viral envelope); exceptions are bunyaviruses & rhabdoviruses.
Figure 3.
A sketch of how different plant viruses appear under the electron microscope. A) Tobacco Mosaic Virus. B) Luteovirus. C) Banana Streak Virus. D) Tomato Spotted Wilt Virus.
Figure 3.
A sketch of how different plant viruses appear under the electron microscope. A) Tobacco Mosaic Virus. B) Luteovirus. C) Banana Streak Virus. D) Tomato Spotted Wilt Virus.
Figure 4.
The shape / morphology of plant viruses: A) Tomato Spotted Wilt Virus, B) Plant Rhabdoviruses C) Alfamovirus D) Tobacco mosaic virus E) Potyvirus F) Badnavirus G) Plant Reoviruses, Partitiviridae, Bromoviridae, Caulimovirus H) Tenuivirus I) Geminiviridae.
Figure 4.
The shape / morphology of plant viruses: A) Tomato Spotted Wilt Virus, B) Plant Rhabdoviruses C) Alfamovirus D) Tobacco mosaic virus E) Potyvirus F) Badnavirus G) Plant Reoviruses, Partitiviridae, Bromoviridae, Caulimovirus H) Tenuivirus I) Geminiviridae.
Figure 5.
A detailed picture of Tobacco mosaic virus depicting the helical capsid architecture.
Figure 5.
A detailed picture of Tobacco mosaic virus depicting the helical capsid architecture.
Figure 6.
A diagram representing the capsid organization of cubical/icosahedral plant viruses with two, three & fivefold axis of symmetry.
Figure 6.
A diagram representing the capsid organization of cubical/icosahedral plant viruses with two, three & fivefold axis of symmetry.
9.2. Nature of Plant Viral Genomes
The sum of the total nucleic acid present in a virus is called its genome. Plant virus can have any one of the nucleic acids but never both. The nature of the virus genome also forms a central point in the classification of plant viruses. Based on the nature of their genome plant viruses are divided into DNA viruses & RNA viruses. The DNA viruses are further divided into single stranded DNA viruses and double stranded DNA viruses. Both groups are further divided into circular DNA viruses & linear DNA viruses. Similarly, the RNA viruses are also classified into double stranded RNA viruses & single stranded RNA viruses. The single stranded RNA viruses can have their RNA genome either as a positive sense strand or a negative sense strand. Within a single capsid the genetic material can be present in a single continuous strand / non segmented form or it can exist as multiple genome segments; such viruses being termed as segmented viruses. In some plant viruses the genome exists as different segments in more than one virus particle. Such viruses are termed as split genome viruses and are nick named as bipartite if 2 genome segments are present (each segment being enclosed separately in a virus particle) & multipartite if more than 2 genomic pieces are present (generating the need for multiple particle components). Some other ssDNA plant viruses like those of the geminiviruses appear as twin particles/geminate as a result of partial fusion of 2 isometric capsids.
Figure 7.
some multipartite viruses. A) Cucumovirus & Bromovirus B) Ilarvirus C) Comovirus D) Tobravirus E) Pomovirus F) Benyvirus.
Figure 7.
some multipartite viruses. A) Cucumovirus & Bromovirus B) Ilarvirus C) Comovirus D) Tobravirus E) Pomovirus F) Benyvirus.
Currently more than 1000 different plant viruses have been accurately described & studied of which 90% have an RNA genome out of which mostly 76% have +ssRNA as their genetic material. This is followed by negative sense ssRNA viruses such as plant infecting Bunyaviruses & Rhabdoviruses which constitute roughly 14% of plant viruses with RNA genomes. Reoviruses which are double stranded RNA viruses come next with 4% & finally plant retroviruses of the pseudoviridae family also make up 2% of the total RNA viruses. DNA viruses such as the single stranded geminiviruses & double stranded DNA RT viruses respectively contribute to 4% & 2% of the total plant viruses.
Figure 8.
A picture showing the nature of plant viral genomes & families fall in each class.
Figure 8.
A picture showing the nature of plant viral genomes & families fall in each class.
9.3. The Organization of Genomes of Plant Viruses
Before we go into the life cycle of these viruses it is necessary to understand the organization of the plant viral genome i.e., the total no of genes, the types of genes & their location. A visual representation of such an information is known as the genetic map of plant viruses. To become a successful plant pathogen a total of 5 genes are predicted for a virus which includes 3 additional genes besides the regular polymerase gene and coat protein gene. These 3 genes include the Gene for movement protein, gene for RNAi suppressor & gene for vector transmission protein. The gene for movement protein & RNAi suppressor proteins have been seen till date only in plant viruses (thus are 2 additional genes for a plant virus which are absent in animal viruses). The product of the CP gene is the coat protein which protects the plant viral genome & also provides antigenicity to it. The RNA dependent RNA polymerase found in RNA viruses on the whole performs the function of replicating the RNA genome and it is made up of mostly 3 subunits i.e., a core polymerase/pol which adds RNA bases, a helicase/hel which unfolds the 2°RNA structure and a methyl transferase/mt which adds a cap structure to the RNA. (likewise DNA viruses have a DNA polymerase which replicates the DNA genome). The movement protein/MP encoded by the movement gene is the plant viral protein required for Cell-to-cell transport within plants. Besides these regular proteins the genomes of some plant viruses code for a protease/pro to process a polyprotein into individual proteins. Some plant viral genomes also encode a terminal genome linked protein/VPg for priming RNA synthesis.
9.4. Life Cycle of a Plant Virus
The plant host, vector & the plant virus itself form the triads of plant virus mediated disease establishment. The generalized infection cycle of a plant virus starts with the inoculation of plant virus into its plant host by a suitable vector. Following this event the other sequential steps include
1. Penetration into a plant cell
2. Uncoating
3. Transcription/translation
4. Replication,
5. Intercellular & long-distance transport
6. Assembly & maturation
7. Arthropod/fungi/nematode (= vector) acquisition.
It is important to note that unlike the animal viruses where receptor mediated endocytosis is known to occur, Plant virus penetrates into a cell not via endocytosis but rather through a transient wound in the cell wall & plasmalemma created either naturally or via means of a vector. Plant RNA viruses similar to their animal counterparts replicate in the cytoplasm or in one of the cytoplasmic organelles. Replication in an organelle often happens in the association with the organelle membrane. Potyvirus, Potexvirus, Tobamovirus replicate in the cytoplasm via the creation of a viroplasm. Tymovirus replicates in the chloroplast, Comovirus & Bromovirus in the endoplasmic reticulum, Tospovirus in Golgi, Tombusvirus in the mitochondria & Cucumovirus & Alfamovirus in the plant vacuole. DNA viruses like the Geminivirus & Plant Nucleorhabdoviruses replicate in the nucleus. For a virus to takeover a cell & synthesize it’s progeny viral proteins have to be made. In order to facilitate the synthesis of viral proteins in a plant cell there is requirement of a viral mRNA for it is only the mRNA that can be translated with the help of plant ribosomes into a protein. Thus whatever be the infecting plant virus genome ultimately an mRNA must be created to fulfil viral genome expression. Based on how an mRNA is generated from a viral genome template Baltimore had classified viral genomes into 7 classes based on genome nature & polarity. viruses which fall under the class I are those with ds DNA genomes. These viruses are rarely seen among plants but are very common in the animals. They produce an mRNA via transcription mediated through the host RNA dependent RNA polymerase. Plant viruses which fall under class II have single stranded DNA genome. These plant viruses first change their ssDNA into a dsDNA (using host DNA polymerase) because the former can’t be recognized by host RNA polymerase II. Plant viruses that fall under the Class III are those with ds RNA genomes. Since there’s no host polymerase which can transcribe a ds RNA template it is mandatory for these plant viruses to have a special self-coded RNA polymerase that can generate an mRNA from dsRNA genome. Viruses which fall under the Class IV are the most predominantly encountered plant viruses. The have their genome as a ssRNA which is itself in the 5’-3’ direction (positive sense) making it capable to directly act as an mRNA. Plant viruses of the Class V category are those with ssRNA genomes in 3’-5’ direction (negative sense). These plant viruses have a self-coded RNA polymerase which they carry within themselves an integral virion component which performs the function of transcription (hence often called transcriptase) to create the mRNA. Plant viruses which fall in class VI are those which have a + ss RNA genome and an RT enzyme as an integral part of their virion. Unlike the members of class IV, these plant viruses synthesize their mRNA only via transcription from a DNA intermediate. The virus encoded reverse transcriptase enzyme first catalyzes the formation of a ssDNA of negative sense from their genomic RNA. The same enzyme which also possesses a RNAse H & DNA pol activity first degrades the RNA strand from the DNA RNA hybrid and then generates a cDNA thus finally giving rise to a dsDNA. This dsDNA is then transcribed within the nucleus by host RNA pol II to give rise to the viral mRNA.
9.5. The Replication/Duplication of Plant Viral Genomes
The replication of the viral genome & formation of progeny genomes is the most significant step in the virus life cycle. RNA plant viruses themselves encode a viral specific RNA polymerase which adds new nucleotide bases in the 5’ to 3’ direction (using gRNA as template) to generate complement/antiparallel strands which are then used as templates to generate many more gRNAs. The helicase activity of RdRP enables multiple complement RNA strands (currently in the synthesis phase) to be sequentially synthesized at a particular time following which they are released orderly as full length single stranded RNA complements. The transient intermediate complent RNA stage which is created as a result of multiple RdRPs association at 3’end of an RNA & progressive movement towards the 5’ end is termed as replicative intermediate stage (complement RNAs being referred to as replicative intermediates). Also a transient dsRNA termed as the Replicative form is also generated which conserves the parent gRNA strand. The full length complement RNA strands which are generated through RI stage are then used as templates for generating progeny RNA strands. With each RNA complement/antigenome multiple RdRPs can associate and through another RI stage, multiple progeny viral gRNAs are finally generated. For dsRNA viruses both the + RNA & - RNA can act as templates for the synthesis of complement strands which then associates together to form progeny RNAs. The replication of dsDNA RT viruses is undertaken by a reverse transcriptase enzyme which has the ability to change mRNAs into first ssDNA & later ds genomic DNA whereas the replication of single stranded DNA plant viruses passes through a transient circular replicative form created by host DNA polymerase III which function as the template for rolling circle replication & creation of single stranded DNA progenies.
Figure 9.
A generalized diagram showing the replication cycle of different plant viruses; A) replication cycle of positive sense single stranded RNA viruses. B) Replication cycle of negative sense single stranded RNA viruses. C) Replication of double stranded DNA RT viruses / Caulimoviridae family. D) Replication cycle of single stranded DNA viruses. E) Replication cycle of double stranded RNA viruses.
Figure 9.
A generalized diagram showing the replication cycle of different plant viruses; A) replication cycle of positive sense single stranded RNA viruses. B) Replication cycle of negative sense single stranded RNA viruses. C) Replication of double stranded DNA RT viruses / Caulimoviridae family. D) Replication cycle of single stranded DNA viruses. E) Replication cycle of double stranded RNA viruses.
9.6. Plant Virus: How Does It Spread Within Its Host?
Following the primary infection the virus has to move from source to the sink. Intercullar movement along with long distance movement is a must to enable systemic infection within the plant. The movement of plant viruses from cell to cell is an active virus function but is rather slow whereas the long-distance movement though happens via a passive transport mechanism is fast. Intercellular transport must ensure that the plasmodesmata is bypassed. But the pore size of plasmodesmata are about 2nm with a size exclusion limit of 1kDa which is not adequate for the plant viruses to pass. Thus plant viruses must modify the plasmodesmata; a function which is often executed by the movement protein via 2 mechanisms i.e., either by increasing the diffusion limits of the desmotubule within the plasmodesmata (as in Tobamovirus) or by creating a hollow transport tubule with the association of individual movement protein molecules (as in Comovirus/Tospovirus). Once the plasmodesmata gets modified plant viruses can either move as genome or virion from cell to cell. Long distance transport of viruses in plants is accomplished through the phloem sieve elements (which function in metabolite transport).
9.7. Plant Viral Diseases: How Do They Get Transmitted?
Viral diseases of plants are always contagious in nature. It is the transmission step that leads to plant viral epidemics following which widespread damage of crops & economic losses are reported. Unlike animal viruses, Plant viruses rarely come outside the plant host spontaneously (as pure virus particles in air) & are often carried in plant sap or debris. Also upon coming in contact with a new host the viruses themselves can’t achieve active infection & for this a wounded area with living cells exposed must be present. This often necessitates the need for an external agency to function between the plant hosts. Transmission of plant viruses can be through vectors, nematodes, soil microbes (= Fungi), dodder etc. (biotic agents) or through infected seeds, pollen & other vegetative parts. Plant virus transmission has been categorized into horizontal transmission (wherein transmission takes place from one plant to another plant in the field) & Vertical transmission (wherein a plant gets the virus from its parent plant). The transmission of the viruses can happen by either natural (mechanical rubbing of leaves under strong wind followed by sap exchange) or artificial / induced means (propagation & experimental transmission). Vectors are biological organisms which act as carriers of pathogen thus involving in transmission of a disease from one plant to another. Within the fields the insect vectors such as aphids, whiteflies, leaf hoppers, mealy bugs, beetles, grasshoppers, thrips, tree hoppers etc. form the most common method of virus transmission. Based on the nature of association of virus with its vector, 4 types of transmission are categorized: a) Non persistent transmission: The plant viruses which follow this kind of transmission are referred to as non-persistent or stylet borne viruses because the viruses remain localized in the insect sucking parts (site of entry) after acquisition. These viruses (on account of low vector specificity) are transmitted just via physical means given their vector becomes infective as soon as they probe/feed on host parenchyma (absence of latent period). The vector loses the viruses upon moulting. B) Semipersistent transmission: These viruses unlike nonpersistent viruses are acquired through deep feeding (usually involves the phloem) & have a persistence in the vector for 10 – 100 hours. These viruses are however don’t require a latent period, don’t circulate or multiply within its vector & the vector loses its infectivity upon moulting. C) Persistent transmission: The viruses which follows this kind of transmission are called circulative viruses; They first accumulate in the insect mouth parts from where they spread to internal tissues & later gets circulated throughout the insect body. The vector usually takes a relatively large time period in virus acquisition & transmission D) Propagative transmission: These are those circulative viruses which also have the ability to multiply/propagate/undertake a development cycle within its host vector. These viruses are retained within the vector for a long period of time (varying from several days to weeks). These viruses are also not lost upon moulting of the vector.
Table 3.
An introduction of common agents of plant virus transmission & some details on important viruses that can be potentially transmitted through them.
Table 3.
An introduction of common agents of plant virus transmission & some details on important viruses that can be potentially transmitted through them.
| Agents of viral transmission |
Description |
Examples |
| Vectors |
| Aphids |
Members of family aphidaceaClass: Homoptera |
Aphid Myzus persicae transmits Potato leaf roll virus, Tobacco ringspots virus etc. |
| Leaf hoppers/plant hoppers |
Active jumping plant bugs belonging to families Jassidae & Fulgoridae |
Nephotettix implicticeps transmits Rice Tungro virusCirculifer tenellus transmits Beet curly top virus |
| Whiteflies |
Members of the family Aleurodidae & class Homoptera |
Trialeuroides vaporarium transmits Closterovirus & Criniviruses of Beet & cauliflowerBemesia tabacci transmits leaf curl of tobacco |
| Thrips |
Tiny insects of family Thripidae & class Homoptera |
Thrip Frankinella occidentalis transmits Tospoviruses:- GRSV, INSV, TCSV |
| Mealy bugs |
Small bugs of family Coccidae & class Homoptera |
Nymphs & adult bugs transmit Badnavirus & Terovirus |
| Mites |
Eriophyid group |
Viruses causing Fig mosaic disease & Pigeon pea sterility are transmitted by mites. |
| Beetle |
Members of order coleoptera & family Chrysomelidae |
Transmits plant virus of genera Tymovirus, Bromovirus, Comovirus & Sobemovirus |
| Grass hoppers |
Ground dwelling insects those of which are members of order Orthoptera & class insecta |
The Grasshopper Melanoplus differentialis transmits Turnip yellow Mosaic Virus & Turnip Crinkle virus |
|
Parasitic plant which obtains nutrition by its root like haustoria. |
Cuscuta subinclusa transmits Sugarbeet curly top virusCuscuta campestris transmits Bushy stunt virus of tomato |
- 2.
Nematodes |
Free living ectoparasitic worms which transmits viruses via root feeding |
Xiphineria transmits polyhedral nepoviruses like Grapevine fan leaf virus |
- 3.
Fungi |
Soil dwelling Fungi with hyphae |
Synchytrium, Polymyxa, Spongospora & Pythium transmits Potato virus X, Wheat mosaic virus, Potato mop Top virus & Beet necrotic yellow vein virus respectively |
- 4.
Seeds |
Seeds derived from virus infected plant transmits viruses |
Tobacco ring spot virus in soyabean show 100% transmission through seeds whereas Barley stripe mosaic virus show >50% transmission through seeds. |
- 5.
Pollen |
When virus infected pollen grains fall on pistil of healthy plant |
Alfalfa mosaic virus & Bean common mosaic virus are transmitted via pollen grains. |
- 6.
Vegetative propagules |
In plants which propagate vegetative viruses may be transmitted via buds, grafts, cuttings, suckers, tubers, rhizomes, bulbs, corns or other propagules |
Bunchy top of Banana is transmitted through infected suckers |
- 7.
Mechanical Transmission |
Artificial introduction of active virus through wounds or abrasions: this method is generally used in lab for experimental purposes & is known as sap inoculation. leaf rub, pinprick, micro injection & wound swabbing are common methods of sap inoculation |
Potato virus X can be transmitted through sap inoculation |
10. Conclusion
Virology as a discipline of science had its evolution through the initial step of discovery of a new plant pathogen different from the pathogens existing at that time. The coinage of the modern term virus & the elucidation of ‘virus concept’ for a pathogen not obeying Koch’s postulates followed immediately. Subsequently, the characteristic nature of disease symptoms induced by this pathogen in plants together with the modes of transmission of this agent were found out. In the coming years the selective purification of this agent via crystallization enabled the exact nature of the pathogen to be revealed first at the biochemical level and later at the molecular level. In due course of time, the upsurge of serological & molecular based techniques also enabled accurate plant viral detection. Understanding the genetics of plant viruses was the next immediate breakthrough. In addition to the detection of viruses within already infected was also important to prevent healthy plants from succumbing to the disease of its neighbour; Besides being a stand alone field in itself, virology also finds applications in the fields of biotechnology, nanotechnology and genome engineering. Present research on virology is emphasizing on the better understanding of viral genetics and protein functions along with improvisation in virus detection techniques.
Acknowledgements
We are grateful to Department of Biotechnology, India for funding research.
Conflict of Interest
The author declares no conflicts of interest.
References
- Saunders, K.; Bedford, I.D.; Yahara, T.; Stanley, J. The earliest recorded plant virus disease. Nature 2003, 422, 831–831. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A. Over demozaiekziekte van de tabak: voorloopige mededeeling. Tijdschr, Landbouwk 1882, 2, 359–364. [Google Scholar]
- Mayer, A. Uber die Mosakkrankheit des Tabaks. Landw, Vers statn 1886, 32, 451–467. [Google Scholar]
- Chamberland, C. Sur un filtre domnant de l’eau physiologiquement pure. C. R. Acad. Sci. 1884, 99, 247–248. [Google Scholar]
- Ivanowski, D. Uber die Mosaikkrankheit der Tabaksplanze. Bull. Acad. Imp. Sci. St Petersb. Nouv. Ser. III 1892, 35, 65–70. [Google Scholar]
- Beijernick, M.W. Uber ein contagium vium fluidum als Ursache der fleckenkrankheit der Tabaksblatter. Verh. K. Akad. Wet. Amsterdam 1898, 65, 3–21. [Google Scholar]
- Smith, R.E.; Boncquet, P.A. New light on curly top of the sugar beet. Phytopathology 1915, 5, 103–107. [Google Scholar]
- Ball, E.M. The leafhoppers of the Sugarbeet and their relation to curly leaf conditions. US Dept. Agr. Entomology. Bull. 1909, 66, 33–52. [Google Scholar]
- Doolittle, S.P. A new infectious mosaic disease of cucumber. Phytopathology 1916, 6, 145–147. [Google Scholar]
- Jagger, C. Experiments with the cucumber mosaic disease,” Phytopathology, vol. 6.2, pp. 149–151, 1916.
- Smith, C.E. Transmission of cowpea mosaic by the bean leaf-beetle. Sci. Plant Pathog. Dis. Diagnosis 1924, 60, 268–268. [Google Scholar]
- Pittman, H. A Spotted wilt of tomatoes. J. Austr. Counc. Sci. Ind. Res. 1927, 1, 74–77. [Google Scholar]
- Kirkpatrick, T.W. Further Studies on Leaf-curl of Cotton in the Sudan. Bull. Èntomol. Res. 1931, 22, 323–363. [Google Scholar] [CrossRef]
- Amos, R. G. Halto, R. C. Knight, A. M. Masse, “ Experiments in the transmission of Reversion in black currants, “ East. Malling Res. Stat. Ann. Rpt. for 1925, pp. 126 – 150.
- Reddick, D.; Stewart, V.B. Transmission of the virus of bean mosaic in seed and observations on thermal death point of seed and virus. Phytopathology 1919, 9, 445–450. [Google Scholar]
- Reddick, D. La transmission du virus de la mosaique du haircot par le pollen. Deux. Congr. Intern. Path. Comp. 1931, 363–366. [Google Scholar]
- Purdy Beale, H.A. Immunological reactions with tobacco mosaic virus. Proc. Soc. Exp. Biol. Med 1928, 25, 702–703. [Google Scholar] [CrossRef] [PubMed]
- McKinney, H.H. Mosaic diseases in the Canary Islands. West Africa and Gibraltar. J. Agric. Res. 1929, 39, 577–578. [Google Scholar]
- Holmes, F.O. Local Lesions in Tobacco Mosaic. Bot. Gaz. 1929, 87, 39–55. [Google Scholar] [CrossRef]
- Smith, K.M. On the composite nature of certain potato virus diseases of the mosaic group as revealed by the use of plant indicators and selective methods of transmission. Proc. R. Soc. London. Ser. B, Contain. Pap. a Biol. Character 1931, 109, 251–267. [Google Scholar] [CrossRef]
- Kunkel, O. Studies on acquired immunity with tobacco and Aucuba mosaics. Phytopathology 1934, 24, 437–66. [Google Scholar]
- Watson, M.A.; Roberts, F.M. A comparative study of the transmission of Hyoscyamus virus 3, potato virus Y and cucumber virus 1 by the vectors Myzus persicae (Sulz), M. circumflexus (Buckton), and Macrosiphum gei (Koch). Proc. R. Soc. London. Ser. B - Biol. Sci. 1939, 127, 543–576. [Google Scholar] [CrossRef]
- Bradley, R.H.E. Studies on the aphid transmission of henbane mosaic virus. Ann. Appl. Biol 1952, 39, 78–97. [Google Scholar] [CrossRef]
- Hoggan, A. Some viruses affecting spinach and certain aspects of insect transmission. Phytopathology 1933, 23, 446–474. [Google Scholar]
- Sylvester, S.E. Transmission of sugar beet yellow net virus by green peach aphids. Phytopathology 1949, 39, 117–132. [Google Scholar]
- Sylvester, S.E. Beet mosaic virus green peach aohids relationship. Phytopathology 1949, 39, 417–424. [Google Scholar]
- Sylvester, S.E. Transmission of Brassica nigra virus by green peach aphids. Phytopathology 1950, 40, 743–745. [Google Scholar]
- Hewitt, W.B.; Raski, D.J.; Goheen, A.C. Nematode vector of soil borne fanleaf virus of grapevines. Phytopathology 1958, 48, 586–595. [Google Scholar]
- Fukushi, T.; Shikata, E.; Kimura, I. Some morphological characters of rice dwarf virus. Virology 1962, 18, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, A.; Black, L. Hereditary variation in the ability of a leafhopper to transmit two unrelated plant viruses. Virology 1962, 16, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Maramorosch, K. SECTION OF BIOLOGY: VIRUSES THAT INFECT AND MULTIPLY IN BOTH PLANTS AND INSECTS*. Trans. New York Acad. Sci. 1958, 20, 383–393. [Google Scholar] [CrossRef]
- Thornbury, D.W.; Hellmann, G.M.; Rhoads, R.E.; Pirone, T.P. Purification and characterization of potyvirus helper component. Virology 1985, 144, 260–267. [Google Scholar] [CrossRef]
- Ammar, E.D. Association of Potyvirus Helper Component Protein with Virions and the Cuticle Lining the Maxillary Food Canal and Foregut of an Aphid Vector. Phytopathology® 1994, 84. [Google Scholar] [CrossRef]
- Woolston, C.J.; Czaplewski, L.G.; Markham, P.G.; Goad, A.S.; Hull, R.; Davies, J.W. Location and sequence of a region of cauliflower mosaic virus gene 2 responsible for aphid transmissibility. Virology 1987, 160, 246–251. [Google Scholar] [CrossRef]
- Chen, B.; Francki, R.I.B. Cucumovirus transmission by the aphid Myzus persicae is determined solely by the viral coat protein. J. Gen. Virol. 1990, 71, 939–944. [Google Scholar] [CrossRef]
- Ullman, D.E. TospovirusReplication in Insect Vector Cells: Immunocytochemical Evidence that the Nonstructural Protein Encoded by the S RNA of Tomato Spotted Wilt Tospovirus Is Present in Thrips Vector Cells. Phytopathology® 1993, 83. [Google Scholar] [CrossRef]
- Wijkamp, I.; van Lent, J.; Kormelink, R.; Goldbach, R.; Peters, D. Multiplication of tomato spotted wilt virus in its insect vector, Frankliniella occidentalis. J. Gen. Virol. 1993, 74, 341–349. [Google Scholar] [CrossRef]
- Heuvel, J.F.J.M.v.D.; Verbeek, M.; van der Wilk, F. Endosymbiotic bacteria associated with circulative transmission of potato leafroll virus by Myzus persicae. J. Gen. Virol. 1994, 75, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Heuvel, J.F.v.D.; Bruyère, A.; A Hogenhout, S.; Ziegler-Graff, V.; Brault, V.; Verbeek, M.; van der Wilk, F.; Richards, K. The N-terminal region of the luteovirus readthrough domain determines virus binding to Buchnera GroEL and is essential for virus persistence in the aphid. J. Virol. 1997, 71, 7258–65. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, W.N.; Rawlins, T.E. Method for Determining Shape of Colloidal Particles; Application in Study of Tobacco Mosaic Virus. Exp. Biol. Med. 1932, 30, 155–157. [Google Scholar] [CrossRef]
- Stanley, W.M. Isolation of a Crystalline Protein Possessing the Properties of Tobacco-Mosaic Virus. Science 1935, 81, 644–645. [Google Scholar] [CrossRef]
- Bawden, F.C.; Pirie, N.W.; Bernal, J.D.; Fankuchen, I. Liquid Crystalline Substances from Virus-infected Plants. Nature 1936, 138, 1051–1052. [Google Scholar] [CrossRef]
- Kausche, G.A.; Pfankuch, E.; Ruska, H. The visualization of plant virus under the microscope. Nat. Sci. 1939, 27, 292–299. [Google Scholar] [CrossRef]
- Williams, R.C.; Wyckoff, R.W.G. Electron Shadow-Micrography of Virus Particles. Exp. Biol. Med. 1945, 58, 265–270. [Google Scholar] [CrossRef]
- Brakke, M.K. Density Gradient Centrifugation: A New Separation Technique1. J. Am. Chem. Soc. 1951, 73, 1847–1848. [Google Scholar] [CrossRef]
- Franklin, R.E. Structure of Tobacco Mosaic Virus. Nature 1955, 175, 379–381. [Google Scholar] [CrossRef]
- Caspar, D.L.D. Structure of Tobacco Mosaic Virus: Radial Density Distribution in the Tobacco Mosaic Virus Particle. Nature 1956, 177, 928–928. [Google Scholar] [CrossRef]
- Franklin, R.E. Structure of Tobacco Mosaic Virus: Location of the Ribonucleic Acid in the Tobacco Mosaic Virus Particle. Nature 1956, 177, 928–930. [Google Scholar] [CrossRef]
- Markham, R.; Smith, K.M. Studies on the virus of turnip yellow mosaic. Parasitology 1949, 39, 330–342. [Google Scholar] [CrossRef]
- Harris, J.I.; Knight, C.A. Action of Carboxypeptidase on Tobacco Mosaic Virus. Nature 1952, 170, 613–614. [Google Scholar] [CrossRef]
- Fraenkel-Conrat, H.; Williams, R.C. RECONSTITUTION OF ACTIVE TOBACCO MOSAIC VIRUS FROM ITS INACTIVE PROTEIN AND NUCLEIC ACID COMPONENTS. Proc. Natl. Acad. Sci. 1955, 41, 690–698. [Google Scholar] [CrossRef]
- Gierer, A.; Schramm, G. Infectivity of Ribonucleic Acid from Tobacco Mosaic Virus. Nature 1956, 177, 702–703. [Google Scholar] [CrossRef]
- Fraenkel-Conrat, H.; Singer, B. Virus reconstitution. Biochim. Biophys. Acta 1957, 24, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Schuster, H.; Schramm, G. Determination of biological effective unit in the ribose nucleic acid of tobacco mosaic virus chemical pathway. Z. Naturforsch 1958, 13, 697–704. [Google Scholar] [CrossRef]
- Gierer, A.; Mundry, K.-W. Production of Mutants of Tobacco Mosaic Virus by Chemical Alteration of its Ribonucleic Acid in vitro. Nature 1958, 182, 1457–1458. [Google Scholar] [CrossRef] [PubMed]
- Tsugita, A.; Gish, D.T.; Young, J.; Fraenkel-Conrat, H.; Knight, C.A.; Stanley, W.M. THE COMPLETE AMINO ACID SEQUENCE OF THE PROTEIN OF TOBACCO MOSAIC VIRUS. Proc. Natl. Acad. Sci. 1960, 46, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, H. Comparison of the tryptic peptides of chemically induced and spontaneous mutants of tobacco mosaic virus. Virology 1960, 12, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Tsugita, A.; Fraenkel-Conrat, H.; Nirenberg, M.W.; Matthaei, J.H. DEMONSTRATION OF THE MESSENGER ROLE OF VIRAL RNA. Proc. Natl. Acad. Sci. 1962, 48, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Mandeles, S. Location of Unique Sequences in Tobacco Mosaic Virus Ribonucleic Acid. J. Biol. Chem. 1968, 243, 3671–3674. [Google Scholar] [CrossRef] [PubMed]
- Zimmern, D. The 5′ end group of tobacco mosaic virus RNA is m7G5′ppp5′Gp. Nucleic Acids Res. 1975, 2, 1189–1202. [Google Scholar] [CrossRef]
- Keith, J.; Fraenkel-Conrat, H. Tobacco mosaic virus RNA carries 5′-terminal triphosphorylated guanosine blocked by 5′-linked 7-methylguanosine. FEBS Lett. 1975, 57, 31–33. [Google Scholar] [CrossRef]
- Zaitlin, M.; Hariharasubramanian, V. Proteins in tobacco mosaic virus-infected tobacco plants. Biochem. Biophys. Res. Commun. 1970, 39, 1031–1036. [Google Scholar] [CrossRef]
- Zaitlin, M.; Hariharasubramanian, V. A gel electrophoretic analysis of proteins from plants infected with tobacco mosaic and potato spindle tuber viruses. Virology 1972, 47, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Beachy, R.N.; Zaitlin, M. Characterization and in vitro translation of the RNAs from less-than-full-length, virus-related, nucleoprotein rods present in tobacco mosaic virus preparations. Virology 1977, 81, 160–169. [Google Scholar] [CrossRef]
- Bruening, G.; Beachy, R.N.; Scalla, R.; Zaitlin, M. In vitro and in vivo translation of the ribonucleic acids of a cowpea strain of tobacco mosaic virus. Virology 1976, 71, 498–517. [Google Scholar] [CrossRef] [PubMed]
- Takebe, I. The Use of Protoplasts in Plant Virology. Annu. Rev. Phytopathol. 1975, 13, 105–125. [Google Scholar] [CrossRef]
- Takebe, I.; Otsuki, Y. INFECTION OF TOBACCO MESOPHYLL PROTOPLASTS BY TOBACCO MOSAIC VIRUS. Proc. Natl. Acad. Sci. 1969, 64, 843–848. [Google Scholar] [CrossRef]
- Takebe, I.; Otsuki, Y.; Aoki, S. Isolation of tobacco mesophyll cells in intact and active state. Plant Cell Physiol. 1968, 9, 115–124. [Google Scholar] [CrossRef]
- Sakai, F.; Takebe, I. Protein synthesis in tobacco mesophyll protoplasts induced by tobacco mosaic virus infection. Virology 1974, 62, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Pelham, H.R.B. Leaky UAG termination codon in tobacco mosaic virus RNA. Nature 1978, 272, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Kassanis, B.; Nixon, H.L. Activation of one Tobacco Necrosis Virus by Another. J. Gen. Microbiol. 1961, 25, 459–471. [Google Scholar] [CrossRef]
- Schneider, I. Characteristics of a satellite-like virus of tobacco ringspot virus. Virology 1971, 45, 108–122. [Google Scholar] [CrossRef]
- Diener, T. Potato spindle tuber “virus”. Virology 1971, 45, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Semancik, J.S.; Weathers, L.G. Exocortis Disease: Evidence for a New Species of “Infectious” Low Molecular Weight RNA in Plants. Nat. New Biol. 1972, 237, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Black, M.; Markham, R. Base-pairing in the ribonucleic acid of wound-tumor virus. Neth. J. Plant Pathol. 1963, 69, 215. [Google Scholar]
- Lister, R. Possible relationships of virus-specific products of tobacco rattle virus infections. Virology 1966, 28, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.; Wakeman, R.; Romanko, R. DNA in cauliflower mosaic virus. Virology 1968, 36, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.M. Infectious DNA from a whitefly-transmitted virus of Phaseolus vulgaris. Nature 1977, 266, 54–55. [Google Scholar] [CrossRef]
- Goodman, R.M. Single-stranded DNA genome in a whitefly-transmitted plant virus. Virology 1977, 83, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.D.; Barker, H.; Bock, K.R.; Guthrie, E.J.; Meredith, G.; Atkinson, M. Plant viruses with circular single-stranded DNA. Nature 1977, 270, 760–762. [Google Scholar] [CrossRef]
- Hadidi, A.; Fraenkel-Conrat, H. Characterization and specificity of soluble RNA polymerase of brome mosaic virus. Virology 1973, 52, 363–372. [Google Scholar] [CrossRef]
- Hariharasubramanian, V.; Hadidi, A.; Singer, B.; Fraenkel-Conrat, H. Possible identification of a protein in brome mosaic virus infected barley as a component of viral RNA polymerase. Virology 1973, 54, 190–198. [Google Scholar] [CrossRef]
- Kong, F.; Sivakumaran, K.; Kao, C. The N-Terminal Half of the Brome Mosaic Virus 1a Protein Has RNA Capping-Associated Activities: Specificity for GTP and S-Adenosylmethionine. Virology 1999, 259, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.; Hayashida, H.; Miyata, T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. Nature 1983, 305, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Stenger, D.C.; Revington, G.N.; Stevenson, M.C.; Bisaro, D.M. Replicational release of geminivirus genomes from tandemly repeated copies: evidence for rolling-circle replication of a plant viral DNA. Proc. Natl. Acad. Sci. 1991, 88, 8029–8033. [Google Scholar] [CrossRef] [PubMed]
- Laufs, J.; Traut, W.; Heyraud, F.; Matzeit, V.; Rogers, S.G.; Schell, J.; Gronenborn, B. In vitro cleavage and joining at the viral origin of replication by the replication initiator protein of tomato yellow leaf curl virus. Proc. Natl. Acad. Sci. 1995, 92, 3879–3883. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Aoyagi, M.; Yamanashi, Y.; Saito, H.; Ikawa, S.; Meshi, T.; Okada, Y. Nucleotide Sequence of the Tobacco Mosaic Virus (Tomato Strain) Genome and Comparison with the Common Strain Genome1. J. Biochem. 1984, 96, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Plaskitt, K.; Wilson, T. Evidence that tobacco mosaic virus particles disassemble contranslationally in vivo. Virology 1986, 148, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Ahlquist, P.; French, R.; Janda, M.; Loesch-Fries, L.S. Multicomponent RNA plant virus infection derived from cloned viral cDNA. Proc. Natl. Acad. Sci. 1984, 81, 7066–7070. [Google Scholar] [CrossRef]
- Dawson, W.O.; Beck, D.L.; Knorr, D.A.; Grantham, G.L. cDNA cloning of the complete genome of tobacco mosaic virus and production of infectious transcripts. Proc. Natl. Acad. Sci. 1986, 83, 1832–1836. [Google Scholar] [CrossRef] [PubMed]
- Kamer, G.; Argos, P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984, 12, 7269–7282. [Google Scholar] [CrossRef]
- Takamatsu, N.; Ishikawa, M.; Meshi, T.; Okada, Y. Expression of bacterial chloramphenicol acetyltransferase gene in tobacco plants mediated by TMV-RNA. EMBO J. 1987, 6, 307–311. [Google Scholar] [CrossRef]
- Deom, C.M.; Oliver, M.J.; Beachy, R.N. The 30-Kilodalton Gene Product of Tobacco Mosaic Virus Potentiates Virus Movement. Science 1987, 237, 389–394. [Google Scholar] [CrossRef]
- Saito, H.; Tomioka, H.; Sato, K.; Tasaka, H.; Tsukamura, M.; Kuze, F.; Asano, K. Identification and partial characterization of Mycobacterium avium and Mycobacterium intracellulare by using DNA probes. J. Clin. Microbiol. 1989, 27, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Abel, P.P.; Nelson, R.S.; De, B.; Hoffmann, N.; Rogers, S.G.; Fraley, R.T.; Beachy, R.N. Delay of Disease Development in Transgenic Plants That Express the Tobacco Mosaic Virus Coat Protein Gene. Science 1986, 232, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K. Effects of Cotton Leaf Crumple Virus on Cotton Inoculated at Different Growth Stages. Plant Dis. 1987, 71. [Google Scholar] [CrossRef]
- Gonsalves, D. CONTROL OF PAPAYA RINGSPOT VIRUS IN PAPAYA: A Case Study. Annu. Rev. Phytopathol. 1998, 36, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Pruss, G.; Ge, X.; Shi, X.M.; Carrington, J.C.; Vance, V.B. Plant viral synergism: the potyviral genome encodes a broad-range pathogenicity enhancer that transactivates replication of heterologous viruses. Plant Cell 1997, 9, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Turpen, T.H.; Reinl, S.J.; Charoenvit, Y.; Hoffman, S.L.; Fallarme, V.; Grill, L.K. Malaria Epitopes Expressed on the surface of Recombinant Tobacco Mosaic Virus. Nat. Biotechnol. 1995, 13, 53–57. [Google Scholar] [CrossRef]
- Clark, F.; Bar-Joseph, M. Enzyme Immunosorbent assays in plant virology, “ Methods in Virology, Academic Press, Newyork, NY, ISBN 0124702082, pp. 51 – 85, 1984. [CrossRef]
- De Boer, S.H.; López, M.M. New Grower-Friendly Methods for Plant Pathogen Monitoring. Annu. Rev. Phytopathol. 2012, 50, 197–218. [Google Scholar] [CrossRef]
- Tsuda, S. A Novel Detection and Identification Technique for Plant Viruses: Rapid Immunofilter Paper Assay (RIPA). Plant Dis. 1992, 76. [Google Scholar] [CrossRef]
- Mullis, K.; Faloona, F.; Scharf, S.; Saiki, R.; Horn, G.; Erlich, H. Specific enzymatic amplification of DNA in vitro: The polymerase chain reaction. Cold Spring Harb. Symp. Quant. Biol. 1986, 51, 263–273. [Google Scholar] [CrossRef]
- Saponari, M.; Loconsole, G.; Liao, H.-H.; Jiang, B.; Savino, V.; Yokomi, R.K. Validation of high-throughput real time polymerase chain reaction assays for simultaneous detection of invasive citrus pathogens. J. Virol. Methods 2013, 193, 478–486. [Google Scholar] [CrossRef]
- Fukuta, S.; Nimi, Y.; Ohishi, K.; Yoshimura, Y.; Anai, N.; Hotta, M.; Fukaya, M.; Kato, T.; Oya, T.; Kanbe, M. Development of reverse transcription loop-mediated isothermal amplification (RT-LAMP) method for detection of two viruses and chrysanthemum stunt viroid. Annu. Rep. Kansai Plant Prot. Soc. 2005, 47, 31–36. [Google Scholar] [CrossRef]
- Haible, D.; Kober, S.; Jeske, H. Rolling circle amplification revolutionizes diagnosis and genomics of geminiviruses. J. Virol. Methods 2006, 135, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; A Armes, N. DNA Detection Using Recombination Proteins. PLOS Biol. 2006, 4, e204. [Google Scholar] [CrossRef] [PubMed]
- Glais, L.; Jacquot, E. Detection and Characterization of Viral Species/Subspecies Using Isothermal Recombinase Polymerase Amplification (RPA) Assays. In Plant Pathology; Humana Press, New York, NY, USA, 2015; Volume 1302, pp. [CrossRef]
- Hadidi, A.; Czosnek, H.; Barba, M. DNA microarrays and their potential applications for detection of plant viruses, viroids and phytoplasmas. J. Plant. Pathol 2004, 86, 97–104. [Google Scholar]
- Tiberini, A.; Barba, M. Optimization and improvement of oligonucleotide microarray-based detection of tomato viruses and pospiviroids. J. Virol. Methods 2012, 185, 43–51. [Google Scholar] [CrossRef]
- Boonham, N.; Kreuze, J.; Winter, S.; van der Vlugt, R.; Bergervoet, J.; Tomlinson, J.; Mumford, R. Methods in virus diagnostics: From ELISA to next generation sequencing. Virus Res. 2014, 186, 20–31. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).